Abstract
Background
Topoisomerase 2α (Topo 2α) is a nuclear enzyme that alters the topology of DNA. It’s essential for normal chromosome segregation during cellular division. We aimed to investigate the association of Topo 2α expression with clinical, pathological parameters and prognosis in surgically resected non-small cell lung cancer (NSCLC) patients.
Methods
The study is comprised of 100 surgically resected NSCLC (squamous cell carcinoma in 50 patients, adenocarcinoma in 50 patients). The paraffin embedded tumor sections were retrieved for expression of Topo 2α. Nuclear and cytoplasmic expression of Topo 2α was determined by immunohistochemistry. Clinical, pathological data and survival of patients were determined from the hospital files. Median follow-up time was 35 (range, 4-120) months.
Results
Nuclear and cytoplasmic expression of Topo 2α was positive in 41 (41%) and 66 (66%) patients, respectively. There was no significant association between nuclear or cytoplasmic expression of Topo 2α and age, gender, smoking history. While nuclear expression was significantly increased in squamous cell carcinoma (P=0.008), OR (95% CI): 3.01 (1.31-6.92), cytoplasmic expression wasn’t different. Both nuclear and cytoplasmic expression didn’t show any association with tumor diameter, pathological stage, tumor differentiation and relapse. There was no significant association between nuclear or cytoplasmic expression of Topo 2α and survival. Tumor diameter (P=0.031) and metastasis to N2 lymph nodes (P=0.005) were independent prognostic factors.
Conclusions
There was no association between Topo 2α expression and prognosis in surgically resected NSCLC patients. Nuclear expression of Topo 2α was significantly higher in patients with squamous cell carcinoma.
Key Words : Non-small cell lung cancer, topoisomerase 2α, prognosis
Introduction
Lung cancer is a group of heterogeneous clinical entities with common molecular and cellular origins, but different accumulated genetic mutations with different clinical behaviors and prognoses (1). The outcome of a lung cancer patient depends on a variety of variables defined as prognostic factors (2). The recurrence rates of surgically resected non-small cell lung cancer (NSCLC) patients are highly variable changing between 20% to 85% (3). The principal factors related to the recurrence are tumor stage, histology, localization, adequacy of mediastinal dissection and administration of adjuvant chemotherapy. There are also new and promising molecular/biologic markers that are not utilized routinely for determination of prognosis such as regulators of cellular growth (kRAS, EGFR, RB), of the metastatic cascade (TPA, Cyclin-D1, cathepsin) and of apoptosis (p53, bcl-2) (1-4).
Topoisomerase 2 (Topo 2) is a nuclear enzyme that alters the topology of DNA and is essential for normal chromosome segregation at mitosis. In mammalian cells, there are two isoforms as Topo 2α and Topo 2β. Topo 2α is considered a specific marker of cell proliferation in both normal and neoplastic tissues (5). It is also a target for some chemotherapeutic agents in clinical use (6). Previous clinical studies have shown that Topo 2α expression reflects several biologic behaviours in human cancers such as lung carcinomas (7,8). In a small study by Guineeet al., Topo 2α expression was higher in frequency in SCLC compared with NSCLC (8). In a study by Dingemanset al., high expression of Topo 2α was predictive of worse survival and high expression of Topo 2β predictive of lower chemotherapy response rates in patients with small cell lung cancer (SCLC) (9).
In this study, we aimed to investigate the association of Topo 2α expression with clinical (age, gender, smoking history, administration of adjuvant chemotherapy), pathological parameters (tumor histology, stage, tumor diameter, involvement of lymph nodes, differentiation) and prognosis in surgically resected NSCLC patients.
Material and methods
Patients
This study is approved by the local ethic comittee of Ataturk Chest Diseases and Chest Surgery Education and Research Hospital. Informed consent waived for this retrospective study. Surgically resected tumor specimens from 100 randomly selected NSCLC patients who were not treated with preoperative chemotherapy were studied. Hospital files were reviewed for clinical data. There were 91 male and 9 female patients with a mean age of 59.7±10.5 years (range, 37-78 years). All patients underwent lobectomy (82 patients) or pneumonectomy (18 patients) with hilar and mediastinal lymph node dissection. Pathological stage was determined according to the 7th edition of TNM staging system (10). Histopathological diagnosis was squamous cell carcinoma and adenocarcinoma in 50 patients each. There were 5 patients with stage 1a, 28 patients with stage 1b, 21 patients with stage 2a, 21 patients with stage 2b, 25 patients with stage 3a. The clinical and pathological characteristics of patients are seen in Table 1. Twenty-nine patients received adjuvant cisplatin based chemotherapy regimens, 15 patients adjuvant radiotherapy and 6 patients both adjuvant chemotherapy and radiotherapy.
Table 1. The clinical and pathological characteristics of patients.
| Variables | Number of patients (n) | Proportion | Ranges |
|---|---|---|---|
| Gender Female Male |
9 91 |
9% 91% |
- - |
| Mean age (years) | - | - | 59.7±10.5 (range, 37-78) |
| Non-smoker® Smoker |
9 90 |
9% 90% |
- - |
| Operation type Pneumonectomy Lobectomy |
18 (14 left/4 right) 82 |
- - |
- - |
| Histopathology Squamous cell carcinoma Adenocarcinoma |
50 50 |
- - |
- - |
| Mean tumor diameter (cm) | 4.9±2.1 (1-12) | ||
| Tumor stage 1A 1B 2A 2B 3A |
5 28 21 21 25 |
5% 28% 21% 21% 21% (N2 involvement in 22 patients) |
- - - - - |
| Differentiation Well Moderate Poor |
57 31 12 |
57% 31% 12 |
- - - |
| Adjuvant therapy Chemotherapy* Radiotherapy Chemotherapy + Radiotherapy |
29 15 6 |
- - - |
- - - |
*Cisplatin-based chemotherapy regimens.
Nine patients who died due to postoperative complications or toxicities due to adjuvant chemotherapies were excluded from survival analysis. Median follow up time was 35 [4-120] months. There were 49 (53.8%) relapses during the follow up period. Mean relapse time was 35.5±25.7 [3-120] months.
Immunohistochemistry
All slides of the patients were reviewed. Representative blocks were selected for immunohistochemistry. Tissue samples were fixed in 10% buffered formalin embedded in parafin and cut at 6 µm for immunohistochemistry. Sections were dewaxed in xylene substitute (ThermoScientific) and hydrated with a graded series of ethanol concentrations and water.
Immunostaining of Topo 2α was performed using the streptavidin-biotin complex kit (ThermoScientific, Fremon, USA). Sections were incubated with primary antibody solution for Topo 2α Ab-4 (ThermoScientific) at a dilution of 1:20 for 30 minutes at room temperature. Diaminobenzidine was used as the chromogen. After incubation, the chromogen specimens were counterstained with Hari shematoxylin and coverslipped. Samples of tonsil tissues were used as positive control. Negative controls were performed by substituting without primary antibody.
The intensity of Topo 2αimmunostaining was evaluated by light microscopy (Labsphot-2; Nikon, Tokyo, Japan). The expression of Topo 2α was evaluated as nuclear and cytoplasmic. More than 10% of nuclear positive cells were considered as nuclear positivity. Cytoplasmic immunoreactivity was evaluated based on the percentage of positive tumor cells and scored as negative: no cytoplasmic Topo 2α staining, weak (1+): 0-30% staining, moderate (2+): 31-60% staining, and intense (3+): more than 60% staining.
Statistical analysis
Statistical analysis was performed using SPSS for windows release 11.5 package program. Univariate Logistic Regression Analysis was performed to analize the relation between expression of nucleer or cytoplasmic expression of Topo 2α and clinical and pathological findings. Survival curves were computed by using method of Kaplan Meier. In order to evaluate the independent prognostic relevance of nuclear and cytoplasmic expression of Topo 2α, we performed multivariate analysis using Cox Regression model. A value of P<0.05 was accepted as statistically significant.
Results
Nuclear and cytoplasmic expression of Topo 2α in tumor tissues
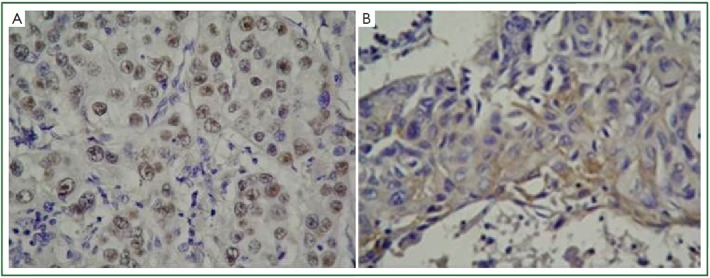
Nuclear expression of Topo 2α was positive in 41 (41%) and negative in 59 (59%) specimens. Cytoplasmic expression of Topo 2α was positive in 66 (66%) and negative in 34 (34%) specimens. Cytoplasmic expression was weak in 33, moderate in 13 and intense in 20 specimens. Figure 1 demonstrates intranuclear expression of Topo 2α in an adenocarcinoma and intracytoplasmic expression of Topo 2α in a squamous cell carcinoma respectively.
Figure 1.
Intranuclear expression of topoisomerase 2α in an adenocarcinoma (A), Intracytoplasmic expression of topoisomerase 2α in a squamous cell carcinoma (B) (Topo 2α ×200).
The association of Topo 2α expression with clinical and pathological characteristics
There was not any significant association between nuclear or cytoplasmic expression of Topo 2α and patient age, gender, smoking history (P>0.05). While cytoplasmic expression of Topo 2α was not different, nuclear expression was significantly increased in squamous cell carcinoma (P=0.008), OR (95% CI): 3.01 (1.31-6.92). Both nuclear and cytoplasmic expression of Topo 2α did not show any association with tumor diameter, pathological stage, tumor differentiation and presence of relapse.
The association of Topo 2α expression and survival
The median overall survival was 34 [4-120] months. At the end of study 47 (51.6%) patients were dead and 44 (48.4%) patients were alive. There wasn’t any significant association between nuclear or cytoplasmic expression of Topo 2α and survival (P>0.05).
Multivariate analysis related to survival using Cox regression is shown in Table 2. Tumor diameter (P=0.031) and involvement of N2 lymph nodes (P=0.005) were independent prognostic factors. They were related with poor prognosis.
Table 2. Multivariate analysis related to survival using Cox regression.
| Variables | OR | 95% CI | P | |
|---|---|---|---|---|
| Age | 0.98 | 0.95-1.01 | 0.24 | |
| Female® Male |
- 1.04 |
- 0.37-2.92 |
- 0.92 |
|
| Non-smoker® Smoker |
- 1.41 |
- 0.50-3.93 |
- 0.51 |
|
| Squamous® Adenocarcinoma |
- 1.40 |
- 0.78-2.51 |
- 0.25 |
|
| Tumor diameter | 1.17 | 1.01-1.35 | 0.031* | |
| Tumor stage 1A® 1B 2A 2B 3A |
- 2.23 4.39 1.49 6.82 |
- 0.28-17.3 0.56-34.1 0.17-12.9 0.89-52.1 |
- 0.44 0.15 0.71 0.06 |
|
| N0® N1 N2 |
- 1.47 2.79 |
- 0.74-2.94 1.36-5.74 |
- 0.26 0.005* |
|
| Differentiation Poor® Moderate Well |
- 0.41 0.73 |
- 0.16-1.06 0.32-1.67 |
- 0.06 0.46 |
|
| Lobectomy® Pneumonectomy |
- 1.57 |
- 0.75-3.26 |
- 0.22 |
|
| Adjuvant therapy Given® Not given |
- 0.66 |
- 0.37-1,20 |
- 0.17 |
|
| Nuclear topo2α Negative® Positive |
- 1.24 |
- 0.69-2.22 |
- 0.46 |
|
| Cytoplasmic topo2α Negative® + ++ +++ |
- 1.04 1.97 1.28 |
- 0.49-1.22 0.86-4.47 0.57-2.90 |
- 0.90 0.10 0.54 |
|
®Reference category, *Statistically significant.
Discussion
Topo 2 is an essential nuclear enzyme that catalyzes the changes in the topology of DNA. It plays a critical role during mitosis for chromosome condensation and segregation, in both neoplastic and nonneoplastic cells (5). There are two isoforms of Topo 2 in mammalian cells: Topo 2α and Topo 2β. In the experimental studies on cell cycles, Topo 2α expression was undetectable until the cells reached to late S phase, peaked in G2-M phase and decreased after mitosis. Topo 2β expression was constant through the cell cycle (11). Studies including specimens of lung carcinoma patients revealed that Topo 2α gene expression was significantly higher in tumor tissues compared to normal tissues. But Topo 2β gene expression was not different between tumor tissues and normal lung tissues (7,12,13). Therefore Topo 2α expression is a specific marker of cell proliferation and is thought to be related to poor prognosis.
There are several studies reporting that high expression of Topo 2α was associated with poor prognosis in lung cancer patients (9,14). In a study including tumor samples derived from 93 previously untreated SCLC patients, survival was shorter in patients with extensive disease, poorer performance status, and in patients whose tumors expressed high Topo 2α and Ki67 levels. High Topo 2β expression was found to be predictive for lower chemotherapy response rates. The authors concluded that immunohistochemical assessment of these markers in diagnostic biopsies may give important prognostic information and may help selecting patients in the worse prognostic categories for novel therapeutic agents (9). Topo 2α was also studied as a drug resistance marker in advanced NSCLC patients. There wasn’t any relation between Topo 2α expression and response to chemotherapy. But they observed a shorter survival in patients with high Topo 2α levels. They explained the shorter survival rate with higher Topo 2α expression, higher proliferation rate and increased aggressiveness (14). In the present study, since we studied patients who had a curative surgery, our hypothesis was “Higher Topo 2α expression might be related to early recurrence or in other words poorer survival”. But we couldn’t find a relation between Topo 2α expression and survival.
Studies investigating the biological difference between NSCLC and SCLC observed a lower Topo 2α expression in NSCLC compared to SCLC patients (7,8,15). This stituation can be an explanation for the difference in chemosensitivity. Higher Topo 2α expressing tumors are more chemosensitive than lower Topo 2α expressing tumors. In a study of Giacconeet al., higher expression of Topo 2α was correlated not only with sensitivity to Topo 2α inhibitors, but also with sensitivity to other classes of chemotherapeutic agents. They postulated that Topo 2α might be an essential component of a common pathway of cell death which is triggered by multiple or all antineoplastic agents (16). In another study involving 103 squamous cell carcinomas of the head and neck, higher Topo 2α expression was significantly related to better response to chemotherapy, despite the cytotoxic drugs used was not Topo 2α antagonists (17). In this study we observed, 41% nuclear expression and 66% cytoplasmic expression of Topo 2α. These ratios are high enough not to be ignored. Whereas most NSCLC patients are resistant to Topo 2α drugs, it may be possible to predict the minority of tumors which are sensitive. Larger prospective studies comparing the chemotherapy responses in Topo 2α expressing and not expressing NSCLC are needed. In contrary to the study of Dingemanset al. (14), the demonstration of higher chemosensitivity in higher Topo 2α expressing tumors would be a milestone in managing NSCLC patients.
There are a few studies investigating the difference of Topo 2α expression in the histological subtypes of NSCLC (13,18). In these studies they analyse Topo 2α gene expression by PCR method. In the study by Liu et al., Topo 2α gene expression was significantly higher in squamous cell carcinomas than in adenocarcinomas (18). Conversely to this study, Mirskiet al. found that Topo 2α levels were lower in squamous cell carcinomas than in adenocarcinomas (13). In the present study we investigate the intensity of immunostaining Topo 2α in tumor tissues. We found a significantly higher nuclear expression of Topo 2α in patients with squamous cell carcinoma.
There are also studies showing the association of Topo 2α gene expression and tumor differentiation in breast carcinomas, head and neck carcinomas and lung cancer (17-19). In a study including surgically resected tumor specimens from 98 NSCLC patients, Topo 2α gene expression was significantly higher in moderately and poorly differentiated tumors compared to well differentiated ones. The overexpression of Topo 2α gene was associated with more aggressive carcinogenesis, accelerated cell proliferation and tumor dedifferentiation. There wasn’t any statistically significant relation between Topo 2α gene expression and gender or pathological tumor stage (18). In our study we couldn’t find a relation between Topo 2α expression and tumoral differentiation.
There are a few studies investigating the association of Topo 2α expression with clinical parameters such as age and gender. As in this study they did not find any relation (18,20). In this study we didn’t find an association between Topo 2α expression and smoking history. In study of Liu et al., Topo 2α expression was higher in smokers (18). The reason for this difference may be a relatively higher ratio of smoking history (90% present study, 63% Liu et al.) in the present study.
In conclusion; in this study we determined both the nuclear and cytoplasmic expression of Topo 2α in tumor specimens of surgically resected NSCLC patients by immunohistochemistry. We investigated the association of both nuclear and cytoplasmic expression of Topo 2α with clinical (age, gender, smoking history, administration of adjuvant chemotherapy), pathological parameters (tumor histology, stage, tumor diameter, involvement of lymph nodes, differentiation) and prognosis. We couldn’t find any association between Topo 2α expression and clinical findings. Nuclear expression of Topo 2α was significantly higher in patients with squamous cell carcinoma. There wasn’t any association between Topo 2α expression and survival. As expected tumor diameter and involvement of N2 lymph nodes were independent prognostic parameters in multivariate analysis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Brundage MD, Mackillop WJ. Prognostic factors in thoracic malignancies. In: Goldstraw P. eds. IASLC Manual - Staging Manual in Thoracic Oncology. Orange Park, FL, USA: Editorial Rx Press. 2009:129-40. [Google Scholar]
- 2.Gospodarowicz MK, O’Sullivan B, Koh ES. Prognostic factors: Principles and applications. In: Peter Goldstraw. eds. IASLC Manual - Staging Manual in Thoracic Oncology. Orange Park, FL, USA: Editorial Rx Press. 2009:111-28. [Google Scholar]
- 3.Brundage MD, Mackillop WJ. Lung cancer. In: Gospodarowicz MK HD, Hutter RVP, O’Sullivan B, et al. eds. Prognostic Factors in Cancer. New York: Wiley-Liss, 2001:351-70. [Google Scholar]
- 4.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 2002;122:1037-57 [DOI] [PubMed] [Google Scholar]
- 5.Heck MM, Earnshaw WC, Topoisomerase II: A specific marker for cell proliferation. J Cell Biol 1986;103:2569-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Froelich-Ammon SJ, Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem 1995;270:21429-32 [DOI] [PubMed] [Google Scholar]
- 7.Syahruddin E, Oguri T, Takahashi T, et al. Differential expression of DNA topoisomerase II alpha and II beta genes between small cell and non-small cell lung cancer. Jpn J Cancer Res 1998;89:855-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinee DG, Jr, Holden JA, Benfield JR, et al. Comparison of DNA topoisomerase II alpha expression in small cell and nonsmall cell carcinoma of the lung. In search of a mechanism of chemotherapeutic response. Cancer 1996;78:729-35 [DOI] [PubMed] [Google Scholar]
- 9.Dingemans AM, Witlox MA, Stallaert RA, et al. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res 1999;5:2048-58 [PubMed] [Google Scholar]
- 10.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71 [DOI] [PubMed] [Google Scholar]
- 11.Woessner RD, Mattern MR, Mirabelli CK, et al. Proliferation-and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ 1991;2:209-14 [PubMed] [Google Scholar]
- 12.Giaccone G, van Ark-Otte J, Scagliotti G, et al. Differential expression of DNA topoisomerases in non-small cell lung cancer and normal lung. Biochim Biophys Acta 1995;1264:337-46 [DOI] [PubMed] [Google Scholar]
- 13.Mirski SE, Voskoglou-Nomikos T, Young LC, et al. Simultaneous quantitation of topoisomerase II alpha and beta isoform mRNAs in lung tumor cells and normal and malignant lung tissue. Lab Invest 2000;80:787-95 [DOI] [PubMed] [Google Scholar]
- 14.Dingemans AC, van Ark-Otte J, Span S, et al. Topoisomerase IIalpha and other drug resistance markers in advanced non-small cell lung cancer. Lung Cancer 2001;32:117-28 [DOI] [PubMed] [Google Scholar]
- 15.Kreisholt J, Sorensen M, Jensen PB, et al. Immunohistochemical detection of DNA topoisomerase IIalpha, P-glycoprotein and multidrug resistance protein (MRP) in small-cell and non-small-cell lung cancer. Br J Cancer 1998;77:1469-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giaccone G, Gazdar AF, Beck H, et al. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res 1992;52:1666-74 [PubMed] [Google Scholar]
- 17.Stathopoulos GP, Kapranos N, Manolopoulos L, et al. Topoisomerase II alpha expression in squamous cell carcinomas of the head and neck. Anticancer Res 2000;20:177-82 [PubMed] [Google Scholar]
- 18.Liu D, Huang CL, Kameyama K, Hayashi E, et al. Topoisomerase IIalpha gene expression is regulated by the p53 tumor suppressor gene in nonsmall cell lung carcinoma patients. Cancer 2002;94:2239-47 [DOI] [PubMed] [Google Scholar]
- 19.Nakopoulou L, Lazaris AC, Kavantzas N, et al. DNA topoisomerase II-alpha immunoreactivity as a marker of tumor aggressiveness in invasive breast cancer. Pathobiology 2000;68:137-43 [DOI] [PubMed] [Google Scholar]
- 20.Chiappori AA, Zheng Z, Chen T, et al. Features of potentially predictive biomarkers of chemotherapeutic efficacy in small cell lung cancer. J Thorac Oncol 2010;5:484-90 [DOI] [PMC free article] [PubMed] [Google Scholar]