Abstract
Oxaloacetate is an intermediate of the citrate fermentation pathway that accumulates in the cytoplasm of Lactococcus lactis ILCitM(pFL3) at a high concentration due to the inactivation of oxaloacetate decarboxylase. An excess of toxic oxaloacetate is excreted into the medium in exchange for citrate by the citrate transporter CitP (A. M. Pudlik and J. S. Lolkema, J. Bacteriol. 193:4049–4056, 2011). In this study, transamination of amino acids with oxaloacetate as the keto donor is described as an additional mechanism to relieve toxic stress. Redirection of the citrate metabolic pathway into the transamination route in the presence of the branched-chain amino acids Ile, Leu, and Val; the aromatic amino acids Phe, Trp, and Tyr; and Met resulted in the formation of aspartate and the corresponding α-keto acids. Cells grown in the presence of citrate showed 3.5 to 7 times higher transaminase activity in the cytoplasm than cells grown in the absence of citrate. The study demonstrates that transaminases of L. lactis accept oxaloacetate as a keto donor. A significant fraction of 2-keto-4-methylthiobutyrate formed from methionine by citrate-driven transamination in vivo was further metabolized, yielding the cheese aroma compounds 2-hydroxy-4-methylthiobutyrate and methyl-3-methylthiopropionate. Reducing equivalents required for the former compound were produced in the citrate fermentation pathway as NADH. Similarly, phenylpyruvate, the transamination product of phenylalanine, was reduced to phenyllactate, while the dehydrogenase activity was not observed for the branched-chain keto acids. Both α-keto acids and α-hydroxy acids are known substrates of CitP and may be excreted from the cell in exchange for citrate or oxaloacetate.
INTRODUCTION
Transamination of amino acids is a key step in the formation of aroma compounds by lactic acid bacteria (LAB) in the food fermentation industry (24, 30). The reaction is a first step in amino acid catabolism and is catalyzed by pyridoxal-5′-phosphate (PLP)-dependent aminotransferases that convert amino acids into the corresponding α-keto acids. At the same time, a keto donor, usually α-ketoglutarate, is converted to the corresponding amino acid (i.e., glutamate). The α-keto acids are the precursors of many flavor compounds, like aldehydes, alcohols, carboxylic acids, or α-hydroxy acids, that are formed by subsequent enzymatic or spontaneous reactions, like decarboxylation, dehydrogenation, or oxidation. Unfortunately, for the dairy industry, conversion of amino acids into aroma compounds by LAB is often limited by lack of sufficient keto donor (27).
Citrate constitutes almost 90% of the organic acids in milk, where it is present at concentrations ranging from 4 to 10 mM (19). Citrate fermentation by LAB leads to the production of two potential keto donors for transamination reactions, i.e., oxaloacetate and pyruvate (8). However, the two best-characterized transaminases from lactic acid bacteria, AraT, specific for aromatic amino acids (20), and BcaT, specific for branched-chain amino acids (29), were reported to accept neither oxaloacetate nor pyruvate as a keto donor in their purified forms (20, 28). The aspartate transaminase AspC, encoded on the genome of Lactococcus lactis IL1403 by the aspB gene (2), did accept oxaloacetate (4), while the closest homolog of the alanine transaminase AlaT (encoded by the aspC gene) (2) found in Corynebacterium glutamicum showed the highest activity with pyruvate as a keto donor (12, 14). Moreover, analysis of L. lactis IL1403 genome sequence (2) revealed the presence of 9 other putative transaminase genes, among which ytjE was reported to be involved in cysteine and methionine metabolism (13). None of the other enzymes was functionally characterized.
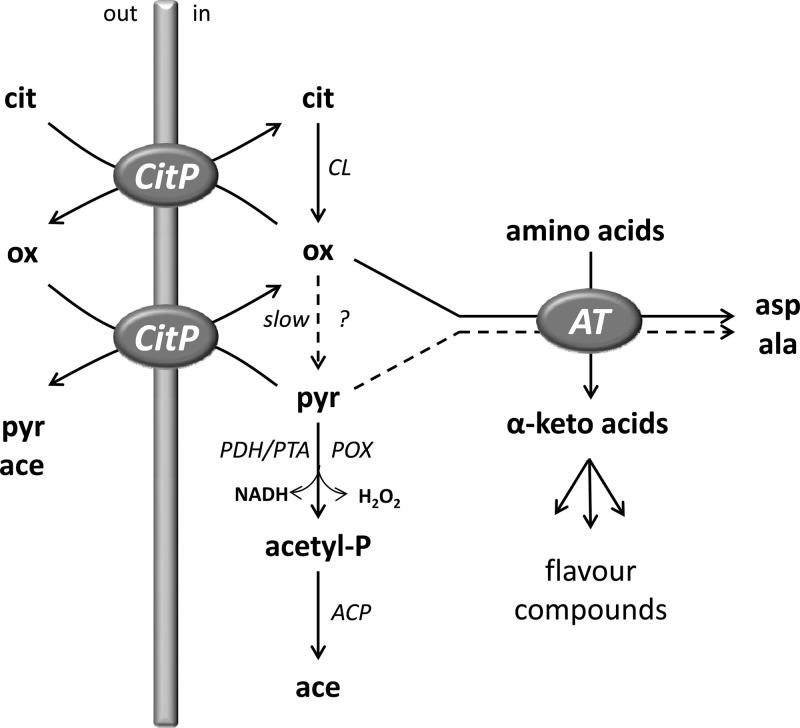
Recently, accumulation of oxaloacetate in the cells of L. lactis, which is widely used as a starter in the dairy industry, was demonstrated (17). In resting cells, citrate fermentation in the oxaloacetate decarboxylase-deficient mutant strain L. lactis ILCitM(pFL3), a derivative of strain IL1403 (1), was shown to proceed in two steps (Fig. 1) (17). The first step consists of a short pathway in which two enzymes are involved: the citrate transporter CitP and citrate lyase (CL). CL converts internal citrate into oxaloacetate (and acetate) with high efficiency. Oxaloacetate accumulates rapidly in the cytoplasm and is excreted in exchange for citrate by CitP. In the second step, which follows upon complete consumption of citrate, oxaloacetate reenters the cell via CitP in exchange for the available intermediates/end products pyruvate and/or acetate. During the whole process, internal oxaloacetate is slowly converted into pyruvate by the activity of a cryptic decarboxylase. Under these conditions, pyruvate from citrate is converted to the end product acetate (17).
Fig 1.
Citrate-driven transamination in L. lactis ILCitM(pFL3). See the text for an explanation. ?, cryptic oxaloacetate decarboxylase; ACP, acylphosphate phosphohydrolase; AT, aminotransferase; cit, citrate; ox, oxaloacetate; pyr, pyruvate; acetyl-P, acetyl phosphate; ace, acetate; asp, aspartate; ala, alanine.
The present study demonstrates that oxaloacetate produced by the oxaloacetate-deficient mutant strain L. lactis ILCitM(pFL3) can be used efficiently to drive transamination in the presence of amino acids. The α-keto acids produced from the amino acids are either exported by CitP into the medium (18) or further metabolized into flavor compounds. The cells use the transamination pathway as a second escape route to relieve the stress caused by the toxic levels of oxaloacetate, in addition to excretion of the intermediate from the cell.
MATERIALS AND METHODS
Chemicals.
2-Hydroxy-4-methylthiobutyrate, 2-keto-4-methylthiobutyrate, acetate, aldehyde dehydrogenase, citrate, diethylethoxymethylenemalonate (DEEMM), l-alanine, l-aminoadipic acid, l-cysteine, l-glutamine, l-histidine, l-isoleucine, l-leucine, l-lysine, l-phenylalanine, l-proline, l-serine, l-threonine, l-tryptophan, l-tyrosine, l-valine, methyl-3-methylthiopropionate, oxaloacetate, phenyllactate, phenylpyruvate, PLP, pyruvate, α-ketoglutarate, α-ketomethylvalerate, α-ketoisocaproate, and α-ketoisovalerate were obtained from Sigma-Aldrich Chemicals. l-Arginine, l-asparagine, l-aspartate, l-glutamate, l-glycine, and l-methionine were obtained from Merck. Solutions of amino acids were prepared in 50 mM potassium phosphate buffer, pH 5.8; if required, the pH was adjusted to 5.8 with 5 M KOH. l-Lactate dehydrogenase (l-LDH), l-malate dehydrogenase (l-MDH), and CL were obtained from Roche Applied Science. 3-Methylthiopropionic acid was obtained by saponification of methyl-3-methylthiopropionate (25) or by enzymatic reaction of methional catalyzed by 1 U of aldehyde dehydrogenase in the presence of 1.3 mM NAD+ at pH 8.0 at 37°C (26).
Bacterial strains and growth conditions.
L. lactis strain IL1403(pFL3) (11) and the oxaloacetate decarboxylase-deficient derivative ILCitM(pFL3) (1) were used in this study. Plasmid pFL3 harbors the lactococcal CRL264 citP gene under the control of the Streptococcus pneumoniae polA promoter (11). Neither the expression nor the plasmid copy number is under the control of citrate or pH in these strains (6). The mutant strain ILCitM(pFL3) was constructed by a deletion of 14 bp between positions 584 and 598 of the oxaloacetate decarboxylase gene mae (1). Precultures were grown overnight at 30°C in M17 broth medium supplemented with 0.5% (wt/vol) glucose (M17G) and 5 μg · ml of tetracycline−1. Cells were grown in M17G medium at an initial pH adjusted to 7.0. When indicated, 20 mM citrate, pH 7.0, was added to the medium. Growth was performed in 100-ml serum bottles without agitation and at 30°C. Growth was followed by measuring the optical density at a wavelength of 660 nm (OD660). Cells were harvested at mid-exponential growth phase when the optical density was 0.6 by spinning for 10 min at 3,000 rpm. The cells were washed two times with 50 mM potassium phosphate buffer, pH 5.8, and finally resuspended in the same buffer at 4°C.
Citrate-, oxaloacetate-, or pyruvate-driven transamination.
Resting cells at an OD660 of 1.5 in 50 mM potassium phosphate buffer, pH 5.8, were incubated at 30°C without agitation for 10 min. The assay was performed in a total volume of 1.5 ml. At time zero, 2 mM citrate, oxaloacetate, or pyruvate was added in the presence or absence of 2 mM amino acid and 50 μM PLP. Samples (50 to 100 μl) were taken at the indicated times and immediately centrifuged for 0.5 min at maximum speed in a tabletop centrifuge. The supernatant was stored on ice or frozen until further analysis by enzymatic assays and/or high-performance liquid chromatography (HPLC).
Enzymatic assays.
Citrate, oxaloacetate, and pyruvate were measured as described previously (16) using the commercially available enzymes CL, l-MDH, and l-LDH. Briefly, an aliquot of 30 μl of the sample was added to 50 mM Tris-HCl buffer, pH 7.8. containing NADH. Oxaloacetate in the sample was converted to l-malate at the expense of NADH after addition of l-MDH. Pyruvate in the sample was measured by addition of l-LDH, which results in the conversion of pyruvate to l-lactate at the expense of NADH. Addition of CL converts citrate in the sample to oxaloacetate (and pyruvate), resulting in an additional decrease in the NADH concentration equivalent to the citrate concentration present in the sample. The assay was performed in 96-well microtiter plates. The decrease in the NADH concentration was measured spectroscopically at 340 nm. Standard deviations were obtained from at least 3 different experiments.
HPLC/reversed phase HPLC (RP-HPLC) analysis.
Samples were run on a Shimadzu high-speed HPLC Prominence UFLC and later analyzed using the LC Solutions 1.24 SP1 software from Shimadzu (Kyoto, Japan). Products of citrate metabolism and transamination (α-keto acids and further metabolites) were determined by loading an aliquot of 10 μl of the supernatant on an Aminex HPX-87H anion-exchange column (300 by 7.8 mm; Bio-Rad Laboratories, Inc., Richmond, CA) operated at 30°C in isocratic mode using 0.005 M H2SO4 as the mobile phase and a flow rate of 0.8 ml/min.
Amino acids were analyzed by RP-HPLC after DEEMM derivatization. Aminoenone derivatives of amino acids were obtained by reaction of 175 μl of 1 M borate buffer, pH 9.0, 75 μl of methanol, 2 μl of 0.1% (wt/vol) l-aminoadipic acid, 3 μl of DEEMM, and 100 μl of supernatant in a 1.5-ml closed tube over 30 min of incubation at room temperature in an ultrasound bath. Then, the sample was incubated at 70°C for 2 h to allow complete degradation of the excess of DEEMM (7). The RP-HLPC protocol for detection of aminoenone derivatives was adapted from Gómez-Alonso et al. (7). An Alltech Platinum EPS C18 column (250 by 4.6 mm) operated at 25°C was used to run the binary gradient shown in Table 1 with a flow rate of 0.8 ml/min. Eluent A was 25 mM acetate buffer, pH 5.8, with 0.02% sodium azide, and eluent B was an 80:20 mixture of acetonitrile and methanol. The target compounds were identified according to the retention times and were quantified using the external-standard method. Measurements of the concentrations of citrate, oxaloacetate, and pyruvate were in good agreement between the enzymatic and HPLC methods. Standard deviations were obtained from at least 3 different experiments.
Table 1.
Eluent gradient for HPLC determination of aminoenone derivatives of amino acids
| Eluent | % at time (min): |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 13 | 13.5 | 17 | 20 | 32 | 40 | 45 | 48 | 50 | |
| A | 94 | 84 | 82 | 82 | 78 | 68 | 5 | 5 | 94 | 94 |
| B | 6 | 16 | 18 | 18 | 22 | 32 | 95 | 95 | 6 | 6 |
RESULTS
Citrate-driven transamination.
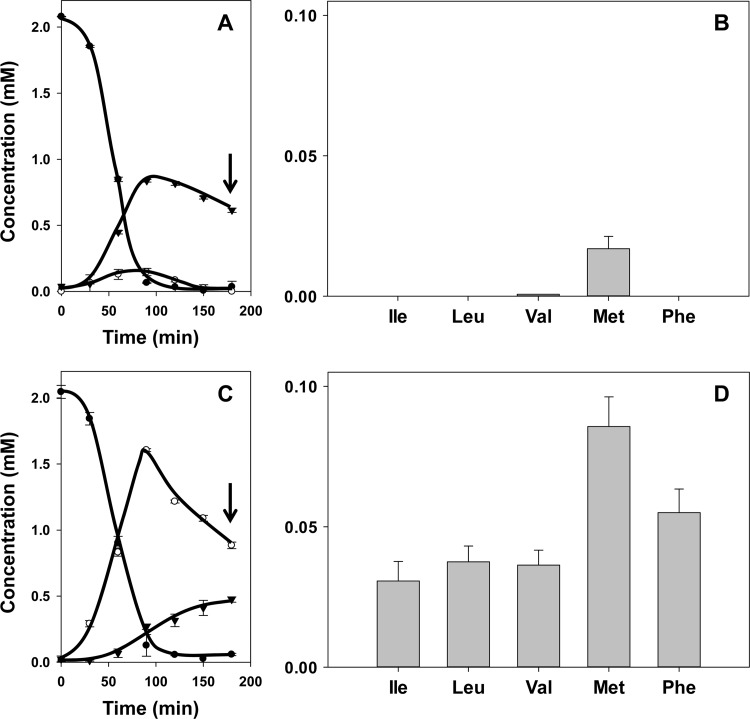
L. lactis strain IL1403(pFL3) harbors plasmid pFL3, which encodes the citrate transporter CitP under the control of a constitutive promoter (see Materials and Methods). Together with the metabolic enzymes encoded on the chromosome, the transporter completes the citrate fermentation pathway. The citrate fermentation pathway in L. lactis IL1403 produces two potential keto donors for transamination activity, i.e., oxaloacetate and pyruvate (Fig. 1) (16). Resting cells of L. lactis IL1403(pFL3) were incubated in 50 mM KPi, pH 5.8, containing the cofactor PLP (50 μM) in the presence of 2 mM citrate and the amino acid Ile, Leu, Val, Met, or Phe (2 mM). The highest concentrations of pyruvate and oxaloacetate produced by the cells, 0.85 and 0.15 mM, respectively, were observed when all citrate was consumed after 90 min. The remainder of the citrate added was converted to acetate (data not shown) (16). Following the depletion of citrate, pyruvate and oxaloacetate were taken up and metabolized again by the cells (16, 17). After another 90 min, when pyruvate was present at a concentration of 0.6 mM and no oxaloacetate was detected any longer (Fig. 2A, arrow), measurement of the corresponding α-keto acid (Fig. 1) of Ile, Leu, Val, Met, or Phe by HPLC revealed very little transamination activity by the cells (Fig. 2B). Only in the presence of methionine was 20 μM 2-keto-4-methylthiobutyrate detected. Control experiments showed that the α-keto acid of methionine was formed only when both citrate and methionine were present.
Fig 2.
Citrate metabolism (A and C) and α-keto acid production (B and D) by resting cells of L. lactis IL1403(pFL3) (A and B) and ILCitM(pFL3) (C and D). Resting cells were incubated with 2 mM citrate and 2 mM amino acids Ile, Leu, Val, Met, and Phe. (A and C) ●, citrate; ○, oxaloacetate; ▼, pyruvate. (B and D) The corresponding α-keto acids of Ile, Leu, Val, Met, and Phe were determined after 3 h of incubation (A and C, arrows). The error bars indicate standard deviations.
L. lactis strain ILCitM(pFL3), a derivative of strain IL1403(pFL3), is deficient in oxaloacetate decarboxylase (1). Consequently, incubation in the presence of 2 mM citrate and one of the five amino acids resulted in the production of 1.6 mM oxaloacetate after complete consumption of citrate, which was a bit slower than in the parent strain (Fig. 2C) (17). Subsequently, oxaloacetate was consumed again to reach a concentration of 0.9 mM after 3 h. The pyruvate concentration slowly increased during the incubation period to reach a concentration of 0.5 mM. In contrast to the parent strain, the oxaloacetate decarboxylase-deficient strain produced significant amounts of the α-keto acids of all five amino acids (Fig. 2D). The highest production was observed from Met (80 to 90 μM), followed by Phe (50 to 60 μM) and the branched-chain amino acids (30 to 40 μM). In all cases, transamination was strictly dependent on the presence of both citrate and the amino acids, while the production of the α-keto acids was strongly reduced when the cofactor PLP was omitted from the buffers (data not shown). Hence, the measured α-keto acids are products of PLP-dependent amino acid transamination driven by metabolism of citrate in the mutant strain. The kinetics of citrate consumption and oxaloacetate and pyruvate formation were not significantly affected by the presence or absence of the amino acids in the mutant strain, indicating that the flux going into the transamination route was minor.
Oxaloacetate- and pyruvate-driven transamination.
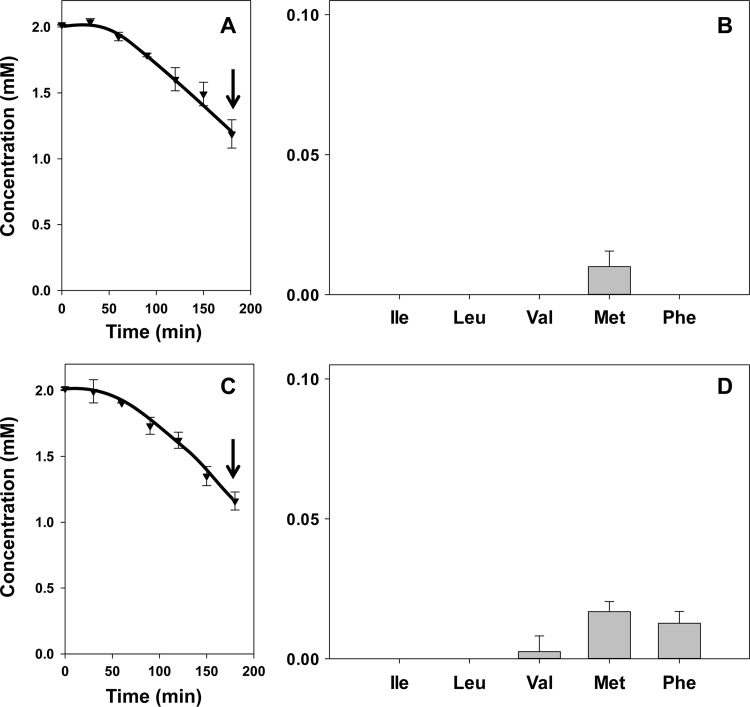
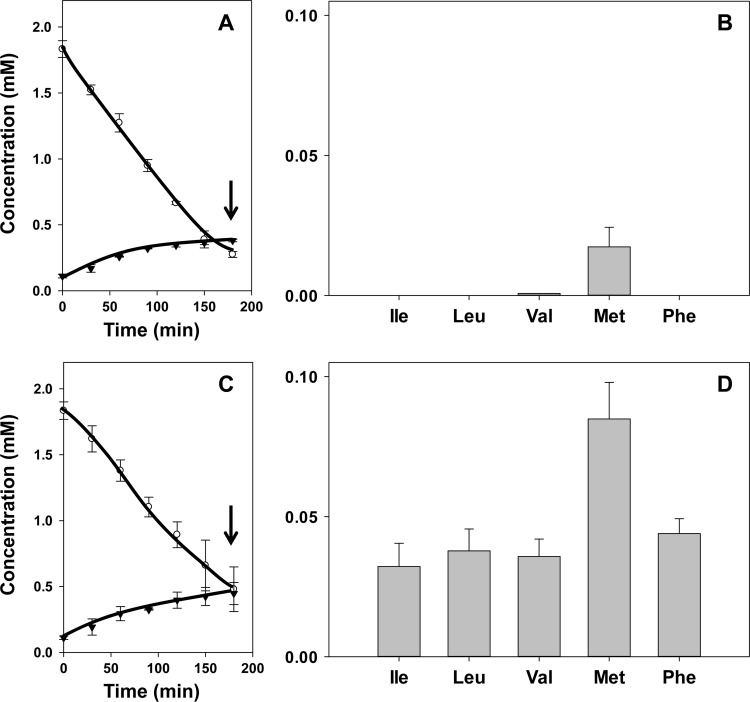
The higher concentration of oxaloacetate and lower concentration of pyruvate produced from citrate by the mutant strain ILCitM(pFL3) suggested that the former is the keto donor in the transamination reactions. In agreement with this, incubation of both the parent and mutant strains in the presence of 2 mM pyruvate and one of the five amino acids did not result in the amounts of α-keto acids that were observed with the mutant strain in the presence of citrate. In fact, for both strains, the result was very similar to that observed for the parent strain in the presence of citrate (Fig. 3). When, after 3 h of incubation, 0.85 mM pyruvate was consumed (Fig. 3A and C), 10 to 20 μM the α-keto acid of methionine was produced (Fig. 3B and D). In addition, the mutant strain produced 15 μM phenylpyruvate, the α-keto acid of Phe, while no α-keto acids formed from the branched-chain amino acids could be detected (Fig. 3D). Apparently, pyruvate is a poor keto donor for transaminases of L. lactis IL1403. In contrast, incubation of the mutant and parent strains in the presence of oxaloacetate and any of the five amino acids resulted in the same pattern of α-keto acid production as observed in the presence of citrate (Fig. 2 and 4). Oxaloacetate is taken up by the cells via the citrate transporter CitP (Fig. 1) (17). In the parent strain, internalized oxaloacetate is rapidly decarboxylated, producing low cytoplasmic oxaloacetate concentrations (16). In agreement with the above results, the decarboxylation product pyruvate does not enter the transamination pathway efficiently (Fig. 4A and B). In the oxaloacetate decarboxylase-deficient strain, the oxaloacetate concentration in the cytoplasm is much higher, supporting significant transamination (Fig. 4C and D). Remarkably, in spite of the different kinetics of cytoplasmic oxaloacetate conversion, the overall kinetics of oxaloacetate consumption and pyruvate excretion were quite similar in the two strains.
Fig 3.
Pyruvate metabolism (A and C) and α-keto acid production (B and D) by resting cells of L. lactis IL1403(pFL3) (A and B) and ILCitM(pFL3) (C and D). Resting cells were incubated with 2 mM pyruvate and 2 mM amino acids Ile, Leu, Val, Met, and Phe. (A and C) ▼, pyruvate. (B and D) The corresponding α-keto acids of Ile, Leu, Val, Met, and Phe were determined after 3 h of incubation (A and C, arrows). The error bars indicate standard deviations.
Fig 4.
Oxaloacetate metabolism (A and C) and α-keto acid production (B and D) by resting cells of L. lactis IL1403(pFL3) (A and B) and ILCitM(pFL3) (C and D). Resting cells were incubated with 2 mM oxaloacetate and 2 mM amino acids Ile, Leu, Val, Met, and Phe. (A and C) ○, oxaloacetate; ▼, pyruvate. (B and D) The corresponding α-keto acids of Ile, Leu, Val, Met, and Phe were determined after 3 h of incubation (A and C, arrows). The error bars indicate standard deviations.
Production of aspartate and alanine by ILCitM(pFL3).
In the transamination reaction, the keto donors oxaloacetate and pyruvate result in the formation of the amino acids aspartate and alanine, respectively (Fig. 1). Production of amino acids by the cells under the same conditions described above was measured by RP-HPLC, as described in Materials and Methods. After 3 h of incubation and in the absence of any further additions, resting cells of both the parent and mutant strains produced 15 μM aspartate and 25 μM alanine (Fig. 5). Most likely, some proteolytic activity resulted in the release of free amino acids from the cells. No significant differences were observed when the parent strain was incubated in the presence of 2 mM citrate, 2 mM isoleucine, or both (Fig. 5A and B), in line with the lack of formation of α-ketomethylvalerate, the α-keto acid formed from isoleucine, as described above (Fig. 2B). Similarly, the alanine concentration was not affected when the mutant strain was incubated in the presence of these additions, in line with the lack of pyruvate-driven transamination (Fig. 5D). In contrast, the aspartate concentration was the same in the presence of 2 mM isoleucine but was raised to 50 μM in the presence of 2 mM citrate and to 95 μM in the presence of both isoleucine and citrate (Fig. 5C). A straightforward explanation for the increase in the presence of citrate alone would be transamination between oxaloacetate and amino acids produced in the cell by proteolytic activity. The additional increase in the presence of isoleucine demonstrates that α-ketomethylvalerate produced by the mutant strain is the result of oxaloacetate-isoleucine transaminase activity. The experiments were repeated with the other amino acids, Leu, Val, Phe, and Met (Table 2). In all cases, aspartate was produced without significant amounts of alanine, demonstrating that oxaloacetate and not pyruvate is the keto group donor in citrate-driven transamination. In addition to aspartate and alanine, low concentrations of Glu, Asn, Gln, Gly, Thr, and Lys were detected after 3 h of incubation under each experimental condition, supporting the proteolytic or autolytic activity of the cells.
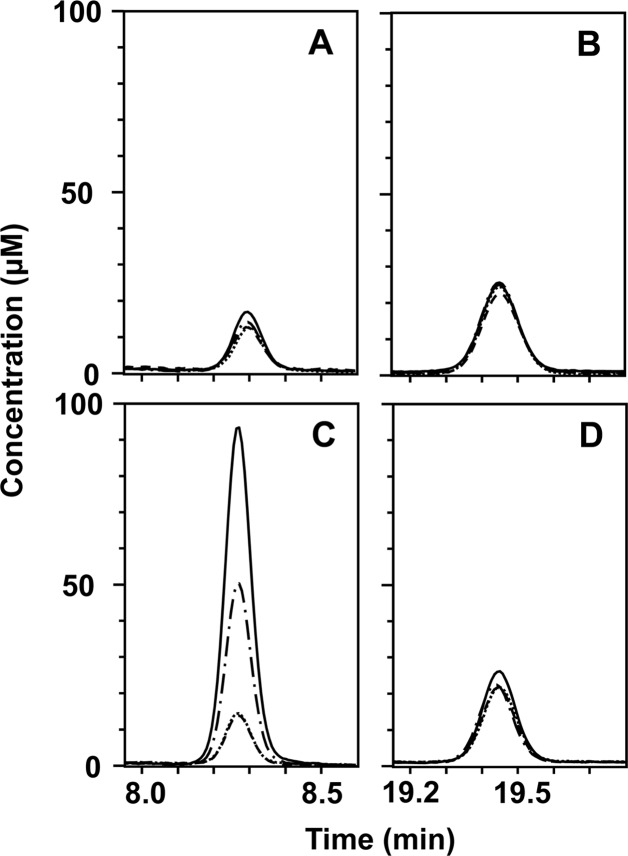
Fig 5.
Aspartate (A and C) and alanine (B and D) production during citrate-driven transamination by resting cells of L. lactis IL1403(pFL3) (A and B) and L. lactis ILCitM(pFL3) (C and D). Resting cells were incubated for 3 h without further additions (long dashes), in the presence of 2 mM citrate (dashes and dots), 2 mM Ile (short dashes), and 2 mM citrate and 2 mM Ile (solid line). The graphs represent the RP-HPLC elution profile.
Table 2.
Transamination of 5 natural amino acids in L. lactis ILCitM(pFL3)
| Amino acida | Amt (μM)b |
|
|---|---|---|
| Asp | Ala | |
| Met | 124 ± 13 | 13 ± 10 |
| Phe | 70 ± 20 | 5 ± 3 |
| Val | 56 ± 13 | 8 ± 7 |
| Ile | 50 ± 6 | 6 ± 4 |
| Leu | 51 ± 9 | 7 ± 3 |
Amino acids were added at a concentration of 2 mM in the presence of 2 mM citrate and 50 μM PLP.
Amounts of Asp and Ala were measured after 3 h of citrate-driven transamination; the amounts of Asp and Ala produced from citrate in the absence of added amino acids were subtracted.
Products of citrate-driven transamination from methionine and phenylalanine.
α-Keto acids are precursors of other flavor compounds (Fig. 1). Further metabolism of 2-keto-4-methylthiobutyrate, the α-keto acid of methionine, by resting cells of L. lactis ILCitM(pFL3) resulted in two additional products that were identified as the reduced form 2-hydroxy-4-methylthiobutyrate and 3-methylthiopropionic acid (MTPA) (Fig. 6A). Phenylpyruvate, the α-keto acid of phenylalanine, was converted to a single compound that was identified as the reduced form phenyllactate (Fig. 6B). The downstream products were detected only in the presence of both citrate and the amino acid. No reduced α-keto acid or any other compounds were produced in the presence of the branched-chain amino acids. The final concentrations of the oxidized and reduced forms produced from methionine were 140 and 50 μM, respectively, and for those from phenylalanine they were 70 and 35 μM, respectively (Fig. 6A and B), which is in good agreement with the amounts of aspartate formed (Table 2). Together with the relatively modest production of 10 μM MTPA, it follows that, under the conditions of the experiment, in the presence of methionine, 10% of the citrate was directed into the transamination route.
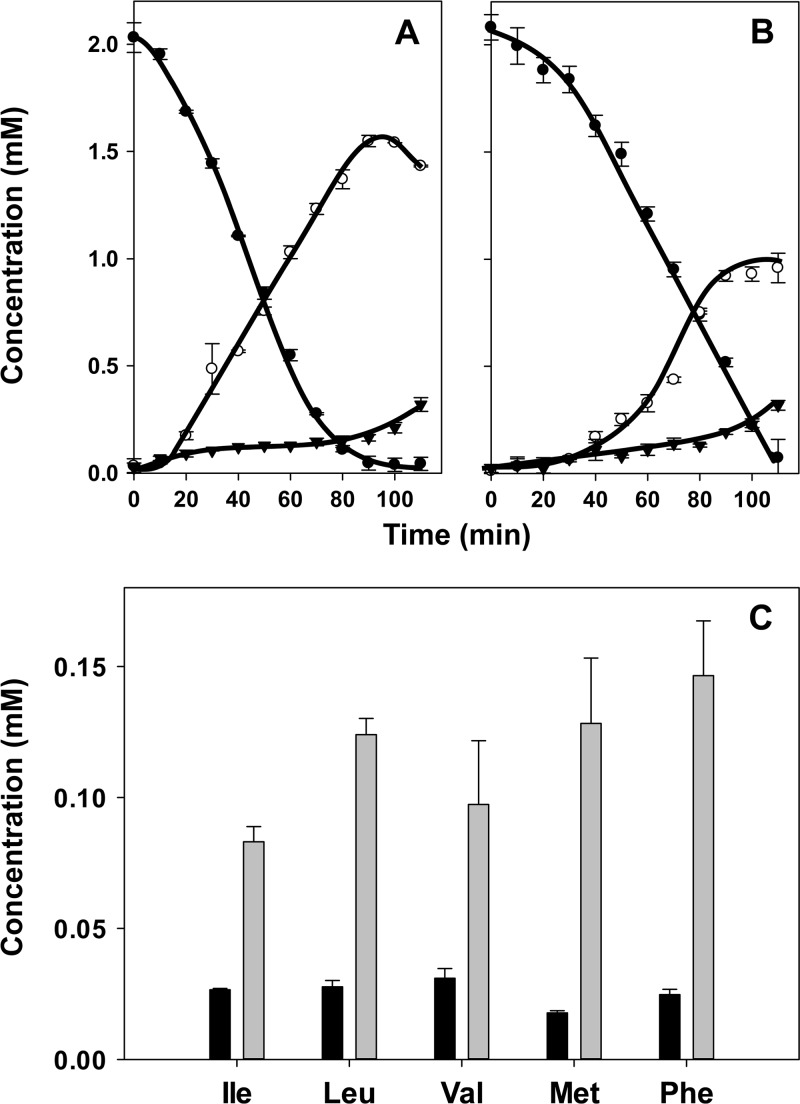
Fig 6.
(A and B) Formation of transamination products of methionine (A) and phenylalanine (B). (C) Further metabolism of 2-keto-4-methylthiobutyrate (α-keto acid of methionine) and phenylpyruvate (α-keto acid of phenylalanine) by citrate metabolism in resting cells of L. lactis ILCitM(pFL3). Resting cells were incubated in the presence of 2 mM citrate and 2 mM Met (A and C) and Phe (B). (A) ●, 2-keto-4-methylthiobutyrate; ○, 2-hydroxy-4-methylthiobutyrate; ▼, MTPA. (B) ●, phenylpyruvate; ○, phenyllactate. (C) ●, citrate, (○) oxaloacetate; ▼, pyruvate; △, acetate (formed in addition to acetate formed by citrate lyase). The error bars indicate standard deviations.
The production of the oxidized and reduced forms from methionine and phenylalanine followed similar kinetics that appeared to be linked directly to the kinetics of citrate metabolism (Fig. 6C). The rate was highest during the first 60 to 70 min, when citrate was consumed and external oxaloacetate reached its maximum (Fig. 6A, B, and C). During the reuptake and consumption of oxaloacetate, the production proceeded at a slower pace to more or less come to a halt when all oxaloacetate was consumed after approximately 6 h. Remarkably, following the depletion of oxaloacetate, the oxidized forms were not further converted into the reduced forms, strongly suggesting that the required reducing equivalents were produced in the citrate metabolic pathway (see Discussion).
Oxaloacetate stress response.
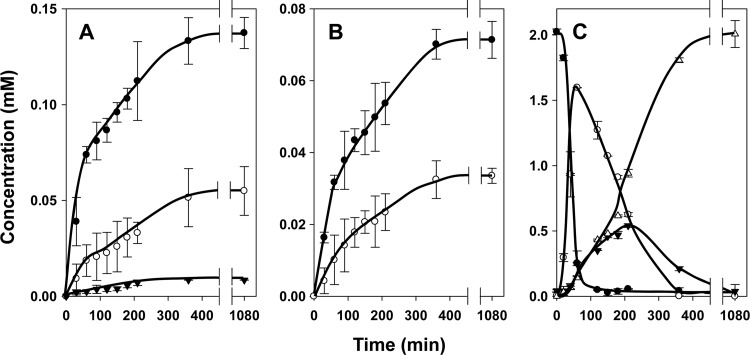
Until now the strains of L. lactis used in the studies were grown in the absence of citrate to prevent oxaloacetate stress in the mutant strain during growth. Addition of citrate to the growth medium reduced the growth rate of the mutant and excretion of oxaloacetate into the growth medium, suggesting toxic effects of a high concentration of the latter in the cytoplasm (1, 17). Rerouting the citrate fermentation pathway into transamination may relieve the stress condition. The kinetics of citrate consumption and oxaloacetate production by resting cells of L. lactis ILCitM(pFL3) grown in the presence of 20 mM citrate were slightly but significantly slower than observed in the absence of citrate (Fig. 7B and A). The highest concentration of oxaloacetate measured outside the resting cells that were grown in the presence of citrate was about 40% lower, 0.95 versus 1.5 mM (Fig. 7B and A, respectively), while the amount of pyruvate produced did not differ significantly. In spite of the lower oxaloacetate concentration produced by the stressed cells, citrate-driven transamination in the presence of the five amino acids was only marginally different than that observed with the cells grown in the absence of citrate (data not shown). Besides transamination activity in the cytoplasm, transamination by the cells involves other steps, such as citrate lyase activity, uptake of citrate and the amino acid, and excretion of aspartate and the α-keto acid product. To isolate the transaminase activity in the cytoplasm from the process as a whole, cells were permeabilized with 0.15% Triton X-100 for 20 min at 30°C to omit the transport steps, and oxaloacetate was used as the keto donor to bypass citrate lyase. The permeabilized cells of the mutant strain grown in the absence of citrate produced approximately 25% less of the corresponding α-keto acids from the three branched-chain amino acids (compare Fig. 7C and 4D). The production of the α-keto acids from phenylalanine and, especially, methionine was significantly more reduced relative to the whole cells (Fig. 7C and 4D), which may be explained at least in part by the lack of formation of the reduced forms of the α-keto acids described above. An inhibitory effect of the Triton X-100 treatment cannot be excluded. The results indicated that the cytoplasmic transamination activity with the branched-chain amino acids was on average 3.5 times higher in the stressed cells. Similarly, the activity with methionine and phenylalanine was found to be about 7 times higher in the stressed cells (Fig. 7C). It follows that oxaloacetate stress sensed by the cells results in an increase of transamination activity, most likely by an upregulation of the expression of the involved transaminases.
Fig 7.
Oxaloacetate stress response in L. lactis ILCitM(pFL3). (A and B) Resting cells of L. lactis ILCitM(pFL3) grown in the absence (A) and presence (B) of 20 mM citrate were incubated with 2 mM citrate and 2 mM amino acids Ile, Leu, Val, Met, and Phe. ●, citrate; ○, oxaloacetate; ▼, pyruvate. (C) The corresponding α-keto acids of Ile, Leu, Val, Met, and Phe were determined after 3 h of incubation of cells grown in the absence (black bars) or presence (gray bars) of 20 mM citrate and treated with 0.15% Triton X-100 for 20 min before addition of 2 mM oxaloacetate and 2 mM amino acids. The error bars indicate standard deviations.
DISCUSSION
Coupling citrate fermentation and amino acid catabolism through transamination.
The aim of the present study was to reroute citrate metabolism in L. lactis into the transamination route to boost amino acid catabolism. The breakdown of amino acids is an important first step in the production of flavor compounds in the cheese industry but is often limited by the availability of a keto donor, while amino acids are in excess in the protein-rich cheese matrix (24, 30). Citrate, which is present in relatively large amounts in milk (19), is split by citrate lyase into acetate and oxaloacetate, a potential keto donor in the transamination reaction. Oxaloacetate was shown to accumulate to high concentrations in the cytoplasm when the next step in the pathway was blocked by inactivation of oxaloacetate decarboxylase in L. lactis strain ILCitM(pFL3) (1, 17). The results presented here clearly show that the mutant strain, but not the parent strain, produced significant amounts of the α-keto acids α-ketomethylvalerate, α-ketoisocaproate, α-ketoisovalerate, phenylpyruvate, and 2-keto-4-methylthiobutyrate from the branched-chain amino acids isoleucine, leucine, and valine; the aromatic amino acid phenylalanine; and the sulfur-containing amino acid methionine, respectively. The keto donor in the transamination reaction was shown to be oxaloacetate, not pyruvate, by the same α-keto acid product profiles obtained when the parent and mutant strains were incubated with oxaloacetate rather than citrate (Fig. 2 and 4). In addition, aspartate, the product of oxaloacetate in the transamination reaction, was formed, and not alanine, the corresponding amino acid of pyruvate (Fig. 5). Clearly, in the mutant strain, part of the oxaloacetate derived from citrate is redirected into the transamination route. In the parent strain, the steady-state concentration in the cytoplasm is too low because of the high activity of the oxaloacetate decarboxylase (16, 17). The fraction of the citrate metabolic flux going into the transamination route is modest, at most 10% in the case of methionine. However, in terms of flavor compounds, this production is highly significant. A yield of 200 μM flavor compounds within 6 h from methionine (Fig. 6A), which is one of the most important precursors of flavors in cheese manufacturing, is much higher than that observed in similar studies using α-ketoglutarate as the keto donor (20, 21).
Oxaloacetate stress response.
The oxaloacetate-deficient strain L. lactis ILCitM(pFL3) mimics the physiological conditions under which oxaloacetate accumulates in the cytoplasm. High concentrations of oxaloacetate are toxic to the cell and result in reduced growth rates and lower biomass yields (1, 17). Possibly, the high concentrations competitively inhibit other metabolic enzymes. Strains expressing the citrate transporter CitP, a very promiscuous carboxylate transporter (18), respond to the stress condition by the excretion of oxaloacetate into the medium in exchange for external citrate (substrate-product exchange), thereby reducing the cytoplasmic concentration of oxaloacetate (Fig. 1) (17). The present study uncovers a second response: the excess of internal oxaloacetate is funneled into the transamination pathway. The required amino acids are readily available because of the high proteolytic activity of L. lactis (15). Stressed cells of the mutant strain ILCitM(pFL3) revealed an upregulation of transamination activity in the cytoplasm (Fig. 7C). In resting-cell experiments, the increased transamination activity is obscured, probably by limitations in the uptake and excretion of substrates and products, but in growing cells, when energy sources are available and transport reactions are not limited, the effect may be substantial. For instance, if 10% of the flux is directed to oxaloacetate-methionine transamination under unstressed conditions (Table 2), upregulation of the involved transaminase by a factor of 7 (Fig. 7) may significantly contribute to the stress response. Then, the production of flavor compounds will be correspondingly higher.
L. lactis transaminases.
The genome sequence of L. lactis IL1403 contains 13 genes encoding putative transaminases (2). AraT is specific for aromatic amino acids (20), BcaT for branched-chain amino acids (29), and AspC for the aspartate transaminase (encoded by aspB) (4); YtjE is involved in cysteine and methionine metabolism (13); and AlaT is the alanine transaminase (encoded by aspC) (14). Other genes annotated as encoding transaminases are serC, argD, glmS, hisC, arcT, nifS, nifZ, and yeiG, but none of them was functionally characterized in L. lactis (2). AraT and BcaT were previously reported to be major transaminases in cheese flavor development from aromatic amino acids and branched-chain amino acids, respectively (20, 21, 29). The purified enzymes showed activity with α-ketoglutarate as the keto donor, and neither AraT nor BcaT showed activity with oxaloacetate or aspartate (20, 28). A double knockout of bcaT and araT still showed transamination of aspartate (21), while inactivation of AspC resulted in no detectable activity with aspartate (4). Studies of the substrate specificities of BcaT, AraT, and AspC were done using α-ketoglutarate as the keto donor that is converted into glutamate. The activity of AspC with aspartate necessarily reveals that oxaloacetate is a substrate of this transaminase.
The present results show that oxaloacetate is an efficient keto donor in the transamination reactions catalyzed by L. lactis IL1403 for the amino acids Ile, Leu, Val, Phe, and Met. In addition, of the 20 naturally occurring amino acids, the mutant cells were capable of producing aspartate from citrate in the presence of tyrosine, tryptophan, and glutamate (unpublished results). The substrate specificities of the oxaloacetate-amino acid transaminases overlap the specificities of the α-ketoglutarate–amino acid transaminases BcaT and AraT. Either the oxaloacetate-specific transaminases are a separate set of enzymes or, more likely, BcaT and AraT in the cytoplasm of L. lactis have affinity for both keto donors.
Metabolic products of α-keto acids.
α-Keto acids produced by aminotransferases are known precursors of flavor compounds converted by dehydrogenation to the corresponding α-hydroxy acids;, decarboxylation to the corresponding aldehydes that are further converted into alcohols, carboxylic acids, and (thio)esters; or dehydrogenation to the corresponding coenzyme A (CoA) esters that are further metabolized to carboxylic acids. These compounds are produced in spontaneous or enzymatic reactions (24, 30). In the resting-cell experiments presented here, only a few of these downstream products showed up because of missing metabolic requirements or missing enzymes or simply because they were not detected. A minor amount of MTPA was formed from methionine. MTPA adds a baked/boiled potato flavor to fermented food products and was identified as an important aroma note in cheddar cheese (23). It is formed directly by spontaneous oxidative decarboxylation of the α-keto acid, which was reported previously in other LAB (10). The most prominent downstream products observed were 2-hydroxy-4-methylthiobutyrate and phenyllactate, formed from methionine and phenylalanine, respectively. α-Hydroxy acids are not major flavor compounds, but they play a role in flavor development in semihard cheeses made with lactococci (24). The conversion of α-keto acids into α-hydroxy acids is catalyzed by hydroxyacid dehydrogenases (HA-DH), which are stereospecific enzymes with broad substrate specificity and are widely distributed in LAB (5). The reduction of α-keto acids by resting cells of L. lactis was previously observed after addition of glucose (22); however, no enzyme has been characterized. Genome analysis of L. lactis IL1403 revealed the putative HA-DH gene hicD, annotated as l-2-hydroxyisocaproate dehydrogenase (2); however, the enzyme has not been studied. More recently, PanE, encoded by panE in L. lactis IL1403, was described as 2-hydroxyisocaproate dehydrogenase, with the highest catalytic efficiencies for α-ketomethylvalerate, α-ketoisocaproate, and α-ketoisovalerate (3). However, in the present studies, no reduction of the branched-chain α-keto acids was observed. A better candidate would be l-lactate dehydrogenase, encoded by the ldh gene, which was shown in Lactobacillus plantarum to be responsible for the reduction of α-keto acids derived from phenylalanine and methionine (3, 9).
The kinetics of production of the reduced and oxidized forms strongly suggested that the required reducing equivalents were produced in the citrate metabolic pathway. Under the conditions of the experiments, pyruvate from citrate is mainly converted to acetate via acetyl phosphate (16). Two operative routes from pyruvate to acetyl phosphate have been proposed: the NADH-producing route catalyzed by the pyruvate dehydrogenase complex (PDH) and phosphotransacetylase (PTA) and the hydrogen peroxide-producing route catalyzed by pyruvate oxidase (POX) (Fig. 1). The two routes are complementary to each other, since H2O2 produced by POX is used to reoxidize NADH produced by PDH. During citrate-driven transamination, reduction of α-keto acids into α-hydroxy acids at the expense of NADH produced by PDH may substitute H2O2 to control the NADH/NAD+ balance in the cell.
Finally, the α-keto acids, and especially α-hydroxy acids, produced in the transamination reactions are substrates of the citrate transporter CitP (18). CitP may be central to a metabolic system of citrate-driven transamination, in which citrate is taken up in exchange for the excretion of the transamination products catalyzed by CitP.
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Augagneur Y, Garmyn D, Guzzo J. 2008. Mutation of the oxaloacetate decarboxylase gene of Lactococcus lactis subsp. lactis impairs the growth during citrate metabolism. J. Appl. Microbiol. 104:260–268 [DOI] [PubMed] [Google Scholar]
- 2. Bolotin A, et al. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambellon E, et al. 2009. The D-2-hydroxyacid dehydrogenase incorrectly annotated PanE is the sole reduction system for branched-chain 2-keto acids in Lactococcus lactis. J. Bacteriol. 191:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dudley EG, Steele JL. 2001. Lactococcus lactis LM0230 contains a single aminotransferase involved in aspartate biosynthesis, which is essential for growth in milk. Microbiology 147:215–224 [DOI] [PubMed] [Google Scholar]
- 5. Fernández M, Zúñiga M. 2006. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 32:155–183 [DOI] [PubMed] [Google Scholar]
- 6. García-Quintáns N, Magni C, de Mendoza D, López P. 1998. The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl. Environ. Microbiol. 64:850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gómez-Alonso S, Hermosín-Gutiérrez I, García-Romero E. 2007. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 55:608–613 [DOI] [PubMed] [Google Scholar]
- 8. Hugenholtz J. 1993. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 12:165–178 [Google Scholar]
- 9. Jia J, Mu W, Zhang T, Jiang B. 2010. Bioconversion of phenylpyruvate to phenyllactate: gene cloning, expression, and enzymatic characterization of D- and L-lactate dehydrogenases from Lactobacillus plantarum SK002. Appl. Biochem. Biotechnol. 162:242–251 [DOI] [PubMed] [Google Scholar]
- 10. Landaud S, Helinck S, Bonnarme P. 2008. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 77:1191–1205 [DOI] [PubMed] [Google Scholar]
- 11. Magni C, Lopez de Felipe F, Sesma F, López P, de Mendoza D. 1994. Citrate transport in Lactococcus lactis subsp. lactis biovar diacetylacis. Expression of the citrate permease. FEMS Microbiol. Lett. 118:78–82 [Google Scholar]
- 12. Marienhagen J, Kennerknecht N, Sahm H, Eggeling L. 2005. Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J. Bacteriol. 187:7639–7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez-Cuesta MC, et al. 2006. YtjE from Lactococcus lactis IL1403 is a C-S lyase with α,γ-elimination activity toward methionine. Appl. Environ. Microbiol. 72:4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oikawa T. 2006. Alanine, aspartate, and asparagine metabolism in microorganisms, p 274–286 In Wendisch VF. Amino acid biosynthesis: pathways, regulation, and metabolic engineering. Microbiology monograph 5. Springer-Verlag, Berlin, Germany [Google Scholar]
- 15. Pillidge CJ, et al. 2002. Autolysis of Lactococcus lactis. Int. Dairy J. 12:133–140 [Google Scholar]
- 16. Pudlik AM, Lolkema JS. 2011. Citrate uptake in exchange with intermediates of citrate metabolic pathway in Lactococcus lactis IL1403. J. Bacteriol. 193:706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pudlik AM, Lolkema JS. 2011. Mechanism of citrate metabolism by an oxaloacetate decarboxylase deficient mutant of Lactococcus lactis IL1403. J. Bacteriol. 193:4049–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pudlik AM, Lolkema JS. 2012. Substrate specificity of the citrate transporter CitP of Lactococcus lactis. J. Bacteriol. 194:3627–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renner E. 1983. Milk and dairy products in human nutrition, p 90–130 Volkswirtschaftlicher Verlag, Munich, Germany [Google Scholar]
- 20. Rijnen L, Bonneau S, Yvon M. 1999. Genetic characterization of the major lactococcal aromatic aminotransferase and its involvement in conversion of amino acids to aroma compounds. Appl. Environ. Microbiol. 65:4873–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rijnen L, et al. 2003. Lactococcal aminotransferases AraT and BcaT are key enzymes for the formation of aroma compounds from amino acids in cheese. Int. Dairy J. 13:805–812 [Google Scholar]
- 22. Roudot-Algaron F, Yvon M. 1998. Le catabolisme des acides aminés aromatiques et des acides aminés à chaîne ramifiée chez Lactoccous lactis. Lait 78:23–30 [Google Scholar]
- 23. Singh TK, Drake MA, Cadwallader KR. 2003. Flavor of cheddar cheese: a chemical and sensory perspective. Compr. Rev. Food Sci. Food Safety 2:166–189 [DOI] [PubMed] [Google Scholar]
- 24. Smit G, Smit BA, Engels WJM. 2005. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 29:591–610 [DOI] [PubMed] [Google Scholar]
- 25. Steele RD, Benevenga NJ. 1978. Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J. Biol. Chem. 253:7844–7850 [PubMed] [Google Scholar]
- 26. Steele RD, Benevenga NJ. 1979. The synthesis of radioactive 3-methylthiopropionate and other alkylthio fatty acids. Anal. Biochem. 98:281–286 [DOI] [PubMed] [Google Scholar]
- 27. Tanous C, Gori A, Rijnen L, Chambellon E, Yvon M. 2005. Pathways for α-ketoglutarate formation by Lactococcus lactis and their role in amino acid catabolism. Int. Dairy J. 15:759–770 [Google Scholar]
- 28. Yvon M, Thirouin S, Rijnen L, Fromentier D, Gripon JC. 1997. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl. Environ. Microbiol. 63:414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yvon M, Chambellon E, Bolotin A, Roudot-Algaron F. 2000. Characterization and role of the branched-chain aminotransferase (BcaT) isolated from Lactococcus lactis subsp. cremoris NCDO 763. Appl. Environ. Microbiol. 66:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yvon M, Rijnen L. 2001. Cheese flavor formation by amino acid catabolism. Int. Dairy J. 11:185–201 [Google Scholar]