Abstract
OBJECTIVE
In a previous study, it was found that a ginseng berry extract with a high content of the ginsenoside Re normalized blood glucose in ob/ob mice. The objective of this study was to evaluate the effect of the ginsenoside Re on insulin resistance of glucose transport in muscles of rats made insulin resistant with a high-fat diet.
MATERIALS/METHOD
Rats were fed either rat chow or a high-fat diet for 5 weeks. The rats were then euthanized, and insulin stimulated glucose transport activity was measured in epitrochlearis and soleus muscle strips in vitro.
RESULTS
Treatment of muscles with Re alone had no effect on glucose transport. The high-fat diet resulted in ~50% decreases in insulin responsiveness of GLUT4 translocation to the cell surface and glucose transport in epitrochlearis and soleus muscles. Treatment of muscles with Re in vitro for 90 min completely reversed the high-fat diet-induced insulin resistance of glucose transport and GLUT4 translocation. This effect of Re is specific for insulin stimulated glucose transport, as Re treatment did not reverse the high-fat diet-induced resistance of skeletal muscle glucose transport to stimulation by contractions or hypoxia.
CONCLUSIONS
Our results show that the ginsenoside Re induces a remarkably rapid reversal of high-fat diet-induced insulin resistance of muscle glucose transport by reversing the impairment of insulin-stimulated GLUT4 translocation to the cell surface.
Keywords: glucose transport, GLUT4 translocation, insulin responsiveness
1. INTRODUCTION
Ginseng preparations are used in traditional Chinese medicine to treat a variety of ailments, including diabetes, and it has been reported that Asian and American ginseng both have anti-hyperglycemic activity in laboratory rodents [1–3] and humans [4,5]. In a previous study, it was found that treatment with a Panax ginseng berry extract normalizes blood glucose levels in diabetic ob/ob mice [6]. Furthermore, the rate of insulin-stimulated glucose disposal during a hyperinsulinemic-euglycemic clamp was increased two-fold in ob/ob mice treated with the ginseng berry extract. This glucose lowering effect appeared to be mediated by the ginsenoside Re, which is present in high concentration in Panax ginseng berries [6].
Insulin resistance associated with the abdominal obesity/metabolic syndrome has become a serious, worldwide public health problem. It is a major cause of type 2 diabetes and greatly increases the risk of developing atherosclerosis. Rodents fed a high-fat diet rapidly develop a syndrome that appears to be the rodent equivalent of the human abdominal-obesity insulin resistance syndrome [7–11]. This model has proved to be useful for studies of muscle insulin resistance. In the present study we used muscles from rats fed a high-fat diet to evaluate the effect of the ginsenoside Re on muscle insulin resistance during the early phase of development of the insulin resistance syndrome.
RESEARCH DESIGN AND METHODS
1.1 Materials
2-Deoxy-D-[1,2 3H]glucose (2-DG) was purchased from American Radiolabeled Chemicals. [14C]-Mannitol was purchased from NEN Life Science Products. Ginsenoside Re was obtained from NetQem (Monmouth Jct., NJ). Antibodies against insulin receptor substrate (IRS)-1, phospha-PkB/Akt (Ser473 and Thr308), and phosphotyrosine were from Upstate Biotechnology. The GLUT4 antibody was a gift from Mike Mueckler (Washington University School of Medicine). Antibodies against AMPK, phospho AMPK, acetyl CoA carboxylase (ACC) and phospo ACC were from Cell Signaling (Beverly, MA). Horseradish peroxidase-conjugated donkey anti-rabbit IgG was purchased from Jackson Immuno Research. Enhanced chemiluminescence reagents were from Amersham. Biotinylated 2N-4-(1-azi-2,2,2-trifluroethyl) benzoyl-1,3-bis (D-mannose-4-yloxy)-2-propylamine (ATB-BMPA) was obtained from Toronto Research Chemicals, Purified pork insulin was from Eli Lilly. Other reagents were purchased from Sigma-Aldrich.
1.2 Treatment of animals
Male (~70g) Wistar rats were obtained from Charles River and placed on either a rat chow or a high-fat diet for 5 wk. The high-fat diet contained, as percent of total calories, lard 32%, corn oil 18%, sucrose 27%, and casein 23%, respectively, of total calories, and was supplemented with vitamins (22 g/kg Teklad vitamin mix no. 40077), minerals (51 g/kg Teklad mineral mix no. 170915) and methionine (4.4 g/kg). The rat chow, Constant Formula Purina Rodent Chow no. 5001, was obtained from Purina Mills (St. Louis, MO); it contains as a percentage of total calories, 58.9% carbohydrate, 12.4% fat, and 28.7% protein. The energy content of the high-fat diet is 5.1 kcal/g, and that of the rat chow diet is 3.3 kcal/g. The rats were provided the diets and water and libitum. This research was approved by the Animals Studies Committee of Washington University.
1.3 Tissue collection
Food was removed at 6:00 pm the day before the experiment. The following morning, rats were anesthetized by an intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt), and the epitrochlearis and soleus muscles [12] were removed. Before incubation, the soleus muscle was split longitudinally into strips with an average weight of 20–25 mg.
1.4 Fat pad weights
After the muscle dissection was completed, the abdominal cavity was opened, and the epididymal, omental, and retroperitoneal fat pads were removed and weighed.
1.5 Re
To solubilize the ginsenoside Re, it was first suspended in 1.0 ml of 0.3% polyvinylpyrrolidone-10 (Sigma-Aldrich) in methanol and dried under vacuum. Dried Re was suspended in KHB and sonicated for 10 min. Re in the extract and in an Re standard were determined using an Agilent liquid chromatograph equipped with a quaternary pump, autosampler and photodiode array detection system. Chromatography was performed using a reverse phase column (Agilent, Zorbax Eclipse XDB-C18, 4.6 × 150 mm, 5 µm). The binary gradient elution system consisted of water (A) and acetonitrile (B); separation was achieved using the following gradient: 0–25 min, 20% B; 25–45 min, 20–40% B; 45–55 min, 40–80%; 55–60 min, 80–100% B. The column temperature was kept constant at 23°C. Flow rate was 0.8 ml/min and injection volume was 5 µL. The UV detection wave length was set at 203 nm and diode array scanning was from 190 nm to 400 nm.
1.6 Muscle incubations
Muscles were incubated for 90 min at 35°C in 2 ml of oxygenated Krebs-Henseleit buffer (KHB) containing 8 mM glucose, 32 mM mannitol, and 0.1% BSA with or without 0.2 mg/ml ginsenoside Re. This concentration of Re is close to the maximal amount that we could get into solution, and is more effective than 0.1 mg/ml. The muscles were then transferred to medium of the same composition with or without the addition of a maximally effective insulin concentration, 2 mU/ml, and incubated for 30 min. Muscles were then washed for 10 min at 30°C in KHB containing 40 mM mannitol and 0.1% BSA, with or without insulin, and with or without Re, to remove glucose from the extracellular space. The flasks were gassed with 95% O2-5% CO2 and shaken continuously in a Dubnoff incubator (Precision Scientific, Chicago, IL) during the incubations.
1.7 Electrical stimulation of muscles
After incubation in the presence or absence of 0.2 mg/ml of Re for 90 min, as described under “muscle incubations”, epitrochlearis and soleus muscles were stimulated to contract, using a Grass stimulator as described previously [12,13]. Re was included in the incubation medium of those muscles that had been exposed to Re during the previous, 90 min incubation.
1.8 Muscle hypoxia
To evaluate the effect of hypoxia, muscles were incubated for 90 min with or without Re in KHB gassed with 95% N2 – 5% CO2 [13,14].
1.9 Measurement of 2-DG transport activity
Glucose transport activity was measured using 2-DG, as described previously [15]. Muscles were incubated for 20 min at 30°C in 2 ml KHB containing 4 mM 2-[1,3-3H]DG (1.5 µCi/ml), 36 mM [14C]mannitol (0.2 µCi/ml, 0.1% BSA, and insulin and/or Re if present in the previous incubation. Extracellular space and intracellular 2-DG concentrations were determined as previously described [16].
1.10 Insulin signaling
Soleus muscles were incubated for 90 min at 35°C with or without 0.2 mg/ml Re followed by 30 min in the same medium with or without addition of 2 mU/ml insulin as described under muscle incubations. The muscles were then clamp frozen and subsequently used for measurement of IRS-1 tyrosine phosphorylation [17], phosphotyrosine bound PI3-kinase activity [18] and phosphorylation of Thr308 and Ser473 of protein kinase B [19].
1.11 Photolabeling of muscle cell surface GLUT4
The quantity of GLUT4 at the cell surface was assessed in epitrochlearis muscles using biotinylated ATB-BMPA exofacial photolabeling as described previously [20].
1.12 Measurement of AMPK and ACC phosphorylation
Total and phosphorylated AMPK and ACC were measured by Western blotting [21].
RESULTS
1.13 Body and fat pad weights
The effects of the high-fat diet on body weight and fat pad weight are shown in Table 1.
Table 1.
Effects of the high-fat diet on body weight and fat pad weight
| Chow | High-fat | |
|---|---|---|
| Body weight, g | 272 ± 4 (36) | 290 ± 4 (36) |
| Fat pad weight, g | 9.91 ± 1.0 (36) | 14.9 ± 1.1 (36)* |
Values are means ± SE for the number of rats shown in parenthesis. The fat pad weight is the sum of the epididymal, omental and retroperitoneal depot weights.
P < 0.005 high-fat vs. chow.
3.2 Reversal of high-fat diet-induced insulin resistance of muscle glucose transport
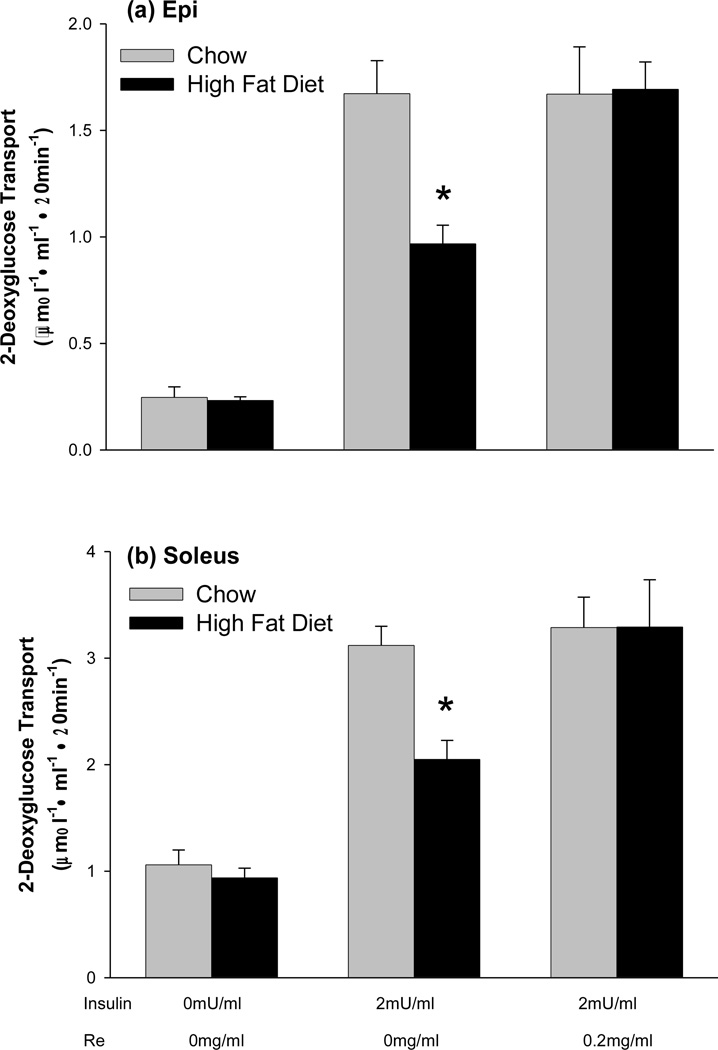
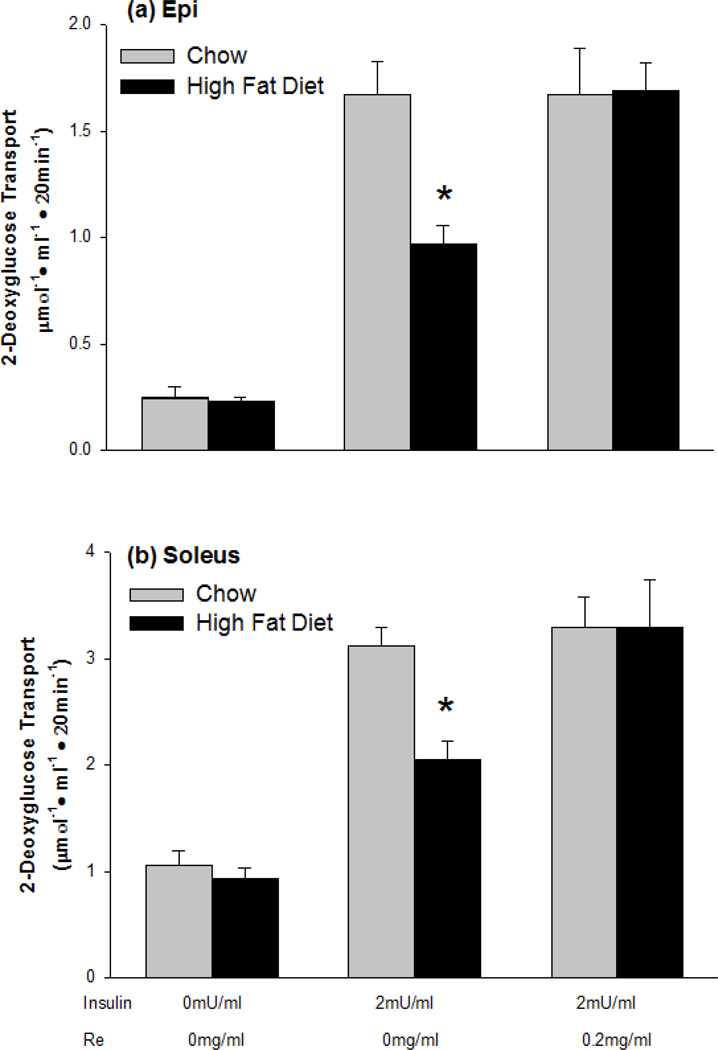
Treatment of muscles with Re alone had no effect on glucose transport, Control 0.23 ± 0.02 µmol 2-DG·ml−1·20min−1, vs. 0.20 ± 0.02 µmol 2-DG·ml−1·20min−1 for Re treated epitrochlearis muscles. The high-fat diet resulted in a ~50% decrease in insulin responsiveness of glucose transport in both the epitrochlearis and soleus muscles (Fig. 1). Treatment of muscles with Re for 90 min completely reversed the high-fat diet induced insulin resistance in both the epitrochlearis muscle, which contains predominantly type 2b, fast-twitch white fibers, and the soleus which contains predominantly type 1, slow twitch red fibers (Fig. 1). Treatment with Re had no effect on insulin responsiveness of glucose transport in muscles of chow fed rats (Fig. 1).
Figure 1.
Pretreatment of muscles with the ginsenoside Re for 90 min reverses high-fat diet-induced insulin resistance of glucose transport in (a) epitrochlearis and (b) soleus muscles. Values are means ± SE for 12 to 24 muscles per group. *P<0.001, Chow versus High-fat Diet.
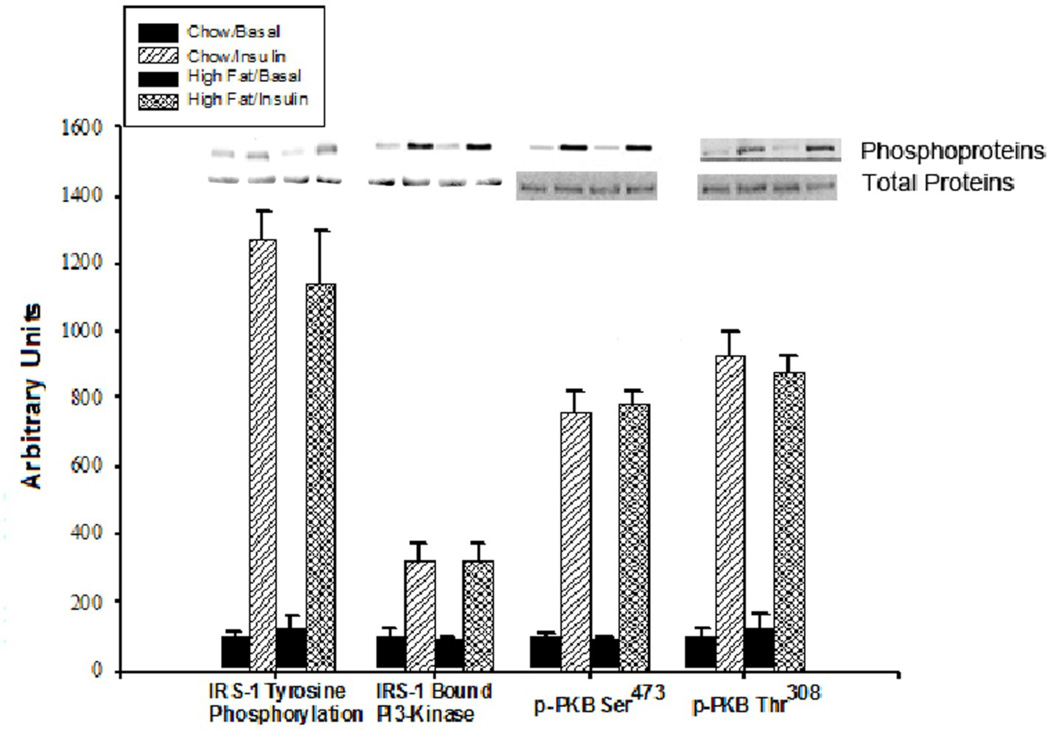
3.3 Insulin signaling
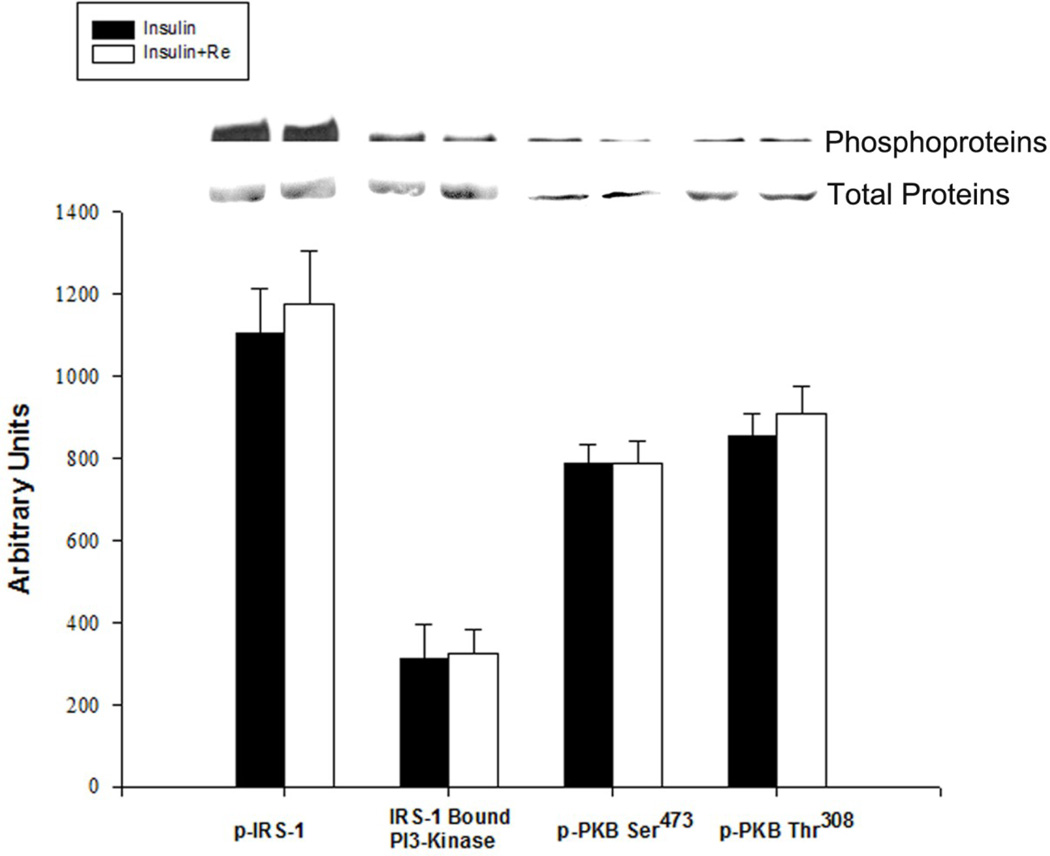
There were no significant differences in basal or insulin-stimulated insulin receptor substrate-1 (IRS-1) phosphorylation, phosphotyrosine bound phosphatidyl inositol-3 kinase (PI3-K) activity, protein kinase B (PKB) serine 473 phosphorylation or PKB/Akt threonine 308 phosphorylation between soleus muscles of chow fed rats and high-fat diet fed rats (Fig. 2). Treatment with Re had no effect on insulin-stimulated IRS-1 phosphorylation, phosphotyrosine bound PI3-K or PKB/Akt phosphorylation in muscles from high-fat diet fed or control rats (Fig. 3).
Figure 2.
Effects of a high-fat diet on insulin signaling in soleus muscles. Results are expressed in arbitrary O.D. units. Bars are means ± SE for 4 muscles in each basal group and 8 muscle for each insulin stimulated group.
Figure 3.
Effects of Re on insulin signaling in soleus muscles of high-fat diet fed rats. Results are expressed in arbitrary O.D. units. Bars are means ± SE for 4 muscles per group.
3.4 Insulin-stimulated increase in cell surface GLUT4 labeling
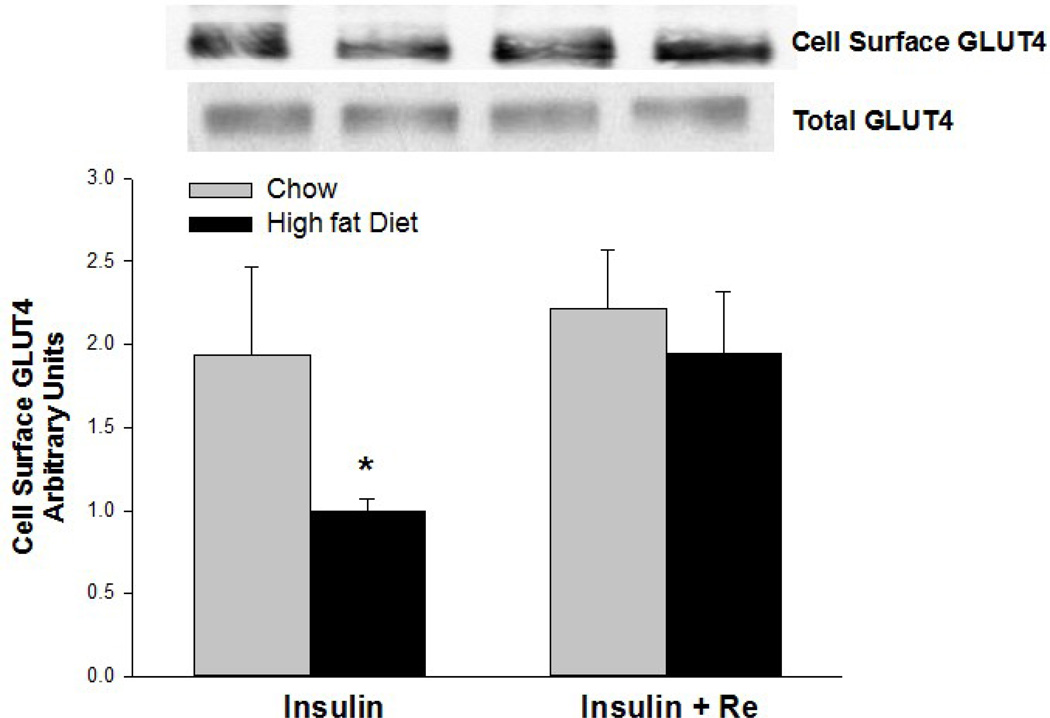
Insulin-stimulated GLUT4 translocation to the cell surface is impaired in muscles of rats fed a high-fat diet for 4–8 wk [11,22,23]. We, therefore, examined the possibility that Re acts by correcting a high-fat diet-induced reduction in GLUT4 translocation. As shown in Fig. 4, insulin-stimulated GLUT4 translocation to the cell surface was markedly decreased in epitrochlearis muscles of the high-fat diet fed rats. This impairment of the insulin-stimulated increase in cell surface GLUT4 was reversed by pretreatment with Re.
Figure 4.
Pretreatment of epitrochlearis muscles with Re for 90 min reverses impairment of the insulin-stimulated increase in cell surface GLUT4 induced by a high-fat diet. Values are means ± SE for 6 or 7 muscles per group. *P<0.01, Chow versus High-fat Diet.
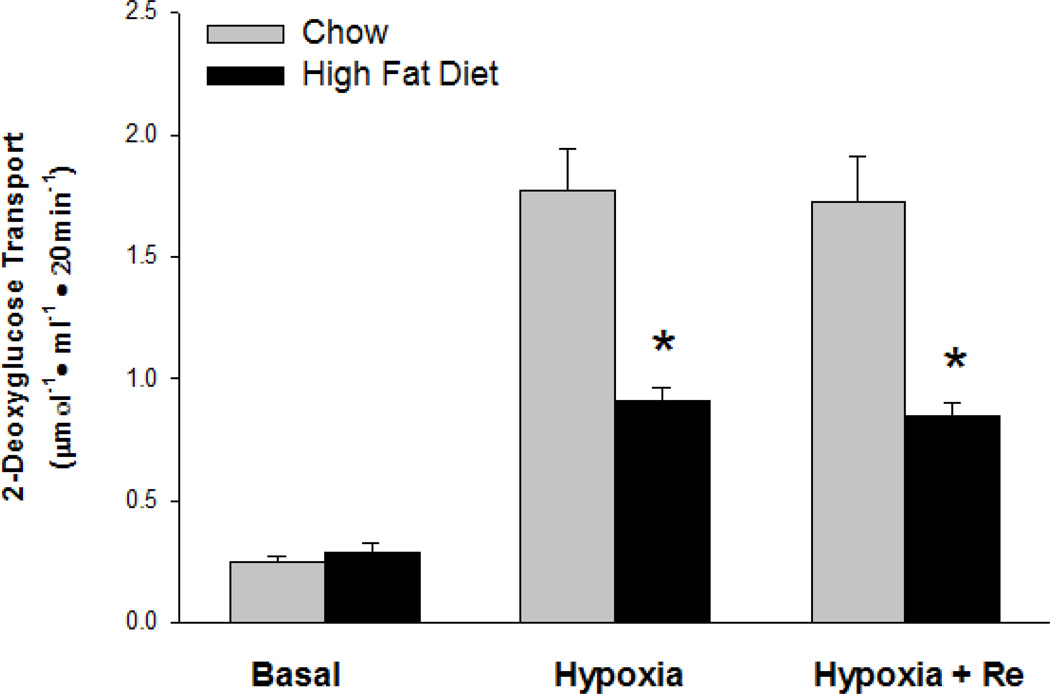
3.5 Re does not reverse high-fat diet-induced resistance of muscle glucose transport to contractions/hypoxia
Muscle contractions and hypoxia have powerful, insulin-like stimulating effects on glucose transport [24]. Like its effect on insulin-stimulated glucose transport, a high-fat diet results in severe resistance of muscle glucose transport to stimulation by contractile activity [10,11,25]. In contrast to the reversal of insulin resistance by Re in muscles from high-fat diet-fed rats, Re did not reverse the effect of the high-fat diet on resistance of muscle glucose transport to contractions (Fig. 5) or hypoxia (Fig. 6). Re did not affect tension development or degree of fatigue.
Figure 5.
Pretreatment of epitrochlearis muscles (a), and soleus muscles (b) for 90 min with Re did not reverse high-fat diet-induced resistance of glucose transport to muscle contractions. Values are means ± SE for 8 to 22 muscles per group. *P<0.01, Chow versus High-fat Diet.
Figure 6.
Pretreatment of epitrochlearis muscles with Re for 90 min did not reverse high-fat diet-induced resistance of glucose transport to stimulation by hypoxia. Values are means ± SE for 8 muscles per group. *P<0.01, Chow versus High-fat Diet.
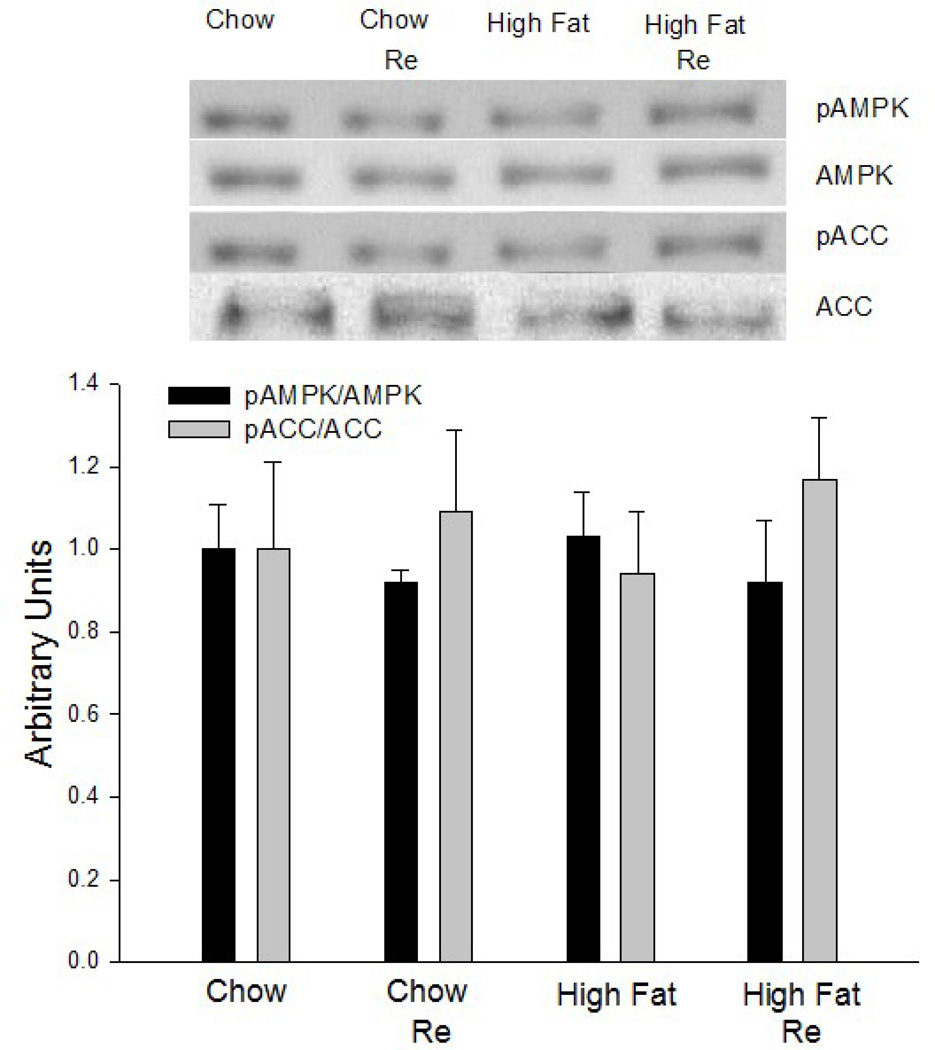
3.6 AMPK Activity
To evaluate the possibility that the effect of Re on high-fat diet-induced insulin resistance is mediated by an increase in AMPK activity, we determined the effect of Re on AMPK and ACC phosphorylation. As shown in Fig. 7, there was no increase in AMPK activity as reflected in the absence of increases in AMPK and ACC phosphorylation. This lack of effect is not surprising in light of the findings that Re did not increase glucose transport in control muscle.
Figure 7.
Pretreatment of epitrochlearis muscles with Re for 90 min did not result in increase in AMPK or ACC phosphorylation. Values are means ± SE for 8 muscles per group.
DISCUSSION
This study provides the new information that in vitro treatment of markedly insulin resistant muscles of high-fat diet fed rats with ginsenoside Re completely normalizes severely impaired insulin-stimulated glucose transport. Insulin-stimulated GLUT4 translocation to the cell surface was decreased to the same extent as glucose transport in the insulin resistant muscles. Pretreatment with Re reversed the impairment of insulin-stimulated GLUT4 translocation, which was likely responsible for the normalization of insulin-stimulated glucose transport by Re. Re appears to act specifically on the high-fat diet induced defect responsible for impairment of insulin-stimulated glucose transport. This is evidenced by the findings that Re had no effect on basal glucose transport, or on insulin-stimulated glucose transport, in muscles of chow fed rats. Furthermore, Re did not correct the resistance of glucose transport to stimulation by muscle contractions or hypoxia. We had assumed that the resistance to stimulation of glucose transport induced by a high-fat diet involves a step common to the insulin-signaling and contraction/hypoxia signaling pathways. However, the finding that Re does not correct the high-fat diet-induced resistance of glucose transport to contractions/hypoxia provides evidence that different steps are involved.
The reversal of insulin resistance in muscle is remarkably rapid, requiring, at the most, 90 min. This finding suggests that Re induces its effect by directly activating, or reversing inhibition, of a step in the insulin-signaling pathway that mediates GLUT4 translocation and/or insertion of GLUT4 into the plasma membrane. However, we have not been able to identify the step at which Re acts. Insulin-signaling is impaired in obese, insulin-resistant humans [26], but there is no way to determine whether this impairment is the cause or the result of the insulin resistance. An advantage of the high-fat diet fed rodent model is that it is possible to study the early events during development of insulin resistance and diabetes. In rats fed a high-fat diet, insulin receptor function and insulin-stimulated IRS-1 tyrosine phosphorylation are normal after 4 wk and 8 wk [11,27,28], but are severely impaired after 30 wk, [11] on the high-fat diet, providing evidence that the impairment of insulin signaling is secondary to the insulin resistance rather than its cause. A plausible mechanism is down regulation of insulin signaling proteins by chronic exposure of muscles to high insulin concentrations. However, some investigators reported that feeding rats a high-fat diet results in decreased activation of IRS-1, PI 3-kinase and PKB (Akt) by insulin in muscles within 4 wk [29–31], which could account for the development of insulin resistance. We have been unable to detect or confirm this impairment.
In conclusion, the results of this study show that ginsenoside Re rapidly, within 90 min, reverses insulin resistance of muscle glucose transport in rats fed a high-fat diet. This effect is mediated by reversal of severe impairment of insulin-induced GLUT4 translocation to the cell surface. The effect of Re is specific to insulin, as it does not correct high-fat diet-induced resistance of muscle glucose transport to stimulation by contraction or hypoxia.
ACKNOWLEDGEMENTS
We thank Victoria Reckamp for expert assistance in preparation of this manuscript and Wole Okunade for the chromatographic analysis of the crude ginseng preparation.
FUNDING
This research was supported by National Institutes of Health Grants DK18986, AT002390, AG000425 and DK56351.
LIST OF ABBREVIATIONS
- GLUT4
Glucose Transporter 4
- ATB-BMPA
Biotinylated 2N-4-(1-azi-2,2,2-trifluroethyl) benzoyl-1,3-bis (D-mannose-4-yloxy)-2-propylamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
AUTHOR’S CONTRIBUTIONS
Dong-Ho Han, Kenneth S. Polonsky, Samuel Klein and John O. Holloszy planned and designed the study; Dong-Ho Han, Sang Hyun Kim, Kazuhiko Higashida, Su-Ryun Jung performed the experiments; Kenneth S. Polonsky and Samuel Klein provided supplies, and John O. Holloszy wrote the manuscript.
REFERENCES
- 1.Kimura M, Waki I, Tanaka O, et al. Pharmacological sequential trials for the fractionation of components with hypoglycemic activity in alloxan diabetic mice from ginseng radix. J.Pharm.Dyn. 1981;4:402–409. doi: 10.1248/bpb1978.4.402. [DOI] [PubMed] [Google Scholar]
- 2.Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutrition, Metabolism & Cardiovascular Diseases. 2005;15:149–160. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kimura M, Suzuki J. The pattern of action of blended Chinese traditional medicines on glucose tolerance curves in genetically diabetic KK-CAy mice. J.Pharm.Dyn. 1981;4:907–915. doi: 10.1248/bpb1978.4.907. [DOI] [PubMed] [Google Scholar]
- 4.Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 5.Vuksan V, Sievenpiper JL, Koo VYY, et al. American Ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch.Int.Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 6.Attele AS, Zhou YP, Xie JT, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 7.Storlien LH, James DE, Burleigh KM, et al. Fat-feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. American Journal of Physiology. 1986;251:E576–E583. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 8.Storlien LH, Oakes ND, Pan DA, et al. Syndromes of insulin resistance in the rat. Inducement by diet and amelioration with Benfluorex. Diabetes. 1993;42:457–462. doi: 10.2337/diab.42.3.457. [DOI] [PubMed] [Google Scholar]
- 9.Kraegen EW, James DE, Storlien LH, et al. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment of euglycaemic clamp plus deoxyglucose administration. Diabetologia. 1986;29:192–198. doi: 10.1007/BF02427092. [DOI] [PubMed] [Google Scholar]
- 10.Han D-H, Hansen PA, Host HH, et al. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: a reevaluation. Diabetes. 1997;46:1761–1767. doi: 10.2337/diab.46.11.1761. [DOI] [PubMed] [Google Scholar]
- 11.Hansen PA, Han D-H, Marshall BA, et al. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. Journal of Biological Chemistry. 1998;273:26157–26163. doi: 10.1074/jbc.273.40.26157. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen EJ, Bourey RE, Rodnick KJ, et al. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. American Journal of Physiology. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- 13.Wright DC, Geiger PC, Han D-H, et al. Are tyrosine kinases involved in mediating contraction-stimulated muscle glucose transport? Am.J.Physiol. 2006;290:E123–E128. doi: 10.1152/ajpendo.00280.2005. [DOI] [PubMed] [Google Scholar]
- 14.Cartee GD, Douen AG, Ramlal T, et al. Stimulation of glucose transport in skeletal muscle by hypoxia. Journal of Applied Physiology. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- 15.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. Journal of Applied Physiology. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 16.Young DA, Uhl JJ, Cartee GD, et al. Activation of glucose transport in muscle by prolonged exposure to insulin: Effects of glucose and insulin concentration. Journal of Biological Chemistry. 1986;261:16049–16053. [PubMed] [Google Scholar]
- 17.Hansen PA, Nolte LA, Chen MM, et al. Increased GLUT4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. Journal of Applied Physiology. 1998;85:1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- 18.Kawanaka K, Nolte LA, Han D-H, et al. Mechanisms underlying impaired GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. American Journal of Physiology. 2000;279:E1311–E1318. doi: 10.1152/ajpendo.2000.279.6.E1311. [DOI] [PubMed] [Google Scholar]
- 19.Kawanaka K, Han D-H, Gao J, et al. Development of glucose-induced insulin resistance in muscle requires protein synthesis. Journal of Biological Chemistry. 2001;276:20101–20107. doi: 10.1074/jbc.M010599200. [DOI] [PubMed] [Google Scholar]
- 20.Geiger PC, Han D-H, Wright DC, et al. How muscle insulin sensitivity is regulated: testing of a hypothesis. Am.J.Physiol. 2006;291:E1258–E1263. doi: 10.1152/ajpendo.00273.2006. [DOI] [PubMed] [Google Scholar]
- 21.Han D-H, Hancock CR, Jung SR, et al. Deficiency of the mitochondrial electron transport chain in muscle does not cause insulin resistance. PLoS ONE. 2011 doi: 10.1371/journal.pone.0019739. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zierath JR, Houseknecht KL, Gnudi L, et al. High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes. 1997;46:215–223. doi: 10.2337/diab.46.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay F, Lavigne C, Jacques H, et al. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (ζ/λ) activities. Diabetes. 2001;50:1901–1910. doi: 10.2337/diabetes.50.8.1901. [DOI] [PubMed] [Google Scholar]
- 24.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. American Journal of Physiology. 2003;284:E453–E467. doi: 10.1152/ajpendo.00463.2002. [DOI] [PubMed] [Google Scholar]
- 25.Rosholt MN, King PA, Horton ES. High-fat diet reduces glucose transporter responses to both insulin and exercise. American Journal of Physiology. 1994;266:R95–R101. doi: 10.1152/ajpregu.1994.266.1.R95. [DOI] [PubMed] [Google Scholar]
- 26.Goodyear LJ, Giorgino F, Sherman LA, et al. Insulin Receptor Phosphorylation, Insulin Receptor Substrate-1 Phosphorylation, and Phosphatidylinositol 3-Kinase Activity are Decreased in Intact Skeletal Muscle Strips from Obese Subjects. Journal of Clinical Investigation. 1995;95:2195–2204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd JJ, Contreras I, Kern M, et al. Effect of a high-fat sucrose diet on in vivo insulin receptor kinase activation. American Journal of Physiology. 1990;259:E111–E116. doi: 10.1152/ajpendo.1990.259.1.E111. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M, Okamoto M, Kono S, et al. Effects of a high-fat diet on insulin receptor kinase and the glucose transporter in rats. J.Nutr.Biochem. 1992;3:241–250. [Google Scholar]
- 29.Frangioudakis G, Ye JM, Cooney GJ. Both saturated and n-6 polyunsaturated fat diets reduce phosphorylation of insulin receptor substrate-1 and protein kinase B in muscle during the initial stages of in vivo insulin stimulation. Endocrinology. 2005;146:5596–5603. doi: 10.1210/en.2005-0481. [DOI] [PubMed] [Google Scholar]
- 30.Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. Journal of Clinical Investigation. 2007;117:1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ropelle ER, Pauli JR, Prada PO, et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J.Physiol. 2006;577:997–1007. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]