Abstract
Epithelial cells of the digestive tracts of most animals are short-lived, and are constantly replenished by the progeny of long-lived, resident intestinal stem cells. Proper regulation of intestinal stem cell maintenance, proliferation and differentiation is critical for maintaining gut homeostasis. Here we review recent genetic studies of stem cell-mediated homeostatic growth in the Drosophila midgut and the mouse small intestine, highlighting similarities and differences in the mechanisms that control stem cell proliferation and differentiation.
Introduction
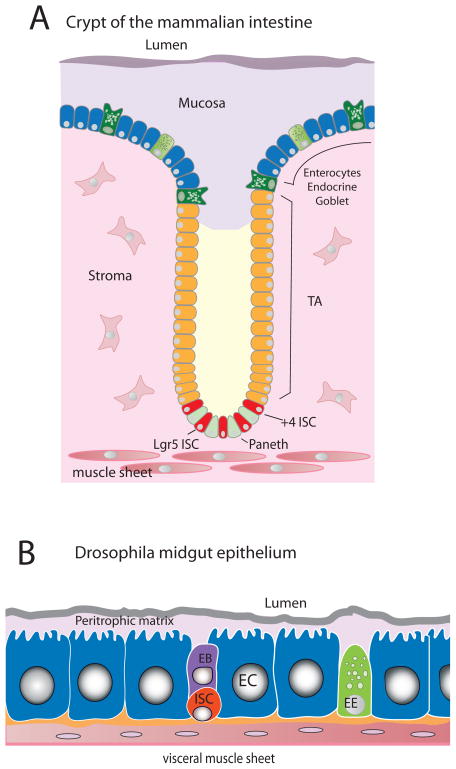
The endodermal portion of the insect intestine, termed the midgut, and its mammalian counterpart, comprising the stomach, small intestine and colon, serve as the animal’s principal organs for digestion and nutrient absorption. In the mammalian small intestine, absorptive enterocytes and secretory goblet and enteroendocrine cells reside in finger-like protrusions known as villi. These cells are short-lived, being constantly shed from the villi and replaced by new cells generated in neighboring invaginations called the Crypts of Lieberkühn. The intestinal epithelium is perhaps the most rapidly turned over tissue in mammals, with enterocyte lifespans averaging a week or less. Intestinal stem cells (ISCs) reside at the basal ends of the crypts, intermingled with long-lived Paneth cells of the secretory lineage (Figure 1A). ISCs proliferate to self-renew and also give rise to transient progeny that amplify through further divisions. As cells exit the crypts and move apically, they differentiate into either absorptive enterocytes or one of three types of secretory cells: Paneth, enteroendocrine, or goblet. The mammalian colon is similarly maintained by ISCs located in crypts, but villi are absent and replaced by a smooth epithelium. In addition to these endodermal cells produced by ISCs, the mammalian intestine has stromal cells of several types – mesenchymal fibroblasts, immune cells and others – and is surrounded by mesodermally derived visceral muscle.
Figure 1.
A) Schematic of a crypt of the adult mammalian small intestine, with villi omitted. B) Schematic of the midgut epithelium of adult Drosophila. EC: enterocyte; EE: Enteroendocrine cell; EB: enteroblast (transient undifferentiated cell); ISC: intestinal stem cell; TA: transient amplifying cells.
The endodermal portion of the Drosophila intestine, termed the midgut, undergoes similar dynamic cell turnover, also mediated by long-lived intestinal stem cells [1,2]. The fly midgut however lacks crypts and villi, instead comprising a cellular monolayer ensheathed by two orthogonal layers of visceral muscle. Intestinal stem cells reside at the basal side of this epithelium, sandwiched between enterocytes and basement membrane produced in part by visceral muscle (Figure 1B). They divide to self-renew and to give rise to committed progenitors (called enteroblasts), which directly differentiate, without cell division, into two functional cell lineages similar to those found in vertebrates: absorptive enterocytes and enteroendocrine cells. Differentiating enterocytes endoreplicate their genome 2–3 times to increase their size and develop a brush border similar to that in mammals. Drosophila lacks the Paneth, Goblet, Stromal, and Dentritic cells found in mammals, but some of their functions – such as immunity and barrier production - are fulfilled by enterocytes. Instead of the thick mucosa produced by mammalian goblet cells, insect intestines have a tough but relatively thin (~200μm) membrane called the peritrophic matrix. This matrix is comprised of the exoskeletal protein chitin and glycoproteins including mucins related to those found in vertebrate mucosa, and it provides an essential barrier against infection by enteric pathogens [3].
Intestinal stem cell niches
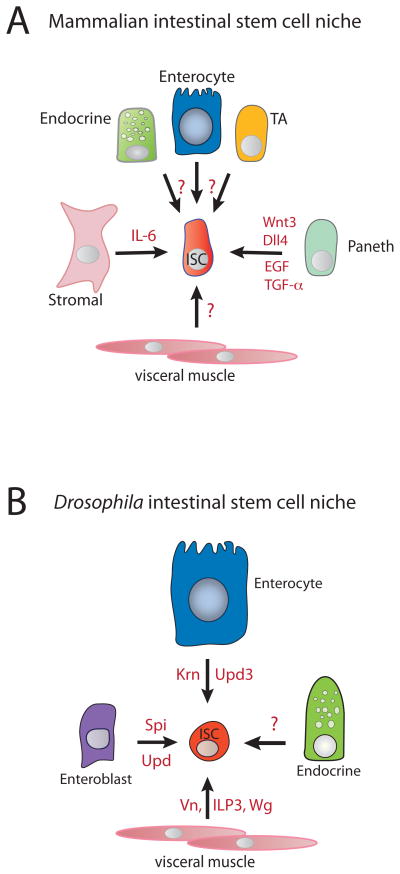
The location and number of intestinal stem cells in the crypts of the mammalian intestine have long been debated. Studies using improved stem cell markers and elegant cell lineage tracing techniques, however, suggest that there may be two inter-convertible stem cell types present in the crypts [4,5]. One cell type, located at the “+4” position in the crypt (see Figure 1A), is marked by Bmi, Tert and Hopx expression. These slow-cycling, label-retaining cells can produce entire intestinal cell lineages [6,7]. Another group of cells with stem properties, located at the bottom of the crypts and interdigitated with Paneth cells, have been called crypt basal columnar cells (CBCs). CBCs are marked by Lgr5, CD133 and Sox9 expression. These are fast-cycling cells that are also capable of giving rise to entire crypts and villi within 3 days [8–10]. Cell ablation and lineage tracing demonstrated that these two stem cell types could interconvert, suggesting that slow-cycling +4 Bmi+ stem cells might function as a reserve stem cell pool for fast-cycling, Lgr5+ CBCs [5]. In the mouse small intestine, the Lgr5+ stem cells reside between Paneth cells and the +4 Bmi+ cells reside just above the Paneth cell zone (Figure 1A). Gene expression profiling of Paneth cells revealed that they express essential regulators of ISC growth and survival, including EGF, TGF-α, Wnt3 and Dll4 [11]. Although early models invoked the intestinal stroma as a source of such niche factors, recent in vitro organoid culture experiments as well as in vivo genetic tests indicate that Paneth cells serve an essential supportive niche role for the ISCs [11,12]. As Paneth cells are long-lived secretory cells of the ISC lineage, it can be concluded that ISCs generate an important component of their own niche. Paneth cells have been reported to be absent from the colon, however, indicating that there may be more than one way to build a niche for ISCs.
The midgut epithelium of Drosophila lacks any cell type obviously analogous to a Paneth cell, but ISCs adhere via integrins to the visceral muscle. The muscle expresses several potential niche factors that are capable of promoting ISC growth, including wingless (a Wnt), vein (an Egfr ligand), and dilp3 (an insulin-like peptide) [13–15]. Hence the visceral muscle has been proposed to serve as the stem cell niche [13–15]. However, cells within the ISC lineage -differentiated enterocytes and transient enteroblasts - also produce survival and growth factors that support Drosophila’s ISCs. As detailed below these factors are especially important during gut epithelial regeneration following damage, but they are also likely to be used for stem cell maintenance. Hence, in so far as stem cell progeny comprise an important part of the niche, insects look somewhat similar to vertebrates. Interesting in this light is that, during the midgut’s development, stem-like progenitor cells proliferate to form clusters, and some cells within these clusters differentiate into peripheral cells that function as a transient niche for the progenitors that build the adult gut and contribute the adult ISCs [16,17]. These peripheral cells produce Dpp, a BMP-type signal that suppresses differentiation, and the EGFR ligands Spitz and Keren [17], potent ISC growth factors. Thus, during midgut development, stem-like progenitor cells also generate an essential part of their own niche. The in vitro self-assembly of intestinal organoids composed of crypts and villi from isolated murine ISCs and Paneth cells [11,12] suggests a similar capability in mice.
Stem cell proliferation, differentiation, and the regenerative response
Genetic analyses identified canonical Wnt signaling as a primary force in maintaining tissue homeostasis in the murine intestine. Mice lacking the Tcf4 transcription factor or β-catenin, positive effectors in Wnt signaling, have reduced proliferation in the intestinal epithelium that depletes transient amplifying cells, and consequent loss of crypts and villi [18–20]. Conversely, activating Wnt signaling drives hyperproliferation in the crypts, as do loss-of-function mutations in APC (adenomatous polyposis coli), a negative effector of Wnt signaling. Such mutations are associated with a majority of CRC, and their relevance as causal initiators of adenoma formation has been confirmed in several mouse models [19,21–24].
In contrast to the central role played by Wnt signaling in maintaining the stem/progenitor cell compartment in the murine intestine, alterations in Drosophila Wnt signaling show relatively mild phenotypes. Although the fly’s visceral muscle produces a Wnt ligand, wingless (wg), that is important in ISC maintenance [14,15], loss of both fly APC homologs or ectopic expression of Wg or ArmS10 (activated β-Catenin) lead only to slow onset dysplasia and modest increases in intestinal stem cell proliferation. In these cases, both differentiated cell types were still produced indicating that, wingless signaling does not control self-renewal or differentiation as in the mouse [14,25]. Further studies are needed, however, to fully appreciate the role of Wnt signaling in the Drosophila midgut.
In mammals, Notch signaling is also essential to maintain cells in the crypt compartment in their undifferentiated, proliferative state [26]. Activation of Notch signaling impairs secretory cell differentiation and leads to an increase in Wnt-dependent proliferating cells that expand to the crypt-villus boundary [27,28]. Consistent with a pro-proliferative function, mice that are mutant for CSL, a transcription factor required together with Notch for target gene activation, display the reciprocal phenotype: the proliferative compartment decreases at the expense of an expanded population of secretory (goblet) cells. Similar phenotypes were obtained with the γ-secretase inhibitor dibenzazepine (DBZ), when the Notch1 and Notch2 receptors were deleted [29], or when Dll1 and Dll4 ligands were deleted [30]. Further supporting Notch’s role in maintaining progenitor cells, the Notch ligand Dll4 is expressed by Paneth cells [11], and lineage tracing of Notch activity has indicated that Notch signaling is active in intestinal stem cells [31].
Notch is also a central regulator in the fly’s intestine, but its precise functions only partially overlap with those in the mouse. In the fly’s midgut, Notch is expressed in progenitor cells, including ISCs and their undifferentiated sisters, the enteroblasts. The Notch ligand, Delta (Dl) is expressed only in stem cells, however, resulting in asymmetric Notch activation only in enteroblasts [32]. Loss of Notch, Dl, or other pathway components such as Su(H) or Neuralized, or treatment with the γ-secretase inhibitor DAPT, all result in defective lineage commitment and a rapid, exponential expansion of stem-like cells that produce endocrine cells, but not enterocytes [1,2,32–34]. Conversely, ectopic expression of an activated Notch (Nintra) drives the rapid, direct differentiation of ISCs into enterocyte-like cells and consequently depletes the gut of stem cells [1]. Thus, although the role for Notch signaling in suppressing secretory cell fate is seen in both systems, Notch signaling has no apparent pro-proliferative function in the fly’s midgut. In considering how to reconcile this striking difference, Fre et al. [26] have noted that in both systems Notch activation may promote the transient cell fate, and that the principle difference may be that the fly’s transient cells, the enteroblasts, are post-mitotic.
Cytokines related to leptins and interleukins (termed Unpaireds, Upd) and ligands for the EGF receptor (Spi, Krn, Vn) act as growth, mitogenic, and survival factors for Drosophila intestinal stem cells [15,35–40]. Ectopic activation of Jak/Stat or EGFR signaling promotes rampant stem cell proliferation, leading to severe midgut hyperplasia, whereas midguts defective in either pathway suffer reduced rates of epithelial renewal, leading in some cases to atrophy. These functions are particularly prominent during regenerative growth following injury or enteric infection. Multiple ligands in each pathway are expressed by different midgut cell types, with some (Upd2, Upd3, Vn) being strongly induced following damage and others (Spi) more constitutive. Positive feedback between cells types and the EGFR, JAK-Stat, and JNK pathways generates a robust response akin to inflammatory signaling in mammals and capable of driving rapid epithelial repair. The activation of negative feedback inhibitors (e.g. SOCS, Puckered) helps to downregulate the response as repair is completed. These same cell- and signaling interactions appear to act at lower levels to maintain basal rates of epithelial replacement. From these observations, a feedback mechanism for regulating midgut homeostasis and regeneration was proposed, in which signaling from spent enterocytes promotes their replacement by activating the ISCs.
Although a similar sort of retrograde feedback from villi to crypts has not yet been rigorously elucidated in mammals, the mouse intestine regenerates rapidly following damage and appears to depend on Cytokines (e.g. IL-6) and Stat signaling to repair itself [41] [42]. Murine ISCs are also known to require EGFR/ErbB signaling for their growth [11,12]. Indeed a recent report has shows that a negative feedback regulator of ErbB/EGFR signaling, Lrig1, is highly expressed in the crypts, and that its deletion leads to excessive ISC proliferation, crypt expansion, and longer villi in the small intestine [43]. Thus Cytokine/Jak/Stat and Receptor Tyrosine Kinase/Ras/MapK signaling appear to have important roles in controlling ISC growth in the mouse. However, other signals such as Bone Morphogenetic Proteins (BMPs), Sonic Hedgehog (Shh) [44] or Ephs and ephrins [45] are also plausible regulators of crypt/villus balance. In any case it is difficult to imagine how the mouse intestine, being subject to extremely dynamic changes in nutrients and enteric ecology, could maintain homeostasis without a sophisticated system of feedback between differentiated cells and stem cells.
Recent work from both Drosophila and the mouse has also highlighted a role for Hippo signaling in intestinal epithelial homeostasis. In the mouse, loss of negative effectors (Mst1, Mst2, Sav) or gain of a positive downstream transcription co-activator (Yap1) in the Hippo signaling pathway results in small intestinal hyperplasia and adenoma formation. Furthermore, Yap was demonstrated to be induced and essential for effective intestinal regeneration following damage [46–48]. Similar results were obtained in Drosophila, with the additional insight that Hippo signaling in differentiated enterocytes was essential to restrain the proliferation of ISCs [49–53]. This surprising finding led to the proposal that Hippo signaling, as a known sensor of cell adhesion and cytoskeletal integrity, might be part of the first-line damage sensing mechanism to control expression of the cytokines and growth factors that regulate ISC proliferation. Such damage sensing functions could be relevant to diseases involving chronic inflammation in the intestine, such as ulcerative colitis and Crohn’s disease, which are risk factors for CRC. Indeed, mutations in the Hippo pathway are now being recognized in CRC. Nevertheless, the fly work showed that Hippo signaling is unlikely to be the only damage sensor in the intestinal epithelium, and other mechanisms, for instance JNK signaling, are believed to play major roles.
Symmetric vs. asymmetric stem cell division
To maintain tissue homeostasis, stem cells must balance self-renewal with differentiation. Early models proposed that stem cells are immortal and always divide asymmetrically, replacing themselves at each division, but quantitative lineage analysis in the murine intestine has ruled out this classical model for tissue maintenance [54]. Rather, lineage tracing showed that fast-cycling intestinal stem cells divide symmetrically and are lost stochastically, mostly to differentiation. At the population level, this loss rate averages 50%, and so a constant stem cell pool is maintained [54,55]. To explain the exact balance of ISC loss and duplication, it was postulated that Lgr5+ stem cells at the crypt base undergo “neutral” competition for niche signals provided by a limited number of Paneth cells, which thereby define the number of stem cells each crypt can support. The mechanisms determining the number of Paneth cells, and how new crypts emerge through crypt fission during regeneration, thus become interesting questions.
For Drosophila, initial clonal assays suggested that nearly all ISC divisions were functionally asymmetric [2,32], but a second look revealed a picture more similar to the mouse, in which stem cells can either be duplicated or lost to differentiation following symmetric divisions [13,56]. In this case it has been proposed that proximity of an ISC to the visceral muscle, which produces growth and survival factors such as an EGFR ligand (Vein), a Wnt (Wingless), and an insulin like peptide (dILP3), may promote stemness or at least ISC survival and maintenance [13,14,40]. It is noteworthy that Drosophila’s enteroblasts (EBs, Fig 1B), transient stem cell daughters that adhere to ISCs, also make an EGFR ligand (Spitz) and a cytokine (Upd) that promote ISC growth. This suggests that fly enteroblasts may function analogously to the mouse’s Paneth cells, as a limiting niche component that controls ISC number. In addition to this, any factor that tips the balance of lateral inhibition during Notch/Delta signaling between ISC/EB pairs will have a critical impact on ISC number in the fly’s midgut. The rate of ISC division is one such factor, since this determines the number of cells in the clusters that participate in Delta/Notch interactions. Also quite interesting in this regard is the finding [13] that increased insulin signaling, a response to feeding that stimulates ISC growth and division, can increase the likelihood of functionally symmetric, duplicative stem cell divisions. This can expand the stem cell pool, and consequently the size of the whole gut. Starvation, which reduces insulin signaling, was found to have the opposite effect, allowing gut shrinkage during fasting [13]. Effects of nutrition on the stem cell lineage have not yet been documented in a mammal, though a similar phenomenon was reported to occur during the establishment of crypts and villi in newborn mice [57]. Further advances in understanding the in vivo rules governing ISC duplication should be highly relevant to regenerative medicine, and also promise to help explain known connections between inflammation, nutrition, and stem cell derived cancers such as colorectal carcinoma.
Figure 2.
Factors that are known to stimulate ISC maintenance and proliferation in the A) mouse small intestine and B) Drosophila midgut and their cellular sources.
Acknowledgments
B.A.E. was supported by NIH R01 GM51186, the ERC, and the DKFZ. H.J. was supported by startup funds from University of Texas, Southwestern Medical Center. We thank Jeffery Rosa and Parthive Patel for comments on the manuscript, and Hanna Reuter and Julien Elric for help with figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult drosophila midgut epithelium. Nature. 2006;439(7075):475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 2*.Ohlstein B, Spradling A. The adult drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–474. doi: 10.1038/nature04333. In these two papers [1, 2], the authors showed that the adult Drosophila midgut undergoes dynamic renewal mediated by intestinal stem cells, and that Notch signaling regulates stem cell self-renewal and differentiation. [DOI] [PubMed] [Google Scholar]
- 3.Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in drosophila melanogaster. Proc Natl Acad Sci U S A. 108(38):15966–15971. doi: 10.1073/pnas.1105994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science (New York, NY. 2011;334(6061):1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. doi: 10.1038/nature10408. In these two papers [4, 5], the authors revealed the relationship between two intestinal stem cell pools, the Lgr5+ CBCs annd the Bmi+ +4 ISCs. While they are capable of giving rise to each other, the +4 stem cell pool are relatively quiescent and function as a reserve for active Lgr5+ CBCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mtert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108(1):179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature genetics. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. The authors [8] identified a new stem cell pool marked by Lgr5 in the mouse intestine. Lineage tracing experiments indicate that the Lgr5+ stem cells are capable of generating all intestinal epithelial cells, and are the likely source for intestinal renewal in mouse. [DOI] [PubMed] [Google Scholar]
- 9.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, et al. Continuous cell supply from a sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. In these two papers [11,12], the authors demonstrated that individual Lgr5+ stem cell could be cultured in vitro to form crypt-villus-like organoids without any mesenchymal cells. Similar to those in vivo, the crypts contain multiple Lgr5+ stem cells that are in direct contact with Paneth cells. They express multiple stem cell regulators and serve as an essential niche for stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 13**.O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147(3):603–614. doi: 10.1016/j.cell.2011.08.048. The authors [13] demonstrated that adaptive fly midgut growth in response to nutrient avaliability is driven not only by increased stem cell proliferation but also by the predominance of symmetric stem cell division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin G, Xu N, Xi R. Paracrine wingless signalling controls self-renewal of drosophila intestinal stem cells. Nature. 2008;455(7216):1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 15.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. Egfr, wingless and jak/stat signaling cooperatively maintain drosophila intestinal stem cells. Developmental biology. 2011;354(1):31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of drosophila intestinal stem cells. Science (New York, NY. 2010;327(5962):210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Edgar BA. Egfr signaling regulates the proliferation of drosophila adult midgut progenitors. Development. 2009;136(3):483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible cre-mediated control of gene expression in the murine gastrointestinal tract: Effect of loss of beta-catenin. Gastroenterology. 2004;126(5):1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Molecular and cellular biology. 2007;27(21):7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking tcf-4. Nature genetics. 1998;19(4):379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 21.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, et al. Loss of apc in vivo immediately perturbs wnt signaling, differentiation, and migration. Genes & development. 2004;18(12):1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, Romagnolo B. Crypt-restricted proliferation and commitment to the paneth cell lineage following apc loss in the mouse intestine. Development. 2005;132(6):1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 23.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, Kanamaru R, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the apc gene. Science (New York, NY. 1997;278(5335):120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 24.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. The EMBO journal. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates drosophila intestinal stem cell proliferation. Development. 2009;136(13):2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 26.Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: The cases of drosophila, zebrafish and the mouse. Exp Cell Res. 2011;317(19):2740–2747. doi: 10.1016/j.yexcr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by notch. Proc Natl Acad Sci U S A. 2005;102(35):12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 29.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both notch1 and notch2 is accompanied by derepression of cdk inhibitors p27kip1 and p57kip2. EMBO reports. 2008;9(4):377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 140(4):1230–1240. e1231–1237. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, Kopan R. Mapping the consequence of notch1 proteolysis in vivo with nip-cre. Development. 2007;134(3):535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Ohlstein B, Spradling A. Multipotent drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science (New York, NY) 2007;315(5814):988–992. doi: 10.1126/science.1136606. In this paper [32], the authors revealed that the expression levels of Notch ligand Dl in the intestinal stem cells dictates the differentiation fate of its progeny. [DOI] [PubMed] [Google Scholar]
- 33.Perdigoto CN, Schweisguth F, Bardin AJ. Distinct levels of notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 138(21):4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- 34.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the drosophila intestine. Development. 2010;137(5):705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biteau B, Jasper H. Egf signaling regulates the proliferation of intestinal stem cells in drosophila. Development. 2011;138(6):1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in drosophila. Genes & development. 2009;23(19):2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila egfr pathway coordinates stem cell proliferation and gut remodeling following infection. BMC biology. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. Egfr/ras/mapk signaling mediates adult midgut epithelial homeostasis and regeneration in drosophila. Cell stem cell. 2011;8(1):84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/jak/stat signaling mediates regeneration and homeostasis in the drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the jak/stat pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. Journal of molecular cell biology. 2009;2(1):37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 41.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. Il-6 and stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (socs3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26(33):4833–4841. doi: 10.1038/sj.onc.1210286. These two papers [41, 42] show that damage induced in the mouse colon by DSS (dextran sodium sulfate, a detergent) promoted IL-6/Stat and NF-kB signaling and cell proliferation in the crypts. These effects as well as colitis-associated tumorigenesis depended upon the STAT signaling inhibitor, SOCS3 and IL-6. [DOI] [PubMed] [Google Scholar]
- 43*.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of erbb signalling. Nat Cell Biol. 2012 doi: 10.1038/ncb2464. This paper [43] demonstrates more clearly than ever before that ErbB (an EGFR homolog) activity is a strong inductive signal for intestinal stem-cell proliferation in the mouse. The deletion of Lrig1, a negative effector of ErbB, increases the size of the crypts and the legnth of the villi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7(5):349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 45.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20(2):194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of yes-associated protein (yap) overabundance. Proc Natl Acad Sci U S A. 108(49):E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24(21):2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. Yap1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 49.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. Drosophila pez acts in hippo signaling to restrict intestinal stem cell proliferation. Curr Biol. doi: 10.1016/j.cub.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Karpowicz P, Perez J, Perrimon N. The hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 137(24):4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 107(49):21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The hippo pathway regulates intestinal stem cell proliferation during drosophila adult midgut regeneration. Development. 2010;137(24):4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staley BK, Irvine KD. Warts and yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 20(17):1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 55*.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science (New York, NY) 2010;330(6005):822–825. doi: 10.1126/science.1196236. In these two papers [54, 55], the authors demonstrated that Lgr5+ stem cells divide symmetrically and then compete for limited niche signals (Paneth cells) in the crypts, and are lost to differentiation stoichastically. [DOI] [PubMed] [Google Scholar]
- 56*.Navascues J. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. The EMBO journal. 2012 doi: 10.1038/emboj.2012.106. in press. This paper uses clonal analysis to demonstrate that Drosophila’s ISCs can divide symmetrically to duplicate, and are also lost at measurable rates. The paper uses a mathematical analusis to show that rates of ISC gain and loss are normally balanced, and so the stem cell pool remains constant. This phenomenon is termed “neutral competition”. It was also shown in the mouse [54, 55], and further demonstrated in Drosophila in [13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itzkovitz S, Blat IC, Jacks T, Clevers H, van Oudenaarden A. Optimality in the development of intestinal crypts. Cell. 148(3):608–619. doi: 10.1016/j.cell.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]