Abstract
We investigated the transcriptional responses of Pseudomonas aeruginosa under phosphate-deficient (0.2 mM) conditions compared to phosphate sufficiency (1 mM). This elicited enormous transcriptional changes in genes related to phosphate acquisition, quorum sensing, chemotaxis, toxin secretion, and regulation. This dysregulation also led to increased virulence-associated phenotypes, including swarming motility and cytotoxicity.
TEXT
A characteristic trait of Pseudomonas aeruginosa is its high versatility, enabling this Gram-negative microbe to colonize a wide range of habitats such as soil, water, plants, and animals (25). It is the third most common nosocomial pathogen, causing serious opportunistic infections in elderly, immunocompromised, and injured individuals as well as chronic infections in the lungs of cystic fibrosis (CF) patients (18, 26). It has a tremendous capacity to adapt to diverse circumstances, and various conditions, including biofilm, quorum, or swarming lifestyles, exposure to subinhibitory antibiotics, and nutritional deprivation, lead to very large changes in gene expression and altered virulence and/or antibiotic resistance (3, 6, 12, 22, 23). To adapt efficiently to the changes in its surroundings, Pseudomonas has evolved sophisticated regulatory networks, and almost 10% of all genes in the P. aeruginosa genome encode proteins with a regulatory function (29).
Phosphate is essential for all living organisms, participating in critical biochemical processes, being an essential component of the energy dynamics of cells and a component of nucleic acids, phospholipids in membranes, and other biomolecules. Therefore, the ability to withstand conditions of phosphate starvation, making use of the available phosphate, is of great importance for cell survival. Consequently, microorganisms possess complex regulatory pathways for the control of the mechanisms involved in sensing phosphate availability as well as phosphate uptake and utilization. Furthermore, these networks usually overlap central metabolic routes because of the importance of phosphate in cellular physiology. Among Gram-negative bacteria, the best-characterized phosphate regulon is that of Escherichia coli, in which a two-component regulatory system, PhoBR, gets activated under phosphate-limiting conditions and binds to a conserved sequence (Pho-box) in the promoters of its target genes, inducing or repressing their expression (13). These genes include those encoding systems for high-affinity uptake of inorganic phosphate as well as acquisition of phosphate from alternative sources such as phosphonates and organic phosphate. P. aeruginosa also possesses homologs to PhoBR (1, 7). Significantly, phosphate limitation in Pseudomonas alters the production of quorum-sensing signals (15) and, consequently, it might have an impact on virulence and social behaviors such as biofilm formation and swarming motility. Indeed, Haddad et al. (10) related the expression of the Pho regulon to biofilm formation and the type III secretion system in P. aeruginosa.
The phosphate deprivation regulon is involved in influencing virulence traits in various microorganisms (2, 31, 27, 28, 30). Long et al. (17) showed that phosphate depletion is commonly observed after surgery and related this to an increase in the virulence of P. aeruginosa. This relationship was confirmed in vivo, as the growth of P. aeruginosa in a low-phosphate medium resulted in enhanced killing of Caenorhabditis elegans due to overexpression of the Pseudomonas quinolone signal (PQS) quorum-sensing signal and the iron chelator pyoverdin (33). Recently, two independent studies showed an upregulation of the genes involved in phosphate uptake in P. aeruginosa upon contact with differentiated human epithelial cells (4, 8), indicating that environmental cues other than low phosphate might induce expression of these genes inside the host. Thus, a better understanding of the phosphate regulon in P. aeruginosa and its correlation with virulence properties would help clarify its potential role in infections.
As a wild-type strain, the sequenced P. aeruginosa PAO1 strain H103 was routinely grown in Luria-Bertani (LB) broth or agar. Defined-phosphate, HEPES-based minimal medium was prepared as described previously (11). To identify the genes dysregulated during phosphate starvation, microarray analysis was performed on bacteria grown under phosphate-sufficient (1 mM phosphate) or phosphate-deficient (0.2 mM) conditions at 37°C with shaking (250 rpm) to an optical density at 600 nm (OD600) ∼ 0.5. Cells from these cultures were harvested, and total RNA was isolated with an RNeasy Midi RNA isolation kit (Qiagen), processed as described previously (19), and hybridized to P. aeruginosa PAO1 DNA microarray epoxy-coated slides from the J. Craig Venter Institute, Pathogen Functional Genomics Resource Center (http://pfgrc.jcvi.org/index.php/microarray.html). Results were analyzed using ArrayPipe (version 1.7). The four biological replicates were averaged to obtain overall changes for the samples grown under low-phosphate compared to phosphate-sufficient conditions. A two-sided one-sample Student's t test was used to determine statistical significance, and changes of 2-fold or greater with a P value of ≤0.05 were used as the cutoffs for reporting expression changes. The microarray data are available at MIAMExpress (see below).
These microarrays revealed a highly complex transcriptional response to phosphate-deficient growth conditions, with a total of 842 dysregulated genes, of which 495 were upregulated and 347 were downregulated (Table 1; see also Table S1 in the supplemental material for the full list). Critically, several of these genes were previously identified in our screening for promoters induced under low-phosphate conditions (16). There was a global upregulation of the genes involved in sensing the extracellular concentration of inorganic phosphate as well as in phosphate acquisition and utilization. Among these genes, we found the operon encoding the conserved two-component system PhoRB and the high-affinity phosphate transport system pstSCAB-phoU operon as well as gene locus PA0688, which has high homology to pstS. Similarly, expression of phosphate-specific porins OprP and OprO was induced under low-phosphate conditions, as was that of the phosphodiesterases glpQ and gene locus PA2352, the putative phosphate transporter PA0450, the alkaline phosphatase-encoding phoA gene, and the genes involved in the utilization of phosphonates (gene loci PA3372 to PA3384). The microarray data also revealed the upregulation of an operon encoding an extracellular DNase and an alkaline phosphatase (PA3909 and PA3910) that can provide Pseudomonas with phosphate and nitrogen from DNA (21). The expression of genes involved in the production of phospholipases, such as plcB, plcH, and plcN, which can lyse the phospholipids in eukaryotic membranes, was also enhanced during phosphate deprivation; presumably to enable phosphate acquisition in the host from organic sources, although phospholipases might additionally play a role in membrane remodeling, as reported for other microorganisms (34).
Table 1.
Selected genes from the microarray comparing the transcriptome of P. aeruginosa H103 grown under phosphate-deficient conditions to that grown under phosphate-sufficient conditions
| Gene identifier | Gene name | Fold change | P value | Descriptiona |
|---|---|---|---|---|
| PA0026 | plcB | 2.4 | 0.0001 | Phospholipase C |
| PA0083 | tssB1 | 4.0 | 4.1E−05 | Type VI secretion system protein |
| PA0084 | tssC1 | 2.7 | 0.0001 | Type VI secretion system protein |
| PA0085 | htp1 | 5.4 | 0.0004 | Type VI secretion system protein |
| PA0087 | tssE1 | 2.1 | 0.0009 | Type VI secretion system protein |
| PA0088 | tssF1 | 3.1 | 6.0E−05 | Type VI secretion system protein |
| PA0089 | tssG1 | 3.9 | 0.0007 | Type VI secretion system protein |
| PA0090 | clpV1 | 5.0 | 0.0001 | Type VI secretion system ATPase |
| PA0091 | vgrG1 | 4.3 | 0.0001 | Type VI secretion system protein |
| PA0173 | 5.6 | 0.0001 | Probable chemotaxis-specific methylesterase | |
| PA0174 | 5.6 | 0.0003 | Conserved hypothetical protein | |
| PA0175 | 10.6 | 7.7E−05 | Probable chemotaxis protein methyltransferase | |
| PA0176 | aer2 | 15.4 | 0.0001 | Aerotaxis transducer methyl-accepting chemotaxis protein |
| PA0177 | 9.1 | 6.7E−05 | Probable purine-binding chemotaxis protein | |
| PA0178 | 4.9 | 0.0011 | Probable two-component sensor | |
| PA0179 | 4.0 | 0.0001 | Probable two-component response regulator | |
| PA0347 | glpQ | 25.0 | 1.3E−07 | Glycerophosphoryl diester phosphodiesterase, periplasmic |
| PA0426 | mexB | 2.6 | 3.2E−05 | RND multidrug efflux transporter |
| PA0450 | 4.1 | 0.0002 | Probable phosphate transporter | |
| PA0677 | hxcW | 4.6 | 3.7E−05 | HxcW putative pseudopilin |
| PA0678 | hxcU | 8.2 | 7.2E−06 | HxcU putative pseudopilin |
| PA0679 | hxcP | 7.3 | 1.9E−05 | Hypothetical protein |
| PA0680 | hxcV | 2.4 | 0.0004 | HxcV putative pseudopilin |
| PA0681 | hxcT | 7.6 | 0.0001 | HxcT pseudopilin |
| PA0682 | hxcX | 13.3 | 3.7E−05 | HxcX atypical pseudopilin |
| PA0683 | hxcY | 8.8 | 1.8E−05 | Probable type II secretion system protein |
| PA0684 | hxcZ | 11.7 | 3.8E−06 | Probable type II secretion system protein |
| PA0685 | hxcQ | 12.3 | 1.1E−05 | Probable type II secretion system protein |
| PA0686 | hxcR | 13.1 | 8.4E−06 | Probable type II secretion system protein |
| PA0687 | hxcS | 3.6 | 5.6E−05 | Probable type II secretion system protein |
| PA0688 | 308 | 1.2E−06 | Probable binding protein component of ABC transporter | |
| PA0763 | mucA | 2.1 | 8.7E−05 | Anti-sigma factor MucA |
| PA0764 | mucB | 2.3 | 0.0074 | Negative regulator for alginate biosynthesis MucB |
| PA0842 | 33.5 | 8.3E−07 | Probable glycosyl transferase | |
| PA0843 | plcR | 9.7 | 4.5E−05 | Phospholipase accessory protein PlcR precursor |
| PA0844 | plcH | 25.7 | 3.2E−05 | Hemolytic phospholipase C precursor |
| PA0996 | pqsA | 4.2 | 0.019 | Probable coenzyme A ligase |
| PA0997 | pqsB | 5.0 | 0.0094 | Homologous to beta-keto-acyl-acyl-carrier protein synthase |
| PA0998 | pqsC | 4.5 | 0.0031 | Homologous to beta-keto-acyl-acyl-carrier protein synthase |
| PA0999 | pqsD | 3.2 | 0.0026 | 3-Oxoacyl-[acyl-carrier-protein] synthase III |
| PA1000 | pqsE | 7.8 | 0.0031 | Quinolone signal response protein |
| PA1001 | phnA | 6.6 | 0.0033 | Anthranilate synthase component I |
| PA1002 | phnB | 4.7 | 0.0047 | Anthranilate synthase component II |
| PA1003 | mvfR | 2.4 | 0.0003 | Transcriptional regulator |
| PA1078 | flgC | 2.1 | 0.0005 | Flagellar basal-body rod protein FlgC |
| PA1082 | flgG | 2.0 | 0.0011 | Flagellar basal-body rod protein FlgG |
| PA1086 | flgK | 2.3 | 0.0003 | Flagellar hook-associated protein 1 FlgK |
| PA1087 | flgL | 2.0 | 4.9E−05 | Flagellar hook-associated protein type 3 FlgL |
| PA1092 | fliC | 2.3 | 0.0016 | Flagellin type B |
| PA1130 | rhlC | 2.6 | 0.0022 | Rhamnosyltransferase 2 |
| PA1249 | aprA | 2.1 | 0.001 | Alkaline metalloproteinase precursor |
| PA1423 | 2.7 | 0.0036 | Probable chemotaxis transducer | |
| PA1456 | cheY | 2.6 | 0.0038 | Two-component response regulator CheY |
| PA1561 | aer | 2.2 | 4.7E−05 | Aerotaxis receptor Aer |
| PA1665 | 2.0 | 0.0047 | Hypothetical protein | |
| PA1712 | exsB | −2.1 | 0.0098 | Exoenzyme S synthesis protein B |
| PA1871 | lasA | 5.5 | 0.028 | LasA protease precursor |
| PA1900 | phzB2 | 11.7 | 0.025 | Probable phenazine biosynthesis protein |
| PA1901 | phzC2 | 6.8 | 0.0106 | Phenazine biosynthesis protein PhzC |
| PA1902 | phzD2 | 11.5 | 0.0025 | Phenazine biosynthesis protein PhzD |
| PA1903 | phzE2 | 3.0 | 0.0305 | Phenazine biosynthesis protein PhzE |
| PA1904 | phzF2 | 9.0 | 0.0064 | Probable phenazine biosynthesis protein |
| PA1905 | phzG2 | 7.2 | 0.0055 | Probable pyridoxamine 5′-phosphate oxidase |
| PA1930 | 3.0 | 0.0006 | Probable chemotaxis transducer | |
| PA1985 | pqqA | 3.2 | 0.001 | Pyrroloquinoline quinone biosynthesis protein A |
| PA1987 | pqqC | 2.2 | 0.0005 | Pyrroloquinoline quinone biosynthesis protein C |
| PA2352 | 5.8 | 2.4E−05 | Probable glycerophosphoryl diester phosphodiesterase | |
| PA2360 | 3.2 | 0.003 | Hypothetical protein | |
| PA2365 | 8.7 | 0.0002 | Conserved hypothetical protein | |
| PA2366 | 7.1 | 0.0055 | Conserved hypothetical protein | |
| PA2367 | 2.9 | 0.0025 | Hypothetical protein | |
| PA2368 | 4.5 | 0.0036 | Hypothetical protein | |
| PA2369 | 3.4 | 0.0017 | Hypothetical protein | |
| PA2370 | 3.5 | 0.0016 | Hypothetical protein | |
| PA2371 | 3.0 | 0.003 | Probable ClpA/B-type protease | |
| PA2372 | 3.2 | 0.0088 | Hypothetical protein | |
| PA2373 | 3.7 | 0.0054 | Conserved hypothetical protein | |
| PA2374 | 2.2 | 0.0089 | Hypothetical protein | |
| PA2396 | pvdF | 2.1 | 0.0449 | Pyoverdine synthetase F |
| PA2426 | pvdS | 4.3 | 0.0095 | Sigma factor PvdS |
| PA2505 | −6.7 | 9.5E−05 | Probable porin | |
| PA2520 | czcA | −2.9 | 0.0014 | RND divalent metal cation efflux transporter |
| PA2521 | czcB | −4.8 | 0.0015 | RND divalent metal cation efflux membrane fusion protein |
| PA2522 | czcC | −3.6 | 0.001 | Outer membrane protein precursor CzcC |
| PA2561 | ctpH | 2.8 | 0.0011 | Probable chemotaxis transducer |
| PA2573 | 5.0 | 0.0022 | Probable chemotaxis transducer | |
| PA2788 | 2.2 | 0.0019 | Probable chemotaxis transducer | |
| PA2803 | 8.8 | 6.1E−05 | Hypothetical protein | |
| PA2804 | 14.9 | 2.9E−06 | Hypothetical protein | |
| PA2920 | 2.2 | 0.0018 | Probable chemotaxis transducer | |
| PA3095 | xcpZ | 2.6 | 8.0E−05 | General secretion pathway protein M |
| PA3096 | xcpY | 2.8 | 0.0001 | General secretion pathway protein L |
| PA3097 | xcpX | 2.9 | 0.0008 | General secretion pathway protein K |
| PA3098 | xcpW | 2.8 | 9.3E−05 | General secretion pathway protein J |
| PA3099 | xcpV | 2.2 | 8.2E−05 | General secretion pathway protein I |
| PA3100 | xcpU | 2.9 | 8.7E−05 | General secretion pathway outer membrane protein H |
| PA3101 | xcpT | 4.9 | 2.1E−06 | General secretion pathway protein G |
| PA3102 | xcpS | 3.0 | 5.1E−05 | General secretion pathway protein F |
| PA3103 | xcpR | 2.7 | 5.1E−05 | General secretion pathway protein E |
| PA3104 | xcpP | 3.9 | 3.5E−05 | Secretion protein XcpP |
| PA3105 | xcpQ | 4.6 | 2.4E−05 | General secretion pathway protein D |
| PA3279 | oprP | 250 | 7.8E−08 | Phosphate-specific outer membrane porin OprP |
| PA3280 | oprO | 16.6 | 3.3E−06 | Pyrophosphate-specific outer membrane porin OprO |
| PA3296 | phoA | 69.1 | 2.3E−09 | Alkaline phosphatase |
| PA3319 | plcN | 16.4 | 1.1E−07 | Nonhemolytic phospholipase C precursor |
| PA3372 | phnP | 5.3 | 1.8E−05 | Conserved hypothetical protein |
| PA3373 | phnN | 6.3 | 8.0E−07 | Conserved hypothetical protein |
| PA3374 | phnM | 25.4 | 1.3E−07 | Conserved hypothetical protein |
| PA3375 | phnL | 18.0 | 6.5−06 | Probable ATP-binding component of ABC transporter |
| PA3376 | phnK | 18.6 | 1.3E−06 | Probable ATP-binding component of ABC transporter |
| PA3377 | phnJ | 25.6 | 4.7E−07 | Conserved hypothetical protein |
| PA3378 | phnI | 20.3 | 1.4E−06 | Conserved hypothetical protein |
| PA3379 | phnH | 13.8 | 2.4E−06 | Conserved hypothetical protein |
| PA3380 | phnG | 19.9 | 5.3E−07 | Conserved hypothetical protein |
| PA3381 | phnF | 6.6 | 4.7E−06 | Probable transcriptional regulator |
| PA3382 | phnE | 52.6 | 9.0E−08 | Phosphonate transport protein PhnE |
| PA3383 | phnD | 91.0 | 2.0E−09 | Binding protein component of ABC phosphonate transporter |
| PA3384 | phnC | 27.2 | 2.5E−07 | ATP-binding component of ABC phosphonate transporter |
| PA3476 | rhlI | 3.0 | 0.0002 | Autoinducer synthesis protein RhlI |
| PA3477 | rhlR | 4.7 | 0.0002 | Transcriptional regulator RhlR |
| PA3478 | rhlB | 9.0 | 0.001 | Rhamnosyltransferase chain B |
| PA3479 | rhlA | 11.5 | 0.0005 | Rhamnosyltransferase chain A |
| PA3540 | algD | 2.3 | 0.0006 | GDP-mannose 6-dehydrogenase AlgD |
| PA3550 | algF | 2.2 | 0.0099 | Alginate o-acetyltransferase AlgF |
| PA3551 | algA | 5.1 | 0.0002 | Alginate biosynthesis enzyme |
| PA3622 | rpoS | 3.1 | 0.0005 | Sigma factor RpoS |
| PA3909 | eddB | 144 | 2.9E−08 | Hypothetical protein |
| PA3910 | eddA | 34.6 | 3.3E−07 | Hypothetical protein |
| PA4190 | pqsL | 2.6 | 3.8E−05 | Probable FAD-dependent monooxygenase |
| PA4209 | phzM | 7.2 | 0.0235 | Probable phenazine-specific methyltransferase |
| PA4210 | phzA1 | 2.5 | 0.0069 | Probable phenazine biosynthesis protein |
| PA4211 | phzB1 | 55.1 | 0.0016 | Probable phenazine biosynthesis protein |
| PA4217 | phzS | 29.6 | 0.0012 | Flavin-containing monooxygenase |
| PA4290 | 3.0 | 0.0094 | Probable chemotaxis transducer | |
| PA4292 | −3.6 | 9.7E−05 | Probable phosphate transporter | |
| PA4302 | 2.7 | 0.0002 | Probable type II secretion system protein | |
| PA4350 | olsB | 29.0 | 2.2E−07 | Conserved hypothetical protein |
| PA4351 | olsA | 18.7 | 2.2E−08 | Probable acyltransferase |
| PA4551 | pilV | 2.4 | 0.009 | Type 4 fimbrial biogenesis protein PilV |
| PA4555 | pilY2 | 2.0 | 0.0126 | Type 4 fimbrial biogenesis protein PilY2 |
| PA4723 | dksA | −3.5 | 2.5E−05 | Suppressor protein DksA |
| PA4844 | ctpL | 7.1 | 0.0001 | Probable chemotaxis transducer |
| PA4853 | fis | −3.8 | 9.8E−05 | DNA-binding protein Fis |
| PA4915 | 2.1 | 0.0016 | Probable chemotaxis transducer | |
| PA5261 | algR | 2.4 | 7.9E−05 | Alginate biosynthesis regulatory protein AlgR |
| PA5360 | phoB | 16.1 | 5.4E−07 | Two-component response regulator PhoB |
| PA5361 | phoR | 9.3 | 5.4E−05 | Two-component sensor PhoR |
| PA5365 | phoU | 18.7 | 9.5E−09 | Phosphate uptake regulatory protein PhoU |
| PA5366 | pstB | 19.0 | 2.2E−08 | ATP-binding component of ABC phosphate transporter |
| PA5367 | pstA | 25.7 | 7.4E−09 | Membrane protein component of ABC phosphate transporter |
| PA5368 | pstC | 27.7 | 3.9E−09 | Membrane protein component of ABC phosphate transporter |
| PA5369 | pstS | 223 | 2.6E−09 | Hypothetical protein |
RND, resistance-nodulation-cell division.
Another group of gene loci significantly dysregulated in response to phosphate depletion comprised those involved in chemotactic responses (PA0173 to PA0179, PA1423, PA1456, PA1930, PA2561, PA2573, PA2788, PA2920, PA4290, PA4844, and PA4915). Of particular significance were the phosphate-specific chemoreceptors CtpL (PA2561) and CtpH (PA4844) identified by Wu et al. (32) as necessary for chemotaxis with different concentrations of phosphate.
A total of 45 genes, including rpoS, fis, gbdR, and algR, that were dysregulated under low-phosphate conditions encoded products with a regulatory function. Other known regulators observed in the microarray were those that participate in quorum-sensing signaling, including mvfR, pvdS, and rhlR, reinforcing the concept that the phosphate regulon and the quorum-sensing network are tightly interconnected. Indeed, we observed dysregulation by phosphate of known quorum-sensing-dependent genes, such as those involved in the biosynthesis of rhamnolipids or phenazines. Jensen et al. (15) previously indicated that the Pseudomonas quinolone signal (PQS) can regulate the expression of rhlRI partly through PhoB. Additionally, Zaborin et al. (33) observed a clear upregulation of quorum-sensing-regulated genes in the complete absence of phosphate compared to a phosphate-rich medium, which we here confirmed in comparisons of phosphate-sufficient to phosphate-deficient conditions. Furthermore, they linked this induction with the phenomenon of red death in C. elegans, which appeared to be mediated by PQS.
The general secretion pathway genes, together with the alternative type II and the type VI secretion systems, were upregulated under low-phosphate conditions, whereas the type III secretion system genes did not show any change or a slight downregulation (exsB). Genes representing all three type VI secretion islands of P. aeruginosa (HSI-1 [PA0074 to PA0091], HSI-2 [PA1656 to PA1671], and HSI-3 [PA2359 to PA2371]) were upregulated in the microarray analysis. Although our understanding of the mechanisms and roles of type VI secretion systems is still somewhat limited, recent evidence in Pseudomonas indicates that they might participate in chronic infection (20, 24). Overall, there was also significant overlap of our results with the microarray analysis of Zaborin et al. (33), who compared cells in the complete absence of phosphate to cells with phosphate.
Before attempting other phenotypical assays, we compared the levels of growth of P. aeruginosa in the minimal medium with 0.2 mM and 1 mM phosphate in order to determine if there was a significant growth defect under the former conditions. Our results showed, however, that growth was not significantly affected under phosphate-deficient conditions (Fig. 1).
Fig 1.
Growth curve of P. aeruginosa PAO1 under phosphate (Pi)-deficient (0.2 mM) and phosphate-sufficient (1 mM) conditions. The results shown in this figure represent the averages and standard deviations of the results of three independent experiments.
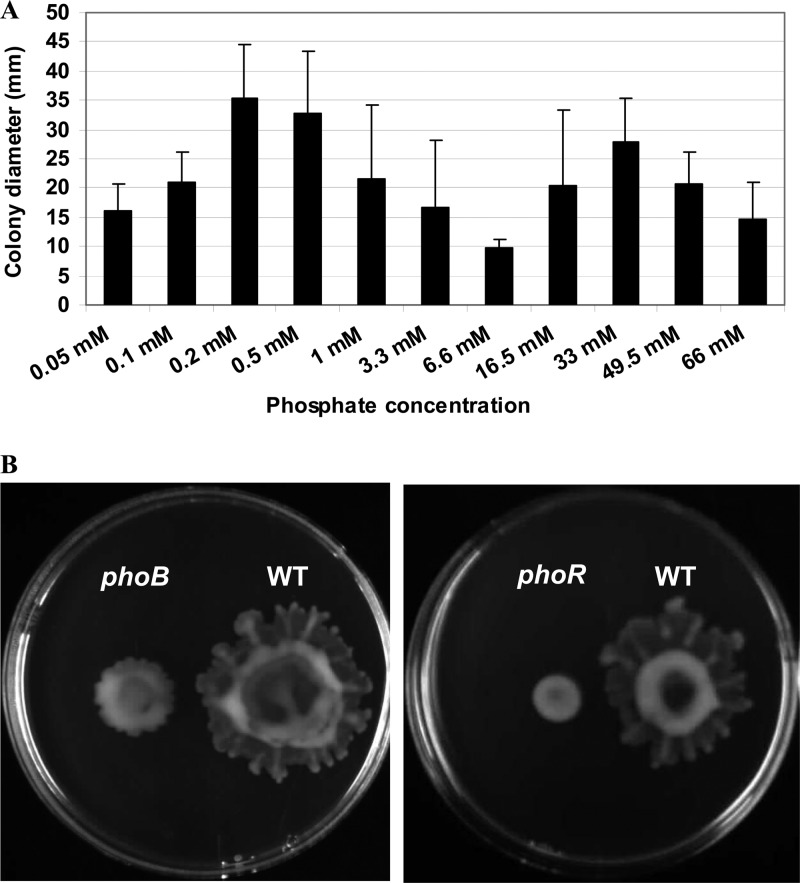
We observed a significant upregulation of many genes related to quorum sensing and motility and thus tested the P. aeruginosa swarming phenotype using various concentrations of phosphate. Swarming motility assays revealed an intriguing bimodal response to the concentration of phosphate in P. aeruginosa. As the level of phosphate in the swarming medium decreased from high (33 mM; the normal concentration in BM2 minimal medium) to sufficient (1 mM), there was a clear reduction in the size of the swarming colonies (Fig. 2A), perhaps due in part to the reduction in the growth rate as the availability of phosphate decreased. However, as the concentration dropped further toward deficient levels (0.2 mM), swarming motility gradually increased again (Fig. 2A). Based on the microarray data, it seems likely that the increase in swarming motility under phosphate-deficient conditions was related to the upregulation of quorum-sensing genes and the subsequent increased production of rhamnolipids that is essential for swarming motility (5). In fact, genes involved in synthesis of rhamnolipids, such as rhlA, rhlB, and rhlC, were also significantly induced under these conditions (Table 1).
Fig 2.
Effect of phosphate availability on swarming motility. (A) Diameters of swarming colonies of wild-type P. aeruginosa on plates containing swarming medium with increasing concentrations of phosphate. (B) Swarming motility phenotypes of the wild type (WT) and the phoB and phoR mutants on plates containing a low (0.2 mM) concentration of phosphate.
To show that this was related to regulatory events, we examined the swarming ability of mutants with mutations in the major regulators that respond to low phosphate, namely, phoB and phoR, which were taken from the University of Washington mutant library (14). Both strains showed a complete loss of swarming motility when grown on plates with a low (0.2 mM) concentration of phosphate (Fig. 2B), indicating that the PhoBR two-component system is necessary to promote swarming under low-phosphate conditions.
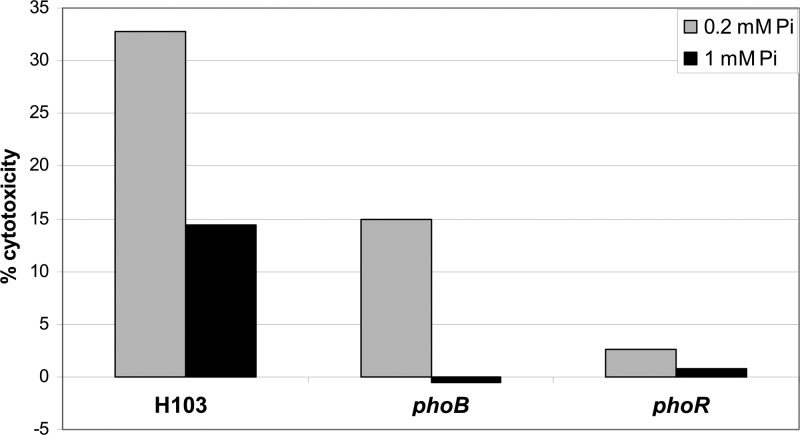
In addition to the effects on motility, we evaluated the impact of phosphate concentration on the cytotoxicity of P. aeruginosa. Cells from the wild-type strain or phoB or phoR mutants were grown in medium with low (0.2 mM) or sufficient (1 mM) phosphate prior to interaction with the human bronchial epithelial cell line 16HBE14o- at a multiplicity of infection of 50, as described previously (9). At 6 h postinfection, the percentage of cell lysis was determined. Two-fold-greater cytotoxicity, determined by increased release of lactate dehydrogenase, was observed for strain H103 grown in a low-phosphate medium before the infection compared to that of cells grown under phosphate-sufficient conditions (Fig. 3). Comparison of the cytotoxicities of the phoB and phoR regulatory mutants showed a clear reduction under both phosphate-sufficient and -deficient conditions. The phoR mutant had the greatest effect on cytotoxicity under phosphate-limiting conditions, but under phosphate-sufficient conditions, both regulatory mutants demonstrated no cytotoxicity at 6 h. A major candidate for the proteins responsible for altered cytotoxicity would be the phosphate-inducible hemolysins.
Fig 3.
Analysis of the effect on cytotoxicity of phosphate deficiency prior to infection of HBE cells. The graph shows percent cytotoxicity compared to total cell lysis inflicted by treatment with Triton X-100 of the wild-type H103 strain and phoB and phoR mutant strains after growth in a sufficient (1 mM) or deficient (0.2 mM) concentration of phosphate. Data represent the results of a single experiment representative of three with the same trends.
In conclusion, these results demonstrate how the opportunistic pathogen P. aeruginosa adapts to even moderate reductions in inorganic phosphate supply by modulating global gene expression. Indeed, phosphate deprivation led to a remarkably intricate transcriptional response that affected genes involved in a wide range of cellular functions, including virulence properties such as swarming and cytotoxicity. The induction of cytotoxicity under adverse conditions would appear to be directed at the acquisition of phosphate from affected cells.
Microarray data accession number.
The microarray data determined in this work are available under MIAMExpress accession number E-MTAB-1170.
Supplementary Material
ACKNOWLEDGMENTS
The work described in this paper was funded by grants from the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation. R.E.W.H. holds a Canada Research Chair.
Footnotes
Published ahead of print 6 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Anba J, Bidaud M, Vasil ML, Lazdunski A. 1990. Nucleotide sequence of the Pseudomonas aeruginosa phoB gene, the regulatory gene for the phosphate regulon. J. Bacteriol. 172:4685–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoyama T, Takanami M, Makino K, Oka A. 1991. Cross-talk between the virulence and phosphate regulons of Agrobacterium tumefaciens caused by an unusual interaction of the transcriptional activator with a regulatory DNA element. Mol. Gen. Genet. 227:385–390 [DOI] [PubMed] [Google Scholar]
- 3. Breidenstein EBM, de la Fuente-Núñez C, Hancock REW. 2011. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19:419–426 [DOI] [PubMed] [Google Scholar]
- 4. Chugani S, Greenberg EP. 2007. The influence of human respiratory epithelia on Pseudomonas aeruginosa gene expression. Microb. Pathog. 42:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Déziel E, Lépine F, Milot S, Villemur R. 2003. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 149:2005–2013 [DOI] [PubMed] [Google Scholar]
- 6. Fernández L, Breidenstein EBM, Hancock REW. 2011. Creeping baselines and adaptive resistance to antibiotics. Drug Resist. Updat. 14:1–21 [DOI] [PubMed] [Google Scholar]
- 7. Filloux A, Bally M, Soscia C, Murgier M, Lazdunski A. 1988. Phosphate regulation in Pseudomonas aeruginosa: cloning of the alkaline phosphatase gene and identification of phoB- and phoR-like genes. Mol. Gen. Genet. 212:510–513 [DOI] [PubMed] [Google Scholar]
- 8. Frisk A, et al. 2004. Transcriptome analysis of Pseudomonas aeruginosa after interaction with human airway epithelial cells. Infect. Immun. 72:5433–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gooderham WJ, et al. 2009. The sensor kinase PhoQ mediates virulence in Pseudomonas aeruginosa. Microbiology 155:699–711 [DOI] [PubMed] [Google Scholar]
- 10. Haddad A, Jensen V, Becker T, Häussler S. 2009. The Pho regulon influences biofilm formation and type three secretion in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 1:488–494 [DOI] [PubMed] [Google Scholar]
- 11. Hancock REW, Poole K, Benz R. 1982. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J. Bacteriol. 150:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Høiby N, Ciofu O, Bjarnsholt T. 2010. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 5:1663–1674 [DOI] [PubMed] [Google Scholar]
- 13. Hsieh YJ, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 13:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs MA, et al. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 100:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen V, et al. 2006. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J. Bacteriol. 188:8601–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewenza S, et al. 2005. Construction of a mini-Tn5-luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res. 15:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J. 2008. Depletion of intestinal phosphate after operative injury activates the virulence of Pseudomonas aeruginosa causing lethal gut-derived sepsis. Surgery 144:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyczak JB, Cannon CL, Pier GB. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051–1060 [DOI] [PubMed] [Google Scholar]
- 19. McPhee JB, et al. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 188:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mougous JD, et al. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mulcahy H, Charron-Mazenod L, Lewenza S. 2010. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ. Microbiol. 12:1621–1629 [DOI] [PubMed] [Google Scholar]
- 22. Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277–1287 [DOI] [PubMed] [Google Scholar]
- 23. Overhage J, Bains M, Brazas MD, Hancock REW. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 190:2671–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Potvin E, et al. 2003. In vivo functional genomics of Pseudomonas aeruginosa for high-throughput screening of new virulence factors and antibacterial targets. Environ. Microbiol. 5:1294–1308 [DOI] [PubMed] [Google Scholar]
- 25. Rahme LG, et al. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902 [DOI] [PubMed] [Google Scholar]
- 26. Rowe SM, Miller S, Sorscher EJ. 2005. Cystic fibrosis. N. Engl. J. Med. 352:1992–2001 [DOI] [PubMed] [Google Scholar]
- 27. Rüberg S, Pühler A, Becker A. 1999. Biosynthesis of the exopolysaccharide galactoglucan in Sinorhizobium meliloti is subject to a complex control by the phosphate-dependent regulator PhoB and the proteins ExpG and MucR. Microbiology 145:603–611 [DOI] [PubMed] [Google Scholar]
- 28. Slater H, Crow M, Everson L, Salmond GP. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol. Microbiol. 47:303–320 [DOI] [PubMed] [Google Scholar]
- 29. Stover CK, et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 30. von Krüger WM, et al. 2006. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics 6:1495–1511 [DOI] [PubMed] [Google Scholar]
- 31. Winans SC. 1990. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J. Bacteriol. 172:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu H, et al. 2000. Identification and characterization of two chemotactic transducers for inorganic phosphate in Pseudomonas aeruginosa. J. Bacteriol. 182:3400–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaborin A, et al. 2009. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc. Natl. Acad. Sci. U. S. A. 106:6327–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zavaleta-Pastor M, et al. 2010. Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc. Natl. Acad. Sci. U. S. A. 107:302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.