Abstract
Objective
To investigate the relationship between respiratory viral load and lung lesion severity of patients with pandemic H1N1 2009 influenza A pneumonia.
Study design
Cross-sectional observation study.
Methods
24 consecutive H1N1 influenza patients with viral pneumonia (13 males, 11 females, mean age: 17.5 years) during their presentation to hospital were retrospectively analysed. Viral load were first measured on average 5.2 days after the onset of symptoms. The initial CT and viral load measurement was carried on the same day in 13 patients. The rest were carried out with a mean interval time of 1.5 days. All patients had viral load follow-up till turned negative. Thirteen patients had radiological follow-up.
Results
There was no significant correlation between the initial lung lesion severity and viral load (P=0.4). Both viral load and lung lesion severity decreased over time, being highest value at initial presentation. The patients had higher initial viral load or higher initial lung lesion severity tended to be slower in resolving. The lung lesion decreased at a slower rate than viral load.
Conclusions
While there was no correlation between the initial viral load and lung lesion severity, these two indices provide valuable information for epidemiological control.
Key Words : Chest X-ray; tomography, computed; pandemic; lung, infection; viral load; influenza A (H1N1)2009; real time RT-PCR
Introduction
In April of 2009, a new strain of human influenza A H1N1 virus was identified in Mexico and was characterized by a unique combination of gene segments that had not been previously identified among human or swine influenza A viruses (1-5). The 2009 H1N1 virus is a triple-reassortant influenza virus containing genes from human, swine, and avian influenza viruses (6,7). The majority of H1N1 influenza cases have been mild influenza-like illnesse (8). However, large-scale reports of hospitalized patients with H1N1 influenza in the United States demonstrated that this strain of H1N1 virus can cause severe illness, including sepsis, pneumonia, and acute respiratory distress syndrome (9,10). Cases of severe illness, such as acute respiratory distress syndrome and death, have been reported in previously healthy persons (2). This phenomenon may be attributed to the lack of pre-existing cross-reactive antibody against pandemic H1N1virus in these subjects. On the contrary, most if not all of these individuals have pre-existing antibody against the prevailing seasonal influenza virus.
Pneumonia is detected in 40% of influenza A H1N1 cases (10). The reported radiologic findings included patchy consolidation and ground-glass opacities consistent with viral pneumonia (11,12). Galit Aviram et al. reported that extensive involvement of both lungs is associated with adverse prognosis, while normal initial radiographs cannot exclude adverse outcome (13). The correlation between the virological profile and clinical characteristics of pandemic H1N1 virus infection can provide important knowledge for epidemiological control and clinical management in terms of antiviral therapy and infection control measures. It has been reported that in influenza A H5N1 virus infection, serum virus level had prognostic significance (14). The relationship between respiratory viral load and lung lesion severity of patients with influenza A H1N1 pneumonia hasn’t been reported. In this study, this correlation was investigated in 24 consecutivepatients with H1N1 viral pneumonia.
Methods
Subjects
The patient data were collected from one single hospital. All the patient data were collected from this hospital. Consecutive 24 patients diagnosed with pandemic H1N1 2009 influenza A from May 2009 to December 2009 and had H1N1 virus induced lung lesions during their presentation to the hospital were retrospectively analysed. The individuals of this study consisted of 13 males and 11 females with age ranging from 3 to 49 years (mean, 17.5 years). During this period, there were additional 20 H1N1 influenza patients without radiological lung lesions during their initial presentation. These 20 cases were not included in this analysis due to the absence of radiological findings of the lung. The institutional review board of the Shenzhen No. 3 People’s Hospital approved this study. Informed consent was waived owing to its observational nature. All the patients presented with an influenza like illness, including fever, headache, dry cough or productive cough, shortness of breath, rhinitis. The patients fulfilled the clinical criteria for confirmed pandemic H1N1 2009 influenza A infection, as established by the U.S. Centers for Disease Control and Prevention. These criteria included influenza-like symptoms and a real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay with positive results for H1N1 virus.
Among these 24 patients, one case had chronic hepatitis and one case had liver cirrhosis; one case had history of gastric ulcer; one case had history of kidney calculus; one case had history of partial thyroidectomy due to hyperthyroidism; one case had bronchial asthma during early childhood. The rest patients had no other significant medical history, and none of the other patients had any symptoms, signs, or laboratory findings indicating the presence of other additional acute illness.
The initial viral load measurement and first time CT were both performed prior to anti-viral treatment. Respiratory viral load was first measured on average 5.2 days after the onset of symptoms (range, 2-9 days). The first CT and first viral load measurement was carried on the same day in 13 patients. For the remaining patients, the first CT and first viral load measurement were carried out with an interval time of one day to 3 days (mean, 1.5 days). Once the diagnosis was confirmed, all the patients were administered orally with Oseltamivir (Tamiflu®, Roche) or Zanamavir (Relenza®, GlaxoSmithKline) and general supportive treatments.
All the 24 patients had viral load follow-up. 13 patients had additional radiological follow-up following the initial examination, with chest X-ray or CT. Radiological follow-up varied from once to four times (average 2 times) and the average follow-up interval was 7.2 days. This 13 patients’ respiratory viral load measurement follow-up varied from twice to 18 times (average 4.4 times), and the average follow-up interval was two days.
One case developed bacterial pneumonia during the course of hospitalization and antibiotic was administered. After treatment, all the patients’ clinical symptoms disappeared and virus load turned negative, and were then transferred to community health centers for further follow-up. No recurrence of symptoms or other illness was observed in the community health centers for these subjects.
Quantitative real- time RT-PCR for detection of influenza A H1N1 virus
Respiratory specimens were collected from nasopharyngeal swab or bronchoalveolar aspirate and viral load was examined by real-time RT-PCR method. Primers and probes specific for 2009 human H1N1 influenza A virus (H1 and H3 subtypes) were developed and quantitative real-time RT-PCR tests were performed for detection of the virus according to published guidelines by World Health Organization (WHO). These reagents allowed for a rapid detection of H1N1 infuenza A virus. Technical details on this assay have been published on the WHO Global Influenza Program Website (www.who.int/csr/disease/influenza/en). In order to establish copy number as a unit of viral load, the primers were designed to establish PCR assay to amplify a part of the M gene of 2009 H1N1 influenza A virus according to the published sequences online by WHO, then the amplified PCR products were cloned into vector PMD18-T and subsequently produced a new plasmid containing M gene. Concentration of the resultant new plasmid was serially diluted from 109to 103 copies/mL and quantitative real-time PCR assay was performed to detect copy number of viral load in nasopharyngeal-swab specimens of the patients using the resultant new plasmid containing M gene as a standard curve. The unit of mol can be transformed into copy number according to the following equation: (6.02×1023 copies/mol) × (concentration of plasmid g/mL)/(MW g/mol) = copies/mL. According to institutional reference, viral load value of less than 1.7 log10 copies/mL was regarded as negative value. Negative viral load was confirmed with results from two consecutive measurements.
Imaging techniques and lung lesion score
Chest CT was performed for all patients diagnosed with H1N1 influenza, including 24 patients with positive findings, and 20 patients with negative results who were not included in this analysis. CT scans were carried out on a dual slice scanner (Philips MX 4000, Philips, the Netherlands). The protocol used was as follows: end-inspiratory acquisition, 120 kV, 150-200 mAs, slice thickness 10mm, interslice gap 0.2 mm. The images were viewed on a PACS system with both lungs (window width, 1,500 HU; level, -500 HU) and mediastinal (window width, 350 HU; level, 40 HU) settings. All the studies were unenhanced. Chest X-ray or CT was used for follow-up. All posteroanterior upright radiographs were acquired with digital radiography (Hologic EPEX/O, Hologic, America), while the anteroposterior portable chest radiographs were acquired with a computed radiography machine (CR 850; Kodak Direct View systems, America).
All chest CT and chest X-ray were assessed blinded to clinical information and in consensus by two experienced radiologists regarding lesion location, lesion extent, and lesion morphology. The two reviewers were blinded to the clinical data and the patients’ outcomes. In order to assess the lung lesion extent, the lungs were classified into three zones (upper, middle, and lower); each zone was evaluated separately. Each of the three zones corresponded to approximately one-third of the images from the lung apex to 1 cm below the domes of the diaphragm (15,16). Each lung zone was assigned a score that was based on the follows: (I) score 0, 0 involvement; (II) score 1, less than 25% involvement; (III) score 2, 25% to less than 50% involvement; (IV) score 3, 50% to less than 75% involvement; and (V) score 4, 75% or greater involvement. Summation of scores provided an evaluation of overall lung involvement (maximal score for both lungs was 24).
Results
According to our inclusion criteria, all the patients had CT evidence of lung abnormalities consistent with influenza H1N1 pneumonia. The lung lesions were of typical virus infection, including ground-glass opacity (hazy areas of increased attenuation without obscuration of the underlying vessels), consolidation (homogeneous opacification of the parenchyma with obscuration of the underlying vessels), reticular opacities, linear opacities, interlobular septal thickening, and mixed pattern (Figures 1,2).
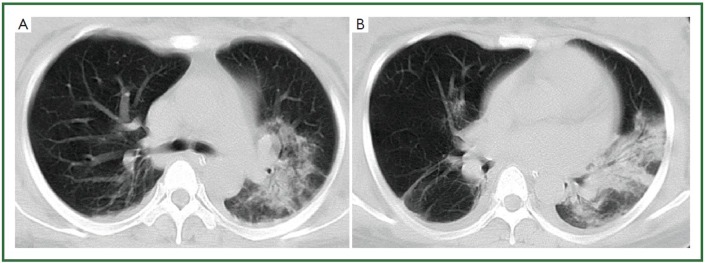
Figure 1.
28-year-old woman with H1N1 viral pneumonia. No history of chronic pulmonary disease. Chest CT was obtained day 6 after onset of influenza symptoms. CT images (A-B) demonstrate ground-glass opacities and consolidation predominantly in the middle zone and lower zone of the left lung, and minor patchy ground-glass opacities in the lower zone of the right lung. There is bilateral mild pleural involvement. The lung lesion score is 8.
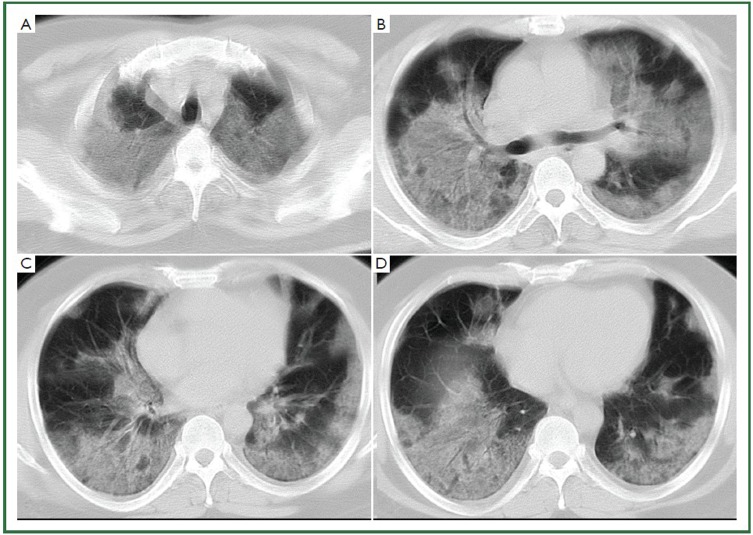
Figure 2.
44-year-old man with H1N1 viral pneumonia in critical condition. No history of chronic pulmonary disease. Chest CT was obtained 6 days after the onset of the influenza symptoms. Bilateral and widespread ground-glass opacities in all six lung zones are demonstrated (A-D). There is bilateral mild pleural effusion. The chest lesion score is 19.
The mean viral load from the first time measurement was 4.56 log10copies/mL (SD =1.62 log10 copies/mL, range, 1.70-8.35 log10 copies/mL). With the five patients whose virus load was measured within 72 hours after the onset of symptom, the viral load was 6.5 log10 copies/mL (SD =1.78, range, 3.96-8.35 log10 copies/mL). The mean lung lesion scores from the first time CT of the 24 patients was 6.6 (SD =6.63, range, 1-24). The correlation between the lung lesion and the virus load viral load was r=–0.14 (n=24 patients, Figure 3). With only the cases’ where first time CT and first time viral load measurement were performed within 48 hours were included for analysis, an r=–0.15 was obtained (n=20 patients). In both cases, the correlation between the lung lesion score and respiratory viral load was not statistically significant (P=0.4).
Figure 3.
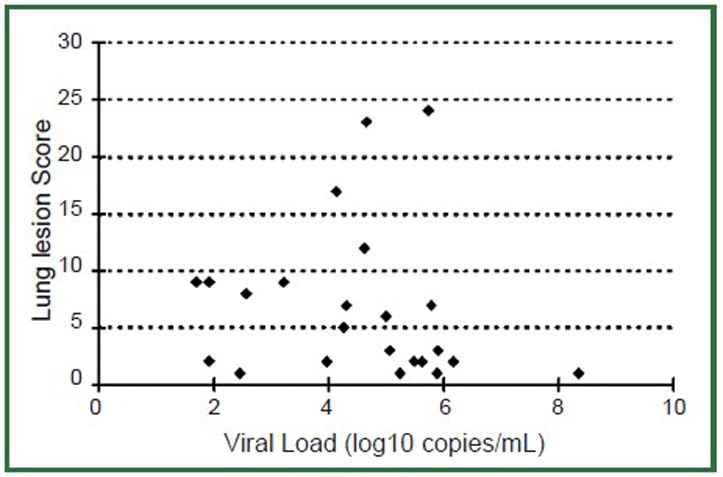
Relationship between initial respiratory viral load (x-axis) and lung lesion score (y-axis) during initial presentation to the hospital (n=24 patients). There is no significant correlation between these two indices (P=0.4).
The viral load time course and lung lesion score time course of the 13 patients with radiological exam and viral load follow-up are shown in Figures 4, 5. Figure 4 showed all the cases’ respiratory viral load decreased over time, with the highest value at the time of initial presentation. The patients had higher initial viral load tended to have longer virus shedding period. Figure 5 showed lung lesion scores were also highest in the initial presentation and decreased over time, except in one case where there is a secondary chest infection. The patients had higher initial lung lesion score were also slower in lesion resolving. Compared with the respiratory viral load decrease rate (mean slope =–0.31), the lung lesion scores decreased at a slower rate (mean slope =–0.27).
Figure 4.
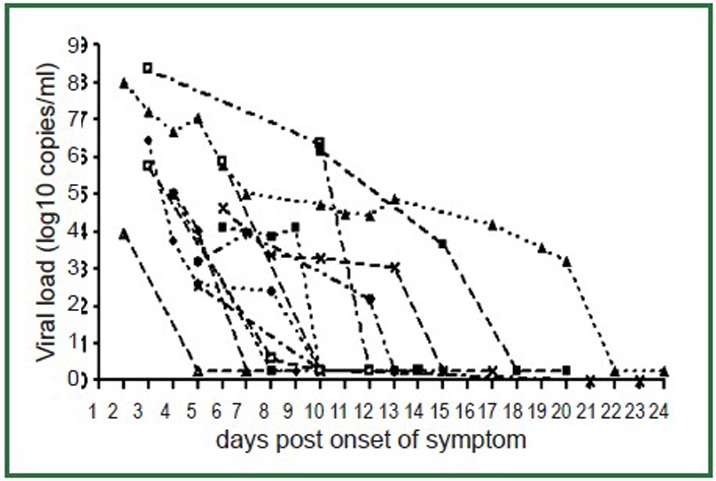
Respiratory viral load temporal change over time. Viral loads are highest in the initial presentation and, with treatment, decrease over time. The patients had higher initial viral load tend to have longer virus shedding period.
Figure5.
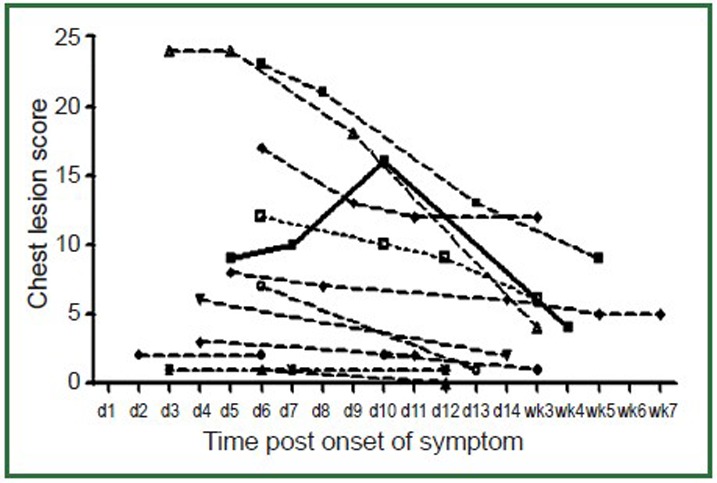
Lung lesion score temporal change over time (d1 = day 1,wk3= week 3). Lung lesion scores are highest in the initial presentation and, with treatment, decrease over time. In one case (solid thick line) there was a secondary bacterial lung infection and chest lesion score increased during the course with an eventual decrease after anti-bacterial treatment. The patients had higher lung lesion score tend to have a longer disease course.
Discussion
Specific clinical features have been reported for infections associated with the influenza A H1N1 2009 virus. Most hospitalized patients are under the age of 18 and very few are over 65 years (10). Our series had a mean age of 17.5 years. This is similar to the series reported by Toet al. where the mean age was 21 years (17). However, the demographics of our series were different from the series reported by Aviram et al., their series had a mean age of 40.4 years. All our patients recovered after treatment. This could be due to most of our patients were in healthy condition prior to this disease. It has been reported that between 40% and 70% of patients with severe symptoms have an underlying medical history (3,10).
Dynamic viral load reflects the interaction between viral replication and clearance by body defense mechanisms. To et al. reported that in patients with pandemic H1N1 virus infection, peak viral load occurred on the day of onset of symptoms, and declined gradually afterwards (17). This finding was similar to the previous studies in seasonal influenza virus infection, in which viral load was generally low or undetectable by day 5 of illness, but could persist for up to 21 days in children (18). This is due to patient’s immune system responded to infection (19,20). Anti-viral treatment may also contribute to the decrease of viral load (21). The mean respiratory viral load during presentation in our patients was lower than the data reported by To et al. (17). This is likely due to our patients presented to the hospital on average later after the symptoms than To et al.’s group. With the patients presented to hospital within 3 days after onset of symptoms (Figure 3), respiratory viral loading of 6.5±1.78 log10 copies/mL was similar to Toet al.’s data (17) (Figure 4). With the 22 pandemic H1N1 cases reported by To et al. no virus being detectable in respiratory specimens by RT-PCR 8 days after the onset of symptoms, except in one patient. Our patients had a slower clearance of respiratory viral loading than To et al.’ s group (17).
Monitoring viral load throughout the disease course has been used as an objective means of checking the clinical progress or response to antiviral therapy. de Jong et al. reported fatal outcome of human influenza A H5N1 is associated with high viral load and hypercytokinemia (14). It has been reported for seasonal influenza patients hospitalized with severe diseases have more active and prolonged viral replication (22). In severe acute respiratory syndrome (SARS) cases, it has been reported that viral load in nasopharyngeal aspirates from day 10 to day 15 after onset of symptoms was associated with oxygen desaturation, mechanical ventilation, diarrhea, hepatic dysfunction, and death (23). In SARS coronavirus and influenza A H5N1 virus infections, there was the inverse correlation between the absolute lymphocyte count and concomitant viral load level in nontreated and treated patients irrespective of the days post symptom onset at the time when the specimens were sampled (21). However, recently Duchamp et al. reported influenza A H1N1 2009 viral load did not correlate with clinical history or specific clinical symptoms (24).
The general radiologic findings of H1N1 pneumonia, similar to all viral pneumonias, are represented by poorly defined nodules, patchy areas of peribronchial ground-glass opacity, and air-space consolidation (11,12,25). In a series of 272 U.S. patients, 40% of the hospitalized patients who underwent chest radiography at admission had radiographic findings consistent with pneumonia (10). In the largest series of patients published, which included 1088 cases of hospitalization or death in California, 833 (66%) patients who underwent chest radiography during their hospitalization had opacities suggestive of pneumonia or acute respiratory distress syndrome (9). Our results agreed with these reports. During the period of May- December 2009, 24 (54.5%) of the 44 patients diagnosed with H1N1 influenza in our hospital had associated lung abnormalities.
There are several limitations with this study. The patient number of this study is small. However, results from this study were consistent among patient subjects, the authors tend to believe even there would be a modest increase in patient number, the conclusions would be the same. The patients were presented to hospital on average 5.2 days after the onset of symptoms. This was later than some other series reported (13). However, our additional analysis showed with the five patients whose virus load and CT were obtained within 72 hours after the onset of symptom, there was no trend to show a correlation between initial viral load and initial lung lesion score. As there was no X-ray exam prior to the onset of symptoms, it could not be certain that in current series there were no lung lesions existed before the H1N1 viral pneumonia; other secondary respiration infections during the treatment course also cannot be excluded. However, our series were mostly young subjects, and all the cases’ disease course was consistent with viral pneumonia except one case with secondary bacterial infection, the chance of these possibilities is small. Even such cases did exist in small number, they would not change the overall conclusion of this study. The value of acquiring CT instead of chest X-ray for all H1N1 influenza patients is questionable. Though ground-glass opacity is a well-defined descriptor on a CT scan, it is a subtle finding with limited demonstrability at chest radiography. With H1N1 pneumonia, similar to various other lung diseases, subtle lung opacities may be detected at chest CT when chest radiographs have normal results (11). The individuals of our series were admitted to our institution when the epidemic, time course and outcome of this disease were uncertain, and some extreme containment and mitigation measures had been taken.
To the best of our knowledge, this is the first time such a study has been carried out. This current study provides valuable information on H1N1 viral pneumonia epidemiology. Our data shows both higher initial respiratory viral load and higher initial lung lesion score are associated slower resolution of those abnormalities. Respiratory viral load decreases faster than chest lesion score. However, there is no correlation between initial respiratory viral load and initial lung lesion score. This result seems to support Duchamp et al.’s recent report that, for influenza A H1N1 2009, viral load does not correlate with clinical history or specific clinical symptoms (24).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Zimmer SM, Burke DS. Historical perspective--Emergence of influenza A (H1N1) viruses. N Engl J Med 2009;361:279-85 [DOI] [PubMed] [Google Scholar]
- 2.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009;361:680-9 [DOI] [PubMed] [Google Scholar]
- 3.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009;302:1880-7 [DOI] [PubMed] [Google Scholar]
- 4.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009;325:197-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen G, Martin J, O'Donnell J, et al. Surveillance of the first 205 confirmed hospitalised cases of pandemic H1N1 influenza in Ireland, 28 April - 3 October 2009. Euro Surveill 2009;14. pii: 19389. [PubMed] [Google Scholar]
- 6.Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360:2605-15 [DOI] [PubMed] [Google Scholar]
- 7.Trifonov V, Khiabanian H, Greenbaum B, et al. The origin of the recent swine influenza A(H1N1) virus infecting humans. Euro Surveill 2009;14; pii: 19193. [PubMed] [Google Scholar]
- 8.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol 2009;45:169-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009;302:1896-902 [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009;361:1935-44 [DOI] [PubMed] [Google Scholar]
- 11.Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol 2009;193:1488-93 [DOI] [PubMed] [Google Scholar]
- 12.Ajlan AM, Quiney B, Nicolaou S, et al. Swine-origin influenza A (H1N1) viral infection: radiographic and CT findings. AJR Am J Roentgenol 2009;193:1494-9 [DOI] [PubMed] [Google Scholar]
- 13.Aviram G, Bar-Shai A, Sosna J, et al. H1N1 influenza: initial chest radiographic findings in helping predict patient outcome. Radiology 2010;255:252-9 [DOI] [PubMed] [Google Scholar]
- 14.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006;12:1203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi GC, Khong PL, Müller NL, et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology 2004;230:836-44 [DOI] [PubMed] [Google Scholar]
- 16.Lu PX, Wang YX, Zhou BP, et al. Radiological features of lung changes caused by avian influenza subtype A H5N1 virus: report of two severe adult cases with regular follow-up. Chin Med J (Engl) 2010;123:100-4 [PubMed] [Google Scholar]
- 17.To KK, Chan KH, Li IW, et al. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol 2010;82:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell DM, World Health Organization Writing Group Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis 2006;12:81-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng VC, Tang BS, Wu AK, et al. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J Infect 2004;49:262-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest 1998;101:643-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li IW, Hung IF, To KK, et al. The natural viral load profile of patients with pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment. Chest 2010;137:759-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009;200:492-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis 2004;10:1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchamp MB, Casalegno JS, Gillet Y, et al. Pandemic A(H1N1)2009 influenza virus detection by real time RT-PCR: is viral quantification useful? Clin Microbiol Infect 2010;16:317-21 [DOI] [PubMed] [Google Scholar]
- 25.Marchiori E, Zanetti G, Hochhegger B, et al. High-resolution computed tomography findings from adult patients with Influenza A (H1N1) virus-associated pneumonia. Eur J Radiol 2010;74:93-8 [DOI] [PubMed] [Google Scholar]