Abstract
In this study, natural fungal diversity in wood-decaying species was explored for biomass deconstruction. In 2007 and 2008, fungal isolates were collected in temperate forests mainly from metropolitan France and in tropical forests mainly from French Guiana. We recovered and identified 74 monomorph cultures using morphological and molecular identification tools. Following production of fungal secretomes under inductive conditions, we evaluated the capacity of these fungal strains to potentiate a commercial Trichoderma reesei cellulase cocktail for the release of soluble sugars from biomass. The secretome of 19 isolates led to an improvement in biomass conversion of at least 23%. Of the isolates, the Trametes gibbosa BRFM 952 (Banque de Ressources Fongiques de Marseille) secretome performed best, with 60% improved conversion, a feature that was not universal to the Trametes and related genera. Enzymatic characterization of the T. gibbosa BRFM 952 secretome revealed an unexpected high activity on crystalline cellulose, higher than that of the T. reesei cellulase cocktail. This report highlights the interest in a systematic high-throughput assessment of collected fungal biodiversity to improve the enzymatic conversion of lignocellulosic biomass. It enabled the unbiased identification of new fungal strains issued from biodiversity with high biotechnological potential.
INTRODUCTION
The magnitude of fungal diversity is estimated to be 1.5 million species, but only 5% of species have been described. This estimate was calculated from the ratio of fungal species to vascular plant species for various ecologically defined groups of fungi in well-studied regions (14, 15). In the light of data from site inventories, the fungus/plant ratio ranges from 5:1 to 33:1. Available evidence also indicates that fungal diversity in the tropics is richer than that in temperate regions (2, 15). The true scale of fungal diversity is still controversial but bears directly upon areas of human enterprise, such as ecology, agriculture, medicine, and industry (5). France is the only Western country to possess a large tropical forest heritage, with 8 million hectares of forests in its overseas territories. Most of these are tropical forests, considered to be the main world reservoir of biological diversity. In particular, French Guiana is of great interest as it has been little modified by human activities and remains largely unexplored. Tropical fungi have traditionally been underresearched, and their taxonomic placement has been confounded, often by misidentification with temperate fungi. The exploration of tropical fungi is thus limited by (i) the extensive training needed for sampling of complex tropical habitats, (ii) the paucity of newly trained systematists specializing in tropical mycology, and (iii) traditional difficulties in delineating species boundaries (3). Therefore, exploration of wood-decaying fungi is challenging.
Lignocellulose is both physically and chemically resistant to degradation even after plant death, as cellulose fibrils are embedded in the hemicellulose and lignin matrix (10). Wood-rotting fungi belonging to the phyla Basidiomycota and Ascomycota play a key role in recycling nutrients in forest ecosystems. They are known to produce a high number and broad variety of extracellular enzymes with different, complementary catalytic activities to degrade lignocellulose-rich materials (8, 9, 38). Fungal lignocellulolytic enzymes have therefore been studied for the hydrolysis of renewable biomass resources available in large amounts, such as plants, plant parts (e.g., seeds and stalks), plant constituents (e.g., starch and fiber), processing by-products (e.g., distiller's grains and corn solubles), and municipal, agricultural (e.g., straw), and industrial wastes, into high-added-value products. Bioconversion of plant cell wall polysaccharides, cellulose, and hemicellulose to simple sugars for subsequent fermentation to bioethanol has been widely studied, as the prospect of its biological production from abundant lignocellulosic feedstocks is attractive (23, 44). However, enzymatic hydrolysis (saccharification) is still the major bottleneck in the biorefinery process. The fungus Trichoderma reesei, an anamorph of the pantropical ascomycete Hypocrea jecorina, originally isolated from cotton canvas in the Solomon Islands during World War II (28), has been identified as a good candidate for monosaccharide release, due to its capacity to secrete high levels of cellulases. T. reesei has undergone several rounds of mutation/selection starting from the QM6a strain to increase its capacity to produce and secrete cellulases in high yields (19). As a result, the engineered T. reesei industrial CL847 strain is able to secrete more than 100 g of proteins per liter of culture and has been proposed to be one of the most promising strains for conversion of lignocellulose to fermentable sugars. However, conversion is still not optimal due to the heterogeneous composition of plant biomass. In addition, analysis of the T. reesei genome has revealed a low number and low level of diversity of enzymes likely to be involved in biomass degradation compared with the number and diversity in other filamentous fungi (24). Thus, there is a need to develop at a significantly reduced cost more effective enzymatic cocktails with a range of properties complementary to current cellulase systems.
In this study, fungal biodiversity in wood-decaying species was explored without a priori knowledge for biomass deconstruction. The strains used were isolated from fresh decaying wood specimens collected in situ in different geographical areas, i.e., tropical forests from French overseas territories and temperate forests from metropolitan France. Isolates were carefully identified by both morphological and molecular methods. Following production of fungal secretomes under similar inductive conditions, conversion of biomass was assessed by taking advantage of an in-house automated methodology. The potential of fungal secretomes to improve a reference cellulolytic cocktail originating from T. reesei was evaluated.
MATERIALS AND METHODS
Collection and identification of fungal specimens.
Fruit bodies growing on dead wood were collected in 2007 and 2008 during the rainy season in both tropical areas (between April and August) and temperate areas (between October and March) in different biotopes (Table 1). Field identification of basidioma was achieved via classical methodology by macro- and micromorphological analyses using taxonomic guides and standard procedures (13, 26, 32–36). For ascoma identification, specimens were examined using the methods described by Rossman et al. (30) and Hirooka et al. (16). Microscopic observations and measurements were made in water, and ascospore ornamentation was observed in lactic cotton blue.
Table 1.
Fungal strains collected in temperate and tropical forests and identified in this study
| Forest type and taxond | Classification | BRFM no. | Voucher specimen | Geographic location (country, county, citya) | ITS GenBank accession no. |
|---|---|---|---|---|---|
| Temperate forests | |||||
| Cyanonectria buxi | Ascomycota, Nectriaceae | 1205 | CLL 7165 | France, Deux-Sèvres | JX082385 |
| Geejayessia sp. | Ascomycota, Nectriaceae | 1015 | CLL 7150 | Spain, NAb | JX082350 |
| Hypocrea lixii | Ascomycota, Hypocreaceae | 1058 | CLL 7132 | France, NA | JX082359 |
| Neonectria discophora | Ascomycota, Nectriaceae | 1206 | CLL 7178 | France, Deux-Sèvres | JX082386 |
| Bjerkandera adusta | Basidiomycota, Meruliaceae | 965 | BEL 160 | France, Orne, Bellême | JX082339 |
| Daedaleopsis confragosa | Basidiomycota, Polyporaceae | 1130 | BEL 08-270 | France, Orne, Bellême | JX082372 |
| Daedaleopsis confragosa | Basidiomycota, Polyporaceae | 1131 | BEC 08-275 | France, Aude, Belcaire | JX082373 |
| Daedaleopsis confragosa | Basidiomycota, Polyporaceae | 1143 | BEC 08-311 | France, Aude, Bellême | JX082375 |
| Daedaleopsis confragosa | Basidiomycota, Polyporaceae | 1145 | BEL 08-240 | France, Orne, Bellême | JX082376 |
| Fomitopsis pinicola | Basidiomycota, Fomitopsidaceae | 882 | LUM 25 | France, Bouches-du-Rhône, Marseille | JX082329 |
| Ganoderma lucidum | Basidiomycota, Ganodermataceae | 885 | BAU 34 | France, Var, Plan d'Aups | JX082330 |
| Ganoderma resinaceum | Basidiomycota, Ganodermataceae | 872 | MRS 26 | France, Bouches-du-Rhône, Marseille | JX082326 |
| Ganoderma resinaceum | Basidiomycota, Ganodermataceae | 875 | MRS 27 | France, Bouches-du-Rhône, Marseille | JX082328 |
| Gloeophyllum sepiarum | Basidiomycota, Gloeophyllaceae | 988 | ARI 08-09 | France, Ariège, Varilhes | JX082348 |
| Inonotus tamaricis | Basidiomycota, Hymenochaetaceae | 880 | LEC 30 | France, Var, Saint-Cyr sur Mer | Only ITS1 was available |
| Inonotus radiatus | Basidiomycota, Hymenochaetaceae | 1153 | BEC 08-277 | France, Aude, Roquefeuil | JX082384 |
| Ischnoderma benzoinum | Basidiomycota, Fomitopsidaceae | 1134 | BEL 08-250 | France, Orne, Bellême | JX082374 |
| Merulius tremellosus | Basidiomycota, Meruliaceae | 968 | BEL 163 | France, Orne, Bellême | JX082340 |
| Polyporus brumalis | Basidiomycota, Polyporaceae | 985 | TAR 08-01 | France, Hautes Pyrénées, Puydarieux | JX082346 |
| Postia stiptica | Basidiomycota, Fomitopsidaceae | 1148 | HYE 08-381 | France, Var, Hyères | JX082379 |
| Postia stiptica | Basidiomycota, Fomitopsidaceae | 1149 | HYE 08-382 | France, Var, Hyères | JX082380 |
| Postia stiptica | Basidiomycota, Fomitopsidaceae | 1150 | HYE 08-386 | France, Var, Hyères | JX082381 |
| Postia stiptica | Basidiomycota, Fomitopsidaceae | 1151 | HYE 08-388 | France, Var, Hyères | JX082382 |
| Postia stiptica | Basidiomycota, Fomitopsidaceae | 1152 | POR 08-370 | France, Var, Porquerolles | JX082383 |
| Skeletocutis nivea | Basidiomycota, Polyporaceae | 987 | ND 167 | France, Pyrénées Atlantiques, Correze | JX082347 |
| Stereum hirsutum | Basidiomycota, Stereaceae | 889 | BAU 42 | France, Var, Plan d'Aups | JX082331 |
| Lenzites warnieri | Basidiomycota, Polyporaceae | 972 | ND 169 | France, Vaucluse, Lumières | JX082341 |
| Lenzites warnieri | Basidiomycota, Polyporaceae | 973 | ND 181 | France, Vaucluse, Opedette | JX082342 |
| Trametes gibbosa | Basidiomycota, Polyporaceae | 873 | BEL 2 | France, Orne, Bellême, Beech tree | JX082327 |
| Trametes gibbosa | Basidiomycota, Polyporaceae | 952 | MOU 147 | France, Ariège, Moulis, Beech Forest | JX082338 |
| Trametes gibbosa | Basidiomycota, Polyporaceae | 983 | ARI 08-03 | France, Ariège, Varilhes | JX082344 |
| Trametes gibbosa | Basidiomycota, Polyporaceae | 1115 | BEL 08-268 | France, Orne, Bellême | JN645064c |
| Trametes gibbosa | Basidiomycota, Polyporaceae | 1147 | BEL 08-255 | France, Orne, Bellême | JX082378 |
| Trametes hirsuta | Basidiomycota, Polyporaceae | 984 | LYO 08-10 | France, Rhône, Rontalon | JX082345 |
| Trametes ochracea | Basidiomycota, Polyporaceae | 1019 | DRO 08-12 | France, Drôme, Bourg de Péage | JX082351 |
| Trametes sp. (Trametes aff. meyenii) | Basidiomycota, Polyporaceae | 1121 | GUY 08-152 | French Guiana, Macouria | JN645083c |
| Trametes versicolor | Basidiomycota, Polyporaceae | 1116 | BEL 08-252 | France, Orne, Bellême | JX082367 |
| Trametes versicolor | Basidiomycota, Polyporaceae | 1146 | BEL 08-253 | France, Orne, Bellême | JX082377 |
| Tropical forests | |||||
| Nectria pseudocinnabarina | Ascomycota, Nectriaceae | 1288 | CLL 8299 | Martinique, NA | JX082392 |
| Hypocrea lixii | Ascomycota, Hypocreaceae | 1285 | CLL 110 | French Guiana, Nouragues | JX082390 |
| Neocosmospora cf. hematococca | Ascomycota, Nectriaceae | 1214 | CLL 8033 | French Guiana, Sinnamary | JX082388 |
| Neocosmospora cf. hematococca | Ascomycota, Nectriaceae | 1286 | CLL 8012 | French Guiana, Kourou | JX082391 |
| Rugonectria cf. rugulosa | Ascomycota, Nectriaceae | 1213 | CLL 8212 | Guadeloupe, Capesterre-Belle-Eau | JX082387 |
| Xylaria curta | Ascomycota, Xylariaceae | 1269 | CLL 8045 | French Guiana, Sinnamary | JX082389 |
| Amauroderma sp. | Basidiomycota, Ganodermataceae | 915 | GUY 74 | French Guiana, Sinnamary | JX082335 |
| Amauroderma sp. | Basidiomycota, Ganodermataceae | 916 | GUY 83 | French Guiana, Sinnamary | JX082336 |
| Amauroderma sp. | Basidiomycota, Ganodermataceae | 1117 | GUY 08-151 | French Guiana, Macouria | JX082393 |
| Artolenzites elegans (Trametes elegans) | Basidiomycota, Polyporaceae | 1122 | GUY 08-145 | French Guiana, Saül | JN645066c |
| Coriolopsis sp. | Basidiomycota, Polyporaceae | 1125 | GUY 08-85 | French Guiana, Sinnamary | JX082370 |
| Coriolopsis sp. | Basidiomycota, Polyporaceae | 1126 | GUY 08-201 | French Guiana, Macouria | JX082371 |
| Cyathus sp. | Basidiomycota, Agaricaceae | 934 | GUY 104 | French Guiana, Sinnamary | JX082337 |
| Earliella scabrosa | Basidiomycota, Polyporaceae | 1106 | GUY 08-137 | French Guiana, Saül | JX082364 |
| Fomes fasciatus | Basidiomycota, Polyporaceae | 1081 | GUY 08-34 | French Guiana, Kourou | JX082362 |
| Ganoderma sp. | Basidiomycota, Ganodermataceae | 1030 | GUY 08-48 | French Guiana, Sinnamary | JX082353 |
| Ganoderma sp. | Basidiomycota, Ganodermataceae | 1035 | GUY 08-107 | French Guiana, Saül | JX082354 |
| Ganoderma subfornicatum | Basidiomycota, Ganodermataceae | 1024 | GUY 08-57 | French Guiana, Sinnamary | JX082352 |
| Grammothele sp. | Basidiomycota, Polyporaceae | 910 | GUY 82 | French Guiana, Sinnamary | JX082334 |
| Gymnopilus sp. | Basidiomycota, Strophariaceae | 1082 | GUY 08-73 | French Guiana, Kourou | JX082363 |
| Lenzites sp. | Basidiomycota, Polyporaceae | 1048 | GUY 08-17 | French Guiana, Kourou | JX082355 |
| Lenzites sp. | Basidiomycota, Polyporaceae | 1049 | GUY 08-18 | French Guiana, Kourou | JX082356 |
| Lenzites sp. (Leiotrametes sp.) | Basidiomycota, Polyporaceae | 1050 | GUY 08-20 | French Guiana, Kourou | GU731566c |
| Lenzites sp. | Basidiomycota, Polyporaceae | 1053 | GUY 08-134 | French Guiana, Saül | JX082357 |
| Lenzites sp. | Basidiomycota, Polyporaceae | 1054 | GUY 08-146 | French Guiana, Saül | JX082358 |
| Lenzites sp. (Leiotrametes sp.) | Basidiomycota, Polyporaceae | 1078 | GUY 08-156 | French Guiana, Macouria | JN645062c |
| Lenzites sp. | Basidiomycota, Polyporaceae | 1079 | GUY 08-159 | French Guiana, Macouria | JX082361 |
| Lenzites sp. (Leiotrametes sp.) | Basidiomycota, Polyporaceae | 1080 | GUY 08-167 | French Guiana, Kourou | JN645063c |
| Phellinus sp. | Basidiomycota, Hymenochaetaceae | 907 | GUY 33 | French Guiana, Sinnamary | JX082332 |
| Phellinus sp. | Basidiomycota, Hymenochaetaceae | 908 | GUY 70 | French Guiana Sinnamary | JX082333 |
| Phellinus sp. | Basidiomycota, Hymenochaetaceae | 1007 | GUY 07-05 | French Guiana, Sinnamary | JX082349 |
| Pycnoporus sanguineus | Basidiomycota, Polyporaceae | 981 | CAL 189 | New Caledonia, NA | JX082343 |
| Pycnoporus sanguineus | Basidiomycota, Polyporaceae | 1114 | GUY 08-215 | French Guiana, Cayenne | JX082366 |
| Tinctoporellus epimiltinus | Basidiomycota, Polyporaceae | 1077 | GUY 08-26 | French Guiana, Kourou | JX082360 |
| Trametes lactinea (Leiotrametes lactinea) | Basidiomycota, Polyporaceae | 1119 | GUY 08-16 | French Guiana, Kourou | JX082368 |
| Trametes lactinea (Leiotrametes lactinea) | Basidiomycota, Polyporaceae | 1120 | GUY 08-131 | French Guiana, Saül | JX082369 |
When available.
NA, not available.
Welti et al. (42).
cf. and aff. indicate members of the indicated genus resembling the listed species.
Isolation of strains.
For each basidiomycetes specimen collected, primary isolation was made from context tissue of fresh fruit bodies on the day of collection, using malt agar medium supplemented with chloramphenicol (50 μg · ml−1). Apparently monomorphic cultures obtained after at least two transfers onto fresh agar plates were further authenticated using molecular tools to check strain purity and identity. With regard to ascomycetes, isolates were obtained from ascospores using potato dextrose agar (PDA; BD Difco, France) supplemented with streptomycin (5 μg · ml−1).
Molecular authentication (DNA extraction, PCR, and sequencing).
Genomic DNA was isolated from mycelial powder (40 to 80 mg) as described by Lomascolo et al. (21). The ITS region was amplified using the ITS1 and ITS4 primers as described by White et al. (43). The ITS1-5.8S rRNA gene-ITS2 were amplified from 50 ng genomic DNA in 50 μl PCR reagent containing 1.5 U Expand High Fidelity PCR system (Roche, France) with a protocol adapted from that of Lomascolo et al. (21). Extension was carried out for 1 min at 51°C. The PCR products were sequenced by Cogenics (Meylan, France). Fungal internal transcribed spacer (ITS) sequences were checked and edited with MEGA (version 5) software (41). Using the BLAST algorithm, ITS sequences were compared with sequences in the GenBank database and FunGene-DB database (for Polyporales fungi; http://www.fungene-db.org). The best BLAST match was reported for each strain, and phylogenetic analysis was used to confirm its relevance. The taxon name was validated only when the morphological identification of the specimen and the molecular identification of the strain were consistent. All authenticated strains were deposited into the fungal culture collection of the International Centre of Microbial Resources (CIRM-CF; http://www.inra.fr/crb-cirm/) at the French National Institute for Agricultural Research (INRA; Marseille, France). The strains were maintained on malt agar (BD Difco) slants, using MA2 (malt extract at 2% [wt/vol]) medium for basidiomycetes and MYA2 (malt extract at 2% [wt/vol] and yeast extract at 0.1% [wt/vol]) medium for ascomycetes.
Culture conditions and supernatant preparation.
On the basis of previous studies (6, 9, 20), the fungal cultures were grown in a liquid medium containing 15 g · liter−1 (based on the dry matter) of the autoclaved maize bran fraction (provided by ARD, Pomacle, France) as a carbon source, 2.5 g · liter−1 of maltose as a starter, 1.842 g · liter−1 of diammonium tartrate as a nitrogen source, 0.5 g · liter−1 yeast extract, 0.2 g · liter−1 KH2PO4, 0.0132 g · liter −1 CaCl2 · 2H2O, and 0.5 g · liter−1 MgSO4 · 7H2O. The sugar content (wt/wt) of the autoclaved maize bran fraction was 16.10% arabinose, 28.73% xylose, 0.17% mannose, 5.65% galactose, and 22.06% glucose. Miniaturized fungal cultures were carried out in 16-well baffled plates as described by Alberto et al. (1) and Couturier et al. (9). The cultures were inoculated with 2 × 105 spores · ml−1 for sporulating fungi or with mycelial fragments generated using a FastPrep-24 system (MP Biomedicals, France) set to 5 m · s−1 for 40 s for nonsporulating fungi, before incubation in 16-well baffled plates at 30°C with orbital shaking at 140 rpm (Infors HT, Switzerland). Each isolate was grown in 16-well baffled plates in triplicate. All the cultures were stopped at 7 and 10 days after inoculation for ascomycetes and basidiomycetes, respectively, as this was the midterm growth for the corresponding fungi under our conditions (16-well baffled plates) and extended growth led to evaporation of the culture medium. The culture broths (secretomes) were harvested and pooled (total volume, 20 to 30 ml), filtered (using a 0.2-μm-pore-size polyethersulfone membrane; Vivaspin; Sartorius, Germany), diafiltered, and concentrated (Vivaspin polyethersulfone membrane with a 10-kDa cutoff; Sartorius) in 50 mM acetate solution buffer, pH 5, to a final volume of 3 ml and then stored in appropriate vials (1.2-ml tubes with septa in the cluster plate; ABgene, Thermo Scientific) at −20°C until use.
Saccharification assays.
The T. reesei CL847 secretome (E508 enzymatic cocktail) obtained from IFPEN (Rueil-Malmaison, France) was used as a reference enzymatic cocktail (8, 9, 40). Enzymatic commercial cocktails were obtained from the manufacturers, as indicated in Table 2. The concentrated secretomes were tested for their ability to hydrolyze micronized wheat straw (WS; Triticum aestivum Apache, France) that was kindly provided by F. Meaux (St. Jean du Salés, Aveyron, France), harvested in 2007, and used for grinding experiments as described by Navarro et al. (27) and Silva et al. (39). WS was stored at 4°C before use. WS particles had an average size of 100 μm. Wheat straw was composed of cellulose at 32.0 ± 0.7, hemicelluloses (arabinoxylans) at 20.5 ± 0.4, lignin at 17.4 ± 0.3, extractives at 9.5 ± 2.2, and ash at 6.1 ± 0.1 g/100 g dry wheat straw (39). A 1% (wt/vol) WS suspension was prepared in 50 mM acetate buffer, pH 5, supplemented with 40 μg · ml−1 of tetracycline as an antibiotic and 30 μg · ml−1 of cycloheximide as an antifungal agent. The resulting suspension was dispensed into 96-well plates by a Tecan Genesis Evo 200 robot (Tecan, Lyon, France), and the plates were frozen at −20°C until needed. The saccharification assay was performed by a previously described high-throughput automated method (27). All the appropriate blanks and controls were run as described by Navarro et al. (27). A substrate-free negative control was set up by filling wells with 50 mM sodium acetate buffer, pH 5, and the background of soluble sugars present in the WS sample was determined by incubating WS in the absence of enzyme. The sugars released were quantified at the saccharification plateau defined by Navarro et al. (27) as 30 μg of CL847 representing 100% sugar-releasing activity at 24 h for WS (reference activity). It is important to note that at the saccharification plateau, a supplementation of T. reesei CL847 enzyme cocktail by itself did not change the yield of total sugars released. To quantify the sugars released at the saccharification plateau, 15 μl of each concentrated secretome was added to the substrate plate either alone or together with 30 μg of the T. reesei CL847 enzymatic cocktail. The reducing sugars released were quantified using the dinitrosalicylic acid (DNS) assay (27). The addition of fungal secretomes was not normalized on the basis of the protein loading since there was a large gap in the amount of total protein secreted by fungal strains. Moreover, based on previous work (9), we noticed that the efficiency of fungal secretomes in supplementation trials is more closely correlated to enzyme diversity rather than total enzyme loading of fungal secretomes.
Table 2.
Fungal commercial cocktails testeda
| Enzyme cocktail | Origin | Fungal species | % sugar |
|
|---|---|---|---|---|
| −CL847 | +CL847 | |||
| E508-CL847 | IFPEN | Trichoderma reesei | 100 | 100 |
| Accelerase 1500 | Genencor | Trichoderma reesei | 91 | 113 |
| Cellobiase 188 | Novozyme | Aspergillus niger | 31 | 113 |
| Celluclast | Novozyme | Trichoderma reesei | 98 | 117 |
| Depol 686L | Biocatalysts | Trichoderma reesei | 98 | 104 |
| Depol 740L | Biocatalysts | Humicola sp. | 41 | 119 |
| Hemicellulase | Sigma | Aspergillus niger | 38 | 115 |
| Pectinex Ultra SP-L | Novozyme | Aspergillus aculeatus | 62 | 102 |
| Viscozyme L | Novozyme | Aspergillus aculeatus | 4 | 102 |
| Xylanase | Sigma | Trichoderma viride | 39 | 113 |
Total solubilized sugars were measured using enzyme cocktails alone (−CL847) and supplemented with the T. reesei secretome (+CL847) and were expressed as a percentage by taking the result for T. reesei CL847 commercial cocktail as a reference. Values are means of at least triplicate measures performed independently. Standard errors of the means were <5%.
Enzyme activity measurements.
para-Nitrophenyl (pNP)-based chromogenic substrates and complex substrates were used to assay the enzymatic activities of the Trametes gibbosa BRFM 952 (Banque de Ressources Fongiques de Marseille) secretome as described by Couturier et al. (9).
Nucleotide sequence accession numbers.
All the nucleotide sequences were deposited in GenBank under the accession numbers given in Table 1.
RESULTS
Fungal diversity.
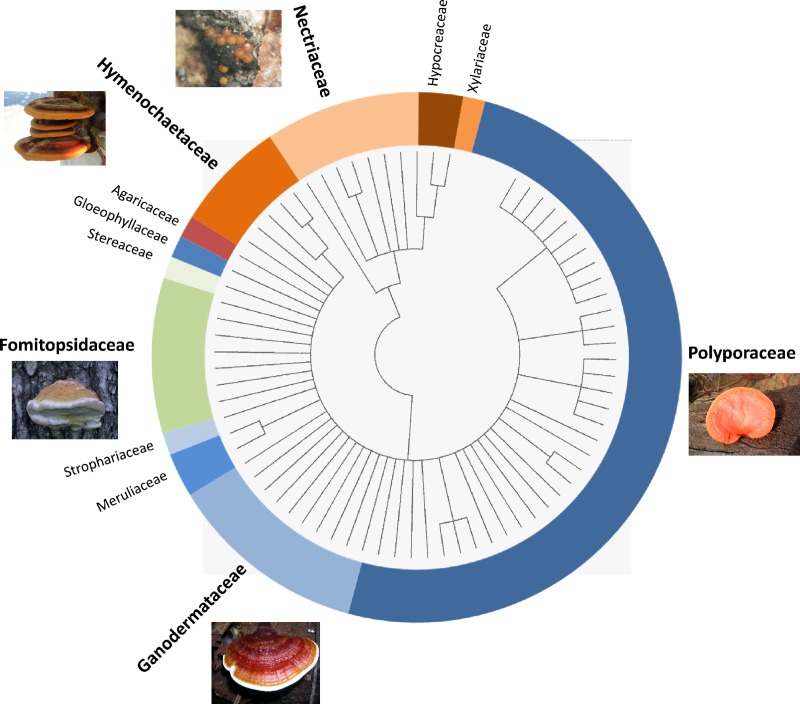
Whatever the climatic zone (tropical or temperate), about half of the isolates did not grow on plates and/or were contaminated by other microorganisms (data not shown). A total of 74 pure cultures was finally recovered (Table 1), from which 69% of the isolates were identified to the species level (34 different species mainly from temperate regions) and 31% were identified to the genus level only (mainly from tropical regions). A cladogram, constructed using ITS sequences, illustrates the fungal diversity thus obtained (Fig. 1). Among the fungal species recovered and authenticated, 64 belonged to basidiomycetes mainly from the Polyporaceae, Ganodermataceae, Fomitopsidaceae, and Hymenochaetaceae and 10 belonged to ascomycetes mainly from the Nectriaceae.
Fig 1.
Natural fungal diversity explored. The cladogram was constructed on the basis of ITS sequences, and pictures illustrate the main fungal classes represented.
Production of fungal secretomes.
Hydrolysis of plant biomass requires the concerted action of a range of lignocellulolytic enzymes. The secretion of these enzymes in vitro is regulated by the type and complexity of the plant material used as an inducer for the fungal cultures. In this study, maize bran was selected as it is a powerful inducer for the expression of a broad range of genes that encode lignocellulolytic enzymes, e.g., endoxylanases, endomannanases, arabinofuranosidases, carbohydrate esterases, and oxidoreductases, as previously shown by Couturier et al. (9). All the fungi tested were able to grow on maize bran with a satisfactory yield of secreted protein and were harvested at a single time point, i.e., 7 days of growth for ascomycetes and 10 days of growth for basidiomycetes (see Materials and Methods). All secretomes were similarly diafiltered and then tested individually for their ability to release sugars from biomass, taking advantage of our in-house automated saccharification method (27). Given the crops produced in France for possible conversion to biofuel, we focused our study on cereal straw from wheat.
Saccharification efficiencies of fungal secretomes.
The release of reducing sugars from wheat straw (WS) was quantified at the saccharification plateau (24 h), taking the T. reesei CL847 enzymatic cocktail as a reference (Table 3). Experiments were performed using fungal secretomes either alone or in combination with the T. reesei CL847 enzymatic cocktail. Data were fitted using a quartile statistical analysis.
Table 3.
Contribution of fungal secretomes to the saccharification of wheat strawa
| Species | BRFM no. | −CL847 |
+CL847 |
||
|---|---|---|---|---|---|
| % sugar | Quartile | % sugar | Quartile | ||
| Ganoderma sp. | 1030 | 87 | 4 | 108 | 1 |
| Artolenzites elegans | 1122 | 84 | 4 | 121 | 3 |
| Hypocrea lixii | 1285 | 84 | 4 | 127 | 4 |
| Trametes gibbosa | 952 | 74 | 4 | 160 | 4 |
| Hypocrea lixii | 1058 | 65 | 4 | 137 | 4 |
| Coriolopsis sp. | 1126 | 61 | 4 | 95 | 1 |
| Neonectria discophora | 1206 | 60 | 4 | 114 | 2 |
| Nectria pseudocinnabarina | 1288 | 56 | 4 | 134 | 4 |
| Rugonectria cf. rugulosa | 1213 | 55 | 4 | 112 | 2 |
| Cyanonectria buxi | 1205 | 53 | 4 | 131 | 4 |
| Tinctoporellus epimiltinus | 1077 | 52 | 4 | 132 | 4 |
| Ganoderma subfornicatum | 1024 | 51 | 4 | 113 | 2 |
| Trametes ochracea | 1019 | 50 | 4 | 124 | 4 |
| Lenzites sp. | 1054 | 46 | 4 | 123 | 4 |
| Postia stiptica | 1152 | 46 | 4 | 117 | 3 |
| Phellinus sp. | 1007 | 45 | 4 | 125 | 4 |
| Trametes sp. | 1121 | 45 | 4 | 105 | 1 |
| Coriolopsis sp. | 1125 | 44 | 4 | 113 | 2 |
| Ganoderma sp. | 1035 | 44 | 4 | 117 | 3 |
| Daedaleopsis confragosa | 1143 | 43 | 3 | 127 | 4 |
| Daedaleopsis confragosa | 1145 | 43 | 3 | 135 | 4 |
| Trametes gibbosa | 1147 | 42 | 3 | 114 | 2 |
| Merulius tremellosus | 968 | 41 | 3 | 120 | 3 |
| Postia stiptica | 1148 | 41 | 3 | 119 | 3 |
| Skeletocutis nivea | 987 | 41 | 3 | 91 | 1 |
| Trametes lactinea | 1119 | 41 | 3 | 95 | 1 |
| Trametes lactinea | 1120 | 41 | 3 | 114 | 2 |
| Postia stiptica | 1150 | 40 | 3 | 114 | 2 |
| Postia stiptica | 1151 | 40 | 3 | 116 | 3 |
| Daedaleopsis confragosa | 1131 | 39 | 3 | 123 | 4 |
| Lenzites sp. | 1053 | 39 | 3 | 112 | 2 |
| Trametes gibbosa | 1115 | 39 | 3 | 104 | 1 |
| Trametes versicolor | 1146 | 39 | 3 | 128 | 4 |
| Amauroderma sp. | 1117 | 38 | 3 | 112 | 2 |
| Amauroderma sp. | 916 | 38 | 3 | 127 | 4 |
| Earliella scabrosa | 1106 | 38 | 3 | 120 | 3 |
| Phellinus sp. | 907 | 38 | 3 | 118 | 3 |
| Daedaleopsis confragosa | 1130 | 37 | 2 | 111 | 2 |
| Geejayessia sp. | 1015 | 37 | 2 | 121 | 3 |
| Inonotus radiatus | 1153 | 37 | 2 | 114 | 2 |
| Ischnoderma benzoinum | 1134 | 37 | 2 | 121 | 3 |
| Lenzites sp. | 1079 | 36 | 2 | 112 | 2 |
| Postia stiptica | 1149 | 36 | 2 | 108 | 1 |
| Trametes gibbosa | 983 | 36 | 2 | 96 | 1 |
| Xylaria curta | 1269 | 36 | 2 | 101 | 1 |
| Pycnoporus sanguineus | 1114 | 35 | 2 | 128 | 4 |
| Trametes versicolor | 1116 | 35 | 2 | 103 | 1 |
| Ganoderma lucidum | 885 | 34 | 2 | 115 | 3 |
| Gymnopilus sp. | 1082 | 34 | 2 | 111 | 2 |
| Lenzites sp. | 1048 | 34 | 2 | 153 | 4 |
| Lenzites warnieri | 972 | 33 | 2 | 113 | 2 |
| Neocosmospora cf. hematococca | 1214 | 33 | 2 | 126 | 4 |
| Phellinus sp. | 908 | 33 | 2 | 123 | 4 |
| Fomes fasciatus | 1081 | 32 | 2 | 108 | 1 |
| Neocosmospora cf. hematococca | 1286 | 32 | 2 | 121 | 3 |
| Lenzites sp. | 1049 | 30 | 1 | 111 | 2 |
| Fomitopsis pinicola | 882 | 29 | 1 | 98 | 1 |
| Inonotus tamaricis | 880 | 29 | 1 | 122 | 3 |
| Lenzites sp. | 1050 | 29 | 1 | 110 | 2 |
| Lenzites sp. | 1078 | 29 | 1 | 117 | 3 |
| Lenzites warnieri | 973 | 28 | 1 | 103 | 1 |
| Trametes hirsuta | 984 | 28 | 1 | 122 | 4 |
| Bjerkandera adusta | 965 | 27 | 1 | 118 | 3 |
| Pycnoporus sanguineus | 981 | 27 | 1 | 95 | 1 |
| Gloeophyllum sepiarum | 988 | 26 | 1 | 117 | 3 |
| Polyporus brumalis | 985 | 26 | 1 | 106 | 1 |
| Amauroderma sp. | 915 | 25 | 1 | 112 | 2 |
| Lenzites sp. | 1080 | 25 | 1 | 120 | 3 |
| Trametes gibbosa | 873 | 24 | 1 | 84 | 1 |
| Cyathus sp. | 934 | 22 | 1 | 104 | 1 |
| Grammothele sp. | 910 | 22 | 1 | 103 | 1 |
| Ganoderma resinaceum | 875 | 20 | 1 | 110 | 2 |
| Ganoderma resinaceum | 872 | 17 | 1 | 102 | 1 |
| Stereum hirsutum | 889 | 0 | 1 | 117 | 3 |
Total solubilized sugars were measured using fungal secretomes alone (−CL847) and in combination with the T. reesei secretome (+CL847) and are expressed in percent by taking the T. reesei CL847 commercial cocktail as a reference. Values are means of at least triplicate measures performed independently. Standard errors of the means were <5%. Results are classified into four categories using a quartile statistical analysis: for fungal secretomes tested alone, quartile 1 ≤ 31, 32 ≤ quartile 2 ≤ 37, 38 ≤ quartile 3 ≤ 43, and quartile 4 ≥ 44; for fungal secretomes tested in supplementation, quartile 1 ≤ 109, 110 ≤ quartile 2 ≤ 114, 115 ≤ quartile 3 ≤ 122, and quartile 4 ≥ 123. Quartile 4 is highlighted in bold.
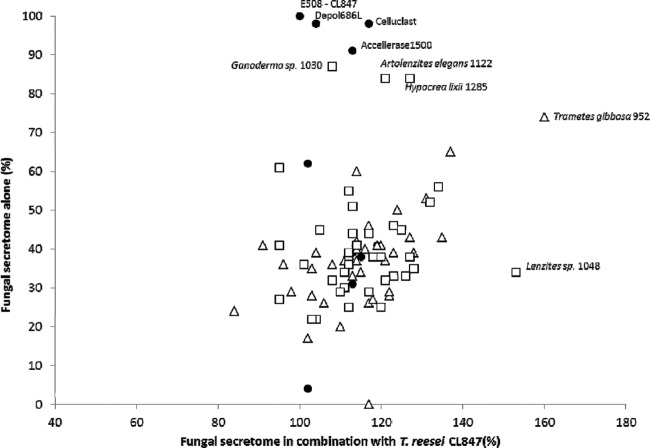
The best-performing secretomes alone were represented by quartile 4, which comprised 6 ascomycetes, including four species of Nectriaceae and two species of Hypocrea lixii (teleomorph of Trichoderma harzianum) and 13 basidiomycetes with 6 fungi of temperate origin and 13 of tropical origin. We noted a significant difference in effectiveness among strains of the genus Ganoderma according to geographical origin. Among the six Ganoderma spp., the three of tropical origin were present in quartile 4 (best performing) and the three of temperate origin were in quartile 1 (worst performing). For comparison, the commercial cocktails originating from fungi were tested under the same experimental conditions (Table 2). The results comparing fungal secretome and commercial cocktails alone are shown in Fig. 2. The most efficient commercial cocktails all originated from Trichoderma strains (CL847-E508, Depol 686L, Celluclast, and Accellerase 1500) with yields of between 91% and 100%. It is striking that three fungi (Ganoderma sp. strain BRFM 1030, Artolenzites elegans BRFM 1122, and Hypocrea lixii BRFM 1285) in quartile 4 yielded amounts of sugars comparable to those of T. reesei commercial cocktails, with, respectively, 87%, 84% and 84%.
Fig 2.
Species distribution following hydrolysis of biomass. Hydrolysis of biomass was performed with fungal secretomes alone (y axis) and in supplementation with the T. reesei commercial cocktail (x axis). Fungal secretomes were from commercial cocktails (full circles), tropical isolates (open squares), or temperate isolates (open triangles). Names of the best-performing fungi and commercial cocktails are indicated.
Each fungal secretome was also tested in combination with T. reesei CL847 enzyme cocktail (Table 3). Of the 19 fungi that performed best under our experimental conditions (above 123%, corresponding to quartile 4), only 4 were ascomycetes (two species of Nectriaceae and two Hypocrea lixii isolates), whereas all the other strains were basidiomycetes (e.g., the genera Daedaleopsis, Phellinus, and Trametes). The three Daedaleopsis confragosa isolates (BRFM 1131, BRFM 1143, and BRFM 1145) were classified in quartile 4. Two fungal secretomes belonging to the Trametes-related genera (Lenzites sp. strain BRFM 1048 from tropical forests and Trametes gibbosa BRFM 952 from temperate forests) stood out from the others by improving the conversion yield by 53% and 60%, respectively (Table 3 and Fig. 2). In quartile 4, no correlation in relation with geographical distribution was observed, i.e., 8 isolates collected in temperate forests and 10 collected in tropical forests. For comparison, the fungal commercial cocktails tested under the same experimental conditions failed to efficiently supplement the T. reesei CL847 cocktail (Table 2; Fig. 2).
Enzymatic characterization of the Trametes gibbosa BRFM 952 secretome.
As the secretome of T. gibbosa BRFM 952 was the best performing one in combination with the T. reesei CL847 commercial cocktail (Table 3; Fig. 2), we assessed its sugar-cleaving capabilities. We quantified its main glycoside hydrolase activities using a microplate assay that contained pNP sugars and complex polysaccharides as the substrates (Table 4). Cellulose degradation was estimated by the quantification of endoglucanase (carboxymethyl cellulose [CMC]), cellulase (Avicelase; Avicel [AVI]), filter paper (FP), cellobiohydrolase (pNP-β-d-cellobioside [pCel] and pNP-β-d-lactobioside [pLac]), and β-glucosidase (pNP-β-d-glucopyranoside [pGlu]) activities. Although the T. gibbosa BRFM 952 secretome did not display any cellobiohydrolase activity, it showed activity 8 times higher on crystalline cellulose (AVI) than the T. reesei CL847 enzymatic cocktail. Also, the overall activity on cellulose of the T. gibbosa BRFM 952 secretome measured using FP was twice that of T. reesei CL847. Hemicellulose degradation was estimated by quantifying the xylanase activities using structurally different xylans (birchwood xylan [BRX], soluble wheat arabinoxylan [SAX], and insoluble wheat arabinoxylan [IAX]) as the substrates, and the exo-acting glycosidase activities were estimated using pNP-α-l-arabinofuranoside (pAra) and pNP-β-d-xylopyranoside (pXyl). The T. gibbosa BRFM 952 secretome displayed some hemicellulase activities, but they were not higher than the hemicellulase activity of the T. reesei CL847 cocktail. Pectic degradation was assessed using pectin, arabinogalactan (AGA), arabinan (ARB), and pNP-α-d-galactopyranoside (pGal). We note that pectin-related activities were present in significantly smaller amounts in the commercial cocktail than in the T. gibbosa BRFM 952 secretome. The overall esterase activity assessed using pNP-acetate (pAc) was not detected in the T. gibbosa BRFM 952 or T. reesei CL847 secretome. We also note that 4.39 U of laccase activity per mg of protein was measured in the T. gibbosa BRFM 952 secretome, whereas no activity was detected in the T. reesei CL847 cocktail.
Table 4.
Lignocellulosic enzyme activities of T. reesei enzyme preparation E508 (CL847 strain) and Trametes gibbosa BFRM 952 secretome
| Secretome source | Lignocellulosic enzyme activity (U · mg−1) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose-degrading enzymes |
Xylan-degrading enzymes |
Pectin-degrading enzymes |
Others |
||||||||||||||
| FP | AVI | CMC | pGlu | pLac | pCel | BRX | SAX | IAX | pXyl | pAra | Pectin | ARB | AGA | pGal | pAc | Laccase | |
| E508 (CL847) | 0.12 | 0.01 | 0.33 | 0.19 | 0.04 | 0.05 | 0.94 | 1.59 | 0.37 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | NDa | ND |
| T. gibbosa BRFM 952 | 0.24 | 0.08 | 0.04 | 0.03 | ND | ND | 0.18 | 0.29 | 0.10 | 0.01 | 0.02 | 0.28 | 0.08 | 0.47 | ND | ND | 4.39 |
ND, no activity detected.
DISCUSSION
Our study is the first to report large-scale trials of supplementation of a commercial enzymatic cocktail with secretomes from fungal strains isolated from natural diversity. In addition, the current literature contains only sparse data on the screening of fungal strains using real lignocellulosic materials. Shrestha et al. (37) have isolated novel fungal species (mainly ascomycetes) from decaying bioenergy grasses, among which some were able to convert Miscanthus biomass. However, the dry weight loss was only 8 to 13% over 4 weeks. Recently, there has also been growing interest in the potential of plant-pathogenic fungi to optimize hydrolysis of lignocellulosic biomass (12). For instance, large-scale screening of ascomycetes revealed that the plant pathogens were more active than the nonpathogens on several lignocellulosic substrates (17).
From the 74 fungi identified and tested in this study, we found both ascomycetes and basidiomycetes strains that significantly improve sugar conversion in combination with the reference T. reesei CL847 commercial cocktail. None of the other fungal commercial cocktails tested under the same experimental conditions resulted in any significant increase in biomass conversion, although they contained a large range of carbohydrate-active enzyme (CAZyme) activities (7; www.cazy.org). The fungi that best performed alone (Ganoderma sp. strain BRFM 1030, Artolenzites elegans BRFM 1122, and Hypocrea lixii BRFM 1285) were not the best-performing ones when tested in combination with the T. reesei CL847 enzymatic cocktail. This suggests that their enzymatic strategies to deconstruct lignocellulose were similar to those T. reesei. Among the best-performing fungal secretomes that significantly supplemented the T. reesei enzymatic cocktail (i.e., quartile 4), we found several genera belonging to the white-rot fungi (e.g., the genera Daedaleopsis, Phellinus, Pycnoporus, and Trametes). White-rot fungi mineralize cell wall components (cellulose, hemicelluloses, and lignins) and extensively degrade lignins (38). For instance, Pycnoporus is known to produce high FP endoglucanase, β-glucosidase, xylanase, mannanase, α-galactosidase, α-arabinofuranosidase, and polygalacturonase activities (11) as well as large amounts of laccases (22) and cellobiose dehydrogenase (CDH) (4), which have been shown to affect biomass conversion (4, 11, 25). It is now clear that fungi convert lignocellulose through a multienzymatic process involving numerous CAZymes and oxidative enzymes.
The Trametes gibbosa BRFM 952 was the best-performing isolate with a 60% improved conversion, a feature that was not universal to the Trametes and related genera. Indeed, some Trametes isolates (e.g., T. gibbosa BRFM 873 and T. versicolor BRFM 1116) were classified in quartile 1 (i.e., corresponding to the worst-performing secretomes). Variability in biomass conversion suggests that enzymatic pools are different following induction of the secretion of lignocellulose-acting enzymes due to genetic differences among the organisms. This feature was observed by King et al. (17) and Russell et al. (31). They indicate that after the identification of promising species, there is still significant variation among isolates which may reveal superior candidates. It thus highlights the importance of a systematic high-throughput bioconversion assessment of collected fungal biodiversity in order to identify promising fungal isolates, particularly if a genus or species is suspected of being hypervariable. The increase in sugar conversion obtained when the T. reesei enzymatic cocktail was supplemented with the T. gibbosa BRFM 952 secretome could be explained by the presence of CAZymes or oxidoreductases absent in T. reesei. The T. gibbosa BRFM 952 secretome displayed a high activity on cellulose, in close agreement with the findings of Couturier et al. (9), with FP activity and WS conversion being correlated following analysis of a set of 20 fungi using activity profiling. Recent data from the literature on the synergy between family GH61 enzymes (copper-dependent polysaccharide monooxygenases) and oxidoreductases acting on lignocellulose components (18) could also explain the efficiency of the T. gibbosa secretome in combination with the T. reesei CL847 enzymatic cocktail, which contains an enzyme from the family GH61 (9).
Obviously, exploration of fungal biodiversity through their secretomes is currently one of the most relevant methods to find new enzymes of industrial interest involved in lignocellulose degradation (9, 29). Any of the top candidates identified in this study would be ideal for closely controlled synergy experiments in future work. The use of proteomics is the next step for these explorations, together with transcriptomics for the dynamic study of enzyme production. A full exploitation of the data requires access to their genomic information, which will be a major breakthrough to gain a better understanding of this fungal biodiversity.
ACKNOWLEDGMENTS
This study was funded by the French National Research Agency (ANR; program E-TRICEL ANR-07-BIOE-006).
We thank X. Rouau and G. Ghizzi for the preparation of micronized wheat straw and Genencor and Biocatalysts for providing samples of commercial cocktails. Fungi were collected during field trips organized in metropolitan France, French Guiana, Guadeloupe, and Martinique. We thank the UMR EcoFog, the DIREN of Guadeloupe and Martinique, the ONF Martinique, and the Parc National de Guadeloupe administration for their assistance. The authors are especially grateful to H. Charlotte, mayor of Saül and president of the Parc National Amazonien de Guyane, for his help during our stay in this locality. B. Rivoire is acknowledged for morphological identification of Postia stiptica.
Footnotes
Published ahead of print 6 July 2012
REFERENCES
- 1. Alberto F, Navarro D, de Vries RP, Asther M, Record E. 2009. Technical advance in fungal biotechnology: development of a miniaturized culture method and an automated high-throughput screening. Lett. Appl. Microbiol. 49:278–282 [DOI] [PubMed] [Google Scholar]
- 2. Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA. 2000. Are tropical fungal endophytes hyperdiverse. Ecol. Lett. 3:267–274 [Google Scholar]
- 3. Arnold AE. 2001. Fungal endophytes in neotropical trees: abundance, diversity, and ecological implications, p 739–743 In Ganeshaiah KN, Shaanker RU, Bawa KS. (ed), Tropical ecosystems: structure, diversity and human welfare. Proceedings of the International Conference on Tropical Ecosystems. Oxford, New Delhi, India. [Google Scholar]
- 4. Bey M, Berrin JG, Poidevin L, Sigoillot JC. 2011. Heterologous expression of Pycnoporus cinnabarinus cellobiose dehydrogenase in Pichia pastoris and involvement in saccharification processes. Microb. Cell Fact. 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blackwell M. 2011. The fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 98:426–438 [DOI] [PubMed] [Google Scholar]
- 6. Bonnin E, et al. 2001. Aspergillus niger I-1472 and Pycnoporus cinnabarinus MUCL39533, selected for the biotransformation of ferulic acid to vanillin, are also able to produce cell wall polysaccharide-degrading enzymes and feruloyl esterases. Enzyme Microb. Technol. 28:70–80 [DOI] [PubMed] [Google Scholar]
- 7. Cantarel BL, et al. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37:D233–D238 doi:10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couturier M, et al. 2011. Podospora anserina hemicellulases potentiate the Trichoderma reesei secretome for saccharification of lignocellulosic biomass. Appl. Environ. Microbiol. 77:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couturier M, et al. 2012. Post-genomic analyses of fungal lignocellulosic biomass degradation reveal the unexpected potential of the plant pathogen Ustilago maydis. BMC Genomics 13:57 doi:10.1186/1471-2164-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Boer W, Folman LB, Summerbell RC, Boddy L. 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol. Lett. 29:795–811 [DOI] [PubMed] [Google Scholar]
- 11. Falkoski DL, et al. 2012. Characterization of cellulolytic extract from Pycnoporus sanguineus PF-2 and its application in biomass saccharification. Appl. Biochem. Biotechnol. 166:1586–1603 [DOI] [PubMed] [Google Scholar]
- 12. Gibson DM, King BC, Hayes ML, Bergstrom GC. 2011. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 14:264–270 [DOI] [PubMed] [Google Scholar]
- 13. Gilbertson RL, Ryvarden L. 1987. North American polypores, p 451 Fungiflora, Oslo, Norway [Google Scholar]
- 14. Hawksworth DL. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol. Res. 95:641–655 [Google Scholar]
- 15. Hawksworth DL. 2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol. Res. 105:1422–1432 [Google Scholar]
- 16. Hirooka Y, Rossman A, Samuels G, Lechat C, Chaverri P. 2012. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Stud. Mycol. 71:1–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. King BC, et al. 2011. Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol. Biofuels 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langston JA, et al. 2011. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl. Environ. Microbiol. 77:7007–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Crom S, et al. 2009. Tracking the roots of cellulase hyperproduction by the fungus Trichoderma reesei using massively parallel DNA sequencing. Proc. Natl. Acad. Sci. U. S. A. 106:16151–16156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lesage-Meessen L, et al. A biotechnological process involving filamentous fungi to produce natural crystalline vanillin from maize bran. Appl. Biochem. Biotechnol. 102–103:141–153 [DOI] [PubMed] [Google Scholar]
- 21. Lomascolo A, et al. 2002. Molecular clustering of Pycnoporus strains from various geographic origins and isolation of monokaryotic strains for laccase hyperproduction. Mycol. Res. 106:1193–1203 [Google Scholar]
- 22. Lomascolo A, Uzan-Boukhris E, Herpoël-Gimbert I, Sigoillot JC, Lesage-Meessen L. 2011. Peculiarities of Pycnoporus species for applications in biotechnology. Appl. Microbiol. Biotechnol. 92:1129–1149 [DOI] [PubMed] [Google Scholar]
- 23. Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F. 2009. New improvements for lignocellulosic ethanol. Curr. Opin. Biotechnol. 20:372–380 [DOI] [PubMed] [Google Scholar]
- 24. Martinez D, et al. 2008. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 26:553–560 [DOI] [PubMed] [Google Scholar]
- 25. Moilanen U, Kellock M, Galkin S, Viikari L. 2011. The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzyme Microb. Technol. 49:492–498 [DOI] [PubMed] [Google Scholar]
- 26. Moncalvo J-M, Ryvarden L. 1997. A nomenclatural study of the Ganodermataceae Donk, p 114 Fungiflora, Oslo, Norway [Google Scholar]
- 27. Navarro D, et al. 2010. Automated assay for screening the enzymatic release of reducing sugars from micronized biomass. Microb. Cell Fact. 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peterson R, Nevalainen H. 2012. Trichoderma reesei RUT-C30—thirty years of strain improvement. Microbiology 158:58–68 [DOI] [PubMed] [Google Scholar]
- 29. Ravalason H, et al. 2012. Fusarium verticillioides secretome as a source of auxiliary enzymes to enhance saccharification of wheat straw. Bioresour. Technol. 114:589–596 [DOI] [PubMed] [Google Scholar]
- 30. Rossman AY, Samuels GJ, Rogerson CT, Lowen R. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 42:1–248 [Google Scholar]
- 31. Russell JR, et al. 2011. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 77:6076–6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryvarden L, Gilbertson RL. 1993. European polypores part 1, p 1–387 Fungiflora, Oslo, Norway [Google Scholar]
- 33. Ryvarden L, Gilbertson RL. 1994. European polypores part 2, p 394–743 Fungiflora, Oslo, Norway [Google Scholar]
- 34. Ryvarden L. 1987. New and noteworthy polypores from tropical America. Mycotaxon 28:525–541 [Google Scholar]
- 35. Ryvarden L. 1991. Genera of polypores. Nomenclature and Taxonomy, p 363 Fungiflora, Oslo, Norway [Google Scholar]
- 36. Ryvarden L. 2004. Neotropical polypores, p 229 Fungiflora, Oslo, Norway [Google Scholar]
- 37. Shrestha P, Szaro TM, Bruns TD, Taylor JW. 2011. Systematic search for cultivatable fungi that best deconstruct cell walls of Miscanthus and sugarcane in the field. Appl. Environ. Microbiol. 77:5490–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sigoillot JC, et al. 2012. Fungal strategies for lignin degradation. In Jouanin L, Lapierre C. (ed). Lignins: biosynthesis, biodegradation and bioengineering, vol 61 Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 39. Silva GG, Couturier M, Berrin JG, Buléon A, Rouau X. 2012. Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 103:192–200 [DOI] [PubMed] [Google Scholar]
- 40. Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot JC. 2006. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme Microb. Technol. 39:897–902 [Google Scholar]
- 41. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Welti S, et al. 2012. Molecular phylogeny of Trametes and related genera, and description of a new genus Leiotrametes. Fungal Diversity 55:47–64 [Google Scholar]
- 43. White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfrand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA [Google Scholar]
- 44. Zaldivar J, Nielsen J, Olsson L. 2001. Fuel ethanol production from lignocelluloses: a challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. l. 56:17–34 [DOI] [PubMed] [Google Scholar]