Abstract
Football participation requires considerable utilization of both aerobic and anaerobic energy systems to match the high energetic demands of the sport. The consequent stresses imposed on the physiological and metabolic systems carries players to the threshold of exhaustion during match-play, from which they are required to recover in preparation for the subsequent game. A high number of players experience fatigue during the high-intensity bouts and a consequent decline in their performance towards the end of the game is a likely outcome during match-play. The current review aims to establish the current understanding that relates to metabolic limitations of performance and the associated mechanisms for the onset of fatigue that may be instrumental in further development of evidence-based nutritional and training interventions in this event.
Keywords: Soccer, Metabolism, Glycogen, Carbohydrate, Intermittent exercise
INTRODUCTION
In a football context, fatigue is defined as a decline in the capacity to sustain muscular work that is manifested as a reduction in work rate towards the end of the game [1, 2]. Recent studies indicated the importance of the comprehension of the energy demands in football to aid in the understanding of substrate utilization during match-play [3, 4]. It has been estimated that the total distance covered in football typically ranges between 10–11 km, with an energy expenditure yield of 1500 kcal during active play [5, 6]. It is important to consider that the amount of energy expended during football participation is greater than mere running to cover a similar distance [7]. While it has been reported that field running is more energy demanding than on a compact terrain [8], the augmented energy expenditure in football is largely attributed to the additional imposed physical demands during the match such as environmental influences and sport-specific movements [5, 9]. The latter would explain the enormous physical demands of football; stressing the majority of the body's internal systems that ultimately succumb players to the threshold of exhaustion during match-play [10].
In terms of performance, a decline in work rate in the second half was consistently found irrespective of the level of competition and the physical fitness of participants [1]. However, debate is ongoing with regards to the validity of the simple comparison of the overall distance run for each half to interpret whether a player had succumbed to fatigue [11]. It has been postulated that teams and players may pace their efforts in order to sustain the work rate throughout the duration of the game, suggesting that players may exert an effort below their physical capacity in the first half as an energy sparing technique [12]. With this in mind, data from various studies (Table 1) comparing the total distance run by elite football players appear to be consistent in finding a decrement in performance in the second half of the game.
Table 1.
Comparison of the distances covered in the first and second halves of football match-play in different leagues
| Reference | League | Distance | Significant decrements in performance? |
|---|---|---|---|
| 13 | Swedish | 3% greater distance in the first half | Yes |
| 14 | Brazilian | 8% greater distance in the first half | Yes |
| 15 | Danish | 5% greater distance in the first half | Yes |
| 2 | Italian | 3% greater distance in the first half | Yes |
| 16 | Euro cup | 1% greater distance in the first half | Yes |
| 17 | English | 2% greater distance in the first half | Yes |
| 18 | South American + English | 4% greater distance in the first half | Yes |
Motion analysis during match-play indicated that fatigue was shown to occur during three different stages in a game:(i) following short-term intense period throughout the game; (ii) the initial phase of the second half; and (iii) towards the end of the game [19]. This review will focus on the likely perturbations for the development of transient fatigue and the ensuing decrement in performance at the latter stages of the game.
PHYSIOIOGICAL AND METABOLIC DEMANDS OF FOOTBALL
Football is broadly characterized as an intermittent aerobic event interspersed with periods of high-intensity activities [20, 21]. Players perform numerous different types of exercise intensities during the game [22]. In accordance, it was indicated that both the aerobic and anaerobic energy systems contribute to the physiological demands of the game [3]. The total duration of active play in football is typically 90 minutes [4], indicating that the primary energy source during the game is supplied via aerobic glycolysis [20], with an average maximal oxygen uptake (VO2max) of around 70 – 80% during the match [5]. The mean and peak heart rates of players were estimated to be around 85 and 98, respectively [21].
Although the energy delivery during a football game is predominantly supplied by the aerobic metabolism, high-intensity anaerobic bouts were indicated to be an essential component of performance in football [2, 7] because they comprise the most crucial events during the game [20]. Thus, the anaerobic effort is a determinant in repeated sprint bouts and other sport-specific maneuvers [23]. It was shown that the ability to utilize the anaerobic system to higher degree increases with the level of competition [20], such that high-intensity anaerobic activity was reported to distinguish between the different standards of players [21], higher and lower levels of competition [22], training status [21] tactical role of players within a team [24] and the success of a team [25]. The anaerobic contribution to the overall energy demand becomes emphasized when direct involvement in play takes place, such as position contention and ball possession [7]. This is evident by means of blood lactate concentrations of 2–10 mmol.l−1 during competitive football play [22, 26]. However, it was observed that blood lactate measurements are variable between players, with a wide range of values that may reach 12 mmol.l−1 in some participants [20, 22, 26]. The fluctuations in the blood lactate measurements in the literature were attributed to the varying collection times, which could be taken following low-intensity activity or high-intensity bouts [27]. This was supported by findings that a high rate of anaerobic glycolysis was observed at short but frequent periods of time during the game, and therefore the high blood lactate concentrations observed in football may be a reflection of an accumulative effect that corresponds to the numerous high-intensity bouts [3, 22, 26].
SUBSTRATE UTILIZATION IN FOOTBALL
The large aerobic energy yield and the pronounced anaerobic energy turnover during periods of a match are associated with a large consumption of substrates [22]. Therefore, a profound comprehension of substrate utilization during the course of a football match is crucial to provide nutritional strategies for a football player and to influence performance during match-play [3]. It was shown, based on match analysis [21] and laboratory studies [28] that a heavy reliance on endogenous carbohydrate (CHO) stores is apparent in football competition [29]. In fact, glycogen stores were indicated to play a central role in energy metabolism during prolonged, intense intermittent activities [30] that resemble the activity profile seen in football [29]. During the game, CHO is primarily obtained by glycogenolysis within the exercising muscles, with a subsidiary contribution arising from extramuscular glucose utilization from the liver [22, 29]. With increased duration, however, the contribution from glycogen degradation declines with a synchronous increase in blood glucose levels at exercise intensities similar to those (∼70% VO2max) observed in a football match [29, 31].
Provided sufficient pre-exercise liver glycogen content [32], the intense nature of football will result in blood glucose levels close to or slightly above resting levels [33]. This indicates the ability of the liver to maintain glucose concentrations during a match [34]. This is further supported by the obtained glucose concentrations towards the end of both match-play conditions [22, 35] and laboratory-based simulation of football activity [36] that ranged between 3.8 and 4.5 mmol.l−1, with only 3 players approaching hypoglycemic levels (∼3.2 mmol.l−1) [35]. The utilization of glycogen stores during a football match was suggested to be 155 – 160 g from the muscle glycogen stores, with an estimated 600 kcal of energy provided, while blood glucose derived from the liver may account for approximately 210 kcal of energy during the game [33]. In accordance, the endogenous CHO stores are suggested to supply ∼55% of the energy requirements of match-play, and a substantial utilization of lipids and proteins must also be taken into account [22]. Nevertheless, muscle glycogen is likely to provide the majority of energy during a football match, as evidenced by the pronounced reliance on CHO metabolism during match-play [37].
It was suggested that lipid oxidation to fuel the aerobic processes of the exercising muscles during the game are derived from intramuscular triglycerides or via the blood as free fatty acids (FFA), with an estimated 40% of the total energy being met from the oxidation of FFA [22]. Accordingly, it was observed that FFA concentrations increase during the game, with marked elevations during the second half [26]. This was attributed to the frequent periods of rest and low-intensity exercise during match-play, that would enable sufficient perfusion of the adipose tissue and subsequently promote the release of FFA [34]. This effect is further illustrated by the high FFA concentrations at half-time and after the game [26]. In contrast to continuous exercise, a less pronounced elevation in glycerol is observed, which may reflect a high turnover of glycerol as a gluconeogenic precursor in the liver [3, 22]. Hormonal changes may also influence the increase in FFA concentrations [26]. It was shown that the progressive lowered insulin levels coincided with elevations in catecholamines concentrations during the game [21], whereby stimulating a higher rate of lipolysis and a subsequent release of FFA into circulation [38]. This is reinforced by the lowered blood lactate levels that are known to antagonize the suppression of FFA mobilization from the adipose tissue [26, 38]. The increase in lipid turnover during the game was suggested to be a compensatory mechanism against the diminished muscle glycogen levels together with elevated concentrations of catecholamine [24]. Accordingly, the utilization of FFA and muscle triglycerides are chosen favorably in maintaining blood glucose during the game [3].
Metabolism of amino acids was proposed to serve as an auxiliary fuel source during prolonged activities that involve substantial physiological strain; similar to football participation to induce significant amino acid oxidation [39]. Furthermore, it was established that amino acid oxidation is inversely related to the diminishing glycogen reserves [40]. Correspondingly, the effort exerted during the game and the likely insufficient CHO intake by players [41] suggests that glycogen depletion is plausible during match-play. The extent of protein metabolism during a football game remains unclear [33]. However, it was shown that a small contribution of the total energy requirement is derived from breakdown of protein, particularly branched-chain amino acids, with an estimated supply of 2 – 3% of total energy metabolism [42].
TRANSIENT FATIGUE DURING THE GAME
The imposed high-intensity maneuvers in football consistently involve repeated sprint bouts and significant long runs with short periods of recovery [23]. The anaerobic metabolism comprises the most crucial events in football, as it is a main determinant in sprinting, jumping, tackling and duel play [20]. Elite football players perform 150–250 discrete intense actions that are brief in nature during a game [2]. This indicates a high anaerobic energy turnover at certain periods during the game, which consequently induce temporary fatigue during match-play [3]. The amount of high-intensity running in the 5 minute period ensuing the most intense period during the game showed a 12% reduction in performance [2]. Furthermore, it was shown that following an intense period of the game, sprint performance was significantly reduced when compared to sprint performance at the end of the first half [26]. Taken together, these findings suggest that football players experience transient fatigue during the game [24]. An exercise induced elevation of lactate within the active muscle was postulated to contribute to the temporary decrement in performance intense periods in the game [26]. Nonetheless, a weak relationship (r2=0.25 and 0.06 in the first and second halves respectively, P>0.05) was found between muscle lactate and the reduction in sprint performance in football [26]. Further studies corroborated that the accumulation of lactate does not cause fatigue [43, 44].
The high-intensity bouts in contemporary football are characterized as explosive, short (≤10 meters) and frequent in nature with an estimated recovery of ∼72 seconds between these bouts [17, 25]. The rapid depletion of ATP-PCr and the relative short time to replenish phosphocreatine (PCr) stores is suggested to influence the ability to perform subsequent bouts of high-intensity activity, and consequently contribute to a decline in performance during exercise [45]. This may not necessarily demonstrate a causal relationship in football, as evidenced by only a 25% reduction in PCr levels following a high-intensity period during the game [26]. It is noteworthy that the values in the aforementioned study may have been underestimated as a result of the rapid recovery of PCr stores and standard delay (∼20 seconds) in obtaining the muscle biopsy. Indeed, PCr may deplete individual fibers almost completely at the point of fatigue subsequent to intense activities [46], which could influence repeated sprint effort [47]. In vivo, metabolic acidosis demonstrated a detrimental effect in muscle performance during intense activity [48]. This could be ascribed to disturbances in the homeostasis of the muscle cell, and the displacement of calcium (Ca2 +) from the sarcoplasmic reticulum within the muscle cell [45]. Nonetheless, it was shown that muscle pH is only moderately reduced (∼6.8) during a game [26]. Moreover, the implementation of a Yo-Yo intermittent recovery test, performed as an intermittent exercise to exhaustion with an intense nature, reported no change in lactate, PCr and pH values at the final stage of the test [44]. Another factor that may contribute to the onset of transient fatigue may be a reduction in intramuscular ATP availability [34]. Recent findings reported higher levels of muscle inosine monophosphate (IMP) and a concomitant elevation in plasma ammonia (NH3) markers during the game and thus sufficient stimulation in adenosine monophosphate (AMP) deaminase reaction; an indicative for sufficient ATP supply [26]. Therefore, the aforementioned findings appear to dismiss high levels of lactate accumulation, increased metabolic acidosis and PCr depletion as contributors to the possible causes that trigger the onset of temporary fatigue and that other factors should be investigated to obtain sufficient understanding of the plausible reasons of transient fatigue in football [34].
Another proposed factor in the onset of temporary fatigue in football was suggested to be linked to an accumulation of potassium (K+) in the muscle interstitial metabolism and the consequent electrical disturbances in the muscle cell [49]. This was concurrent with another observation, which reported a significant increase in interstitial K+ concentrations during exhaustive exercise with a simultaneous decline in muscle pH [50]. When extrapolated to football, the accumulation of extracellular K+ in conjunction with electrical disturbances may contribute to subsequent transient fatigue [24]. Nevertheless, the average plasma K+ levels in football were shown to be ∼5 mmol.l−1, exhibiting lower K+ concentrations in football than those following a 30-second incremental exercise to exhaustion [26, 44]. It should be recognized that the plasma K+ concentrations were drawn from the arm of participants and could not provide an explicit view of the concentrations in the contracting muscle fibers [3]. Thus, K+ turnover in the muscle during a game remains equivocal [24].
FATIGUE TOWARDS THE END OF THE GAME
The capacity to perform intense efforts is markedly reduced towards the end of the game in football, as evidenced by a reduction in high-intensity running in the final 15 minutes in a large number (40%) of players during a top-level game [2]. It was argued that the majority of players succumb to fatigue at the end stage of a game and consequently their ability to perform repeated sprint bouts was shown to deteriorate [24]. This was further substantiated by an increase in high-intensity exertion (+65% sprinting; +25% running) by substitutes who came on the second half in comparison to players who started the game, with the decline in performance experienced independent of the positions held by players within the team [2]. Despite that a number of nutritional interventions demonstrated that the elevation of muscle glycogen prior to a prolonged intermittent exercise through CHO ingestion improves performance during such activities [28], this was not universal [51]. In this regard, the underlying mechanisms behind the likely correlation between muscle glycogen concentration and fatigue in prolonged intermittent exercise remains to be elucidated [3].
The energy sources in high-intensity bouts depend predominantly on muscle glycogen rather than extracellular glucose [52]. It is imperative, therefore, that endogenous CHO stores are an essential substrate to supply the energetic requirements for football participation [37]. It was observed that muscle glycogen stores were almost depleted by 50% at the end of the match with reported concentrations of ∼200 mmol.kg dry weight−1 [51]. However, these values are likely to be sufficient to maintain maximal glycolytic rate, demonstrating that muscle glycogen is not significantly diminished in football [43]. However, histochemical analysis to investigate single muscle fibers revealed that a substantial number (∼50%) of the individual fibers were depleted or partially depleted of glycogen, particularly fast twitch (FT) fibers [26]. Interestingly, the depletion of glycogen in FT fibers to a critically low level where maximal glycolytic rate cannot be maintained [43] was shown to determine the point of fatigue during a simulation of football [53] and real match conditions [26]. Therefore, the significant reduction of muscle glycogen observed in individual fibers could debilitate maximal effort and consequently decline single and repeated sprint performance, which may provide support for the notion CHO availability may become a limiting factor in performance [3, 54].
Other proposed candidates to induce fatigue at the end of a football game were dehydration and hyperthermia [55]. It was shown that players lose more than 2 L of fluids during a match under temperate thermal environments [56]; under hot and humid environments the fluid loss was estimated to reach between 4–5 L [57]. A moderate decrement in the hydration status of players may have a negative impact on performance [58], and that fluid loss of 1–2% of body mass could augment a rise in core temperature [59]. The mean core temperatures in football were reported to be within 39–39.5°C [60]. However, a number of individual values were found to exceed 40°C [61], which may instigate central fatigue [62]. Thus, it was indicated that players reduce their exercise intensity towards the end of the game as a coping mechanism to counter the effects of fluid loss [7]. In addition, it was reported that a significant decline in high-intensity sprinting ability was observed in concurrence with fluid loss equivalent to 1% of body mass [26]. In football, no differences in core temperatures were observed between the end of each half during the game [63], suggesting that the association between fluid loss and impaired performance at the end of the game could not be consistently established [3].
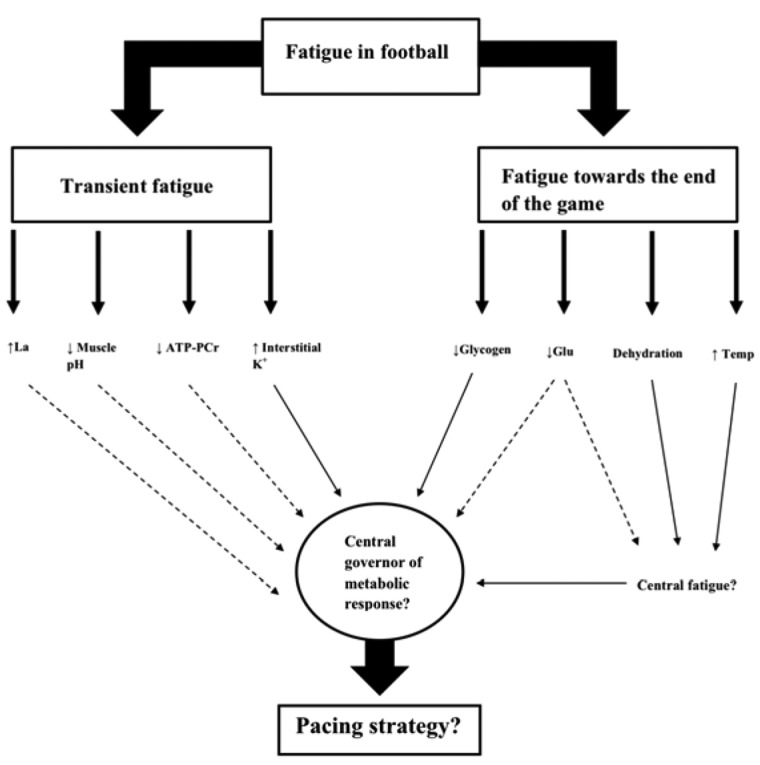
Fatigue and the subsequent decline in performance during football is an intricate phenomenon with several contributing factors [34]. The causes of fatigue during participation in football remain ambiguous, notwithstanding that impaired exercise performance is likely to incorporate numerous factors whose relative roles are subject to inter-individual variability [52, 64]. Regardless of these complexities, the current premise behind the likely mechanisms of fatigue during prolonged intermittent exercise (i.e. football) are presented in the current review (Fig. 1). Recent evidence has undermined the involvement of lactate accumulation, muscle pH disturbances, PCr depletion and energy deficiency in development of transient fatigue following intense periods in football [26]. One proposed candidate remains to be the accumulation of plasma K+ and the subsequent electrical disturbances on the muscle membrane structures [34], which appears to be concurrent with recent single-fiber investigations as a plausible cause of exercise fatigue [65]. This possibility in football, however, requires further investigations as only one study had been conducted under methodological limitations [26].
Fig. 1.
Flow diagram illustrating the proposed causes of fatigue occurring transiently and towards the end of a football match. Dashed arrows indicate an unlikely cause of fatigue. Closed arrows indicated possible causes of fatigue in football. Adapted from [3, 7, 11, 19, 26, 55, 58, 66].
The comprehension of the underlying mechanisms behind the onset of fatigue towards the end of the game are arguably more critical, given that the majority of football players succumb to this type of fatigue [2]. Accumulating evidence parallels fatigue at the latter stages of the game to a glycogen-depleted state [13, 28, 53]. This comes in agreement with prolonged cycling-based exercise with similar intensities to football (70% Vo2max), where glycogen depletion coincided with the development of fatigue [67]. An intriguing observation in football-specific exercise is that fatigue in such activities concurred with a precipitous decline of glycogen to critically low levels [43, 53] further reinforcing the notion that glycogen depletion is a major factor in the decrement of performance towards the final stages of the game. It is also noteworthy that central fatigue may substantially influence a decline in performance during match-play [66]. The exercise-induced stress of football has been shown to include factors that are related to the deteriorations in central nervous system (CNS) function such as hyperthermia, hypoglycemia and hyperammoniaemia[62, 68]. Therefore, it is premature to discount the central component in the fatigue process in football [66].
CONCLUSION
In conclusion, the determination of a single metabolic factor that can be causally related to the development of fatigue during football remains to be established. Rather, it has been recently speculated that a central metabolic control system (CNS) may govern the peripheral physiological responses (i.e. fluid loss, metabolite accumulation, core temperature), such that players may be adopting pacing strategies during the game to counteract the potential failure of any peripheral physiological system and thus may ascribe for the absence of a single metabolic factor to fatigue [11].
ACKNOWLEDGMENTS
The author would like to express his gratitude to the Saudi Arabian Ministry of Higher Education for their funding of the postgraduate program.
REFERENCES
- 1.Reilly T, Drust B, Clarke N. Muscle fatigue during football match-play. Sports Med. 2008;38:357–67. doi: 10.2165/00007256-200838050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Mohr M, Krustrup P, Bangsbo J. Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci. 2003;21:519–28. doi: 10.1080/0264041031000071182. [DOI] [PubMed] [Google Scholar]
- 3.Bangsbo J, Mohr M, Krustrup P. Physical and metabolic demands of training and match-play in the elite football player. Journal of Sports Sciences. 2006;24:665–74. doi: 10.1080/02640410500482529. [DOI] [PubMed] [Google Scholar]
- 4.Osgnach C, Poser S, Bernardini R, Rinaldo R, di Prampero PE. Energy cost and metabolic power in elite soccer: a new match analysis approach. Med Sci Sports Exerc. 2010;42:170–8. doi: 10.1249/MSS.0b013e3181ae5cfd. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong LE. Nutritional strategies for football: counteracting heat, cold, high altitude, and jet lag. J Sports Sci. 2006;24:723–40. doi: 10.1080/02640410500482891. [DOI] [PubMed] [Google Scholar]
- 6.Dellal A, Chamari K, Wong DP, et al. Comparison of physical and technical performance in European soccer match-play: FA Premier League and La Liga. Eur J Sport Sci. 2011;11:51–9. [Google Scholar]
- 7.Reilly T. The Science of Training – Soccer. London: Routledge; 2007. [Google Scholar]
- 8.Pinnington HC, Dawson B. The energy cost of running on grass compared to soft dry beach sand. J Sci Med Sport/Sports Med Aust. 2001;4:416–30. doi: 10.1016/s1440-2440(01)80051-7. [DOI] [PubMed] [Google Scholar]
- 9.Fauno P, Jakobsen WB. Mechanism of anterior cruciate ligament injuries in soccer. Int J Sports Med. 2006;27:75–79. doi: 10.1055/s-2005-837485. [DOI] [PubMed] [Google Scholar]
- 10.Reilly T, Ekblom B. The use of recovery methods post-exercise. J Sports Sci. 2005;23:619–27. doi: 10.1080/02640410400021302. [DOI] [PubMed] [Google Scholar]
- 11.Edwards AM, Noakes TD. Dehydration: cause of fatigue or sign of pacing in elite soccer? Sports Med. 2009;39:1–13. doi: 10.2165/00007256-200939010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Carling C, Bloomfield J, Nelsen L, Reilly T. The role of motion analysis in elite soccer: contemporary performance measurement techniques and work rate data. Sports Med. 2008;38:839–62. doi: 10.2165/00007256-200838100-00004. [DOI] [PubMed] [Google Scholar]
- 13.Saltin B. Metabolic fundamentals in exercise. Med Science Sports. 1973;5:137–46. [PubMed] [Google Scholar]
- 14.Barros RML, Misuta MS, Menezes RP, et al. Analysis of the distances covered by first division Brazilian soccer players obtained with an automatic tracking method. J Sports Sci Med. 2007;6:233–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Bangsbo J, Norregaard L, Thorso F. Activity profile of competition soccer. Can J Sport Sci. 1991;16:110–6. [PubMed] [Google Scholar]
- 16.Di Salvo V, Baron R, Tschan H, et al. Performance characteristics according to playing position in elite soccer. In J Sports Med. 2007;28:222–7. doi: 10.1055/s-2006-924294. [DOI] [PubMed] [Google Scholar]
- 17.Bradley PS, Sheldon W, Wooster B, et al. High-intensity running in English FA Premier League soccer matches. J Sports Sci. 2009;27:159–68. doi: 10.1080/02640410802512775. [DOI] [PubMed] [Google Scholar]
- 18.Rienzi E, Drust B, Reilly T, et al. Investigation of anthropometric and work-rate profiles of elite South American international soccer players. J Sports Med Phys Fitness. 2000;40:162–9. [PubMed] [Google Scholar]
- 19.Mohr M, Krustrup P, Bangsbo J. Fatigue in soccer: a brief review. J Sports Sci. 2005;23:593–9. doi: 10.1080/02640410400021286. [DOI] [PubMed] [Google Scholar]
- 20.Stolen T, Chamari K, Castagna C, Wisloff U. Physiology of soccer: an update. Sports Med. 2005;35:501–36. doi: 10.2165/00007256-200535060-00004. [DOI] [PubMed] [Google Scholar]
- 21.Krustrup P, Mohr M, Ellingsgaard H, Bangsbo J. Physical demands during an elite female soccer game: importance of training status. Med Sci Sports Exerc. 2005;37:1242–8. doi: 10.1249/01.mss.0000170062.73981.94. [DOI] [PubMed] [Google Scholar]
- 22.Bangsbo J. The physiology of soccer–with special reference to intense intermittent exercise. Acta Physiol Scand. 1994;619:1–155. [PubMed] [Google Scholar]
- 23.Rhea MR, Lavinge DM, Robbins P, et al. Metabolic conditioning among soccer players. J Strength Cond Res. 2009;23:800–6. doi: 10.1519/JSC.0b013e3181a330b6. [DOI] [PubMed] [Google Scholar]
- 24.Rampinini E, Coutts AJ, Castagna C, et al. Variation in top level soccer match performance. Int J Sports Mede. 2007;28:1018–24. doi: 10.1055/s-2007-965158. [DOI] [PubMed] [Google Scholar]
- 25.Di Salvo V, Gregson W, Atkinson G, et al. Analysis of high intensity activity in Premier League soccer. Int J Sports Med. 2009;30:205–12. doi: 10.1055/s-0028-1105950. [DOI] [PubMed] [Google Scholar]
- 26.Krustrup P, Mohr M, Steensberg A, Bencke J, et al. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38:1165–74. doi: 10.1249/01.mss.0000222845.89262.cd. [DOI] [PubMed] [Google Scholar]
- 27.Åstrand P-O, Rodahl K, Dahl HA, Strømme SB. Textbook of Work Physiology 4th edition. 4th ed. Champaign; IL: Human Kinetics; 2003. [Google Scholar]
- 28.Balsom PD, Gaitanos GC, Soderlund K, Ekblom B. High-intensity exercise and muscle glycogen availability in humans. Acta Physiol Scand. 1999;165:337–345. doi: 10.1046/j.1365-201x.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves M. Carbohydrate and lipid requirements of soccer. J Sports Sci. 1994;12:S13–6. [PubMed] [Google Scholar]
- 30.McInerney P, Lessard SJ, Burke LM, et al. Failure to repeatedly supercompensate muscle glycogen stores in highly trained men. Med Sci Sports Exerc. 2005;37:404–11. doi: 10.1249/01.mss.0000155699.51360.2f. [DOI] [PubMed] [Google Scholar]
- 31.Romijn J, Coyle E, Sidossis L, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metabol. 1993;265:E380. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 32.Leatt PB, Jacobs I. Effect of glucose polymer ingestion on glycogen depletion during a soccer match. Canadian J Sport Sci. 1989;14:112–6. [PubMed] [Google Scholar]
- 33.Shephard RJ. Biology and medicine of soccer: an update. J Sports Sci. 1999;17:757–86. doi: 10.1080/026404199365498. [DOI] [PubMed] [Google Scholar]
- 34.Bangsbo J, Iaia FM, Krustrup P. Metabolic response and fatigue in soccer. Int J Sports Physiol Perform. 2007;2:111–27. doi: 10.1123/ijspp.2.2.111. [DOI] [PubMed] [Google Scholar]
- 35.Ekblom B. Applied physiology of soccer. Sports Med. 1986;3:50–60. doi: 10.2165/00007256-198603010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Alghannam AF. Carbohydrate-protein ingestion improves subsequent running capacity towards the end of a football-specific intermittent exercise. Applied Physiol Nutr Metabol. 2011;36:748–57. doi: 10.1139/h11-097. [DOI] [PubMed] [Google Scholar]
- 37.Hawley JA, Tipton KD, Millard-Stafford ML. Promoting training adaptations through nutritional interventions. J Sports Sci. 2006;24:709–21. doi: 10.1080/02640410500482727. [DOI] [PubMed] [Google Scholar]
- 38.Galbo H. Hormonal and metabolic adaptation to exercise. New York: Thieme- Stratton.; 1983. pp. 1–144. [Google Scholar]
- 39.Lemon PW. Protein requirements of soccer. J Sports Sci. 1994;12:S17–22. [PubMed] [Google Scholar]
- 40.Wagenmakers AJ, Beckers EJ, Brouns F, et al. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am J Physiol. 1991;260:E883–90. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- 41.Maclaren D. Nutrition. In: Reilly T, Williams AM, editors. Science and Soccer. 2nd ed. London: Routledge; 2003. pp. 1–144. [Google Scholar]
- 42.Wagenmakers AJM, Brookes JH, Conley JH, et al. Exercise-induced activities of the branched-chain 2-oxo acid dehydrogenase in human muscle. Exerc Sport Sci Reviews. 1989;59:159–67. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- 43.Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–27. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krustrup P, Mohr M, Amstrup T, et al. The yo-yo intermittent recovery test: physiological response, reliability, and validity. Med Sci Sports Exerc. 2003;35:697–705. doi: 10.1249/01.MSS.0000058441.94520.32. [DOI] [PubMed] [Google Scholar]
- 45.Van Someren KA. The physiology of anaerobic endurance training. In: Whyte G, editor. The Physiology of Training. UK: Churchill Livingstone; 2006. pp. 85–115. [Google Scholar]
- 46.Soderlund K, Hultman E. ATP and phosphocreatine changes in single human muscle fibers after intense electrical stimulation. Am J Physiol. 1991;261:E737–41. doi: 10.1152/ajpendo.1991.261.6.E737. [DOI] [PubMed] [Google Scholar]
- 47.Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol. 1996;80:876–84. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 48.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–96. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordsborg N, Mohr M, Pedersen LD, et al. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–8. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs I, Westlin N, Karlsson J, et al. Muscle glycogen and diet in elite soccer players. Eur J Appl Physiol Occup Physiol. 1982;48:297–302. doi: 10.1007/BF00430219. [DOI] [PubMed] [Google Scholar]
- 52.Sahlin K. Metabolic Factors in Fatigue. In: Hargreaves MS L, editor. Exercise Metabolism. 2nd ed. Champaign; IL: Human Kinetics; 2006. pp. 163–86. [Google Scholar]
- 53.Nicholas CW, Tsintzas K, Boobis L, Williams C. Carbohydrate-electrolyte ingestion during intermittent high-intensity running. Med Sci Sports Exerc. 1999;31:1280–6. doi: 10.1097/00005768-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery. J Sports Sci. 2004;22:15–30. doi: 10.1080/0264041031000140527. [DOI] [PubMed] [Google Scholar]
- 55.Mohr M, Mujika I, Santisteban J, et al. Examination of fatigue development in elite soccer in a hot environment: a multi-experimental approach. Scand J Med Sci Sports. 2010;20:125–32. doi: 10.1111/j.1600-0838.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- 56.Shirreffs SM, Sawka MN, Stone M. Water and electrolyte needs for football training and match-play. J Sports Sci. 2006;24:699–707. doi: 10.1080/02640410500482677. [DOI] [PubMed] [Google Scholar]
- 57.Mustafa KY, Mahmoud NE. Evaporative water loss in African soccer players. J Sports Med Physical Fitness. 1979;19:181–3. [PubMed] [Google Scholar]
- 58.Edwards AM, Mann ME, Marfell-Jones MJ, et al. Influence of moderate dehydration on soccer performance: physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br J Sports Med. 2007;41:385–91. doi: 10.1136/bjsm.2006.033860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman JR, Maresh CM, Armstrong LE, et al. Effects of hydration state on plasma testosterone, cortisol and catecholamine concentrations before and during mild exercise at elevated temperature. Eur J Appl Physiol Occup Physiol. 1994;69:294–300. doi: 10.1007/BF00392033. [DOI] [PubMed] [Google Scholar]
- 60.Ozgunen KT, Kurdak SS, Maughan RJ, et al. Effect of hot environmental conditions on physical activity patterns and temperature response of football players. Scand J Med Sci Sports. 2010;20:140–7. doi: 10.1111/j.1600-0838.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- 61.Maughan RJ, Shirreffs SM, Ozgunen KT, et al. Living, training and playing in the heat: challenges to the football player and strategies for coping with environmental extremes. Scand J Med Sci Sports. 2010;20:117–24. doi: 10.1111/j.1600-0838.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 62.Nybo L, Secher NH. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol. 2004;72:223–61. doi: 10.1016/j.pneurobio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Mohr M, Krustrup P, Nybo L, et al. Muscle temperature and sprint performance during soccer matches–beneficial effect of re-warm-up at half-time. Scand J Med Sci Sports. 2004;14:156–62. doi: 10.1111/j.1600-0838.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- 64.Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39:389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- 65.Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol. 2010;110:1–15. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- 66.Meeusen R, Watson P, Dvorak J. The brain and fatigue: new opportunities for nutritional interventions? J Sports Sci. 2006;24:773–82. doi: 10.1080/02640410500483022. [DOI] [PubMed] [Google Scholar]
- 67.Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–50. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 68.Nybo L, Dalsgaard MK, Steensberg A, et al. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J Physiol. 2005;563:285–90. doi: 10.1113/jphysiol.2004.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]