Oral tolerance is a long-recognized mechanism of inducing antigen-specific peripheral immune tolerance. In the past decade a large number of investigators have successfully applied oral tolerance to the treatment of autoimmune diseases in animal models by feeding autoantigens, and human trials have begun with this approach as well (1). In this issue of the Proceedings, Teitelbaum et al. (2) report that oral tolerance using Copolymer 1 (Cop 1, Copaxone, glatiramer acetate), which simulates myelin basic protein (MBP) immunologically, is effective in both the rat and mouse models of experimental allergic encephalomyelitis (EAE) and indeed may be more effective than MBP itself. Injectible Cop 1 has been used successfully to treat both EAE (3) and the human disease multiple sclerosis (MS) (4). These results raise the possibility that orally administered Cop 1 may be an effective treatment for MS.
Although the phenomenon of oral tolerance has been known since the turn of the century, it is only recently that the mechanisms underlying oral tolerance have been elucidated (5). Orally administered proteins and oral peptides are active immunologically and have clear immunologic effects on the gut-associated lymphoid tissue (6), which constitutes approximately 70% of the immune reactive cells in the body. There are multiple mechanisms by which orally administered antigen induces tolerance, the primary determining factor being the dose of antigen administered (7). Low doses of antigen induce regulatory T cells that act by secreting anti-inflammatory cytokines such as transforming growth factor (TGF) type β, IL-10, and IL-4 (8, 9). Higher doses induce anergy or deletion of cells specific for the fed antigen (10, 11). These multiple mechanisms most probably evolved to maintain tolerance to the large variety of proteins that are ingested over a wide dose range.
Cop 1 is a synthetic basic copolymer of l-alanine, l-glutamic acid, l-lysine, and l-tyrosine in a residue molar ratio of 4.6:1.5:3.6:1.0 with a molecular mass between 4,700 and 11,000 Da. It initially was designed to mimic MBP and induce EAE. However, it was not encephalitogenic, but instead, suppressed MBP-induced EAE (3), a finding observed in a number of species. It has been more than 25 years since this initial observation and the following immunologic properties of Cop 1 have emerged: (i) Cop 1 exhibits crossreactivity both on the cellular (12) and humoral (13) level with MBP though the crossreactivity is partial; (ii) Cop 1 binds in a promiscuous fashion to HLA-DR molecules (14) and can serve as a T cell antigen receptor antagonist of the 82–100 epitope of MBP (15); (iii) in animals, Cop 1 induces regulatory cells that can adoptively transfer protection not only to MBP-induced EAE (16) but EAE induced by proteolipid protein (PLP) (17) and spinal cord homogenate (18); (iv) in humans, but not in other species, Cop 1 induces proliferation of peripheral blood lymphocytes from normal individuals not treated with Cop 1 (19, 20); (v) Cop 1 treatment does not suppress other autoimmune diseases in animals, including uveitis, myasthenia gravis, thyroiditis, diabetes, and lupus models though it recently has been reported to prevent murine graft-vs.-host disease in vivo, but at much higher doses (21) and has been reported to inhibit type II collagen-reactive T cells clones in vitro (22); (vi) Cop 1 prepared from d-amino acids is as efficient as Cop 1 in competing for binding to MHC class II and in preventing graft-vs.-host disease (23), but it is ineffective in suppressing EAE (24), suggesting that the action of Cop 1 in EAE involves an additional step of specificity; and (vii) when mixed in a complete Freund’s adjuvant emulsion containing either PLP (25) or myelin oligodendrocyte glycoprotein (26) it suppresses EAE.
Two mechanisms of action have been proposed for the in vivo effects of Cop 1. The first is that it binds to MHC and inhibits immune responses by competitively affecting binding of the encephalitogenic protein MBP. The second is that it induces MBP crossreactive regulatory cells, which act to suppress ongoing inflammatory processes by the release of anti-inflammatory cytokines when they encounter MBP in vivo. Although Cop 1 binds to MHC in a class II restricted fashion and has pronounced MHC and antigen binding properties in vitro, it is unlikely that its in vivo effects after injection in humans or animal model is via this mechanism. It is unlikely that a daily injection of 20 mg of Cop 1 s.c. in MS patients could displace binding of endogenous MBP and other myelin antigens from MHC binding sites on antigen-presenting cells in the brain and throughout the immune system. It appears that the in vivo immunologic mechanism of Cop 1 relates to it acting as an altered peptide ligand that preferentially induces regulatory cells that crossreact with MBP and potentially with other myelin antigens, and this mechanism occurs either when Cop 1 is given by injection or orally.
The results reported by Teitelbaum et al. (2) with oral Cop 1 are consistent with our current understanding of the mechanisms of oral tolerance and with the large body of data showing that Cop 1 can induce regulatory cells. The authors demonstrate suppression of EAE in both mice and rats that can be adoptively transferred and that is dose dependent. Disease suppression is better seen at lower rather than higher doses of oral Cop 1 and is associated with generation of immune responses that favor secretion of IL-10 and TGF-β as opposed to IFN-γ. There is no crossreactivity between Cop 1-specific T cell lines and MBP in terms of IFN-γ secretion whereas there is in terms of IL-10 and TGF-β secretion. Rat Cop 1 lines themselves do secrete IFN-γ when activated with Cop 1, although murine lines do not. This is a unique property of Cop 1-induced cells. Specifically, when Cop 1-specific T cells are activated with MBP there is no induction of Th1 cytokines like IFN-γ but these T cells continue to produce Th2 and Th3 cytokines upon activation with the crossreactive ligand. In these terms MBP acts as a partial agonist for Cop 1-specific T cells.
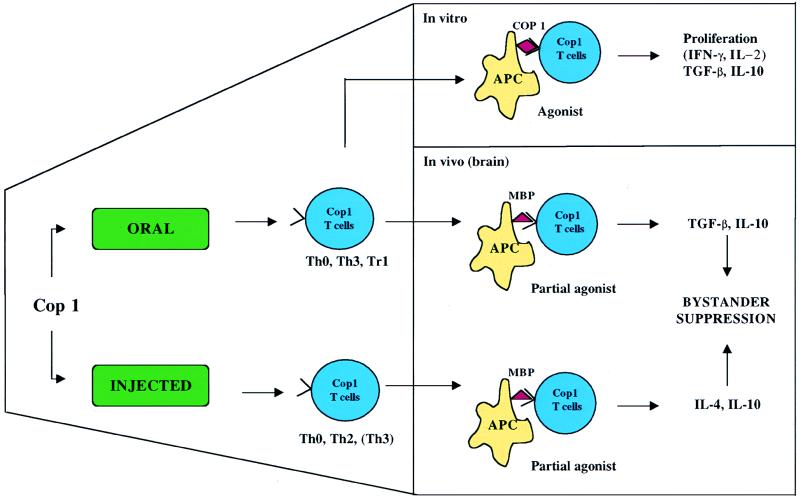
A proposed mechanism of action of oral Cop 1 is presented in Fig. 1. Oral Cop 1 may be more effective than oral MBP because it expands crossreactive T cells but not the autoantigen-specific T cells per se, and when these cells are activated by MBP they produce Th2 and TGF-β cytokines. Because Cop 1 regulatory cells secrete anti-inflammatory cytokines, they also suppress EAE induced by other myelin antigens via bystander suppression. A similar situation has been reported by injecting a well-defined altered peptide ligand (L144/R147) derived by the substitution of two T cell antigen receptor contact residues of the encephalitogenic PLP peptide 139–151 (27, 28). In humans, 85–99 reactive MBP-specific Th0 T cell clones when stimulated with an altered peptide ligand are induced to secrete TGF-β and no longer secrete IL-2, IFN-γ, IL-10, or IL-4 (29).
Figure 1.
Immunologic properties of Cop 1 T cells induced orally or by injection. Oral Cop 1 is processed in the gut-associated lymphoid tissue where it preferentially induces T cells that secrete TGF-β and IL-10, whereas parenteral administration induces IL-4 and IL-10, though there may be overlap. In vitro, restimulation of orally induced Cop 1 T cells with Cop 1, which acts as an agonist, proliferate, and secrete TGF-β and IL-10 and in some instances IFN-γ and IL-2. In vivo, MBP, endogeneously expressed in the brain, acts as a partial agonist to stimulate Cop 1 T cells to secrete TGF-β and IL-10/IL-4 and to mediate bystander suppression.
When Cop 1 is injected, it also acts as an altered peptide ligand and preferentially generates Th2 type responses characterized by IL-4, IL-5, IL-10, and in some instances TGF-β, whereas when it is processed in the gut the regulatory cells are induced that preferentially secrete TGF-β and IL-10. Such cells may have stronger disease-suppressing properties than cells that secrete IL-4 (30). Of note, in patients injected with Cop 1 there appears to be induction of Th2 and Th3 type cells (31) although they were not characterized for Cop 1 specificity.
Regulatory cells mediating active suppression play an important role in immune tolerance (32). One of the major mechanisms by which regulatory cells act is via the secretion of anti-inflammatory cytokines after antigen-specific triggering. New T cell types have been described that have strong regulatory properties: Th3 cells that secrete TGF-β (9) and Tr1 cells whose regulation is mediated by the secretion of IL-10 and TGF-β (33). TGF-β-secreting Th3 cells are preferentially generated after orally administered antigen, although they play a role in other instances such as transplantation tolerance (34), and they are associated with natural recovery from EAE (35, 36). TFG-β has an important role in the gut as a switch factor for IgA (37), and IL-4 is both an important cytokine in the gut (38) and a differentiation factor for Th3 cells (39, 40). The gut epithelium is also rich in IL-10, which may favor induction of IL-10-secreting regulatory cells (Tr1 cells). When administered orally, Cop 1 appears to be inducing both TGF-β- and IL-10-secreting cells. IL-4-secreting Th2 cells also may serve as regulatory cells for Th1 responses and are induced by injected Cop 1.
The induction of crossreactive Th3, Tr1, or Th2 type regulatory cells by Cop1 leads to the phenomenon termed “bystander suppression,” which solves a major conceptual problem in the treatment of organ-specific inflammatory autoimmune disease (41). It now is clear that there are multiple reactivities against autoantigens in the target organ, even though an autoimmune response may begin against a single autoantigen. Thus, treatments designed to suppress, delete, or inactivate a population of cells with a single specificity are problematic. A cell reactive with MBP that secretes IL-10 or TGF-β is able to suppress reactivity against other myelin antigens in the microenvironment. Indeed oral MBP can suppress PLP-induced disease (42), and similar effects of bystander suppression have been demonstrated for Cop 1 (17, 18). Perhaps one of the most dramatic examples of bystander suppression is the suppression of viral-induced diabetes in the lymphocytic choriomeningitis virus model by the oral administration of insulin and the associated up-regulation of IL-4 and IL-10 and the TGF-β in the pancreas (43).
Although oral tolerance has been shown to be effective in a large number of animal models, it has not yet led the development of an approved drug for the treatment of a human autoimmune disease. The most promising results to date have been in the treatment of rheumatoid arthritis (RA) with oral type II collagen (44, 45) in which dose ranging studies (5–2,500 μg) have identified a lower dose (60 μg) as the most efficacious and a phase III trial of oral type II collagen in 770 patients with RA is scheduled for completion in September. Thus, the clinical results in RA are consistent with low-dose oral tolerance and the induction of regulatory cells and what would be predicted from the mechanism of oral tolerance defined in animal models. Five studies of oral and nasal insulin for the prevention and treatment of type 1 diabetes are ongoing, and although preliminary results from one of these suggests some positive effects in older patients treated with oral insulin (46) it will be 3–4 more years until results of the long-term trials with oral insulin are completed. In MS, a large phase III trial of a bovine myelin mixture vs. placebo did not show positive clinical effects. Failure of the bovine myelin trial probably relate to two factors: (i) dose ranging studies were not performed because of the nature of the trial design, and (ii) a myelin mixture was used. In both human and animal studies, crude mixtures of proteins were not as effective when given orally. In a pilot trial of patients with uveitis, purified retinal S-Ag showed positive trends whereas a retinal mixture did not (47). In animal studies, a crude acetylcholine mixture was not as efficacious as purified acetylcholine receptor (48) and a myelin mixture was not as efficacious as MBP (49).
Does oral Cop 1 have advantages over orally administered MBP or previously tested myelin mixtures? It appears the answer may be yes. In animal models, Cop 1 is more effective than MBP, something we ourselves have observed in studying oral Cop 1 in MBP T cell receptor transgenic mice (50). Furthermore, Cop 1 has the unique property of inducing crossreactive regulatory cells and not encephalitogenic cells. Although virtually all studies in animal models have shown safety and efficacy of orally administered antigens, there are exceptions in which an oral antigen may induce or exacerbate an autoimmune process (51). Although worsening of autoimmune disease has not been seen in the large-scale trials in MS and rheumatoid arthritis this possibility must be taken into account. Because Cop 1 is not encephalitogenic, it may well have a better safety profile.
A unique feature of the use of oral Cop 1 to treat MS is that it is currently efficacious when given by injection. The immune system works via amplification, and most vaccines involve priming and boosting. Because Cop 1 can be given safely by injection the possibility exists that its oral effect could be enhanced or boosted by periodic injections that would serve to boost the Th2 or Th3 responses. This approach then would be a true vaccination paradigm for the treatment of autoimmune disease.
It takes as long as 15–30 years from the time of a basic science observation for that finding to be reduced to practice in the clinic. This time frame has been true for Cop 1, which was first described in 1970, and also may be true for the optimal application of oral tolerance to human disease. Two of the major ways in which orally administered antigens may be made more efficacious are changing the structure of the antigen to make it a stronger tolerogen and coupling it to or administrating it with an adjuvant to enhance its activity. Oral Cop 1 appears to be addressing the first issue as its unique structure and pronounced MHC-binding properties appear to make it an antigen with greater tolerogenic capacity when given orally.
MS has been a difficult disease to understand and treat because of the multifactorial nature of the disease, our only recent understanding of mechanisms of tolerance and autoimmunity, and a lack of monitoring techniques for the disease. These issues are in the process of being resolved as we now have drugs that have shown efficacy in MS and appear to be working according to current immunologic theory (52). With the advent of MRI scanning it is now possible to directly visualize the pathologic process in the nervous system to assess the response to therapeutic agents (53). New data concerning the effect of Cop 1 on MRI will be available in the coming year and if positive could serve as a basis by which oral Cop 1 could rapidly be tested for efficacy in MS.
The work of Teitelbaum et al. (2) on oral Cop 1 opens an exciting new chapter in the treatment of MS. Currently approved INF-β drugs as well as Cop 1 are given by injections, which are difficult for patients and often meet resistance. Furthermore, as is the case with malignant and other infectious diseases such as AIDS, combination therapy with multiple drugs may be required to control disease processes. It is not practical for patients to give themselves multiple injections of different drugs for MS. The prospect of oral Cop 1 as the first orally active drug specific for MS may herald the development of other orally active MS drugs that then could be used in combination and would be enthusiastically prescribed by physicians and received by MS patients.
Footnotes
The companion to this Commentary is published on page 3842.
References
- 1.Weiner H L. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum D, Arnon R, Sela M. Proc Natl Acad Sci USA. 1999;96:3842–3847. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 4.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B, et al. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 5.Weiner H L, Mayer L F. Oral Tolerance: Mechanisms and Applications. New York: New York Acad. Sci.; 1996. [DOI] [PubMed] [Google Scholar]
- 6.Gonnella P A, Chen Y, Inobe J-I, Quartulli M, Weiner H L. J Immunol. 1998;160:4708–4718. [PubMed] [Google Scholar]
- 7.Friedman A, Weiner H. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller A, Lider O, Roberts A B, Sporn M B, Weiner H L. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo V K, Inobe J-I, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Whitacre C C, Gienapp I E, Orosz C G, Bitar D. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 11.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo V K, Weiner H L. Nature (London) 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 12.Webb C, Teitelbaum D, Arnon R, Sela M. Eur J Immunol. 1973;3:279–286. doi: 10.1002/eji.1830030506. [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum D, Aharoni R, Sela M, Arnon R. Proc Natl Acad Sci USA. 1991;88:9528–9532. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridkis-Hareli M, Strominger J L. J Immunol. 1998;160:4386–4397. [PubMed] [Google Scholar]
- 15.Aharoni R, Teitelbaum D, Arnon R, Sela M. Proc Natl Acad Sci USA. 1999;96:634–639. doi: 10.1073/pnas.96.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharoni R, Teitelbaum D, Arnon R. Eur J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 17.Aharoni R, Teitelbaum D, Sela M, Arnon R. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 18.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brosnan C F, Litwak M, Neighbor P A, Lyman W D, Carter T H, Bornstein M B, Bloom B R. Neurology. 1985;35:1754–1759. doi: 10.1212/wnl.35.12.1754. [DOI] [PubMed] [Google Scholar]
- 20.Burns J, Krasner L J, Guerrero F. Neurology. 1986;36:92–94. doi: 10.1212/wnl.36.1.92. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel P G, Aharoni R, Chen Y, Chen J, Teitelbaum D, Arnon R, Sela M, Chao N J. Proc Natl Acad Sci USA. 1996;93:5061–5066. doi: 10.1073/pnas.93.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridkis-Hareli M, Rosloniec E F, Fugger L, Strominger J L. Proc Natl Acad Sci USA. 1998;95:12528–12531. doi: 10.1073/pnas.95.21.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aharoni R, Schlegel P, Teitelbaum D, Roikhel-Karpov O, Chen Y, Arnon R, Sela M, Chao N. Immunol Lett. 1997;58:79–87. doi: 10.1016/s0165-2478(97)00032-1. [DOI] [PubMed] [Google Scholar]
- 24.Webb C, Teitelbaum D, Herz A, Arnon R, Sela M. Immunochemistry. 1976;13:333–337. doi: 10.1016/0019-2791(76)90344-x. [DOI] [PubMed] [Google Scholar]
- 25.Teitelbaum D, Fridkis-Hareli M, Arnon R, Sela M. J Neuroimmunol. 1996;64:209–217. doi: 10.1016/0165-5728(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Nun A, Mendel I, Bakimer R, Fridkis-Hareli M, Teitelbaum D, Arnon R, Sela M, Kerlero de Rosbo N. J Neurol. 1996;243:S14–S22. doi: 10.1007/BF00873697. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson L A, Murtaza A, Hafler B P, Sette A, Kuchroo V K. Proc Natl Acad Sci USA. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santambrogio L, Lees M B, Sobel R A. J Neuroimmunol. 1998;81:1–13. doi: 10.1016/s0165-5728(97)00138-0. [DOI] [PubMed] [Google Scholar]
- 29.Windhagen A, Scholz C, Höllsberg P, Fukaura H, Sette A, Hafler D A. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Das M P, Howard E D, Weiner H L, Sobel R A, Kuchroo V K. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 31.Miller A, Shapiro S, Gershtein R, Kinarty A, Rawashdeh H, Honigman S, Lahat N. J Neuroimmunol. 1998;92:113–121. doi: 10.1016/s0165-5728(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 32.Mason D, Powrie F. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 33.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. Nature (London) 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 34.Josien R, Douillard P, Guillot C, Müschen M, Anegon I, Chetritt J, Menoret S, Vignes C, Soulillou J P, Cuturi M C. J Clin Invest. 1998;102:1920–1926. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury S J, Hancock W W, Weiner H L. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Hancock W W, Marks R, Gonnella P A, Weiner H L. J Neuroimmunol. 1998;82:149–159. doi: 10.1016/s0165-5728(97)00193-8. [DOI] [PubMed] [Google Scholar]
- 37.Kim P-H, Kagnoff M F. J Immunol. 1990;144:3411–3416. [PubMed] [Google Scholar]
- 38.Daynes R, Araneo B, Dowell T, Huang K, Dudley D. J Exp Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inobe J, Slavin A J, Komagata Y, Chen Y, Liu L, Weiner H L. Eur J Immunol. 1998;28:2780–2790. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Seder R A, Marth T, Sieve M C, Strober W, Letterio J J, Roberts A B, Kelsall B. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- 41.Miller A, Lider O, Weiner H L. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Sabbagh A, Miller A, Santos L M B, Weiner H L. Eur J Immunol. 1994;24:2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 43.Von Herrath M G, Dyrberg T, Oldstone M B A. J Clin Invest. 1996;98:1324–1331. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trentham D, Dynesius-Trentham R, Orav E, Combitchi D, Lorenzo C, Sewell K, Hafler D, Weiner H. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 45.Barnett M L, Kremer J M, St. Clair E W, Clegg D O, Furst D, Weisman M, Fletcher M J F, Lavin P T, Finger E, Morales A, et al. Arthritis Rheum. 1998;41:290–297. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Coutant, R., Zeidler, A., Rappaport, R., Schatz, D., Schwartz, S., Raskin, P., Rogers, D., Bode, B., Crockett, S., Marks, J., et al. (1998) Diabetes47, Suppl. 1, A97 (abstr.).
- 47.Nussenblatt R B, Gery I, Weiner H L, Ferris F, Shiloach J, Ramaley N, Perry C, Caspi R, Hafler D A, Foster S, Whitcup S M. Am J Ophthalmol. 1997;123:583–592. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]
- 48.Okumura S, McIntosh K, Drachman D B. Ann Neurol. 1994;36:704–713. doi: 10.1002/ana.410360504. [DOI] [PubMed] [Google Scholar]
- 49.Benson, J. M., Stuckman, S. S., Cox, K. L., Wardrop, R. M., Gienapp, I. E., Cross, A. H., Trotter, J. L. & Whitacre, C. C. (1999) J. Immunol., in press. [PubMed]
- 50.Maron R, Slavin A, Weiner H L. J Neuroimmunol. 1998;90:82. (abstr.). [Google Scholar]
- 51.Faria, A. & Weiner, H. L. (1999) Adv. Immunol. in press. [DOI] [PubMed]
- 52.Weiner H L. Can J Neurol Sci. 1998;25:93–101. doi: 10.1017/s0317167100033680. [DOI] [PubMed] [Google Scholar]
- 53.Miller D H, Grossman R I, Reingold S C, McFarland H F. Brain. 1998;121:3–24. doi: 10.1093/brain/121.1.3. [DOI] [PubMed] [Google Scholar]