Abstract
The success of Mycobacterium tuberculosis depends on its ability to withstand and survive the hazardous environment inside the macrophages that are created by reactive oxygen intermediates, reactive nitrogen intermediates, severe hypoxia, low pH, and high CO2 levels. Therefore, an effective detoxification system is required for the pathogen to persist in vivo. The genome of M. tuberculosis contains a new family of hemoproteins named truncated hemoglobin O (trHbO) and truncated hemoglobin N (trHbN), encoded by the glbO and glbN genes, respectively, important in the survival of M. tuberculosis in macrophages. Mycobacterial heat shock proteins are known to undergo rapid upregulation under stress conditions. The expression profiles of the promoters of these genes were studied by constructing transcriptional fusions with green fluorescent protein and monitoring the promoter activity in both free-living and intracellular milieus at different time points. Whereas glbN showed an early response to the oxidative and nitrosative stresses tested, glbO gave a lasting response to lower concentrations of both stresses. At all time points and under all stress conditions tested, groEL2 showed higher expression than both trHb promoters and expression of both promoters showed an increase while inside the macrophages. Real-time PCR analysis of trHb and groEL2 mRNAs showed an initial upregulation at 24 h postinfection. The presence of the glbO protein imparted an increased survival to M. smegmatis in THP-1 differentiated macrophages compared to that imparted by the glbN and hsp65 proteins. The comparative upregulation shown by both trHb promoters while grown inside macrophages indicates the importance of these promoters for the survival of M. tuberculosis in the hostile environment of the host.
The success of Mycobacterium tuberculosis depends on its ability to withstand and survive the hazardous environment inside macrophages that are created by reactive oxygen intermediates (ROI), reactive nitrogen intermediates (RNI), severe hypoxia, low pH, and high CO2 levels (5). It has already been reported that activation of macrophages induces the synthesis of an inducible NO synthase (iNOS), resulting in the production of various RNI (7, 21). Activated macrophages can also produce an oxidative burst which is responsible for the killing of intracellular M. tuberculosis. ROI and RNI can damage DNA and other chemical moieties on which their propagation and protection depend, including Fe-S clusters, tyrosyl radicals, hemes, sulfhydryls, thioethers, and alkenes (22). Macrophages can produce superoxide (O2−) and nitric oxide (NO) in almost equimolar amounts and thus can be abundant generators of peroxynitrite (OONO−), a highly oxidizing agent (41, 45). NO limits the survival and growth of microbial pathogens by inhibiting enzymes like respiratory oxidases and iron sulfur centers of key enzymes such as aconitase (11, 13).
Since early events of M. tuberculosis infection involve entry and multiplication within the bacteriostatic environment of macrophages, which is characterized mainly by ROS and RNS, an effective detoxification system is required for the pathogen to persist in vivo. The genome of M. tuberculosis contains glbO and glbN genes, encoding truncated hemoglobin O (trHbO) and truncated hemoglobin N (trHbN), respectively, which belong to a new family of hemoproteins that are extensively distributed in eubacteria, cyanobacteria, protozoans, and plants (27, 29, 38). The amino acid sequences of trHbs are shorter and share very low similarity to the sequences of classical vertebrate hemoglobins and myoglobins. The mycobacterial trHb length varies from 215 amino acid residues for M. vanbalenii trHbN to 121 amino acid residues for M. smegmatis trHbN, with the majority being ≤136 amino acid residues. Homology between members of different trHb classes is insignificant (<20% identity) (3, 40). The genetic organization of glbO is largely conserved across various mycobacterial species in comparison to that of glbN. Among mycobacteria, adaptation to obligate parasitism has resulted in the loss of trHb genes, as in the case of M. leprae, which retained only glbO (4, 6, 27). Since M. leprae has retained only a minimalistic repertoire of functional genes, trHbO can be regarded as an essential product for intracellular parasitism, while trHbN appears to be dispensable.
The mechanism by which trHbN protects M. tuberculosis from the nitrosative stress and microbicidal activities of macrophages relies on its oxygen-dependent NO dioxygenase (NOD) activity. The oxygenated form of trHbN can very efficiently convert NO to harmless nitrate (25, 28). The inactivation of the glbN gene of M. bovis BCG impaired the ability of stationary-phase cells of the organism to protect aerobic respiration from NO (24). Dikshit and colleagues (14, 25) and Ouellet et al. (24) showed that trHbN of M. tuberculosis has a potent ability to relieve the toxicity of NO and nitrosative stress in Escherichia coli and M. smegmatis. The aerobic organism M. tuberculosis can use trHbO to collect and deliver available O2 to the components of the electron transport chain in order to survive in the granulomas (16, 26). Ferrous oxygenated M. tuberculosis trHbN and trHbO as well as M. leprae trHbO have been shown to facilitate NO scavenging (2, 24). Oxygenated trHbN catalyzes fast NO oxidation compared to the speed of catalysis for trHbO, which likely makes the NO detoxification role for trHbN higher and which shows that trHbO plays only a marginal role in the protection of mycobacteria against NO (3). Mycobacterial trHbO is present during all stages of growth in the host, and the expression of several oxidative defense genes was found to be constitutive (37). In addition, exposure to low NO concentrations triggers the initiation of mycobacterial dormancy (44).
Heat shock proteins (HSPs) are generally responsible for preventing damage to proteins in response to high levels of heat. HSPs stabilize cellular proteins in response to diverse sources of stress or injury. The mycobacterial Hsp family includes proteins such as DnaK, DnaJ, GrpE, GroES, GroEL, and other low-molecular-weight HSPs (31). HSPs also have a number of immunological effects, including the induction of proinflammatory cytokines (23, 30, 36, 43). Mycobacterial HSPs may also participate in cytokine expression resulting from infection by M. tuberculosis (31). A temperature increase from 37°C to 42°C induced elevated synthesis of three major proteins corresponding to the DnaK, GroEL, and GroES proteins of M. tuberculosis previously identified to be prominent antigens (42). In addition to its role as a heat shock protein, GroEL functions as a chaperonin to assist in folding linear amino acid chains into their respective three-dimensional structure.
Global analysis of gene expression in several pathogenic bacteria, including M. tuberculosis (9), has revealed that large-scale changes occur upon in vitro exposure to environmental conditions that simulate the intracellular milieu (15, 19). The expression pattern of mycobacterial trHb and groEL2 promoters under various oxidative and nitrosative stress conditions will give an idea about the expression of the corresponding genes inside the hostile macrophage environment. Since little is known about the expression and regulation of mycobacterial trHb and groEL2 promoters, we decided to study the response of the promoters by constructing transcriptional fusions with green fluorescent protein (GFP) and monitoring the promoter activity under various stress conditions and also in THP-1 differentiated macrophages postinfection. Here we have compared the expression profile of mycobacterial trHb and groEL2 promoters at different time points under both free-living and intracellular conditions. We have also looked into the mRNA profiles of the genes in M. smegmatis and M. tuberculosis and carried out survival assays in M. smegmatis.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
M. smegmatis mc2155 was grown in Luria-Bertani broth (USB) with 0.05% Tween 80 and plated in the same medium with 1.5% agar supplemented with kanamycin (25 μg/ml) wherever appropriate. All transformations in Escherichia coli were performed with strain JM109. E. coli was grown in Luria-Bertani broth at 37°C in shaking cultures. Kanamycin (25 μg/ml) was added to the medium whenever required. Plasmid pFPV27 was a kind gift from Lalitha Ramakrishnan, Department of Microbiology, University of Washington.
Cloning of mycobacterial glbO, glbN, and groEL2 promoters and promoters with full genes.
All promoter sequences were amplified from M. tuberculosis H37Rv genomic DNA, which was isolated using a previously published protocol (33). The sequences encompassing positions −240/+26, −410/+70, and −375/+18 relative to the start codons of glbN, glbO, and groEL2, respectively, were PCR amplified using custom-synthesized primers that incorporated 5′ NotI and 3′ BamHI restriction sites (Table 1). The amplified products were then cloned into pFPV27 that had previously been digested with the same restriction enzymes. The glbO, glbN, and groEL2 transcriptional fusions were termed as pFPVO, pFPVN, and pMRm, respectively. The glbO, glbN, and groEL2 genes, along with the promoters, were also amplified from M. tuberculosis H37Rv genomic DNA and cloned into pFPV27, and these constructs were named pFPVOpg, pFPVNpg, and pMRmpg, respectively. The sequences of all the cloned fragments were confirmed using BigDye Terminator cycle sequencing chemistry for the ABI BioPrism apparatus (Applied Biosystems). Electrocompetent M. smegmatis mc2155 cells were electroporated with these plasmids as described previously (39).
Table 1.
Nucleotide sequences of primers used in this study
| No. | Primer | Sequence |
|---|---|---|
| 1 | GlbO-F | 5′-AAATATGCGGCCGCCTTCGTGCACCACGTCGGCC-3′ |
| 2 | GlbO-R | 5′-GGATCCGCATAGAAACGCGACACGATCGC-3′ |
| 3 | GlbN-F | 5′-AAATATGCGGCCGCCACCAAGATTCATCGTGGGTAG-3′ |
| 4 | GlbN-R | 5′-CGGGATCCTTGCGCAAGCGTGACAGTAGTCC-3′ |
| 5 | GroEL2-F | 5′-TTGCGGCCGCAACGGTGACCACAACGACGC-3′ |
| 6 | GroEL2-R | 5′-CAATGGCCAAGACAATTGCGGGATCC-3′ |
Analysis of expression of transcriptional fusions under various stress conditions.
M. smegmatis cells containing pFPV27, pFPVO, pFPVN, and pMRm were grown for 24 h and were then inoculated in fresh medium at an optical density at 610 nm (OD610) of 0.05 and allowed to grow by shaking at 37°C for another 10 h. The cells were exposed to different physiological stresses, viz., 1 mM and 5 mM H2O2 for oxidative stress and 10 mM and 30 mM NaNO2 for nitrosative stress, and we also checked the influence of THP-1-derived macrophage cell lysates on the expression of these transcriptional fusions. These concentrations of H2O2 and NaNO2 were used, as they had previously been determined to be minimally lethal to mycobacteria (34). The cultures were then incubated at 37°C in a shaking incubator. One hundred microliters of each culture was transferred to 96-well plates at 24 h, 48 h, and 72 h. The fluorescence was measured in a Tecan fluorescent microplate reader at excitation/emission wavelengths of 488 nm/520 nm. Relative fluorescence units (RFU) were determined to be the number of fluorescence units/OD600. Background fluorescence due to read-through transcription of the transcriptional fusion vector was determined by measuring the RFU of M. smegmatis harboring pFPV27. All the experiments were carried out in triplicate.
Differentiation and in vitro infection of THP-1 cells.
The monocytic cell line THP-1 was cultured in RPMI 1640 (RPMI) supplemented with 10% fetal bovine serum. Cells (2.5 × 105) were seeded in 12-well plates and allowed to adhere and differentiate in the presence of phorbol myristate acetate (PMA; 25 ng/ml) at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Cells were then washed three times with Hanks balanced salt solution, and antibiotic-free culture medium was added. Adherent cells were infected with M. smegmatis containing pFPV27, pFPVO, pFPVN, and pMRm at a multiplicity of infection (MOI) of 1:20 (macrophages to bacilli). After 4 h of infection, the cells were washed 3 times with warm RPMI to remove extracellular bacteria, and cells were incubated with complete RPMI containing 10 μg/ml gentamicin for 2 h to prevent growth of extracellular bacteria. Subsequently, the cells were washed and maintained in complete RPMI for the rest of the experiment.
Analysis of intracellular expression of transcriptional fusions.
To analyze the intracellular expression of transcriptional fusions, macrophage monolayers infected with M. smegmatis containing pFPV27, pFPVO, pFPVN, and pMRm were further incubated for 4 h, 24 h, and 48 h. The intracellular mycobacteria were processed using a previously published protocol (10) with slight modifications. Briefly, the macrophage monolayer was detached from 12-well plates using cell dissociation solution (Sigma), collected by centrifugation (2,000 rpm for 5 min at 4°C), and broken by 20 passages through a 23-gauge needle. Intact cells and nuclei were removed by pelleting at 3,000 rpm for 5 min, and the supernatant containing bacteria was again centrifuged at 12,000 rpm for 10 min. The pellet was then resuspended in 100 μl phosphate-buffered saline (PBS), the suspension was added to a 96-well plate, and the RFU and absorbance were recorded as described in the previous section. All measurements were corrected for background fluorescence by subtracting the RFU for the control strain carrying plasmid pFPV27.
Extraction of RNA and RT-PCR.
In 25-cm2 tissue culture flasks (Nunc), 40 × 105 cells were seeded and allowed to adhere and differentiate in the presence of PMA (25 ng/ml) at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Infection was performed with M. smegmatis containing pFPV27, pFPVOpg, pFPVNpg, and pMRmpg (MOI = 1:20). After 4 h, 24 h, and 48 h of incubation, the culture supernatants were removed and the macrophage monolayer was washed twice with 1× PBS and detached with a cell scraper. The cell suspension was pelleted and resuspended in 350 μl of RA1 buffer (GE Healthcare) and 3.5 μl of β-mercaptoethanol, and the suspension was transferred to a sterile 1.5-ml tube. The cells were lysed in a Mini-BeadBeater (2,500 rpm for 10 s; Biospec), after addition of 5-mm glass beads (Biospec). Total RNA was isolated using an RNA Spin minikit (GE Healthcare) following the manufacturer's instructions. First-strand cDNA was synthesized from total RNA using a first-strand synthesis kit (Fermentas) with random oligonucleotides. The total RNA concentration for each sample was measured using a NanoDrop spectrophotometer (Thermo) at 260 nm. The quality of RNA was determined at a 260- and 280-nm absorbance ratio (A260/A280). 16S rRNA was used as an endogenous control to normalize expression levels. Real-time PCR (RT-PCR) was done using gene-specific primers. The SYBR green method was used, and all reactions were performed in triplicate. Each reaction tube contained 10 μl iQSYBR green supermix (Bio-Rad), 300 nM gene-specific forward and reverse primers, and 50 ng of cDNA template, made up to a final volume of 20 μl with nuclease-free water. The RT-PCR cycling conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 58°C for 30 s, and the cycling was done using a Bio-Rad iCycler 5 apparatus with an iQ multicolor real-time PCR detection system (Bio-Rad). Data analysis was performed with the iQ multicolor real-time PCR detection system optical software system (Bio-Rad), version iQ5. Relative standard curves for the target and endogenous control primer pairs were performed to verify that both PCR efficiencies were comparable.
Another experiment was done according to the above-described protocol, where THP-1-derived macrophages were infected with M. tuberculosis rather than recombinant M. smegmatis, and the levels of mycobacterial trHb and groEL2 mRNAs in THP-1-derived macrophages at 4 h, 24 h, and 48 h postinfection were analyzed with real-time PCR.
Confocal microscopy.
In a 96-well glass-bottom tissue culture plate (Nunc), 50 × 104 cells were seeded and allowed to adhere and differentiate in the presence of PMA (25 ng/ml) at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Infection was performed with M. smegmatis containing pFPV27, pFPVO, pFPVN, and pMRm (MOI = 1:20). After 4 h, 24 h, and 48 h of incubation, the cells were fixed with 4% paraformaldehyde in the dark for 10 min at room temperature. Glycerol (90%) was added as the mounting medium, and the cells were visualized in a confocal microscope.
Survival assay.
Sixty thousand cells were seeded in a 96-well flat-bottom tissue culture plate (Tarson) and allowed to adhere and differentiate in the presence of PMA (25 ng/ml) at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Infection was performed with recombinant M. smegmatis containing pFPV27, pFPVOpg, pFPVNpg, and pMRmpg (MOI = 1:20). After 4 h of infection, the cells were washed three times with warm RPMI and treated with RPMI containing gentamicin (10 μg/ml) for 2 h at 37°C to kill extracellular bacteria. Those monolayers in which survival for 24 h and 48 h was to be measured were incubated in complete RPMI containing gentamicin (5 μg/ml) to prevent extracellular growth of bacteria that might be released by premature lysis of macrophages. Cells in duplicate wells were analyzed at 0 h, 24 h, and 48 h postinfection by adding 200 μl of water and vigorously pipetting several times to ensure cell lysis and release of surviving intracellular bacteria. The lysates were serially diluted in Luria-Bertani broth with 0.05% Tween 80 and plated onto Luria-Bertani agar plates containing 0.05% Tween 80 and kanamycin (25 μg/ml). The numbers of CFU were counted after incubation at 37°C for 3 days, and the mean value obtained was plotted along with the value for the control.
Statistical analysis.
Results were expressed as the mean plus or minus the standard deviation. Statistical comparison was performed using one-way analysis of variance. A probability (P) value of less than 0.05 was considered statistically significant.
RESULTS
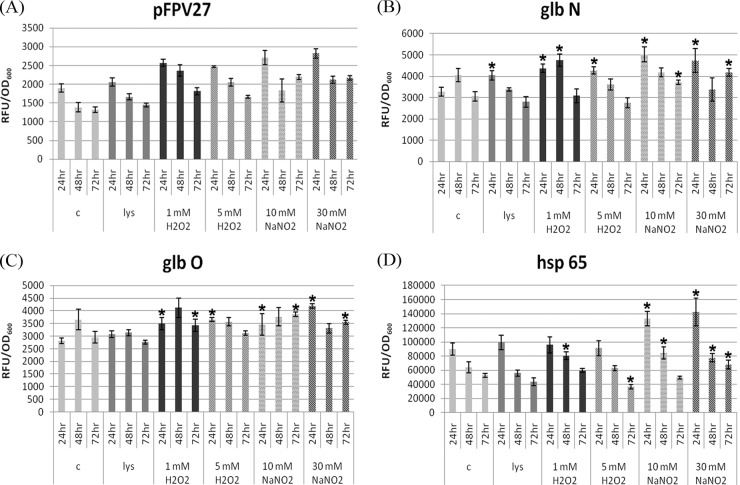
The activities of the promoters of the three M. tuberculosis genes glbO, glbN, and groEL2 were studied by subjecting recombinant M. smegmatis to various stress conditions (Fig. 1). Among the various stress conditions tested, 10 mM NaNO2 was the most potent inducer of the glbN promoter, and for glbO, it was 30 mM NaNO2, followed by 5 mM H2O2. glbN showed an early response to both oxidative and nitrosative stress, with the highest being at the 24-h time point. The glbO promoter responded more to the lower concentrations of both stresses, and the effects were more lasting. In the absence of any stress, the activities of glbN and glbO promoters increased from the 24-h to 48-h time points, and the activities of both showed a downward trend at 72 h. For the groEL2 promoter, 30 mM NaNO2 elicited maximal expression at all the time points tested. In the absence of any stress, the level of groEL2 promoter expression was higher at the 24-h time point and decreased from the 24-h to the 72-h time point, unlike the expression profile shown by the trHb promoters.
Fig 1.
Differential regulation of the M. tuberculosis trHb and groEL2 promoters under various stress conditions at different time points. Plasmid pFPV27 (A) and promoter glbN (B), glbO (C), and groEL2 (D) activities in M. smegmatis in the control and in the presence of THP-1 macrophage cell lysate, 1 and 5 mM H2O2, and 10 and 30 mM NaNO2 at the 24-h, 48-h, and 72-h time points. *, the mean difference was significant at a P value of <0.05. The statistical significance was calculated by comparing the control with various stress conditions at each time point.
The activities of the mycobacterial trHb and groEL2 promoters in M. smegmatis were determined under intracellular conditions in THP-1 differentiated macrophages (Fig. 2). All the promoters showed considerable increases in activity with time, and the maximal activity was at the 48-h time point. The glbN promoter showed higher activity than the glbO promoter at all time points tested, but the activity of the groEL2 promoter was higher than that of both trHb promoters. This finding indicates that the groEL2 promoter is stronger than both of the globin promoters under intracellular conditions. Confocal microscopy of the infected THP-1-derived macrophages showed distinct fluorescence in phagocytosed M. smegmatis carrying glbO, glbN, and groEL2 transcriptional fusions compared to the control (Fig. 3A and B).
Fig 2.
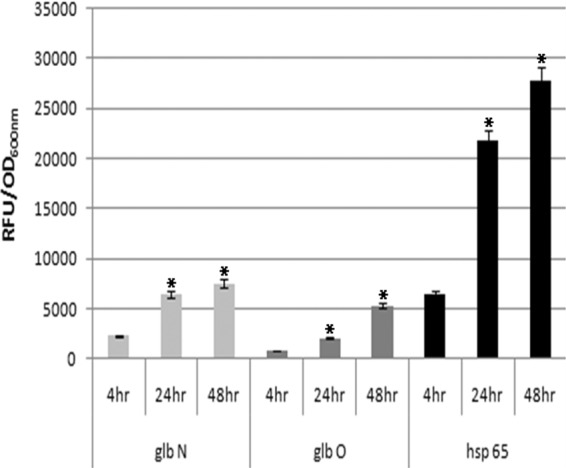
Activities of M. tuberculosis trHb and groEL2 gene promoters during intracellular infection. glbN, glbO, and groEL2 promoter activities of intracellular M. smegmatis at 4 h, 24 h, and 48 h postinfection of PMA-differentiated THP-1 macrophages. *, the mean difference was significant at a P value of <0.05 level.
Fig 3.

Activities of M. tuberculosis trHb and groEL2 gene promoters during infection of PMA-differentiated THP-1 macrophages. Infected cells and intracellular mycobacteria were visualized at 4 h, 24 h, and 48 h postinfection by confocal laser scanning fluorescence microscopy (Sp2; Leica). Fluorescent images (fl) and overlay images (ol) of mycobacterial glbO, glbN, and groEL2 promoters at 4 h postinfection (A) and 24 h postinfection (B).
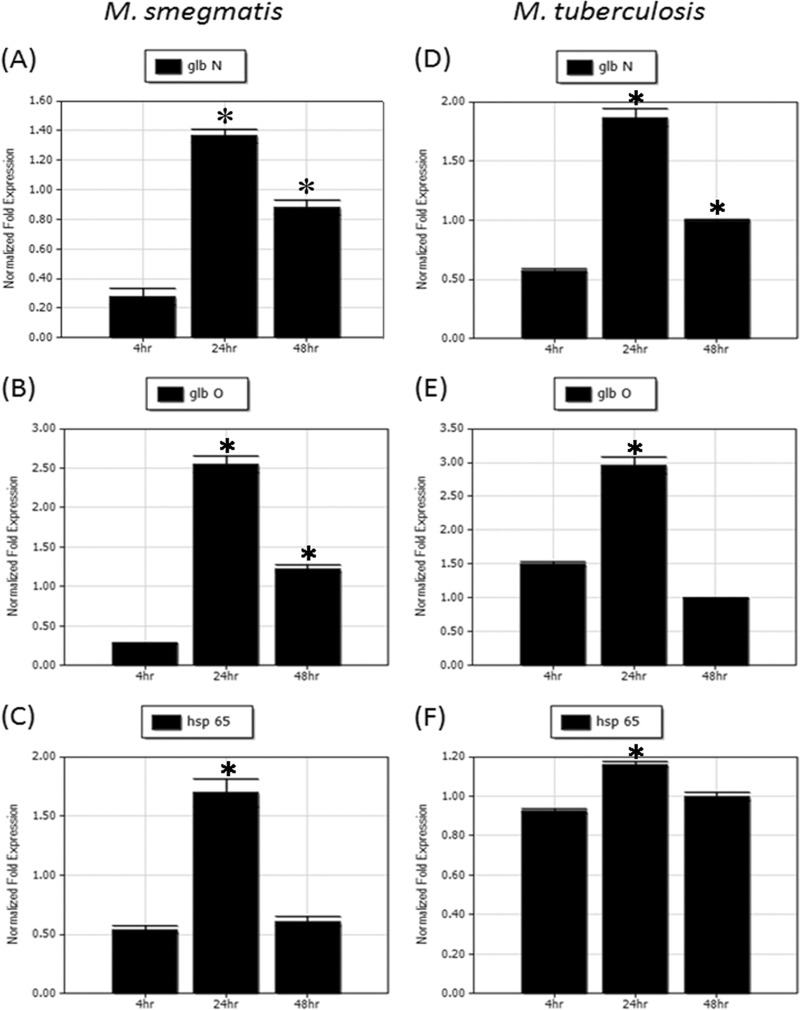
In order to analyze the levels of mycobacterial trHb and groEL2 mRNAs in macrophages following infection, we cloned the full lengths of both the mycobacterial trHb gene and groEL2 into pFPV27 along with their respective promoters. Total RNA was isolated from THP-1-derived macrophages infected with recombinant M. smegmatis at various time points after infection, and real-time PCR was carried out with gene-specific primers. Mycobacterial trHb and groEL2 mRNAs showed an increase in expression from 4 h to 24 h and showed a downward trend at 48 h. The extent of upregulation shown by both trHb mRNAs from 4 h to 24 h was higher than that of groEL2 mRNA at the same time points (Fig. 4A to C).
Fig 4.
Real-time PCR analysis of mycobacterial trHb and groEL2 mRNAs in recombinant M. smegmatis and in M. tuberculosis after THP-1 differentiated macrophage infection. Normalized expression analysis of glbN (A), glbO (B), and groEL2 (C) mRNAs in recombinant M. smegmatis and glbN (D), glbO (E), and groEL2 (F) mRNAs in M. tuberculosis after 4 h, 24 h, and 48 h of infection with THP-1 differentiated macrophages. *, the mean difference was significant at a P value of <0.05. The statistical significance was calculated by comparing the control (values at 4 h) with the values at 24 and 48 h.
After the studies in recombinant M. smegmatis, we analyzed the expression of mycobacterial trHb and groEL2 mRNAs in M. tuberculosis H37Rv-infected THP-1-derived macrophages by real-time PCR. The expression profiles of mycobacterial trHb and groEL2 mRNAs in THP-1-derived macrophages infected with recombinant M. smegmatis and M. tuberculosis H37Rv were comparable. Mycobacterial trHb and groEL2 mRNAs showed an upregulation from 4 h to 24 h, and both showed a downward trend at 48 h (Fig. 4D to F). Altogether the expression of both trHb promoters and the groEL2 promoter showed an increase in expression when inside macrophages.
The intracellular survival rate of recombinant M. smegmatis harboring the trHb and groEL2 proteins was studied. It was observed that compared to the control, the presence of trHbO helped M. smegmatis to survive better in macrophages than trHbN and Hsp65 (Fig. 5).
Fig 5.
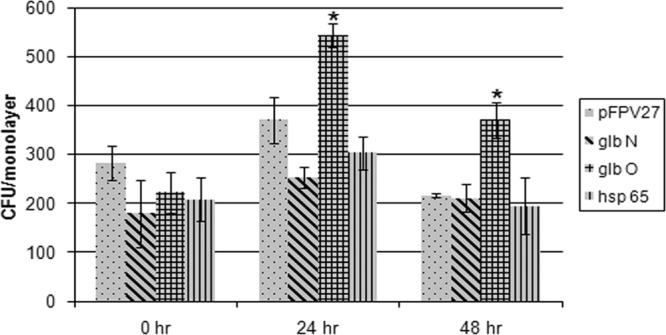
Survival of recombinant M. smegmatis overexpressing mycobacterial trHb and groEL2 proteins in THP-1 differentiated macrophages. (A) The retrieved surviving intracellular bacteria were counted at 0 h, 24 h, 48 h, and 72 h postinfection. *, the mean difference was significant at a P value of <0.05. The statistical significance was calculated by comparing the values for M. smegmatis with the plasmid vector (pFPV27) with the values for M. smegmatis with the recombinant vectors at each time point.
DISCUSSION
The first line of host defense of intracellular M. tuberculosis is by subverting intracellular antimicrobial mechanisms, and under such conditions, M. tuberculosis induces an appropriate protective response. trHbO and trHbN produced at different stages of growth (8, 20, 26) could contribute significantly to overcoming the intracellular host defense mechanisms. The transcriptional activities of the glbN and glbO promoters during the aerobic growth of M. smegmatis in our study were consistent with the protein expression pattern observed for trHbN and trHbO in M. bovis (20, 26). The involvement of M. tuberculosis trHbN in protection from the reactive nitrogen intermediates, particularly NO, has substantially been ascribed to its efficient NOD activity (8, 24, 25). Like the glbO promoter, the expression of the glbN promoter also showed a biphasic response: both promoters showed upregulation from 24 h to 48 h and a decrease in expression at the 72 h. Nitrosative stress, especially 10 mM nitrate, was a more potent inducer of glbN expression than oxidative stress. This observation can be correlated to the previously observed function of trHbN to detoxify NO and therefore should be available to the mycobacteria whenever toxic levels of RNI are present. Studies on transcriptional regulation of M. tuberculosis trHbN have shown a significant level of trHbN synthesis during macrophage infection (27), and its NOD activity is sustained under low oxygen (28). In order to combat the rising levels of RNI shortly after infection, mycobacteria have to respond immediately, and our finding of the highest response at 24 h for the glbN promoter supports this. In addition, macrophage cell lysate also induced higher levels of glbN promoter expression at 24 h.
The physiological role of M. tuberculosis trHbO has primarily been related to O2 metabolism. trHbO is a membrane-associated protein which interacts with the CyoB subunit of the E. coli terminal oxidase cytochrome O complex and sustains aerobic respiration under microaerobic conditions by facilitating O2 delivery to components of the electron transfer chain. Hence, trHbO was hypothesized to be endowed with O2 uptake or delivery properties during mycobacterial hypoxia and latency (16, 25). The finding that the glbO promoter was upregulated by both H2O2 and NaNO2 stresses suggests the induction of trHbO biosynthesis during exposure to ROI and RNI. Also, the glbO response to the lower concentrations of both stresses was more lasting and better than the early response, which indicates the crucial involvement of trHbO in mycobacterial survival in the presence of various stresses and for O2 delivery. trHbO is required for aerobic respiration, and its levels are enhanced by the general stresses that the bacteria would come across once inside the phagosomes, which include RNI, ROI, and hypoxia (26). Consistent with the observation made above, we observed higher glbO promoter expression from the 24-h to the 48-h time point. Since 30 mM NaNO2 followed by 5 mM H2O2 induced maximal glbO expression, this can be correlated with the poor protection against .NO toxicity by mycobacterial trHbO compared to that by trHbN (28), indicating that the primary role of trHbO is not in .NO oxidation.
The first promoters used to drive protein expression in mycobacteria were derived from genes encoding the mycobacterial heat shock proteins GroEL2 and Hsp70, and these promoters were used since heat shock genes are expressed at a high level. Stover et al. (35) found that these promoters were able to drive the expression of foreign genes to produce 10% or more of total mycobacterial proteins, if used on an extrachromosomal plasmid, which was due to the deregulation of the promoter. In our study, the expression pattern shown by the groEL2 promoter was different from that shown by the globin promoters. The expression of groEL2 promoter activity was reduced from the 24-h time point to the 72-h time point in the absence of any stress. Previously, Stover et al. (35) showed upregulation of the groEL2 promoter under heat (42°C), acidic pH (pH 4), and oxidative (H2O2) stress. However, we report the response of the groEL2 promoter to oxidative and nitrosative stresses at different time points. Since nitrosative stress, especially 30 mM nitrite, induced the maximal expression of the groEL2 promoter at all time points tested, it can be used as an effective inducer of the groEL2 promoter. Aravindhan et al. (1) recently reported a high level of induction of the groE promoter during heat shock, followed by osmotic, dehydration, and oxidative stress conditions, and a moderate increase in the activity was observed under pH stress. Even though various stresses given under in vitro conditions are artificial and cannot be equated to those that occur under in vivo conditions, study of the differential regulation of trHb and groEL2 promoters provides us with a clue about the complex regulatory network that operates under in vivo conditions. Like glbN promoter expression, nitrosative stress induced early groEL2 promoter activity, with the maximum occurring at the 24-h time point.
While M. tuberculosis attempts to reside inside the macrophage to continue the infection indefinitely, the host tries to eliminate the pathogen via the activation of microbicidal mechanisms. To combat this, mycobacteria upregulate both trHb promoters and the groEL2 promoters, as shown by the significant increase in intracellular promoter activity from the 4-h time point to 48 h. In vitro experiments have demonstrated the induction of NO both in human alveolar macrophages (12) and in human monocytes and the direct correlation between the growth inhibition of M. tuberculosis and the presence of NO (32). Infection of iNOS-knockout mice with M. tuberculosis indicated an extensive susceptibility of these mice, as measured by increases in bacterial loads and decreased survival times (17, 18). Consistent with previous observations (29), we observed that the glbN promoter exhibited a much higher activity than the glbO promoter, and both promoters were found to be active after intracellular growth for about 48 h, with a slow but steady increase in activity. The role of superoxide radical in the survival of M. tuberculosis inside the macrophages has also been shown by using mice lacking the cytosolic p47 (phox) gene, which is essential for NADPH-dependent production of superoxide radicals. phox−/− mice showed an increase in bacterial loads during the early period of infection with M. tuberculosis (22). The results obtained suggest that the stresses that M. tuberculosis faces during growth inside macrophages keep the trHb gene promoters active and that there might be a requirement for constant expression of both glbO and glbN proteins during the intracellular growth of M. tuberculosis. However, we report the intracellular expression profile of the M. tuberculosis groEL2 promoter at different time points after infection. It has already been reported that the mycobacterial groEL2 promoter showed constitutive expression under stress conditions like heat shock but that the groE promoter might be more active during infection (1). The mycobacterial groEL2 promoter emitted higher GFP fluorescence and was thus stronger than both of the globin promoters at all time points tested. Even though the expression of the glbN promoter was comparatively higher than that of the glbO promoter in the intracellular milieu, the survival assay indicated a survival advantage for recombinant M. smegmatis bearing trHbO rather than trHbN. This could be due to the dual function carried out by the trHbO protein, such as sustainment of aerobic respiration under microaerobic conditions and slow oxidation of deleterious .NO (3). The presence of the Hsp65 protein did not provide any survival advantage to intracellular M. smegmatis, and it possibly plays little role in the intracellular survival of mycobacteria.
Within a few hours following phagocytosis, mycobacteria have to encounter ROS and RNS. In order to scavenge these radicals, mycobacteria start to upregulate their stress-related gene expression. Analysis of glbN, glbO, and groEL2 gene transcription in both M. smegmatis and M. tuberculosis by real-time PCR analysis showed that it was comparable and showed an upregulation from the 4-h to the 24-h time point, but the expression of these genes showed downregulation at the subsequent 48-h time point. This pattern of mRNA expression obtained can be correlated with the early response shown by globin and trHb promoters against oxidative and nitrosative stresses.
M. tuberculosis has evolved the mechanisms needed to precisely regulate the expression of promoters and, thereby, genes that allow it to survive in the dynamic macrophage environment. Here, we have created transcriptional fusions of the mycobacterial trHb promoters glbO and glbN and the mycobacterial groEL2 promoter with GFP and analyzed the patterns of expression of these promoters under various stress conditions in both the free-living and intracellular milieus at different time points. The expression of these promoters was first studied under in vitro conditions that simulated the major environmental challenges faced by mycobacteria. glbN showed an early response to the oxidative and nitrosative stresses tested, and the glbO responses to the lower concentrations of both stresses were more lasting and better than the early response. At all time points and under all stress conditions tested, the activity of the groEL2 promoter was stronger than that of both the glbO and glbN promoters, and nitrosative stress elicited the highest expression of the groEL2 promoter. The intracellular expression of both of the trHb promoters and the groEL2 promoter showed an increase when inside the macrophages. Among the trHb promoters, the glbN promoter showed higher activity than the glbO promoter, but it showed less activity than the groEL2 promoter. Real-time PCR analysis of total RNA isolated from recombinant M. smegmatis- and M. tuberculosis H37Rv-infected THP-1-derived macrophages with gene-specific primers indicated comparable profiles of expression of both trHb promoters and the groEL2 promoter in the intracellular milieu. The levels of both trHb and groEL2 mRNAs showed an initial upregulation at the 24-h time point, followed by a downregulatory trend at the 4-h time point. It was also observed that the presence of the glbO protein imparted an increased survival advantage to M. smegmatis compared with that of the glbN and hsp65 proteins. The comparative upregulation shown by both trHb promoters while growing inside macrophages and the survival assay indicate the importance that these promoters have for survival of M. tuberculosis in the hostile environment inside the host.
ACKNOWLEDGMENT
S.V.J. and G.K.M. are grateful to the Council of Scientific and Industrial Research, Government of India, for financial support.
Footnotes
Published ahead of print 6 July 2012
REFERENCES
- 1. Aravindhan V, et al. 2009. Mycobacterium tuberculosis groE promoter controls the expression of the bicistronic groESL1 operon and shows differential regulation under stress conditions. FEMS Microbiol. Lett. 292:42–49 [DOI] [PubMed] [Google Scholar]
- 2. Ascenzi P, et al. 2006. Nitric oxide scavenging by Mycobacterium leprae GlbO involves the formation of the ferric heme-bound peroxynitrite intermediate. Biochem. Biophys. Res. Commun. 339:450–456 [DOI] [PubMed] [Google Scholar]
- 3. Ascenzi P, Bolognesi M, Milani M, Guertin M, Visca P. 2007. Mycobacterial truncated hemoglobins: from genes to functions. Gene 398:42–51 [DOI] [PubMed] [Google Scholar]
- 4. Brosch R, Pym AS, Gordon SV, Cole ST. 2001. The evolution of mycobacterial pathogenicity: clues from comparative genomics. Trends Microbiol. 9:452–458 [DOI] [PubMed] [Google Scholar]
- 5. Casadevall A. 2008. Evolution of intracellular pathogens. Annu. Rev. Microbiol. 62:19–33 [DOI] [PubMed] [Google Scholar]
- 6. Cole ST, et al. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011 [DOI] [PubMed] [Google Scholar]
- 7. Collins HL, Kaufmann SH. 2001. The many faces of host responses to tuberculosis. Immunology 103:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couture M, et al. 1999. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 96:11223–11228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowley SC, Av-Gay Y. 2001. Monitoring promoter activity and protein localization in Mycobacterium spp. using green fluorescent protein. Gene 264:225–231 [DOI] [PubMed] [Google Scholar]
- 10. Dhandayuthapani S, et al. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17:901–912 [DOI] [PubMed] [Google Scholar]
- 11. Gardner PR, Costantino G, Salzman AL. 1998. Constitutive and adaptive detoxification of nitric oxide in Escherichia coli. Role of nitric-oxide dioxygenase in the protection of aconitase. J. Biol. Chem. 273:26528–26533 [DOI] [PubMed] [Google Scholar]
- 12. Jagannath C, Actor JK, Hunter RL., Jr 1998. Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide 2:174–186 [DOI] [PubMed] [Google Scholar]
- 13. Lama A, et al. 2009. Role of pre-A motif in nitric oxide scavenging by truncated hemoglobin, HbN, of Mycobacterium tuberculosis. J. Biol. Chem. 284:14457–14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lama A, Pawaria S, Dikshit KL. 2006. Oxygen binding and NO scavenging properties of truncated hemoglobin, HbN, of Mycobacterium smegmatis. FEBS Lett. 580:4031–4041 [DOI] [PubMed] [Google Scholar]
- 15. Lee BY, Horwitz MA. 1995. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J. Clin. Invest. 96:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C, He Y, Chang Z. 2004. Truncated hemoglobin o of Mycobacterium tuberculosis: the oligomeric state change and the interaction with membrane components. Biochem. Biophys. Res. Commun. 316:1163–1172 [DOI] [PubMed] [Google Scholar]
- 17. MacMicking JD, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641–650 [DOI] [PubMed] [Google Scholar]
- 18. MacMicking JD, et al. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mekalanos JJ. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mukai M, Savard PY, Ouellet H, Guertin M, Yeh SR. 2002. Unique ligand-protein interactions in a new truncated hemoglobin from Mycobacterium tuberculosis. Biochemistry 41:3897–3905 [DOI] [PubMed] [Google Scholar]
- 21. Nathan C. 1992. Nitric oxide as a secretory product of mammalian cells. FASEB J. 6:3051–3064 [PubMed] [Google Scholar]
- 22. Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97:8841–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohashi K, Burkart V, Flohe S, Kolb H. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558–561 [DOI] [PubMed] [Google Scholar]
- 24. Ouellet H, et al. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 99:5902–5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pathania R, Navani NK, Gardner AM, Gardner PR, Dikshit KL. 2002. Nitric oxide scavenging and detoxification by the Mycobacterium tuberculosis haemoglobin, HbN in Escherichia coli. Mol. Microbiol. 45:1303–1314 [DOI] [PubMed] [Google Scholar]
- 26. Pathania R, Navani NK, Rajamohan G, Dikshit KL. 2002. Mycobacterium tuberculosis hemoglobin HbO associates with membranes and stimulates cellular respiration of recombinant Escherichia coli. J. Biol. Chem. 277:15293–15302 [DOI] [PubMed] [Google Scholar]
- 27. Pawaria S, Lama A, Raje M, Dikshit KL. 2008. Responses of Mycobacterium tuberculosis hemoglobin promoters to in vitro and in vivo growth conditions. Appl. Environ. Microbiol. 74:3512–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pawaria S, et al. 2007. Intracellular growth and survival of Salmonella enterica serovar Typhimurium carrying truncated hemoglobins of Mycobacterium tuberculosis. Microb. Pathog. 42:119–128 [DOI] [PubMed] [Google Scholar]
- 29. Pesce A, Nardini M, Milani M, Bolognesi M. 2007. Protein structure in the truncated (2/2) hemoglobin family. IUBMB Life 59:535–541 [DOI] [PubMed] [Google Scholar]
- 30. Pockley AG. 2003. Heat shock proteins as regulators of the immune response. Lancet 362:469–476 [DOI] [PubMed] [Google Scholar]
- 31. Qamra R, Mande SC, Coates AR, Henderson B. 2005. The unusual chaperonins of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 85:385–394 [DOI] [PubMed] [Google Scholar]
- 32. Rich EA, et al. 1997. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber. Lung Dis. 78:247–255 [DOI] [PubMed] [Google Scholar]
- 33. Sarojini S, Soman S, Radhakrishnan I, Mundayoor S. 2005. Identification of moaA3 gene in patient isolates of Mycobacterium tuberculosis in Kerala, which is absent in M. tuberculosis H37Rv and H37Ra. BMC Infect. Dis. 5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Springer B, et al. 2001. Silencing of oxidative stress response in Mycobacterium tuberculosis: expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect. Immun. 69:5967–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stover CK, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460 [DOI] [PubMed] [Google Scholar]
- 36. Vabulas RM, et al. 2002. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277:15107–15112 [DOI] [PubMed] [Google Scholar]
- 37. Voskuil MI, Bartek IL, Visconti K, Schoolnik GK. 2011. The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol. 2:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vuletich DA, Lecomte JT. 2006. A phylogenetic and structural analysis of truncated hemoglobins. J. Mol. Evol. 62:196–210 [DOI] [PubMed] [Google Scholar]
- 39. Wards BJ, Collins DM. 1996. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol. Lett. 145:101–105 [DOI] [PubMed] [Google Scholar]
- 40. Wittenberg JB, Bolognesi M, Wittenberg BA, Guertin M. 2002. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J. Biol. Chem. 277:871–874 [DOI] [PubMed] [Google Scholar]
- 41. Xia Y, Zweier JL. 1997. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. U. S. A. 94:6954–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young DB, Garbe TR. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu H, Yang YH, Rajaiah R, Moudgil KD. 2011. Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum. 63:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yukl ET, et al. 2011. Nitric oxide dioxygenation reaction in DevS and the initial response to nitric oxide in Mycobacterium tuberculosis. Biochemistry 50:1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zingarelli B, O'Connor M, Wong H, Salzman AL, Szabo C. 1996. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J. Immunol. 156:350–358 [PubMed] [Google Scholar]