Abstract
Bacillus anthracis is one of the most dangerous potential biological weapons, and it is essential to develop a rapid and simple method to detect B. anthracis spores in environmental samples. The immunoassay is a rapid and easy-to-use method for the detection of B. anthracis by means of antibodies directed against surface spore antigens. With this objective in view, we have produced a panel of monoclonal antibodies against B. anthracis and developed colorimetric and electrochemiluminescence (ECL) immunoassays. Using Meso Scale Discovery ECL technology, which is based on electrochemiluminescence (ECL) detection utilizing a sulfo-Tag label that emits light upon electrochemical stimulation (using a dedicated ECL plate reader, an electrical current is placed across the microplate with electrodes integrated into the bottom of the plate, resulting in a series of electrically induced reactions leading to a luminescent signal), a detection limit ranging between 0.3 × 103 and 103 CFU/ml (i.e., 30 to 100 spores per test), depending on the B. anthracis strain assayed, was achieved. In complex matrices (5 mg/ml of soil or simulated powder), the detection level (without any sample purification or concentration) was never altered more than 3-fold compared with the results obtained in phosphate-buffered saline.
INTRODUCTION
Bacillus anthracis, the etiological agent of anthrax, is a Gram-positive bacterium capable of forming endospores when vegetative cells are deprived of an essential nutrient. The core of the B. anthracis spore is surrounded by several integuments, the outermost of which is the exosporium (12). Spores are highly resistant to temperature, pressure, and UV radiation and to a wide variety of chemical toxins (2, 25). These properties allow the spores to survive in soil for many years until an appropriate environment allows the spore to germinate and grow as vegetative cells (17). B. anthracis has long been recognized as a potential bioterrorism weapon, and since its use in the 2001 attack in the United States, there has been a growing need for a rapid and accurate test to detect B. anthracis spores.
Most current rapid tests are based on nucleic acid detection, which has the advantage of being specific and highly sensitive, with a detection limit of between 1 and 30 spores per reaction (1, 3, 11, 15). However, the main drawback of these methods is the need for a clean starting sample concentrated in a small volume. In addition, these technologies generally use sophisticated equipment reserved for laboratory analysis, although small hand-held PCR assays are now becoming available for field testing. Immunoassays based on detection of surface spore antigens can provide a first-line, easy-to-use, and rapid method for detection of B. anthracis spores. Specific immunodetection of B. anthracis spores is challenging because of possible cross-reactivity of the antibodies (Abs) with near-neighbor species such as Bacillus cereus and Bacillus thuringiensis. Antibodies against the exosporium (28), the vegetative cells (19), oligosaccharide epitopes of the BclA spore surface protein (16, 32, 33), and extracellular antigen I (EAI) have been raised and used in immunoassays for B. anthracis detection (35). B. anthracis spores were successfully detected by immunofluorescence and cytometry techniques (20, 21, 27), but not with high specificity, because polyclonal antibodies were used in both cases and these methods are not suitable for samples containing a small quantity of target spores overwhelmed by other organisms in a complex matrix such as soil. Few immunoassays have been evaluated for detection of B. anthracis spores in environmental samples (4, 10, 34). Using immunomagnetic beads, a detection limit of between 102 and 105 spores, depending on the strain, was achieved by Bruno et al., but assay sensitivity was compromised in the soil matrix (4). Sensitive detection of B. anthracis was reported for assays using an evanescent wave fiber-optic biosensor (34) and the integrating waveguide Biosensor (10), with detection limits of 4 × 104 and 104 spores/ml, respectively. For all these immunoassays, sensitivity and specificity are highly dependent on the antibodies used. Here we describe the production and characterization of new monoclonal antibodies (MAbs) raised against surface epitopes of the B. anthracis spore. The resulting sandwich immunoassay allowed sensitive and specific detection of B. anthracis spores. Using the A1 monoclonal antibody as the capture antibody and R93 MAb as the tracer antibody, colorimetric detection and electrochemiluminescence (ECL) detection were compared. Furthermore, the effect of different white powder matrices and soils on the detection of B. anthracis spores was evaluated.
MATERIALS AND METHODS
Monoclonal antibody production.
Three Biozzi mice were immunized by intraplantary injection of 107 formaldehyde-inactivated B. anthracis spores (incubated in 4% formaldehyde for 4 h at 37°C) from two strains (7702 Sterne and RA3R) in complete Freund adjuvant. At 4-week intervals, three subsequent injections were done with the same dose of spores. Two weeks after each injection, the immune response, i.e., the levels of anti-B. anthracis spore antibodies, was evaluated by enzyme-linked immunosorbent assay (ELISA) (see below). Mice with the highest ELISA titer were selected for preparation of monoclonal antibodies. Three days before fusion, selected mice received an intravenous injection of 107 spores. Spleen cells from mice were fused with myeloma NS1 cells as previously described (7). After fusion, in the first screening by ELISA using spore-coated plates, 80 of 870 (9.2%) hybridomas and 110 of 812 (13.5%) hybridomas appeared to react with 7702 and ra3R spores, respectively. Following subcloning by limiting dilution, 28 and 20 clones expressing anti-7702 spore MAbs and anti-RA3R spore MAbs, respectively, were stabilized.
Spore preparation.
B. anthracis strains (7702, RA3R, and 9602 strains), recombinant strain PF09 (7702 ΔbclA), B. cereus strains (B. cereus 569, B. cereus 9241, and B. cereus 10987), B. thuringiensis strains (B. thuringiensis 407 and B. thuringiensis 9727), and B. subtilis strain 168 were obtained from M. Mock (Institut Pasteur). The B. anthracis Vollum strain was from the Health Protection Agency Culture Collection.
Spores were prepared from NBY (nutrient broth yeast extract) agar incubated for 7 days at 30°C. After three washes in distilled water, spores were purified by differential centrifugation for 30 min at 6,000 × g at 4°C through layers of 45% to 55% Radioselectan (Schering) (76% renografin) prepared in distilled water and washed three times in cold distilled water (18).
Screening of hybridoma culture supernatants and spore ELISA.
The presence of antibody in the hybridoma culture supernatant was detected using ELISA. Briefly, 50 μl of 107 spores per ml in water was added to each well of a poly-l-lysine-pretreated 96-well Maxisorb (Nunc) plate and incubated overnight at 37°C. The plates were then saturated with enzyme immunoassay (EIA) buffer (0.1 M phosphate buffer [pH 7.4], 0.1% bovine serum albumin [BSA], 0.15 M NaCl, 0.001% sodium azide) for 4 h at room temperature. Then, 50 μl/well of hybridoma culture supernatant was added for reaction overnight at 4°C. After three washes, 50 μl/well of goat anti-mouse antibody covalently coupled to acetylcholinesterase (AChE) was added for 4 h at room temperature. After 6 washes, AChE activity was revealed by the colorimetric method of Ellman et al. (5).
Determination of binding complementarity for each pair of MAbs.
The binding of the different MAbs to B. anthracis spores was analyzed using immunometric tests, with one capture MAb immobilized on the solid phase and the other biotin-labeled MAb used as a tracer. Briefly, 100 μl of spore suspension (2 × 105 inactivated 7702 spores in duplicate) was added to a microtiter plate coated with one of the MAbs. After an 18-h incubation at 4°C, the plates were washed before addition of 100 μl of biotin-labeled MAb (0.5 μg/ml). After a 3-h reaction at 20°C, plates were washed and reacted with 100 μl of AChE-labeled streptavidin (2 Ellman units/ml) for 1 h at 20°C. Plates were then extensively washed, and solid-phase bound AChE was revealed by addition of 200 μl of Ellman's reagent.
Immunometric assays using MAb-biotin or MAb-AChE conjugates.
Immunometric assays were performed in 96-well Maxisorb immunoplates (Nunc) coated with purified antibody. Simultaneously, 100 μl of spore suspension and biotin-labeled or AChE-labeled MAb was loaded into the wells; plates were centrifuged at 1,500 × g for 5 min and reacted for 18 h or 2 h, respectively, at room temperature. In the case of biotin-labeled MAb, plates were washed and further reacted with 100 μl of AChE-labeled streptavidin (2 Ellman units/ml) for 1 h at 20°C. After several washes, plate results were revealed by addition of 200 μl of Ellman's reagent.
Electrochemiluminescence assays.
Standard 96-well Sector PR 100 plates (Meso Scale Discovery) were coated with 50 μl of MAb (80 μg/ml)–50 mM phosphate buffer (pH 7.4). After an 18-h incubation at 20°C, plates were washed and blocked with 5% BSA–phosphate-buffered saline (PBS) for 1 h. A 100-μl volume of spore suspension and 20 μl of ruthenium-labeled MAb (prepared with Meso Scale Discovery Sulfo-TAG N-hydroxysuccinimide [NHS]-ester according to the manufacturer's instructions [Meso Scale Discovery]) were loaded into the wells, centrifuged at 1,500 × g for 5 min, and reacted for 2 h at room temperature. The wells were washed 5 times in 50 mM phosphate buffer (pH 7.4), and 150 μl of 1× T buffer (Meso Scale Discovery) was added to each well. The plates were read with a Meso Scale Discovery PR100 plate reader.
Western blot analysis of spore extracts and BclA protein.
Spores (109) were heated for 15 min at 90°C with 50 μl of extraction buffer (50 mM Tris HCl [pH 10], 8 M urea, 2% 2-mercaptoethanol) and centrifuged at 13,000 rpm for 10 min. The supernatant was diluted 10 times in Laemmli buffer, heated for 5 min at 100°C, and electrophoresed using 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% nonfat dry milk and then probed with 1 μg/ml of anti-Bacillus anthracis MAbs. Goat anti-mouse immunoglobulin coupled to horseradish peroxidase was used as a secondary antibody at a 1/500 dilution, and peroxidase activity was detected by chemiluminescence (ECL Plus Western Blotting Detection system; GE Healthcare-Amersham).
For BclA analysis, the polyhistidine-tagged recombinant BclA protein was produced in Escherichia coli strain M15(pREP4) and purified using a nickel-nitrilotriacetic acid (Ni-NTA) affinity column (chelating Sepharose Fast Flow; GE Healthcare).
Limit of detection of anti-B. anthracis spore immunoassays.
The four-parameter logistic (4-PL) function was used to model the characteristic curve for the immunoassays. The limit of detection was calculated by interpolating the average background signal (measured in eight wells) plus 3 standard deviations on the standard curve.
RESULTS
Characterization of monoclonal antibodies to B. anthracis spores.
A total of 28 and 20 hybridoma clones expressing monoclonal antibodies against B. anthracis 7702 spore (MAbs of the A series) and B. anthracis RA3R spore (MAbs of the R series), respectively, were stabilized, and antibodies were produced (see Materials and Methods).
MAb specificity was first characterized using ELISA to test the capacity of MAbs to bind a panel of selected strains from the Bacillus cereus group and Bacillus subtilis: 3 B. anthracis spore strains, a BclA deletion mutant of strain 7702 (PF09), 3 B. cereus and 2 B. thuringiensis spore strains very closely related to B. anthracis species, and the B. subtilis 168 strain (see Materials and Methods and Table 1). B. subtilis spores differ structurally from those of the B. cereus group, since the spores are not surrounded by the exosporium. All the strains were formaldehyde treated before being tested with biotinylated antibodies by ELISA (see Materials and Methods).
Table 1.
Immunoreactivity of anti-Bacillus anthracis MAbs with closely related inactivated strains from B. cereus, B. thuringiensis, and B. subtilisa
| Group | MAb |
B. anthracis |
B. cereus |
B. thuringiensis |
B. subtilis Bs 168 | WB BclA | Isotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba 9602 | Ba 7702 | Ba RA3R | Ba PF09 | Bc 569 | Bc 9241 | Bc 10987 | Bt 407 | Bt 9727 | |||||
| I | R12 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | +++ | + | − | IgM |
| R-73 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | − | IgM | |
| A6 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | − | IgG | |
| R94 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | − | IgM | |
| A20 | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − | IgG | |
| A23 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | − | IgG | |
| A40 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | − | IgG | |
| A77 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | +++ | ++ | − | IgG | |
| II | A49 | ++ | ++ | + | +++ | +++ | +++ | + | ++ | +++ | − | − | IgM |
| A65 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | − | − | IgM | |
| A70 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | − | − | IgG | |
| III | R31 | +++ | +++ | +++ | +++ | − | +++ | +++ | − | +/− | − | − | IgM |
| R44 | +++ | +++ | +++ | +++ | − | +++ | − | − | +/− | − | − | IgM | |
| R61 | +++ | +++ | +++ | +++ | − | +++ | − | − | +/− | − | − | IgM | |
| R63 | +++ | +++ | +++ | +++ | − | +++ | − | − | +/− | − | − | IgM | |
| R71 | +++ | +++ | +++ | +++ | − | +++ | − | − | − | − | − | IgM | |
| R97 | +++ | +++ | +++ | +++ | − | +++ | − | − | +/− | − | − | IgM | |
| R109 | +++ | +++ | +++ | +++ | − | +++ | ++ | − | +/− | − | − | IgM | |
| IV | R16 | − | − | + | − | − | − | − | − | − | − | − | IgG |
| R45 | − | − | +++ | − | + | ++ | +++ | − | +++ | − | − | IgM | |
| R68 | − | − | +++ | − | − | − | +++ | − | + | − | − | IgM | |
| V | A39 | +++ | +++ | +++ | − | +++ | − | + | +++ | +++ | − | + | IgG |
| R30 | +++ | ++ | +++ | − | + | ++ | +++ | + | + | − | + | IgG | |
| R74 | +++ | +++ | +++ | − | +++ | +++ | ++ | +++ | +++ | − | + | IgG | |
| A1 | +++ | ++ | +++ | − | + | ++ | + | + | ++ | − | + | IgG | |
| A12 | ++ | + | +++ | − | + | +++ | +++ | + | + | − | + | IgG | |
| A18 | ++ | + | + | − | ++ | + | ++ | + | + | − | + | IgG | |
| A53 | ++ | ++ | ++ | − | + | ++ | +++ | + | ++ | − | + | IgG | |
| A55 | +++ | +++ | +++ | − | + | ++ | +++ | ++ | ++ | − | + | IgG | |
| A80 | +++ | ++ | +++ | − | + | ++ | +++ | + | ++ | − | + | IgG | |
| VI | R54 | ++ | ++ | +++ | − | − | − | − | + | ++ | − | + | IgG |
| R78 | ++ | +++ | +++ | − | − | − | ++ | − | +++ | − | + | IgG | |
| A15 | +++ | +++ | +++ | − | + | − | − | − | +++ | − | + | IgG | |
| VII | R13 | ++ | ++ | ++ | − | − | − | − | − | ++ | − | + | IgG |
| R17 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| R93 | +++ | ++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A3 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A4 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A7 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A8 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A14 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A36 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A42 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A44 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A50 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A52 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A69 | +++ | +++ | +++ | − | − | − | − | − | +++ | − | + | IgG | |
| A71 | +++ | ++ | ++ | − | − | − | − | − | +++ | − | + | IgG | |
Reaction of 0.5 μg/ml of MAbs using ELISA (see Materials and Methods). −, negative signals; +, positive by Western blot analysis or signals > 0.1 absorbance units by ELISA; ++, signals > 0.3 absorbance units by ELISA; +++, signals > 0.5 absorbance units by ELISA. Isotype (IgM or IgG) and reactivity of monoclonal antibodies with BclA by Western blot analysis are indicated.
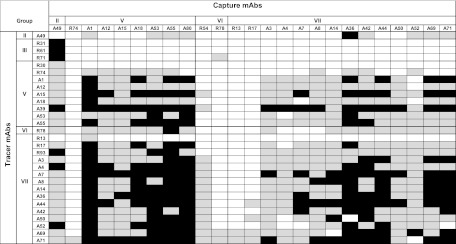
These experiments classified the 48 MAbs into 7 groups as shown in Table 1. Group I included 8 MAbs that showed a complete lack of specificity, since they bound all bacillus strains tested, including B. subtilis spores. Group II (3 MAbs) also exhibited broad recognition of the Bacillus genus, with the exception of B. subtilis. Groups III and IV comprised 7 and 3 MAbs, respectively, all resulting from the immunization with strain RA3R. Group III bound strongly to the 4 B. anthracis strains and to B. cereus strain 9241. Some of these MAbs may recognize B. thuringiensis 9727 and/or B. cereus 10987. Group IV recognized only RA3R from the 4 B. anthracis strains and also some of the B. cereus strains and B. thuringiensis strain 9727. The 27 remaining MAbs (groups V to VII) bound wild-type strain 7702 but not BclA mutant strain PF09, suggesting that they recognize the BclA protein. Interestingly, all these MAbs were IgG whereas IgM appeared to predominate in the 4 other groups. Western blot experiments with exosporium extracts of strains 7702 and PF09 and the BclA recombinant protein further demonstrated this clear difference in reactivity between the 21 MAbs of groups I to IV and the 27 MAbs of groups V to VII. As illustrated in Fig. 1 for MAb A36 (representative of group 6 and 7 MAbs), only the 27 antibodies binding strain 7702 and not strain PF09 recognized both the BclA protein present at the surface of spores and the recombinant BclA (Table 1), indicating that these antibodies are directed more precisely against the protein part of BclA at the spore surface. Among these antibodies, 15 (group VII) bound all the B. anthracis spores tested and only one of the related B. thuringiensis strains, strain 9727, which is phylogenetically very close to B. anthracis. This restricted specificity was not observed for group V, whose MAbs bound several B. cereus and B. thuringiensis strains, while group VI (3 MAbs) presented intermediate specificity.
Fig 1.
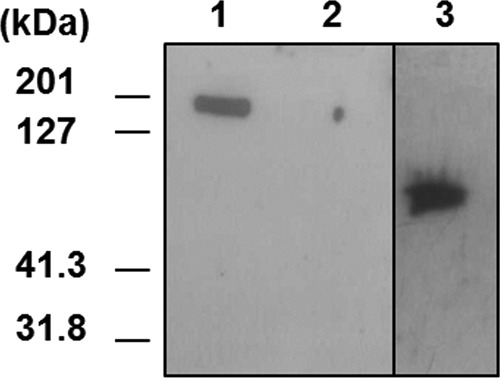
Reactivity of anti-spore MAbs with exosporium and recombinant BclA. Proteins were separated by SDS-PAGE and analyzed by immunoblotting with MAb 77A-36. Lane 1, exosporium extract from a B. anthracis (strain 7702) spore; lane 2, exosporium extract from BclA mutant strain PF09; lane 3, purified recombinant BclA.
Detection of B. anthracis spores by a two-site immunometric assay.
Complementary binding studies were performed to establish a two-site immunometric site assay for B. anthracis spores. The 2,304 combinations resulting from the 48 MAbs obtained were tested using a sequential procedure and 2 × 105 formaldehyde-inactivated 7702 spores. More than 571 combinations provided good detection, and 184 pairs appeared most efficient (signal > 0.2 absorbance units [AU] and > 0.5 AU, respectively, after 1 h of staining; see Fig. 2). It is worth noting that most of these combinations involved group V to VII MAbs. The best assays, those giving a good ratio of signal to noise, were further evaluated for a dilution series using strain 7702. Figure 3 shows the results for the eight more sensitive and specific combinations (18-h reaction). All these immunoassays had similar sensitivities of close to 104 CFU/ml. To optimize the assay, the combination that included MAb A1 as the capture antibody and MAb R93 labeled with AChE as the tracer was selected. The best sensitivity was obtained by using a simultaneous procedure (simultaneous reaction of the sample and the AChE-R93 tracer with the capture MAb). The formation of the immune complex was accelerated by performing a 5-min centrifugation step before a 2-h immunoreaction at room temperature. Figure 4 presents the standard curves obtained for live spores from B. anthracis, B. cereus, B. thuringiensis, and B. subtilis. Very good sensitivity was obtained for all B. anthracis strains tested, with a limit of detection between 103 and 2 × 103 CFU/ml, depending on the B. anthracis strain. As expected, the B. thuringiensis 9727 strain was also well detected, with a limit of detection close to 3 × 103 CFU/ml, whereas other non-B. anthracis strains were not detected even with a 107 CFU/ml concentration. The combination using MAb R45 as the capture antibody and AChE-labeled R-93 as the tracer allows the specific detection of strain RA3R versus the B. cereus and B. thuringiensis strains tested and 5% cross-reactivity with some other B. anthracis strains (data not shown). The detection limit was close to 5 × 103 CFU/ml.
Fig 2.
Colorimetric signal obtained for combinations of mAbs used as capture and tracer antibodies. Tests were performed with the inactivated 7702 strain (2 × 105 spores/ml). Black squares, signals > 0.2 absorbance units; light grey squares, signals > 0.05 absorbance units; white squares, low or nonspecific signals.
Fig 3.
Capacity of selected ELISA for the detection of B. anthracis spores. Different concentrations of inactivated B. anthracis Sterne spores were detected with various combinations of MAbs. In order to allow a direct comparison, the nonspecific binding of each pair of MAbs was subtracted. OD, optical density.
Fig 4.
Standard curves for various Bacillus strains in PBS–0.1% BSA buffer. Immunoassays were performed with A1 as the capture antibody and R93 as the AChE tracer antibody. The bacillus concentration range was 103 to 106 spores per ml. The inset shows the low-concentration part of the curve. The nonspecific binding of each MAbs was subtracted. OD, optical density.
Comparison of ECL assay with colorimetric ELISA.
To compare the two detection techniques, the same capture and tracer antibodies (A1 MAb and R93 MAb, respectively) were used. For colorimetric ELISA, the tracer antibody was coupled to AChE (one tetrameric AChE form for one antibody) (8), while for the ECL assay, the same antibody was coupled to ruthenium (see Materials and Methods). Both ELISA and ECL were optimized in terms of the concentration of capture antibody and the concentration of detection antibodies. For both assays, samples were reacted for 2 h at 20°C, with the solid-phase antibody and the tracer antibody being reacted simultaneously. After a washing step, the reaction solution was added and the plates were read 30 min afterward for the colorimetric assay and immediately for the ECL reagent. In both assays, we observed that a 5-min centrifugation step before the 2-h immunoreaction step increased the sensitivity by 1 order of magnitude (data not shown).
As shown in Fig. 5, the curves were mainly linear, allowing detection of the tested strains over a large concentration range. The slope of the ECL curve was greater. The limit of detection of the AChE-colorimetric assay ranged between 103 and 4 × 103 CFU/ml for the B. anthracis strain, while the ECL assay provided sensitivity ranging between 0.3 × 102 and 103 CFU/ml, thus appearing three to four times more sensitive than the colorimetric ELISA.
Fig 5.
Comparison of ELISA (A) and ECL assay (B) for the detection of various B. anthracis strains and B. thuringiensis strain 9727. Spores were diluted in EIA buffer and assayed using simultaneous procedures with a 2-h incubation time. The same pair of antibodies was used, with A1 as the capture antibody and R93 as the tracer antibody. The nonspecific binding of each MAb was subtracted. OD, optical density.
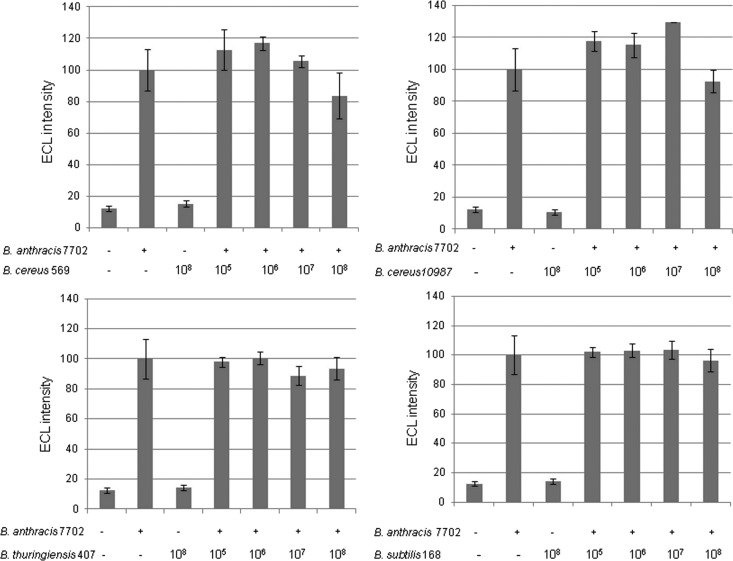
Detection of B. anthracis spores in the presence of other Bacillus strains as a masking agent.
The possible interference of other Bacillus strains in the detection was evaluated by mixing B. anthracis strain Sterne 7702 (at a final concentration of 104 CFU/ml) with various Bacillus spores at increasing concentrations from 105 to 108 CFU/ml. As shown in Fig. 6, the presence of low or high concentrations of spore from B. cereus, B. thuringiensis, or B. subtilis did not significantly affect the detection of the Sterne 7702 spores. In fact, the presence of such related spores induced only a 20% variation of the signal for a concentration of B. anthracis of 104 spores/ml.
Fig 6.
Detection of B. anthracis strain Sterne 7702 (+, 104 spores; −, no spore) in the presence of spores (concentration range, 105 to 108 spores per ml) from B. cereus, B. thuringiensis, and B. subtilis strains. Data represent the results of a Meso Scale Discovery immunoassay performed with A1 MAb as the capture antibody and R93 MAb as the AChE tracer.
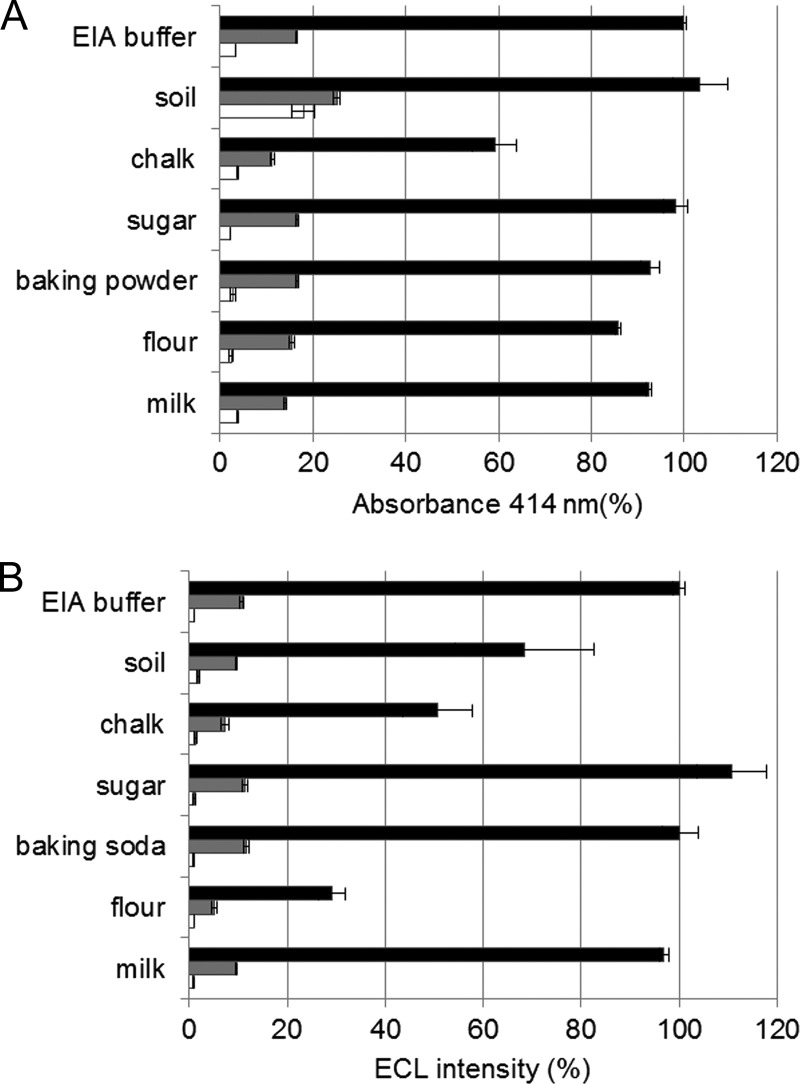
Spore detection in domestic powder and environmental samples.
Samples were prepared as a suspension of 5 mg/ml powder in PBS supplemented with 0.1% BSA. Dried milk, flour, baking soda, and chalk were selected as representative domestic powders, and soil was chosen as an environmental sample. The effect of these suspensions on the detection of 2.5 × 104 and 2.5 × 105 B. anthracis spores (7702 strain) was evaluated using both the AChE colorimetric ELISA and the ECL assay. As shown in Fig. 7, taking the results obtained for EIA buffer as a reference, few differences were observed for colorimetric ELISA, with a 40% decrease of the specific signal upon adding chalk and a 5-fold increase of the nonspecific binding observed with the soil solution. The effects appeared greater for the ECL assay, with large variations in specific signals for soil and chalk (30% and 50% decreases, respectively), while for flour only 30% of the specific signal remained. ECL detection thus seems more affected by the medium than the colorimetric enzyme assay. Similar results were obtained with the Ames and the Vollum B. anthracis strains (data not shown).
Fig 7.
Comparison of ELISA (A) and ECL assay (B) for the detection of B. anthracis strain Sterne 7702 spores spiked into various suspensions of domestic powders (chalk, sugar, baking soda, flour, and milk) and soil. Black bars, signals with 2 × 105 CFU/ml of B. anthracis spores; gray bars, signals with 2 × 104 CFU/ml of B. anthracis spores; white bars, signal in the absence of spore.
DISCUSSION
PCR is unambiguously the most sensitive of the rapid methods used to detect pathogens such as B. anthracis spores, reaching a sensitivity of 1 to 10 equivalent genomes per reaction. However, the application of PCR to environmental samples has many pitfalls due to its susceptibility to inhibitors and contamination, as outlined in recent papers (22–24). In addition, due to the rigid wall of bacillus spores, additional treatments are required for DNA isolation.
Immunoassays based on the detection of surface exosporium should be considered an alternative method for detection of B. anthracis spores, even if performing such assays is challenging, because of the presence of cross-reactive antigens in closely related strains, such as the B. cereus strains. In the present study, a large panel of 48 MAbs directed against the surface of spores from two B. anthracis strains, i.e., the Sterne 7702 and RA3R strains, was obtained. Several of the MAbs were highly specific for B. anthracis spores and recognized BclA, the major surface protein of the spore. This protein has a central collagen-like region formed by polymorphic GXX repeats that are strain specific (26, 29, 30). BclA is also found in B. cereus and B. thuringiensis but not in B. subtilis. Very specific and sensitive immunoassays for B. anthracis strains were developed, exhibiting cross-reactivity only with the very closely related B. thuringiensis 9727 strain. It is worth noting that, unlike the other B. thuringiensis strains, strain 9727 is associated with severe disease, and its pathogenicity has been confirmed by infection of both immunosuppressed and immunocompetent mouse (13, 14). Swiecki et al. (28) raised monoclonal antibodies against B. anthracis spores that all cross-reacted with B. thuringiensis strain 9727, which confirms the difficulty of obtaining antibodies that specifically recognize all B. anthracis strains. In the present study, B. anthracis strain RA3R was included because it belongs to subgroup B2, which is the most common subgroup in France but extremely rare elsewhere in the world (6).
To date, few colorimetric ELISAs that detect B. anthracis spores have been developed. Using antibodies directed against the anthrose tetrasaccharide, Kuehn et al. developed a specific and sensitive ELISA able to detect 1 × 104 to 5 × 104 inactivated B. anthracis CDC1014 spores/ml (16). The most sensitive spore detection performed by means of a two-site immunometric assay was achieved using Luminex technology with a detection limit of 103 to 104 spores per ml (31).
After optimization, the present colorimetric ELISA had a limit of detection ranging between 103 CFU/ml (strains Vollum, RA3R, and 9602) and 4 × 103 CFU/ml (strain 7702). Using our best pair of antibodies and the same assay conditions (time of incubation, centrifugation step), the ECL Meso Scale Discovery technology was three to four times more sensitive than ELISA, with a limit of detection of between 3 × 102 and 103 spores/ml (30 to 100 spores per well), making ECL the most sensitive immunoassay described so far for detection of B. anthracis spores. Similar differences between the two technologies in terms of sensitivity were reported for the detection of ricin B chain by Guglielmo and Thullier (9), with ECL outperforming ELISA. It is worth noting that during the present study, we consistently observed that the immunodetection of spores can be very significantly improved if the immunoreaction (2 h at room temperature) is preceded by a short centrifugation step (5 min, 1,500 × g). This very likely reflects the more rapid access of spores to the surface-bound antibodies. In fact, the signals measured under these conditions were equivalent to those observed after an overnight reaction (data not shown). This centrifugation step was routinely included in the procedure. Both the ELISA and the ECL assay described here are able to detect B. anthracis spores in different powders and in soil with, in the worst case, only a 3-fold decrease of the signal. Furthermore, even in the presence of a 1,000-fold excess of closely related strains possibly found in soil, B. anthracis spores were still detectable. These assays therefore appear the most sensitive immune methods for detection of B. anthracis spores (strains Sterne, RA3R, 9602, Ames, and Vollum) in complex matrices, with at least 2.5 × 104 CFU/5 mg of powder or soil detected without pretreatment or purification. These experiments demonstrate that these two immunometric assays, coupled to either colorimetric or ECL detection, are robust and sensitive techniques for detection of B. anthracis spores in the environment.
ACKNOWLEDGMENTS
This work was supported by the Commissariat à l'Energie Atomique et aux Energies Alternatives (CEA, France).
We thank Karine Devilliers and Marc Plaisance for expert technical assistance.
Footnotes
Published ahead of print 6 July 2012
REFERENCES
- 1. Adone R, et al. 2002. Sequence analysis of the genes encoding for the major virulence factors of Bacillus anthracis vaccine strain ‘Carbosap’. J. Appl. Microbiol. 93:117–121 [DOI] [PubMed] [Google Scholar]
- 2. Aronson AI, Fitz-James P. 1976. Structure and morphogenesis of the bacterial spore coat. Bacteriol. Rev. 40:360–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baeumner AJ, Leonard B, McElwee J, Montagna RA. 2004. A rapid biosensor for viable B. anthracis spores. Anal. Bioanal Chem. 380:15–23 [DOI] [PubMed] [Google Scholar]
- 4. Bruno JG, Yu H. 1996. Immunomagnetic-electrochemiluminescent detection of Bacillus anthracis spores in soil matrices. Appl. Environ. Microbiol. 62:3474–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellman GL, Courtney KD, Andres V, Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88–95 [DOI] [PubMed] [Google Scholar]
- 6. Fouet A, et al. 2002. Diversity among French Bacillus anthracis isolates. J. Clin. Microbiol. 40:4732–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grassi J, Frobert Y, Lamourette P, Lagoutte B. 1988. Screening of monoclonal antibodies using antigens labeled with acetylcholinesterase: application to the peripheral proteins of photosystem 1. Anal. Biochem. 168:436–450 [DOI] [PubMed] [Google Scholar]
- 8. Grassi J, et al. 1989. Production of monoclonal antibodies against interleukin-1 alpha and -1 beta. Development of two enzyme immunometric assays (EIA) using acetylcholinesterase and their application to biological media. J. Immunol. Methods 123:193–210 [DOI] [PubMed] [Google Scholar]
- 9. Guglielmo-Viret V, Thullier P. 2007. Comparison of an electrochemiluminescence assay in plate format over a colorimetric ELISA, for the detection of ricin B chain (RCA-B). J. Immunol. Methods 328:70–78 [DOI] [PubMed] [Google Scholar]
- 10. Hang J, et al. 2008. Development of a rapid and sensitive immunoassay for detection and subsequent recovery of Bacillus anthracis spores in environmental samples. J. Microbiol. Methods 73:242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartley HA, Baeumner AJ. 2003. Biosensor for the specific detection of a single viable B. anthracis spore. Anal. Bioanal Chem. 376:319–327 [DOI] [PubMed] [Google Scholar]
- 12. Henriques AO, Moran CP. 2000. Structure and assembly of the bacterial endospore coat. Methods 20:95–110 [DOI] [PubMed] [Google Scholar]
- 13. Hernandez E, Ramisse F, Cruel T, le Vagueresse R, Cavallo JD. 1999. Bacillus thuringiensis serotype H34 isolated from human and insecticidal strains serotypes 3a3b and H14 can lead to death of immunocompetent mice after pulmonary infection. FEMS Immunol. Med. Microbiol. 24:43–47 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez E, Ramisse F, Ducoureau JP, Cruel T, Cavallo JD. 1998. Bacillus thuringiensis subsp. konkukian (serotype H34) superinfection: case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 36:2138–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffmaster AR, et al. 2002. Evaluation and validation of a real-time polymerase chain reaction assay for rapid identification of Bacillus anthracis. Emerg. Infect. Dis. 8:1178–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehn A, et al. 2009. Development of antibodies against anthrose tetrasaccharide for specific detection of Bacillus anthracis spores. Clin. Vaccine Immunol. 16:1728–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth. John Wiley and Sons, New York, NY [Google Scholar]
- 19. Phillips AP, Ezzell JW. 1989. Identification of Bacillus anthracis by polyclonal antibodies against extracted vegetative cell antigens. J. Appl. Bacteriol. 66:419–432 [DOI] [PubMed] [Google Scholar]
- 20. Phillips AP, Martin KL. 1988. Limitations of flow cytometry for the specific detection of bacteria in mixed populations. J. Immunol. Methods 106:109–117 [DOI] [PubMed] [Google Scholar]
- 21. Phillips AP, Martin KL, Capey AJ. 1987. Direct and indirect immunofluorescence analysis of bacterial populations by flow cytometry. J. Immunol. Methods 101:219–228 [DOI] [PubMed] [Google Scholar]
- 22. Rao SS, Mohan KV, Atreya CD. 2010. Detection technologies for Bacillus anthracis: prospects and challenges. J. Microbiol. Methods 82:1–10 [DOI] [PubMed] [Google Scholar]
- 23. Schneider S, Enkerli J, Widmer F. 2009. A generally applicable assay for the quantification of inhibitory effects on PCR. J. Microbiol. Methods 78:351–353 [DOI] [PubMed] [Google Scholar]
- 24. Schriewer A, Wehlmann A, Wuertz S. 2011. Improving qPCR efficiency in environmental samples by selective removal of humic acids with DAX-8. J. Microbiol. Methods 85:16–21 [DOI] [PubMed] [Google Scholar]
- 25. Setlow P. 1994. Mechanisms which contribute to the long-term survival of spores of Bacillus species. Soc. Appl. Bacteriol. Symp. Ser. 23:49S–60S [DOI] [PubMed] [Google Scholar]
- 26. Steichen C, Chen P, Kearney JF, Turnbough CL. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stopa PJ. 2000. The flow cytometry of Bacillus anthracis spores revisited. Cytometry 41:237–244 [DOI] [PubMed] [Google Scholar]
- 28. Swiecki MK, Lisanby MW, Shu F, Turnbough CL, Kearney JF. 2006. Monoclonal antibodies for Bacillus anthracis spore detection and functional analyses of spore germination and outgrowth. J. Immunol. 176:6076–6084 [DOI] [PubMed] [Google Scholar]
- 29. Sylvestre P, Couture-Tosi E, Mock M. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169–178 [DOI] [PubMed] [Google Scholar]
- 30. Sylvestre P, Couture-Tosi E, Mock M. 2003. Polymorphism in the collagen-like region of the Bacillus anthracis BclA protein leads to variation in exosporium filament length. J. Bacteriol. 185:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamborrini M, Holzer M, Seeberger PH, Schürch N, Pluschke G. 2010. Anthrax spore detection by a luminex assay based on monoclonal antibodies that recognize anthrose-containing oligosaccharides. Clin. Vaccine Immunol. 17:1446–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tamborrini M, et al. 2009. Immuno-detection of anthrose containing tetrasaccharide in the exosporium of Bacillus anthracis and Bacillus cereus strains. J. Appl. Microbiol. 106:1618–1628 [DOI] [PubMed] [Google Scholar]
- 33. Tamborrini M, Werz DB, Frey J, Pluschke G, Seeberger PH. 2006. Anti-carbohydrate antibodies for the detection of anthrax spores. Angew. Chem. Int. Ed. Engl. 45:6581–6582 [DOI] [PubMed] [Google Scholar]
- 34. Tims TB, Lim DV. 2004. Rapid detection of Bacillus anthracis spores directly from powders with an evanescent wave fiber-optic biosensor. J. Microbiol. Methods 59:127–130 [DOI] [PubMed] [Google Scholar]
- 35. Wang DB, et al. 2009. Detection of B. anthracis spores and vegetative cells with the same monoclonal antibodies. PLoS One 4:e7810 doi: 10.1371/journal.pone.0007810 [DOI] [PMC free article] [PubMed] [Google Scholar]