Abstract
Mannheimia haemolytica, Pasteurella multocida, and Bibersteinia trehalosi have been identified in the lungs of pneumonic bighorn sheep (BHS; Ovis canadensis). Of these pathogens, M. haemolytica has been shown to consistently cause fatal pneumonia in BHS under experimental conditions. However, M. haemolytica has been isolated by culture less frequently than the other bacteria. We hypothesized that the growth of M. haemolytica is inhibited by other bacteria in the lungs of BHS. The objective of this study was to determine whether P. multocida inhibits the growth of M. haemolytica. Although in monoculture both bacteria exhibited similar growth characteristics, in coculture with P. multocida there was a clear inhibition of growth of M. haemolytica. The inhibition was detected at mid-log phase and continued through the stationary phase. When cultured in the same medium, the growth of M. haemolytica was inhibited when both bacteria were separated by a membrane that allowed contact (pore size, 8.0 μm) but not when they were separated by a membrane that limited contact (pore size, 0.4 μm). Lytic bacteriophages or bactericidal compounds could not be detected in the culture supernatant fluid from monocultures of P. multocida or from P. multocida-M. haemolytica cocultures. These results indicate that P. multocida inhibits the growth of M. haemolytica by a contact- or proximity-dependent mechanism. If the inhibition of growth of M. haemolytica by P. multocida occurs in vivo as well, it could explain the inconsistent isolation of M. haemolytica from the lungs of pneumonic BHS.
INTRODUCTION
Pneumonia is an important disease of domestic and wild ruminants (17, 19, 27, 38). The morbidity and mortality, however, varies among the different ruminant species. Bighorn sheep (BHS; Ovis canadensis) are particularly susceptible to pneumonia, which is considered the primary disease responsible for the drastic decline of these animals in North America, from an estimated two million animals at the beginning of the 19th century to less than 70,000 at this time (8, 37). The bacteria detected in the pneumonic lungs of BHS include Mannheimia haemolytica, Bibersteinia trehalosi, Pasteurella multocida, and Mycoplasma ovipneumonia. M. haemolytica produces an exotoxin known as leukotoxin (Lkt). Although it is cytolytic to all subsets of ruminant leukocytes, polymorphonuclear leukocytes (PMNs) are the most susceptible subset (10, 24). Lkt-induced cytolysis of PMNs is responsible for the acute inflammation and lung injury characteristics of this disease. Based on the fact that lktA deletion mutants of M. haemolytica A1 cause no mortality in BHS (12) and reduced mortality and mild lung lesions in calves, Lkt has been accepted as the most important virulence factor of M. haemolytica (22, 30, 34). More than 90% of B. trehalosi isolates of BHS origin do not produce leukotoxin (33, 39). P. multocida has not been shown to produce virulence factors that can cause the type of lesions observed in pneumonic BHS lungs, particularly dead and dying PMNs and macrophages. Hence, B. trehalosi and P. multocida are unlikely to be the major pathogens of pneumonia in BHS (35). Experimental inoculation studies have shown that M. ovipneumoniae does not cause fatal pneumonia in BHS lambs (5). Nevertheless, it can predispose adult BHS to M. haemolytica pneumonia (6, 14). It is not clear whether M. ovipneumoniae is a necessary predisposing agent for BHS pneumonia. M. haemolytica has been shown to consistently cause fatal pneumonia in BHS under experimental conditions (12, 17, 19, 29), but M. haemolytica has been isolated from pneumonic lungs much less frequently than the other organisms (13). However, species-specific PCR assays have identified M. haemolytica in the lung tissues of pneumonic BHS much more frequently than the other organisms (31, 33). Consequently, we hypothesized that the growth of M. haemolytica is inhibited by other bacteria in the lungs. B. trehalosi has been shown to inhibit the growth of M. haemolytica in vitro (13). Therefore, the objectives of the present study were (i) to characterize the in vitro growth curves of M. haemolytica and P. multocida, (ii) to determine whether P. multocida inhibits the growth of M. haemolytica and, if it does, (iii) to characterize the possible mechanism(s) of inhibition.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Four field isolates of P. multocida (BHS 3272, 07/1920/139, 96/65/2, and 95/1545/4) and four isolates of Moraxella bovoculi obtained from the Washington Animal Disease Diagnostic Laboratory were used in the present study. An ampicillin-resistant leukotoxin (lktA) deletion mutant of M. haemolytica A1 previously developed by Murphy et al. (28) and an lktA+ M. haemolytica isolate (10/2/3) previously tagged with green fluorescent protein by us (25) were also used in the present study. From the freezer stock, all isolates of P. multocida, M. haemolytica, and M. bovoculi were plated in brain heart infusion (BHI) agar plates and incubated overnight at 37°C. Single colonies from BHI plates were cultured in 5 ml of BHI broth in individual tubes overnight at 37°C with shaking (225 rpm). These bacterial cultures were then spread on individual BHI agar plates containing ampicillin (25 μg/ml), rifampin (10 μg/ml), nalidixic acid (30 μg/ml), or tetracycline (15 μg/ml). Each antibiotic-resistant isolate was spread on other antibiotic-containing BHI agar plates to determine resistance to other antibiotics. In this manner, four rifampin-resistant (Rifr) P. multocida isolates (BHS 3272, 07/1920/139, 96/65/2, and 95/1545/4), one nalidixic acid-resistant (Nalr) P. multocida isolate (07/1920/213), and one Nalr M. haemolytica isolate (91N-10) were obtained and stored as frozen stocks in 30% glycerol in BHI at −80°C. These antibiotic-resistant isolates were used here because of the ease of detection of these strains (by growth in appropriate antibiotic-containing media).
In order to determine the specificity of inhibition of growth of M. haemolytica by P. multocida, the coculture experiment was also performed with killed P. multocida. For this experiment, approximately 5 × 107 CFU of P. multocida were killed by incubating with 1 ml of 70% isopropyl alcohol for 1 h. The bacteria were then washed and resuspended in 1 ml of BHI.
CFU assay.
The number of CFU of P. multocida, M. haemolytica, and M. bovoculi in monocultures and cocultures were determined by CFU assay as described previously by us (13). The M. bovoculi strain used here was not resistant to any antibiotic. The number of CFU of M. bovoculi in coculture was determined by subtracting the number of CFU of M. haemolytica, which was determined by enumerating the number of CFU of this bacterium on ampicillin-resistant BHI plates.
Proximity-dependent inhibition assay.
This assay was performed as previously described (13). To determine whether P. multocida-mediated growth inhibition of M. haemolytica was consistent with contact- or proximity-dependent inhibition, cell culture inserts with two different pore sizes (0.4 and 8.0 μm) were used. Bactericidal compounds and phages are able to diffuse through 0.4-μm pores. Generally, bacteria do not pass though 0.4-μm pores, but they can readily pass through 8.0-μm pores. The 0.4- and 8.0-μm-pore-size polyethylene terephthalate (PET) track-etched membrane cell culture inserts (BD Falcon; BD Biosciences, Franklin Lakes, NJ) were placed in six-well cell culture plates creating upper and lower chambers. Approximately 106 CFU of Rifr P. multocida in 10 μl of BHI were added into the upper chambers containing 2.5 ml of antibiotic-free BHI, and ∼105 CFU of ampicillin-resistant (Ampr) M. haemolytica (10:1 inhibitor/target ratio) in 10 μl of BHI were added into the lower chambers containing the same volume of antibiotic-free BHI. Evaporation of the culture medium was minimized by covering the culture plates with the lids and wrapping with parafilm. The plates were incubated at 37°C with shaking at 100 rpm. Aliquots were collected from both upper and lower chambers at 24 h, serially diluted with BHI broth, and placed on each type of agar plates (ampicillin- or rifampin-containing BHI agar plates). Rifr P. multocida and Ampr M. haemolytica individually cultured in six-well culture plates with the same culture volume were used as treatment controls.
Bactericidal assay.
Bactericidal compounds in P. multocida monoculture and P. multocida-M. haemolytica coculture supernatant fluids were collected at 6 and 24 h postinoculation and evaluated by solid BHI agar and broth assays, as described previously (13). Culture supernatant fluids were filtered through low protein binding filters (Acrodisc 0.2- or 0.45-μm-pore-size Supor membrane syringe filters; Pall Life Sciences) to minimize the loss of bactericidal compounds (if any) during filtration. M. haemolytica (∼106 CFU in 100 μl of BHI broth) gently mixed with BHI soft molten agar (3 ml) was poured onto preheated (37°C) BHI agar plates with a swirling movement and kept at room temperature for 5 min. A total of 20 μl of culture supernatant fluid from P. multocida or M. haemolytica monoculture or P. multocida and M. haemolytica coculture was placed on solidified soft agar, followed by incubation overnight at 37°C. The plates were examined the following day for zones of inhibition on the agar plates. This assay was repeated with 10 μl of P. multocida culture instead of the culture supernatant fluid. In another experiment ∼106 CFU of M. haemolytica in 5 ml of 2× BHI broth was mixed with 5 ml of mono- or coculture filter-sterilized supernatant fluid, followed by incubation for 24 h at 37°C with shaking (225 rpm). Samples were then collected, and CFU assay was performed to calculate the number of bacteria in each of the culture tubes. M. haemolytica without the culture supernatant fluid was used as a negative control.
If a soluble inhibitor is involved, it is possible that its concentration in the supernatant fluid is too low to observe the inhibition. Therefore, the supernatant fluids were concentrated by ammonium sulfate precipitation (4). Approximately 100 to 150 μg of 40% ammonium sulfate-precipitated proteins was used for the detection of bacteriocin activity on M. haemolytica plates by a “spot-on-lawn assay.” M. haemolytica with BHI broth alone was used as a negative control.
PCR.
Multiplex PCR assay was performed to confirm the identity of M. haemolytica and P. multocida colonies in each plate and broth culture. The previously designed M. haemolytica species-specific forward primer MhgcpF (5′-AGA GGC CAA TCT GCA AAC CTC G-3′) and reverse primer MhgcpR (5′-GTT CGT ATT GCC CAA CGC CG-3′) (13) and the P. multocida species-specific primer KMT1T7 (5′-ATC CGC TAT TTA CCC AGT GG-3′) and primer KMT1SP6 (5′-GCT GTA AAC GAA CTC GCC AC-3′) (36) were used. PCR was performed with GoTaq green master mix (Promega, Inc., Madison, WI) in a 25-μl final volume as described previously (13).
Statistical analysis.
Bacterial numbers were calculated and are expressed as the mean log10 CFU/ml of culture media ± the standard deviation determined at different time points. The data were statistically analyzed by repeated-measures analysis of variance (ANOVA) and Student t test using GraphPad online software (GraphPad, La Jolla, CA). A P value of <0.05 was considered statistically significant.
RESULTS
P. multocida inhibits the growth of M. haemolytica in coculture.
When P. multocida and M. haemolytica were cultured individually, both species exhibited similar growth characteristics and yielded overlapping growth curves (data not shown). The average doubling time for M. haemolytica and P. multocida was 26 min. Rifr P. multocida and Ampr M. haemolytica strains did not differ from the respective wild-type strains with respect to growth in culture (Fig. 1).
Fig 1.
The antibiotic-resistant isolates of M. haemolytica and P. multocida exhibit comparable growth rates as their respective parent strains. The CFU/ml for antibiotic-sensitive (♢) and antibiotic-resistant (△) M. haemolytica and antibiotic-sensitive (○) and antibiotic-resistant (□) P. multocida when grown in BHI broth as monocultures were determined. The results are the mean CFU/ml from three independent experiments (±the standard deviations [SD]). There was no statistically significant difference (P > 0.05 based on repeated measures of ANOVA) between the CFU/ml for the antibiotic-resistant and antibiotic-sensitive isolates.
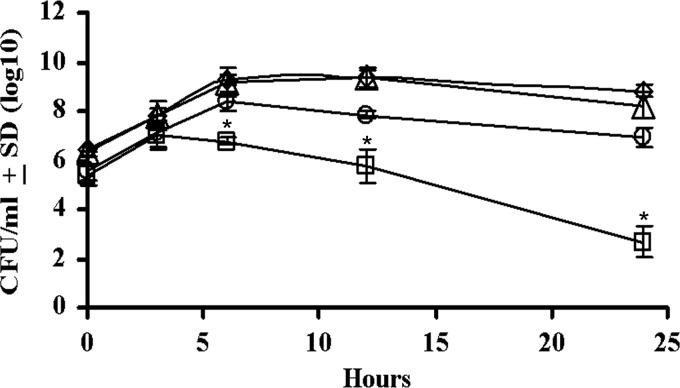
When Rifr P. multocida and Ampr M. haemolytica A1 lktA deletion mutants were cocultured, the CFU count of Ampr M. haemolytica began to decrease at 3 h and continued to decrease through the stationary phase (Fig. 2). At 24 h, the CFU count of Ampr M. haemolytica in coculture was 4 logs lower than that in monoculture. In contrast, the CFU count of Rifr P. multocida in coculture was almost identical to that in monoculture. A similar inhibition of growth was observed with another Ampr M. haemolytica strain of unknown serotype (Fig. 3), except that the difference in CFU counts between monoculture and coculture was 3 logs at 24 h.
Fig 2.
P. multocida inhibits the growth of M. haemolytica A1 in coculture in vitro. The CFU/ml for P. multocida (BHS3272) when grown as monoculture (♢) or coculture (△) with M. haemolytica were determined at the indicated time points. Similarly, the CFU/ml for M. haemolytica when grown as monoculture (○) or coculture (□) with P. multocida were determined at the indicated time points. The results are the mean CFU from three independent experiments (±the SD). The CFU/ml for M. haemolytica in monoculture and M. haemolytica in coculture are statistically significantly different at 6, 12, and 24 h postinfection (*, P < 0.0001 based on repeated measures of ANOVA). There was no statistically significant difference between the CFU/ml for P. multocida in monoculture and P. multocida in coculture at all time points (P > 0.05).
Fig 3.
P. multocida inhibits the growth of M. haemolytica 10/2/3 in coculture in vitro. The CFU/ml for P. multocida (96/65/2) when grown as a monoculture (♢) or coculture (△) with M. haemolytica were determined at indicated time points. Similarly, the CFU/ml for M. haemolytica when grown as monoculture (○) or coculture (□) with P. multocida were determined at the indicated time points. The results are the mean CFU from three independent experiments (±the SD). The CFU/ml for M. haemolytica in monoculture and M. haemolytica in coculture are statistically significantly different at 6, 12, and 24 h postinfection (*, P < 0.05 based on repeated measures of ANOVA). There was no statistically significant difference between the CFU/ml for P. multocida in monoculture and P. multocida in coculture at all time points (P > 0.05).
To examine the generality of the inhibitory effect of P. multocida on M. haemolytica, we performed coculture experiments involving different field isolates of Rifr Nalr P. multocida and Ampr Nalr M. haemolytica. Similar results were obtained with all of these pairs of P. multocida and M. haemolytica (Table 1). The reduction in the CFU counts of the M. haemolytica isolates in coculture ranged from 2 to 4 logs, and the reduced CFU counts were significantly different from the CFU counts of M. haemolytica in monoculture (P < 0.0001). The CFU counts of P. multocida strains in coculture were not different from the CFU counts in monoculture, indicating that the growth of P. multocida was not inhibited by M. haemolytica. P. multocida- and M. haemolytica-specific PCR assays confirmed the specificity of the selective plating protocol.
Table 1.
Inhibition of the growth of M. haemolytica by P. multocida
| Bacterial strain combination (M. haemolytica strain, P. multocida strain)a | Mean no. of bacteria (log10 CFU/ml)b |
|||
|---|---|---|---|---|
|
M. haemolytica |
P. multocida |
|||
| Monoculture | Coculture | Monoculture | Coculture | |
| A1 mutant, BHS3272 | 6.95 | 2.87 | 8.87 | 8.83 |
| A1 mutant, 07/1920/139 | 7.21 | 2.68 | 9.29 | 8.65 |
| A1 mutant, 95/1545/4 | 7.32 | 4.15 | 8.47 | 7.94 |
| 10/2/3 (gfp), 96/65/2 | 8.04 | 4.96 | 8.09 | 8.17 |
| 91N-10, 07/1920/139 | 8.34 | 5.57 | 9.50 | 8.44 |
| 91N-10, BHS3272 | 8.05 | 4.23 | 7.70 | 7.60 |
Three different isolates of M. haemolytica were cocultured with four different isolates of P. multocida in different combinations.
The numbers represent the log10 CFU/ml of the coculture at after 24 h. The difference in the CFU/ml for M. haemolytica in monocultures and cocultures is statistically significant (P < 0.0001). Results are based on two independent experiments, except for the first two combinations of bacterial strains, which are based on three independent experiments.
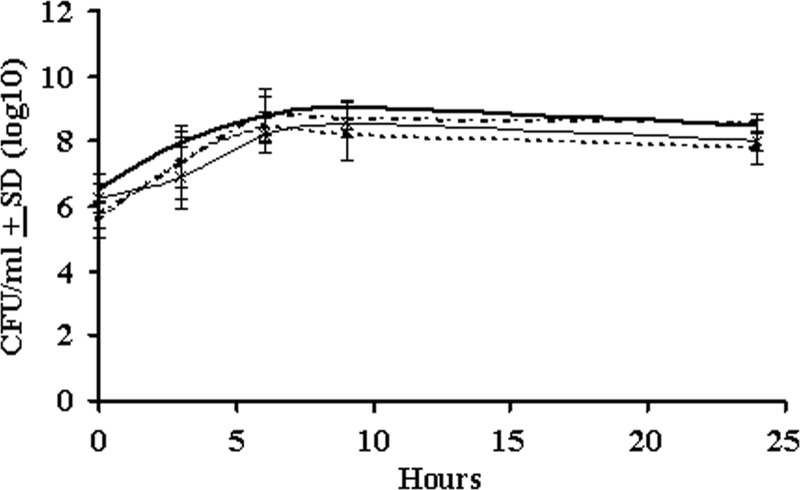
To determine the specificity of the inhibition of growth of M. haemolytica by P. multocida, M. haemolytica was also cocultured with M. bovoculi. As shown in Fig. 4, the growth of M. haemolytica was not inhibited by M. bovoculi, indicating that the inhibition of growth of M. haemolytica by P. multocida was specific. Furthermore, coculture of M. haemolytica with killed P. multocida did not result in the inhibition of growth of M. haemolytica, suggesting the involvement of a secreted inhibitory molecule in the growth inhibition (data not shown).
Fig 4.
The growth of M. haemolytica A1 is not inhibited by M. bovoculi in coculture. The CFU/ml for M. bovoculi when grown as monoculture (–×–) or coculture (—) with M. haemolytica were determined at the indicated time points. Similarly, the CFU/ml for M. haemolytica when grown as a monoculture (dotted line) or coculture (dashed line) with M. bovoculi were determined at the indicated time points. The results are the mean CFU from three independent experiments (±the SD). M. haemolytica exhibited similar growth curves in monocultures and cocultures with M. bovoculi.
P. multocida inhibition of M. haemolytica is consistent with a proximity-dependent mechanism.
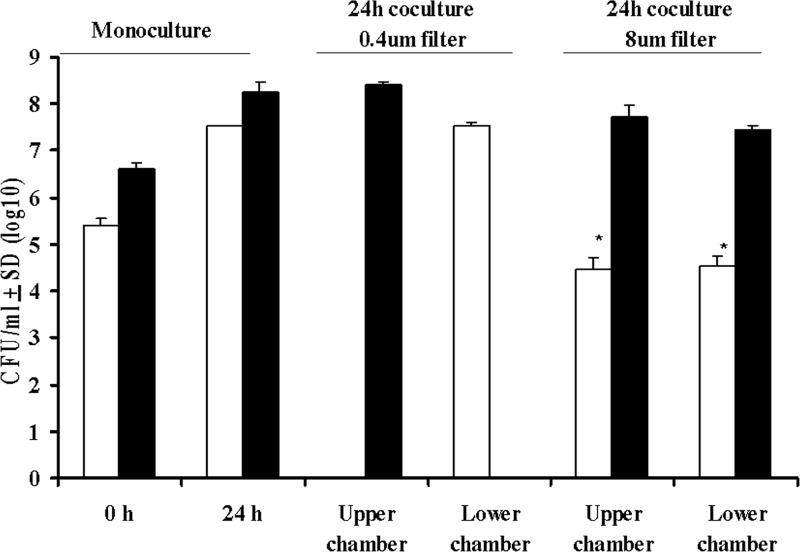
We performed a proximity-dependent inhibition assay using cell culture inserts with pore-size of either 0.4 or 8.0 μm. When P. multocida in the upper chamber was separated from M. haemolytica in the lower chamber using a 0.4-μm-pore-size membrane, the CFU counts of P. multocida in the upper chamber and M. haemolytica in the lower chamber were similar to that observed in monocultures of P. multocida and M. haemolytica, respectively (Fig. 5). When these cultures were separated by using an 8.0-μm-pore-size membrane, the CFU counts of P. multocida in the upper and lower chambers were similar to those observed in monocultures of this bacterium (P > 0.05). In contrast, the average CFU counts of M. haemolytica in the upper and lower chambers were significantly lower than those observed in monocultures for this bacterium (P < 0.001). Identical results were obtained when P. multocida was placed in the lower chamber and M. haemolytica was placed in the upper chamber (data not shown). Taken together, these results indicate that P. multocida inhibits the growth of M. haemolytica by a contact- or proximity-dependent mechanism.
Fig 5.
P. multocida inhibits the growth of M. haemolytica by a proximity-dependent mechanism. Ampr M. haemolytica (□) and Rifr P. multocida (■) were cultured in six-well culture plates to establish baseline CFU (CFU/ml) counts at 0 and 24 h when grown as monocultures. Both strains were then cultivated in six-well plates with either a 0.4- or a 8.0-μm-pore-size filter to separate cells and with Rifr P. multocida placed in the top chamber and Ampr M. haemolytica placed in the bottom chamber. Samples from each chamber were analyzed for CFU/ml at 24 h of culture on ampicillin- as well as rifampin-containing BHI agar plates. Compared to the expected number of Ampr M. haemolytica grown in monoculture, there was a significant reduction in the number of Ampr M. haemolytica (*, P < 0.0001 [Student t test]) when cultivated with 8.0-μm-pore-size filters, where there was clear migration of both species between the top and bottom chambers. The results are the mean CFU counts from four independent experiments. The bars indicate the SD of the means and are based on four independent experiments.
Inhibition of M. haemolytica by P. multocida is not mediated by observable soluble bactericidal compounds.
To assess the potential for any bactericidal compounds produced by P. multocida, culture supernatant fluid from this bacterium was placed in contact with M. haemolytica, and no inhibition of growth of M. haemolytica was observed. It is plausible that the lack of inhibition of the growth of M. haemolytica could be due to the low concentration of the bactericidal compound, although even 150 μg of proteins (in 50 μl) precipitated from the culture fluid by ammonium sulfate did not inhibit the growth of M. haemolytica. To determine whether the coculture of both bacteria is necessary for P. multocida to produce the bactericidal compound, culture supernatant fluid from a P. multocida-M. haemolytica coculture was also used in this assay, but there was no inhibition of growth of M. haemolytica. Direct plating of P. multocida on M. haemolytica plates yielded the same results. Taken together, these results suggest that inhibition of growth of M. haemolytica by P. multocida is not mediated by a soluble bactericidal compound unless it is very labile or it is present at exceedingly low concentrations.
DISCUSSION
M. haemolytica, B. trehalosi, P. multocida, and M. ovipneumoniae have been identified as pathogens associated with pneumonia in BHS (5, 9, 20, 23, 27). Of these, M. haemolytica has been shown to consistently induce fatal pneumonia in BHS under experimental conditions (12, 19, 29). However, M. haemolytica has been isolated by culture much less frequently than the others (31, 33). We hypothesized that the growth of M. haemolytica is inhibited by other bacteria in the lungs. In an earlier study, Dassanayake et al. (13) found that B. trehalosi inhibits the growth of M. haemolytica in vitro. In the present study we determined whether P. multocida inhibits the growth of M. haemolytica.
In monocultures, P. multocida and M. haemolytica exhibited similar growth characteristics as evidenced by overlapping growth curves, and this did not change when strains were resistant to selective antibiotics (Fig. 1). Based on the results of coculture experiments, it is clear that P. multocida inhibits the growth of M. haemolytica (Fig. 2 and 3) and that close proximity is required for the inhibition (Fig. 5). Furthermore, this phenotype was evident in experiments with several strains (Table 1), indicating that the susceptibility to inhibition is not unique to a particular serotype of M. haemolytica and that the inhibitory phenotype is not unique to a particular isolate of P. multocida. In all of the coculture experiments, the CFU counts of M. haemolytica and P. multocida were determined by plating an aliquot of the culture on ampicillin- or rifampin-containing agar plates, respectively. A subset of the colonies growing on the ampicillin and rifampin plates was confirmed to be M. haemolytica and P. multocida, respectively, by species-specific PCR assays (data not shown).
B. trehalosi inhibits the growth of M. haemolytica by a proximity-dependent mechanism (13). P. multocida and B. trehalosi are closely related bacterial species within the family Pasteurellaceae. Therefore, we tested the hypothesis that P. multocida also utilizes a contact- or proximity-dependent inhibition mechanism. Inhibition of growth of M. haemolytica by P. multocida when the two bacteria were separated by a membrane with a pore size of 8.0 μm (which allows the passage of bacteria), but not when they were separated by a membrane with a pore size of 0.4 μm (which allows the passage of secreted proteins and bacteriophages, but not bacteria) is consistent with a contact- or proximity-dependent mechanism. Certain strains of uropathogenic Escherichia coli (EC93) have been shown to inhibit the growth of other strains of genetically different E. coli (E. coli K-12) by a contact-dependent mechanism (2). Specific ligand interaction between CdiAB from the inhibitory strains and BamA (YaeT) on the surface membranes of target strains mediates the inhibition (3). A similar mechanism may be involved in the inhibition of M. haemolytica by P. multocida. This speculation is supported by the observation that a potential BamA homologue has been annotated for the Pasteurella species (26). Further experiments are necessary to determine whether the mechanism of inhibition by P. multocida is similar to that of E. coli EC93. E. coli 25 and E. coli 264 also inhibit several other strains of E. coli, including E. coli O157:H7 (32). The mechanism of inhibition is proximity dependent, but the molecular basis for the inhibition has not been described in the literature.
Several bacteriophages have been isolated from members of the genera Pasteurella and Mannheimia, and some of these phages form plaques with indicator strains of the same species (1, 15). Sawant et al. (32) have shown that the 0.4-μm-pore-size filters used in the present study are permissive to the diffusion of phages. The lack of inhibition of growth of M. haemolytica by P. multocida when they were separated by a membrane with a pore size of 0.4 μm is consistent with the absence of bacteriophage in the culture supernatant fluid. Furthermore, certain bacterial strains of E. coli and Klebsiella have been shown to produce bactericidal compounds that inhibit competitors in the vicinity (11, 16, 21). We could not demonstrate inhibition of growth of M. haemolytica on agar plates using the culture supernatant fluid from P. multocida monoculture or P. multocida and M. haemolytica coculture. Neither the addition of concentrated proteins from the culture supernatant fluid nor direct plating of P. multocida caused inhibition of growth of M. haemolytica, indicating that an insufficient concentration of any bactericidal compounds, if any, could be detected by our assay. Collectively, these results indicate that the inhibition of growth of M. haemolytica by P. multocida is mediated by a contact- or proximity-dependent mechanism. It is likely that the contact- or proximity-dependent mechanism involves bactericidal compounds or quorum-sensing molecules that are needed only at very low concentrations to achieve inhibition. The possibility of the “inhibitory molecule” being secreted directly into the target cells cannot be discounted.
B. trehalosi inhibits the growth of M. haemolytica (13). B. trehalosi grows faster than M. haemolytica and achieves a higher final cell density when grown in monocultures. The CFU count for B. trehalosi at 24 h is 3 logs higher than that for M. haemolytica. However, the growth rate of P. multocida in monoculture is comparable to that of M. haemolytica, and P. multocida very effectively inhibits the growth of M. haemolytica. It is tempting to speculate that the mechanism of inhibition of M. haemolytica by P. multocida is more potent than that of B. trehalosi. However, further studies are necessary to confirm this notion. In summary, our study clearly demonstrates that P. multocida inhibits the growth of M. haemolytica. The inhibition is mediated by a contact- or proximity-dependent mechanism.
ACKNOWLEDGMENTS
This research was partially supported by funds from the Wild Sheep Foundation and its Eastern, Idaho, Oregon, Washington, and Wyoming chapters; Wyoming Wildlife/Livestock Disease Partnership funds; Wyoming Governor's Big Game License Coalition; the U.S. Forest Service; and the WA Agricultural Research Center (D.R.C.).
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Ackermann HW, Karaivanov L. 1984. Morphology of Pasteurella multocida bacteriophages. Can. J. Microbiol. 30:1141–1148 [DOI] [PubMed] [Google Scholar]
- 2. Aoki SK, et al. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248 [DOI] [PubMed] [Google Scholar]
- 3. Aoki SK, et al. 2008. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol. Microbiol. 70:323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avonts L, Uytven EV, Vuyst LD. 2004. Cell growth and bacteriocin production of probiotic Lactobacillus strains in different media. Int. Dairy J. 14:947–955 [Google Scholar]
- 5. Besser TE, et al. 2008. Association of Mycoplasma ovipneumoniae infection with population-limiting respiratory disease in free-ranging Rocky Mountain bighorn sheep (Ovis canadensis canadensis). J. Clin. Microbiol. 46:423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besser TE, et al. 2012. Causes of pneumonia epizootics among bighorn sheep, Western United States, 2008–2010. Emerg. Infect. Dis. 18:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Buechner HK. 1960. The bighorn sheep in the United States, its past, present, and future. Wildl. Monogr. 4:3–174 [Google Scholar]
- 9. Coggins VL. 1988. The Lostine Rocky Mountain bighorn sheep die off and domestic sheep. Proc. Biennial Symp. Northern Sheep Goat Council 6:57–64 [Google Scholar]
- 10. Confer AW, Panciera RJ, Clinkenbeard KD, Moiser DA. 1990. Molecular aspects of virulence of Pasteurella haemolytica. Can. J. Vet. Res. 54:S48–S52 [PubMed] [Google Scholar]
- 11. Cursino LD, et al. 2006. Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. J. Appl. Microbiol. 100:821–829 [DOI] [PubMed] [Google Scholar]
- 12. Dassanayake RP, et al. 2009. Mannheimia haemolytica serotype A1 exhibits differential pathogenicity in two related species, Ovis canadensis and Ovis aries. Vet. Microbiol. 133:366–371 [DOI] [PubMed] [Google Scholar]
- 13. Dassanayake RP, et al. 2010. Bibersteinia trehalosi inhibits the growth of Mannheimia haemolytica by proximity-dependent mechanism. Appl. Environ. Microbiol. 76:1008–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dassanayake RP, et al. 2010. Mycoplasma ovipneumoniae can predispose bighorn sheep to fatal Mannheimia haemolytica pneumonia. Vet. Microbiol. 145:354–359 [DOI] [PubMed] [Google Scholar]
- 15. Davies RL, Lee I. 2006. Diversity of temperate bacteriophages induced in bovine and ovine Mannheimia haemolytica isolates and identification of a new P2-like phage. FEMS Microbiol. Lett. 260:162–170 [DOI] [PubMed] [Google Scholar]
- 16. De Lorenzo V. 1984. Isolation and characterization of microcine E492 from Klebsiella pneumoniae. Arch. Microbiol. 139:72–75 [DOI] [PubMed] [Google Scholar]
- 17. Foreyt WJ. 1989. Fatal Pasteurella haemolytica pneumonia in bighorn sheep after direct contact with clinically normal domestic sheep. Am. J. Vet. Res. 50:341–344 [PubMed] [Google Scholar]
- 18. Reference deleted.
- 19. Foreyt WJ, Snipes KP, Kasten RW. 1994. Fatal pneumonia following inoculation of healthy bighorn sheep with Pasteurella haemolytica from healthy domestic sheep. J. Wildl. Dis. 30:137–145 [DOI] [PubMed] [Google Scholar]
- 20. George JL, Martin DJ, Lukas PM, Miller MW. 2008. Epidemic pasteurellosis in a bighorn sheep population coinciding with the appearance of a domestic sheep. J. Wildl. Dis. 44:388–403 [DOI] [PubMed] [Google Scholar]
- 21. Gillor O, Kirkup BC, Riley MA. 2004. Colicins and microcins: the next generation antimicrobials. Adv. Appl. Microbiol. 54:129–146 [DOI] [PubMed] [Google Scholar]
- 22. Highlander SK, et al. 2000. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect. Immun. 68:3916–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaworski MD, Hunter DL, Ward ACS. 1998. Biovariants of isolates of Pasteurella from domestic and wild ruminants. J. Vet. Diagn. Investig. 10:49–55 [DOI] [PubMed] [Google Scholar]
- 24. Jeyaseelan S, Sreevatsan S, Maheswaran SK. 2002. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim. Health Res. Rev. 3:69–82 [DOI] [PubMed] [Google Scholar]
- 25. Lawrence PK, et al. 2010. Transmission of Mannheimia haemolytica from domestic sheep (Ovis aries) to bighorn sheep (Ovis canadensis): unequivocal demonstration with green fluorescent protein-tagged organisms. J. Wildl. Dis. 46:706–717 [DOI] [PubMed] [Google Scholar]
- 26. Mahasreshti PJ, et al. 1997. Purification and partial characterization of the OmpA family of proteins of Pasteurella haemolytica. Infect. Immun. 65:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller MW. 2001. Pasteurellosis, p 330–339 In Williams ES, Barker IK. (ed), Infectious diseases of wild mammals, Iowa State University Press, Ames, IA: [Google Scholar]
- 28. Murphy GL, Whitworth LC, Clinkenbeard KD, Clinkenbeard PA. 1995. Hemolytic activity of the Pasteurella haemolytica leukotoxin. Infect. Immun. 63:3209–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onderka DK, Rawluk SA, Wishart WD. 1988. Susceptibility of Rocky Mountain bighorn sheep and domestic sheep to pneumonia induced by bighorn and domestic livestock strains of Pasteurella haemolytica. Can. J. Vet. Res. 52:439–444 [PMC free article] [PubMed] [Google Scholar]
- 30. Petras SF, et al. 1995. Antigenic and virulence properties of Pasteurella haemolytica leukotoxin mutants. Infect. Immun. 63:1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Safaee S, Weiser GC, Cassirer EF, Ramey RR, Kelley ST. 2006. Microbial diversity in bighorn sheep revealed by culture-independent methods. J. Wildl. Dis. 42:545–555 [DOI] [PubMed] [Google Scholar]
- 32. Sawant AA, Casavant NC, Call DR, Besser TE. 2011. Proximity-dependent inhibition in Escherichia coli isolates from cattle. Appl. Environ. Microbiol. 77:2345–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shanthalingam S, et al. 2011. Species-specific PCR assay Mannheimia haemolytica not detected by cultural methods, from the lungs of pneumonic bighorn sheep, abstr. P37 Proc. Int. Pasteurellaceae Conf. [Google Scholar]
- 34. Tatum FM, et al. 1998. Construction of an isogenic leukotoxin deletion mutant of Pasteurella haemolytica serotype 1: characterization and virulence. Microb. Pathog. 24:37–46 [DOI] [PubMed] [Google Scholar]
- 35. Tomassini L, Gonzales B, Weiser GC, Sischo W. 2009. An ecologic study comparing distribution of Pasteurella trehalosi and Mannheimia haemolytica between Sierra Nevada bighorn sheep, White Mountain bighorn sheep, and domestic sheep. J. Wildl. Dis. 45:930–940 [DOI] [PubMed] [Google Scholar]
- 36. Townsend KM, Frost AJ, Lee CW, Papadimitriou JM, Dawkins HJS. 1998. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J. Clin. Microbiol. 36:1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valdez R, Krausman PR. 1999. Mountain sheep of North America, p 19 University of Arizona Press, Tucson, AZ: [Google Scholar]
- 38. Weiser GC, et al. 2003. Characterization of Pasteurella multocida associated with pneumonia in bighorn sheep. J. Wildl. Dis. 39:536–544 [DOI] [PubMed] [Google Scholar]
- 39. Weiser GC, Miller DS, Drew ML, Rhyan JC, Ward AC. 2009. Variation in Pateurella (Bibersteinia) and Mannheimia spp. following transport and antibiotic treatment in free-ranging and captive Rocky Mountain bighorn sheep (Ovis canadensis canadensis). J. Zoo Wildl. Med. 40:117–125 [DOI] [PubMed] [Google Scholar]