Abstract
Water quality was assessed at two marine beaches in California by measuring the concentrations of culturable fecal indicator bacteria (FIB) and by library-independent microbial source tracking (MST) methods targeting markers of human-associated microbes (human polyomavirus [HPyV] PCR and quantitative PCR, Methanobrevibacter smithii PCR, and Bacteroides sp. strain HF183 PCR) and a human pathogen (adenovirus by nested PCR). FIB levels periodically exceeded regulatory thresholds at Doheny and Avalon Beaches for enterococci (28.5% and 31.7% of samples, respectively) and fecal coliforms (20% and 5.8%, respectively). Adenoviruses were detected at four of five sites at Doheny Beach and were correlated with detection of HPyVs and human Bacteroides HF183; however, adenoviruses were not detected at Avalon Beach. The most frequently detected human source marker at both beaches was Bacteroides HF183, which was detected in 27% of samples. Correlations between FIBs and human markers were much more frequent at Doheny Beach than at Avalon Beach; e.g., adenovirus was correlated with HPyVs and HF183. Human sewage markers and adenoviruses were routinely detected in samples meeting FIB regulatory standards. The toolbox approach of FIB measurement coupled with analysis of several MST markers targeting human pathogens used here demonstrated that human sewage is at least partly responsible for the degradation of water quality, particularly at Doheny Beach, and resulted in a more definitive assessment of recreational water quality and human health risk than reliance on FIB concentrations alone could have provided.
INTRODUCTION
The emerging paradigm in assessment of recreational water quality includes the concept that knowledge of the dominant source(s) of microbial contamination is crucial for protection of human and ecosystem health, for accurate risk assessment, and for remediation of water bodies with impaired water quality. Determination of whether water is contaminated by human sources, such as municipal sewage or onsite disposal (septic) systems, is a first and important step in assessing human health risk and devising appropriate remediation strategies for a given water body.
Bacteroides spp. are Gram-negative, strictly anaerobic, non-spore-forming bacilli that outnumber conventional fecal indicator bacteria (FIB), such as coliforms and enterococci, in both human and animal feces; i.e., they occur at concentrations of 109 to 1011 organisms · g−1 in feces (26, 59) and 109 Bacteroides organisms · 100 ml−1 in sewage (16). In contrast, conventional FIB concentrations in untreated sewage are orders of magnitude lower, e.g., approximately 107 CFU · 100 ml−1 for total coliforms and 106 CFU · 100 ml−1 for fecal coliforms and enterococci (24). Due to its relatively great sensitivity (23) and its position as one of the first library-independent microbial source tracking (MST) methods directed against human fecal sources, the human-associated Bacteroides assay has been widely used to assess pollution sources in both PCR and quantitative PCR (qPCR) formats (4, 8, 9, 19–21, 28, 33, 34, 43, 49).
Methanobrevibacter smithii is the most prominent methanogen in the human gastrointestinal tract and has been found at concentrations of 107 to 1010 organisms · g−1 in feces (12, 35). The use of the nifH gene of M. smithii to identify human-associated fecal pollution has been limited but successful in MST studies conducted to date (23, 55), as it is relatively host specific compared to some other MST markers (3, 23, 31).
Adenovirus types 40 and 41 are etiological agents of viral gastroenteritis. These viruses have been utilized to indicate human fecal pollution in water (14, 29, 46). Because they are pathogens, these viruses directly inform risk assessment models for human health. In contrast, human polyomaviruses (HPyVs) are generally nonpathogenic and are excreted in the urine and feces of healthy individuals (57, 60). The HPyV PCR and qPCR methods used in this study targeted the species JC virus (JCV) and BK virus (BKV) (37, 38), which are both widespread in sewage (2, 11, 36, 47). Both viruses are genetically stable, distributed worldwide, and maintain high seropositive rates in the human population (1, 11, 18, 45, 53, 54, 62). The use of PCR methods that target both JCV and BKV in order to detect human sewage pollution has been successful in a number of laboratory and field studies (5, 10, 23, 37, 38).
Research to define the most useful MST method(s) for human and other pollution sources is ongoing, and several researchers have suggested use of a toolbox or multitooled approach (41, 42, 48, 58). Studies using the toolbox approach incorporate multiple indicators or markers to assess water quality and pollution sources (41, 58). To date, several studies have determined nonpoint or point source contributions to water quality degradation utilizing an MST toolbox approach (9, 25, 27, 37, 42).
This study incorporated data from two beaches on the U.S. West Coast with various potential fecal inputs and different hydrologies: Doheny State Beach (Dana Point, CA) and Avalon Beach (Catalina Island, CA). See the Materials and Methods section for more detail. Established and emerging methods for detecting sewage contamination from human sources at Doheny and Avalon Beaches were employed here, including PCR for human-associated Bacteroides sp. strain HF183 (8), Methanobrevibacter smithii PCR (55), nested PCR for adenovirus (46), and PCR and qPCR for HPyVs (37, 38). The goals of this study were to (i) assess the presence and absence of several MST markers targeting human sources, (ii) enumerate FIB by conventional, culture-dependent methods, (iii) determine any correlations among indicators, markers, and pathogens, and (iv) examine the differences in occurrence of indicators and markers among the locations. We hypothesized that the amalgamation of bacterial, viral, and methanogen-based MST data would allow a more complete perspective of microbial contamination sources and a better interpretation of water quality and human health risks at beaches thought to be impacted by nonpoint source pollution.
MATERIALS AND METHODS
Construction of recombinant plasmid for HPyV controls and qPCR standard curve.
BK virus (ATCC VR-837) was obtained from the American Type Culture Collection (Manassas, VA), and propagated in HEL-299 cells (ATCC CCL-137). The cell line was grown in Eagle minimum essential medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Inc., Carlsbad, CA). Cell lines were maintained in Eagle minimum essential medium containing 2% FBS. DNA was extracted from 0.1 ml of the BK virus culture using a DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA) and used as the template in the HPyV PCR assay (37). The resulting 176-bp amplicon was purified using a QIAquick PCR purification kit (Qiagen, Inc.) and then cloned into the pCR4-TOPO vector (Invitrogen, Inc.). The vector was then transferred into Escherichia coli One Shot chemically competent cells and plated on LB agar containing 100 μg · ml−1 ampicillin. Recombinant plasmids with a single copy of the amplicon were purified using a GenElute 5-min plasmid miniprep kit (Sigma, St. Louis, MO) following the manufacturer's instructions. Purified recombinant plasmid DNA containing the insert was quantified using a Qubit fluorometer (Invitrogen, Inc., Carlsbad, CA). DNA quantification was performed in triplicate and averaged to determine the estimated total DNA concentration. Insert copy numbers were estimated by multiplying the average DNA concentration by Avogadro's number and then dividing by the product of the entire length of the plasmid (in base pairs) and average weight of a base pair (61).
Positive PCR controls.
All primers and probes used in this study are described in Table 1. To construct clones for use as positive controls, a specific gene fragment for each MST marker (human-associated 525-bp region of the 16S rRNA gene of Bacteroides or 221-bp region of the mnif gene of M. smithii) was amplified using the primers described below. The 525-bp and 221-bp amplicons were then cloned into a pCR4-TOPO vector and transformed into E. coli One Shot chemically competent cells as described above. Recombinant plasmids were propagated and purified as described above. Plasmids containing inserts were confirmed by sequencing at Macrogen USA (Rockville, MD). All sequences were subjected to a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST) for comparison with published sequences. All constructed plasmids used as PCR or qPCR positive controls contained the correct sequence.
Table 1.
Sequences of primers and probe used in this study
| Assay | Primer or probe | Sequence | Reference(s) |
|---|---|---|---|
| Human Bacteroides PCR | HF183 | 5′-ATC ATG AGT TCA CAT GTC CG-3′ | 8 |
| Bac708R | 5′-CAA TCG GAG TTC TTC GTG-3′ | ||
| M. smithii PCR | Mnif-342f | 5′-AAC AGA AAA CCC AGT GAA GAG-3′ | 55 |
| Mnif-363r | 5′-ACG TAA AGG CAC TGA AAA ACC-3′ | ||
| Adenovirus nested PCR | hexAA1885 | 5′-GCC GCA GTG GTC TTA CAT GCA CAT C-3′ | 46 |
| hexAA1913 | 5′-CAG CAC GCC GCG GAT GTC AAA GT-3′ | ||
| nehexAA1893 | 5′-GCC ACC GAG ACG TAC TTC AGC CTG-3′ | ||
| nehexAA1905 | 5′-TTG TAC GAG TAC GCG GTA TCC TCG CGG TC-3′ | ||
| Human polyomavirus PCR/qPCRa | SM2 | 5′-AGT CTT TAG GGT CTT CTA CCT TT-3′ | 37, 38 |
| P6 | 5′-GGT GCC AAC CTA TGG AAC AG-3′ | ||
| KGJ3 | 5′-(FAM)-TCA TCA CTG GCA AAC AT-(MGBNFQ)-3′ |
The SM2 and P6 primers were used in both the PCR and qPCR assays.
Negative controls.
The absence of contamination in each DNA extraction was tested using sterile, DNA-free water, which was processed in parallel with all samples through DNA extraction and PCR or qPCR. In addition, contamination of water samples during collection or filtration was ruled out using field and method blanks. Method blanks were processed in parallel with all water samples starting with filtration of sterile buffered water through to DNA extraction and PCR or qPCR. All DNA extraction blanks and method blanks were negative in all assays.
Human polyomavirus qPCR and standard curve.
The HPyV qPCR mixtures were prepared using SM2 and P6 primers and KGJ3 probe (Table 1). The previously published reaction mixture and thermocycling conditions were used (38). To produce a standard curve, the recombinant plasmid DNA was serially diluted in nuclease-free reagent-grade water to a final concentration ranging from 102 to 106 gene copies · μl−1. Five microliters of each dilution was used as the template in the TaqMan real-time standard curve PCRs. Each dilution was run in duplicate. A standard curve was run with every qPCR assay. Applied Biosystems default settings for the threshold cycle (CT) were used for data analysis. The CT values were plotted against copy number to generate the standard curve. Linear regression was used to assess the relationship between CT values and copy number.
Doheny Beach sites and sampling schedule.
All samples analyzed in this study were collected as part of a larger epidemiological study in southern California (15). This study incorporated data from two West Coast beaches with various potential fecal inputs and different hydrologies: Doheny State Beach (Dana Point, CA) and Avalon Beach (Catalina Island, CA). FIB concentrations in the waters of Doheny State Beach frequently exceed regulatory standards for microbial water quality (104 and 400 CFU/100 ml for enterococci and fecal coliforms, respectively) (40). The poor water quality has been attributed to several factors, including limited water circulation caused by a jetty located at the northwest end of the beach, high-density seagull populations releasing fecal matter into the water and sediments, and urban runoff from the San Juan Creek watershed (40, 44). Occasionally, under high-tide or high-flow conditions in San Juan Creek, the San Juan Creek Lagoon breaches a confining berm of sand that forms during summer and discharges into the Pacific Ocean (39). Water quality monitoring around the outflow area has reported consistently higher concentrations of FIB in the creek than the ocean (40) and higher enterococci levels at the beach when the berm is open than when it is closed (15). In a 2003 study, antibiotic resistance analysis and ribotyping of E. coli and enterococci in the area suggested that the high concentrations of indicator bacteria were due to intervening storm drains, direct fecal contamination by avian sources (e.g., seagulls), and survival and proliferation of fecal bacteria adapted to the secondary habitat (44). A wastewater treatment plant is located near the San Juan Creek less than a mile upstream of Doheny Beach; however the effluent is discharged into the San Juan Creek Ocean Outfall (32a). The Ocean Outfall is located approximately 2.1 km offshore in a southwest direction from Doheny Beach at San Juan Creek and has a flow rate of 19.1 million gallons per day (51, 52).
Water was collected every weekend at five sites along Doheny Beach over a 4-month period (May to September 2008). The five sites were designated A to E (Fig. 1A). Site A, B, D, and E samples were collected along the beach. Site A samples were collected adjacent to the jetty. Site B samples were collected at the north beach between Sites A and D. Site C samples were collected in the San Juan Creek Lagoon approximately 50 m prior to its discharge across the beach into the ocean. Site D samples were collected on the north end of the south beach adjacent to the lagoon. Site E samples were collected at the south end of the south beach.
Fig 1.
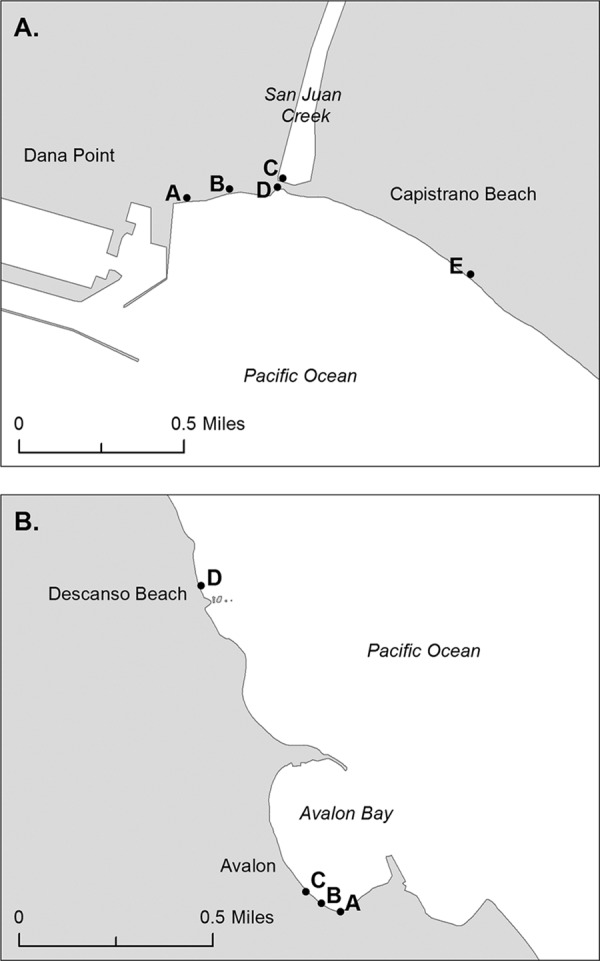
Sample sites. (A) Site designations at Doheny State Beach and San Juan Creek Lagoon. Sites A and B were located north of San Juan Creek, site C was the San Juan Creek Lagoon, site D was located on the beach side of the San Juan Creek Lagoon, and site E was located south of the lagoon. (B) Site designations at Avalon Beach at Catalina Island. Sites A to C were located along Avalon Beach. Site D was located north of Avalon Beach at Descanso Beach.
Avalon Beach sites and sampling schedule.
The FIB concentrations in the waters of Avalon Beach frequently exceed regulatory standards (40). However, in contrast to Doheny Beach, Avalon Beach waters circulate relatively freely (32). Sewer lines run parallel to and within 20 m of the beach, and storm water runoff is channeled into the sewer lines using low-flow diverters (9, 22). When the low-flow diverters reach maximum capacity, runoff enters small drains that discharge into the ocean through the sand (22). By way of these drains, untreated runoff and sewage can enter the ocean and degrade water quality. In addition, Avalon Beach suffers from aging sewer infrastructure, much of which dates from the early 20th century. The situation is exacerbated by the limited availability of freshwater on the island, which necessitates the use of seawater to run the sanitary collection system and makes iron and steel sewer pipes more susceptible to failure due to corrosion. Large numbers of sea gulls and pigeons accumulate around restaurants near the beach and may also contribute to inputs of fecal bacteria (9). In addition, the area is frequented by boat traffic, with many boats anchoring off the beach for hours and even days.
Water was collected every Thursday through Sunday at four sites along Catalina Island over a 2-month period (June to August 2008). The four sites were designated A to D (Fig. 1B). Samples from sites A to C were collected along Avalon Beach inside Avalon Harbor. Site A samples were collected at the south beach in the southernmost corner of the beach. Site B samples were collected on the south side of the pier (located between sites B and C). Site C samples were collected on the north side of the pier. Avalon Beach site D (Descanso Beach), which is north of Avalon Beach and outside Avalon Harbor, was used as a control site due to the historically low levels of fecal bacteria there (40).
Sample collection.
Samples were collected between 8:00 to 9:00 a.m. All samples were taken at a 0.5-m depth. Samples were collected in sterile polyethylene buckets and transported to the laboratory on ice. Water samples were processed within 3 h of collection. All samples were analyzed for enterococci, total coliforms, fecal coliforms, HPyVs (quantified by qPCR and presence/absence of detection by PCR), human-associated Bacteroides, M. smithii, and adenovirus (as described below).
Enumeration of culturable indicator organisms.
Culturable concentrations of all indicator organisms were obtained using standard methods. Enterococci were enumerated by membrane filtration on mEI agar, with incubation at 41 ± 0.5°C for 24 h (56). Fecal coliform concentrations were determined by membrane filtration using mFC agar, with incubation at 44.5 ± 0.5°C for 24 h (6). Total coliform concentrations were enumerated by membrane filtration on mEndo agar, with incubation at 35 ± 0.5°C for 24 h (6).
Concentration of microbes for PCR.
Bacteria, methanogens, and viruses from 500-ml samples were concentrated simultaneously on a 0.45-μm-pore-size nitrocellulose filter. Bacteria and methanogens were concentrated by physical capture on the filter. To concentrate the viruses, the pH of the water was adjusted to 3.5 using 2.0 N HCl prior to filtration (6, 23, 37). The low pH does not affect the concentration or detection of the bacteria and methanogens (23). After filtration, each filter was placed into a 2-ml microcentrifuge tube. The tube containing the filter was placed on dry ice and shipped from California to the University of South Florida lab (Tampa, FL). DNA was extracted from the filter using a modified MoBio and Qiagen DNA extraction protocol as described in the supplemental material and was used as the template for human-associated Bacteroides PCR, M. smithii PCR, adenovirus nested PCR, HPyV PCR, and HPyV qPCR assays.
Detection of human-associated Bacteroides.
Previously published primers specific for a region of the 16S rRNA gene of human-associated Bacteroides (8), including the HF183 forward primer, were used in a touchdown PCR (23) (Table 1). PCR mixtures were prepared as previously published (23). The touchdown PCR conditions were as follows: DNA polymerase activation at 95°C for 3 min, followed by 43 cycles of DNA melting at 94°C for 45 s and then annealing for 45 s and extension at 72°C for 30 s. Annealing temperatures ranged from 65 to 55°C. Cycles were performed twice at temperatures of 65 to 63°C, once at temperatures of 62 to 56°C, and finally, 30 times at 55°C, followed by a final elongation at 72°C for 5 min (Eppendorf Mastercycler thermocycler). A plasmid containing the 525-bp target fragment of the 16S rRNA gene was used as the PCR positive control. PCR products were separated and visualized using SYBR gold staining.
Detection of human-associated M. smithii.
Previously published primers specific for the nifH gene of human-associated M. smithii (55) were used in the touchdown PCR (23) (Table 1). PCR mixtures were prepared as previously published (23). The touchdown PCR conditions were the same as those described above for the human-associated Bacteroides assay. A plasmid containing the 221-bp target fragment of the nifH gene was used as the PCR positive control. PCR products were separated and visualized as described above.
Adenovirus nested PCR.
Previously published primers specific for the hexon gene of human adenoviruses were used in the nested PCR (46) (Table 1). Initial amplification was carried out in a 50-μl reaction mixture containing 25 μl GoTaq green master mix (Promega Corporation), 0.8 μM concentrations of each primer (hexAA1885 and hexAA9113), and 5 μl of template DNA. In both PCRs, the first round of denaturation was carried out for 4 min at 94°C, followed by 30 cycles of denaturation at 94°C for 90 s, annealing at 55°C for 90 s, and extension at 72°C for 120 s and by a final elongation at 72°C for 5 min. For the nested PCR, the 50-μl reaction mixture contained 25 μl GoTaq green master mix (Promega Corporation), 0.4 μM concentrations of each primer (nehexAA1893 and nehexAA1905), and 1 μl of template from the first round of PCR.
Detection of human polyomaviruses.
Primers specific for a partial region of the HPyV T-antigen gene were used for PCR (38) (Table 1). PCR preparations and thermocycling conditions were those previously published (23). A plasmid containing the 173-bp target fragment of the T-antigen gene was used as the PCR positive control. PCR products were separated and visualized as described above.
Quantification of human polyomaviruses in beach samples.
All qPCRs were carried out in a 25-μl volume, essentially as described by McQuaig et al. (37). Amplification conditions and standard curve material were as described above for PCR. Standard curve reactions were run in duplicate for each qPCR run performed over a range of from 102 to 106 gene copies · μl−1, and the average R2 was 0.9879 ± 0.0103.
Statistical analysis.
Summary statistics were computed for variables of interest using GraphPad InStat software, version 3.00 (GraphPad Software, San Diego, CA). Bacterial concentrations and HPyV copy numbers were log10 transformed, and differences among concentrations and copy numbers were determined using paired or unpaired t tests. Means were considered significantly different at the alpha level of 0.05. Relationships between indicators and markers were determined by calculating Pearson correlation coefficients. Differences were considered significant when P was <0.05, and two-sided tests were performed for all analyses. Observations of human-associated markers were converted to binary data, and binary logistic regression models (SPSS, version 17.0) were used to assess relationships between HPyV or FIB concentrations and the presence or absence of human-associated markers. Nagelkerke's R square, which can range from 0.0 to 1.0, denotes the effect size (the strength of the relationship); stronger associations have values closer to 1.0. Relationships were considered significant when the P value for the model chi-square was ≤0.05 and the confidence interval for the odds ratio did not overlap 1.0. The odds ratio is the measure of the effect size and an estimation of the probability of the same response of the two variables. Fisher's exact test was used to assess significant differences in the frequency of observation of binary marker data. Differences were considered significant at an alpha level of 0.05.
RESULTS
Bacterial water quality indicator concentrations at Doheny Beach sites.
One hundred thirty samples were collected from five Doheny Beach sites over the study period (26 samples per site). The average log10-transformed concentrations of all FIB at each Doheny Beach site are summarized in Fig. 2A. The average concentration of each FIB at site C was significantly greater than that at any other Doheny Beach site (P < 0.001). Enterococci at site C exceeded regulatory standards for one-time sampling (104 CFU/100 ml) in 88.5% of the samples (40). Fecal coliform concentrations at site C exceeded regulatory standards (400 CFU/100 ml) in 76.9% of the samples (40). California has a total coliform regulatory standard of 10,000 CFU/100 ml, and at site C, total coliform concentrations exceeded the California standard in 10.0% of the samples.
Fig 2.

Average log10-transformed concentrations of enterococci, fecal coliforms, total coliforms, and HPyVs at the Doheny Beach (A) and Avalon Beach (B) sites. Error bars represent standard deviations. Indicator bacteria are reported as log10 CFU · 100 ml−1. HPyVs are reported as log10 copy number · 100 ml−1.
The average concentrations of both enterococci and fecal coliforms at site D were significantly larger than those at sites A, B, and E (P < 0.05). FIB concentrations in water samples at site D exceeded enterococci and fecal coliform regulatory standards in 30.8% and 15.4% of the samples, respectively. The average concentrations of enterococci and fecal coliforms among sites A, B, and E were not significantly different. The individual enterococcus concentrations at sites A, B, and E exceeded regulatory standards in only 11.5%, 7.7%, and 3.8% of the samples, respectively. Fecal coliform concentrations at sites B and E exceeded regulatory standards in only 3.8% of the samples, while no samples with concentrations that exceeded regulatory standards were detected at site A. Aside from site C, total coliform concentrations did not exceed 10,000 CFU/100 ml at any site.
Correlations of bacterial indicators among Doheny Beach sites.
Throughout this report, correlations are noted only when P is <0.05. The concentrations of enterococci at site A were positively and significantly correlated with those at site C (R2 = 0.1692) and site E (r = 0.6390, R2 = 0.4084). Enterococcus concentrations at site E were also positively correlated with those at site B (R2 = 0.2076). Moreover, the concentrations at site C were positively correlated with those at site D (R2 = 0.3407). The concentrations of fecal coliforms at site D were positively correlated with those at site B (R2 = 0.2225), site C (R2 = 0.1748), and site E (R2 = 0.1699). The concentrations of total coliforms were positively correlated between all sites (R2 = 0.2449 to 0.4330) except sites C and E (P = 0.1237). In addition, the concentrations of enterococci, fecal coliforms, and total coliforms were positively correlated with each other at each site (R2 = 0.2489 to 0.7310).
qPCR detection of HPyVs at Doheny Beach sites.
HPyVs were rarely detected by qPCR at any Doheny Beach site (Fig. 2A). HPyVs were not detected at sites C and D, were detected once each at site A and site E, and were detected 3 times at site B. The quantities of HPyVs detected ranged from 125 to 2,884 copies · 100 ml−1. There were no significant differences in the HPyV copy numbers detected among the sites. In addition, there were no significant correlations of HPyV copy numbers between sites where HPyVs were detected.
PCR detection of human-associated water quality indicators at Doheny Beach sites.
The frequency of human marker detection at each site is summarized in Fig. 3A. HPyVs were detected by PCR in 4 samples at site A, 3 samples at site B, and 1 sample each at sites D and E and were not detected at site C. Human-associated Bacteroides organisms were detected in 9 samples at site C, 8 samples at site D, 4 samples at site B, and 2 samples at both sites A and E. The M. smithii marker was detected in Doheny Beach only once, at site C. Adenovirus was detected in 3 samples at both sites B and D, 2 samples at site A, and 1 sample at site C and was not detected at site E. The frequency of human-associated Bacteroides detection was significantly more frequent than that of M. smithii detection (P < 0.01). For all other organisms, detection frequencies were not significantly different.
Fig 3.
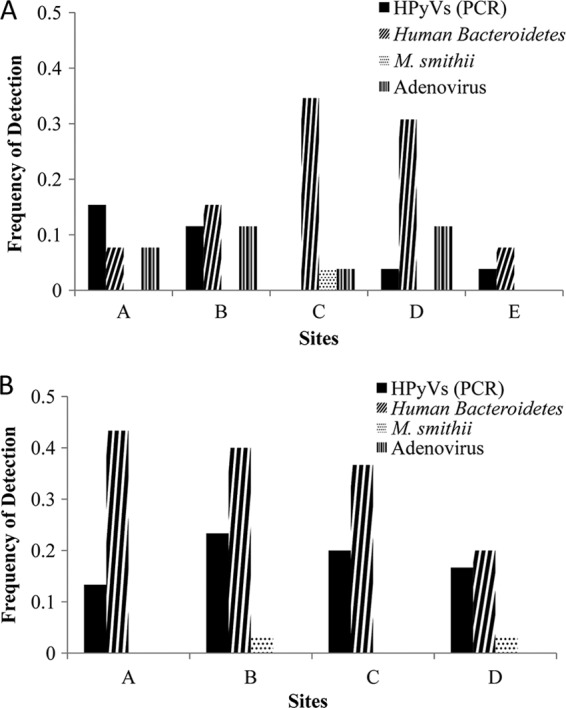
Frequency of detection of human-associated MST markers and adenoviruses at the Doheny Beach (A) and Avalon Beach (B) sites. HumBac, human-associated Bacteroides marker.
Relationships among indicators and markers at Doheny Beach.
A correlation between analytes at each site was frequently but not always noted. At site A, PCR detection of HPyVs was strongly correlated with the concentrations of fecal coliforms (Nagelkerke's R2 = 0.567, P < 0.001; odds ratio = 246.100, P = 0.039). At site B, the concentrations of HPyVs were moderately correlated with the presence of adenovirus (Nagelkerke's R2 = 0.413, P < 0.013; odds ratio = 3.653, P = 0.025). In addition, the detection of HPyVs was significantly correlated with the occurrence of adenovirus (odds ratio = 15.333; R2 = 0.3884, P = 0.0269) at site B. Several positive correlations were documented at site C, including enterococcus concentrations and the occurrence of the human-associated Bacteroides HF183 marker (Nagelkerke's R2 = 0.204, P = 0.041), M. smithii (Nagelkerke's R2 = 1.000, P = 0.004), and adenovirus (Nagelkerke's R2 = 1.000, P = 0.004). The occurrence of the human-associated Bacteroides marker was also significantly correlated with fecal coliform concentrations (Nagelkerke's R2 = 0.191, P = 0.049). No significant relationships were observed at site D or E.
Bacterial indicator concentrations and marker detection at each Doheny Beach site were compiled for all sites combined (Table 2). Enterococcus concentrations were strongly correlated with fecal coliforms (r = 0.8620, R2 = 0.7430), total coliforms (r = 0.8480, R2 = 0.7180), and M. smithii presence (Nagelkerke's R2 = 1.000). Fecal coliforms were also strongly correlated with total coliforms (r = 0.8780, R2 = 0.771). Total coliforms were moderately correlated with M. smithii (Nagelkerke's R2 = 0.4660, P = 0.021). The human-associated Bacteroides marker was weakly correlated with enterococci (Nagelkerke's R2 = 0.1920, P = 0.004), fecal coliforms (Nagelkerke's R2 = 0.1900, P < 0.001; odds ratio = 2.383, P < 0.0005), and total coliforms (Nagelkerke's R2 = 0.1370, P = 0.001; odds ratio = 1.858, P = 0.001). HPyVs detected by PCR and HPyV copy numbers were significantly correlated (Nagelkerke's R2 = 0.5210, P < 0.001). The presence of adenovirus was correlated with both HPyV concentrations (Nagelkerke's R2 = 0.0870, P < 0.033) and detection of human-associated Bacteroides (likelihood ratio = 2.307; R2 = 0.1078, P = 0.0016).
Table 2.
Correlations of microbial targets at Doheny Beach and Avalon Beacha
| Beach and microbial target (correlation type) | Correlation |
|||||
|---|---|---|---|---|---|---|
| Total coliforms | Fecal coliforms | Enterococci | HPyVs | H-Bac | M. smithii | |
| Doheny | ||||||
| Fecal coliforms (r) | 0.8780 | |||||
| Enterococci (r) | 0.8480 | 0.8620 | ||||
| HPyV | NS | NS | NS | |||
| H-Bac (R2) | 0.1370 | 0.1900 | 0.1920 | NS | ||
| M. smithii (R2) | 0.4660 | NS | 1.000 | NS | NS | |
| Adenovirus (R2) | NS | NS | NS | 0.0870 | 0.1078 | NS |
| Avalon | ||||||
| Fecal coliforms (r) | 0.8926 | |||||
| Enterococci (r) | 0.6277 | 0.7282 | ||||
| HPyVs | NS | NS | NS | |||
| H-Bac (R2) | 0.061 | 0.074 | NS | NS | ||
| M. smithii | NS | NS | NS | NS | NS | |
Data are from all sites combined. Comparisons between quantitative data were performed using Pearson's correlation coefficient and are reported as r. Comparisons between the quantitative data set and the binary data set were performed using binary logistic regression and are reported as Nagelkerke's R2. Only correlations in which P values were <0.05 are noted. NS, nonsignificant correlations. No significant relationships with adenovirus were observed in the Avalon Beach samples. HPyVs, human polyomaviruses detected by qPCR; H-Bac, human-associated Bacteroides marker.
Overall, human-associated Bacteroides was the most frequently detected marker of human sewage pollution at all the Doheny Beach sites (n = 25). The M. smithii marker was the least frequently detected marker (n = 1). Adenovirus and HPyVs were detected by PCR in 9 samples. HPyVs were detected by qPCR in 5 samples. The distribution of the various markers in discrete samples is summarized in Table 3. For most comparisons, presence-absence results matched in over 80% of samples (Table 3). More co-observations occurred for human-associated Bacteroides and HPyVs than other combinations of markers, and human-associated Bacteroides and adenoviruses were co-observed in 4.6% of all samples at Doheny Beach.
Table 3.
Co-occurrence of human-associated markers in the Doheny and Avalon Beach samples
| Beach | Marker A | Marker B | % matching results | No. of samples positive |
|
|---|---|---|---|---|---|
| Marker A | Marker B | ||||
| Doheny | HPyVsa | M. smithii | 95.4 | 5 | 1 |
| HPyVsa | HPyVsb | 93.9 | 5 | 9 | |
| M. smithii | Adenovirus | 93.8 | 1 | 9 | |
| HPyVsb | M. smithii | 92.3 | 9 | 1 | |
| HPyVsa | Adenovirus | 90.8 | 5 | 9 | |
| HPyVsb | Adenovirus | 89.2 | 9 | 9 | |
| H-Bacc | Adenovirus | 83.1 | 25 | 9 | |
| H-Bac | M. smithii | 81.5 | 25 | 1 | |
| HPyVsb | H-Bac | 80.0 | 9 | 25 | |
| HPyVsa | H-Bac | 78.5 | 5 | 25 | |
| Avalon | M. smithii | Adenovirus | 98.3 | 2 | 0 |
| HPyVsa | HPyVsb | 87.5 | 21 | 22 | |
| HPyVsa | Adenovirus | 82.5 | 21 | 0 | |
| HPyVsb | Adenovirus | 81.7 | 22 | 0 | |
| HPyVsb | M. smithii | 81.7 | 22 | 2 | |
| HPyVsa | M. smithii | 80.8 | 21 | 2 | |
| H-Bac | Adenovirus | 65.0 | 42 | 0 | |
| H-Bac | M. smithii | 65.0 | 42 | 2 | |
| HPyVsb | H-Bac | 56.7 | 22 | 42 | |
| HPyVsb | H-Bac | 55.8 | 21 | 42 | |
HPyVs, human polyomaviruses detected by qPCR.
HPyVs, human polyomaviruses detected by PCR.
H-Bac, human-associated Bacteroides marker.
Bacterial water quality indicator concentrations at Avalon Beach sites.
One hundred twenty samples were collected from Avalon Beach over the entire study period, with 30 samples collected from each of the sites. The average concentrations of all FIB at each Avalon Beach site are summarized in Fig. 2B. Enterococcus, fecal coliform, and total coliform concentrations at site C were significantly greater than those at sites A and D (P < 0.01). The concentrations of enterococci at site C exceeded regulatory standards in 60.0% of the samples. Fecal coliform concentrations at site C exceeded regulatory standards in 10.0% of the samples. Enterococcus and total coliform concentrations at sites A and B were not significantly different; however, fecal coliform concentrations at site B were significantly higher than those at site A (P < 0.05). The concentrations of enterococci and fecal coliforms at site B exceeded regulatory standards in 46.7% and 13.3% of samples, respectively. The concentrations of total coliforms exceeded 10,000 CFU · 100 ml−1 in 6.7% of the samples. At site A, fecal and total coliform concentrations did not exceed regulatory limits, while enterococcus concentrations that exceeded regulatory standards were observed in only 20.0% of the samples. The average concentrations of all FIB at site D were significantly lower than those at all other Avalon Beach sites (P < 0.001). Concentrations that exceeded regulatory standards were not noted for any bacterial indicators at site D.
Correlations of bacterial indicators among Avalon Beach sites.
The concentrations of enterococci at site D were negatively correlated with those at site A (r = −0.4813, R2 = 0.2316). No significant correlations were found for fecal coliforms among the Avalon Beach sites. Total coliform concentrations were positively correlated at sites B and C (r = 0.4171, R2 = 0.1739). In addition, the concentrations of enterococci, fecal coliforms, and total coliforms were positively correlated with each other at sites A, B, and C (r = 0.5431 to 0.9239, R2 = 0.3404 to 0.5315). At site D, only fecal and total coliform concentrations were significantly correlated (r = 0.6211, R2 = 0.3858).
qPCR detection of HPyVs at Avalon Beach sites.
HPyVs were detected by qPCR at every Avalon Beach site (Fig. 3B). HPyVs were detected 7 times at site C, 6 times at site B, and 4 times at both sites A and D. The quantities of HPyVs detected ranged from 50 to 35,481 copies · 100 ml−1. There were no significant differences in the HPyV copy numbers detected among the sites. In addition, there were no significant correlations of HPyV copy numbers among the sites.
PCR detection of human-associated water quality indicators at Avalon Beach sites.
The frequency of human marker detection at each site is summarized in Fig. 3B. HPyVs were detected by PCR in 7 samples at site B, 6 samples at site C, 5 samples at site D, and 4 samples at site A. Human-associated Bacteroides organisms were detected in 13 samples at site A, 12 samples at site B, 11 samples at site C, and 6 samples at site D. The M. smithii marker was detected once at sites B and D and was not detected at either site A or D. Adenovirus was not detected at any Avalon Beach site. The frequency of human-associated Bacteroides detection was significantly greater than that of HPyV, M. smithii, and adenovirus detection (P < 0.01). The frequency of HPyV detection was significantly greater than that of M. smithii and adenovirus detection (P < 0.01).
Relationships among indicators and markers at Avalon Beach.
Unlike the Doheny Beach sites, there were no significant relationships between indicators or human-associated markers at any of the sites at Avalon Beach. When FIB concentrations and marker detection at all Avalon Beach sites were compiled, enterococcus concentrations were strongly correlated with those of fecal coliforms (r = 0.7282, R2 = 0.5303) and total coliforms (r = 0.6277, R2 = 0.3940). Fecal coliform concentrations were also strongly correlated with total coliform concentrations (r = 0.8926, R2 = 0.7967). The presence of the human-associated Bacteroides marker was weakly correlated with the concentrations of fecal coliforms (Nagelkerke's R2 = 0.074, P = 0.010; odds ratio = 1.827, P = 0.014) and total coliforms (Nagelkerke's R2 = 0.061, P = 0.020; odds ratio = 1.774, P = 0.027). HPyV detection by PCR and HPyV copy numbers were significantly correlated (Nagelkerke's R2 = 0.361, P < 0.001; odds ratio = 2.777, P < 0.0005). All correlations among analytes are summarized in Table 2.
Overall, human-associated Bacteroides organisms were the most frequently detected at all the Avalon Beach sites (n = 42). HPyVs were detected by PCR in 22 samples, M. smithii was detected in 2 samples, and adenoviruses were not detected at any site. The co-occurrence of human markers and pathogens is summarized in Table 3.
Comparison of Doheny and Avalon Beach indicators and markers.
The concentrations of enterococci, fecal coliforms, and total coliforms were not significantly different between Doheny and Avalon Beaches. In contrast, HPyV copy numbers were significantly larger at Avalon Beach (P = 0.0006). In addition, the frequency of PCR detection of HPyVs was significantly higher at Avalon Beach sites (P = 0.0181). The frequency of human-associated Bacteroides and M. smithii detection was not significantly different between Avalon and Doheny Beaches. Adenovirus was not detected at any Avalon Beach site, and therefore, statistical analysis could not be performed; however, it was detected in 9 out of 130 samples at Doheny Beach, indicating a higher frequency of occurrence than at Avalon Beach.
For samples in which MST markers were detected, the frequency of samples meeting FIB regulatory standards was calculated (Table 4). Both enterococcus and fecal coliform concentrations were consistently below regulatory standards (104 CFU/ml and 400 CFU/ml, respectively) when human markers were detected in samples. In Doheny Beach samples, fecal coliform concentrations met regulatory standards in 60 to 100% of the samples in which adenovirus, HPyVs, and human-associated Bacteroides were detected, whereas enterococcus concentrations met regulatory standards in 36 to 80% of the samples positive for the three human markers. When M. smithii was detected at Doheny Beach (n = 1), FIB levels in the sample exceeded regulatory standards for both enterococci and fecal coliforms. In Avalon Beach samples, fecal coliform concentrations met regulatory standards in 76 to 100% of the samples that were positive for HPyVs, human-associated Bacteroides, or M. smithii. Enterococcus concentrations met regulatory standards in a smaller percentage (57 to 100%) of the samples positive for at least one human marker (Table 4).
Table 4.
Frequency of samples positive for human-specific markers and adenoviruses for which concentrations in water met FIB regulatory standards
| Beach | Marker | Frequency of detection in samples not exceeding regulatory standards for: |
|
|---|---|---|---|
| Enterococci (<104 CFU/ml) | Fecal coliforms (<400 CFU/ml) | ||
| Doheny | Adenovirus | 0.44 | 0.78 |
| H-Baca | 0.36 | 0.60 | |
| HPyVsb | 0.78 | 1.00 | |
| HPyVsc | 0.80 | 1.00 | |
| M. smithii | 0 | 0 | |
| Avalon | Adenovirus | NAd | NA |
| H-Bac | 0.62 | 0.76 | |
| HPyVsb | 0.59 | 0.89 | |
| HPyVsc | 0.57 | 0.81 | |
| M. smithii | 1.00 | 1.00 | |
H-Bac, human-associated Bacteroides marker.
HPyVs, human polyomaviruses detected by PCR.
HPyVs, human polyomaviruses detected by qPCR.
NA, marker was not detected in any samples.
DISCUSSION
This study is among the first to compare the magnitude and frequency of observation of culturable FIB, multiple MST markers, and a viral pathogen in recreational waters. Among the most important findings were the correlations of human-associated MST markers with each other and with adenovirus detection. The Doheny State Beach sites were distinguishable in terms of FIB concentrations, the frequency of MST markers, and pathogen detection. The San Juan Creek Lagoon (site C) just upstream of the discharge to the ocean (site D) consistently had the highest concentrations of indicator bacteria, which were highly correlated with the observations at the nearest beach site (site D). Despite the high concentrations of FIB at sites C and D, adenoviruses and HPyVs were detected at a higher frequency at the northern beach sites (sites A and B), and the presence and concentration of the HPyV marker were the most strongly correlated with the presence of adenoviruses at site B. In addition, the presence of human-associated Bacteroides, HPyVs, and adenovirus co-occurred in 2 of the 26 samples collected at site B but not at any other Doheny Beach site.
The nearshore water sites at north Doheny Beach are shallow and commonly used by children. Craun et al. (17) compiled sources of recreational water contamination leading to waterborne illness outbreaks and reported that 25% of the outbreaks were attributed to children in diapers and 34% were due to bather overcrowding (17), which could be a factor in the more frequent detection of viruses at sites A and B. On the other hand, Bacteroides HF183 was detected the most frequently at site C (lagoon) and the adjacent beach site, site D. The epidemiology study conducted in conjunction with this study found that Enterococcus concentrations at Doheny Beach were higher when the berm that periodically blocks the lagoon's entry to the ocean was open and that more relationships between the frequency of highly credible gastrointestinal illness and various methods for enumerating FIB (including qPCR) were found when the berm was open (15). These findings certainly implicate San Juan Creek as an important, but not the only, source of FIB and pathogens at Doheny Beach.
At Avalon Beach, fecal coliform concentrations did not show any geographic relationship (correlation of values between proximal sites). The same observation was true of enterococci. This lack of correlation suggests a separate or disproportionate fecal input(s) that affects each site individually and/or a greater level of mixing of the waters at Avalon Beach than at Doheny Beach. At least two of the three human markers were frequently detected at the Avalon Beach sites (sites A to C). The frequency of human marker detection at these sites strongly suggests contamination from a human source(s). While adenoviruses were not detected at any Avalon Beach site, the relatively small volume of sample analyzed (500 to 1,000 ml) may have limited the detection frequency. Jiang et al. (29) suggest concentrating adenoviruses from 20 liters of water for a representative analysis of indirect anthropogenic input (e.g., leaking sewers). However, the absence of the adenovirus marker and the infrequent detection of the M. smithii marker may be due to differential decay rates of the organisms under environmental conditions and/or may indicate human fecal pollution from a noncommunal source (e.g., septic tanks or boat discharge), because both adenoviruses and M. smithii are excreted by a minority of the population and are less likely to be detected in noncommunity wastes (38).
The Avalon Beach control site (site D), chosen for its historically low levels of fecal bacteria, maintained low levels of culturable FIBs which did not exceed regulatory standards. However, human-associated Bacteroides, M. smithii, and HPyVs were detected by PCR at this site. Moreover, all three markers were simultaneously detected in one of the samples. The detection of the human-associated markers coupled with the low levels of culturable bacterial indicators indicates the disconnect between FIB levels and contamination with human sewage under certain conditions. The low levels of FIB and frequent human-associated marker detection may indicate minimal fecal contributions from wildlife and other natural sources and a strong human fecal input(s). However, recent sewage contamination would result in high levels of indicator bacteria. The relatively low FIB concentrations may be caused by the general inability of fecal bacteria to persist in a culturable state under conditions of high salinity and exposure to solar radiation (7).
Overall, the human-associated Bacteroides HF183 marker was the most frequently detected indicator of human sewage at both Doheny and Avalon Beach sites. This marker also showed the highest co-occurrence with the adenovirus marker detected at the Doheny Beach sites; however, since the human-associated Bacteroides marker was detected at the highest frequency, the incidence of coabsence with adenovirus was the lowest among all the markers (Table 3). Recent studies have documented the detection of the human-associated Bacteroides marker (HF183) in a small percentage of animal fecal samples, including those of chickens, cats, and particularly dogs, indicating the incomplete specificity of this marker (23, 38, 50). Although dogs are not allowed on the beach, Doheny State Beach Park is dog friendly and also maintains a large seagull population near San Juan Creek (John F. Griffith, personal communication). However, the levels of human-associated Bacteroides spp. in raw sewage are several orders of magnitude higher than those of the other human-associated markers used here, giving the marker a potentially higher sensitivity (23). The presence of the human-associated Bacteroides marker should therefore be interpreted carefully, preferably in a quantitative format (16, 33, 34) and in conjunction with other human-associated markers.
Recent studies have also reported that the human specificity of the M. smithii assay is less than 100% (although it is well over 90%) (23, 38). Despite this caveat, the M. smithii marker is ubiquitous in sewage; for instance, Harwood et al. (23) reported the detection of M. smithii in 10−3 and 10−4 dilutions of sewage and in all sewage samples collected from south Florida, northwest Florida, and Mississippi (23). Throughout this study, the M. smithii marker was infrequently detected. Compared to the human-associated Bacteroides marker, which has been detected in sewage diluted to as low as 10−6 (23), the M. smithii marker is relatively insensitive. However, the M. smithii and adenovirus markers had the highest percentage of matching results at both beaches, although the majority of the matching results were coabsences.
The presence of HPyVs detected by PCR and the quantity of HPyVs detected by qPCR were highly correlated at both Doheny and Avalon Beaches, which is not surprising, considering that both assays utilize the same primers. However, agreement among the methods in terms of presence or absence was less than 100%. We hypothesize that the discrepancies in detection frequency can be attributed to the different template volumes used in each assay (2 μl was used in the qPCR assay and 5 μl was used in the PCR assay). The interpretation of HPyV data for recreational water quality assessment may be influenced by the fact that these viruses are excreted in urine; however, the high percentage of analogous results with adenovirus at Doheny Beach (90.2%) and the correlation with adenovirus detection suggest that HPyVs can be predictive of human health risks.
The presence of adenoviruses is a direct assessment of human health risks; however, only a small percentage of the population excretes these viruses (30), which can lead to inconsistent detection in sewage-impacted waters. The lack of adenovirus detection at the Avalon Beach sites when the three human MST markers were present demonstrates that the absence of adenoviruses does not necessarily imply a lack of human health concerns. Therefore, it is recommended that the adenovirus marker be used in conjunction with other pathogen assays and MST markers for detection of sewage contamination.
The potential of HPyVs to be excreted in the urine of swimmers and the incomplete specificity of both the human-associated Bacteroides and M. smithii markers can mean ambiguous results when only one marker is detected. However, the predictive power of each marker is increased when more than one marker is detected at the same site. For all markers utilized in this study, an epidemiological study assessing the human health risks associated with the presence or absence of the marker would more precisely define the usefulness of each assay. In addition, determining the concentrations of each marker by qPCR in future studies may provide a better understanding of the relationships of one marker with other MST markers and waterborne pathogens and ultimately provide a better perspective on the proportion of microbial contamination from human sources.
This study has provided insight on the usefulness of standard, culture-dependent methods for measurements of FIB and several human-associated microbial source tracking markers to assess water quality at beaches impacted by nonpoint and point source pollution. Determining water quality is a complex assessment of various indicators, and careful consideration of the location, climate, historical water quality data, and possible sources of contamination should be made before water quality indicators are selected. In addition, we strongly recommend the use of a multi-indicator toolbox approach when assessing water quality.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the Southern California Coastal Research Project (SCCWRP) personnel involved with the sample collection. We also thank John Paul for the use of the ABI 7500 instrument. In addition, we thank Jenny Delaney, Dave John, Lauren McDaniel, Robert Ulrich, Beth Young, and Brian Zielinski for technical and logistical support.
Funding for this study was provided in part by the U.S. Environmental Protection Agency Gulf of Mexico Alliance Regional Partnership Projects MX-96478707-0.
Footnotes
Published ahead of print 6 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Agostini HT, et al. 2001. Genotypes of JC virus in east, central and southwest Europe. J. Gen. Virol. 82:1221–1331 [DOI] [PubMed] [Google Scholar]
- 2. Agostini HT, Ryschkewitsch CF, Stoner GL. 1996. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J. Clin. Microbiol. 34:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed W, Sidhu JP, Toze S. 2012. Evaluation of the nifH gene marker of Methanobrevibacter smithii for the detection of sewage pollution in environmental waters in southeast Queensland, Australia. Environ. Sci. Technol. 46:543–550 [DOI] [PubMed] [Google Scholar]
- 4. Ahmed W, Stewart J, Powell D, Gardner T. 2008. Evaluation of Bacteroides markers for the detection of human faecal pollution. Lett. Appl. Microbiol. 46:237–242 [DOI] [PubMed] [Google Scholar]
- 5. Ahmed W, Wan C, Goonetilleke A, Gardner T. 2010. Evaluating sewage-associated JCV and BKV polyomaviruses for sourcing human fecal pollution in a coastal river in southeast Queensland, Australia. J. Environ. Qual. 39:1743–1750. [DOI] [PubMed] [Google Scholar]
- 6.American Public Health Association 1998. Standard methods for the examination of water and wastewater, 20th ed, p 9.137–9.141 American Public Health Association, Washington, DC [Google Scholar]
- 7. Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernhard AE, Field KG. 2000. A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl. Environ. Microbiol. 66:4571–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boehm AB, Fuhrman JA, Mrse RD, Grant SB. 2003. Tiered approach for identification of a human fecal pollution source at a recreational beach: case study at Avalon Bay, Catalina Island, California. Environ. Sci. Technol. 37:673–680 [DOI] [PubMed] [Google Scholar]
- 10. Bofill-Mas S, et al. 2006. Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl. Environ. Microbiol. 72:7894–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bofill-Mas S, Pina S, Girones R. 2000. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl. Environ. Microbiol. 66:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bond JH, Jr, Engel RR, Levitt MD. 1971. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J. Exp. Med. 133:572–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reference deleted. [Google Scholar]
- 14. Chapron CD, Ballester NA, Fontaine JH, Frades CN, Margolin AB. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colford JM, et al. 2012. Using rapid indicators for Enterococcus to assess the risk of illness after exposure to urban runoff contaminated marine water. Water Res. 46:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Converse RR, Blackwood AD, Kirs M, Griffith JF, Noble RT. 2009. Rapid QPCR-based assay for fecal Bacteroides spp. as a tool for assessing fecal contamination in recreational waters. Water Res. 43:4828–4837 [DOI] [PubMed] [Google Scholar]
- 17. Craun GF, Calderon RL, Craun MF. 2005. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 15:243–262 [DOI] [PubMed] [Google Scholar]
- 18. Del Valle L, et al. 2004. Primary central nervous system lymphoma expressing the human neurotropic polyomavirus, JC virus, genome. J. Virol. 78:3462–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dick LK, et al. 2005. Host distributions of uncultivated fecal Bacteroidales bacteria reveal genetic markers for fecal source identification. Appl. Environ. Microbiol. 71:3184–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dick LK, Field KG. 2004. Rapid estimation of numbers of fecal Bacteroidetes by use of a quantitative PCR assay for 16S rRNA genes. Appl. Environ. Microbiol. 70:5695–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gourmelon M, et al. 2007. Evaluation of two library-independent microbial source tracking methods to identify sources of fecal contamination in French estuaries. Appl. Environ. Microbiol. 73:4857–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grant SB, et al. 2002. Sources of surf zone water impairment in Avalon Bay, Catalina Island, California. Technical report. City of Avalon, Avalon, CA [Google Scholar]
- 23. Harwood VJ, et al. 2009. Validation and field testing of library-independent microbial source tracking methods in the Gulf of Mexico. Water Res. 43:4812–4819 [DOI] [PubMed] [Google Scholar]
- 24. Harwood VJ, et al. 2005. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71:3163–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harwood VJ, Whitlock J, Withington V. 2000. Classification of antibiotic resistance patterns of indicator bacteria by discriminant analysis: use in predicting the source of fecal contamination in subtropical waters. Appl. Environ. Microbiol. 66:3698–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holdeman LV, Good IJ, Moore WE. 1976. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl. Environ. Microbiol. 31:359–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hundesa A, Maluquer de Motes C, Bofill-Mas S, Albinana-Gimenez N, Girones R. 2006. Identification of human and animal adenoviruses and polyomaviruses for determination of sources of fecal contamination in the environment. Appl. Environ. Microbiol. 72:7886–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenkins MW, Tiwari S, Lorente M, Gichaba CM, Wuertz S. 2009. Identifying human and livestock sources of fecal contamination in Kenya with host-specific Bacteroidales assays. Water Res. 43:4956–4966 [DOI] [PubMed] [Google Scholar]
- 29. Jiang S, Noble R, Chu W. 2001. Human adenoviruses and coliphages in urban runoff-impacted coastal waters of Southern California. Appl. Environ. Microbiol. 67:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang SC. 2006. Human adenoviruses in water: occurrence and health implications: a critical review. Environ. Sci. Technol. 40:7132–7140 [DOI] [PubMed] [Google Scholar]
- 31. Johnston C, Ufnar JA, Griffith JF, Gooch JA, Stewart JR. 2010. A real-time qPCR assay for the detection of the nifH gene of Methanobrevibacter smithii, a potential indicator of sewage pollution. J. Appl. Microbiol. 109:1946–1956 [DOI] [PubMed] [Google Scholar]
- 32. Jones B, Bogucki D. 2002. Bay circulation and exchange. Technical report. City of Avalon, Avalon, CA [Google Scholar]
- 32a. Katagi W, Johnson T, Sutherland G. 2008. Steelhead recovery in the San Juan and Trabuco Creeks Watershed, section 3—watershed conditions. In Proceedings of the American Society of Civil Engineers Research and History Symposium, Honolulu, HI. CDM Smith, Cambridge, MA [Google Scholar]
- 33. Kildare BJ, et al. 2007. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: a Bayesian approach. Water Res. 41:3701–3715 [DOI] [PubMed] [Google Scholar]
- 34. Layton A, et al. 2006. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 72:4214–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin C, Miller TL. 1998. Phylogenetic analysis of Methanobrevibacter isolated from feces of humans and other animals. Arch. Microbiol. 169:397–403 [DOI] [PubMed] [Google Scholar]
- 36. Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. 1993. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 167:13–20 [DOI] [PubMed] [Google Scholar]
- 37. McQuaig SM, Scott TM, Harwood VJ, Farrah SR, Lukasik JO. 2006. Detection of human derived fecal pollution in environmental waters using a PCR based human polyomavirus assay. Appl. Environ. Microbiol. 72:7567–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McQuaig SM, Scott TM, Lukasik JO, Paul JH, Harwood VJ. 2009. Quantification of human polyomaviruses JC virus and BK virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl. Environ. Microbiol. 75:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moore D, Ferguson D, Gonzalez E. 2009. San Juan Creek watershed bacterial study: final report. Orange County Public Health Laboratory, Santa Ana, CA [Google Scholar]
- 40.Natural Resources Defense Council 2009. California. In Testing the water 2009. Natural Resources Defense Council, New York, NY: http://www.nrdc.org/water/oceans/ttw/sumcal.pdf [Google Scholar]
- 41. Noble RT, et al. 2003. Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial source tracking comparison study. J. Water Health 1:195–207 [PubMed] [Google Scholar]
- 42. Noble RT, et al. 2006. Multitiered approach using quantitative PCR to track sources of fecal pollution affecting Santa Monica Bay, California. Appl. Environ. Microbiol. 72:1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okabe S, Okayama N, Savichtcheva O, Ito T. 2007. Quantification of host-specific Bacteroides-Prevotella 16S rRNA genetic markers for assessment of fecal pollution in freshwater. Appl. Microbiol. Biotechnol. 74:890–901 [DOI] [PubMed] [Google Scholar]
- 44.Orange County 2003. San Juan Creek Watershed Chapter. In Drainage area management plan. Orange County Public Health Laboratory, Santa Ana, CA [Google Scholar]
- 45. Pavesi A. 2005. Utility of JC polyomavirus in tracing the pattern of human migrations dating to prehistoric times. J. Gen. Virol. 86:1315–1326 [DOI] [PubMed] [Google Scholar]
- 46. Pina S, Puig M, Lucena F, Jofre J, Girones R. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Polo C, et al. 2004. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin. Microbiol. Infect. 10:640–644 [DOI] [PubMed] [Google Scholar]
- 48. Santo Domingo JW, Bambic DG, Edge TA, Wuertz S. 2007. Quo vadis source tracking? Towards a strategic framework for environmental monitoring of fecal pollution. Water Res. 41:3539–3552 [DOI] [PubMed] [Google Scholar]
- 49. Seurinck S, Defoirdt T, Verstraete W, Siciliano SD. 2005. Detection and quantification of the human-specific HF183 Bacteroides 16S rRNA genetic marker with real-time PCR for assessment of human faecal pollution in freshwater. Environ. Microbiol. 7:249–259 [DOI] [PubMed] [Google Scholar]
- 50. Shanks OC, et al. 2010. Performance of PCR-based assays targeting Bacteroidales genetic markers of human fecal pollution in sewage and fecal samples. Environ. Sci. Technol. 44:6281–6288 [DOI] [PubMed] [Google Scholar]
- 51.South Orange County Wastewater Authority 2007. Agency boundary map. South Orange County Wastewater Authority, Dana Point, CA [Google Scholar]
- 52.South Orange County Wastewater Authority 2005. San Juan Creek Outfall. South Orange County Wastewater Authority, Dana Point, CA [Google Scholar]
- 53. Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. 2003. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 84:1499–1504 [DOI] [PubMed] [Google Scholar]
- 54. Takasaka T, et al. 2004. Subtypes of BK virus prevalent in Japan and variation in their transcriptional control region. J. Gen. Virol. 85:2821–2827 [DOI] [PubMed] [Google Scholar]
- 55. Ufnar JA, et al. 2006. Detection of the nifH gene of Methanobrevibacter smithii: a potential tool to identify sewage pollution in recreational waters. J. Appl. Microbiol. 101:44–52 [DOI] [PubMed] [Google Scholar]
- 56.US Environmental Protection Agency 1997. Method 1600: membrane filter test methods for enterococci in water. Report EPA-821/R-97/004 US Environmental Protection Agency, Washington, DC [Google Scholar]
- 57. Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. 2005. Frequent detection of polyomaviruses in stool samples from hospitalized children. J. Infect. Dis. 192:658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogel JR, et al. 2007. Identifying fecal sources in a selected catchment reach using multiple source-tracking tools. J. Environ. Qual. 36:718–729 [DOI] [PubMed] [Google Scholar]
- 59. Wexler HM. 2007. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 20:593–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong AS, Cheng VC, Yuen KY, Kwong YL, Leung AY. 2009. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant. 43:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yun JJ, et al. 2006. Genomic DNA functions as a universal external standard in quantitative real-time PCR. Nucleic Acids Res. 34:e85 doi:10.1093/nar/gkl400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhong S, et al. 2009. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J. Gen. Virol. 90:144–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


