Abstract
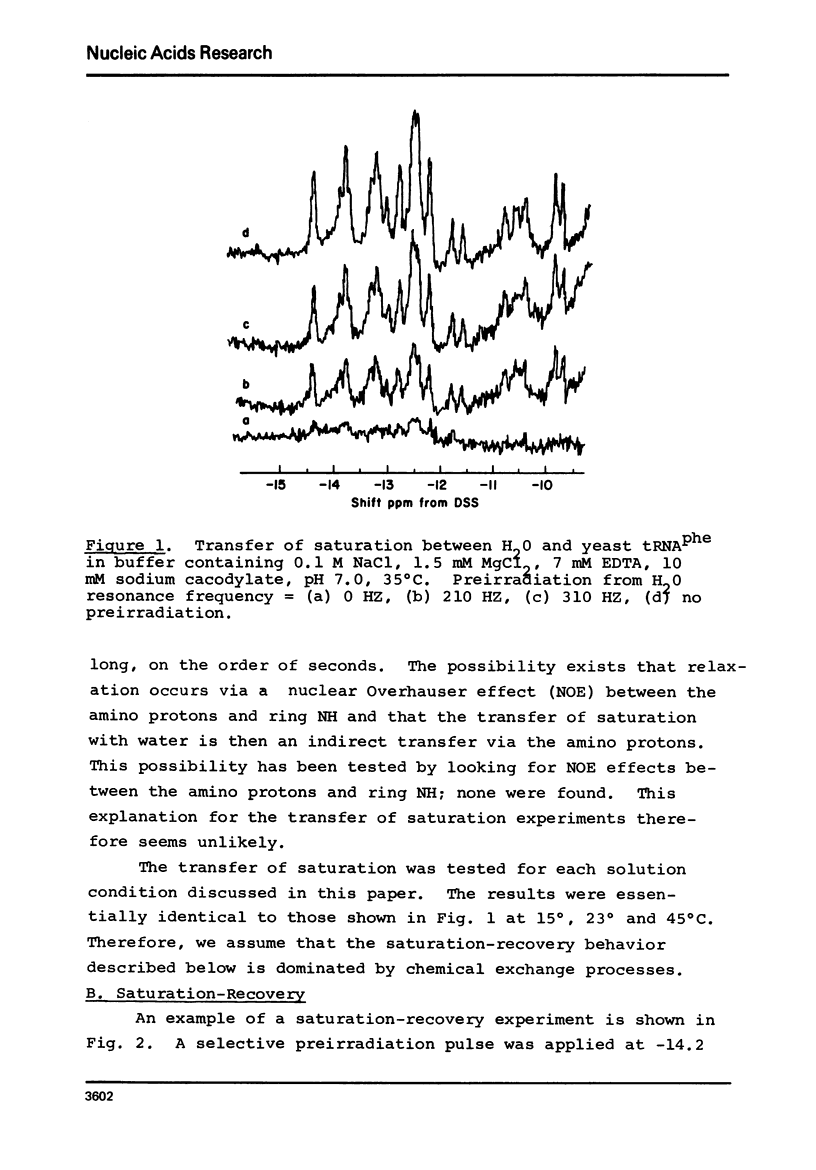
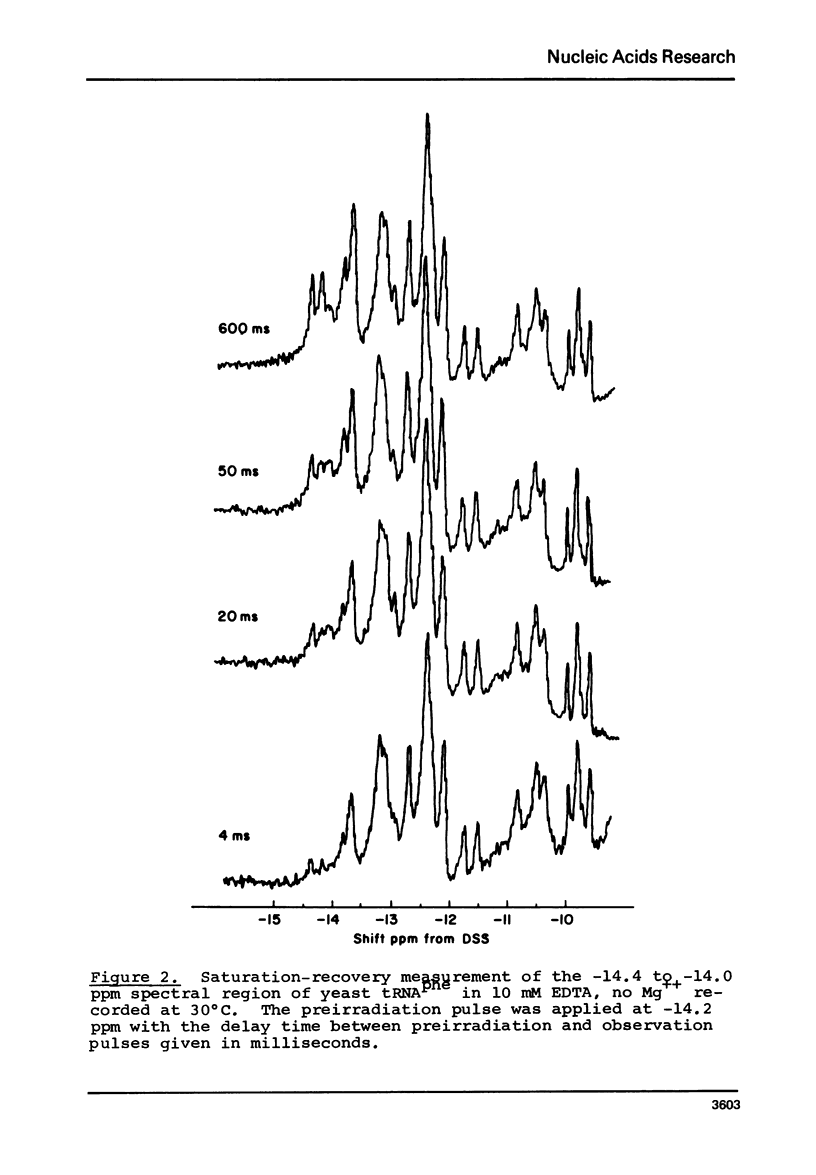
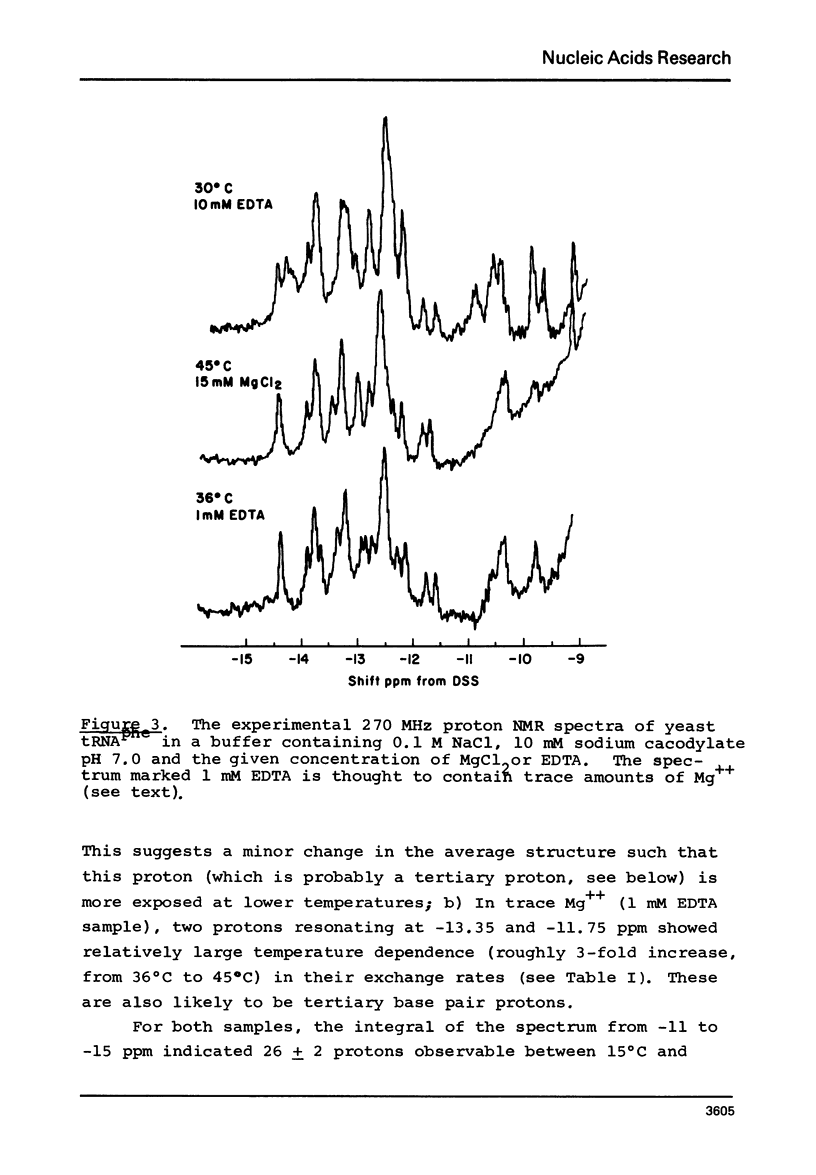
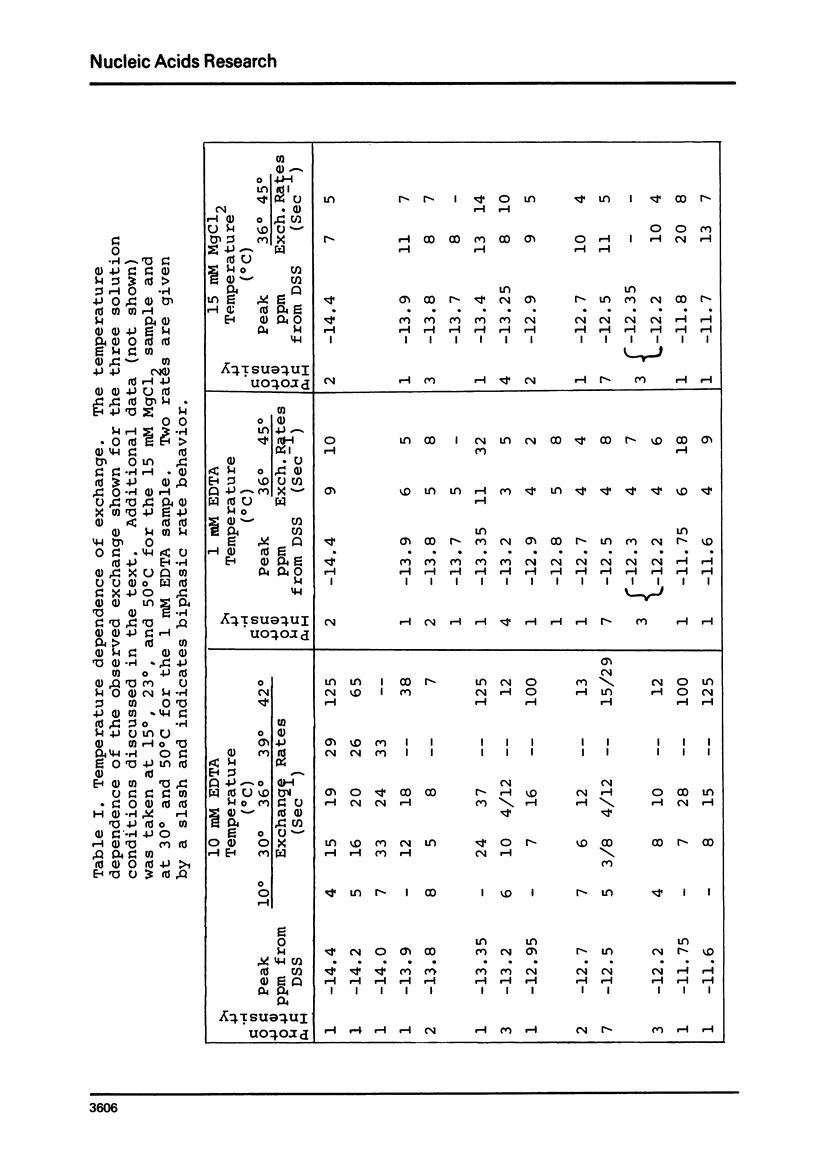
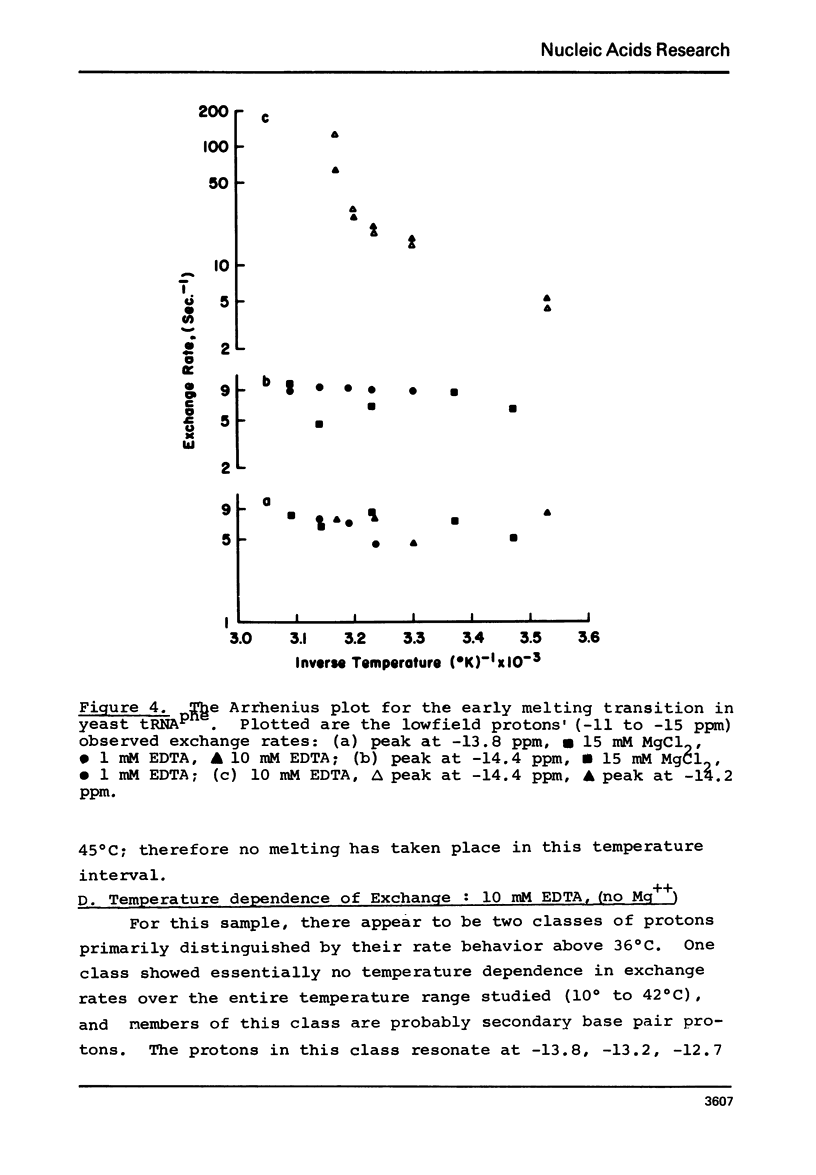
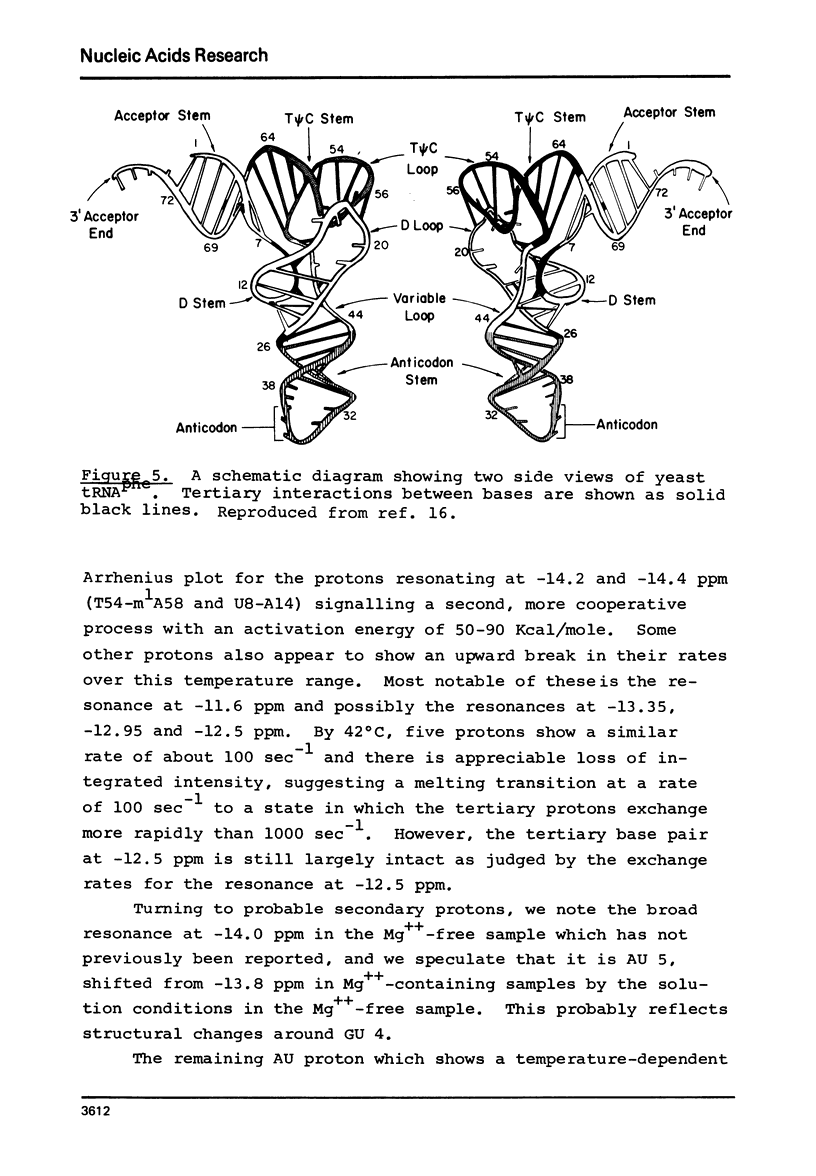
Solvent exchange rates of all the protons of yeast tRNAphe resonating in the lowfield NMR region (-11 to-15 ppm from DSS) have been measured by saturation-recovery long-pulse Fourier transform NMR. All these protons in yeast tRNAphe are in the fast exchange limit with H2O relative to their intrinsic longitudinal relaxation processes. Most rates show very little temperature dependence; however, tertiary base pair protons are preferentially destabilized in the absence of Mg++ at higher temperatures. The measured exchange rates are between 2 and 125 sec-1 for a temperature range from 10 degrees C to 45 degrees C and MgCl2 concentrations between 0 and 15 mM.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton P. H., Jones C. R., Bastedo-Lerner D., Wong K. L., Kearns D. R. Quantitative determination of the number of secondary and tertiary structure base pairs in transfer RNA in solution. Biochemistry. 1976 Oct 5;15(20):4370–4377. doi: 10.1021/bi00665a004. [DOI] [PubMed] [Google Scholar]
- Coutts S. M., Riesner D., Römer R., Rabl C. R., Maass G. Kinetics of conformational changes in tRNA Phe (yeast) as studied by the fluorescence of the Y-base and of formycin substituted for the 3'-terminal adenine. Biophys Chem. 1975 Oct;3(4):275–289. doi: 10.1016/0301-4622(75)80020-2. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Daniel W. E., Jr, Cohn M. Proton nuclear magnetic resonance of spin-labeled Escherichia coli tRNAf1MET. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2582–2586. doi: 10.1073/pnas.72.7.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J. J., Kallenbach N. R., Englander S. W. Hydrogen exchange study of some polynucleotides and transfer RNA. J Mol Biol. 1972 Jan 14;63(1):153–169. doi: 10.1016/0022-2836(72)90527-x. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- Geerdes H. A., Hilbers C. W. The iminoproton NMR spectrum of yeast tRNA-Phe predicted from crystal coordinates. Nucleic Acids Res. 1977 Jan;4(1):207–221. doi: 10.1093/nar/4.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Goldstein R. N., Stefanovic S., Kallenbach N. R. On the conformation of transfer RNA in solution: dependence of denaturation temperature and structural parameters of mixed and formylmethionyl Escherichia coli transfer RNA on sodium ion concentration. J Mol Biol. 1972 Aug 21;69(2):217–236. doi: 10.1016/0022-2836(72)90227-6. [DOI] [PubMed] [Google Scholar]
- Hilbers C. W., Shulman R. G., Kim S. H. High resolution NMR study of the melting of yeast tRNA Phe. Biochem Biophys Res Commun. 1973 Dec 10;55(3):953–960. doi: 10.1016/0006-291x(73)91235-7. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Ts'o P. O. 1H NMR studies of transfer RNA III: the observed and the computed spectra of the hydrogen-bonded NH resonances of baker's yeast transfer-RNA Phe. Nucleic Acids Res. 1977;4(5):1633–1647. doi: 10.1093/nar/4.5.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R. High-resolution nuclear magnetic resonance investigations of the structure of tRNA in solution. Prog Nucleic Acid Res Mol Biol. 1976;18:91–149. doi: 10.1016/s0079-6603(08)60587-5. [DOI] [PubMed] [Google Scholar]
- Kearns D. R., Patel D., Shulman R. G., Yamane T. High resolution nuclear magnetic resonance study of base pairing in four purified transfer RNA molecules. J Mol Biol. 1971 Oct 14;61(1):265–270. doi: 10.1016/0022-2836(71)90224-5. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Hilbers C. W. Proton nuclear magnetic resonance investigations of fraying in double-stranded d-ApTpGpCpApT in H2O solution. Biochemistry. 1975 Jun 17;14(12):2651–2656. doi: 10.1021/bi00683a014. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. R., Ribeiro N. S., Gould G., Robillard G., Hilbers C. W., Shulman R. G. Tertiary hydrogen bonds in the solution structure of transfer RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2049–2053. doi: 10.1073/pnas.72.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. R., Ribeiro N. S., McCollum L., Abbate J., Hurd R. E. High-resolution nuclear magnetic resonance determination of transfer RNA tertiary base pairs in solution. 1. Species containing a small variable loop. Biochemistry. 1977 May 17;16(10):2086–2094. doi: 10.1021/bi00629a006. [DOI] [PubMed] [Google Scholar]
- Reid B. R., Robillard G. T. Demonstration and origin of six tertiary base pair resonances in the NMR spectrum of E. coli tRNA1Val. Nature. 1975 Sep 25;257(5524):287–291. doi: 10.1038/257287a0. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Tarr C. E., Vosman F., Berendsen H. J. Similarity of the crystal and solution structure of yeast tRNAPhe. Nature. 1976 Jul 29;262(5567):363–369. doi: 10.1038/262363a0. [DOI] [PubMed] [Google Scholar]
- Rordorf B. F., Kearns D. R. NMR investigation of proton exchange in transfer RNA by high resolution NMR. Biochem Biophys Res Commun. 1975 Aug 4;65(3):857–862. doi: 10.1016/s0006-291x(75)80464-5. [DOI] [PubMed] [Google Scholar]
- Römer R., Hach R. tRNA conformation and magnesium binding. A study of a yeast phenylalanine-specific tRNA by a fluorescent indicator and differential melting curves. Eur J Biochem. 1975 Jun 16;55(1):271–284. doi: 10.1111/j.1432-1033.1975.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Römer R., Riesner D., Maass G. Resolution of five conformational transitions in phenylalaninespecific tRNA from yeast. FEBS Lett. 1970 Oct;10(5):352–357. doi: 10.1016/0014-5793(70)80471-9. [DOI] [PubMed] [Google Scholar]
- Römer R., Riesner D., Maass G., Wintermeyer W., Thiebe R., Zachau H. G. Cooperative helix-coil transitions in half molecules of phenylalanine specific tRNA from yeast. FEBS Lett. 1969 Sep;5(1):15–19. doi: 10.1016/0014-5793(69)80281-4. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Webb P. K., Fresco J. R. Tritium exchange studies of transfer RNA in native and denaturated conformations. J Mol Biol. 1973 Mar 5;74(3):387–402. doi: 10.1016/0022-2836(73)90379-3. [DOI] [PubMed] [Google Scholar]



