Abstract
Objective
Obesity and joint injury are both primary risk factors for osteoarthritis (OA) that involve potential alterations in the biomechanical and inflammatory environments of the joint. Post-traumatic arthritis (PTA) is a frequent long-term complication of intra-articular fractures. Obesity has been linked to primary OA and may potentially contribute to the development of PTA by a variety of mechanisms. The objectives of this study were to determine if diet-induced obesity influences the severity of PTA in mice and to examine interrelationships between joint degeneration and serum levels of inflammatory cytokines and adipokines in this response.
Methods
C57BL/6 mice were fed either normal chow (13% fat) or a high-fat diet (60% fat) starting at 4 weeks of age. At 16 weeks, half of each group received closed intra-articular fracture of the left knee. At 8 weeks post-fracture, knee osteoarthritis was assessed by cartilage and synovium histology in addition to bone morphology. Serum cytokine concentrations were determined with multiplex assay.
Results
Fractured knee joints of mice on a high-fat diet showed significantly increased osteoarthritic degeneration compared to non-fractured contralateral controls, while fractured knee joints of low-fat mice did not demonstrate significant differences from non-fractured contralateral controls. High-fat diet increased serum concentrations of interleukin-12p70, interleukin-6, and keratinocyte-derived chemokine, while decreasing adiponectin concentrations. Systemic levels of adiponectin were inversely correlated with synovial inflammation in control limbs.
Conclusion
Diet-induced obesity significantly increased the severity of osteoarthritis following intra-articular fracture. Obesity and joint injury together can alter systemic levels of inflammatory cytokines such as IL-12p70.
Keywords: cartilage, adipokine, obesity, inflammation, biomarker, trauma, post-traumatic arthritis, osteoarthritis
Osteoarthritis (OA) is a painful and debilitating disease of synovial joints characterized by degeneration and inflammation of the articular cartilage and other joint tissues. Post-traumatic arthritis (PTA) is the broad term given to OA-like changes that arise following joint trauma. Even with optimal treatment, it is estimated that more than 40% of patients experiencing joint trauma will go on to develop PTA (1), ultimately accounting for 12% of the 26.9 million Americans who suffer from OA (2, 3). PTA poses a challenging and unique clinical problem, with the factors of direct chondrocyte injury, altered biomechanical loading due to articular step-offs, joint instability from ligamentous injury, and resulting acute and chronic inflammation all likely playing a role in its development (4, 5). Additionally, because of its well-defined inciting event, PTA represents an excellent opportunity to study pathophysiologic mechanisms and interventions that also may be broadly applicable to primary idiopathic OA (1).
Obesity is an increasingly common and modifiable condition characterized by increased body composition of adipose tissue. Adipose tissue is now recognized as playing a role in many diseases through secretion of adipose-derived cytokines (i.e., “adipokines”) and hormones resulting in a chronic state of low-grade systemic inflammation (6–8). Specifically, interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) are elevated in human studies of obesity (9–13) in addition to diet-induced mouse models of obesity (14, 15).
The association between obesity and OA has been long-recognized (16), but the mechanisms underlying this relationship are not well-understood. Obesity has been proposed to influence the development of OA primarily through increased joint loading due to increased body weight, but this does not explain the association between obesity and OA in non-weight-bearing joints such as the hand (17). Thus, there is growing support for additional mechanisms wherein the systemic effects of obesity may promote the progressive degradation of joint tissues (18, 19). Pro-inflammatory cytokines linked to obesity, such as IL-1, TNF-α, and IL-6, are likewise associated with the conditions of PTA and OA, and may serve as mediators of catabolic processes in chondrocytes that lead to extracellular matrix degradation (20). Thus, the influences of obesity on PTA may be multi-factorial, including, but not limited to, altered biomechanical loading, as well as local and systemic inflammation. These mechanisms remain untested, however, and the influence of obesity on PTA is largely unknown.
In this study, we hypothesized that diet-induced obesity would increase the severity of PTA. Using a mouse model of closed intra-articular fracture, we investigated the effects of diet and fracture on articular cartilage, joint synovitis, bone morphology, and serum cytokine levels to characterize degenerative changes and gain insight into the systemic, tissue-level, and cellular mechanisms leading to PTA.
Materials and Methods
Animals
All animal procedures were approved by the Duke University IACUC. C57BL/6 male mice (n=14; Charles River Laboratories, Wilmington, MA) were obtained and fed a high-fat (HF) diet (D12492, 60% kcal fat; Research Diets, New Brunswick, NJ) starting at 4 weeks of age. Low Fat (LF) control mice were C57BL/6 male mice (n=18; Charles River) fed a standard diet (5001, 13% kcal fat; PicoLab, Brentwood, MO) starting at 4 weeks of age. All mice were group-housed in filter-top cages with ad libitum access to water and chow. Animal weights were recorded weekly, and mice remained on their respective diets until the completion of the study.
Intra-articular Fracture Model
At 16–19 weeks of age, 7 HF mice and 9 LF mice were randomized to receive moderate articular fractures of the left tibial plateau using a custom apparatus, as previously described (21). This age was chosen since it represents the time at which active growth has decreased and peak bone mass is achieved in mice (22). Briefly, animals were anesthetized and placed in a custom cradle with their left hind limb in neutral position (90° flexion). A custom indenter composed of wedge-shaped stainless steel was attached to a materials testing system (ElectroForce ELF3200, Bose Corp., Minnetonka MN), which applied a 10N compressive pre-load to the anterior aspect of the proximal tibial plateau of the left hind limb. The tibia was then loaded at a rate of 20 N/s to induce fracture. In contrast to previous protocols that did not use a displacement limit on the indenter (21), or used a limit of 3.2 mm (23), a displacement limit of 2.7 mm was used to induce a “moderate” injury against which to test the effects of obesity. After fracture, mice were allowed free cage activity and no surgical interventions were performed in order to examine the natural sequelae of the fractures.
Body Fat Composition Measurements
The body fat content of the mice, excluding the head, was determined within 1 week prior to fracture and immediately prior to sacrifice using a DEXA scanner (GE Lunar PIXImus, GE Healthcare, Madison, WI).
Evaluation of OA severity
All mice were sacrificed 8 weeks after the fracture date. Hind limbs were harvested and fixed in 10% buffered formalin in 90 degrees of flexion (neutral limb alignment). Limbs then underwent standard histologic preparation, including decalcification, dehydration, and paraffin-embedding. The entire joint was sectioned in the coronal plane at a thickness of 8 µm. Sections were stained with hematoxylin, fast green, and Safranin-O. Three independent, blinded graders then assessed sections using a modified Mankin scoring scale. Scores were averaged for individual joint quadrants as well as for the whole joint. The modified Mankin score included the same scoring components described in previous studies with the exclusion of the hypertrophic chondrocyte measure, thus making 108 the maximum possible score per joint (21, 24–26). Internal consistency of the scale was eventually determined to be higher without the hypertrophic chondrocyte measure, as statistical testing showed an increase in the Cronbach’s alpha term (which measures degree of correlation among items) from 0.523 to 0.572 when the hypertrophic chondrocyte measure was excluded (27).
Evaluation of Bone Morphology
Prior to histologic preparation, hind limbs were scanned by a desktop micro-computed tomography system (microCT 40, Scanco Medical AG, Bassersdorf, Switzerland). A hydroxyapatite calibration phantom was used to calibrate bone density values (mg/cm3). Morphometric bone parameters were investigated in the distal femoral condyles, the tibial epiphysis immediately distal to subchondral bone, and the tibial metaphysis, as previously described (21). Parameters reported in the femoral condyles were bone mineral density (BMD; mg/cm3) and cancellous bone fraction (bone volume/total volume, excluding the cortex), while BMD and bone volume (mm3) were reported in the tibial epiphysis and metaphysis.
Evaluation of Synovitis
Histologic sections containing the synovial attachments to bone were stained with hematoxylin and eosin. Three blinded, independent graders assessed sections for synovial lining cell thickness (0–3) and synovial stroma density (0–3) in each joint quadrant using a previously described scoring scale, and scores were averaged for individual joint quadrants as well as for the whole joint (maximum score 24 per joint) (23, 28).
Serum Adipokine and Cytokine Concentrations
Blood was collected from anesthetized mice prior to sacrifice via retro-orbital bleed and cardiac stick for analyses of systemic levels of cytokines. Collected blood was centrifuged at 2450 × g for 15 minutes, and the serum was stored at −80°C until analysis. Serum levels of interleukin (IL)-1β, IL-6, IL-10, IL-12p70, keratinocyte-derived chemokine (KC; which is a human IL-8 analog), interferon-gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) were assessed using a Mouse Pro-inflammatory 7-plex Multiplex ELISA (Meso Scale Discovery, Gaithersburg, MD), while serum levels of adiponectin and resistin were assessed with Quantikine Mouse Immunoassays (R&D Systems, Minneapolis, MN) as directed by the manufacturer.
Statistical Analysis
Body weight (g), body fat (g) and body fat (%) were compared using a multifactorial ANOVA testing for effects of diet, fracture cohort, and diet*fracture. Mankin and synovitis scores from each quadrant and from the total joint required non-parametric testing, as they were not normally distributed. A Wilcoxon signed-rank test was used to compare differences between R (right; control) and L (left; experimental) limbs within each experimental group, and a Kruskal-Wallis ANOVA was used to independently compare L limbs and R limbs among the experimental groups.
MicroCT measurements were analyzed among the four experimental groups by a Repeated Measures ANOVA. R & L limbs were compared as repeated measures with diet (LF or HF) and fracture cohort (non-fx or fx) as dependent variables. Significant effects of diet, fracture, and diet*fracture were noted with a Tukey HSD post-hoc test.
To investigate the effects of fracture and diet, serum cytokine levels were compared among the four experimental groups using a Kruskal-Wallis ANOVA, while diet and fracture groupings were separately assessed using Wilcoxon rank-sum tests. Correlations of cytokines to outcome measures were then investigated using a Spearman Ranked Order Correlation test. Significance was reported at the 95% confidence level (p < 0.05). Analyses were conducted using Statistica (StatSoft Inc., Tulsa, OK).
Results
Animals
Two mice died due to unrelated medical complications throughout the course of the study: one from the HF non-fractured group, and one from the LF fractured group. One mouse from the HF fractured group died due to complications of anesthesia shortly after fracture.
Effects of diet on weight and body composition
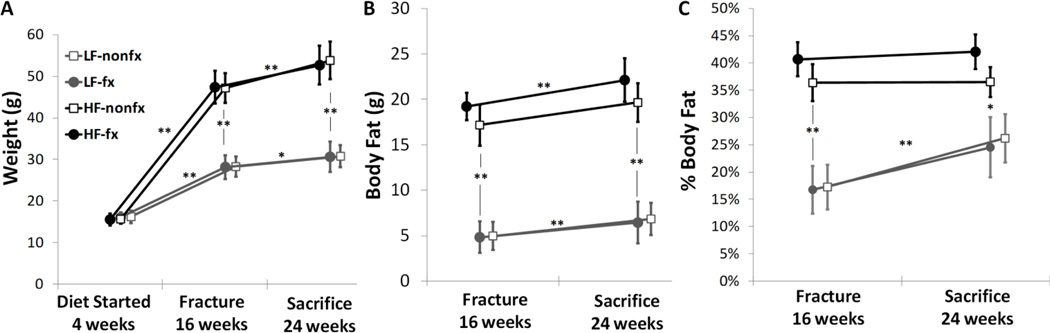
At 16 and 24 weeks of age, the mice fed a high-fat (HF) diet had significantly higher weight than the mice fed a low-fat (LF) diet (p<0.001; Fig 1A). There were no statistically significant differences in weight between the fractured (fx) and non-fractured (nonfx) cohorts overall or within the diet groupings, both at the 16-week fracture time point as well as the 24-week endpoint (Fig 1A). The body fat mass and percentage for these mice demonstrated similar trends with significant differences between the HF and LF groups at 16 and 24 weeks, again with no statistically significant differences seen as a result of fracture (Fig 1B, 1C).
Figure 1.
(A) Weight, (B) Body Fat mass, and (C) Body Fat percentage of each experimental group over time. Mice on a high-fat diet gained more weight and body fat over the course of the experiment. There were no differences within the diet groups based on fracture condition. (LF=low-fat diet, HF=high-fat diet, nonfx=non-fractured, fx=fractured. LF-nonfx: n=9, LF-fx: n=8, HF-nonfx: n=6, HF-fx: n=6. Mean ± SD, **p<0.001, *p<0.05)
Creation and characteristics of joint fracture
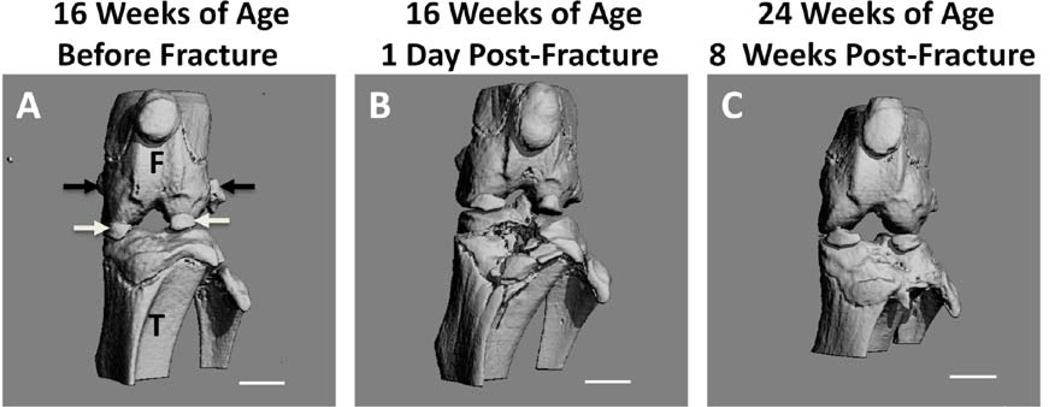
The L hind limbs of mice randomized to the fracture groups were successfully fractured in 16/16 animals. A 3-dimensional reconstructed microCT image from the mouse that expired under anesthesia demonstrates a representative anterolateral fracture of the tibial plateau, which appears to reach intra-articularly (Fig 2B). An additional representative microCT image of a fractured joint after 8 weeks of healing reveals only moderate joint deformity, as intended, despite receiving no surgical reduction or fixation (Fig 2C).
Figure 2.
Representative micro-computed tomography (microCT) 3-D reconstructions of mouse limbs: (A) left limb prior to fracture at 16 weeks. F=femur, T=tibia, white arrows indicate partially calcified menisci, black arrows indicate accessory ossicles (B) 1 day after fracture event, and (C) 8 weeks after fracture event. (Scale bar = 1mm)
Histological evaluation of tibiofemoral articular cartilage
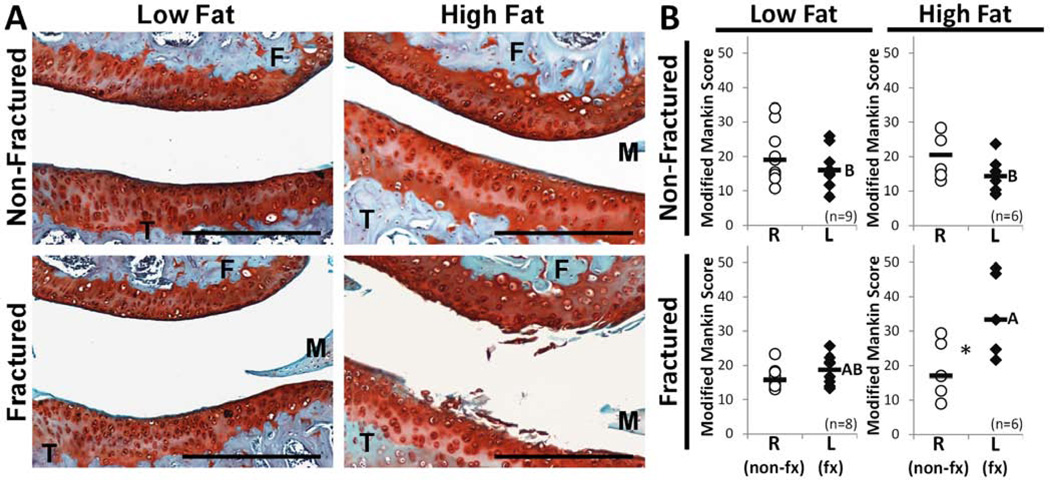
Histologic assessment of the lateral cartilage contact regions demonstrated severe articular cartilage fibrillation and loss of Safranin-O staining in L limbs of the HF-fx group (Fig 3A). L joints exhibited markedly worse cartilage degeneration by modified Mankin score than R joints in the HF-fx group (p=0.0464) but not in any other groups (Fig 3B). These L limbs from the HF-fx group demonstrated worse OA compared to the L limbs of LF-nonfx and HF-nonfx groups (p=0.0245 and p=0.0068, respectively). This effect was strongest in the lateral tibia region, with the HF-fx group exhibiting more severe arthritis than the HF-nonfx cohort (p = 0.0191; regional data not shown). There were no differences of R limbs among experimental groups. Even though there was no global joint effect among control R limbs (Fig 3B), a regional effect in the medial tibia showed increased OA in the HF-nonfx group compared to the LF-fx group (p=0.0188).
Figure 3.
Assessment of osteoarthritis. (A) Representative cartilage histology among experimental groups demonstrating the lateral tibiofemoral contact region in left (experimental) hind limbs. (F=femur, M=lateral meniscus, T=tibia; Scale bar = 100 µm) (B) Mankin scores representative of OA severity. Among L (experimental) limbs, scores were significantly higher in the HF-fractured group compared to all non-fractured groups, whereas the LF-fractured group did not statistically differ from other groups (A,B designation; groups not sharing a common letter are statistically different). There were no differences in OA score observed among the R (control) limbs. The L limb showed significantly worse OA than the R (control) limb in the HF-fractured group only (*p=0.046), whereas there was no effect of fracture seen in the LF-fractured group. (Bars represent median values)
Morphologic assessment of adjacent bone
Cancellous bone fraction in the femur decreased with fracture, with the L limbs from the fractured cohorts showing significantly less BV/TV than the R limbs in those cohorts (p=0.012; Table 1). This effect seems to be driven primarily by the HF diet, as there were significant differences between R and L limbs within the HF-fx group (p=0.001), but not the LF-fx group (p=1.00; Table 1). There was no overall effect of diet on cancellous bone fraction in the femoral condyles (p=0.166). Nonetheless, the HF diet did increase bone volume in both the tibial epiphysis and metaphysis compared to the LF diet, independent of fracture condition (p=0.001 and p=0.006, respectively; Table 1). Bone mineral density (BMD) did not demonstrate significant trends of diet or fracture.
Table 1.
Bone morphology measures of each experimental group at 24 weeks
| Location and Measurement | Low-fat diet | High-fat diet | ANOVA (p-value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LF-nonfx (n=9) |
LF-fx (n=8) |
HF-nonfx (n=6) |
HF-fx (n=6) |
Diet | Fracture | Diet* Fracture |
||||||
| R | L | R (non-fx) | L (fx) | R | L | R (non-fx) | L (fx) | |||||
| Femoral Condyles | ||||||||||||
| Cancellous Bone Fraction (BV/TV) | 0.61 (0.05) |
0.61 (0.07) |
0.58 (0.04) |
0.58 (0.04) |
0.69 (0.09) |
0.69 (0.09) |
0.67 (0.08) | 0.50 (0.20) |
0.166 | 0.012# | 0.016 ǂ | |
| Bone Mineral Density (mg/cm3) | 1106 (17) | 1098 (18) | 1116 (10) | 1093 (15) | 1116 (50) |
1083 (41) |
1085 (48) | 1103 (42) |
0.467 | 0.252 | 0.040 | |
| Tibial Epiphysis | ||||||||||||
| Bone Volume | 0.53 (0.04) |
0.52 (0.04) |
0.51 (0.02) |
0.57 (0.05) |
0.63 (0.09) |
0.62 (0.06) |
0.61 (0.07) | 0.58 (0.09) |
0.001 | 0.512 | 0.275 | |
| Bone Mineral Density (mg/cm3) | 1107 (13) | 1102 (14) | 1108 (13) | 1114 (15) | 1124 (62) |
1121 (58) |
1121 (39) |
1080 (70) |
0.781 | 0.261 | 0.040 | |
| Tibial Metaphysis | ||||||||||||
| Bone Volume | 0.65 (0.09) |
0.58 (0.10) |
0.62 (0.12) |
0.67 (0.13) |
0.91 (0.30) |
0.67 (0.21) |
0.70 (0.38) | 0.92 (0.17) |
0.006 | 0.005 | 0.081 | |
| Bone Mineral Density (mg/cm3) | 1008 (18) | 1033 (33) | 1026 (45) | 1032 (21) | 1003 (41) | 1052 (47) | 1040 (35) | 997 (47) | 0.873 | 0.003 | 0.035 | |
p-values in bold considered significant; overall effects p-values in italics were under the 0.05 threshold for significance, yet yielded no significant individual effects
Tukey HSD post-hoc testing: ǂ HF*fx*L < HF*fx*R (p = 0.001);
L*fx < R*fx (p = 0.012)
Histologic evaluation of knee joint synovitis
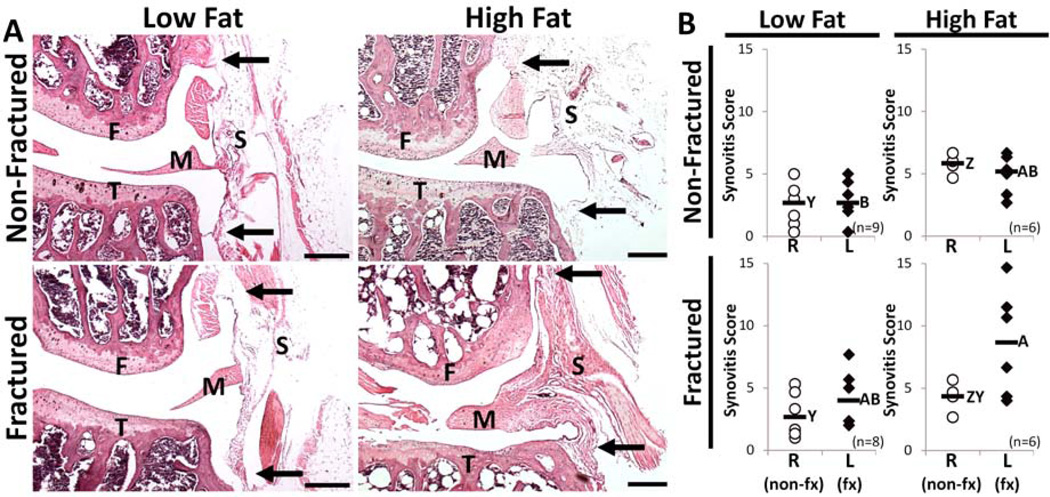
Histologic assessment of the lateral synovium demonstrated increased stromal density and lining-layer thickness in L limbs of the HF-fx group (Fig 4A). No difference in synovitis score for the whole joint was detected between R and L limbs within any of the experimental groups (Fig 4B). Regionally, however, synovitis near the lateral femur was significantly higher in L limbs compared to R limbs within the HF-fx group (p=0.0464; not shown).
Figure 4.
Assessment of synovitis. (A) Representative synovial histology from the lateral side of the left (experimental) hind limb. Synovitis scoring was based on degree of cell thickness in the synovial lining layer near the bone attachments (approximately marked by black arrows) and the cell density of the synovial stroma (approximately marked by letter “S”). F=femur, T=tibia, M=lateral meniscus, Scale bar = 100 µm) (B) Total Joint synovitis scores are representative of the degree of chronic synovial inflammation. Among L (experimental) limbs, scores were significantly higher in the HF-fractured group compared to the LF non-fractured group (A,B designation; groups not sharing a common letter are statistically different). Among R (control) limbs, the HF non-fractured had higher synovial inflammation than both LF groups (Z,Y designation; groups not sharing a common letter are statistically different). There were no differences observed between R and L limbs within experimental groups, including cohorts that received L limb fracture. (Bars represent median values)
Compared to the LF-nonfx group that represents baseline synovial pathology, neither fracture (LF-non vs. LF-fx) nor high-fat diet (LF-nonfx vs. HF-nonfx) led to significantly more synovial inflammation in L limbs. However, the combination of fracture with high-fat diet did induce worse synovial inflammation, as the HF-fx group had significantly higher L limb synovitis scores than the LF-nonfx group (p=0.02; Fig 4B). Individual quadrant analysis revealed the aforementioned effects between experimental groups to be localized primarily to the femoral synovial attachments, both medially and laterally (p=0.0145 and p=0.0079, respectively), where the increased synovial tissue makes the inflammation more pronounced.
Among R limbs, the HF-nonfx group had higher whole-joint synovitis scores than both the LF-nonfx (p=0.0026; Fig 4B) and LF-fx (p=0.0228) groups. Quadrant analysis showed these effects to be driven by the medial tibia, where both HF-fx and HF-nonfx groups have increased synovitis when compared to the LF-nonfx group (p=0.0145 and p=0.0059, respectively).
Serum Adipokine and Cytokine Concentrations
IL-6, KC, and IL-12p70 were all elevated in the HF diet groups compared to LF diet groups, while adiponectin was decreased in HF diet compared to LF diet (Table 2). Serum cytokine levels were not significantly different among the fracture groups regardless of diet. However, in considering the effect of diet and fracture combined, IL-12p70 was significantly higher in the serum of HF-fx mice compared to LF-nonfx (p=0.048; Table 2).
Table 2.
Serum cytokine/adipokine concentrations of each experimental group at 24 weeks
| Low-fat diet | High-fat diet | Kruskal-Wallis ANOVA (p value) | |||||
|---|---|---|---|---|---|---|---|
| LF-nonfx (n=9) |
LF-fx (n=8) |
HF-nonfx (n=6) |
HF-fx (n=6) |
Diet | Fracture | Diet+Fracture | |
| Interleukin-1β (pg/mL) | 3.01 (1.02) | 1.97 (0.855) | 3.84 (2.62) | 2.99 (1.18) | 0.214 | 0.116 | 0.198 |
| Interleukin-6 (pg/mL) | 13.4 (7.83) | 10.2 (115) | 56.4 (218) | 51.2 (93.6) | 0.013 | 0.793 | 0.090 |
| KC (pg/mL) | 105 (24.0) | 71.4 (10.2) | 112 (342) | 107 (26.6) | 0.030 | 0.206 | 0.054 |
| Interleukin-10 (pg/mL) | 31.9 (12.5) | 30.7 (364) | 61.2 (112) | 86.2 (244) | 0.101 | 0.513 | 0.377 |
| Interleukin-12p70 (pg/mL) | 20.4 (7.02) | 36.1 (611) | 92.8 (125) | 194 (552)* | 0.009 | 0.219 | 0.041 |
| Interferon-γ (pg/mL) | 1.52 (2.36) | 1.54 (12.8) | 1.72 (2.77) | 5.57 (10.4) | 0.707 | 0.513 | 0.783 |
| Adiponectin (ng/mL) | 7474 (696) | 7238 (1472) | 5865 (775) | 6076 (450) | 0.00002 | 0.845 | 0.177 |
LF=low-fat, HF=high-fat, nonfx=non-fractured, fx=fractured, KC = “Keratinocyte-derived Chemokine“). Values represent Median (25%–75% Interquartile Range). Multiple Comparisons post-hoc testing for Diet+Fracture effect:
p = 0.048 vs LF-nonfx.
Body Fat (g) at the time of fracture was positively correlated with serum levels of IL-6 (r=0.51) and IL-12p70 (r=0.60), while negatively correlated with serum adiponectin (r=−0.63). No cytokine was associated with total joint Mankin score or total joint synovitis score in L limbs, although adiponectin was negatively correlated with synovitis score in the R limb (r = −0.51). There were no significant effects of IL-1β, IL-10, or IFN-γ found in this study. Mean TNF-α and resistin serum concentrations were below the lower limit of the detectable ranges provided by the manufacturer and were excluded from analysis.
Discussion
In recent years, it has become apparent that obesity is one of the greatest risk factors for a variety of musculoskeletal diseases (29), particularly OA (16). In the present study, mice on a high-fat diet subjected to an intra-articular fracture showed significantly more osteoarthritic degeneration, synovial inflammation, and adaptive bone changes in the experimental fractured limb that were not present in low-fat diet mice subjected to an articular fracture. The findings of this study suggest that high-fat diet-induced obesity is a risk factor that increases PTA severity after intra-articular fracture.
In addition to noting that the HF-fx group had the most pronounced differences between Mankin scores of R (control) and L (experimental) limbs, the L limbs in the HF-fx group showed higher Mankin scores relative to L limbs in the non-fractured comparison groups. However, fracture in mice on a LF control diet did not induce significant differences in Mankin scores between fractured and non-fractured limbs. We attribute this finding to the more moderate displacement limit placed on the indenter for this study compared to our previous studies (21, 26). This moderate displacement limit was selected to prevent more severe fractures so that the potential interaction between diet and fracture could be investigated. We have previously shown a significant correlation between acute joint pathology and increased fracture severity when a displacement limit is not used in this intra-articular fracture model and hypothesized that severe fractures would have had the potential to mask the effects of diet-induced obesity on the joint (23).
When Mankin scores of the L limbs were analyzed by joint region, the lateral tibia was the only region where any effect of fracture could be detected, and here it was again limited to the high-fat diet cohorts. This regional effect is likely due to the fracture location, as microCT reconstructions of the joints and the associated histology show that the fractures most commonly occurred in the lateral plateau of the tibia. A prior study utilizing this model likewise demonstrated more severe changes in the lateral tibia where the fracture occurred (26). In comparing R (control) limbs, although there was no overall effect when analyzing the whole joint, the HF-nonfx group had a higher Mankin score in the medial tibia than the LF-fx group. One proposed mechanism for this localized effect occurring in the media tibia would be a change in activity or gait in the injured (fractured) groups, which may lead to altered loading of both limbs in injured animals. Others have suggested that mechanical load in the knee joint is distributed more medially during gait in mice (30). Future studies may include gait and activity monitoring to further investigate the role of diet and injury in experimental and contralateral limbs.
This study did not show any differences in Mankin score based on diet alone. This is in contrast to a previous C57BL/6 mouse study demonstrating worsening knee OA in mice on a 60% kcal fat diet (31). One important difference in the present study was the fact that the diet was started at a very early age (4 weeks) in the present study, which may potentially lead to adaptation or compensation of the diet-induced effects. Despite not detecting an independent effect of diet, it should be noted that the finding of increased OA with fracture was dependent on the presence of a high-fat diet.
MicroCT analysis revealed post-traumatic changes in bone morphology that were accentuated by a high-fat diet. Cancellous bone fraction of the femoral condyles decreased after fracture of the tibial plateau in the HF group, but not the LF (control diet) group. Previous studies showed a similar decrease in cancellous bone fraction after fracture (21, 26), demonstrating that this is an expected post-traumatic change and that obesity exaggerates this response to fracture. Decreased bone fraction may be a degenerative response related to the development of PTA, or may be due to altered loading or activity following fracture, as has been shown with immobilization (32). MicroCT analysis also confirmed diet-induced obesity’s effect on bone volume (BV), demonstrating increased volume as has been previously observed (33).
Synovial inflammation is frequently observed in human OA (34) and has also been linked to increased severity of trauma in this mouse model (23). The trends for synovial inflammation in this study paralleled those for cartilage degeneration and bone morphology—demonstrating worse disease when a high-fat diet and fracture were combined in L limbs. The R limb synovitis results also closely resembled those seen in the Mankin scoring with the HF-nonfx group having the worst joint synovitis, being statistically increased over both LF groups with a medial tibia regional effect. Again, we see that the R limbs within fractured cohorts may be somewhat protected from joint pathology—possibly from altered loading and activity levels. Additionally, the significance of the comparison between HF-nonfx and LF-nonfx shows that high-fat diet had an overt effect on synovitis scores independent of fracture.
This finding led us to consider the mechanism of obesity-driven synovitis in non-fractured limbs. One possible explanation is that adipose tissue in the high-fat mice secretes systemic pro-inflammatory cytokines that directly affect the synovial tissues (7, 19, 35). Additionally, it is possible that the synovium may be aggravated by local factors originating from the cartilage (36) as a result of the local joint pathology resulting from obesity. Considering that we detected a significant effect of obesity on synovitis, but did not observe such differences with diet in the other measures of joint pathology such as cartilage degeneration or bone morphology, it follows that this increase in synovial inflammation in non-injured limbs is a result of systemic factors related to obesity, not local pathology.
Obesity is associated with a chronic state of low-grade systemic inflammation (6). Systemic increases in pro-inflammatory cytokines in obesity have been attributed to macrophage infiltration into adipose tissue (37, 38). IL-6, which has both pro-inflammatory and anti-inflammatory properties within the joint (20), has been positively correlated with obesity in numerous studies in both humans (12) and mice (14). Mouse KC (human IL-8 analog), which is a known chemoattractant for neutrophils (20), has also been positively correlated with obesity in mice and humans (15, 39, 40). These observations are consistent with our findings of elevated serum levels of IL-6 and KC in mice fed a HF diet. Although previous studies demonstrated elevated levels of IL-6 and KC in the serum of patients with OA (41, 42), serum levels of IL-6 and KC did not correlate with measurements of post-traumatic arthritis in this model.
IL-12p70 (the active form of IL-12) is produced by activated immune cells and is considered a major factor in promoting a Th1 (pro-inflammatory) cytokine profile as opposed to a Th2 (anti-inflammatory) profile (43). Our results indicate that systemic levels of IL-12p70 were elevated with a high-fat diet, much like IL-6 and KC. Furthermore, IL-12p70 was the only systemic cytokine associated with fracture, as serum levels were increased over baseline when high-fat diet and fracture were combined. This response of IL-12p70 to obesity is supported by a recent study that indicates increased numbers of IL-12p70-expressing macrophages in the adipose tissue of C57BL/6 mice on a 60% fat diet compared to mice on a low fat diet (43). Therefore, IL-12p70 may be one such mediator in a mechanism whereby excess adipose tissue primes the body for a more severe inflammatory reaction in response to injury, elevating the risk of eventual PTA.
Adiponectin has been negatively correlated with BMI and body fat in humans (44) and may play a role in the modulation of cartilage destruction in OA (45). In this study, we similarly found that adiponectin negatively correlated with body fat, while also being negatively correlated to the degree of synovitis in R limbs. This trend may have emerged only in the R limbs because it is here that the systemic effects of circulating factors are not confounded by the presence of injury. Lower levels of adiponectin, which were present in obese mice, are therefore coincident with higher levels of synovial inflammation in this study. This therapeutic role of adiponectin is supported by a study showing that an intra-articular injection of adiponectin mitigated the severity of OA in a collagen-induced arthritis mouse model (46).
The interaction between obesity and joint injury could be mediated by other systemic mediators related to high fat diet and obesity, such as leptin. Diet-induced obesity in mice is associated with increased systemic levels of leptin, which has been proposed to serve as a pro-inflammatory adipokine, particularly in the context of osteoarthritis (47). In previous studies, we have shown that diet-induced obesity increases leptin levels in a manner that correlates with joint degeneration (48) and leptin-deficient mice exhibit morbid obesity but no joint degeneration (39).
In interpreting the contribution of inflammatory cytokines to the development of post-traumatic arthritis in combination with a high fat diet, it’s important to note that our measures of cytokine levels were determined systemically from serum collected eight weeks after joint injury, when the acute inflammatory phase had likely resolved. Also, we did not assess local intra-articular levels of cytokines in this study. Therefore, many of these cytokines that did not significantly correlate to arthritic changes in this study may still exert degenerative effects. Despite these limitations, our results confirm the relationship between obesity and systemic inflammatory changes, in addition to showing a systemic response to fracture. Most importantly, they demonstrate a relationship between synovial inflammation of the contralateral joint and systemic levels of an adipokine (adiponectin) that persists 8 weeks after fracture. This conclusion supports the theoretical effect that obesity can have on joint pathology through systemic pro-inflammatory mediators.
These findings may not be limited to PTA after intra-articular fracture, and may indeed be broadly generalizable to OA as a whole, as injury provides an inciting event for study (1). As such, a relationship of inflammatory cytokines to the development of joint pathology may indicate future treatments of PTA or OA using anti-cytokine therapy, once more comprehensive studies are completed that elucidate the molecular environment within the joint following acute injury. This is not to say that biomechanical effects of obesity do not have a role in the development of OA after intra-articular fracture, as this is a well-characterized component of obesity’s relationship to OA (29). However, a biomechanical contribution to OA was not the focus of this investigation, and the effect of obesity on joint loading was not measured. In future studies, assessments of gait, joint stress analysis, and activity may provide additional insight into the effects of trauma or obesity on the joint biomechanics (49, 50).
In summary, this study identifies diet-induced obesity as a significant risk factor for increasing the severity of PTA. Increased cartilage degradation, decreased cancellous bone fraction, and increased synovial inflammation of the fractured limb indicate “whole joint” PTA, which occurred in diet-induced obese mice but not in control diet mice. Serum analysis reveals that obesity and trauma can influence systemic cytokine levels, and that some cytokines are related to the degree of joint pathology. Further investigation into the mechanisms governing the development of PTA in obesity may provide new insights into the pathogenesis, prevention, and treatment of this debilitating disease.
Acknowledgments
The authors thank Brian Diekman, Daniel Mangiapani, and Chia-Lung Wu for their assistance in histological grading, and Karsyn Bailey for assistance in generating and compiling data from microCT scans. We also thank Dr. Tim Griffin for advice and insights in the initial aspects of this study. This study was supported in part by the Arthritis Foundation and NIH grants AR50245, AG15768, AR48852, and AR48182.
References
- 1.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20:719–725. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 5.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 6.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 7.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 9.Bunout D, Munoz C, Lopez M, de la Maza MP, Schlesinger L, Hirsch S, et al. Interleukin 1 and tumor necrosis factor in obese alcoholics compared with normal-weight patients. Am J Clin Nutr. 1996;63:373–376. doi: 10.1093/ajcn/63.3.373. [DOI] [PubMed] [Google Scholar]
- 10.Visser M. Higher levels of inflammation in obese children. Nutrition. 2001;17:480–481. doi: 10.1016/s0899-9007(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 11.Aygun AD, Gungor S, Ustundag B, Gurgoze MK, Sen Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005;2005:180–183. doi: 10.1155/MI.2005.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 13.Straczkowski M, Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87:4602–4606. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Leeman SE, Amar S. Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proc Natl Acad Sci U S A. 2011;108:2867–2872. doi: 10.1073/pnas.1019270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neels JG, Badeanlou L, Hester KD, Samad F. Keratinocyte-derived chemokine in obesity: expression, regulation, and role in adipose macrophage infiltration and glucose homeostasis. J Biol Chem. 2009;284:20692–20698. doi: 10.1074/jbc.M109.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Chaisson CE. Understanding the relationship between body weight and osteoarthritis. Baillieres Clin Rheumatol. 1997;11:671–681. doi: 10.1016/s0950-3579(97)80003-9. [DOI] [PubMed] [Google Scholar]
- 18.Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2010.123. [DOI] [PubMed] [Google Scholar]
- 19.Iannone F, Lapadula G. Obesity and inflammation--targets for OA therapy. Curr Drug Targets. 2010;11:586–598. doi: 10.2174/138945010791011857. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 21.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 22.Sheng MH, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KH, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25:421–429. doi: 10.1016/s8756-3282(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19:864–873. doi: 10.1016/j.joca.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. J Orthop Res. 2002;20:92–100. doi: 10.1016/S0736-0266(01)00066-3. [DOI] [PubMed] [Google Scholar]
- 25.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 26.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58:744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 27.Custers RJ, Creemers LB, Verbout AJ, van Rijen MH, Dhert WJ, Saris DB. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthritis Cartilage. 2007;15:1241–1248. doi: 10.1016/j.joca.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Krenn V, Morawietz L, Haupl T, Neidel J, Petersen I, Konig A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 29.Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) 2008;32:211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 30.Bendele AM. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;2:501–503. [PubMed] [Google Scholar]
- 31.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rantakokko J, Uusitalo H, Jamsa T, Tuukkanen J, Aro HT, Vuorio E. Expression profiles of mRNAs for osteoblast and osteoclast proteins as indicators of bone loss in mouse immobilization osteopenia model. J Bone Miner Res. 1999;14:1934–1942. doi: 10.1359/jbmr.1999.14.11.1934. [DOI] [PubMed] [Google Scholar]
- 33.Ma H, Turpeinen T, Silvennoinen M, Torvinen S, Rinnankoski-Tuikka R, Kainulainen H, et al. Effects of diet-induced obesity and voluntary wheel running on the microstructure of the murine distal femur. Nutr Metab (Lond) 2011;8:1. doi: 10.1186/1743-7075-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–882. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Myers SL, Brandt KD, Ehlich JW, Braunstein EM, Shelbourne KD, Heck DA, et al. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662–1669. [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 39.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 41.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–79. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 43.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 45.Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762:711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Lee SW, Kim JH, Park MC, Park YB, Lee SK. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand J Rheumatol. 2008;37:260–268. doi: 10.1080/03009740801910346. [DOI] [PubMed] [Google Scholar]
- 47.Otero M, Lago R, Gomez R, Lago F, Gomez-Reino JJ, Gualillo O. Leptin: a metabolic hormone that functions like a proinflammatory adipokine. Drug News Perspect. 2006;19:21–26. doi: 10.1358/dnp.2006.19.1.966243. [DOI] [PubMed] [Google Scholar]
- 48.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2011 doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costello KE, Guilak F, Setton LA, Griffin TM. Locomotor activity and gait in aged mice deficient for type IX collagen. J Appl Physiol. 2010;109:211–218. doi: 10.1152/japplphysiol.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45:387–398. [PMC free article] [PubMed] [Google Scholar]