Abstract
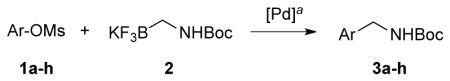
Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions were studied with potassium Boc-protected aminomethyltrifluoroborate through C-O activation of various mesylate derivatives to afford the corresponding products in moderate to good yields.
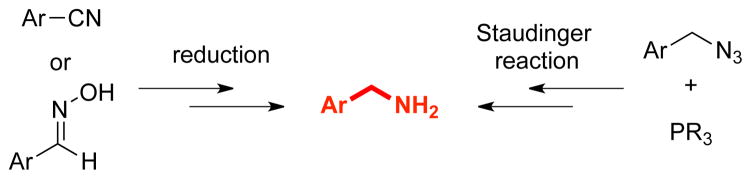
Aminomethylarenes are encountered in many bioactive materials.1 Primary aminomethyl subunits are particularly important targets for synthesis because they exhibit useful properties as drugs and inhibitors. Despite their importance, syntheses of aminomethyl substructures are not general. Reduction of aryl cyanides2 or oximes3 and the Staudinger reaction4 of azides have often been used to build aminomethyl moieties (Scheme 1). However, these approaches have limitations because of their sensitivity to reducible functional groups and the instability of azides,5 respectively.
Scheme 1.
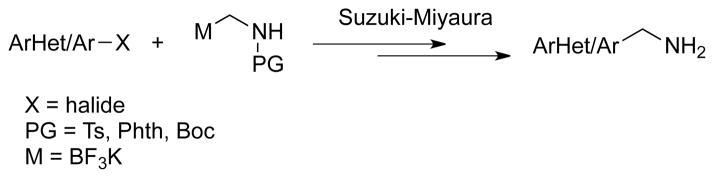
Transition metal mediated cross-coupling reactions of aminomethylmetallic species are one of the most straightforward strategies for preparation of primary aminomethyl moieties. To the best of our knowledge, three methods of Suzuki–Miyaura cross-coupling reactions to afford primary aminomethyl arenes using different protecting groups have been reported (Scheme 2).6 Among them, p-toluenesulfonyl (Ts)6a or N-phthalimido (Phth)6b,6c groups have been utilized, but are not ideal because the amine protecting groups are difficult to remove, requiring relatively harsh reaction conditions.7 Recently, we demonstrated that potassium Boc-protected aminomethyltrifluoroborate can be used as the coupling partner in Suzuki–Miyaura reactions.6d In that contribution, we reported the synthesis and cross-coupling reactions of potassium Boc-protected aminomethyltrifluoroborate 2. Potassium Boc-protected aminomethyltrifluoroborate, which is a primary aminomethyl equivalent, was synthesized through a ‘one-pot’ synthesis in good yield, and is now commercially available. Primary aminomethylarenes are readily available using this method after deprotection of the Boc group. The Boc protecting group is known to be easier to deprotect, compared to Ts or Phth groups, in acidic or even basic conditions.8 Therefore, using the Boc group is a more general approach to the primary amines.
Scheme 2.
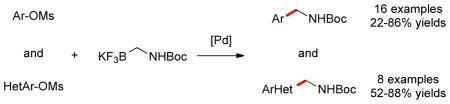
All of the previous reports employed aryl- and hetaryl halides as electrophilic partners in cross-coupling reactions. As alternative coupling partners, aryl- and hetaryl sulfonate derivatives have been utilized in Suzuki–Miyaura couplings.9,10,11 Sulfonate groups are generally easy to handle and are derived from a complementary set of starting materials. Mesylates are of special interest in terms of atom economy, low cost, and stability, even though they show the lowest reactivity among sulfonate derivatives.10 Recently, our group has demonstrated the feasibility of Suzuki–Miyaura cross-coupling reactions of aryl and hetaryl mesylates with tertiary ammoniomethyltrifluoroborates and amidomethyltrifluoroborates.11c To extend the scope of the this transformation, we investigated the Suzuki–Miyaura cross-coupling reaction of potassium Boc-protected primary aminomethyltrifluoroborate with various aryl- and hetaryl mesylates, providing an alternative entry to primary aminomethyl-substituted aromatics.
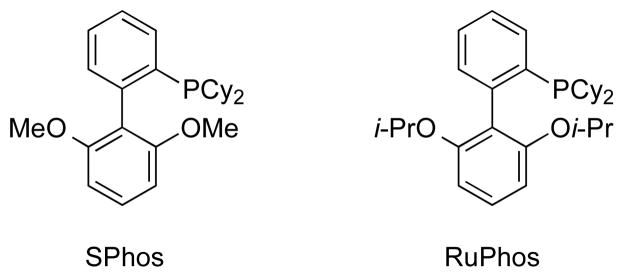
Initially, when the optimal conditions [Pd(OAc)2, SPhos or XPhos, K2CO3, and toluene/H2O, 85 °C, 22 h] for aryl- and hetaryl chlorides with potassium Boc-protected aminomethyltrifluoroborate 2 were applied to mesylates, only trace amounts of products were obtained.6d Keeping these results in mind, we screened the Suzuki–Miyaura cross-coupling with the mesylated 1-naphthol 1a based on the conditions related to C-O activation with potassium organotrifluoroborates reported previously.11 Potassium phosphate tribasic was chosen as a base in a mixture of t-BuOH/H2O. The coupling reactions were screened with different sources of palladium catalysts and ligands. Moreover, the reaction concentrations, ratio of two solvents, and temperature were studied extensively. After the optimization process, the combination of 1 equiv of mesylate, 1.1 equiv of trifluoroborate, 5 mol % of PdCl2(cod), 10 mol % of SPhos or RuPhos (Figure 1), and 7 equiv of K3PO4 in t-BuOH/H2O (1:1, 0.2 M) at 95 °C for 22 h turned out to be the best reaction conditions. Two different ligands were used because neither was general across the entire range of substrates.
Figure 1.
SPhos and RuPhos
With these optimized conditions in hand, we first studied the scope of the coupling reactions with various aryl mesylates (Tables 1 and 2).
Table 1.
Cross-Coupling of Aminomethyltrifluoroborate 2 with Various Electron-Neutral and Electron-Rich Aryl Mesylates
| |||
|---|---|---|---|
| entry | product | ligand | isolated yield (%) |
| 1 |
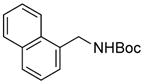 3a |
RuPhos | 79 (87)b |
| 2 |
 3b |
RuPhos | 66 |
| 3 |
 3c |
RuPhos | 72 |
| 4 |
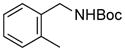 3d |
RuPhos | 72 |
| 5 |
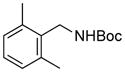 3e |
RuPhos | 22 |
| 6 |
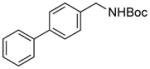 3f |
RuPhos | 86 |
| 7 |
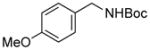 3g |
RuPhos | 59 |
| 8 |
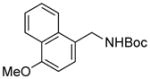 3h |
RuPhos | 84 |
Reaction conditions: 1.0 equiv of aryl mesylate, 1.1 equiv of trifluoroborate, 5 mol % of PdCl2(cod), 10 mol % of ligand, 7 equiv of K3PO4, t-BuOH/H2O (1:1, 0.2 M), 95 °C, 22 h.
4.0 mmol of mesylate, 3 mol % of PdCl2(cod), 6 mol % of RuPhos.
Table 2.
Cross-Coupling of Aminomethyltrifluoroborate 2 with Various Electron-Poor Aryl Mesylates
| |||
|---|---|---|---|
| entry | product | ligand | isolated yield (%) |
| 1 |
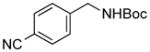 3i |
SPhos | 72 |
| 2 |
3j |
RuPhos | 46 |
| 3 |
3k |
SPhos | 42 |
| 4 |
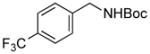 3l |
RuPhos | 83 |
| 5 |
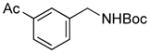 3m |
RuPhos | 82 |
| 6 |
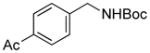 3n |
SPhos | 80 |
| 7 |
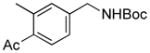 3o |
RuPhos | 81 |
| 8 |
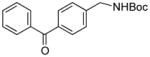 3p |
RuPhos | 86 |
Reaction conditions: 1.0 equiv of aryl mesylate, 1.1 equiv of trifluoroborate, 5 mol % of PdCl2(cod), 10 mol % of ligand, 7 equiv of K3PO4, t-BuOH/H2O (1:1, 0.2 M), 95 °C, 22 h.
Electrophiles with both electron-neutral and electron-rich substituents on the aryl rings represented good coupling partners in the desired reactions (Table 1). The reactions revealed that RuPhos was the most efficient ligand for all substrates containing electron-neutral and electron-donating groups on the aryl ring. With more sterically demanding substituents ortho to the mesylate group, the coupling yields dropped dramatically (Table 1, entries 4 and 5). The more sterically encumbered di-ortho substituted electrophile gave only a 22% isolated yield, perhaps because of a slow oxidative addition step (Table 1, entry 5).12 The reactions were also successful with electron-donating groups on the aryl ring (Table 1, entries 7 and 8). By increasing the reaction scale to 4 mmol of mesylated 1-naphthol 1a, the reaction could be carried out with a lower catalyst loading [3 mol % of PdCl2(cod), and 6 mol % of RuPhos] to obtain the corresponding product 3a with an 87% isolated yield (Table 1, entry 1).
We then investigated electron-poor aryl mesylates as electrophilic coupling partners (Table 2). All electron-deficient aryl mesylates gave the desired products 3i–p in moderate to good yields. In these cases, two different ligands (RuPhos and SPhos) were utilized to obtain the products, the yields of which depended on the nature of the functional groups and ligand utilized. As shown, a wide variety of functional groups, such as nitriles, aldehydes, esters, and ketones, were compatible with the reaction condition (Table 2). However, lower yields were observed with the aldehyde and methyl ester substituents on the aryl ring compared to other substrates (Table 2, entries 2 and 3).
We next expanded the array of electrophiles to hetaryl mesylates (Table 3). Various hetaryl mesylates were coupled with potassium Boc-protected aminomethyltrifluoroborate 2 in moderate to good yields. Again, two ligands (RuPhos and SPhos) were required to give better results depending on the nature of the hetaryl coupling partners. Nitrogen-containing hetaryl mesylates, such as pyridine, quinoline, isoquinoline, indole and thiazole, all provided the expected products in good yields (Table 3, entries 1–7). Interestingly, indole was successfully coupled without any protecting group in a 75% isolated yield (Table 3, entry 6). Moreover, sulfur-containing hetaryls also proved to be good coupling partners under the set of reaction conditions developed (Table 3, entries 7 and 8).
Table 3.
Cross-Coupling of Aminomethyltrifluoroborate 2 with Various Hetaryl Mesylates
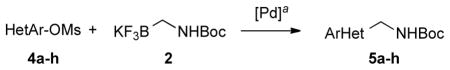
| |||
|---|---|---|---|
| entry | product | ligand | isolated yield (%) |
| 1 |
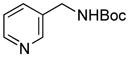 5a |
SPhos | 52 |
| 2 |
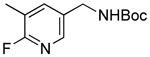 5b |
RuPhos | 78 |
| 3 |
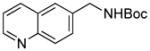 5c |
SPhos | 84 |
| 4 |
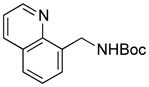 5d |
RuPhos | 87 |
| 5 |
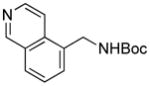 5e |
SPhos | 57 |
| 6 |
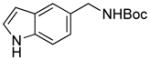 5f |
SPhos | 75 |
| 7 |
5g |
RuPhos | 86 |
| 8 |
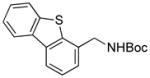 5h |
RuPhos | 88 |
Reaction conditions: 1.0 equiv of hetaryl mesylate, 1.1 equiv of trifluoroborate, 5 mol % of PdCl2(cod), 10 mol % of ligand, 7 equiv of K3PO4, t-BuOH/H2O (1:1, 0.2 M), 95 °C, 22 h.
In summary, we have shown that potassium Boc-protected aminomethyltrifluoroborate, an equivalent of a primary aminomethyl subunit, was a good coupling partner in Suzuki–Miyaura cross-coupling reactions with aryl- and hetaryl mesylates. A broad array of electrophiles, such as functionalized aryl mesylates and hetaryl mesylates, were coupled efficiently under standard coupling reactions. Further efforts to expand the scope of aminomethyl moieties are currently under study.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIGMS R01 81376) for their support of this research. We acknowledge Dr. Rakesh Kohli (University of Pennsylvania) for obtaining HRMS data.
Footnotes
Supporting Information Available Experimental procedures and spectral data of all compounds synthesized. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Musher DM, Fainstein V, Young EJ. Antimicrob Agents Chemother. 1980:254. doi: 10.1128/aac.17.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Campoli-Richards D, Lackner T, Monk J. Drug. 1987;34:411. doi: 10.2165/00003495-198734040-00001. [DOI] [PubMed] [Google Scholar]; (c) Kaczanowska K, Wiesmüller KH, Schaffner A-P. ACS Med Chem Lett. 2010;1:530. doi: 10.1021/ml100200c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Nystrom RF, Brown WG. J Am Chem Soc. 1948;70:3738. doi: 10.1021/ja01191a057. [DOI] [PubMed] [Google Scholar]; (b) Soffer LM, Katz M. J Am Chem Soc. 1956;78:1705. [Google Scholar]
- 3.a) Chandrasekharan J, Ramachandran PV, Brown HC. J Org Chem. 1985;50:5448. [Google Scholar]; (b) Bair KW, Tuttle RL, Knick VC, Cory M, McKee DD. J Med Chem. 1990;33:2385. doi: 10.1021/jm00171a012. [DOI] [PubMed] [Google Scholar]
- 4.a) Knölker H-J, Filali S. Synlett. 2003;11:1752. [Google Scholar]; (b) Gololobov YG, Zhmurova IN, Kasukhin LF. Tetrahedron. 1981;37:437. [Google Scholar]; (c) Gololobov YG, Kaukhin LF. Tetrahedron. 1992;48:1353. [Google Scholar]
- 5.(a) Agrawal JP, Hodgson R. Organic Chemistry of Explosives. Wiley; Chichester: 2007. [Google Scholar]; (b) Huynh MHV, Hiskey MA, Chavez DE, Naud DL, Gilardi RD. J Am Chem Soc. 2005;127:12537. doi: 10.1021/ja0509735. [DOI] [PubMed] [Google Scholar]
- 6.a) Molander GA, Fleury-Brégeot N, Hiebel M-A. Org Lett. 2011;13:1694. doi: 10.1021/ol200202g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tanaka K. WO 2008007670. PCT Int Appl. 2008; (c) Devulapally R, Fleury-Brégeot N, Molander GA, Seapy DG. Tetrahedron Lett. 2012;53:1051. doi: 10.1016/j.tetlet.2011.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Molander GA, Shin I. Org Lett. 2011;13:3956. doi: 10.1021/ol2014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3. John Wiley & Sons; New York: 1999. [Google Scholar]; (b) Kocienski PJ. Protecting Groups. 3. Georg Thieme; Stuttgart, New York: 2005. [Google Scholar]; (c) Hasan I, Marinelli ER, Lin LCC, Fowler FW, Levy AB. J Org Chem. 1981;46:157. [Google Scholar]; (d) Ravinder K, Reddy V, Mahesh KC, Narasimhulu M, Venkateswarlu Y. Synth Commun. 2007;37:281. [Google Scholar]
- 8.a) du Vigneaud V, Behrens OK. J Biol Chem. 1937;117:27. [Google Scholar]; (b) Kharasch MS, Priestley HM. J Am Chem Soc. 1939;61:3425. [Google Scholar]; (c) Snyder HR, Heckert RE. J Am Chem Soc. 1952;74:2006. [Google Scholar]; (d) Li S, Gortler LB, Waring A, Battisti A, Bank S, Closson WD, Wriede PJ. Am Chem Soc. 1967;89:5311. [Google Scholar]
- 9.For recent examples of Suzuki–Miyaura cross-coupling with sulfonate derivatives, see: Fan X-H, Yang L-M. Eur J Org Chem. 2011:1467.So CM, Lau CP, Chan ASC, Kwong FYJ. Org Chem. 2008;73:7731. doi: 10.1021/jo8014819.Petersen MD, Boye SV, Nielsen EH, Willumsen J, Sinning S, Wiborg O, Bols M. Bioorg Med Chem. 2007;15:4159. doi: 10.1016/j.bmc.2007.03.069.Zhang LA, Meng TH, Wu J. J Org Chem. 2007;72:9346. doi: 10.1021/jo7019064.Lipshutz BH, Butler T, Swift E. Org Lett. 2008;10:697. doi: 10.1021/ol702453q.
- 10.For recent examples of Suzuki–Miyaura cross-coupling with mesylates, see: Leowanawat P, Zhang N, Resmerita A-M, Rosen BM, Percec V. J Org Chem. 2011;76:9946. doi: 10.1021/jo202037x.Chow WK, So CM, Lau CP, Kwong FY. J Org Chem. 2010;75:5109. doi: 10.1021/jo100846t.Molander GA, Beaumard F. Org Lett. 2010;12:4022. doi: 10.1021/ol101592r.Kuroda JI, Inamoto K, Hiroya K, Doi T. Eur J Org Chem. 2009:2251.Bhayana B, Fors BP, Buchwald SL. Org Lett. 2009;11:3954. doi: 10.1021/ol9015892.So CM, Lau CP, Kwong FY. Angew Chem, Int Ed. 2008;47:8059. doi: 10.1002/anie.200803193.So CM, Kwong FY. Chem Soc Rev. 2011;40:4963–4972. doi: 10.1039/c1cs15114b.
- 11.a) Molander GA, Beaumard F. Org Lett. 2011;13:3948. doi: 10.1021/ol201469r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Beaumard F, Niethamer TK. J Org Chem. 2011;76:8126. doi: 10.1021/jo2015246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA, Beaumard F. Org Lett. 2011;13:1242. doi: 10.1021/ol200128y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alami M, Amatore C, Bensalem S, Choukchou-Brahim A, Jutand A. Eur J Inorg Chem. 2001:2675. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.