Abstract
The Visual Word Form System (VWFS), located in the occipito-temporal cortex, is involved in orthographic processing of visually presented words (Cohen et al., 2002). Recent fMRI studies in children and adults have demonstrated a gradient of increasing word-selectivity along the posterior-to-anterior axis of this system (Vinckier et al., 2007), yet whether this pattern is modified by the increased reading experience afforded by age is still in question. In this study, we employed fMRI and an implicit word-processing task, and then used a region of interest analysis approach along the occipito-temporal cortex to test the prediction that the selectivity for words along the extent of the VWFS differs between older experienced and younger novice readers. Our results showed differences between children and adults during word processing in the anterior left occipito-temporal cortex, providing evidence of developmental refinement for word recognition along the VWFS.
Keywords: Neural development, ventral stream, occipito-temporal cortex, reading acquisition, fMRI, regions of interest
Introduction
Two decades of in vivo brain imaging studies of reading have been able to significantly advance our understanding of the functional neuroanatomy of the components of the reading process in typical and atypical readers (for reviews, see: Price et al., 2003; Pugh et al, 2001; Sandak et al., 2004; Schlaggar and McCandliss, 2007). Specifically, a dorsal-ventral schema has been derived for the left hemisphere under which the ventral region (occipito-temporal cortex) has been functionally assigned as the visual orthographic system, specialized for fast processing of visually presented word forms, and relying on familiarity of word representations (Petersen et al., 1990; Salmelin et al., 1996; Tarkiainen et al., 1999; Cohen et al., 2002; Dehaene et al., 2004; Maurer et al., 2006; Baker et al., 2007). The dorsal aspects on the other hand, host phonological and semantic processing in a distributed manner: temporo-parietal cortex subserves phonological manipulations (phonological awareness), phonological decoding (linking orthography to phonology), as well as semantic processing, while inferior frontal regions are involved in these same processes, together with articulation (Fiez and Petersen, 1998; Poldrack et al., 1999; Jobard et al., 2003; Mechelli et al., 2003).
How these regions change developmentally as a consequence of reading experience has been a topic of recent research interest. Though methodological and practical challenges exist in examining the developmental trajectory of neural changes that occur with reading acquisition, several studies have been able to shed some light on the issue through cross-sectional studies involving beginning readers and adults (see Schlaggar and McCandliss, 2007 for a review). Consistent with models of typical reading acquisition (Pugh et al., 2001), younger normal readers have been observed to rely primarily on left temporo-parietal (dorsal) regions during word processing, while left inferior frontal regions are not observed until readers reach adulthood (Booth et al., 2001; Schlaggar et al., 2002; Shaywitz et al., 2002, 2004; Gaillard et al., 2003; Turkeltaub et al., 2003; Brown et al., 2005). What is less clear is whether there is a developmental course in those ventral stream regions involved in orthographic processing, especially the so called “visual word form area” (VWFA – Cohen et al., 2000, 2002; McCandliss et al., 2003; Cohen and Dehaene, 2004).
In adult readers the VWFA consistently exhibits activation during word processing tasks, and has been shown to demonstrate selectivity for print over other types of stimuli such as checkerboards (Cohen et al., 2002), with an invariance for non-essential properties of the visual input such as case, font, size and location (Dehaene et al., 2001, 2004). It is considered to exhibit sensitivity to orthographic familiarity at the whole-word level (Kronbichler et al., 2004, 2007; Bruno et al., 2008) and would therefore seem to be a good candidate for showing modulation during the prolonged stages of reading acquisition. Specifically, the dorsal-ventral model of reading acquisition (Pugh et al., 2001) predicts that early readers rely primarily on the dorsal system during word processing (utilizing phonological assembly), with progressive development of the ventral system being hypothesized to occur with greater reading experience (providing direct lexical access through orthographic processing). However, studies by Schlaggar and colleagues (Schlaggar et al., 2002; Brown et al., 2005) which observed age-related increases in the left inferior frontal region during a task requiring subjects to provide a verbal response to a single presented word, concurrently observed greater activation for children than adults in the left extrastriate cortex, with these regions seemingly becoming less engaged in the more experienced readers. A study by Turkeltaub et al., (2003), utilizing an implicit word processing task (i.e. detection of a tall character within a visually presented real word or false-font), made a similar observation, reporting disengagement of right hemisphere ventral extrastriate regions in concurrence with developmental increases in the left inferior frontal region.
It has been suggested that developmental changes in the occipito-temporal region may not have been captured in this work by not studying populations that are young enough to demonstrate developmental changes here (Turkeltaub et al., 2003). Another explanation however, might be that these studies, focusing on the entire brain, and/or being bound to very specific anatomical locations, failed to capture more fine-grained developmental changes that occur within smaller regions of the ventral stream. A recent longitudinal study by Ben-Shachar and colleagues utilized a region-of-interest approach to examine developmental changes in visual word processing within the left occipito-temporal cortex (Ben-Shachar et al., 2011). Specifically, using an implicit reading task, they presented their pediatric participants with real words at different levels of visibility, demonstrating age-related changes in sensitivity to words in the left occipito-temporal sulcus, an area of cortex close to the classical VWFA. Based on the volume of activation observed in this region when contrasting visible vs. less/non- visible conditions, they noted that sensitivity in this area increases between the ages of 7 – 9 years, decreases after the age of 13 years, and stabilizes to adult levels at the age of 15 years. Thus, this study provided valuable information about developmental changes occurring around the vicinity of the VWFA. However, maturational cortical changes may extend beyond these specific patches of cortex, potentially occurring systematically across a larger portion of the occipito-temporal region. For example, a notable observation is the discovery that within the ventral stream there exists a posterior-to-anterior gradient of word-selectivity (i.e. greater differential activation in anterior regions between words and symbol strings), and a move towards a terminology that describes the “visual word form system” (VWFS). This gradient of word-selectivity has been demonstrated in adults (Vinckier et al., 2007) as well as in adolescents and children (Brem et al., 2006, 2009; van der Mark et al., 2010), primarily using region of interest (ROI) analysis. This approach has also been used to examine potential developmental differences. Brem et al., (2009) utilized a region of interest analysis within the ventral stream to examine whether any differences in the nature of the VWFS gradient of word-selectivity would be observed in children when compared to adolescents and adults in a Swiss-German speaking population. Their study employed a multimodal approach, using both event related potential (ERP) and functional magnetic resonance imaging (fMRI) to take advantage of these technologies' respective strengths (i.e. ERP being more sensitive to fast transient responses and fMRI being more sensitive to sustained responses). The ERP results from this study suggest the existence of a tuning of the VWFS in more experienced readers, with greater preference for word processing being narrowed to more anterior portions of the occipito-temporal cortex in adults relative to children. However, this developmental specialization of word-selective regions was not observed in the fMRI data, potentially due to sustained responses from top-down processes elicited by the task demands. Interestingly, this study reports a negative correlation between reading fluency and word-selective fMRI activity in posterior occipito-temporal regions, thereby at least suggesting less engagement of posterior regions in more experienced readers.
The present study revisits the question about the spatial pattern of brain activity in the left occipito-temporal cortex as a function of reading development. If progressive selectivity for words in the anterior ventral stream is demonstrated in children, yet differs from the adults based on strength or location of selectivity, it does not only provide informed theoretical models of how the adult pattern is established (Vinckier et al., 2007), but it also provides a normative baseline for children by which to understand developmental disorders such as dyslexia, where a gradient of print selectivity has been shown to be lacking (van der Mark et al., 2009). We employed the same implicit reading task reported in Turkeltaub et al., (2003) and took steps to optimize our study for the question at hand: data were acquired at a higher field strength (3T as opposed to 1.5T) and similar to the study of Brem et al., (2009), we used a region of interest (ROI) analysis (rather than whole brain analysis) which allowed us to focus on the nature of the gradient of word-selectivity in the VWFS, and to explore whether greater specialization might be observed in the anterior portions of this region in more mature readers. In addition to within- and between-group analyses, we also examined how activation within this region related to measures of reading ability.
2. Materials and Methods
2.1. Participants
All subjects were monolingual, native speakers of English. No individuals reported a previous diagnosis of developmental disability, severe language or psychiatric disorder. Twenty-six subjects (15 adults, 11 children) were included in final analysis after 17 (10 children) were excluded based on excessive head motion and poor in-scanner performance as described below. All subjects underwent a battery of behavioral tests measuring IQ, reading ability and skills known to support reading (Wagner and Torgesen, 1987) in order to ensure performance was in the normal range for both groups, and that children and adults had comparable performance levels. Tests included the Wechsler Abbreviated Scale of Intelligence (WASI) verbal and performance tests (Wechsler, 1999) and the Woodcock-Johnson Tests of Achievement (WJ-III) Basic Reading cluster to evaluate real and pseudoword reading (Woodcock, McGrew & Mather, 2001). The Lindamood-Bell Auditory Conceptualization test (LAC) was employed to measure phonemic awareness (Lindamood & Lindamood, 2004) and Rapid Automatized Naming (RAN) for naming fluency (Denckla & Rudel, 1976a,b; Denckla & Cutting, 1999). Group performance data on these measures as well as demographic information for the final group of subjects is listed in Table 1. Prior to the experiments, written informed consent was obtained from all participants, as well as from a legal guardian for the children. All experimental procedures were approved by the Georgetown University Institutional Review Board.
Table 1. Subject Demographics.
| Children | Adults | p-value | |
|---|---|---|---|
| N | 11 | 15 | - |
| Sex (Male/Female) | 4/7 | 9/6 | - |
| Age (years) | 10.2 ± 3.0 | 21.5 ± 2.5 | p < 0.0001 |
| Range (years) | 6.7–14.9 | 18.7–25.3 | - |
| Verbal IQ | 119 ± 20 | 120 ± 11 | n.s. |
| Performance IQ | 114 ± 16 | 118 ± 9 | n.s. |
| WJ-III Basic Reading | 116 ± 12 | 109 ± 8 | n.s. |
| LAC-3 | 115 ± 16 | 112 ± 5 | n.s. |
| RAN (Letters/Numbers) | 105 ± 20 | 109 ± 12 | n.s. |
| RAN (Colors/Objects) | 102 ± 22 | 103 ± 13 | n.s. |
Standard scores reported for behavioral measures; WJ-III: Woodcock Johnson III Tests of Achievement; LAC: Lindamood-Bell Auditory Conceptualization Test; RAN: Rapid Automatized Naming (L/N: Letter and Number naming; C/O: Color and Object naming); n.s.: non-significant. For the standardized tests, a score of 100 represents the average, with a standard deviation of 15 points.
2.2. fMRI Acquisition and Task
During fMRI data acquisition, subjects performed an implicit word processing task (Price et al., 1996; Turkeltaub et al., 2003; Turkeltaub et al., 2004) involving the detection of a tall character within a visually presented real word (RW). Subjects were instructed to press a button in their right hand if there was an ascending character in the presented stimulus (e.g. ‘l’ in ‘solve’) and a button in their left hand if there was no ascender (e.g. ‘cease’). Though the participants are not instructed to read the word during this task, reading occurs implicitly and without conscious effort in skilled readers (Price et al., 1996). Various studies involving children and adults have demonstrated activation for this type of task in reading-related brain regions, including those thought to be involved in orthographic, phonological and semantic processing (Price et al., 1996; Turkeltaub et al., 2003; Turkeltaub et al., 2004; Ben-Shachar et al., 2011). The ease of the implicit reading task renders it useful for studies involving children because it avoids confounds driven by differences in task performance. False-font (FF) strings were utilized as an active visual control stimulus and here, subjects again had to pay attention to the presence or absence of an ascender. The false-fonts were matched with the word stimuli in size, number of characters, and location of ascenders and descenders. All stimuli were displayed in black on a white background. A block-design paradigm in which alternating active condition periods (i.e. RW or FF) were interspersed with passive fixation periods (Fix) was utilized in each run (Figure 1). The intervening fixation period lasted for 18s during which a crosshair was displayed in the center of the screen. For the active conditions each task block lasted for 42s, with each run consisting of two blocks each of the RW and FF conditions. Each subject completed two runs, and both runs were included in the analysis for all subjects (10 stimuli per block, 40 total stimuli/56 images per task for each session). Stimulus presentation and recording of responses was controlled using the Presentation software (Neurobehavioral Systems Inc., Albany, CA, USA). To familiarize the subjects with the MRI environment (thus minimizing the potential for motion artifacts), pediatric participants underwent a training session in a mock scanner prior to the experiment.
Figure 1.
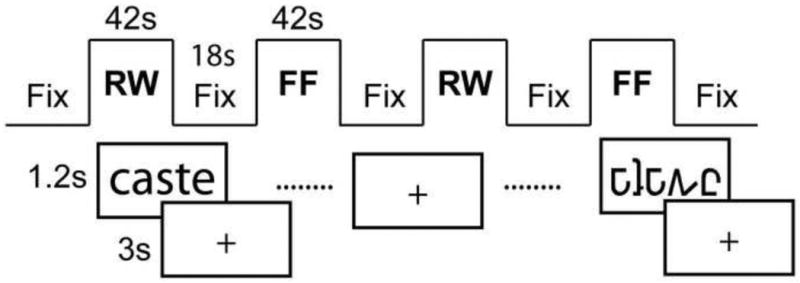
fMRI Implicit Reading feature detection task. Each run consisted of alternating epoch of real-word (RW) and false-font (FF) feature detection. Task epochs were interspersed with fixation periods (Fix) during which a cross-hair was displayed.
Data acquisition was performed on a 3T Siemens Trio scanner located in the Center for Functional and Molecular Imaging at the Georgetown University Medical Center, Washington, DC. For each functional run, 89 images consisting of 50 contiguous axial slices covering the whole brain were acquired with the following parameters: FOV = 192mm, Slice thickness = 2.8mm (0.2mm inter-slice gap), In-plane resolution = 64×64 (voxels size = 3mm isotropic), Flip Angle = 90°, TE = 30ms, TR = 3s.
2.3 Analysis
Pre-processing and analysis of functional datasets was performed using SPM8 (http://fil.ion.ucl.ac.uk/spm/). To prevent T1 saturation effects, the first 5 scans of each run were discarded prior to pre-processing. The resulting datasets were subsequently motion corrected, normalized to the Montreal Neurological Institute (MNI) EPI template, re-sampled to 2mm3 isotropic voxels, and smoothed with a Gaussian kernel of 8mm full width at half maximum. Previous studies have demonstrated the validity of comparing adult and pediatric activation maps normalized to the same stereotactic space for image resolutions greater than 5mm3 (Burgund et al., 2002; Kang et al., 2003). Datasets were subsequently examined for head motion artifacts. Time-points for which the scan-to-scan motion was greater than a pre-determined threshold of 0.75mm (25% of the voxel size) were removed from analysis. Subjects for whom more than 20% of the images in the run exhibited scan-to-scan motion beyond the 0.75mm threshold were excluded from further analysis.
Following pre-processing, subsequent analysis was performed in three steps. First, whole brain activation maps were generated for within-group and between-group comparisons. Next, to examine the spatial layout of sensitivity to real words and false-fonts, gradient images of differential activation were generated within each group at the whole-brain level (similar to Vinckier et al., 2007 and van der Mark et al., 2010). Finally, spherical regions of interest were functionally defined, and applied within the bilateral occipito-temporal regions to statistically test for differences in the nature of relative word-selectivity between the two groups in this region, and also to examine correlations between brain activity in some of these ROIs and reading performance. Details about the procedures employed in each step are presented in the following sections.
2.3.1. Whole-Brain Activation Maps for Within-group and Between-group comparisons
As in our earlier study (Turkeltaub et al., 2003), statistical analysis at the first level involved generating parametric activation maps for the individual subjects for the real word and false-font conditions relative to baseline (RW vs. Fix and FF vs. Fix), and for the direct contrast between the two conditions (RW vs. FF). Stimulus onsets were modeled using the canonical SPM hemodynamic response function. Functional datasets at the first level were high-pass filtered with a cut-off of 128s and corrected for auto-correlations using an AR(1) model (Friston et al., 2002). Group random effects activation maps for each condition were generated using the subject-specific contrast images in a one-sample t-test. Significantly active clusters were considered to be those that survived a cluster-size whole brain correction implemented using the CorrClusTh algorithm by Nichols at a cluster-defining threshold of p < 0.001 (http://www.sph.umich.edu/∼nichols/JG2/CorrClusTh.m).
To test for regions which exhibited reliable differences between children and adults, two-sample t-tests were conducted at the second level for the afore-mentioned contrasts. In each case, we probed for regions in which children exhibited statistically greater activation than adults and vice-versa. Activation maps resulting from the within-group contrast of RW vs. FF, and for all between-group comparisons were presented at an uncorrected p < 0.001 level (k > 20), comparable to those employed in other studies examining ventral stream activations in children (Brown et al., 2005; Booth et al., 2001, 2007; van der Mark et al., 2010; Brem et al., 2010).
2.3.2 Gradient Images
To examine the profile of word-selectivity (i.e. the posterior-to-anterior gradient) within the ventral occipito-temporal cortex in the pediatric and adult groups, we generated maps of differential activation between RWs and FFs relative to baseline (Fix). For each group, we used the statistical maps described above (i.e. p < 0.001; cluster-size corrected) and subtracted the FF vs. Fix map from the RW vs. Fix map. For visualization purposes, the resulting difference maps were masked to display activation specific to the bilateral occipito-temporal network. The mask was generated using MARSBAR (http://marsbar.sourceforge.net), and included the fusiform gyrus, lingual gyrus and inferior occipital gyrus.
2.3.3. ROI Analysis in the Visual Word Form System
Regions of interest were generated within the occipito-temporal region (using MARSBAR) to statistically test for differences in word-selectivity between our children and adult groups along the posterior-to-anterior axis. To avoid the effects of blurring, ROI analysis was performed on unsmoothed data. ROIs were functionally chosen to target regions that most reliably exhibited activation in response to either of the active conditions, while avoiding a bias towards a specific group or condition. To this effect, real word and false-font activations were combined at the first level to obtain an Active vs. Fix activation map for each individual subject. Subsequent to this, a single group map was generated by using a one-sample t-test at the second level to combine activations over all twenty-six individuals across both groups. This map was defined at a cluster-size corrected threshold of p < 0.001. Next, the posterior half of the fusiform gyrus (as defined in the MARSBAR toolbox) was divided into sub-sections along the antero-posterior axis. These subsections were used as bounding boxes to locate eight activation peaks within the ventral occipito-temporal region of the afore-mentioned single-group map that were used as the centers of 4mm3 (approximately 33 voxels) ROI spheres. Eight homotopic spheres were placed at the same locations in the contra-lateral hemisphere for a controlled comparison. For each subject, the percent signal change was computed within the defined ROIs for the RW-FF contrast and subsequently entered into an analysis of variance (ANOVA) using ROI and Hemisphere as within-subject factors, and Group as a between-subject factor. For comparison with the study of Brem et al., (2009), we additionally examined group differences in activity for each of the conditions relative to the fixation baseline (i.e. RW vs. Fix and FF vs. Fix), to determine whether any observed differences were driven by responses to real-words or the false-font control, or both.
Finally, we performed linear regression analysis to examine the relationship between percent signal change for word-selective activity (RW–FF) for some ROIs and standardized measures of real word reading (WJ-III Word Identification - WID), and naming fluency (RAN Letter and Number naming – L/N; RAN Color and Object naming – C/O subtests). These age-normed measures were acquired outside of the scanner and chosen because they emphasize orthographic recognition (rather than phonological decoding or phonemic coding, which are associated with temporo-parietal and less with inferior-temporal cortex).
3. Results
3.1 Task Performance
In-scanner performance measures for both groups are presented in Table 2. All subjects performed with high accuracy on both the real word and false-font conditions of the feature detection task. As in our previous study (Turkeltaub et al., 2003) adults responded overall with greater accuracy and shorter response time than children. Importantly, however, there were no age-related differences when comparing the difference score between real words and false-fonts attained for each group for either accuracy or response time measures. As in Turkeltaub et al., (2003), we found no significant correlation of RW-FF accuracy (r = 0.053; p = 0.792) or reaction time (r = -0.027; p = 0.897) with age, thus this particular task comparison was not susceptible to developmental effects.
Table 2. In-Scanner Performance.
| Children | Adults | p-value | |
|---|---|---|---|
| RW Accuracy (% Correct) | 88.2 ± 14 | 98.8 ± 1.6 | p < 0.05 |
| RW Response Time (ms) | 944 ± 127 | 688 ± 78 | p < 0.001 |
| FF Accuracy (% Correct) | 88.1 ± 12 | 99.0 ± 1.8 | p < 0.05 |
| FF Response Tim e (ms) | 960 ± 111 | 716 ± 83 | p < 0.001 |
| Real Word/False-Font Accuracy Difference (% correct) | 0.03 ± 5.3 | -0.17 ± 2.0 | n.s. |
| Real Word/False-Font Response Time Difference (ms) | -15.6 ± 75 | -28.6 ± 29 | n.s. |
3.2 fMRI Results
3.2.1. Whole Brain Activation Maps
Whole brain activation maps are displayed in Figure 2 for all comparisons. Within-group maps were rendered on the standardized MNI SPM brain surface template to observe regions that most-reliably exhibited activation across the whole brain for each contrast (i.e. RW vs. Fix, FF vs. Fix and RW vs. FF). Axial slices within the occipito-temporal cortex are presented for the between-group maps in order to focus on activations within the VWF region. The plane of each displayed slice was chosen to match the location corresponding to the activation peak within the occipito-temporal region for the specific contrast. A full list of activation peaks for all computed contrasts is presented in Table 3.
Figure 2.
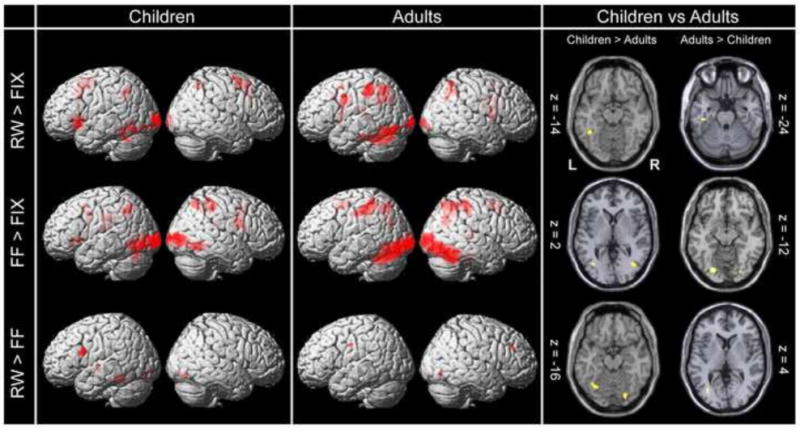
Group Whole Brain Activation maps. Left/Middle Within group activation maps for each of the task conditions relative to fixation (top and middle rows – p < 0.001; cluster-size corrected) and differential activation between real words and false-fonts (bottom – p < 0.001; uncorrected) for children and adults, surface rendered on the standardized MNI SPM template. Right: Between-group activations within the bilateral occipito-temporal cortices for each of the computed contrasts (p < 0.001 uncorrected). Z-coordinates were chosen based on the location of the peak activation within this region. L: denotes the left hemisphere, R: Right hemisphere. A full list of activation peaks is presented in Table 3.
Table 3. MNI Co-ordinates of Whole-Brain Activation Maxima.
| MNI Co-ordinates | MNI Co-ordinates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Task | Group | x | y | z | Anatomical Region | BA | k | Z | Group | x | y | z | Anatomical Region | BA | k | Z |
| RW > Fix | Children | -24 | -96 | 4 | L. Cuneus | 18 | 439 | 5.47 | Adults | -18 | -98 | -4 | L. Cuneus | * | 265 | 4.18 |
| -38 | -64 | -14 | L. Fusiform Gyrus | 19 | 523 | 4.15 | -48 | -70 | -14 | L. Middle Occipital Gyrus | 19 | 1061 | 5.32 | |||
| -26 | -54 | 42 | L. Precuneus | 7 | 197 | 3.87 | -30 | -54 | 32 | L Superior Temporal Gyrus | 39 | 683 | 5.39 | |||
| -38 | 14 | 2 | L. Insula | 13 | 371 | 4.27 | -46 | -40 | 46 | L. Inferior Parietal Lobule | 40 | 455 | 5.50 | |||
| -18 | -26 | 18 | L. Caudate | * | 106 | 4.07 | ||||||||||
| -22 | -10 | 38 | L. Cingulate Gyrus | 24 | 505 | 4.32 | ||||||||||
| 32 | -60 | 52 | R. Superior Parietal Lobule | 7 | 87 | 3.75 | 18 | -94 | -8 | R. Lingual Gyrus | 17 | 133 | 3.90 | |||
| 16 | -2 | 66 | R. Medial Frontal Gyrus | 6 | 109 | 4.48 | 30 | -68 | 32 | R. Precuneus | 39 | 261 | 3.76 | |||
| 2 | 12 | 50 | R. Medial Frontal Gyrus | 6 | 974 | 5.23 | 20 | 2 | 20 | R. Caudate | * | 376 | 4.48 | |||
| Children > Adults | -42 | -50 | -14 | L. Fusiform Gyrus | 37 | 56 | 3.97 | Adults > Children | -40 | -24 | -24 | L. Parahippocampal Gyrus | 36 | 29 | 4.40 | |
| -2 | 10 | 54 | L. Superior Frontal Gyrus | 6 | 105 | 3.76 | 0 | 32 | -8 | L. Anterior Cingulate | 24 | 39 | 3.51 | |||
| 16 | -4 | 66 | R. Medial Frontal Gyrus | 6 | 33 | 4.08 | 10 | 50 | 22 | R. Medial Frontal Gyrus | 9 | 216 | 4.53 | |||
| 4 | 24 | 12 | R. Anterior Cingulate | 24 | 35 | 4.40 | ||||||||||
|
| ||||||||||||||||
| FF > Fix | Children | -42 | -62 | -8 | L. Middle Temporal Gyrus | 37 | 1479 | 5.43 | Adults | -40 | -76 | -12 | L. Inferior Occipital Gyrus | 19 | 3201 | 5.66 |
| -26 | -58 | 44 | L. Superior Parietal Lobule | 7 | 322 | 4.73 | -30 | -56 | 50 | L. Superior Parietal Lobule | 7 | 422 | 4.25 | |||
| 0 | -18 | 22 | L. Cingulate Gyrus | 23 | 135 | 3.99 | -46 | -32 | 44 | L. Inferior Parietal Lobule | 40 | 702 | 4.29 | |||
| -20 | -16 | 16 | L. Thalamus | * | 300 | 5.22 | -4 | 0 | 56 | L. Medial Frontal Gyrus | 6 | 640 | 4.75 | |||
| -32 | 28 | -6 | L. Insula | 13 | 117 | 4.53 | ||||||||||
| 38 | -94 | 4 | R. Middle Occipital Gyrus | 19 | 939 | 4.86 | 42 | -86 | -10 | R. Inferior Occipital Gyrus | 18 | 3140 | 5.79 | |||
| 26 | -62 | 56 | R. Precuneus | 7 | 247 | 4.13 | 4 | -76 | -18 | R. Declive | * | 224 | 4.30 | |||
| 50 | -2 | 20 | R. Inferior Frontal Gyrus | 9 | 113 | 4.12 | 32 | -68 | 32 | R. Precuneus | 39 | 1261 | 4.65 | |||
| 28 | 2 | 28 | R. Cingulate Gyrus | 24 | 645 | 4.93 | 22 | -30 | 10 | R. Thalamus | * | 198 | 4.56 | |||
|
| ||||||||||||||||
| Children > Adults | -36 | -70 | 2 | L. Middle Occipital Gyrus | 19 | 36 | 4.15 | Adults > Children | -22 | -80 | -12 | L. Lingual Gyrus | 18 | 82 | 4.20 | |
| -40 | 48 | 12 | L. Middle Frontal Gyrus | 10 | 203 | 4.31 | ||||||||||
| 38 | -68 | 2 | R. Middle Occipital Gyrus | 19 | 35 | 3.85 | 26 | -82 | -16 | R. Middle Occipital Gyrus | 18 | 65 | 3.93 | |||
| 44 | -58 | -6 | R. Middle Temporal Gyrus | 37 | 37 | 3.84 | 14 | -14 | -28 | R. Parahippocampal Gyrus | 34 | 63 | 3.57 | |||
| 16 | -20 | 34 | R. Cingulate Gyrus | 24 | 98 | 4.09 | ||||||||||
| 32 | 6 | 28 | R. Precentral Gyrus | 6 | 66 | 3.90 | ||||||||||
|
| ||||||||||||||||
| RW > FF | Children | -40 | -44 | -18 | L. Fusiform Gyrus | 37 | 43 | 3.97 | Adults | -14 | -10 | 36 | L. Cingulate Gyrus | 24 | 42 | 6.46 |
| -34 | -14 | -2 | L. Lentiform Nucleus | * | 22 | 4.15 | ||||||||||
| -48 | 10 | 24 | L. Inferior Frontal Gyrus | 9 | 69 | 4.52 | ||||||||||
| 2 | -90 | -8 | R. Lingual Gyurs | 18 | 29 | 3.51 | 12 | -74 | -10 | R. Lingual Gyrus | 18 | 20 | 5.20 | |||
| 14 | -78 | -12 | R. Lingual Gyrus | 18 | 40 | 4.48 | 14 | 36 | 30 | R. Medial Frontal Gyrus | 9 | 21 | 4.99 | |||
|
| ||||||||||||||||
| Children > Adults | -32 | -64 | -16 | L. Fusiform Gyrus | * | 73 | 4.08 | Adults > Children | -32 | -74 | 4 | L. Lingual Gyrus | 19 | 21 | 3.65 | |
| -46 | -8 | 52 | L. Precentral Gyrus | 6 | 28 | 3.80 | -22 | -12 | 38 | L. Cingulate Gyrus | 24 | 45 | 3.77 | |||
| 24 | -80 | -16 | R. Lingual Gyrus | 18 | 37 | 4.00 | 24 | 64 | 16 | R. Superior Frontal Gyrus | 10 | 21 | 3.48 | |||
| 40 | -70 | 16 | R. Middle Temporal Gyrus | 39 | 25 | 3.66 | 14 | 34 | 32 | R. Medial Frontal Gyrus | 9 | 33 | 3.69 | |||
| 20 | -44 | -26 | R. Culmen | * | 26 | 3.78 | ||||||||||
| 46 | -28 | 22 | R. Insula | 13 | 53 | 4.36 | ||||||||||
All co-ordinates listed in the table correspond to the MNI stereotaxic space. Anatomical locations of activated brain areas were identified by converting these MNI co-ordinates into the Talairach anatomical space (Talairach and Tournoux, 1998) using the Yale non-linear (MNI2TAL) transformation (Lacadie et al., 2008) and the Talairach Daemon available online at www.talairach.org/daemon.html. RW: Real Words; FF: False-Fonts; k: cluster size. Boldface values indicate effects surviving an FWE whole-brain corrected threshold of p<0.05.
Real Words vs. Fixation
Within-Groups
Implicit reading of real words compared to fixation (RW vs. Fix) elicited activation in the left occipito-temporal region within the VWF system in both groups. Activation was also observed within the inferior frontal gyrus/insula for the children, while adults revealed activation that extended into the inferior frontal gyrus, though the peak was situated superior to this location. Both groups exhibited activation in bilateral parietal regions during implicit reading of real words, including superior parietal lobes and precuneus (BA 7).
Between-Groups
In the children, greater activation was observed in the left fusiform gyrus, slightly anterior to the reported peak of the VWFA (MNI co-ordinates: -42,-50,-14). For the reverse comparison, i.e. greater activation for adults relative to children, a focus was found in the left parahippocampal gyrus (MNI: -40,-24,-24).
False-Fonts vs. Fixation
Within-Groups
Activity associated with the active control condition of false-font processing was examined in a statistical map of FF greater than Fix and revealed regions within bilateral occipito-temporal and parietal cortices in both the pediatric and adult groups. These included inferior and middle occipital gyrus, middle temporal gyrus, and inferior and superior parietal lobules. Medial and inferior frontal gyri were also activated during the false-font condition.
Between-Groups
Direct comparison between the two groups revealed greater activation for children in bilateral middle occipital gyri, left middle temporal gyrus, and the left middle frontal gyrus. Regions exhibiting greater activation for adults relative to children were restricted to bilateral occipital regions.
Real Words vs. False-Fonts
Within-Groups
Direct comparison between conditions (RW vs. FF) revealed activation peaks in the left inferior frontal and fusiform (VWF) regions for the children. This pattern was not observed in the adults, for whom activations were primarily limited to right hemisphere frontal and occipital regions.
Between-Groups
Between group comparisons yielded greater activation for the children in the left fusiform gyrus close to the VWFA, though the peak occurred at a more posterior-medial location (MNI: -32,-64,-16) than what has been traditionally reported. Adults revealed greater activity than the children in left lingual gyrus (See Table 3).
3.2.2. Gradient of Word-Selectivity
The gradient image revealed the expected pattern of differential activity between words and false-font strings (Figure 3) in the occipito-temporal region. In both children and adults, word-selective activation (red) was observed in the more anterior portions of the left hemisphere, with false-fonts (blue) predominantly driving activation in more posterior regions. This pattern of anterior word-selectivity was not observed in the right hemisphere which predominantly exhibited false-font activation throughout the extent of the occipito-temporal region. Closer inspection of the ventral stream activation pattern in both groups reveals that word-selectivity in the adults occurred at a more anterior location than in children. For reference purposes in Figure 3, cursors were placed at y = -50 for two x (-38, -42) and two z (-14, -22) co-ordinates. While children seemed to be exhibiting strong word-selective activation at this y-co-ordinate, the same was not observed for the adults. Rather, stronger real word activation for the adult group occurred at more anterior and slightly lateral locations.
Figure 3.
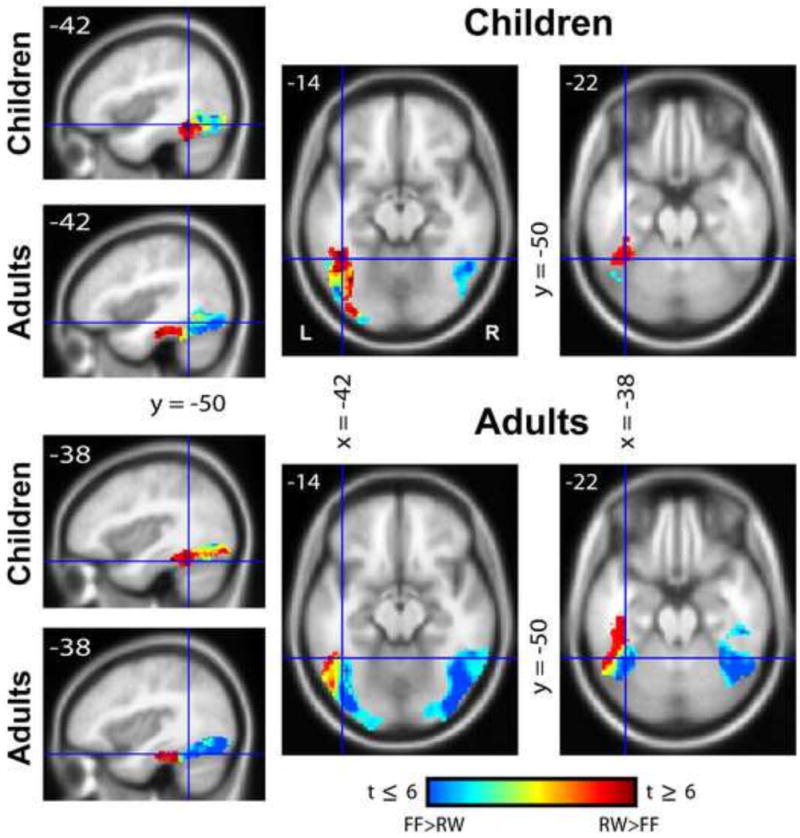
Gradient images illustrating the lay-out of spatial sensitivity to real words (red) and false-fonts (blue) in children and adults. An image of differential activity between words and false-fonts was obtained and masked within the occipito-temporal cortex including the lingual gyrus, fusiform gyrus and the inferior occipital gyrus.
3.2.3 ROI Analysis in the VWF System
Region of interest analysis was used to further explore the differences in the patterns of word-selectivity between the groups, and to submit the above observations to statistical analysis. MNI co-ordinates of the resulting eight spherical regions of interest selected within the bilateral occipito-temporal cortices are displayed in Figure 4 (top). Within each region, group mean percent signal change was calculated for the contrast of RW-FF. These results are displayed in Figure 4A.
Figure 4.
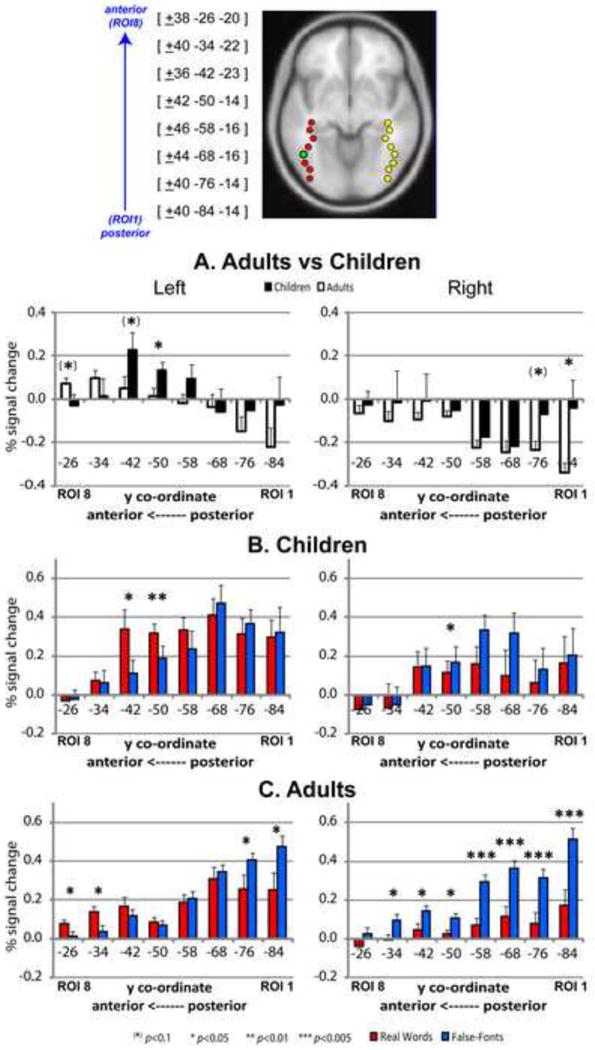
Region of interest analysis in the occipito-temporal cortex. Top: Spherical regions of interest selected in bilateral occipito-temporal regions. Each 4mm3 sphere contained approximately 33 voxels. ROI 4 (third from top - green) represents the co-ordinates located closest to the reported peak of the Visual Word Form Area. Middle/Bottom: Mean percent signal change values within the eight 4mm3 spherical regions of interest within left and right occipito-temporal regions for real words minus false-fonts in children (solid) and adults (clear - A) and for real-words (red) and false-fonts (blue) in children (B) and adults (C) separately. Error bars represent 1 standard error measurement.
The ANOVA yielded a significant main effect of Hemisphere (F(1,25) = 17.7; P < 0.0001), ROI (F(7,19) = 5.01; P < 0.0001) and Group (F(1,25) = 10.7; P = 0.0012), as well as an interaction of ROI × Group (F(7,19) = 3.28; P = 0.019). In the left hemisphere, post-hoc t-tests (two-tailed) revealed that this pattern was driven by greater RW-FF activation for children relative to adults in ROI 5 (i.e. y = -50; p = 0.034) and ROI 6 (i.e. y = -42; p = 0.074), both locations being slightly anterior to what would be considered the classical VWFA peak (located at ROI 4 based on Cohen et al., 2000; McCandliss et al., 2003). In the ROIs further anterior to these locations, RW-FF activity was greater for adults than children, with the difference being marginally significant in ROI 8 (p = 0.083). In all right hemisphere regions, false-fonts elicited greater activation than real words for both groups. This difference was greater for adults than children, particularly in the two most posterior regions (y = -76: p = 0.053; y = -84: p = 0.023).
To further examine the patterns of activity in the occipito-temporal cortex (similar to Brem et al., 2009) we separately plotted percent signal change values for real words relative to fixation and false-fonts relative to fixation for each group (Fig. 4B and 4C). Based on patterns exhibited within these plots, afore-mentioned differences for RW-FF activity between the two groups in the anterior left occipito-temporal cortex appeared to be driven by differences in activation for real words (relative to fixation).
Finally, regression analyses performed in the most anterior four ROIs of the left hemisphere (Figure 5) revealed that for both groups a positive relationship exists between percent signal change for word-selective activation (RW-FF) and letter/number naming speed (RAN; L/N) in the most anterior left ROI (ROI 8: r = 0.402; p = 0.042). Further, in the adults, but not children, we also observed a relationship between word-selective activity and single real word reading (WID) in this same location (r = 0.601; p = 0.018), and comparison of correlation coefficients between the two groups yielded a significant difference (p = 0.022), providing additional confirmation that the relationship between these two variables is different for the adult and pediatric groups.
Figure 5.
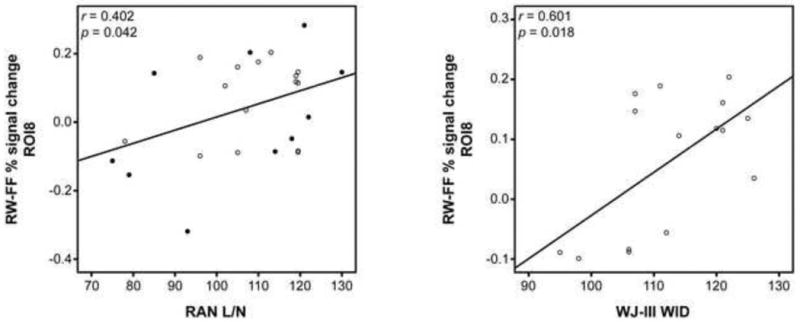
Relationship between measures of reading ability and real word activation. Correlation of word-selective fMRI activity in ROI 8 with naming fluency across the span of subjects (left; children: solid; adults: clear) and with real word reading ability in adults (right).
4. Discussion
Most prior reports on neurodevelopmental changes associated with reading acquisition have examined changes across the entire brain, thereby capturing processes associated with orthographic, phonologic and semantic processing (Booth et al., 2001, 2004; Brem et al., 2006, 2009; Brown et al., 2005; Shaywitz et al., 2002; Turkeltaub et al., 2003) and most of these studies did not find differences in the left hemisphere occipito-temporal regions in and around the visual word form area. As the interest in reading-specific processes in the ventral stream in the adult literature has increased (especially the notion of a word-selective gradient in the VWFS; Vinckier et al., 2007), the question arises about how the organization of the ventral stream may differ between adults and children, given their profound differences in reading experience. Using event-related potentials, a study by Brem et al., (2009) observed developmental differences in the occipito-temporal gradient, however these differences were not observed in the same sample using fMRI under the same task condition, raising the question of the different sensitivities of these techniques and their interactions with specific paradigms. The goal of the present study was to address the question of whether differences exist between children and adults in the occipito-temporal gradient using fMRI, using an implicit reading task in an English speaking population. We employed the same implicit reading paradigm previously reported in a pediatric sample (Turkeltaub et al., 2003), which allows for a comparison of real words and false-font characters, to examine gradients in the visual ventral stream (Vinckier et al., 2007; van der Mark et al., 2009). The current investigation used a higher field strength than our previous work as well as most of the developmental studies cited above, which might help differentiating task-related activity in the ventral stream of children and adults.
In accordance with previous literature, whole-brain analysis revealed activation in traditional left-hemisphere language areas for both the adult and pediatric samples when contrasting real words with fixation, and to a lesser extent when real words were contrasted with false-fonts. As revealed by the gradient images and ROI analysis, we also observed the previously reported occipito-temporal gradient in the left hemisphere for both groups. That is, for both children and adults, there was a relative increase in activity in response to viewing real words when contrasted to false-fonts as one moves from posterior to anterior ROIs in the ventral stream. Our main finding was that the nature of this gradient was not identical for the two groups. ROI analysis revealed that children exhibited between-task differences favoring real words in a location posterior to where the adults exhibited greater activity for words relative to false-fonts. We also found that adults, as has been previously reported (Brem et al., 2006, 2009), showed greater activity in posterior occipito-temporal cortex for the control condition relative to the words, while this was not observed in the children. Our results provide evidence for developmental differences, and suggest the need for further fine grained analysis of this ventral stream system in more narrowly defined age groups and under a variety of task conditions.
4.1 Whole Brain Activation Patterns
Our first analysis concerned examining activity in the entire brain. Contrasting the real word and false-font tasks relative to fixation in both children and adults revealed that while real word activations were primarily left hemisphere lateralized (as shown in previous studies of reading: Tagamets et al., 2000; Gaillard et al., 2003; Schlaggar et al., 2002; Turkeltaub et al., 2003, Brem et al., 2006, 2009), false-fonts elicited activation in a bilateral network including occipital, parietal, middle and inferior frontal regions similar to previous studies that utilized this paradigm (Price et al., 1996; Turkeltaub et al., 2003). The real words greater than false-font contrast revealed a focus in the left fusiform gyrus in the children, but not the adults. Direct between-group comparison for words greater than false-fonts using whole brain analysis yielded greater activation for children than adults in the occipito-temporal cortex, with the peak difference located posterior and medial to the traditionally reported VWFA (y = -64). This finding for the whole-brain analysis differs from our previous study (Turkeltaub et al., 2003), where no activation clusters were observed in the occipito-temporal cortex when comparing children and adults for the RW-FF contrast. The difference between the two findings may be a consequence of the higher field strength utilized in the current study, providing greater signal-to-noise ratio to observe these subtle differences.
Failure to show activity in the classical VWFA in the adults using the real word greater than false-font comparison may at first seem surprising. However, it is noteworthy that the original whole-brain analysis imaging study by Price et al. (1996) using the implicit reading paradigm utilized in the present study also did not report significant activity in what has since been termed the VWFA (which according to Cohen et al., 2000 is around Talairach co-ordinates (Tal: -43,-54,-12). Instead, Price and colleagues reported on a more anterior location (Tal: -48,-42,-12). Interestingly, this location is relatively close to that reported for the whole brain analysis in the children in the present study (MNI: -40,-44,-18; Tal: -38,-45;-11), with the adults in the present study showing no activity that surpassed our threshold. Other studies using whole brain analysis and contrasting real words with false-fonts (e.g. van der Mark et al., 2009; Turkeltaub et al., 2003) or real words with symbols (Brem et al., 2009) also did not find the VWFA as reported by Cohen and colleagues. This could simply be an issue of whole brain analysis not being sufficiently constrained to allow the detection of this area. However, as will be discussed below, it appears that the location of a word sensitive region is somewhat sensitive to the exact nature of the task being used.
4.2 Gradient Images and Regions of Interest
Since the inception of brain imaging studies of reading conducted with positron emission tomography (Petersen et al, 1988), whole brain analysis has served the purpose of detecting the location of disparate regions involved in different aspects of lexical processing (e.g. semantics versus orthography). It has also provided a mechanism by which to obtain support for one model of reading over another, for example, addressing parallel versus connectionist models (Rumsey et al., 1997a), and to examine disorders of reading within these networks (Rumsey et al., 1997b). In recent years, a specific interest in the ventral stream of the left hemisphere has emerged, and more specifically the use of fMRI to examine the hierarchy that subserves visual word recognition in the occipito-temporal cortex with an anterior-to-posterior progression (Vinckier et al., 2007). The local combination detector model has been put forward by Dehaene et al. (2005) to best describe the internal organization of this region. As such, this model provides an additional layer of specificity to more global models of reading development (e.g. Pugh et al., 2001) that describe a principal role for the occipito-temporal region in identifying frequently seen words, most likely invoked in the more advanced stages of reading (i.e. following phonological assembly in dorsal brain regions at earlier stages of reading acquisition). Since the local combination detector model is guided by the principles that govern the organization of the primate visual system, one would assume that the hierarchy exists for young children, including pre-readers, but that the use of the system for words is experience-dependent.
In the present study we generated gradient images to show patterns of preferential activity along the occipito-temporal axis, highlighting differences between the two groups in the nature of the “word-selective gradient”. Both children and adult groups in our study exhibited the previously observed gradient of word-selectivity in the left-hemisphere VWFS (Brem et al., 2006, 2009; Vinckier et al., 2007, van der Mark et al., 2010). However, word-selective regions in the adults occurred at a more anterior location and extended into more anterior regions within the VWFS than in children, suggesting the utilization of more anterior regions for word processing with age-related advanced reading skills.
The gradient images further revealed that word-selective activation for the children appeared to follow a more medial trajectory (See Fig. 3), z = -14, whereas for adults this occurred in more lateral portions of the mid-fusiform region (and in this group false-fonts appeared to have greater activation in more medial areas). This profile for adults is similar to that reported by Vinckier et al., (2007) also in an adult population. Lateral portions of the occipito-temporal cortex have been reported to be involved in multi-modal (visual and auditory) word processing (Cohen et al., 2003), spelling (Rapp and Lipka., 2010; Purcell et al., 2011), and in single letter processing (Flowers et al., 2004). As adults are expected to be more familiar with the internal (letter) structure of the word representation, increased response may be elicited in these lateral regions for this group during the word processing task.
For the ROI analysis, word-selective activity (i.e. significantly greater activation for words relative to false-fonts) was observed in the two most anterior ROIs for adults, as already foreshadowed by the gradient images. This anterior increase in real word activation for the adults was accompanied by a relative decrease in more posterior regions: false-font activations were significantly greater than real words in the two most posterior ROIs for adults but not children. Our findings therefore again emphasize the separation between false-font processing regions in the posterior occipito-temporal cortex, and word processing in the more anterior regions for adults. The children also demonstrated word-selective activity in two anterior left occipito-temporal ROIs, located directly behind those two seen in adults. Children did not exhibit a greater response to false-fonts in the posterior brain regions as the adults did, suggesting that earlier aspects of the visual system are also modulated by age and reading experience.
One surprising finding is that the two stimulus categories elicited similar activation levels at the site of ROI 4, prompting us to ask why both the children and adult groups failed to show the classical VWFA. Mid-portions of the fusiform gyrus (i.e. the VWFA and regions directly anterior and posterior to it) are thought to be involved in accessing sub-lexical (orthographic-to-phonologic) information of visually presented print stimuli (Cohen et al., 2002; Dehaene et al., 2005; Vinckier et al., 2007). Instead of finding selectivity for words in the VWFA proper, we observed increasing word-selectivity in regions anterior to the classical VWFA (for both children and adults). Interestingly, our findings are consistent with van der Mark et al.'s pediatric study (2009), which also found that the real words greater than false-fonts contrast elicited a significant differential signal in their ROI located anterior to the VWFA proper (but not the VWFA itself). This ROI was located at MNI: -42,-44,-18 and ours is at MNI: -42,-50,-14. Monitoring words compared to symbol strings for repetition also did not elicit a differential response in the ROI aligned with the VWFA proper in either studies reported by Brem and colleagues, but again, the ROI anterior to it (MNI: -42,-42,-18) for both children and adults (Brem et al., 2009), and likewise, both ROIs located anterior to the ROI aligned with the VWFA in their adolescent/adult sample (Brem et al., 2006), showed a strong preference for words over the control stimulus.
It is therefore tempting to speculate, as have others (Brem et al., 2006), that there is a profound difference in this paradigm from that used by Cohen and colleagues, who instead contrasted words with low level stimuli such as checkerboards (e.g. Cohen et al., 2002). For example, the false-font task does induce widespread activity in the posterior portion of the ventral visual stream, and, as these infringe the middle portion of the fusiform gyrus, this provides less of a difference (than a checkerboard control condition) in the regions considered to be the VWFA. Notably, we provide further evidence for the idea that using a symbolic/symbol-like control task results in an anterior “shift” for the foci that shows the greatest differential response for words when compred to the control stimulus, thereby effectively identifying a different position for the VWFA along the anterior-posterior axis based on the demands of the control stimulus. It is worth noting that the publication by Vinckier et al. (2007) did employ real words contrasted to false-fonts and elicited a relatively greater response for real words than false-fonts in ROIs anterior to the VWFA. Future studies comparing these two types of paradigms (i.e. symbol-like vs. checkerboard control stimuli) within the same group could help resolve this issue.
Returning to the observation that while for children, real words were significantly greater than false-fonts in the two ROIs directly anterior to the VWFA (ROI 5: y = -50; ROI 6: y = -42), adults did not demonstrate a pattern of word-selectivity in these regions, but instead in the two ROIs anterior to this. This observation does not necessarily rule out that adults demonstrate word-selectivity in any of the locations directly anterior to the VWFA. After all, the observation is a consequence of the ROIs utilized in this study. As discussed above, gradient images demonstrated that word-selectivity for the adults followed a more lateral trajectory relative to the children. While our ROIs in these locations more closely matched those of Brem and colleagues in their study examining developmental effects (Brem et al., 2009), they are located more medial than the ROIs of Vinckier et al., (2007) examining the gradient of word-selectivity in an adult sample. In fact, when we selected ROIs at more lateral locations (MNI: x, y, z = -50,-42,-23; more closely matching those of Vinckier and colleagues), we indeed observed significantly greater word activation for the adults relative to false-fonts (p = 0.02). Thus, we find that adults did exhibit word-selective activation in the regions directly anterior to the classical VWFA, but similar to Vinckier and colleagues, we observe that for this group, word-selectivity appears to follow a more lateral trajectory.
While we have argued above that those studies using paradigms similar to the one used in the present study also show word-related activity anterior to the “traditional” VWFA location, unlike the afore-mentioned studies, we report for the first time recruitment of more anterior locations in the adults for real word processing relative to the children. These regions were quite anterior to the classical VWFA, extending into the parahippocampal gyrus. It behooves us therefore to contemplate what “selectivity to words” means in this context, given that the implicit processing of words elicits a range of processes (orthographic, semantic, phonological) none of which can be isolated with this experimental paradigm. One possible interpretation is that the recruitment of these anterior regions may be the result of adults accessing semantic representations of the presented words, as described in the dual-route cascaded computational model, where orthographic input proceeds to either phonological output or semantics (Coltheart et al., 2001).
Various studies have demonstrated involvement of the fusiform/parahippocampal regions (at co-ordinates close to those reported in the current study) using tasks that involve semantic representation. These have been summarized most effectively by Binder et al., (2009) in a meta-analysis of 120 studies involving semantic processing. Tasks included in the meta-analysis were those that revealed differences in the degree to which stored knowledge was accessed or the type of knowledge accessed. Activation likelihood was observed in the fusiform and parahippocampal gyri, notably close to the position on the posterior-anterior axis that coincides with the ROIs identified in the current study to be more active for words than false-fonts in adults. The exact function of this region is still poorly understood, and it seems activity underlying semantic processing is more commonly reported for object rather than word stimuli. In that context it is of note that even though our subjects were not given a task that evaluated semantic processing, the fact that we observe a correlation of activation in these regions with the measure of naming fluency suggests that some level of semantic processing may be occurring here.
Finally, developmental disengagement of right hemisphere regions reported by Turkeltaub et al., (2003) was also observed in the present study. While seven out of the eight regions in the right hemisphere exhibited significantly greater activation for false-fonts than real words in the adults, only one region showed this pattern in children. In addition, Turkeltaub et al., (2003) reported a negative correlation of word-selective BOLD activity with age in the posterior fusiform gyrus. This pattern was also observed in our current sample for the most posterior ROI in the right hemisphere (ROI 1: r = -0.41; p = 0.037), providing further evidence of developmental disengagement of right hemisphere posterior regions for word processing with greater reading experience.
4.3 Further Considerations
Results from our study support observed ERP results from the study of Brem et al., (2009) showing that specialized word processing is narrowed to more anterior regions in more experienced readers (i.e. adults). While the study of Brem and colleagues did not demonstrate this difference when data were acquired with fMRI, they did observe a negative correlation between word-selective activity and reading speed across their full sample in the posterior VWFS, suggesting that fast readers do not rely on the posterior aspect of this system. Our study observed a positive correlation between word-selective activity and naming speed in the most anterior region of the VWFS. The combination of results from these two studies supports the idea that less proficient readers may rely more on posterior aspects of the VWF system during word processing, with individuals exhibiting greater reading proficiency (which may in part be a function of greater reading experience) relying more on the anterior aspects of the system. In the adults only, we also observed a positive correlation between word-selective activity and word identification. This correlation was not observed in the children, suggesting that development of this anterior region for word processing may occur later in the reading trajectory. Future longitudinal studies may be utilized to track how the specific neural changes occur with the progression of reading development.
Worth noting is the difference in language backgrounds between individuals in our study and that of Brem and colleagues. The German language has a shallow orthography compared to English, which contains a larger number of phoneme-to-grapheme representations, and greater inconsistencies between spoken and written representations (see Erickson et al., 2010 for a review). Thus the rate of reading and reading acquisition is slower in English learners relative to their German counterparts, or those learning other more transparent languages (Wimmer and Goswami, 1994; Landerl et al., 1997; Seymour et al., 2003; Ziegler and Goswami, 2005). As such, development of regions involved in language processing may occur at a slower rate in English speakers, with German children at a similar age/grade level being closer to their adult counterparts in terms of neural development. Whether this may in part account for the unobserved differences in Brem et al, (2009) and the observed differences in the current study requires greater investigation, but remains worthy of consideration.
One weakness of the present study is the wide age range of the subjects in the pediatric sample. It is not known at what age the gradient of selectivity is established. Failure to see developmental changes in the VWFA in children in earlier reports using a whole brain analysis approach raised the possibility (Turkeltaub et al., 2003) that these changes may occur at a younger age. At the same time, previous studies have focused on an older age group (Brem et al., 2009; van der Mark et al., 2009) and there is good reason to believe that these changes are not apparent until a few years after formal schooling has begun. Future studies using larger samples of children at each age group and preferably employing a longitudinal design like that used by Ben-Shachar and colleagues (2011) will be able to home in on the specifics of the developmental trajectory. The goal of the present study was to provide the initial step of asking if in general, there are differences between children and adults, especially in the light of the discrepant fMRI and EEG finding reported by Brem and colleagues (2009).
Nevertheless, our wide age range raises the question as to whether the differences between children and adults may be driven by the younger participants within the pediatric sample. To address this question we interrogated homogeneity within our pediatric sample. To probe for an age-related effect in the pediatric sample, we examined whether activity in our ROIs was modulated by age. Specifically, we correlated fMRI activity for RW vs. Fix, FF vs. Fix and RW vs. FF in all (left and right) occipito-temporal ROIs with age for the pediatric sample and found no significant correlation. When these analyses were repeated using measures of reading ability (Word ID, Word Attack) instead of chronological age, we obtained the same negative results. This suggests that observed effects are unlikely to be driven by a particular subset (based on age or reading ability) of the pediatric sample.
Finally, the dissociation between observations of our study and that of Brem et al., (2009) might also be due to the differences in the tasks employed within each study. Brem and colleagues utilized a task that required subjects to press a button after immediate repetition of a presented word or symbol string. This task – commonly referred to as a 1-back task – engages a wide cortical network (Owen et al., 2005), and thus, elicited responses may be susceptible to top-down activations due to task demand. Indeed, this was highlighted by the authors as a possible explanation for the dissociation between their observed ERP and fMRI results. Also, between-task differences in performance and reaction time were reported in their study. Our task on the other hand simply involved feature detection within the visually presented stimuli, and thus may be less susceptible to afore-mentioned top-down activations. Further, the relative performance on false-fonts and real words was constant across our adult and pediatric samples, suggesting the validity of the task for examining developmental differences. We posit that while ERP is still more sensitive to the transient responses highlighting these subtle differences in response profiles, the combination of higher field fMRI as well as the use of less demanding tasks might provide the sensitivity required to observe these differences, and speaks to the importance of conducting more focused ROI analyses in the ventral stream, not unlike the work conducted for face processing in the “Fusiform Face Region” (for review see Kanwisher and Voyel, 2006).
Conclusion
We examined whether developmental differences exist in the anterior-to-posterior gradient of word-selectivity previously observed separately in typically reading children and adults in the left occipito-temporal visual word form system. Our study utilized an implicit word processing task with fMRI at 3T, applying both whole-brain and region of interest analysis techniques. We observed gradients of word-selectivity in the left occipito-temporal cortex (but not in the right hemisphere) for both pediatric and adult groups. Both whole-brain activation patterns and region of interest analysis revealed recruitment of more anterior occipito-temporal regions for word-selective processing in the adults. Further, results suggested that anterior word specialization in adults was driven by increased activation to real words, and activation in these anterior regions was correlated with measures of whole word reading in the adults but not in the children. Overall, results suggest development of specialized word processing regions in the anterior visual word form system with greater reading experience. These findings in typical pediatric and adult readers not only further inform our understanding of the local combination model for the left occipito-temporal cortex put forward by Dehaene and colleagues (2005), but also contributes to a foundation of normal reading acquisition by which to eventually compare atypical reading, such as developmental dyslexia.
Research Highlights.
Adults and children exhibited a left occipito-temporal gradient of word specificity
Word specific regions were more anterior in adults relative to children
Specificity in these regions for adults was driven by increased real word activity
Anterior word-specific activity correlated with measures of reading fluency
Results add to existing knowledge about neural changes during reading development
Acknowledgments
This work was supported by NIH grants P50 HD40095 and R01 HD056107. We would like to thank Emma Cole, Iain DeWitt, Megan Luetje, Alison Merikangas, Ashley Wall-Piche and Jenni Rosenberg, for their assistance. We would also like to thank Jeremy Purcell for providing constructive comments on the manuscript, and our subjects for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Olumide A. Olulade, Email: oao24@georgetown.edu.
D. Lynn Flowers, Email: lflowers@triad.rr.com.
Eileen M. Napolielo, Email: emn5@geogetown.edu.
Guinevere F. Eden, Email: edeng@georgetown.edu.
References
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proclamations of the National Academy of Sciences, USA. 2007;104(21):9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TB, Marsel Mesulam M. The development of specialized brain systems in reading and oral-language. Child Neuropsychology. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007;45:775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Human Brain Mapping. 2009;30:1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proclamations of the National Academy of Sciences, USA. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bruno JL, Zumberge A, Manis FR, Lu ZL, Goldman JG. Sensitivity to orthographic familiarity in the occipito-temporal region. Neuroimage. 2008;39:1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. NeuroImage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Naming of objects-drawings by dyslexic and other learning disabled children. Brain and Language. 1976a;3:1–15. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Rapid “automatized” naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976b;14(4):471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Cutting LE. History and significance of rapid automatized naming. Annals of Dyslexia. 1999;49:29–42. [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin JF, Poline JB, Rivière D. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Erickson K, Sachse S. Reading acquisition, AAC and the transferability of English research to languages with more consistent or transparent orthographies. Augmentative and Alternative Communication. 2010;26(3):177–190. doi: 10.3109/07434618.2010.505606. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proclamations of National Academy of Sciences, USA. 1998;95:914–21. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21(3):829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RNA, Kiebel S, Philips C, Ashburner J. Classical and Bayesian Inference in Neuroimaging: Applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai1 R, Humphries C, Seidenberg MS, Binder JR. Neural Systems for Reading Aloud: A Multiparametric Approach. Cerebral Cortex. 2010;20(8):1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19:1–11. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetrix X. More accurate Talairach coordinates or neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K, Wimmer H, Frith U. The impact of orthographic consistency on dyslexia: A German-English comparison. Cognition. 1997;63:315–334. doi: 10.1016/s0010-0277(97)00005-x. [DOI] [PubMed] [Google Scholar]
- Lindamood PC, Lindamood P. Lindamood-Bell Auditory Conceptualization Test. Austin, TX: Pro-Ed Incorporated; 2004. [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, Steinhausen HS, Brandeis D. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33:749–58. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–99. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Sartori G, Orlandi P, Price CJ. Semantic relevance explains category effects in medial fusiform gyri. Neuroimage. 2006;30:992–1002. doi: 10.1016/j.neuroimage.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-Back Working Memory Paradigm: A Meta-Analysis of Normative Functional Neuroimaging Studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, Noppeney U. Normal and pathological reading: converging evidence from lesion and imaging studies. Neuroimage. 2003;20:s30–s41. doi: 10.1016/j.neuroimage.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee J, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–92. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Purcell JJ, Napoliello EM, Eden GF. A combined fMRI study of typed spelling and reading. NeuroImage. 2011;55:750–762. doi: 10.1016/j.neuroimage.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Lipka K. The Literate Brain: The Relationship between Spelling and Reading. Journal of Cognitive Neuroscience. 2010;23(5):1–18. doi: 10.1162/jocn.2010.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997a;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997b;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Annals of Neurology. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Sandak R, Einar Mencl W, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8(3):273–292. [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–79. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. The Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Seymour PHK, Aro M, Erskine JM. Foundation literacy acquisition in European orthographies. British Journal of Psychology. 2003;94:143–174. doi: 10.1348/000712603321661859. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Einar Mencl W, Todd Constable R, Holahan JM, Marchione KE, Fletcher JM, Reid Lyon G, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: An fMRI study. Journal of Cognitive Neuroscience. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system – an approach to cerebral imaging. New York, NY: Thieme Medical Publishers; 1998. [Google Scholar]
- Tarkiainen A, Helenius P, Hansen P, Cornelissen P, Salmelin R. Dynamics of letter string perception in the human occipito-temporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, Eden GF. The neural basis of hyperlexic reading: An fMRI case study. Neuron. 2004;41:11–25. doi: 10.1016/s0896-6273(03)00803-1. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmuller J, Kronbichler M, Loenneker T, Klaver P, Martin E, Brandeis D. Children with dyslexia lack multiple specializations along the visual word form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101(2):192–212. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wimmer H, Goswami U. The influence of orthographic consistency on reading development: Word recognition in English and German children. Cognition. 1994;51:91–103. doi: 10.1016/0010-0277(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychological Bulletin. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]


