Abstract
Except for several conspicuous cases, very little is known about sulfur oxidizers living in natural freshwater environments. Sulfuricella denitrificans skB26 is a psychrotolerant sulfur oxidizer recently isolated from a freshwater lake as a representative of a new genus in the class Betaproteobacteria. In this study, an approximately 3.2-Mb draft genome sequence of strain skB26 was obtained. In the draft genome, consisting of 23 contigs, a single rRNA operon, 43 tRNA genes, and 3,133 coding sequences were identified. The identified genes include those required for sulfur oxidation, denitrification, and carbon fixation. Comparative proteomic analysis was conducted to assess cold adaptation mechanisms of this organism. From cells grown at 22°C and 5°C, proteins were extracted for analysis by nano-liquid chromatography–electrospray ionization–tandem mass spectrometry. In the cells cultured at 5°C, relative abundances of ribosomal proteins, cold shock proteins, and DEAD/DEAH box RNA helicases were increased in comparison to those at 22°C. These results suggest that maintenance of proper translation is critical for growth under low-temperature conditions, similar to the case for other cold-adapted prokaryotes.
INTRODUCTION
Chemolithoautotrophic sulfur-oxidizing bacteria, which are widely distributed in aquatic environments, have attracted interest among microbial ecologists. With their functions of inorganic carbon fixation and sulfur oxidation, they thrive as primary components of ecosystems fueled by reduced sulfur species, represented by those in terrestrial hot springs and hydrothermal vents. On the other hand, significant influences of sulfur oxidizers on cycles of nitrogen and phosphorus have been shown for several nitrate-storing bacteria supplied with sulfide from sulfate reduction in organic-rich marine sediments (20, 27, 28). Except for these conspicuous cases, however, the ecology of chemolithoautotrophic sulfur oxidizers in natural environments remains largely unknown. In marine environments, several key players in sulfide oxidation were identified only recently, in intertidal sediment (15) and water columns (7, 14, 32). A trial to identify major planktonic sulfur oxidizers was also performed in a stratified freshwater lake (1), but very little is known about chemolithotrophic sulfur oxidizers living in general freshwater environments.
Recently, a chemolithotrophic sulfur oxidizer, Sulfuricella denitrificans skB26, was isolated from a stratified freshwater lake (13) as a representative of a new genus in the class Betaproteobacteria. Occurrences of close relatives of this bacterium have been reported in several studies, in the form of 16S rRNA gene clones detected in libraries constructed with general primer pairs (3, 16, 19, 24). Strain skB26 was isolated from a cold anoxic hypolimnion and has the ability to grow at temperatures as low as 0°C. In general aquatic ecosystems, the primary source of reduced sulfur species is sulfate reduction in anoxic nitrate-depleted zones, which are often associated with low temperature. Therefore, cold adaptation may have essential significance for sulfur oxidizers in natural environments for access to sufficient supplies of growth substrate. Although several sulfur oxidizers have been isolated from low-temperature environments (7, 12, 26), their cold adaptation mechanisms are poorly understood. Here we report the draft genome sequence of this novel psychrotolerant chemolithoautotroph, along with results of a proteomic analysis to gain insight into cold adaptation.
MATERIALS AND METHODS
Genome sequence and annotation.
Sulfuricella denitrificans skB26 was grown in a previously described NaCl-free defined medium (13) containing 20 mM thiosulfate and 20 mM nitrate at 22°C. Genomic DNA was extracted by using an AquaPure genomic isolation kit (Bio-Rad Laboratories, CA) and then fragmented by shearing with a Hydroshear instrument (GeneMachines, CA). From the resulting DNA fragments, an 8-kbp paired-end library was prepared using GS Titanium paired-end library adaptors (Roche Diagnostics). The library was sequenced by using a Genome Sequencer FLX system (Roche Diagnostics). The paired-end reads were assembled by using GS De Novo Assembler, version 2.3, with default parameters. Gaps between contigs belonging to the same scaffold were closed by Sanger sequencing of PCR products. The draft genome sequence was automatically annotated using the Microbial Genome Annotation Pipeline (www.migap.org/). In the pipeline, open reading frames (ORFs) were identified by MetaGene Annotator, and then predicted ORFs were used to search reference databases, including RefSeq, TrEMBL, and the COGs (clusters of orthologous groups of proteins) data set. Genes for tRNAs and rRNAs were identified by tRNAscan-SE and rRNAmmer, respectively (30). Further genome analysis was performed using IMC-GE software (In Silico Biology, Japan). In the draft genome, homologous genes involved in sulfur oxidation, denitrification, and carbon dioxide fixation were identified by a BLASTP search using the sequence data of Thiobacillus denitrificans as the query (threshold, >30%), and normalized gene names were assigned. To predict the functions of two homologous genes encoding Csp family proteins, the amino acid sequences were subjected to PSI-BLAST searches, with an identity threshold of 90%, and then manually annotated.
Protein extraction and SDS-PAGE.
S. denitrificans was cultured in the same medium at the temperature for optimum growth (22°C) and the in situ temperature of the isolation source (5°C). Cells were harvested at stationary phase (35 and 68 days at 22°C and 5°C, respectively) and washed once with phosphate-buffered saline by centrifugation at 10,000 × g for 20 min at 4°C. The pellets were frozen at −80°C until protein extraction. Protein extraction was performed using a kit intended to extract total cellular proteins, including membrane proteins, i.e., a ReadyPrep protein extraction kit for total protein (Bio-Rad Laboratories, CA). The cell pellets were suspended in a buffer (included in the kit) which contained a strong chaotropic extraction reagent. After 10 sonications for 10 s each at 30-s intervals, resulting lysates were centrifuged for 20 min at 10,000 rpm at 20°C. The soluble fractions were recovered and used for further analysis. The protein content was quantified by the Bradford method, using a Bio-Rad protein assay kit (Bio-Rad Laboratories). The protein samples were mixed with sodium dodecyl sulfate sample buffer. The resulting samples were incubated at 99°C for 5 min, and then 50 μg of each denatured protein sample was subjected to SDS-PAGE using a 12.5% SuperSep Ace precast gel (Wako Pure Chemical Industries, Japan). After electrophoresis, the gel was stained with Coomassie brilliant blue R-250 and destained, and the lanes were cut out for in-gel protein digestion with trypsin.
In-gel trypsin protein digestion and mass analysis.
Proteins were digested with trypsin as previously described (29). Nano-liquid chromatography–electrospray ionization–tandem mass spectrometry (nano-LC-ESI-MS/MS) was performed on a multidimensional HPLC Paradigm MS2 chromatograph (AMR Inc., Japan) and nanospray electrospray ionization device (Michrom Bioresources Inc., CA) connected to an LTQ ion-trap MS (Thermo Fisher Scientific, MA). The digested peptides were loaded on an L-column 2 ODS column (Chemicals Evaluation & Research Institute, Japan) packed with C18 (5 μm by 12-nm pore size). The solvent system consisted of solvent A (2% acetonitrile and 0.1% formic acid in H2O) and solvent B (90% acetonitrile and 0.1% formic acid in H2O). The flow rate was maintained at 1 μl/min, and solvent B was increased in a linear gradient from 5% to 65% over 40 min. It was then further increased to 95% and kept at that concentration for 5 min before returning to 5% for analysis of the next sample. Peptide spectra were recorded in the range of m/z 450 to 1,800. Mass spectra were acquired in data-dependent scan mode. One MS/MS spectrum of the most intense peak was collected following the full-spectrum scan.
MASCOT search and semiquantitative analysis of proteins.
For peptide identification from MS/MS data, a protein sequence database was constructed from the S. denitrificans draft genome. By using MASCOT (ver.2.3.01), MS/MS spectra were searched against the generated database. Search parameters were set as follows: tryptic digest with a maximum of two missed cleavages; fixed modifications, carbamidomethyl cysteine; variable modifications, methionine oxidation; peptide masses, monoisotopic; positive charge ( +1, +2, or +3) of peptide; and mass tolerances of 1.2 Da for the precursor ion and 0.8 Da for product ions. To assess false-positive identifications, an automatic decoy search was performed against a randomized database with a significance threshold of <0.05 for the P value, and the estimated false discovery rate was below 3.4%. Protein identification was judged as positive when two or more different peptides derived from it were detected.
To evaluate protein content, the exponentially modified protein abundance index (emPAI) was determined as previously described (10). For comparative analysis, the normalized protein content (PC) value was calculated as follows: PC = emPAI/ΣemPAI × 100 (%), where ΣemPAI is the summation of emPAI values for all identified proteins.
Nucleotide sequence accession numbers.
The Sulfuricella denitrificans skB26 draft genome sequence data have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers BAFJ01000001 to BAFJ01000023. The version described in this paper is the first version.
RESULTS AND DISCUSSION
Genome sequence and annotation.
By pyrosequencing, we sequenced 77,574,798 bp from 386,840 reads. The generated sequences were assembled into 177 contigs, and two scaffolds containing multiple contigs were constructed. There were 124 gaps within the scaffolds, and 117 of them were closed by Sanger sequencing of PCR products. As a result, an S. denitrificans skB26 draft genome consisting of 23 contigs was obtained. Basic characteristics of the draft genome are shown in Table 1. The functional classification of the identified coding sequences (CDSs) based on the COG designations is shown in Fig. S1 in the supplemental material.
Table 1.
Basic characteristics of S. denitrificans skB26 draft genome
| Feature | Value |
|---|---|
| Size (bp) | 3,238,714 |
| GC content (%) | 56.1 |
| No. of contigs | 23 |
| Maximum contig length (bp) | 737,595 |
| Total no. of coding sequences | 3,133 |
| Coding sequence density (gene/kbp) | 0.905 |
| Total CDS length (bp) | 2,931,503 |
| Average gene length (bp) | 935.7 |
| No. of rRNA operons | 1 |
| No. of tRNA genes | 43 |
Genes required for sulfur oxidation, denitrification, and carbon fixation are summarized in Table S1 in the supplemental material. As reported previously, strain skB26 oxidizes thiosulfate and elemental sulfur to sulfate. The draft genome contains the soxXYZAB gene cluster but lacks soxCD, suggesting the generation of elemental sulfur as an intermediate of thiosulfate oxidation (18). In Allochromatium vinosum, elemental sulfur is oxidized to sulfite by the dissimilatory sulfite reductase system, encoded by dsr genes (2). Accordingly, the gene cluster dsrABEFHCMKLJOPNR occurs in the genome of skB26. We also identified a putative hdrAABC gene cluster encoding the heterodisulfide reductase complex, hypothesized to oxidize disulfide intermediates to sulfite (21). It has been proposed that adenosine-5′-phosphosulfate reductase (apr) and ATP sulfurylase (sat) oxidize sulfite to sulfate (17); the genes for these enzymes were also found in the strain. Furthermore, the gene for sulfide:quinone oxidoreductase (sqr), which catalyzes sulfide oxidation (22), occurred in the genome.
Strain skB26 contains genes necessary for denitrification, including nitrite reductase (nir), nitric oxide reductase (nor), and nitrous oxide reductase (nos) genes (23). The draft genome lacks a cytoplasmic membrane nitrate reductase (nar) gene, thought to generally be possessed by denitrifiers (23), but contains a periplasmic nitrate reductase (nap) gene.
Effects of low temperature on relative protein abundance.
For proteomic analysis, S. denitrificans was grown on thiosulfate under denitrifying conditions. Cells cultured at 22°C (MT sample) and 5°C (LT sample) were harvested in stationary phase and subjected to comparative proteomic analysis using nano-LC-ESI-MS/MS. Using MASCOT searches, 1,450 and 1,579 proteins were identified in MT and LT, respectively. The detected proteins were classified into functional categories based on COG designations, but an effect of temperature was not apparent in this qualitative comparison (see Fig. S1 in the supplemental material). For the comparisons based on semiquantitative analysis, normalized PC values were calculated.
The most abundant protein in LT was the ribosomal protein L5 (PC = 47%), which increased about 5-fold compared to that in MT (see Table S2 in the supplemental material). Similarly, a 20-fold increase was observed for the ribosomal protein L7/L12 in LT, and 6 ribosomal proteins were identified only in LT. The protein L7/L12 has been reported to be a cold acclimation protein (6), and increased ribosomal protein abundance at low temperatures has been observed in other prokaryotes (11, 33). The high cellular levels of ribosomal proteins observed in S. denitrificans may be associated with cold adaptation.
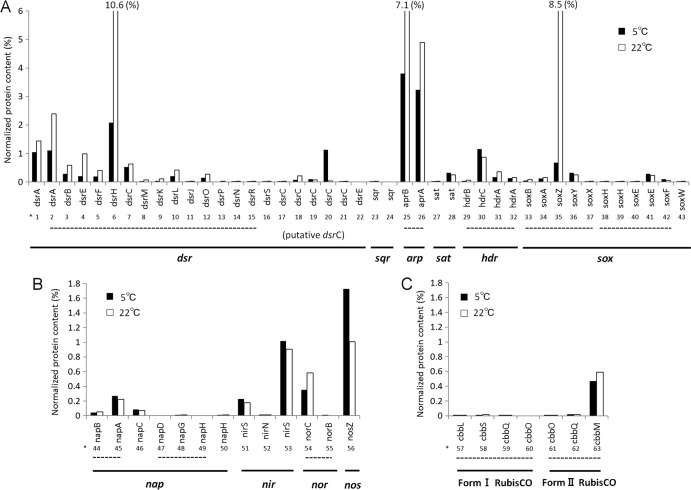
To eliminate interference from abundant ribosomal proteins, PC values were recalculated after excluding these proteins for comparison of other proteins. In order to examine effects of temperature specifically observed in anaerobic sulfur oxidizers, PC values of proteins putatively involved in sulfur oxidation, denitrification, and carbon dioxide fixation were compared between LT and MT (Fig. 1). Among these three functions, differences in relevant proteins were apparent for sulfur oxidation, with generally lower contents in LT (Fig. 1A). These sulfur oxidation-related proteins, including heterodisulfide reductase (Hdr), which lacks direct evidence for involvement in thiosulfate oxidation, were the second most abundant proteins (following the ribosomal proteins) in both MT and LT. The sum of the PC values of the proteins shown in Fig. 1A reached 40% in MT but decreased to 17% in LT. These results may be relevant to suppressed energy production at low temperatures, as shown for other cold-adapted bacteria (31). It should be noted, however, that the decrease in total sulfur oxidation-related proteins was attributed largely to exceptionally drastic changes in DsrH and SoxZ (Fig. 1). In the process of sulfur oxidation, DsrH and SoxZ work in conjunction with DsrEF and SoxY, respectively (4). The imbalanced changes of DsrH and SoxZ may have no connection to energy production.
FIG 1.
Normalized protein contents of products of genes associated with sulfur oxidation (A), denitrification (B), and carbon fixation via RubisCO (C), calculated by excluding all ribosomal proteins. Normalized gene names are shown with identification numbers corresponding to those in Table S1 in the supplemental material. Dashed lines indicate putative gene clusters.
As candidates for components of the cold adaptation mechanism, proteins with greater PC values in LT (more than 5 times those in MT) after the recalculation are listed in Table 2. One of the candidate proteins for cold adaptation, the cold shock protein CspD, has been reported as a cold-inducible protein in Bacillus subtilis (5). Another cspD homolog was also found in the genome (SCD_00462), and its product was identified only in LT, as the most abundant LT-specific protein (see Table S3 in the supplemental material). One of the major negative effects of low temperature is the inhibition of translation caused by stabilization of the mRNA secondary structure. It has been proposed that Csp proteins work in conjunction with the DEAD/DEAH box helicase to maintain the proper conformation of mRNA in B. subtilis (8). In the draft genome of strain skB26, 3 copies of the DEAD/DEAH box helicase gene were identified. The protein encoded by one of these genes was more abundant (about 9-fold) at 5°C (Table 2), and the product of another copy was identified only in LT (see Table S3). The greatest change (32-fold) was observed in the sulfur relay protein DsrC (SCD_00181), although other putative sulfur relay proteins, encoded by five genes, were not increased in LT (see Table S4). In Escherichia coli, the DsrC homolog is involved in thiolation of tRNA, which facilitates ribosome binding (9). Increased abundances of these proteins and ribosomal proteins consistently suggest that maintenance of proper translation at low temperatures is important for cold adaptation of S. denitrificans skB26.
Table 2.
Proteins that were more abundant (change of >5-fold) at 5°C than at 22°C
| Locus taga | Protein description | Functional categoryb | Normalized PCc |
PC ratio (5°C/22°C) | |
|---|---|---|---|---|---|
| 22°C | 5°C | ||||
| SCD_00181 | Sulfur relay protein, TusE/DsrC/DsvC family | P | 0.03548 | 1.12914 | 31.8 |
| SCD_01927 | Glutaredoxin-like protein | O | 0.03040 | 0.57336 | 18.9 |
| SCD_00850 | Flagellin and related hook-associated proteins | N | 0.00107 | 0.01660 | 15.5 |
| SCD_01247 | Fe-S cluster assembly scaffold IscU | C | 0.00835 | 0.08731 | 10.5 |
| SCD_00930 | DEAD/DEAH box helicase domain-containing protein | LKJ | 0.00281 | 0.02504 | 8.9 |
| SCD_01712 | Hypothetical protein | S | 0.17117 | 1.39561 | 8.2 |
| SCD_02129 | Anaerobic ribonucleoside-triphosphate reductase | F | 0.00207 | 0.01552 | 7.5 |
| SCD_01704 | Hydrogenase maturation factor | O | 1.45679 | 10.89136 | 7.5 |
| SCD_02710 | Fe(II) trafficking protein YggX | CO | 0.01884 | 0.12361 | 6.6 |
| SCD_01982 | Nitroreductase | C | 0.00247 | 0.01570 | 6.4 |
| SCD_00192 | Guanylate kinase | F | 0.00909 | 0.05573 | 6.1 |
| SCD_02164 | Cytochrome c553 | C | 0.09928 | 0.60598 | 6.1 |
| SCD_03038 | Protein tyrosine phosphatase | X | 0.00882 | 0.05151 | 5.8 |
| SCD_00637 | RNA chaperone (CspD [CspA family protein]) | K | 0.01951 | 0.10436 | 5.3 |
| SCD_02624 | Pilus protein | T | 0.01971 | 0.10504 | 5.3 |
| SCD_00876 | Beta-hydroxyacyl-(acyl carrier protein) dehydratase FabZ | I | 0.00354 | 0.01772 | 5.0 |
Locus tags indicate gene localizations in the first version of the draft genome (GenBank accession no. BAFJ01000001 to BAFJ01000023).
One-letter abbreviations indicate the COG functional categories.
PC values were calculated by excluding all ribosomal proteins.
Another candidate protein for cold adaptation was beta-hydroxyacyl-(acyl carrier protein) dehydratase (FabZ), a protein involved in fatty acid biosynthesis (Table 2). It is well known that modification of membrane fatty acid composition is critical for maintaining membrane fluidity under cold conditions, and various bacteria tend to increase the content of unsaturated fatty acids at low temperatures (25). In a cycle of hydrocarbon chain elongation for fatty acid biosynthesis, FabZ catalyzes dehydration to generate unsaturated fatty acids (34). Although the fatty acid profile of strain skB26 has not been revealed, it may change in response to low temperatures by involvement of FabZ.
Other candidate proteins shown in Table 2 were classified into various functional categories, including cell motility, nucleic acid metabolism, energy production, and signal transduction. For these proteins, it was impossible to deduce how they contribute to growth or survival at low temperatures. Their functional diversity may reflect universal effects of temperature on various biological processes and respective responses of this organism to cope with them.
From the comparative proteomic analysis, it was suggested that mechanisms to maintain proper translation and membrane fluidity play central roles in cold adaptation of S. denitrificans. It should be noted that the protein identification was performed on the basis of the draft genome, and some important genes and proteins may thus still be undiscovered. For further detailed analyses, a project to obtain the complete genome sequence of strain skB26 is ongoing. Considering that studies on sulfur oxidizers tend to place a disproportionate emphasis on extremophiles living in harsh environments, knowledge from studies on S. denitrificans will be valuable for a better understanding of the sulfur cycle in general freshwater environments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Kasahara and R. Kadoya for assistance in proteomic analysis.
This study was supported by a grant (no. 2237005) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, to Manabu Fukui.
Footnotes
Published ahead of print 6 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Biderre-Petit C, et al. 2011. Identification of sulfur-cycle prokaryotes in a low-sulfate lake (Lake Pavin) using aprA and 16S rRNA gene markers. Microb. Ecol. 61:313–327 [DOI] [PubMed] [Google Scholar]
- 2. Dahl C, et al. 2005. Novel genes of the dsr gene cluster and evidence for close interaction of Dsr proteins during sulfur oxidation in the phototrophic sulfur bacterium Allochromatium vinosum. J. Bacteriol. 187:1392–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Field EK, et al. 2010. Application of molecular techniques to elucidate the influence of cellulosic waste on the bacterial community structure at a simulated low-level-radioactive-waste site. Appl. Environ. Microbiol. 76:3106–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghosh W, Dam B. 2009. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 6:999–1043 [DOI] [PubMed] [Google Scholar]
- 5. Graumann P, Marahiel MA. 1996. Some like it cold: response of microorganisms to cold shock. Arch. Microbiol. 166:293–300 [DOI] [PubMed] [Google Scholar]
- 6. Graumann PL, Marahiel MA. 1999. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:203–209 [PubMed] [Google Scholar]
- 7. Grote J, et al. 2012. Genome and physiology of a model epsilonproteobacterium responsible for sulfide detoxification in marine oxygen depletion zones. Proc. Natl. Acad. Sci. U. S. A. 109:506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunger K, Beckering CL, Wiegeshoff F, Graumann PL, Marahiel MA. 2006. Cold-induced putative DEAD box RNA helicases CshA and CshB are essential for cold adaptation and interact with cold shock protein B in Bacillus subtilis. J. Bacteriol. 188:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeuchi Y, Shigi N, Kato J, Nishimura A, Suzuki T. 2006. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 21:97–108 [DOI] [PubMed] [Google Scholar]
- 10. Ishihama Y, et al. 2005. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4:1265–1272 [DOI] [PubMed] [Google Scholar]
- 11. Kaan T, Homuth G, Mäder U, Bandow J, Schweder T. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441–3445 [DOI] [PubMed] [Google Scholar]
- 12. Knittel K, et al. 2005. Thiomicrospira arctica sp. nov. and Thiomicrospira psychrophila sp. nov., psychrophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacteria isolated from marine Arctic sediments. Int. J. Syst. Evol. Microbiol. 55:781–786 [DOI] [PubMed] [Google Scholar]
- 13. Kojima H, Fukui M. 2010. Sulfuricella denitrificans gen. nov., sp. nov., a sulfur-oxidizing autotroph isolated from a freshwater lake. Int. J. Syst. Evol. Microbiol. 60:2862–2866 [DOI] [PubMed] [Google Scholar]
- 14. Lavik G, et al. 2009. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature 457:581–584 [DOI] [PubMed] [Google Scholar]
- 15. Lenk S, et al. 2011. Novel groups of gammaproteobacteria catalyse sulfur oxidation and carbon fixation in a coastal, intertidal sediment. Environ. Microbiol. 13:758–774 [DOI] [PubMed] [Google Scholar]
- 16. Li D, et al. 2010. Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl. Environ. Microbiol. 76:7171–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer B, Kuever J. 2007. Molecular analysis of the distribution and phylogeny of dissimilatory adenosine-5′-phosphosulfate reductase-encoding genes (aprBA) among sulfur-oxidizing prokaryotes. Microbiology 153:3478–3498 [DOI] [PubMed] [Google Scholar]
- 18. Mussmann M, et al. 2007. Insights into the genome of large sulfur bacteria revealed by analysis of single filaments. PLoS Biol. 5:e230 doi:10.1371/journal.pbio.0050230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson DM, Ohene-Adjei S, Hu FS, Cann IK, Mackie RI. 2007. Bacterial diversity and distribution in the Holocene sediments of a northern temperate lake. Microb. Ecol. 54:252–263 [DOI] [PubMed] [Google Scholar]
- 20. Otte S, et al. 1999. Nitrogen, carbon, and sulfur metabolism in natural Thioploca samples. Appl. Environ. Microbiol. 65:3148–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quatrini R, et al. 2009. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genomics 10:394 doi:10.1186/1471-2164-10-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reinartz M, Tschäpe J, Brüser T, Trüper HG, Dahl C. 1998. Sulfide oxidation in the phototrophic sulfur bacterium Chromatium vinosum. Arch. Microbiol. 170:59–68 [DOI] [PubMed] [Google Scholar]
- 23. Richardson DJ, Watmough NJ. 1999. Inorganic nitrogen metabolism in bacteria. Curr. Opin. Chem. Biol. 3:207–219 [DOI] [PubMed] [Google Scholar]
- 24. Rotaru C, Woodard LT, Choi S, Nevin PK. 2012. Spatial heterogenity of bacterial communities in sediment from an infiltration basin receiving highway runoff. Microb. Ecol. 64:461–473 [DOI] [PubMed] [Google Scholar]
- 25. Russell N, Fukunaga N. 1990. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. FEMS Microbiol. Lett. 75:171–182 [Google Scholar]
- 26. Sattley WM, Madigan MT. 2006. Isolation, characterization, and ecology of cold-active, chemolithotrophic, sulfur-oxidizing bacteria from perennially ice-covered Lake Fryxell, Antarctica. Appl. Environ. Microbiol. 72:5562–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sayama M. 2001. Presence of nitrate-accumulating sulfur bacteria and their influence on nitrogen cycling in a shallow coastal marine sediment. Appl. Environ. Microbiol. 67:3481–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schulz HN, Schulz HD. 2005. Large sulfur bacteria and the formation of phosphorite. Science 307:416–418 [DOI] [PubMed] [Google Scholar]
- 29. Shevchenko A, Wilm M, Vorm O, Mann M. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68:850–858 [DOI] [PubMed] [Google Scholar]
- 30. Sugawara H, Ohyama A, Mori H, Kurokawa K. 2009. Microbial genome annotation pipeline (MiGAP) for diverse users. Software Demonstrations S001-1–2. 20th Int. Conf. Genome Inform. (GIW2009) Posters and Software Demonstrations, Yokohama, Japan [Google Scholar]
- 31. Ting L, et al. 2010. Cold adaptation in the marine bacterium, Sphingopyxis alaskensis, assessed using quantitative proteomics. Environ. Microbiol. 10:2658–2676 [DOI] [PubMed] [Google Scholar]
- 32. Walsh DA, et al. 2009. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326:578–582 [DOI] [PubMed] [Google Scholar]
- 33. Williams TJ, et al. 2011. Defining the response of a microorganism to temperatures that span its complete growth temperature range (−2°C to 28°C) using multiplex quantitative proteomics. Environ. Microbiol. 13:2186–2203 [DOI] [PubMed] [Google Scholar]
- 34. Zhang YM, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6:222–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.