Abstract
Purpose
To determine the change in intraocular pressure (IOP) after cataract extraction in the Observation Group of the Ocular Hypertension Treatment Study (OHTS).
Design
Comparative case series
Participants
Forty-two participants (63 eyes) who underwent cataract surgery in at least one eye during the study and a control group of 743 participants (743 eyes) who did not undergo cataract surgery
Methods
We defined the “split date” as the study visit date that cataract surgery was reported in the cataract surgery group, and a corresponding date in the control group. Preoperative IOP was defined as the mean IOP of up to 3 visits prior to split date. Postoperative IOP was the mean IOP of up to 3 visits including the split date (0, 6, and 12 months with ‘0 months’ equaling the split date). In both groups, we censored data after initiation of ocular hypotensive medication, or glaucoma surgery of any kind.
Main outcome measures
Difference in preoperative and postoperative IOP.
Results
In the cataract group, postoperative IOP was significantly lower than the preoperative IOP (19.8 ± 3.2 mmHg vs. 23.9 ± 3.2 p<0.001). The postoperative IOP remained lower than preoperative IOP for at least 36 months. The average decrease in postoperative IOP from preoperative IOP was 16.5%, and 39.7% of eyes had postoperative IOP ≥ 20% below preoperative IOP. A greater reduction in postoperative IOP occurred in the eyes with the highest preoperative IOP. In the control group, the corresponding mean IOP’s were 23.8 ± 3.6 prior to the split date and 23.4 ± 3.9 after the split date.
Conclusion
Cataract surgery decreases IOP in ocular hypertensive patients over a long period of time.
INTRODUCTION
Many individuals with ocular hypertension or glaucoma develop cataracts and require cataract surgery. Recent studies suggest that modern clear-cornea phacoemulsification cataract surgery lowers intraocular pressure (IOP) among many of these patients, and that this reduction is generally proportional to pre-surgical IOP.1–8 As helpful as these reports have been to guide clinical practice, they share a number of limitations – most are retrospective; most used only single baseline IOP and/or follow-up measurements; and most included a mix of treated and untreated patients. In addition, most were single-center studies, limiting the ability to generalize their results. Furthermore these studies were not designed to minimize regression to the mean9 or differential bias due to IOP lowering from ocular hypotensive medications. In contrast, the Ocular Hypertension Treatment Study protocol10 measured IOP according to a rigorous protocol at baseline and throughout follow-up in order to minimize regression to the mean and measurement bias.
The Ocular Hypertension Treatment Study (OHTS) began recruitment in 1994 and continued systematic follow-up for over a decade. The OHTS demonstrated that medical lowering of IOP decreases the risk of developing primary open-angle glaucoma among individuals with ocular hypertension.11 Some OHTS participants required cataract surgery in one or both eyes; and the OHTS protocol permitted them to undergo cataract surgery at the clinical center or by a community ophthalmologist. For this reason, the OHTS dataset provides a unique opportunity to evaluate the effect of cataract surgery on IOP with numerous masked measurements of IOP both before and after surgery in a well-characterized cohort. The present study describes the changes in IOP following cataract extraction among participants of the OHTS Observation group.
MATERIALS AND METHODS
Ocular Hypertension Treatment Study
The OHTS is a multicenter, randomized clinical trial to determine the safety and efficacy of ocular hypotensive medication in delaying or preventing the onset of primary open-angle glaucoma in individuals with elevated IOP. Eligibility criteria included age between 40 to 80 years, IOP between 24 mm Hg and 32 mm Hg in one eye and between 21 mm Hg and 32 mm Hg in the other eye, no evidence of either glaucomatous structural or functional damage by standard clinical measures, and best-corrected visual acuity of at least 20/40 in both eyes with no evidence of visually significant cataract. All participants provided written informed consent for participation in the study. The protocol for the OHTS is described in detail elsewhere.10 From February 1994 to October 1996, participants (n=1,636) were randomly assigned to a Medication Group (using topical ocular hypotensive medications) or to the Observation Group (no ocular hypotensive medications). The Institutional Review Boards at each OHTS center approved this study, and all participants signed an informed consent. The study was conducted in accordance with the tenets of the Declaration of Helsinki. In addition, a data and safety monitoring committee monitored the ethical conduct of the study and the accumulating data for adverse and beneficial treatment effects.
Racial classification of participants was by self-identification. OHTS measured central corneal thickness (CCT) 2 years after the start of randomization with a calibrated ultrasonic pachymeter (Pachette 500, DGH Technologies, Exton, PA). A previously published article12 describes the protocol for measurement of CCT.
Inclusion and Exclusion Criteria
This report only includes data from the observation group of OHTS, and excludes data from the medication group because the medication group underwent changes in ocular hypotensive medications during the study to maintain a specific protocol-derived target intraocular pressure. This report excluded eyes if: 1) they were not eligible for cataract surgery because of aphakia or pseudophakia at the enrollment visit; 2) history of trabeculectomy surgery after enrollment; 3) use of topical ocular hypotensive medication use; or 4) less than 1 year of follow-up. In addition, our analysis excluded data from OHTS II, in which all patients were offered ocular hypotensive medications.
Cataract surgery group
At each clinical center, the clinic coordinator recorded a history of cataract surgery or any other ocular surgery at each 6-month visit after enrollment. The coordinators did not collect the exact date of the cataract surgery, information regarding the method of cataract surgery (e.g., clear cornea or scleral tunnel), whether the operation had complications (e.g., vitreous loss), nor whether postoperative complications occurred (e.g., postoperative uveitis). A previous study13 reports the visual acuity, Lens Opacity Classification Score, visual field results, as well as other characteristics of those participants that underwent cataract surgery. Because the OHTS database did not include the exact date of surgery, we used a “split date”, which was defined as the study visit date that the participant reported cataract surgery. The visit considered the split date and subsequent follow-up visits are defined as “postoperative”.
We excluded eyes from the cataract surgery group if their history included: 1) use of topical ocular hypotensive medications prior to the split date; 2) no visits prior to or after the split date; and 3) laser peripheral iridotomy within 12 months prior to the split date.
Control group
A control group was created by randomly selecting one eye from observation group participants who had not undergone cataract in either eye, and met the same inclusion and exclusion criteria. After determining the first and last visit for each eye, we stratified the control eyes by follow-up time and randomly selected eyes within follow-up strata so that the distribution of follow-up visits of the control eyes corresponded to the split dates in the cataract surgery group.
Intraocular Pressure
An operator used a calibrated Goldmann applanation tonometer to measure IOP by looking through the slit-lamp and adjusting the tonometer dial without looking at the measurement. A separate person read and recorded the measurement. They repeated the process once; and if the two measures differed by more than 2 mm Hg, they performed a third measurement. Visit IOP was the mean of these 2, or the median of 3 IOP measurements. A similar process was completed for the contralateral eye. Every attempt was made to keep subsequent IOP measurements within a four-hour time window to reduce diurnal fluctuation.
Preoperative IOP was the mean IOP of up to 3 visits prior to split date (e.g., 18, 12 and 6 months prior). Postoperative IOP was the mean IOP of up to 3 visits starting with the visit of the split date (e.g., 0, 6, and 12 months after [with ‘0 months’ equaling the split date]).
Statistical Methods
We compared the baseline ocular, clinical, and demographic characteristics of the control group to those in the cataract surgery group. Differences in baseline variables were tested with chi-square tests and mixed model analysis of variance (ANOVA) as applicable.
Time to cataract surgery was defined as the time in months from randomization to the split date. Time from cataract surgery was defined as months from the split date. We used Kaplan-Meier methods to estimate the median time to cataract surgery for the cataract group and adjust for different follow-up times. Data were censored after events that could alter the natural history of post-operative IOP. These included: 1) laser iridotomy; 2) laser-assisted in situ keratomileusis (LASIK); and 3) initiation of topical ocular hypotensive medication.
To test for differences in preoperative and postoperative IOP and to construct the 95% confidence interval for mean change, we used a mixed model repeated measures ANOVA which adjusted for repeated IOP measures over time for a given eye and the inter-correlation between the two eyes of a single participant. To calculate the slope of IOP change after cataract surgery in the cataract group, we used a mixed model repeated measures ANOVA, to adjust for repeated postoperative IOP measures over time and the inter-correlation between two eyes of a single participant.
Pearson correlation and multiple regression analyses were used to analyze the association of demographic and clinical factors with postoperative IOP in the surgical participants. For participants who had bilateral cataract surgery, the first eye to undergo surgery was used in the multiple regression analysis.
RESULTS
Demographic and clinical characteristics of the cataract and control group
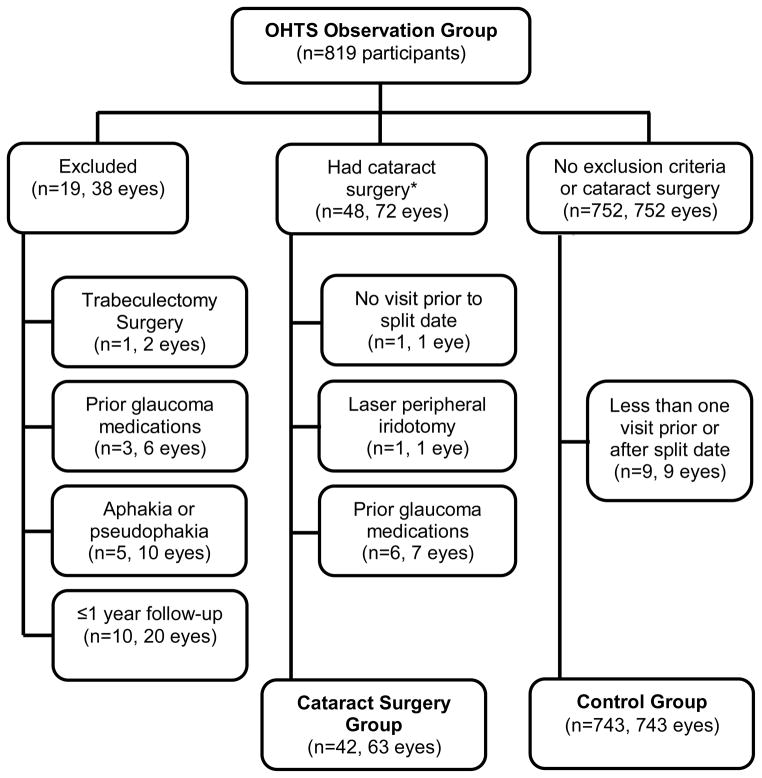
Figure 1 is a flow diagram describing the patients included, and excluded in this report. We excluded 19 participants from the analysis dataset because of a history of trabeculectomy surgery, aphakia or pseudophakia at the enrollment visit, topical ocular hypotensive medication use, or less than 1 year of follow-up from enrollment. Cataract surgery was performed in 72 eyes of 48 participants. In the cataract surgery group, we excluded 9 eyes of 6 participants for: no visits prior to or after the visit in which cataract surgery was reported; laser peripheral iridotomy within 12 months of the visit cataract surgery was reported; and/or use of topical ocular hypotensive medications prior to cataract surgery. Therefore, the cataract surgery group includes 63 eyes of 42 participants (n=21 unilateral cataract surgery and n=21 bilateral cataract surgery). The median time was 57 months from enrollment to the split date (first study visit after cataract surgery in the first eye).
Figure 1.
Flowchart of participants included into the current report of the Intraocular pressure (IOP) change after cataract surgery in the Ocular Hypertension Treatment Study (OHTS). The cataract group includes 63 eyes of 42 participants who had cataract surgery and the control group includes 743 eyes of 743 participants. Split date is the date that the participant reported cataract surgery. *Two participants in the cataract group had more than one exclusion criteria.
Seven hundred fifty two participants were eligible for the control group. We excluded 9 participants because they had no visits prior to or after the randomly determined split date. Therefore, the control group includes 743 eyes of 743 participants.
Table 1 compares the demographic and ocular characteristics of the cataract surgery group to the control group. Compared to the control group, participants undergoing cataract surgery were older at baseline (64.1 ± 8.9 vs. 55.0 ± 9.4 years, p<0.0001) and had thicker central corneal thickness measurements (584.7 ± 33.7 vs. 574.3 ± 38.4, p=0.04). No statistical significant differences between groups (p<0.05) were detected for race, gender, marital status, highest education, baseline horizontal or vertical cup/disc ratio, or mean pre-operative IOP.
Table 1.
Baseline characteristics in the cataract surgery and control group in the Ocular Hypertension Treatment Study. Data are presented in mean ± standard deviation unless otherwise noted.
| Cataract surgery group (n=42, 63 eyes) | Control group (n=743, 743 eyes) | p-value* | |
|---|---|---|---|
| Age, years | 64.1 ± 8.9 | 55.0 ± 9.4 | <0.001 |
| Female, % | 66.7 | 57.6 | 0.25 |
| African American, % | 14.3 | 25.6 | 0.10 |
| Some College, % | 59.5 | 67.3 | 0.30 |
| Central corneal thickness** (microns) | 584.7 ± 33.7 | 574.3 ± 38.4 | 0.04 |
| Horizontal cup to disc ratio | 0.39 ± 0.22 | 0.36 ± 0.18 | 0.31 |
| Vertical cup to disc ratio | 0.39 ± 0.23 | 0.39 ± 0.20 | 0.99 |
| Preoperative intraocular pressure† (mmHg) | 23.9 ± 3.2 | 23.8 ± 3.6 | 0.88 |
Comparison of the cataract surgery group to the control group using a mixed model analysis of variance or chi-square test as applicable. Eye specific data were adjusted for inter-correlation between the two eyes of a single participant using this mixed effects model with subject number as a random effect.
Central corneal thickness measurements were missing for 3 participants in the cataract surgery group and 82 participants in the control group (n=60 in cataract surgery group, and n=661 in control group)
Preoperative intraocular pressure (IOP) was the mean IOP of up to 3 visits prior to the visit that cataract surgery was reported, or the mean IOP of up to three visits before the split date in the control group.
Post-operative IOP
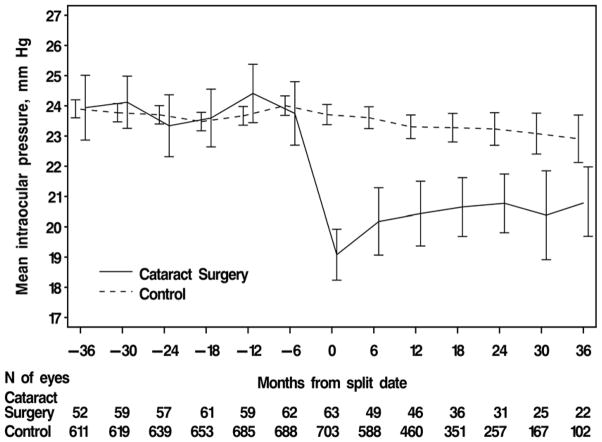
Figure 2 shows the mean IOP in the cataract group at pre-and postoperative visits and mean IOPs in the control group at visits corresponding to pre-postoperative visits. The split date in which cataract surgery was reported is indicated by “0” and visits thereafter are indicated by positive values. Visits prior to the split date are indicated by negative values. The mean postoperative IOP in the cataract surgery group was significantly lower compared to the mean preoperative IOP (19.8 ± 3.2 vs. 23.9 ± 3.2 mm Hg, p<0.001). The estimated mean decrease in IOP postoperatively in the cataract surgery group was 4.0 mm Hg (95% CI: 3.4–4.7, mixed model ANOVA). Figure 2 also shows a significant lowering of mean IOP that had not returned to the baseline IOP by 36 months. However, a significant trend (Slope=0.05 mm/month, p<0.001, 95% CI: 0.02–0.07) for increasing postoperative IOP is shown.
Figure 2.
Intraocular pressure (IOP) before and after cataract surgery in the Ocular Hypertension Treatment Study. Month 0 is the split date or the study visit that the participant reported cataract surgery, or a randomly selected, corresponding date in the control group. Preoperative IOP was the mean IOP of up to 3 visits prior to the split date. Postoperative IOP was the mean IOP of up to 3 visits including the split date (0, 6, and 12 months). In the cataract surgery group, the mean postoperative IOP was lower than the mean preoperative IOP (23.9 ± 3.2 vs.19.8 ± 3.2 mm Hg, p<.0001, mixed model analysis of variance). In the control group, the mean IOP before and after the split date IOP were 23.8 ± 3.6 vs. 23.4 ± 3.9 mm Hg, p<.002, respectively. Error bars are ± two standard errors of the mean.
In the control group, the mean IOP for visits corresponding to pre-and postoperative visits in the cataract group were 23.8 ± 3.6 and 23.4 ± 3.9 respectively. There was a slight mean decrease in IOP in the control group of 0.3 mm Hg (95% CI 0.1 to 0.4 mm Hg, p<.002, mixed model ANOVA).
In the cataract group, the average percent decrease from preoperative IOP was 16.5% (95%CI: 13.2–19.9%), and 39.7% of eyes had postoperative IOP ≥ 20% below preoperative IOP. Table 2 shows the distribution of percent change in the mean postoperative IOP from the mean preoperative IOP.
Table 2.
Distribution of percent change in mean postoperative intraocular pressure (IOP) from mean preoperative IOP. Overall, the cataract surgery group had a 16.5% decrease in mean postoperative IOP from preoperative IOP in the Ocular Hypertension Treatment Study cataract surgery group (n= 63 eyes of 42 participants).
| Percent Change in Mean Postoperative IOP from Mean Preoperative IOP* | Number of eyes | Percent of Eyes |
|---|---|---|
| ≥ 30% Decrease | 10 | 15.9% |
| 20% to 29% Decrease | 15 | 23.8% |
| 10% to 19% Decrease | 20 | 31.7% |
| 0% to 9% Decrease | 11 | 17.5% |
| Increase from preoperative IOP** | 7 | 11.1% |
Preoperative IOP was the mean IOP of up to 3 visits prior to split date (e.g., 18, 12 and 6 months prior). Postoperative IOP was the mean IOP of up to 3 visits starting with the visit of the split date (e.g., 0, 6, and 12 months after [with ‘0 months’ equaling the split date]). Percent change is calculated: ((mean postoperative IOP – mean preoperative IOP)/mean preoperative IOP)*100%.
7 eyes had an increase in mean postoperative IOP compared to mean preoperative IOP. Percentage increase was 0.7%, 2.8%, 3.2%, 6.4%, 12.3%, 13.4%, and 18.3%.
Postoperative IOP data in the cataract group and corresponding IOP data in the control group were censored after events that could affect postoperative IOP including laser iridotomy, laser-assisted in situ keratomileusis (LASIK), or initiation of topical ocular hypotensive medication. Fewer eyes in the cataract surgery group were censored when compared to the control group for starting ocular hypotensive medications (6.3% (4/63) vs. 16.3% (121/743), p =0.04). No eyes in the cataract surgery group were censored for laser iridotomy or LASIK whereas in the control group, a total of 13 (1.7%) eyes were censored- 7 eyes for LASIK (n=7) and 6 eyes for laser iridotomy.
Factors associated with postoperative IOP
Pearson correlation coefficients between the postoperative IOP of the first eye to undergo cataract surgery (n=42) and demographic/clinical factors were as follows: mean preoperative IOP (r=0.53, p<0.001), central corneal thickness (r=0.19, p=0.24), baseline age (r=−0.14, p=0.37), gender (r=0.05, p=0.73) and race (r=−0.14, p=0.38). The only factor statistically significantly associated with postoperative IOP in the multivariate regression equation that included the aforementioned variables was preoperative IOP (p<.001). Higher preoperative IOP was associated with higher postoperative IOP and conversely lower preoperative IOP was associated with lower postoperative IOP.
We analyzed the percent change in postoperative IOP from preoperative IOP in 63 eyes stratified by tertiles of mean preoperative IOP with approximately 21 eyes each. In the lowest tertile of preoperative IOP (IOP less than 22.3 mm Hg), the mean percent change in postoperative IOP was −11.0% ± 13.1% (95% CI: −17.1% to −4.9%); in the 2nd tertile of preoperative IOP (22.3 and 25.0 mm Hg), the mean percent change in postoperative IOP was −16.2 ± 11.9 % (95% CI: −21.3% to −11.1%) and; in the 3rd tertile of preoperative IOP (greater than 25 mm Hg), the mean change in postoperative IOP was −22.5 ± 12.7 % (95% CI: −28.4% to −16.5%).
DISCUSSION
We have confirmed, in a well-characterized ocular hypertensive cohort, that cataract surgery with intraocular lens implantation lowers IOP. Cataract surgery decreased postoperative IOP by 4.0 mm Hg, resulting in a 16.5% decrease from preoperative IOP, which was sustained at that level for at least a year. The effect persisted, but diminished over the next 2 years. The group with the highest preoperative IOP had the largest percentage drop in postoperative IOP.
Bigger and Becker suggested in the 1970’s that cataract surgery lowered IOP.14 However, a systematic review of surgical strategies for coexisting glaucoma and cataract published as recently as 2002 found no randomized clinical trials or cohort studies that addressed the question of whether cataract surgery had a long-term effect upon IOP in glaucoma patients.15
Two retrospective studies reported the long-term effect of cataract surgery. Shingleton6 reviewed the records of approximately 150 patients, evenly divided between those with glaucoma, those who were glaucoma suspects, and those without glaucoma, who underwent cataract surgery and were followed for at least 3 years. They reported a mean decrease of about 1.5 mm Hg in all 3 groups at 3 years. Many eyes were treated with ocular hypotensive medications both before and after surgery, and many did not have elevated IOP. Poley3, 4 reported IOP changes after cataract surgery for up to 10 years with follow-up of 4 years or greater in 50% of eyes. They stratified the eyes according to the level of pre-operative IOP, and reported that the higher the pre-operative IOP, the greater the reduction in IOP after cataract surgery. For example, the IOP reduction was 6.5 mm Hg in 19 eyes with pre-operative IOP between 23 and 31 mm Hg, but only 1.6 mm Hg in eyes with preoperative IOP in the 15–17 mm Hg range with a median follow-up of 4 years.
A prospective study by Samuelson16 reported the results of a regulatory trial including a ‘cataract surgery only’ group. At 12 months, they found an IOP reduction of 8.5 ± 4.3 mm Hg with cataract surgery alone in a group of ocular hypertension and early glaucoma patients. This result is greater than Shingleton6 and Poley3, 4, and twice the absolute change in IOP than in our current report. However, 35% of these eyes were back on ocular hypotensive medications at 12 months. Overall, these previous studies are difficult to compare to our current report, which censored data after initiating of ocular hypotensive medications.
Although highly suggestive of the IOP lowering effect of cataract surgery, the methods of the studies listed above did not include multiple measurements of IOP. The World Glaucoma Association published guidelines for measuring IOP in clinical trials.17 These included using a calibrated Goldmann tonometer, averaging of at least two IOP measurements during an exam, and using the mean of 3 IOP measurements taken on at least two separate days. We conformed to these guidelines, which is not surprising since the guidelines were generally adapted from the OHTS protocol and other randomized trials. Another strength of our study included analyzing eyes only in the untreated, OHTS observation group, eliminating the possibility of IOP-lowering effects from ocular hypotensive medications.
Shrivastava and Singh18 in a recently published review of the literature on IOP lowering after cataract surgery pointed out that the anterior chamber angle configuration may influence the amount of IOP lowering following cataract surgery. In particular, their review suggested that eyes with narrower anterior chamber angles experience a greater decrease in IOP after cataract surgery than eyes with open angles. All the eyes in the OHTS had gonioscopically open angles at the baseline visit, so the IOP lowering observed was not likely to be due to the conversion of a narrow angle to a more open angle. Another explanation may come from a post mortem study19 in human eyes, which demonstrated an association between increased facility of outflow and tension on the lens zonule in eyes with open angles. Cataract surgery with lens implantation may increase mechanical tension on the zonule with widening trabecular spaces and decreased outflow resistance. Similarly to the latter study, Meyer20 and Kee21 demonstrated increased outflow facility by tonography after phacoemulsification in eyes without glaucoma. Overall, the exact mechanism of IOP lowering after cataract surgery is unknown.
Using the OHTS data set, with its careful method for measuring IOP, we have confirmed the general results of previous studies, and provide important new information on the magnitude and duration of the IOP lowering effect of phacoemulsification in eyes with elevated IOP. The estimated mean drop of 0.3 mm Hg in the control group at visits corresponding to pre- and postoperative visits in the cataract group was statistically significant because of the large sample size, but is not likely to be clinically significant given its small magnitude. In comparison to the actual date of surgery (which is not known in the current report), we used a “split date”-an interval measure of time. Since OHTS visits were every 6 months; the split date represents an interval of time ranging from 0 to 6 months since surgery. Therefore, our methods may underestimate the duration of the reduction in intraocular pressure after cataract extraction by as much as 6 months.
Caution must be used in extrapolating our findings to eyes with lower IOP, higher IOP, and to eyes with glaucoma. Similarly, the results of this report should not be used to recommend a particular treatment (e.g., medications, laser, surgery) for ocular hypertension because the participants were not randomized to surgery and we are unable to compare differing treatments for their ability to decrease IOP. The OHTS database does not include cataract surgery complications. Since complications may increase IOP, our database may have a higher IOP when compared to a database that excluded eyes with complications. Fewer eyes in the cataract surgery group were censored when compared to the control group for starting ocular hypotensive medications (6.3% vs. 16.3%, p =0.04). We do not know whether the investigators started IOP medications because these eyes reached a protocol-derived treatment threshold (35 mm Hg), whether they developed glaucoma, or needed lower IOP for other reasons. Therefore, we are unable to state whether cataract surgery decreases the risk of developing glaucoma in patients with ocular hypertension; only that it decreases IOP.
Acknowledgments
The sponsors or funding agency had no role in the design or conduct of this research.
This work was supported by awards from the National Eye Institute, the National Center on Minority Health and Health Disparities, National Institutes of Health (grants EY09341, EY09307) and unrestricted grants from Merck Research Laboratories and Research to Prevent Blindness, Inc.
Footnotes
Disclosures:
S.L. Mansberger: C. Consultant/Advisor: Allergan; S. Grant Support: National Eye Institute; L: Merck
M.O. Gordon: S. Grant Support: National Eye Institute
H Jampel: S. Grant Support: National Eye Institute
A. Bhorade: None
J.D. Brandt: C. Consultant /Advisor: Alcon, MSD (Merck-Europe) ; S. Grant Support: National Eye Institute.
B.S. Wilson: S. Grant Support: National Eye Institute.
M.A. Kass: S. Grant Support: National Eye Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jahn CE. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J Cataract Refract Surg. 1997;23:1260–4. doi: 10.1016/s0886-3350(97)80325-2. [DOI] [PubMed] [Google Scholar]
- 2.Mathalone N, Hyams M, Neiman S, et al. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg. 2005;31:479–83. doi: 10.1016/j.jcrs.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 3.Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735–42. doi: 10.1016/j.jcrs.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 4.Poley BJ, Lindstrom RL, Samuelson TW, Schulze R., Jr Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–55. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Shingleton BJ, Gamell LS, O’Donoghue MW, et al. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25:885–90. doi: 10.1016/s0886-3350(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 6.Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494–8. doi: 10.1097/01.ijg.0000212294.31411.92. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R, Kuroki S, Fujiwara N. Ten-year follow-up of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation performed by the same surgeon. Ophthalmologica. 1997;211:79–83. doi: 10.1159/000310763. [DOI] [PubMed] [Google Scholar]
- 8.Tong JT, Miller KM. Intraocular pressure change after sutureless phacoemulsification and foldable posterior chamber lens implantation. J Cataract Refract Surg. 1998;24:256–62. doi: 10.1016/s0886-3350(98)80208-3. [DOI] [PubMed] [Google Scholar]
- 9.McDonald CJ, Mazzuca SA, McCabe GP., Jr How much of the placebo ‘effect’ is really statistical regression? Stat Med. 1983;2:417–27. doi: 10.1002/sim.4780020401. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 11.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 12.Brandt JD, Beiser JA, Kass MA, Gordon MO Ocular Hypertension Treatment Study (OHTS) Group. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–88. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 13.Herman DC, Gordon MO, Beiser JA, et al. Ocular Hypertension Treatment Study (OHTS) Group. Topical ocular hypotensive medication and lens opacification: evidence from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2006;142:800–10. doi: 10.1016/j.ajo.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigger JF, Becker B. Cataracts and primary open-angle glaucoma: the effect of uncomplicated cataract extraction on glaucoma control. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:260–72. [PubMed] [Google Scholar]
- 15.Friedman DS, Jampel HD, Lubomski LH, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology. 2002;109:1902–13. doi: 10.1016/s0161-6420(02)01267-8. [DOI] [PubMed] [Google Scholar]
- 16.Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–67. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Parrish RK, II, Minckler DS, Lam D, et al. Guidelines of design and reporting of glaucoma surgical trials. In: Shaarawy TM, Sherwood MB, Grehn F, editors. Guidelines on Design and Reporting of Glaucoma Surgical Trials. World Glaucoma Association; The Hague, Netherlands: Kugler; 2008. [Accessed February 22, 2012]. pp. 8–9. Available at: http://www.icoph.org/dynamic/attachments/resources/guidelinesglaucomasurgicaltrials.pdf. [Google Scholar]
- 18.Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21:118–22. doi: 10.1097/ICU.0b013e3283360ac3. [DOI] [PubMed] [Google Scholar]
- 19.Van Buskirk EM. Changes in the facility of aqueous outflow induced by lens depression and intraocular pressure in excised human eyes. Am J Ophthalmol. 1976;82:736–40. doi: 10.1016/0002-9394(76)90011-8. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology. 1997;104:1221–7. doi: 10.1016/s0161-6420(97)30154-7. [DOI] [PubMed] [Google Scholar]
- 21.Kee C, Moon SH. Effect of cataract extraction and posterior chamber lens implantation on outflow facility and its response to pilocarpine in Korean subjects. Br J Ophthalmol. 2000;84:987–9. doi: 10.1136/bjo.84.9.987. [DOI] [PMC free article] [PubMed] [Google Scholar]