Abstract
With the advent of the key discovery in the mid-1980s that the amyloid β-protein (Aβ) is the core constituent of the amyloid plaque pathology found in Alzheimer disease (AD), an intensive effort has been underway to attempt to mitigate its role in the hope of treating the disease. This effort fully matured when it was clarified that the Aβ is a normal product of cleavage of the amyloid precursor protein, and well-defined proteases for this process were identified. Further therapeutic options have been developed around the concept of anti-Aβ aggregation inhibitors and the surprising finding that immunization with Aβ itself leads to reduction of pathology in animal models of the disease. Here we review the progress in this field toward the goal of targeting Aβ for treatment and prevention of AD and identify some of the major challenges for the future of this area of medicine.
There are three main ways to target amyloid β-protein for treating Alzheimer disease: inhibiting its production, preventing its aggregation (or promoting its disaggregation), and promoting its clearance.
Treatment of Alzheimer disease (AD) through a biological and molecular understanding of the disease has been the cornerstone of research in the field for the past 20 years. In this article we will review some of the therapeutic efforts that are being pursued and have been attempted over this period during which the amyloid β (Aβ) peptide has been the primary target. These efforts can generally be divided into three areas: β- and γ-secretase inhibition, Aβ aggregation inhibitors, and active and passive Aβ immunotherapy approaches (Fig. 1).
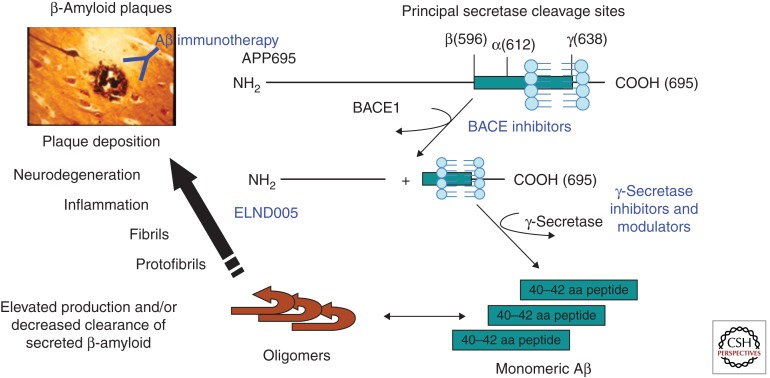
Figure 1.
Amyloidogenic processing of amyloid precursor protein (APP) by BACE1 and γ-secretase. The figure depicts the principal proteolytic processing steps of APP leading to the production of 40–42-residue amyloid β (Aβ) peptide, the subsequent steps ultimately culminating in compaction and deposition of the peptide in β-amyloid plaques in brain of AD patients (and transgenic AD mouse models), and the primary point of intervention by the different therapeutic antiamyloid approaches discussed in this article.
β- AND γ-SECRETASE INHIBITORS FOR AD
The identification of Aβ as the primary constituent of amyloid plaques in Alzheimer brain presented a tangible target for developing therapies for the disease (Fig. 2). The three fundamental approaches currently in play targeting Aβ for treatment and prevention of AD involve inhibiting its production, preventing its aggregation (or promoting its disaggregation), and promoting its clearance. Therapeutic advances with the latter two approaches are discussed in the following sections of this article. The focus of this section is therapeutic advances on inhibiting production of Aβ.
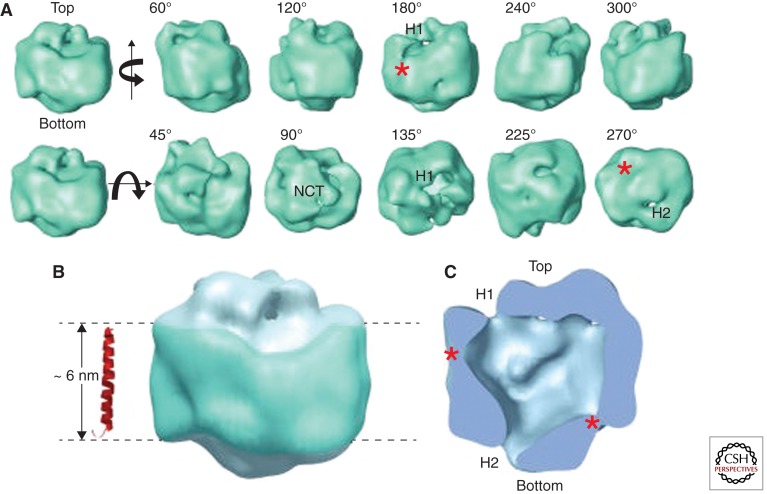
Figure 2.
Electron micrograph based 3D structure of the γ-secretase complex. (A) Surface rendering of the 3D reconstruction. The first row displays side views generated by rotating the map around a vertical axis, and the second row shows tilted views by rotating around a horizontal axis. The rotation angles are shown within each view. Two openings at the top and bottom are labeled H1 and H2, respectively, where visible. The top density is labeled NCT because the lectin labeling showed that the NCT ectodomain is located at this surface. (B) The potential transmembrane segment with the belt-like structure is outlined in blue by two parallel dashed lines, 60 Å apart. For size comparison, a typical transmembrane α-helix, taken from the rhodopsin structure (Protein Data Bank ID code 1GZM), is shown to the left of the structure. (C) A cut-open view of the γ-secretase complex from the side, revealing a large central chamber and one opening (H1) at the top and one at the bottom (H2). Two weak-density lateral regions are labeled with asterisks. (Image is from Lazarov et al. 2006; printed with permission from D. Selkoe.)
The pathologic accumulation of Aβ in plaques is postulated to result from an imbalance between production and clearance during aging. Transgenic mouse models overexpressing human amyloid precursor protein (APP) bearing certain familial AD mutations have validated overproduction of Aβ as driving AD-type amyloid pathology. The mouse models have also provided insights into the interrelationship between related pathologies characteristic of the disease, for example, tau, inflammatory end points (e.g., microgliosis, astrocytosis), neuritic dystrophy, and Aβ-related behavioral deficits (presumed preclinical surrogates of the cognitive deficits in patients). Nevertheless, the Aβ overproduction mouse models do not exhibit robust neuronal loss characteristic of the advanced human disease as judged postmortem. An additional caveat of current mouse models in the context of therapeutics targeting production of amyloid is that, in contrast to rare familial forms of AD (Scheuner et al. 1996), there is limited evidence that sporadic AD is a driven by overproduction of Aβ (Fukumoto et al. 2002; Holsinger et al. 2002; Yang et al. 2003; Li et al. 2004). Indeed, evidence in support of the alternative possibility—that accumulation of β amyloid in sporadic AD results from reduced clearance and/or turnover of the peptide—has been reported (Mawuenyega et al. 2010). Irrespective of whether overproduction or reduced clearance causes amyloid pathology in sporadic AD, clinical evaluation of the benefits of inhibiting production as a therapeutic strategy for sporadic AD is still warranted from two perspectives. First is the ample evidence for pathology attributable to toxic effects of excess soluble Aβ (Gong et al. 2003; Kayed et al. 2003; Lacor et al. 2004; Cleary et al. 2005; Lesne et al. 2006; Townsend et al. 2006b; Cheng et al. 2007; Walsh and Selkoe 2007; Klyubin et al. 2008; Shankar et al. 2008) as well as pathology attributable to plaque-associated Aβ (Spires et al. 2005; Kuchibhotla et al. 2008; Meyer-Luehmann et al. 2008, 2009). Second, inhibiting production offers a logical approach for restoring homeostasis between production and clearance if retarded clearance mechanisms are a widespread contributor to sporadic AD. Interestingly, the findings of Mawuenyega et al. are consistent with the conclusion that a partial inhibition of production (common target ∼30%) could be therapeutically efficacious, as the observed in vivo clearance of Aβ40 and Aβ42 was reduced by ∼35% in sporadic AD patients in comparison with healthy controls.
The constitutive production of Aβ from APP, a type I transmembrane protein, revealed the central role of two distinct proteases, and provided a cornerstone for drug discovery efforts. The two primary amyloidogenic proteases are commonly referred to as BACE1 (β-site APP cleaving enzyme 1) and γ-secretase. The initial cleavage of APP leading to Aβ generation is mediated by BACE1 (Fig. 3), and results in two products, an amino-terminal fragment of APP termed sAPPβ that is released into the luminal/extracellular compartment, and a membrane embedded carboxy-terminal fragment, termed C99. C99 is the immediate substrate for a series of cleavages by γ-secretase. The primary products of γ cleavage of C99 are the 40- or 42-residue β-amyloid peptide, and the APP intracellular domain (AICD). Detailed analyses of the cleavage events mediated by both enzymes on APP have revealed additional minor sites of cleavage by BACE1, as well as a complex processive proteolytic activity by γ-secretase (see Harrison et al. 2004; John 2006; Haass et al. 2011).
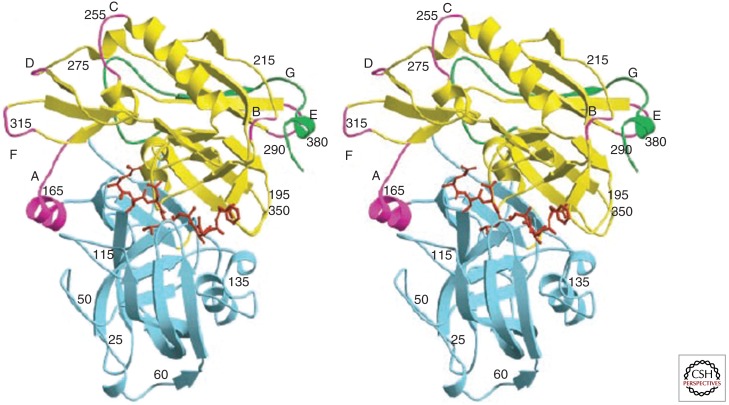
Figure 3.
Crystal structure of BACE1 complexed with a small molecule inhibitor. The crystal structure of BACE1 complexed to inhibitor OM99-2. Stereo view of the polypeptide backbone of BACE1 is shown as a ribbon diagram. The amino-terminal and carboxy-terminal lobes are blue and yellow, respectively, insertion loops (relative to pepsin) designated A–G in the carboxy-terminal lobe are magenta, and the COOH-terminal extension unique to BACE is green. The inhibitor bound between the lobes is shown in red. (Modified from Hong et al. 2000.)
BACE1 and γ-secretase presented obvious drug targets for inhibiting production of Aβ, and they have been the subjects of intensive research. Properties distinguishing these enzymes from one another include the fact that BACE1 is a monomeric protein displaying relative specificity for cleavage site sequence, and that it catalyzes substrate cleavage in an aqueous environment. In contrast, γ-secretase is a multisubunit protein complex comprising four subunits, with little apparent specificity for sequence, and it performs water-mediated catalysis of the transmembrane domains of substrates in the hydrophobic environment of the plasma membrane. Features in common between the two enzymes include the fact that both are membrane-bound aspartyl proteases, both have been demonstrated to process multiple substrates in addition to APP, and at least in the case of APP, both cleave the substrate at multiple sites (in common with most proteases).
These common properties have presented challenges for development of small-molecule inhibitors targeting either enzyme. In the case of γ-secretase, its many substrates, particularly Notch, have made clinical development of substrate-selective inhibitors difficult. In the case of BACE1, the conventional aspartyl protease nature of this target (i.e., an extended substrate binding groove) shows limited discovery of central nervous system (CNS)-permeable small molecule inhibitors. Nevertheless, progress has been made on both fronts, as we will now discuss.
γ-Secretase Inhibitors
Three principal classes of γ-secretase inhibitors (GSIs) have progressed into AD clinical trials: nonselective inhibitors, cleavage site modulators, and APP-selective/Notch-sparing inhibitors. Expert reviews of the rich medicinal chemistry underlying the three approaches have been published over the years (Josien 2002; Harrison et al. 2004; Churcher and Beher 2005; Pissarnitski 2007; Olson and Albright 2008; Garofalo 2009), and the interested reader is directed to those publications for further background, as this article will review clinical properties and results from GSIs in AD trials. Progress with, and limitations of, GSIs have been intertwined with the unfolding story of the complex biology of this target. To appreciate the rationale for testing each of these inhibitor classes, a review of the complex biology of γ-secretase accompanies the review of the progress with each inhibitor.
Biochemical identification of presenilin 1 (PS1) as the target of substrate-based transition state isosteres (Esler et al. 2000; Li et al. 2000), as well as inhibitors of Aβ production discovered from cell-based screens (Seiffert et al. 2000), provided conclusive evidence to settle the argument, based on reverse genetic approaches supporting PS1 and PS2 as a critical component of γ-secretase (De Strooper et al. 1998; Herreman et al. 1999, 2000; Steiner et al. 1999), that two transmembrane aspartyl residues in the presenilin amino-terminal fragment (NTF) and carboxy-terminal fragment (CTF) comprised the active site of γ-secretase (Wolfe et al. 1999). This discovery culminated in the recognition of γ-secretase as the first example of intramembrane cleaving aspartyl proteases (iClip). The recognition of PS as the catalytic subunit of γ-secretase established the molecular identity of the target of ongoing efforts toward clinical development of what subsequently came to be appreciated as nonselective γ-secretase inhibitors.
The most advanced nonselective GSI, Semagacestat (LY450139, IC50 of ∼60 nm for cellular Aβ; reviewed in Henley et al. 2009) progressed into phase 3 human testing by Eli Lilly and Co. before further development was halted (Eli Lilly 2010). Semagacestat’s origins stemmed from a collaboration between Athena Neurosciences and Eli Lilly & Co. that provided two seminal demonstrations: first, that acute pharmacologic inhibition of γ-secretase by an early lead in the series (known as DAPT) could effectively lower brain Aβ production in PDAPP mice (a mutant APP transgenic model of AD pathology; Dovey et al. 2001); and second, that inhibition for 3 mo of γ-secretase in PDAPP with LY411575 (a picomolar optimized GSI lead derived from DAPT) decreased plaques and related amyloid pathologies (May et al. 2001). Single-dose studies explored the safety and tolerability of Semgacestat (i.e., LY450139) in healthy volunteers given 5–140 mg of the compound and revealed the pharmacokinetic–pharmacodynamic relationship of Aβ as a biomarker in plasma and cerebrospinal fluid (CSF; Siemers et al. 2005, 2007). Single doses of semagacestat were safe and well tolerated. Pharmacokinetic and pharmacodynamic characterization revealed a plasma t1/2 of 2.5 h and a dose-dependent reduction of plasma Aβ over the first 6 h (ranging from 40% at 50 mg to 73% at 140 mg), followed by a well-documented transient increase in Aβ above baseline. Repeat-dose studies in AD patients explored safety and tolerability and Aβ biomarker effects as primary end points and clinical measures of cognition as secondary end points in patients dosed with 5–50 mg/d semagacestat for 2 or 6 wk (Siemers et al. 2006). A subsequent study explored doses ranging up to 140 mg in AD patients treated for a total of 14 wk in an ascending dose design (Fleisher et al. 2008). As in the single-dose studies, a dose-proportional increase in drug exposure with concomitant reduction of plasma Aβ was observed in the repeat dose studies, with good overall tolerability. No significant effects on the secondary clinical end points (ADAS-Cog and ADAS-ADL) were observed in the 14-wk study, which was not powered to see an effect on these end points. Although a reduction in steady-state CSF Aβ level (considered a more proximal biomarker of brain Aβ) was not observed in the studies cited above, a decline in the rate of synthesis of brain Aβ, monitored in CSF using a novel stable isotope labeling kinetic method, was effected by semagacestat in a dose-responsive manner in healthy volunteers given single doses of 100, 140, or 280 mg of drug (Bateman et al. 2009). Based on the cumulative evidence just reviewed, a phase 3 trial of semagacestat in AD was launched by Eli Lilly and Co. Although generally well tolerated in the single-dose studies, drug-related adverse events were noted in the repeat-dose studies, consistent with its mechanism of action for inhibition of Notch signaling in peripheral tissues (e.g., skin rash, hair color change, and gastrointestinal effects). These observations are consistent with the effects of nonselective GSIs reported in preclinical studies (Searfoss et al. 2003; Milano et al. 2004; Wong et al. 2004; Hyde et al. 2006). A decline in cognition of patients on drug relative to placebo, as well the emergence of skin neoplasms in the LY-450139 phase 3 study, noted in the press release announcing early termination of the trial, are reminiscent of phenotypes noted in partial or conditional PS loss of function models (Fig. 4; Saura et al. 2004; Tournoy et al. 2004; Li et al. 2007).
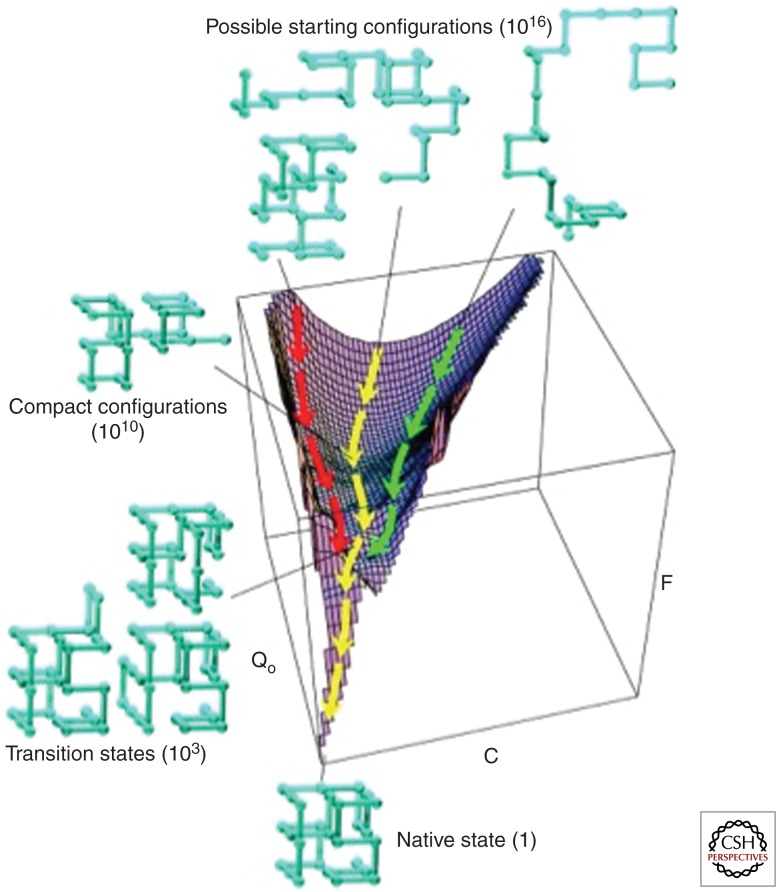
Figure 4.
Spatial energy flow diagram of protein folding. The diagram depicts how, following immediate synthesis, a polypeptide initially has a very high level of possible folding and conformational states (1010). The drive toward a lower energy state results in a reduced number, although still extremely high, of possible conformational states. Eventually with time, these various states, by different routes, converge toward a small number of possibilities, shown on the bottom of the figure. (Modified from Dinner et al. 2000.)
Other nonselective GSIs—for example, MK-0752 and PF-03084014 (Wei et al. 2010)— are being tested in certain cancer indications by Merck and Pfizer, respectively, based on the rationale that dysregulated Notch signaling leads to oncologic transformation. Limited data is available with MK-0752 from the perspective of AD: In phase 1 studies reported by Rosen et al. (2006), MK-0752 exhibited CSF concentrations equivalent to estimated free plasma concentrations, a 2 h delayed Tmax in CSF relative to plasma, and a dose-related reduction of CSF Aβ40 over a 4–12-h period following single doses >300 mg. The highest dose tested, 1000 mg, produced a sustained reduction in CSF Aβ over 24 h. No clinical findings have been reported to date with PF-03084014, and as oncological testing of GSIs falls outside the scope of this article, MK-0752 and PF-03084014 will not be reviewed further here.
Owing to challenges associated with the inhibition of Notch signaling by nonselective GSIs, drug development targeting this enzyme branched into two parallel strategies, cleavage site modulators and APP-selective inhibitors. The discovery of the protoype γ-secretase cleavage site modulators such as Tarenflurbil (Flurizan, R-flurbuprofen) arose from cell culture experiments examining the effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on APP processing (Weggen et al. 2001), which appeared to relate to epidemiological studies, suggesting a reduced prevalence of AD associated with prolonged use of select NSAIDs, particularly ibuprofen (Breitner et al. 1995; Stewart et al. 1997; McGeer and McGeer 1998). Although preclinical studies with ibuprofen in transgenic mouse models of AD showed efficacy against β-amyloid and inflammatory end-points (Lim et al. 2000), consistent with the epidemiological studies, prospective treatment studies of NSAIDs in mild–moderate AD failed to show benefit on primary outcome measures (Aisen et al. 2003; Thal et al. 2005). However, the in vitro studies investigating the mechanism of action revealed that certain NSAIDs, namely ibuprofen, indomethacin, and sulindac sulfide, selectively lowered secretion of Aβ42 (at concentrations of 100–250 µm) with a concomitant increase in Aβ38, without decreasing production of Aβ40 or the Notch intracellular domain, in a cyclooxygenase 1 (COX1)-independent manner (Weggen et al. 2001). This mechanistic insight into a possible effect of certain NSAIDs on γ-secretase, combined with an early recognition that the 42-residue isoform of Aβ conferred a higher risk of developing AD owing to its increased amyloidogenic potential versus shorter isoforms (e.g., Aβ40), provided support for clinical testing of these so-called γ-secretase modulators as AD therapeutics. The demonstration that R-enantiomers of ibuprofen and flurbuprofen retained Aβ42-lowering activity of the parent without inhibiting COX1 (Morihara et al. 2002; Eriksen 2003) suggested R-enantiomers as the preferred clinical candidate for testing in AD. However, the drug concentrations required to lower brain Aβ42 (100–250 µm, based on in vitro activity) are not achieved following typical daily oral dosing regimens of 10–50 mg/kg R-flurbuprofen (Eriksen et al. 2003). Hence, the observed reduction of brain Aβ42 in preclinical studies is difficult to extend to man.
R-Flurbuprofen represented the first selective Aβ-lowering agent/γ-secretase modulator advanced into clinical testing, namely by Myriad Genetics Inc. In a 21-d study in healthy elderly (55–80 year) volunteers given placebo, 400, 800, or 1600 mg/d in a twice-per-day regimen, R-flurbuprofen was safe and well tolerated, and displayed dose-dependent pharmacokinetics in brain and plasma with respect to Cmax, whereas exposure in plasma appeared saturated at 800 mg/d dose and above, and had a 0.5%–1% CSF-to-plasma ratio (consistent with preclinical observations). Drug levels in plasma at Cmax for the three dose groups (83, 158, and 185 µm, respectively) were in the range for lowering of plasma Aβ42 (Galasko et al. 2007). No changes from baseline to day 21 were observed in plasma Aβ42 (sampled at trough drug levels following last dose), CSF Aβ42 (sampled circa plasma Tmax), or shorter species of Aβ. Bioinversion of the R- to S-enatiomer of drug was observed rarely in the treated population. Tarenflurbil was then tested at 400 or 800 mg on a twice-per-day schedule in a 12 mo (+12 mo extended treatment phase) phase 2 double-blind placebo-controlled, multicenter study of mild–moderate AD patients (a curious dosing choice in light of the phase 1 data showing saturation at doses above 800 mg/d). A prespecified analysis showed a treatment effect, manifested as lower rates of decline in activities in daily living (ADAS-ADL) and global function (CDR-sb) just in mild AD patients (baseline Mini-Mental-State Exam, MMSE, 20–26) in the 800 mg b.i.d. dose group relative to placebo at 12 and 24 mo (Wilcock et al. 2008). The treatment effect of the 800 mg b.i.d. dose in mild AD patients at 24 mo was retained in benefits on activities of daily living and global function and also extended to include benefits in cognitive function (ADAS-cog), relative to placebo at 12 mo + 400 mg b.i.d. tarenflurbil 12 mo, or else placebo for 12 mo + 800 mg b.i.d. tarenflurbil for 12 mo. No benefits on primary outcome measures were noted for any time point in moderate AD patients (baseline MMSE 15–19) treated with either dose of tarenflurbil relative to placebo. In fact, moderate AD patients on 800 mg b.i.d. drug showed a faster rate of decline in CDR sum of boxes relative to placebo. No effects on the most proximal mechanistic biomarker, CSF Aβ42, nor drug exposures associated with the different treatment arms, were reported. Clinical testing in mild AD patients treated with 800 mg flurizan b.i.d. for 18 mo in a large multicenter pivotal phase 3 trial failed to meet primary endpoints (ADCS-ADL and ADAS-Cog; Green et al. 2009). Secondary analysis did not reveal any association between primary outcomes and drug pharamcokinetic parameters (Cmax and AUC). Although further development of tarneflurbil was discontinued, subsequent preclinical progress with more potent NSAID-based GSMs is encouraging (Kounnas et al. 2010). Because tarenflurbil did not affect Aβ42 levels in the phase 3 patients as anticipated from its preclinical mechanism of action, the lack of clinical efficacy remains difficult to interpret.
Cinnamide-based compounds originating from Torrey Pines Therapeutics represent a second class of GSMs, now under development at Eisai Corp. Clinical development of the lead in this class, E2012, initially halted owing to a safety observation in a preclinical model, was resumed following satisfactory resolution of the findings (Nagy et al. 2011). No information regarding clinical or preclinical data with E2012 has been formally reported in a scientific forum by Eisai. However, Portelius et al. using IP-MALDI-TOF mass spectrometry, observed a dose-dependent increase in CSF Aβ37 in dogs over a 24-h period following a single dose treatment with 20 and 80 mg/kg E2012, along with a concomitant decrease in Aβ1–39, Aβ1–40, and Aβ1–42 (Portelius et al. 2010).
Systematic studies into the mechanism of substrate cleavage by γ-secretase indicate a processive activity of the enzyme, with an initiating cleavage near the cytosolic/membrane leaflet interface and subsequent cleavages every two or three residues into the transmembrane domain of the substrate (Takami et al. 2009; Xu 2009; Fukumori et al. 2010). These insights have bolstered the rationale underlying the cleavage site modulation by NSAIDs and hopefully will aid in the development of improved small molecules for clinical testing. Additional paths for modulating substrate cleavage by γ-secretase based on a nucleotide binding site (Feng et al. 2001; Netzer et al. 2003; Fraering et al. 2005) are in early stages of investigation. Hence, although the initial clinical outcome with cleavage site modulators has been disappointing, the mechanistic underpinning for continued efforts in this area has been strengthened in the interim.
Notch-sparing or APP substrate selective γ-secretase inhibitors represent the third class of compounds to have advanced to clinical testing in AD. As a class, all compounds in this category are based on a sulfonamide pharmacophore, demonstrate equipotent inhibition of Aβ40 and Aβ42, and display all the signatures of classic nonselective GSIs (Martone et al. 2009; Basi et al. 2010; Gillman et al. 2010). Clinical development of BMS-299897, a 7 nm inhibitor of Aβ production in cells that is a 15-fold less potent inhibitor of Notch cleavage in cells (Barten et al. 2005), was discontinued by Bristol-Myers Squib owing to a combination of pharmacokinetic liabilities (autoinduction of clearance mediated via pregnane X-receptor transactivation) and lack of Aβ reduction in man (Gillman et al. 2010).
The lack of Notch-associated gastrointestinal (GI) toxicity following repeated dosing with BMS-299897 in preclinical models (Milano et al. 2004; Barten et al. 2005) supported renewed efforts for discovery of improved analogs, and BMS-708163 is being advanced through clinical studies by Bristol-Myers Squib at the time of writing. BMS-708163, a 0.3 nm and 193-fold selective inhibitor of Aβ production versus Notch signaling in cells, lowers plasma and brain Aβ in rats in a dose-dependent manner, and a >25% reduction of CSF and brain Aβ relative to baseline levels is sustained for >24 h following a single dose of 2 mg/kg in dogs (Gillman et al. 2010). Results from early clinical studies in healthy young and elderly volunteers as well as early AD/MCI patients indicated that BMS-708163 is generally safe and well tolerated in single doses up to 800 mg, and up to 200 mg following multiple doses for 28 d (Albright et al. 2008; Tong et al. 2010). Pharmacokinetic parameters of BMS-708163 revealed a Tmax at 1–1.5 h, dose-related increases in Cmax and AUC, and a plasma half-life of ∼40 h following a single dose. An approximately twofold accumulation in exposure was observed in subsequent multiple-dose studies. Pharmacodynamic analyses revealed a sustained (72-h) dose-dependent reduction of plasma Aβ following single oral doses ≥400 mg, and a reduction over the initial 6–8 h followed by an increase above baseline between 8 and 72 h at doses ≤200 mg. Serial monitoring of CSF on an hourly basis over 40 h following single oral doses of 50, 200, or 400 mg revealed dose-dependent reductions of all Aβ species measured (38, 40, and 42), with reductions of ∼25% at 24 h after single doses of 200 mg (40% reduction at nadir, 12 h) and 400 mg (55% reduction at nadir). A sustained reduction in CSF Aβ similar to that observed following a single 200-mg dose was also observed following 28 days of treatment with 100 mg. Phase 2 trials of BMS-708163 in patients with mild to moderate AD as well as prodromal AD are in progress (ClinicalTrials.gov, identifierNCT00890890).
Begacestat (GSI-953), discovered at Wyeth (Mayer et al. 2008; Cole et al. 2009; Pu et al. 2009) and currently undergoing clinical development by Pfizer, represents the third of four Notch-sparing GSIs to have advanced into clinical testing. In preclinical safety models (rat and dog), Begacestat, a 12 nm (IC50 for cellular Aβ) approximately 17-fold selective (Aβ < Notch) GSI, manifested histological evidence of slight to mild Notch inhibition-related changes in GI cell populations at doses well above the therapeutic dose (Martone et al. 2009). In man, target inhibition by begacestat was evidenced by a dose-dependent, transient (from 1 to 4 h postdose) reduction of plasma Aβ in healthy volunteers following a single dose at doses between 10 and 600 mg (Martone et al. 2009). The current status of clinical development of begacestat is not publicly known. ELND006, a fourth APP-selective GSI, was progressed into clinical development by Elan Pharmaceuticals. The compound was well tolerated in single-dose studies ranging from 3 to 100 mg in healthy volunteers, with a typical dose-dependent initial decrease followed by rebound above baseline of plasma Aβ (Liang et al. 2011a). A dose-limiting liver toxicity with ELND006 was observed at 30 mg following a daily 21-d oral dosing study investigating a range of daily doses between 3 and 30 mg. In this multidose study, Aβ1–x in plasma was reduced by 27% at 5 h after the last dose in the 30 mg cohort, whereas CSF Aβ1–x showed a linear dose-responsive decrease of ∼10% at 3 mg to ∼38% at 30 mg (Liang et al. 2011b). Further development of ELND006 has been halted.
BACE Inhibitors
The discovery of soluble Aβ peptide in biological fluids (Haass et al. 1992; Seubert et al. 1992) consistent with the constitutive processing of APP was followed by a nearly decade-long effort to molecularly identify the responsible enzyme. The simultaneous reports of cloning BACE1 (β-site APP cleaving enzyme) and its closely related homolog, BACE2, by a variety of approaches (Hussain et al. 1999, 2000; Saunders et al. 1999; Sinha et al. 1999; Vassar et al. 1999; Yan et al. 1999; Acquati et al. 2000; Bennett et al. 2000; Lin et al. 2000) delivered the second molecular target for discovery of drugs to inhibit amyloid production. Knockout mouse models provided in vivo validation of the long-suspected pivotal role for β-secretase in Aβ production and the apparent safety of this target based on the relatively benign phenotype of BACE1-deficient mice (Cai et al. 2001; Luo et al. 2001, 2003; Roberds et al. 2001). Beneficial effects of BACE inhibition modeled in knockout (KO) mice for rescuing Aβ-driven cholinergic dysfunction (Ohno et al. 2004) and memory deficits (Ohno et al. 2006) in APP transgenic mice were also reported. Subsequent characterization of BACE1, as well as BACE1/BACE2 double KO mice, however, revealed roles for this enzyme in cellular pathways involved in myelination and behavior (Harrison et al. 2003; Dominguez et al. 2005; Laird et al. 2005; Hu et al. 2006; Willem et al. 2006; Kobayashi et al. 2008; Savonenko et al. 2008). In addition, the appreciation of an expanded list of BACE1 substrates beyond APP (Kitazume et al. 2001, 2005; Lichtenthaler et al. 2003; Wong et al. 2005; Spoelgen et al. 2006; Kuhn et al. 2007; Woodard-Grice et al. 2008; Hemming et al. 2009; Kihara et al. 2010), many of which are consistent with in vivo phenotypes observed in BACE1-deficient mice, serve as cautionary notes regarding potential safety issues associated with BACE1 inhibitors. Delayed onset of AD pathology in APP × BACE1+/− mice suggest that, as with GSIs, partial inhibition of BACE1 may be a solution toward mitigating the potential safety issues associated with inhibiting this protease (Laird et al. 2005; McConlogue et al. 2007).
The reports of BACE1 cloning were followed in rapid succession by solution of the X-ray crystal structure of BACE1 in complex with a peptidic inhibitor (Hong et al. 2000), and provided a barometer of the industry-wide rational design-based approaches that were in progress to discover potent small molecule inhibitors. The crystal structure illuminated the challenge that lay ahead: reducing the peptidic inhibitor observed in the long substrate-binding grove of BACE1 into a CNS-active small (MW < 500) molecule with favorable drug-like properties. Furthermore, selectivity for inhibition of BACE1 over related aspartyl proteases, such as BACE2 and cathepsin-D, was an additional constraint imposed on potential clinical candidates, in light of the antagonistic amyloidogenic activity of BACE2 (Farzan et al. 2000; Yan et al. 2001; Fluhrer et al. 2002; Basi et al. 2003) and the essential role of cathepsin-D in lysosomal function (Saftig et al. 1996; Benes et al. 2008). The interested reader is directed to expert medicinal chemistry reviews for structure–activity relationship studies toward the objectives of CNS-active, BACE1-selective, drug-like inhibitors (Hussain 2004; Thompson et al. 2005; Durham and Shepherd 2006; John 2006; Ziora et al. 2006; Ghosh et al. 2008; Silvestri 2008; Hamada and Kiso 2009; Stachel 2009). Incompatibility between MW constraints (imposed by potency requirements) and size (for CNS permeability) have proven to be significant challenges in overcoming the p-gylcoprotein-mediated efflux of BACE1 inhibitors from the CNS and has slowed advancement into the clinic of the many research efforts that were launched to discover and develop BACE inhibitors for treatment of AD. Nevertheless, progress to the clinic has been achieved, with several entrants from the pharmaceutical industry believed to be in or near the clinic, and some known examples are detailed below.
Whereas most investigators focused on solving the molecular weight versus potency and selectivity requirements of a BACE1 inhibitor using an iterative rational design approach combining medicinal chemistry and structural elucidation of inhibitor:BACE1 cocrystals, Chang et al. (2004) reported in vivo activity with a cell-penetrant carrier peptide conjugated to a potent peptidic isostere inhibitor. Their result provided a short cut around the limiting chemical properties and a path to clinical development by Comentis of an otherwise CNS incompatible molecule (reviewed in Ghosh et al. 2008). Thus, CTS-21166, arising from collaboration between the Ghosh group at University of Illinois, Chicago, and the Tang group at Oklahoma Medical Research Foundation, is in clinical development for AD at Comentis. Very little has been published on this molecule, and it is unclear if CTS-21116 is still in clinical development today.
As ongoing optimization of leads from rational design-based approaches continues, investigators from Eli Lilly have reported preclinical and clinical findings with a novel BACE inhibitor, LY2811376 (MW 320), discovered and optimized from a fragment-based approach (Boggs et al. 2010; May et al. 2010). The relatively modest in vitro potency of LY2811376 (∼100 nm in primary neuronal cultures, 250 nm in a FRET in vitro BACE assay, and 300 nm in HEK293 cells), combined with ∼10-fold selectivity over BACE2 and ∼60-fold selectivity over cathepsin-D, delivered robust in vivo efficacy at relatively low doses. Dose-responsive and concordant acute reduction of cortical sAPPβ (lowered 33%–43%), C99 (lowered 56%–78%), and Aβ1–x (lowered 47%–68%) were observed following oral gavage of PDAPP mice with 10, 30, or 100 mg/kg LY2811476. A single oral dose of 10 mg produced a sustained effect on these same biomarkers in CSF, plasma, and hippocampus of PDAPP mice for 6–9 h, with a nonsignificant transient elevation of sAPPα in CSF at 3 h. In beagle dogs, a dose-related reduction in plasma Aβ was achieved following single oral doses of 0.5–5 mg/kg, followed by a gradual return to baseline over 36–48 h without a rebound above baseline (as seen with GSIs). Also in dogs, CSF Aβ1–x and Aβ1–42 were reduced from 3 to 24 h following a single oral dose of 5 mg/kg, with return to baseline by 48 h (May et al. 2010). The safety, pharmacokinetics, and pharmacodynamics of LY2811476 were studied in phase 1 single ascending dose (SAD) and a 14-d multiple ascending dose (MAD) studies investigating 10 and 25 mg doses in healthy volunteers (Martenyi et al. 2010). LY2811476 was generally safe and well tolerated in single-dose studies ranging between 5 and 90 mg. Treatment-emergent adverse events were mostly mild and manageable, with no clinically meaningful changes in vital signs or bioanalytes. In the SAD study, LY2811476 demonstrated dose-related increases in Cmax and exposure, with a terminal t1/2 >24 h. The t1/2 based on two cohorts dosed in the MAD study was 66–81 h for the two doses (10 and 25 mg). Dose-dependent pharmacodynamic effects on Aβ in plasma ranging between 45% and 82% reduction at nadir (6–8 h postdose) were observed in individuals treated with 15–90 mg LY2811476, with sustained reduction at 120 h postdose and no rebound above baseline during this time period. In the 14-d MAD study, reduction of plasma Aβ1–40 reached steady state with 1 wk of daily dosing of both 10 and 25 mg. Similar dose-related biomarker changes in CSF (monitored via an indwelling catheter) of Aβ species (reduction of Aβ1–40, 1–42) and APP metabolite (elevation in sAPPα and reduction in sAPPβ) were observed in the 30 and 90 mg cohorts of the SAD study at 36 h post- versus 4 h predose. Unfortunately, despite these encouraging biomarker effects, further clinical development of LY2811376 has been discontinued owing to preclinical toxicity, the nature of which as not yet been disclosed (Martenyi et al. 2010).
Summary and Prospects for β- and γ-Secretase Inhibitors
Inhibiting Aβ generation as a strategy for AD therapy has been a productive avenue of research. The key proteases mediating production of Aβ from APP, BACE1, and γ-secretase have been identified at the molecular level, and their study has revealed novel biology and mechanistic insights into the unique catalytic activities carried out by each enzyme. However, progress toward inhibiting their activity in man and achieving lowered Aβ production in AD patients remains an unrealized objective. The challenges associated with this ultimate objective have been detailed above, and future progress will benefit from rapid and timely dissemination of preclinical and clinical data associated with inhibition of each target, to the ultimate benefit of patients and family members affected. The emerging consensus for earlier treatment (Aisen et al. 2011; Golde et al. 2011) emphasizes the need for safer therapies, earlier diagnosis, and the further validation of preclinical biomarkers predictive of clinical conversion to AD. In the case of γ-secretase, research into the mechanism of activity of this enzyme suggests more potent cleavage site modulators (Kounnas et al. 2010) or perhaps subunit-selective inhibitors (Zhao et al. 2008; Serneels et al. 2009; He et al. 2010) could provide safer drugs for earlier treatment. In the case of BACE1, additional candidates are suspected to be advancing into or through clinical testing based on the patent literature; the publication of clinical findings with these additional BACE1 inhibitors is eagerly awaited. Furthermore, just as the GI toxicity of nonselective GSIs highlighted the in vivo importance of Notch as a γ-substrate, disclosure of the preclinical toxicity observed with LY2811376 is awaited to better understand the limitations of BACE1 inhibitors. Looking forward, as with diseases such as cancer, hypertension, atherosclerosis, and HIV–AIDS, the treatment of AD will probably be best managed through combinatorial approaches, either multimodal anti-Aβ therapies (e.g., immunotherapy + secretase inhibitor; secretase inhibitor + aggregation inhibitor) or cross-modal (e.g., anti-Aβ + anti-tau).
Overview of Aβ Aggregation and Related Treatment Strategies
With the discovery of Aβ as the core constituent of vascular and plaque depositss (Glenner and Wong 1984), the search for Aβ aggregation inhibitors began. The hypothesis is that disaggregating amyloid plaques and/or preventing Aβ aggregation should have potential therapeutic benefits by reducing the pathology of the disease. Despite the simplicity of the hypothesis, turning this vision into a reality has been daunting. Reasons for the difficulties associated with this therapeutic approach range from biophysical challenges associated with the inherently extremely low energy state of amyloid fibrils to basic understanding of the process of Aβ aggregation. Added to these hurdles is the multitude of challenges associated with the development of orally bioavailable small molecules with the characteristic pharmaceutical qualities required for success.
Despite these difficulties, a number of compounds continue in clinical testing that have the potential to succeed as Aβ aggregation inhibitors. Lessons learned from this effort should prove invaluable for future therapeutic efforts aimed at AD as well as possible treatments for other diseases associated with amyloid formation, which are quite numerous.
The Biophysics of Aβ Aggregation
Amyloid is a generic term describing tissue deposits of an aggregated proteins with the common characteristic of ∼8–10 nm-diameter fibrils composed of polymers of β-pleated sheet conformation that exhibit birefringence under polarized light after staining with the dye Congo red. The β-pleated sheet structure is due to the amino acid backbone and not to the side-chains (R groups), although the latter do play a role in intermediate structures of amyloidogenic proteins prior to amyloid formation and hence are relevant. Although different amyloid fibrils vary somewhat in absolute width and length depending upon the subunit protein in question, they share the common feature of being extremely stable and resistant to disaggregation (Calamai et al. 2005). The stability of these structure in terms of ΔG can be enormous (Calamai et al. 2005). As an example of this stability, amyloid fibrils of Aβ isolated from the brains of patients who died with AD are insoluble to both heat and SDS detergent. It is only with extraordinary measures such as the utilization of highly chaotropic salts (e.g., guanidine HCl) or formic acid that they dissolve into their peptide subunits (Roher et al. 1993).
From a biophysical perspective, fibrillization of Aβ can be easily studied in vitro. At concentrations at or above low micromolar levels, the peptide will form stabile fibrils. The kinetics of this process, although variable, are dependent upon the buffer conditions as well as the absolute concentrations of Aβ. The reaction can be easily monitored either by a change in the circular dichroism spectrum owing to the formation of β-pleated sheet-rich assemblies or by the fluorescence of thioflavin, which binds amyloid fibrils and increases as they accumulate. It is important to note that both of these methods provide little or no information about intermediate structures of potential relevance but rather inform the investigator about the final stage of the process, namely amyloid formation. This caveat is extremely important, because unique individual folding steps of diverse peptides must occur for amyloid fibril formation to ultimately take place, and each step can be biophysically unique. The early step is termed the latency phase (Lee et al. 2007). This describes a poorly understood process wherein a small number of amyloidogenic peptides of one type (e.g., Aβ1–42) come together to form tiny nuclei that are subsequently assembled into protofibrils (often ∼4 nm diameter) that will serve as the core of the mature amyloid fibril (∼8 nm). This nucleation step, fortunately for humans, is energetically extremely disfavored under normal circumstances and hence seldom occurs randomly in nature. One can view the process as having a high transition-state energy barrier. There are at least three ways of driving this process forward. One is by increasing the concentration of the amyloidogenic protein. The second is by introducing mutations into the protein that reduce its energy barrier to achieve a misfolded, β-rich conformer that can lead to the initiation of a fibril (Chiti et al. 2003). The third is…?
In Vitro Efforts to Identify Aβ Aggregation Inhibitors
A relatively simple approach to follow the fibrillization of Aβ was introduced in the mid-1990s by several groups that involved monitoring the fluorescence of thioflavin T (e.g., Wood et al. 1996). Thioflavin T has enhanced fluorescence when it binds to amyloid fibrils composed of many different amyloidogenic proteins. The precise reasons for this binding are not completely understood but probably have to do with interaction with the β-pleated sheet structure and intercalation into the growing fibrils. This assay ushered in a series of mechanistic publications as well as numerous screens for compounds by both academic and pharmaceutical laboratories. Indeed, proteins, peptides, herbs, natural products and a wide range of small molecules of both known and unknown activities have been shown to block Aβ fibrillization (see Table 1 and Lorenzo and Yankner 1994; Tomiyama et al. 1994; Bronfman et al. 1996; Higaki et al. 1997; Kihara et al. 1999; Reixach et al. 2000; Thomas et al. 2001; Ono et al. 2002, 2004; Khurana et al. 2003; Blanchard et al. 2004; Hirohata et al. 2005; Fujiwara et al. 2006; Kokkoni et al. 2006; Durairajan et al. 2008; Ryu et al. 2008; Wiesehan et al. 2008; Hong et al. 2009; Rodriguez-Rodriguez et al. 2009). The list of agents is clearly very diverse. Despite this structural diversity, there are some general properties shared by many of the compounds or small molecules that have been identified to inhibit Aβ fibrillization. First, their potency is typically low, with inhibitory activity seen only in the micromolar range. This suggested that stoichiometric amounts of these inhibitors were required to block Aβ fibrillization. Second, with only a few exceptions, these compounds are symmetrical and often planar, with large ring structures. Taken together, these facts have made them difficult starting points for classic medicinal chemistry lead optimization. As a second-tier experimental follow-up to in vitro aggregation assays such as with thioflavin T, a number of these putative Aβ aggregation inhibitors were tested in cellular assays of Aβ neurotoxicity to see if they could effectively block the biological effects. Some of the early compounds were indeed effective, although generally at micromolar concentrations (Blanchard et al. 2004), again making them difficult to progress toward in vivo drug studies. Despite these drawbacks, a few of the early compounds were pursued as far as animal testing in APP transgenic mice to examine whether they would have an effect on plaque burden. Unfortunately, all but a very few were not effective in reducing or retarding plaque pathology (Fig. 5; Yang et al. 2005). As a result of the largely negative outcome of these studies, the general approach of identifying useful Aβ aggregation inhibitors via thioflavin screens has fallen out of favor.
Figure 5.
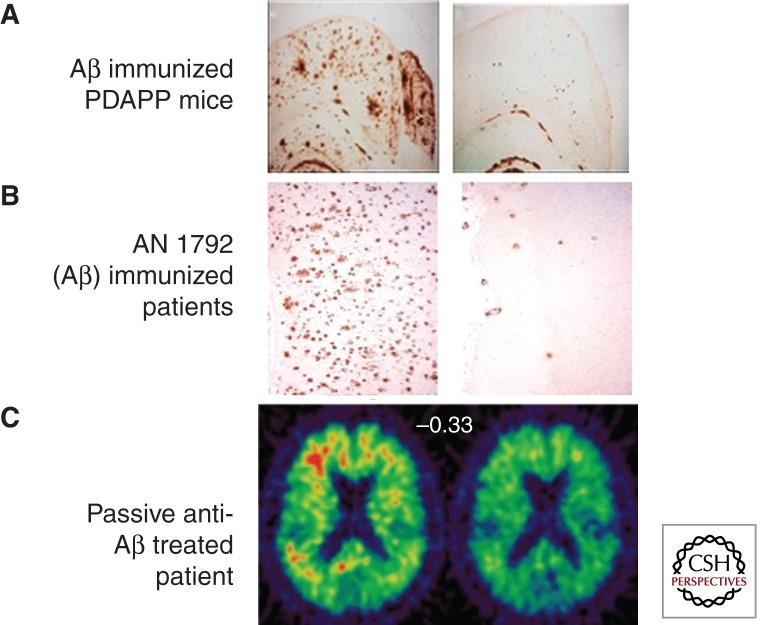
Reduction of Aβ plaque pathology by Aβ immunotherapy in mouse APP transgenic mice and patients suffering from Alzheimer disease. The left panel in each image illustrates amyloid pathology in control (A), in reference (B), or at a baseline (C) and the right panel in each image illustrates amyloid pathology following immunotherapy. (A) Mice immunized with full-length Aβ peptide at mid-age (when plaques are already present) 6 months later show an actual reduction in plaque burden relative to vehicle-treated controls. (From Schenk et al. 1999; reprinted with permission from the author.) (B) Patients who were immunized with AN 1792 (full-length Aβ peptide) from a phase 1 study who eventually died and went to autopsy exhibited low levels of absence of Aβ plaques. (Modified from Nicoll et al. 2006; reprinted with permission from the author.) (C) Living patients from a phase 2 study treated with bapinuezumab (a humanized monoclonal antibody directed to the amino-terminal region of Aβ) showed a reduced level of PET-PIB (positron-emission tomography-Pittsburgh compound B) retention following treatment, suggesting reduced Aβ plaque burden. (From Rinne et al. 2010; reprinted with permission from the author.)
Research to better understand all aspects of the biophysics of amyloid formation of multiple proteins has progressed in the past 10 years in several directions. Most importantly, a more complete understanding of fibril formation and factors involved in the process began to emerge from the use of NMR analyses and calorimetric approaches. The conceptualization of intermediate structures, in particular, led to a refinement of hypotheses and models regarding amyloid formation in general (Lashuel et al. 2002). One significant step forward was the development of a model with high predictive value by Dobson and colleagues (Chiti et al. 2003). This model proposed that the likelihood of a given protein or peptide to form amyloid was dependent upon its charge, hydrophibicity, and β-pleated sheet propensity. Testing of the model by insertion of novel amino acids to increase or decrease the kinetics of amyloid fibril formation was highly predictive. In addition, the model also predicted that a number of known pathogenic mutations in Aβ and other amyloid-forming proteins potentially did so by this mechanism. This concept, together with that of intermediate energy states predisposing to amyloid formation, has changed our current thinking of how proteins become amyloidogenic. Specifically, rather than a few proteins with the capability of forming amyloid in our genome, an alternative view is that nearly all proteins can do so under the appropriate circumstances (Dobson 2003). The idea is that most proteins avoid amyloidogenesis by either never reaching high enough monomer concentrations or have a sufficiently high-energy barrier that amyloid formation is extraordinarily unlikely to occur. The inverse of this concept is that if unusual, adverse environments or circumstances exist, amyloid can form as a consequence.
With these new concepts in mind, alternative ideas and thoughts have arisen about what steps an inhibitor of fibrillization might be able to interfere with. For example, an inhibitor might be able to bind to or stabilize a nonamyloid form of Aβ, representing an intermediate, and in this way indirectly block fibril formation (Liu et al. 2006). Less binding stability of such an inhibitor would be required, because it would not be required to block an extremely highly favored forward reaction of fibril formation in the presence of micromolar levels of Aβ and thioflavin, as had been previously attempted. This general approach and insight has led to a renewed interest and effort in attempts to indirectly block Aβ aggregation—both in vitro and in vivo.
Animal Studies Exploring Aβ Aggregation Inhibitors
Tramiposate is an example of a small molecule that blocks Aβ fibrillization experimentally and was ultimately pursued both in animal models and through large-scale pivotal clinical trials in mild to moderate AD. Tramiposate is a derivative of proprionic acid and is thus nonsymmetrical and small. Biophysically, it is capable of blocking Aβ aggregation and fibrillization effectively at low micromolar concentrations. Whereas the exact form of Aβ that tramiposate interacts with is not known (Gervais et al. 2007), it is likely to be an oligomeric or protofibrillar form of the peptide. The consequence is that fibrils of the peptide are inhibited from forming in the presence of the compound. Atomic force microscopy also suggests that small, globular forms of the peptide are stabilized in its presence (Gervais et al. 2007). The compound was tested in APP transgenic mice to see if it would have any effects on Aβ plaque burden. Results from this study suggested that it does reduce amyloid burden in Tg2576 mice by about 50%, reaching statistical significance. In addition to these effects, the compound also reduced other Aβ-related pathologies in these mice (Gervais et al. 2007).
Recently, animal evidence for another molecule has suggested its potential role in blocking Aβ fibrillization and promoting its decrease in brain. In the early 1990s, McLaurin and colleagues at the Univerisity of Toronto became interested in the possibility that Aβ might interact with phospholipid moieties (McLaurin and Chakrabartty 1996). This work led to several publications that suggested that a key manipulable interaction might involve Aβ binding to inositol (McLaurin et al. 1998, 2000; Fung et al. 2005). Inositol exists in multiple isoforms biologically with the most common form being myo-inositol, a precursor used by all cells for a variety of other organic molecules. Notably, it was shown that scyllo-inositol but not most other inositol stereoisomers were very effective in blocking Aβ aggregation and fibrillization via a number of independent approaches. These findings led to a key series of experiments which demonstrated that scyllo-inositol, in particular, is effective in reducing amyloid burden and improving behavior and survival in the TgCRND8 transgenic mouse model (McLaurin et al. 2006). One interesting feature of the inositols is that they are actively taken up by glucose transporters at the blood–brain barrier, suggesting that, unlike many compounds emerging from medicinal chemistry, they can achieve relatively high concentrations within the brain following peripheral administration. At the neuronal level, scyllo-inositol has been demonstrated to prevent the inhibition of hippocampal LTP caused by cell-secreted oligomers of Aβ (Townsend et al. 2006a). This finding suggests that its mode of action is to impede or block the toxicity of oligomers—thought by some to be key neurotoxic forms of Aβ in the brain.
Clinical Studies Investigating Aβ Aggregation Inhibitors
As a result of the above findings, tramiposate entered phase 1 and 2 trials for mild to moderate AD. These studies showed that the agent was well tolerated and had a good safety profile that supported further clinical development. Exploratory clinical end points were also examined in the phase 2 study (Aisen et al. 2006). Although none of the prespecified endpoints demonstrated benefit, some secondary analyses suggested the possibility of improvement in some subgroups. The effects of tramiposate on CSF Aβ levels were also investigated; the compound appeared to increase CSF Aβ, although very modestly. Pharmacokinetic studies suggested that, whereas the compound could be found in the CSF of treated patients, the amounts were below those required to block Aβ fibrillization in vitro. Taken together, these findings resulted in tramiposate entering large phase 3 pivotal trials. Ultimately, the agent did not meet its primary end points of improvement in the ADAS-COG or Clinical Global Impression of Change. Although the study was not published, it was observed by the company that there was very high variability among the clinical sites in terms of extent of decline of patient groups, which resulted in higher than expected variance in the placebo group decline. No changes in biomarkers were described as a result of treatment. Unfortunately, because of the lack of change in biomarkers, the interpretation of the negative findings from the tramiposate phase 3 study are not interpretable. Did the compound even reach its brain target of Aβ in sufficient concentrations to have an effect? If it did, was it pharmacologically ineffective? Or was it potentially effective but the variance of the population was so great that this masked its benefits? This example of the inability to interpret the results of putatively disease-modifying agents emphasizes the critical requirement for biomarkers in all AD clinical trials. The need is twofold: first, for biomarkers that provide information on whether a given agent has reached its target and had the predicted molecular effect; second, for biomarkers that provide information on whether a downstream feature of the disease was affected. Without these types of data, clinical studies entailing enormous patient effort and risk, to say nothing of time and cost, are expended without any significant provision of knowledge and insight.
Based upon the preclinical studies reviewed in the previous section, scyllo-inositol appeared to be a reasonable candidate for clinical investigation of the effects of an Aβ aggregation inhibitor in mild to moderate AD. Multiple phase 1 studies were conducted, and the compound was found to be reasonably safe and well tolerated. These studies resulted in a phase 2 trial that was recently completed under the sponsorship of Elan Pharmaceuticals and Transition Therapeutics; it examined safety, tolerability and possible efficacy of three doses (0.25, 1, or 2 g b.i.d.) of ELND005 (scyllo-inositol) over an 18 month period. The study was amended by the sponsors to limit the active arm to only the 0.25 g b.i.d. dose, owing to possible safety and tolerability issues of the two higher doses. This amendment indicates that scyllo-inositol, like most investigational molecules, will need to be thoroughly tested clinically before a clear safety profile can be fully established. The 18 month study was completed in mid-2010 (Salloway et al. 2009).
Future Efforts for Aβ Aggregation Inhibitors
Aside from scyllo-inositol, few other molecules that block Aβ aggregation are currently in clinical development. From the perspective of medical hypothesis testing, because the molecule appears to capable of neutralizing Aβ oligomers cytotoxicity, if enough compound enters the brain, it will be a good test of whether toxic oligomers play a role in the clinical manifestations of AD. This is critical because, to date, almost all findings about Aβ oligomers are either in tissue culture or involve mouse models.
In summary, although antiaggregation is an intuitively logical target for neutralizing the effects of amyloid plaques and Aβ oligomer toxicity, only two such agents have progressed to advanced clinical testing in AD patients. Of these, one has failed in pivotal clinical trials for less than clear reasons, and the data for the second are in press at this writing. Nevertheless, our improved understanding of the biophysics and biology of Aβ fibrillization is likely to identify additional molecules worthy of human testing in the future.
Aβ IMMUNOTHERAPY
Crossing the blood–brain barrier remains one of the major obstacles in CNS drug discovery and has historically limited our efforts to targets amenable to medicinal chemistry-driven, small molecule drug discovery. The blood–brain barrier cannot, however, be solely blamed for this small molecule-centric view, and with hindsight, more thought was needed (and indeed is still needed) to be applied to non-small-molecule platforms, whether they be antibody-, peptide-, vaccine-, cell-, or oligonucleotide-based. In 1999, work from Schenk and colleagues at Elan Pharmaceuticals spurred this area of discovery with a potentially transformational approach. These investigators reported striking results following immunization of PDAPP transgenic mice with an intraperitoneal injection of aggregated Aβ once a month for nearly a year (Schenk et al. 1999). This immunization with Aβ1–42 peptide, subsequently referred to as “active immunization,” generated a polyclonal antibody response to Aβ, resulting in significantly reduced amyloid plaque burden, neuritic dystrophy and associated inflammatory changes in the brains of these animals, even when active immunization was started in older animals with pre-existing amyloid pathology. These unexpected findings have since been replicated in hundreds of reports, expanding both our understanding of potential mechanisms and the biological responses that result. Importantly, this work has also spurred a new field of “active” and “passive” immunotherapy for neurodegenerative diseases aimed at slowing or perhaps even arresting the neurodegenerative process (Schenk 2002; Pangalos et al. 2005).
Preclinical Observations
Following the seminal 1999 paper on active Aβ immunization, the same group first demonstrated that one could circumvent the immune response (i.e., not rely on the animal’s ability to generate anti-Aβ antibodies following active immunization) by direct administration of anti-Aβ antibodies into transgenic APP mice. This “passive immunization” approach was likewise very effective at clearing amyloid plaques and reversing neuritic and glial pathology (Bard et al. 2000), to a degree similar to that seen in the original active peptide immunization. Plaque lowering appeared to be dependent on the antibody being able to recognize and bind aggregated Aβ in the brain, as antibodies unable to bind to amyloid plaques in vitro had no significant impact on plaque pathology in vivo (Bard et al. 2000). It is important to note that immunotherapy has an impact on disease pathology beyond amyloid plaque clearance. Amino-terminal specific anti-Aβ antibodies reduce neuritic dystrophy as early as 4 days after treatment, with beneficial effects lasting for over a month (Lombardo et al. 2003; Brendza et al. 2005). Passive and active immunization have both been reported to reduce early tau hyperphosphorylation and cytopathology (Oddo et al. 2004; Wilcock et al. 2009), supporting human genetic evidence that Aβ pathology lies upstream of the alteration of (invariably wild-type) tau in AD. The benefits of immunotherapy on cognition in preclinical models have also been repeated in many studies since the original observations on this aspect (Janus et al. 2000; Morgan et al. 2000). Active and passive immunization which targets amino-terminal and central domains of Aβ have dramatically and sometimes very rapidly improved performance in a variety of cognition assays independent of plaque clearance, suggesting that mechanisms such as sequestration of toxic soluble Aβ species may be critical for improving behavioral deficits observed in transgenic mouse models of AD (Dodart et al. 2002; Kotilinek et al. 2002; Sigurdsson et al. 2004). Both approaches also rescue the abnormal hippocampal synaptic plasticity that occurs in rodents exposed to soluble Aβ oligomers (Klyubin et al. 2005).
Alternative modalities and routes of administration have also been investigated following the first active and passive immunotherapy studies. Intranasal administration of Aβ in a mouse model of AD significantly lowered Aβ and realted cytopathology in the mouse brain (Weiner et al. 2000; Leverone et al. 2003), and others have effectively treated mice with Aβ sequences either using phage display or expressed on adeno-associated virus (Zhang et al. 2003; Lavie et al. 2004). Irrespective of the approach taken, results have consistently demonstrated that active and passive immunization strategies, targeting specific sequences within the Aβ peptide, are able to slow or reverse disease pathology and reverse memory deficits in preclinical models of AD.
Potential Mechanisms of Action
There is generally good agreement as to the preclinical effectiveness of both active and passive immunization approaches in APP transgenic mice. In contrast, the precise mechanism(s) by which anti-Aβ antibodies elicit their beneficial effects in rodents are less well understood and ardently debated. Early mouse immunization studies reported a decrease in MAC-1 (CD11b) positive activated microglia around amyloid plaques but an increase in MHC class II cells associated with punctate Aβ staining around the walls or lumens of blood vessels. These cells phenotypically resembled activated microglia or monocytes (Schenk et al. 1999). Subsequent ex vivo experiments showed that microglia bound to and phagocytosed amyloid plaques decorated with anti-Aβ antibodies in an Fc-dependent manner. Furthermore, microglial-mediated phagocytosis of plaques required antibody binding to the amyloid plaque itself rather than to fibrillar or oligomeric Aβ species (Bard et al. 2000; Wilcock et al. 2003, 2004). Therefore, one proposed mechanism of action is that anti-Aβ antibodies enter the brain following treatment with either an active or passive immunization protocol, bind to parenchymal plaque-bound Aβ and recruit phagocytosing microglia via their cell surface expressed Fc-receptors. However, in contrast to these data, some studies have reported that amyloid plaque clearance can occur in a non-Fc-dependent manner. Anti-Aβ F(ab′)2 fragments, that is, lacking an Fc-region, rapidly cleared amyloid plaques in vivo in a manner indistinguishable from the parental full-length antibody (Bacskai et al. 2002). In addition, two studies have demonstrated that immunotherapy can robustly clear amyloid plaque pathology either when Fc receptors are knocked out (Das et al. 2003) or when microglia have been ablated for four continuous weeks (Grathwohl et al. 2009). These contradictory data suggest at least two mechanisms of amyloid clearance, one that is microglia-dependent and one that is microglia-independent.
A second potential mechanism of action centers on the ability of anti-Aβ antibodies to bind and block the formation and/or neurotoxic activity of soluble Aβ species. This idea is supported by the experiments described above with anti-Aβ F(ab′)2 fragments and suggest that antibodies binding to Aβ can shift an Aβ equilibrium away from more toxic aggregated states to less toxic monomeric states. This is further suggested by in vitro data in which anti-Aβ antibodies blocked the formation of Aβ fibrils and dissolved pre-existing fibrils (Solomon et al. 1997). Subsequent studies identified amino-terminal residues of Aβ (amino acids 3–6 [EFRH]) as the minimally effective epitope for this activity. In support of this, antibodies directed to residues 4–10 of Aβ inhibit decrease both fibril formation and Aβ-mediated neuronal death (McLaurin et al. 2002). More recently, Aβ neutralizing antibodies have been shown to reverse LTP and cognitive deficits by blocking the synaptotoxic activity of soluble Aβ oligomers, including those isolated directly from AD brain (Klyubin et al. 2005; Shankar et al. 2008). Taken together, these findings suggest immunotherapy can have a rapid effect on learning and memory through removal of a variety of soluble toxic Aβ species, independently of any effects on amyloid pathology, at least in APP transgenic models. This concept is consistent with data generated in our and other laboratories in which treatments with a variety of amyloid-lowering drugs can rapidly and robustly reverse cognitive deficits in the absence of effects on plaque pathology (Comery et al. 2005).
The mechanisms described above would theoretically require anti-Aβ antibodies to enter the CNS. Although studies have demonstrated systemically administered antibodies do cross the blood–brain barrier and decorate a large portion of amyloid plaques, the level of central exposure is very low, with only 0.1%–0.5% of a systemically administered antibody reaching the brain (Bard et al. 2000; Choe et al. 2007). This observation raises questions as to how anti-Aβ immunotherapy has such dramatic and widespread effects in the CNS while antibody levels in the brain remain so low. The amyloid “sink” hypothesis is based on the finding that Aβ is actively transported out of the CNS by the low-density lipoprotein receptor (LRP-1; Deane et al. 2004) and actively pumped into the CNS by the receptor for advanced glycation end products (RAGE; Schmidt et al. 2009), among other mechanisms. These transport mechanisms are thought to keep Aβ in a complex equilibrium between the brain and periphery. Using m266, a high-affinity antibody binding to a central epitope of Aβ, De Mattos and colleagues were able to sequester peripheral pools of Aβ, prevent transport of Aβ back into the brain and shift the Aβ equilibrium to one of net Aβ clearance out of the brain (DeMattos et al. 2001, 2002a,b). Importantly, this antibody is highly specific for soluble Aβ and does not bind well to amyloid plaques. This “peripheral sink” hypothesis is obviously attractive, as it highlights a potential mechanism by which immunotherapy can enhance Aβ clearance without requiring central penetration of peripherally circulating antibodies. More recently, investigators have tried to elucidate the relative contribution of Aβ clearance via the periphery (Vasilevko et al. 2007). They used a transgenic mouse carrying Swedish, Dutch, and Iowa APP mutations. The “DI” Aβ peptide derived from these mice has negligible affinity for the LRP receptor and, as a result, undergoes little active transport across the blood–brain barrier. Immunotherapy had no effect on parenchymal or vascular amyloid deposition in this mouse model, whereas central administration of antibodies into the brains of these mice produced a rapid and robust clearance of amyloid deposits. These results support a role for the “peripheral sink” hypothesis and suggest that intact Aβ transport mechanisms can enhance antibody-mediated clearance of Aβ. However, whereas some have noted that acute treatment with m266 is able to trap Aβ in the periphery and acutely reverse cognitive deficits in APP transgenic mice (Dodart et al. 2002), others have shown that chronic treatment with m266 has no impact on cortical plaque pathology (Seubert et al. 2008). Interestingly, a recent report has questioned the “peripheral sink” hypothesis by studying the dynamics and efflux of 125I-labeled Aβ from the CNS and suggesting that peripherally administered m266 sequesters soluble Aβ in the brain, resulting in a reduced rather than enhanced Aβ efflux (Yamada et al. 2009).
At the end of the day, the robustness of the preclinical immunotherapy on Aβ-mediated physiology, behavior, and pathology are strong, and none of the mechanisms described above are mutually exclusive. It is possible and in fact likely that centrally and peripherally mediated effects work in tandem to help lower the levels of toxic Aβ species and, over time, enhance clearance of amyloid plaques in the CNS.
Learning from the First Clinical Trial (of AN1792) and Some Related Concerns
Based on the strong preclinical efficacy data described above and an IND enabling safety studies in mice, rabbits, guinea pigs, and nonhuman primates, an active immunization trial was initiated in patients using full-length Aβ1–42 in combination with a previously validated adjuvant, QS-21. This therapeutic mixture will be referred to as AN1792 for the remainder of this article. The initial single dose phase 1 trial in 24 patients demonstrated good tolerability, leading to the initiation of a larger, multidose phase 1 study with 64 patients given AN1792 and 16 given the adjuvant alone. Dosing intervals of this active immunization were 0, 4, 12, and 24 wk. The study drug was reasonably well tolerated with treatment-related adverse events (AEs) reported in 23% of patients, but with no relationship between dose and AE incidence. One patient in the study had developed a meningoencephalitis, diagnosed after death. Approximately 20% of this elderly phase 1 patient population had an initially positive anti-Aβ antibody titer, increasing to nearly 60% with further immunizations. No treatment differences were observed across efficacy measures in this safety trial, apart from less decline on the Disability Assessment of Dementia scores in active versus placebo treated patients (Bayer et al. 2005).
Based on these two small phase 1 trials, a phase 2a trial was initiated in 372 mild to moderate AD patients to better understand the immunogenicity, safety, and tolerability profile of AN1792. The trial was not powered to show a treatment effect on cognitive decline. This trial was terminated early following four reports of acute meningoencephalitis that later were shown to have affected a total of 6% of all the actively immunized patients (18 out of 300; Orgogozo et al. 2003). Sixteen of the 18 patients who developed the sterile meningioencephalitis had received two doses of AN1792, and the median latency from the last injection to symptoms was 40 days. Almost all of the 18 patients who developed clinically diagnosed meningoencephalitis gradually improved to baseline, but at the time of autopsy patients were left with cerebrovascular lesions. Analysis of this interrupted trial (patients received between one and three of the six planned AN1792 immunizations) showed that ∼20% of patients developed a good antibody response to Aβ, with no correlation between antibody titter and the development of meningoencephalitis.
The first efficacy report from the phase 2a trial with AN1792 came from a subset-analysis of 30 patients treated in Switzerland, and as the results are based on a single site only, any interpretation should be treated with caution. The data suggested a slowing in cognitive decline, as measured by ADAS-COG and MMSE, particularly in those patients generating the highest antibody titers (Hock et al. 2003). The full analysis of all patients treated in this phase 2a trial were reported in 2005 and yielded quite different results (Gilman et al. 2005). No significant effects were found between antibody responders and placebo groups on a number of exploratory measures of cognition or disability (including ADAS-Cog, Disability Assessment for Dementia [DAD], CDR, or MMSE). However in a nine-component neuropsychological test battery (NTB), antibody responders performed significantly better than placebo-treated patients. Furthermore, improvements in some memory components of the NTB, including immediate and delayed memory, were associated with an increased antibody response, suggesting a possible dose-dependent effect of the treatment. In a subset of patients, CSF tau, a potential measure of neurodegeneration, was significantly decreased in antibody responders compared with placebo treated patients, whereas Aβ42 levels remained unchanged. Volumetric MRI was also used to examine cerebral changes in patients treated with AN1792. Comparison of scans predosing and 12 mo after two or three AN1792 doses demonstrated that antibody responders had greater total brain volume loss, ventricular enlargement, and hippocampal volume loss. This apparent loss in brain and hippocampal volume was not associated with a worsening in cognitive function and indeed an improvement in NTB (Fox et al. 2005). A number of factors could explain this surprising finding. For example, increased removal of amyloid deposits, changes in plaque composition or changes in CSF dynamics resulting from increased Aβ outflow could each alter the water content of the brain, resulting in an apparent loss of brain volume (Fox et al. 2005). Another plausible explanation for this observed loss in brain volume could be an acceleration of neuronal degeneration. However, this is unlikely given that antibody responders with increased volume loss showed less cognitive decline on the NTB, and antibody responders had reduced rather than increased CSF tau (Gilman et al. 2005). Finally, the mean loss of cortical volume could have been related to the development of subclinical meningoencephalitis in some of the AN1792-treated patients, associated with shifts in brain fluid and electrolyte balance.
Independent neuropathological assessments of trial subjects who died of unrelated causes many months or years after receiving AN1792 has yielded interesting findings. The first patient studied at autopsy came from the multidose phase 1 trial and was found to have had meningoencephalitis as detected by CD4+ T-cell infiltrates in the leptomeninges, most densely associated with amyloid laden blood vessels, as well as sparse lymphocyte infiltration in the cortex and perivascular spaces (Nicoll et al. 2003). Interestingly, however, there were areas of the neocortex with significant evidence of plaque removal, reduced neuritic dystrophy and reduced astrocyte clusters. Furthermore, in some areas devoid of amyloid deposits, there was evidence of Aβ immunoreactivity in microglia (Nicoll et al. 2003), resembling the microglial staining seen in preclinical mouse experiments. A second report of an AN1792-treated patient with meningoencephalitis also highlighted a decrease in diffuse and neuritic plaques with accompanying activated microglia immunoreactive for Aβ surrounding small “collapsed” plaques (Ferrer et al. 2004). Interestingly, this decrease in amyloid burden was accompanied by an attenuated local stress response, as measured by reduced levels of stress-activated kinase and c-jun amino-terminal kinase. Both enzymes have been implicated in the hyperphosphorylation of tau, and indeed, collapsed plaques were devoid of phospho-tau immunoreactivity. Finally, there was no apparent reduction in vascular Aβ in this patient, and there were multiple, small cortical hemorrhages that may or may not have been related to the presence of severe small vessel disease (Ferrer et al. 2004). Studies from three more AN1972 recipients examined at autopsy, two with meningoencephalitis and one without, corroborated the findings reported by Nicoll et al. and Ferrer et al. and highlight that cortical amyloid plaque pathology can be reduced following Aβ vaccination in the absence of meningoencephalitis (Masliah et al. 2005; Patton et al. 2006b). A more detailed analysis focusing on the cerebrovasculature of nine patients who died between 4 months and 5 years after their first AN1792 immunization showed that treated patients had significantly more Aβ40 and Aβ42 in their blood vessels and a higher density of cortical microhemorrhages (Boche et al. 2008). However, it should be noted that two of the longest survivors had an almost complete absence of vascular Aβ. This finding is consistent with a hypothesis in which Aβ immunization results in solublization of amyloid plaques and exit of Aβ via the perivascular space, leading to a transient increase in cerebrovascular Aβ that is ultimately cleared over time (Boche et al. 2008). Finally, immunohistochemistry of the Aβ species cleared from the brains of patients treated with AN1792 revealed that all major species were impacted (Aβ40, Aβ42, and amino-terminally truncated Aβ species; Nicoll et al. 2006; Patton et al. 2006a).
Long-term follow-up of 129 patients from the phase 2a trial at a mean of 4.6 yr after their AN1792 immunization revealed that numerous patients previously classified as antibody responders still had a low but detectable anti-Aβ antibody titer. Overall, the responder group had significantly less decline as measured on DAD and dependency scales. In addition, MRI brain volume measures now identified no differences between AN1792 and placebo-treated patients, in contrast to the original phase 2 trial findings reported by Fox and colleagues in 2005 (Vellas et al. 2009). In contrast to the above findings, a study of a subset of 15 U.K. patients treated with AN1792 followed to autopsy suggested continued disease progression and cognitive decline. In eight of these progressing subjects who were autopsied, two showed no apparent decrease in plaque burden, four had intermediate decreases, and two had marked decreases (Holmes et al. 2008). Although all AN1792 follow-up studies have shown variable reductions in amyloid plaque burden, data from these long-term follow-up studies reporting cognitive end points need to be treated with care. The phase 2 AN1792 study was halted early with most patients receiving only one or two doses of study drug and most never reaching an optimal or sustained anti-Aβ antibody titer.
Two of the major adverse events observed in the trials with AN1792 were meningoencephalitis and cerebral microhemorrhage. Examination of some of the encephalitis cases postmortem revealed a marked CD4+ T-cell infiltration suggestive of a T-cell response to Aβ (Nicoll et al. 2003; Ferrer et al. 2004; Masliah et al. 2005). The principal T-cell epitopes on Aβ have been mapped to its carboxyl terminus (Glaser et al. 1998; Monsonego et al. 2003) and can therefore be avoided by immunizing with Aβ sequences devoid of these T-cell epitopes in the next generation of active vaccines. Currently, a number of newer vaccines employing only a fragment of Aβ peptide to circumvent a T-cell response are in clinical development. Thus far, by employing this new strategy, no new reports from these follow-on vaccines of meningoencephalitis have been reported. Concerns have also been raised about the potential for active and passive immunotherapy to cause microhemorrhages, an event that has been reported to occur in AD patients as part of the natural history of the disease (Yates et al. 2011). Some efforts have been made to model these events in APP transgenic mice, although the results have been variable. Passive immunizations with antibodies recognizing a variety of Aβ epitopes in transgenic APP mice with CAA have resulted in an increased incidence of CAA-associated microhemorrhages (Pfeifer et al. 2002; Wilcock et al. 2004; Racke et al. 2005). The implications of these mouse findings remain unclear given that the doses of antibody used were sometimes high and the animals had very severe and pre-existing cerebral amyloid angiopathy. Furthermore, these findings have not been observed by others in clinical trials to date (Goni and Sigurdsson 2005).
Current Clinical Approaches to Immunotherapy
Passive Immunization
Although active immunization produces an oligoclonal response and is more convenient to administer, passive immunotherapy using humanized monoclonal anti-Aβ antibodies confers the potential advantage of being easier to stop should serious adverse events be observed during the course of a clinical trial. Two humanized monoclonal antibodies, bapineuzumab and LY2062430, are currently in late-stage clinical development in large multinational trials. Bapineuzumab is an amino-terminal specific antibody derived from the murine monoclonal antibody 3D6. 3D6 has been reported to enhance the clearance of amyloid plaques and significantly reduce total levels of brain Aβ, inflammation, neuritic dystrophy, and synapse loss in the brains of APP transgenic mice (Bard et al. 2000). LY2062430, which is derived from the murine antibody m266, is specific to amino acids 16–24 of Aβ. m266 has been shown to lower brain Aβ levels in the CNS, although there are conflicting data in regard to its ability to clear amyloid pathology from the brains of APP transgenic animals (DeMattos et al. 2001; Seubert et al. 2008). Both antibodies have been reported to acutely reverse memory deficits in APP transgenic mice, with m266 appearing more potent than 3D6 perhaps owing to its preferential binding to soluble Aβ species.
A 234 patient, phase 2a safety and tolerability trial with bapineuzumab has recently been reported. Patients were randomly assigned one of four bapineuzumab doses (0.15, 0.5, 1.0, or 2.0 mg/kg), with intravenous infusions spaced 13 wk apart and final patient assessment completed at week 78. No significant differences were found in primary efficacy endpoints, although post-hoc analyses suggested some cognitive and functional benefit in treatment completers and in non-ApoE4 carriers. No significant decreases were observed in exploratory biomarkers. MRI analyses showed no treatment differences in brain or ventricular volume and CSF Aβ4x-42 and total tau remained unchanged but with a trend toward decreased phopho-tau181 (Salloway et al. 2009). Twenty-eight patients in a separate phase 2 trail of bapineuzimab were assessed for cortical amyloid load using the 11C-PiB PET tracer (19 patients were on study drug and seven on placebo). Treatment with bapineuzumab for 78 wk significantly reduced PiB retention across cortical brain areas compared with the patients’ baseline scans and to placebo. Patients on babpineuzumab had a decrease in PiB retention of 8.5% compared with an increase in PiB retention of 16.9% with placebo, suggesting that bapineuzumab can reduce cortical fibrillar Aβ by ∼25% over a 78-wk period (Rinne et al. 2010). These data are critically important, as they help establish that the ongoing phase 3 bapineuzumab trials will test whether amyloid plaque reduction over 78 wk has any impact on cognitive decline or disease progression in mild to moderate AD patients. The rate of AEs was higher for bapineuzumab than placebo at respective means of 7.5 events compared with 5.7 events, with more than 90% of AEs being mild to moderate in severity. Vasogenic edema (VE) was the only dose-dependent AE and was detected by brain MRI in 10% of treated patients (12/124) versus no placebo patients. VE incidence increased with dose (3.2% for 0.15 mg/kg compared with 26.7% for 2.0 mg/kg) and with ApoE4 gene dose (4.3% with no copies of ApoE4 compared with 33.3% with two copies of ApoE4; Salloway et al. 2009).
Data from clinical trials with LY2062430 patients have yet to be published, but recent data from the initial clinical trials has been orally presented by Ron DeMattos and Eric Siemers (http://www.quintiles.com/information-library/videos/icad-eric-siemers/). In a phase 2a trial, four doses of antibody were tested in patients with mild to moderate AD, with approximately 10 patients per dose arm (100 mg/wk, 400 mg/wk, 100 mg every 4 wk, and 400 mg every 4 wk). None of the 42 treated patients showed any evidence of VEs, microhemorrhage or inflammation, and four patients showed antibody titers against the LY2062430 antibody, although these did not appear to be neutralizing antibodies. Plasma and CSF Aβ levels were significantly elevated, and 0.1% of the antibody was detected in the CSF. No changes were reported in CSF tau. Interestingly, levels of free and unbound CSF Aβ40 appeared to go down, whereas free and unbound Aβ42 increased with rising antibody doses. These changes were suggested to reflect a “leaching” from the brain of plaque bound Aβ42. In addition, data were presented suggesting that amino-terminally truncated fragments of Aβ, probably derived from the brain, were also increased in the CSF following drug treatment. Two additional monoclonal antibodies, GSK933776A (GSK) and gantenerumab (Roche), are in early development, but no data are available in the public domain reporting clinical findings.
Another approach currently in mid-stage development and somewhat related to passive immunotherapy is infusion of intravenous immunoglobulin (IVIg) antibody, an FDA-approved purified immunoglobulin pool from normal donors used for the treatment of inflammatory diseases. In a small seven-patient study of mild to moderate AD patients, IVIg infusions once a month for 6 months resulted in increased plasma levels of Aβ and an improvement in cognitive function. These results mirror a previous report in five AD patients treated with IVIg in which decreased CSF Aβ and increased peripheral Aβ were observed. The latter study also reported stabilization of cognitive decline but no improvement (Dodel et al. 2004).
Active Immunization
Following on from the original AN1792 active immunization trial, three clinical trials are currently testing active immunization in mild to moderate AD patients. ACC-001 is a novel immunoconjugate being developed by Pfizer (formerly Wyeth) and Janssen AIP (J&J and Elan) that links an amino-terminal Aβ fragment to the carrier protein CRM, used for many years in pediatric vaccines such as Prevnar (http://www.theodora.com/drugs/prevnar_for_injection_wyeth.html). CAD-106 is being developed in a collaboration between Novartis and Cytos. Similar to ACC-001, this vaccine is devoid of any Aβ T-cell epitopes and is made up of the first six amino acids of Aβ conjugated to a virus like particle. V950 is an active immunization being developed by Merck, although no information on its design is currently available. Finally, a company called Affiris had developed an Affitope vaccine technology using short six-amino acid peptides that mimic the native Aβ sequence.
THE FUTURE: OPPORTUNITIES AND CHALLENGES
As active and passive immunization approaches continue to bring together investigators from two of the most complex and incompletely understood fields of science, neuroscience and immunology, there is real hope that further improvements to our mechanistic knowledge of AD will enable the successful clinical implementation of these approaches for the treatment of this devastating neurodegenerative disorder. If a safe and well-tolerated immunization strategy—whether active or passive—can be successfully developed, one can envisage a scenario where improving diagnosis of the disease will allow patients to be treated ever earlier, preventing progression of neuropathology and the development of progressive memory impairment.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Patrick L. May for graciously sharing results with us prior to publication.
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
- Acquati F, Accarino M, Nucci C, Fumagalli P, Jovine L, Ottolenghi S, Taramelli R 2000. The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the down critical region. FEBS Lett 468: 59–64 [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ 2003. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 289: 2819–2826 [DOI] [PubMed] [Google Scholar]
- Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D 2006. A Phase II study targeting amyloid-β with 3APS in mild-to-moderate Alzheimer disease. Neurology 67: 1757–1763 [DOI] [PubMed] [Google Scholar]
- Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, Feldman HH, Petersen RC, Siemers E, Doody RS, et al. 2011. Report of the task force on designing clinical trials in early (predementia) AD. Neurology 76: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright CF, Dockens R, Olson RE, Meredith JE, Siemmon R, Lentz KJW, Denton R, Pilcher G, Zaczek R 2008. BMS-708163, a potent and selective γ-secretase inhibitor, decreases CSF Ab at safe and tolerable doses in animals, and humans. In International Conference on Alzheimer’s Disease, 2008, HT-01-05 Alzheimer’s Association, Chicago [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT 2002. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J Neurosci 22: 7873–7878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. 2000. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916–919 [DOI] [PubMed] [Google Scholar]
- Barten DM, Guss VL, Corsa JA, Loo A, Hansel SB, Zheng M, Munoz B, Srinivasan K, Wang B, Robertson BJ, et al. 2005. Dynamics of β-amyloid reductions in brain, cerebrospinal fluid, and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J Pharmacol Exp Ther 312: 635–643 [DOI] [PubMed] [Google Scholar]
- Basi G, Frigon N, Barbour R, Doan T, Gordon G, McConlogue L, Sinha S, Zeller M 2003. Antagonistic effects of β-site amyloid precursor protein-cleaving enzymes 1 and 2 on β-amyloid peptide production in cells. J Biol Chem 278: 31512–31520 [DOI] [PubMed] [Google Scholar]
- Basi GS, Hemphill S, Brigham EF, Liao A, Aubele DL, Baker J, Barbour R, Bova M, Chen XH, Dappen MS, et al. 2010. Amyloid precursor protein selective γ-secretase inhibitors for treatment of Alzheimer’s disease. Alzheimer’s Res Ther 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, Yarasheski KE, Friedrich SW, Demattos RB, May PC, et al. 2009. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann Neurol 66: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S 2005. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology 64: 94–101 [DOI] [PubMed] [Google Scholar]
- Benes P, Vetvicka V, Fusek M 2008. Cathepsin D—Many functions of one aspartic protease. Crit Rev Oncol Hematol 68: 12–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R 2000. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem 275: 20647–20651 [DOI] [PubMed] [Google Scholar]
- Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM 2004. Efficient reversal of Alzheimer’s disease fibril formation and elimination of neurotoxicity by a small molecule. Proc Natl Acad Sci 101: 14326–14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA 2008. Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain 131: 3299–3310 [DOI] [PubMed] [Google Scholar]
- Boggs LN, Lindstrom T, Watson B, Sheehan S, Audia JE, May PC 2010. Proof-of-concept pharmacodynamic assessment of a prototypic BACE1 inhibitor at steady-state using IV infusion dosing in the PDAPP transgenic mouse model of Alzheimer’s Disease. In International Conference on Alzheimer’s Disease, P3–304, S541 Alzheimer’s Association, Honolulu, HI [Google Scholar]
- Breitner JC, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, Pericak-Vance MA, Saunders AM 1995. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging 16: 523–530 [DOI] [PubMed] [Google Scholar]
- Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, et al. 2005. Anti-Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest 115: 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman FC, Garrido J, Alvarez A, Morgan C, Inestrosa NC 1996. Laminin inhibits amyloid-β-peptide fibrillation. Neurosci Lett 218: 201–203 [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC 2001. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci 4: 233–234 [DOI] [PubMed] [Google Scholar]
- Calamai M, Chiti F, Dobson CM 2005. Amyloid fibril formation can proceed from different conformations of a partially unfolded protein. Biophys J 89: 4201–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh AK, et al. 2004. In vivo inhibition of Aβ production by memapsin 2 (β-secretase) inhibitors. J Neurochem 89: 1409–1416 [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, et al. 2007. Accelerating amyloid-β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem 282: 23818–23828 [DOI] [PubMed] [Google Scholar]
- Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM 2003. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424: 805–808 [DOI] [PubMed] [Google Scholar]
- Choe L, D’Ascenzo M, Relkin NR, Pappin D, Ross P, Williamson B, Guertin S, Pribil P, Lee KH 2007. 8-Plex quantitation of changes in cerebrospinal fluid protein expression in subjects undergoing intravenous immunoglobulin treatment for Alzheimer’s disease. Proteomics 7: 3651–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher I, Beher D 2005. γ-Secretase as a therapeutic target for the treatment of Alzheimer’s disease. Curr Pharm Des 11: 3363–3382 [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH 2005. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci 8: 79–84 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov A multicenter, double blind, placebo-controlled, safety and tolerability study of BMS-708163 in patients with prodromal Alzheimer's disease. Identifier NCT00890890. Last updated September 15, 2011 [Google Scholar]
- Cole DC, Stock JR, Kreft AF, Antane M, Aschmies SH, Atchison KP, Casebier DS, Comery TA, Martone RL, Aschmies S, et al. 2005. Acute γ-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 25: 8898–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Chapoval S, Howard V, David CS, Golde TE 2003. Immune responses against Aβ1–42 in HLA class II transgenic mice: Implications for Aβ1–42 immune-mediated therapies. Neurobiol Aging 24: 969–976 [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. 2004. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43: 333–344 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM 2001. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. [See comment.] Proc Natl Acad Sci 98: 8850–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM 2002a. Brain to plasma amyloid-β efflux: A measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295: 2264–2267 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Parsadanian M, O’Dell MA, Foss EM, Paul SM, Holtzman DM 2002b. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem 81: 229–236 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391: 387–390 [DOI] [PubMed] [Google Scholar]
- Dinner AR, Sali A, Smith LJ, Dobson CM, Karplus M 2000. Understanding protein folding via free-energy surfaces from theory and experiment. Trends Biochem Sci 25: 331–339 [DOI] [PubMed] [Google Scholar]
- Dobson CM 2003. Protein folding and misfolding. Nature 426: 884–890 [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, et al. 2002. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci 5: 452–457 [DOI] [PubMed] [Google Scholar]
- Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A, Hemmeter U, Paulsen S, Teipel SJ, Brettschneider S, et al. 2004. Intravenous immunoglobulins containing antibodies against β-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiat 75: 1472–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, et al. 2005. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280: 30797–30806 [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, et al. 2001. Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem 76: 173–181 [DOI] [PubMed] [Google Scholar]
- Durairajan SS, Yuan Q, Xie L, Chan WS, Kum WF, Koo I, Liu C, Song Y, Huang JD, Klein WL, et al. 2008. Salvianolic acid B inhibits Aβ fibril formation and disaggregates preformed fibrils and protects against Aβ-induced cytotoxicty. Neurochem Int 52: 741–750 [DOI] [PubMed] [Google Scholar]
- Durham TB, Shepherd TA 2006. Progress toward the discovery and development of efficacious BACE inhibitors. Curr Opin Drug Discov Devel 9: 776–791 [PubMed] [Google Scholar]
- Eli Lilly 2010. Lilly halts development of semagacestat for Alzheimer’s disease based on preliminary results of phase III clinical trials. Press release, Eli Lilly, Indianapolis. [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, et al. 2003. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ 42 in vivo. J Clin Invest 112: 440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS 2000. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat Cell Biol 2: 428–434 [DOI] [PubMed] [Google Scholar]
- Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H 2000. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc Natl Acad Sci 97: 9712–9717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, et al. 2001. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32: 911–926 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F 2004. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer’s disease. Brain Pathol 14: 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, et al. 2008. Phase 2 safety trial targeting amyloid β production with a γ-secretase inhibitor in Alzheimer disease. Arch Neurol 65: 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R, Capell A, Westmeyer G, Willem M, Hartung B, Condron MM, Teplow DB, Haass C, Walter J 2002. A non-amyloidogenic function of BACE-2 in the secretory pathway. J Neurochem 81: 1011–1020 [DOI] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M 2005. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64: 1563–1572 [DOI] [PubMed] [Google Scholar]
- Fraering PC, Ye W, LaVoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS 2005. γ-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site. J Biol Chem 280: 41987–41996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Iwasaki K, Furukawa K, Seki T, He M, Maruyama M, Tomita N, Kudo Y, Higuchi M, Saido TC, et al. 2006. Uncaria rhynchophylla, a Chinese medicinal herb, has potent antiaggregation effects on Alzheimer’s β-amyloid proteins. J Neurosci Res 84: 427–433 [DOI] [PubMed] [Google Scholar]
- Fukumori A, Fluhrer R, Steiner H, Haass C 2010. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of γ-secretase-mediated intramembrane proteolysis. J Neurosci 30: 7853–7862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC 2002. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 59: 1381–1389 [DOI] [PubMed] [Google Scholar]
- Fung J, Darabie AA, McLaurin J 2005. Contribution of simple saccharides to the stabilization of amyloid structure. Biochem Biophys Res Commun 328: 1067–1072 [DOI] [PubMed] [Google Scholar]
- Galasko DR, Graff-Radford N, May S, Hendrix S, Cottrell BA, Sagi SA, Mather G, Laughlin M, Zavitz KH, Swabb E, et al. 2007. Safety, tolerability, pharmacokinetics, and Aβ levels after short-term administration of R-flurbiprofen in healthy elderly individuals. Alzheimer Dis Assoc Disord 21: 292–299 [DOI] [PubMed] [Google Scholar]
- Garofalo AW 2009. Patents targeting γ-secretase inhibition and modulation for the treatment of Alzheimer’s disease: 2004–2008. Expert Opin Ther Patents 187: 639–703 [Google Scholar]
- Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, Lacombe D, Kong X, Aman A, Laurin J, et al. 2007. Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 28: 537–547 [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Kumaragurubaran N, Hong L, Koelsh G, Tang J 2008. Memapsin 2 (β-secretase) inhibitors: Drug development. Curr Alzheimer Res 5: 121–131 [DOI] [PubMed] [Google Scholar]
- Gillman KW, Starrett JE, Parker MF, Xie K, Bronson JJ, Marcin LR, McElhone KE, Bergstrom CP, Mate RA, Williams R, et al. 2010. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable Î3-secretase inhibitor. ACS Med Chem Lett 1: 120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, et al. 2005. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64: 1553–1562 [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF 1998. The influence of psychological stress on the immune response to vaccines. Ann NY Acad Sci 840: 649–655 [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW 1984. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890 [DOI] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH 2011. Anti-aβ therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron 69: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL 2003. Alzheimer’s disease-affected brain: Presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci 100: 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni F, Sigurdsson EM 2005. New directions towards safer and effective vaccines for Alzheimer’s disease. Curr Opin Mol Ther 7: 17–23 [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. 2009. Formation and maintenance of Alzheimer’s disease β-amyloid plaques in the absence of microglia. Nat Neurosci 12: 1361–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH, Tarenflurbil Phase 3 Study G 2009. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA 302: 2557–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. 1992. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322–325 [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kiso Y 2009. Recent progress itne the discovery of non-peptidic BACE1 inhibitors. Expert Opin Drug Discov 4: 391–416 [DOI] [PubMed] [Google Scholar]
- Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, et al. 2003. BACE1 (β-secretase) transgenic and knockout mice: Identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci 24: 646–655 [DOI] [PubMed] [Google Scholar]
- Harrison T, Churcher I, Beher D 2004. γ-Secretase as a target for drug intervention in Alzheimer’s disease. Curr Opin Drug Discov Devel 7: 709–719 [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, et al. 2010. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature 467: 95–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming ML, Elias JE, Gygi SP, Selkoe DJ 2009. Identification of β-secretase (BACE1) substrates using quantitative proteomics. PLoS One 4: e8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DB, May PC, Dean RA, Siemers ER 2009. Development of semagacestat (LY450139), a functional γ-secretase inhibitor, for the treatment of Alzheimer’s disease. Expert Opin Pharmacother 10: 1657–1664 [DOI] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. 1999. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci 96: 11872–11877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B 2000. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol 2: 461–462 [DOI] [PubMed] [Google Scholar]
- Higaki J, Murphy GM Jr, Cordell B 1997. Inhibition of β-amyloid formation by haloperidol: A possible mechanism for reduced frequency of Alzheimer’s disease pathology in schizophrenia. J Neurochem 68: 333–336 [DOI] [PubMed] [Google Scholar]
- Hirohata M, Ono K, Naiki H, Yamada M 2005. Non-steroidal anti-inflammatory drugs have anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. Neuropharmacology 49: 1088–1099 [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, et al. 2003. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. [See comment.] Neuron 38: 547–554 [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, et al. 2008. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet 372: 216–223 [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G 2002. Increased expression of the amyloid precursor β-secretase in Alzheimer’s disease. Ann Neurol 51: 783–786 [DOI] [PubMed] [Google Scholar]
- Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J 2000. Structure of the protease domain of memapsin 2 (β-secretase) complexed with inhibitor. Science 290: 150–153 [DOI] [PubMed] [Google Scholar]
- Hong HS, Rana S, Barrigan L, Shi A, Zhang Y, Zhou F, Jin LW, Hua DH 2009. Inhibition of Alzheimer’s amyloid toxicity with a tricyclic pyrone molecule in vitro and in vivo. J Neurochem 108: 1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R 2006. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci 9: 1520–1525 [DOI] [PubMed] [Google Scholar]
- Hussain I 2004. The potential for BACE1 inhibitors in the treatment of Alzheimer’s disease. IDrugs 7: 653–658 [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, et al. 1999. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol Cell Neurosci 14: 419–427 [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell DJ, Howlett DR, Chapman GA, Gilmour L, Murdock PR, Tew DG, Meek TD, Chapman C, Schneider K, et al. 2000. ASP1 (BACE2) cleaves the amyloid precursor protein at the β-secretase site. Mol Cell Neurosci 16: 609–619 [DOI] [PubMed] [Google Scholar]
- Hyde LA, McHugh NA, Chen J, Zhang Q, Manfra D, Nomeir AA, Josien H, Bara T, Clader JW, Zhang L, et al. 2006. Studies to investigate the in vivo therapeutic window of the γ-secretase inhibitor N2-[(2S)-2-(3,5-difluorphenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-l-alaninamide (LY411,575) in the CRND8 mouse. J Pharmacol Exp Ther 319: 1133–1143 [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, et al. 2000. A β peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. [See comment.] Nature 408: 979–982 [DOI] [PubMed] [Google Scholar]
- John V 2006. Human β-secretase (BACE) and BACE inhibitors: Progress report. Curr Top Med Chem 6: 569–578 [DOI] [PubMed] [Google Scholar]
- Josien H 2002. Recent advances in the development of γ-secretase inhibitors. Curr Opin Drug Discov Devel 5: 513–525 [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG 2003. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489 [DOI] [PubMed] [Google Scholar]
- Khurana R, Ionescu-Zanetti C, Pope M, Li J, Nielson L, Ramirez-Alvarado M, Regan L, Fink AL, Carter SA 2003. A general model for amyloid fibril assembly based on morphological studies using atomic force microscopy. Biophys J 85: 1135–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Akaike A 1999. Effects of nicotinic receptor agonists on β-amyloid β-sheet formation. Jpn J Pharmacol 79: 393–396 [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimmyo Y, Akaike A, Niidome T, Sugimoto H 2010. Aβ-induced BACE-1 cleaves N-terminal sequence of mPGES-2. Biochem Biophys Res Commun 393: 728–733 [DOI] [PubMed] [Google Scholar]
- Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y 2001. Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci 98: 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazume S, Nakagawa K, Oka R, Tachida Y, Ogawa K, Luo Y, Citron M, Shitara H, Taya C, Yonekawa H, et al. 2005. In vivo cleavage of α2,6-sialyltransferase by Alzheimer β-secretase. J Biol Chem 280: 8589–8595 [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, et al. 2005. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat Med 11: 556–561 [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, et al. 2008. Amyloid β protein dimer-containing human CSF disrupts synaptic plasticity: Prevention by systemic passive immunization. J Neurosci 28: 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS 2008. BACE1 gene deletion: Impact on behavioral function in a model of Alzheimer’s disease. Neurobiol Aging 29: 861–873 [DOI] [PubMed] [Google Scholar]
- Kokkoni N, Stott K, Amijee H, Mason JM, Doig AJ 2006. N-Methylated peptide inhibitors of β-amyloid aggregation and toxicity. Optimization of the inhibitor structure. Biochemistry 45: 9906–9918 [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH 2002. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci 22: 6331–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, et al. 2010. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron 67: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ 2008. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59: 214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Marjaux E, Imhof A, De Strooper B, Haass C, Lichtenthaler SF 2007. Regulated intramembrane proteolysis of the interleukin-1 receptor II by α-, β-, and γ-secretase. J Biol Chem 282: 11982–11995 [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. 2004. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci 24: 10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, et al. 2005. BACE1, a major determinant of selective vulnerability of the brain to amyloid-β amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci 25: 11693–11709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ 2002. New class of inhibitors of amyloid-β fibril formation. Implications for the mechanism of pathogenesis in Alzheimer’s disease. J Biol Chem 277: 42881–42890 [DOI] [PubMed] [Google Scholar]
- Lavie V, Becker M, Cohen-Kupiec R, Yacoby I, Koppel R, Wedenig M, Hutter-Paier B, Solomon B 2004. EFRH-phage immunization of Alzheimer’s disease animal model improves behavioral performance in Morris water maze trials. J Mol Neurosci 24: 105–113 [DOI] [PubMed] [Google Scholar]
- Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H 2006. Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci 103: 6889–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Nayak A, Sethuraman A, Belfort G, McRae GJ 2007. A three-stage kinetic model of amyloid fibrillation. Biophys J 92: 3448–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH 2006. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440: 352–357 [DOI] [PubMed] [Google Scholar]
- Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA 2003. Aβ1–15 is less immunogenic than Aβ1–40/42 for intranasal immunization of wild-type mice but may be effective for “boosting.” Vaccine 21: 2197–2206 [DOI] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. 2000. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405: 689–694 [DOI] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, et al. 2004. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci 101: 3632–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Laird FM, Ma G, Peng S, Placanica L, Wu TC, Crain BJ, Price DL, et al. 2007. Moderate reduction of γ-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci 27: 10849–10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang E, Lohr L, Munson ML, Crans G, Cedarbaum J 2011a. A phase 1 dose escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of ELND006 in healthy elderly subjects. In International Conference on Alzheimer’s Disease Alzheimer’s Association, Paris [Google Scholar]
- Liang E, Wenzhong Liu, Lohr L, Nguyen V, Lin HH, Munson ML, Crans G, Cedarbaum J 2011b. A phase 1, dose escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple oral daily doses of ELND006 in healthy elderly subjects. In International Conference on Alzheimer’s Disease Alzheimer’s Association, Paris [Google Scholar]
- Lichtenthaler SF, Dominguez DI, Westmeyer GG, Reiss K, Haass C, Saftig P, De Strooper B, Seed B 2003. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J Biol Chem 278: 48713–48719 [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, et al. 2000. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 20: 5709–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J 2000. Human aspartic protease memapsin 2 cleaves the β-secretase site of β-amyloid precursor protein. Proc Natl Acad Sci 97: 1456–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu Y, Feng Y, Liu H, Shen X, Chen K, Ma J, Jiang H 2006. Inhibitor discovery targeting the intermediate structure of β-amyloid peptide on the conformational transition pathway: Implications in the aggregation mechanism of β-amyloid peptide. Biochemistry 45: 10963–10972 [DOI] [PubMed] [Google Scholar]
- Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT 2003. Amyloid-β antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci 23: 10879–10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA 1994. β-Amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci 91: 12243–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, et al. 2001. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci 4: 231–232 [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M 2003. BACE1 (β-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis 14: 81–88 [DOI] [PubMed] [Google Scholar]
- Martenyi F, Lowe S, Dean RA, Monk SA, Gonzales CR, Friedrich S, May PC, Audia JE, Citron M, LaBell ES, et al. 2010. Central and peripheral pharmacokinetic and pharmacodynamic effects of the β-site cleavage enzyme (BACE1) inhibitor LY2811376 in humans. In International Conference on Alzherimer’s Disease, P4-008 Alzheimer’s Association, Honolulu, HI [Google Scholar]
- Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, Gong X, Jin M, Kreft A, Harrison B, et al. 2009. Begacestat (GSI-953): A novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein γ-secretase for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther 331: 598–608 [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D 2005. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 64: 129–131 [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ 2010. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Altstiel LD, Bender MH, Boggs LN, Calligaro DO, Fuson KS, Gitter BD, Hyslop PA, Jordan WH, Li WY, et al. 2001. Marked reduction of Ab accumulation and b-amyloid plaque pathology in mice upon chronic treatment with a functional γ-secretase inhibitor. Soc Neurosci Abstr 27: 1806 [Google Scholar]
- May PC, Boggs LN, Yang Z, Lindstrom T, Calligaro D, Citron M, Sheehan S, Audia JE 2010. Central and peripheral pharmacodynamiceffects of BACE1 inhibition following oral administration of LY2811376 to PDAPP mice and beagle dog. In International Conference on Alzheimer’s Disease, P3-467, S590 Alzheimer’s Associaiton, Honolulu, HI [Google Scholar]
- Mayer SC, Kreft AF, Harrison B, Abou-Gharbia M, Antane M, Aschmies S, Atchison K, Chlenov M, Cole DC, Comery T, et al. 2008. Discovery of begacestat, a Notch-1-sparing γ-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem 51: 7348–7351 [DOI] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, et al. 2007. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem 282: 26326–26334 [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL 1998. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol 33: 371–378 [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A 1996. Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem 271: 26482–26489 [DOI] [PubMed] [Google Scholar]
- McLaurin J, Franklin T, Chakrabartty A, Fraser PE 1998. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-β fibril growth and arrest. J Mol Biol 278: 183–194 [DOI] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE 2000. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid β peptide and inhibit aβ-induced toxicity. J Biol Chem 275: 18495–18502 [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, et al. 2002. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. [See comment.] Nat Med 8: 1263–1269 [DOI] [PubMed] [Google Scholar]
- McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, et al. 2006. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med 12: 801–808 [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT 2008. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature 451: 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Mielke M, Spires-Jones TL, Stoothoff W, Jones P, Bacskai BJ, Hyman BT 2009. A reporter of local dendritic translocation shows plaque-related loss of neural system function in APP-transgenic mice. J Neurosci 29: 12636–12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ 2004. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358 [DOI] [PubMed] [Google Scholar]
- Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL 2003. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J Clin Invest 112: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, et al. 2000. A β peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease.[See comment.] [Erratum appears in Nature 2001; 412: 660.] Nature 408: 982–985 [DOI] [PubMed] [Google Scholar]
- Morihara T, Chu T, Ubeda O, Beech W, Cole GM 2002. Selective inhibition of Aβ42 production by NSAID R-enantiomers. J Neurochem 83: 1009–1012 [DOI] [PubMed] [Google Scholar]
- Nagy C, Schuck E, Ishibashi A, Nakatini Y, Rege B, Logevinsky V 2011. Neurodegenerative diseases. 10th International Conference, AD/PD: Advances, Concepts and Challenges. Barcelona, Spain [Google Scholar]
- Netzer WJ, Dou F, Cai D, Veach D, Jean S, Li Y, Bornmann WG, Clarkson B, Xu H, Greengard P 2003. Gleevec inhibits β-amyloid production but not Notch cleavage. Proc Natl Acad Sci 100: 12444–12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JAR, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO 2003. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nat Med 9: 448–452 [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, et al. 2006. Aβ species removal after aβ42 immunization. J Neuropathol Exp Neurol 65: 1040–1048 [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM 2004. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43: 321–332 [DOI] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF 2004. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron 41: 27–33 [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF 2006. Temporal memory deficits in Alzheimer’s mouse models: Rescue by genetic deletion of BACE1. Eur J Neurosci 23: 251–260 [DOI] [PubMed] [Google Scholar]
- Olson RE, Albright CF 2008. Recent progress in the medicinal chemistry of γ-secretase inhibitors. Curr Top Med Chem 8: 17–33 [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Yamada M, Naiki H 2002. Nicotine breaks down preformed Alzheimer’s β-amyloid fibrils in vitro. Biol Psychiat 52: 880–886 [DOI] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M 2004. Vitamin A exhibits potent antiamyloidogenic and fibril-destabilizing effects in vitro. Exp Neurol 189: 380–392 [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, et al. 2003. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 61: 46–54 [DOI] [PubMed] [Google Scholar]
- Pangalos MN, Jacobsen SJ, Reinhart PH 2005. Disease modifying strategies for the treatment of Alzheimer’s disease targeted at modulating levels of the β-amyloid peptide. Biochem Soc Trans 33: 553–558 [DOI] [PubMed] [Google Scholar]
- Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, Kuo YM, Lopez J, Brune D, Ferrer I, et al. 2006a. Amyloid-β peptide remnants in AN-1792-immunized Alzheimer’s disease patients: A biochemical analysis. Am J Pathol 169: 1048–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, Kuo YM, Lopez J, Brune D, Ferrer I, et al. 2006b. Amyloid-β peptide remnants in AN-1792-immunized Alzheimer’s disease patients: A biochemical analysis. [See comment.] Am J Pathol 169: 1048–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M 2002. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science 298: 1379. [DOI] [PubMed] [Google Scholar]
- Pissarnitski D 2007. Advances in γ-secretase modulation. Curr Opin Drug Discov Devel 10: 392–402 [PubMed] [Google Scholar]
- Portelius E, Van Broeck B, Andreasson U, Gustavsson MK, Mercken M, Zetterberg H, Borghys H, Blennow K 2010. Acute effect on the Aβ isoform pattern in CSF in response to γ-secretase modulator and inhibitor treatment in dogs. J Alzheimer’s Dis 21: 1005–1012 [DOI] [PubMed] [Google Scholar]
- Pu J, Kreft AF, Aschmies SH, Atchison KP, Berkowitz J, Caggiano TJ, Chlenov M, Diamantidis G, Harrison BL, Hu Y, et al. 2009. Synthesis and structure–activity relationship of a novel series of heterocyclic sulfonamide γ-secretase inhibitors. Bioorg Med Chem 17: 4708–4717 [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, et al. 2005. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J Neurosci 25: 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reixach N, Crooks E, Ostresh JM, Houghten RA, Blondelle SE 2000. Inhibition of β-amyloid-induced neurotoxicity by imidazopyridoindoles derived from a synthetic combinatorial library. J Struct Biol 130: 247–258 [DOI] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, et al. 2010. 11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9: 363–372 [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, et al. 2001. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet 10: 1317–1324 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez C, Sanchez de Groot N, Rimola A, Alvarez-Larena A, Lloveras V, Vidal-Gancedo J, Ventura S, Vendrell J, Sodupe M, Gonzalez-Duarte P 2009. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J Am Chem Soc 131: 1436–1451 [DOI] [PubMed] [Google Scholar]
- Roher AE, Palmer KC, Yurewicz EC, Ball MJ, Greenberg BD 1993. Morphological and biochemical analyses of amyloid plaque core proteins purified from Alzheimer disease brain tissue. J Neurochem 61: 1916–1926 [DOI] [PubMed] [Google Scholar]
- Rosen LB, Stone JA, Plump A, Yuan J, Harrison T, Flynn M, Dallob A, Matthews C, Stevenson D, Schmidt D, et al. 2006. The γ secretase inhibitor MK-0752 acutely and significantly reduces CSF Aβ40 concentrations in humans. In International Conference on Alzheimer’s Disease, S79, O74-p03-02 Alzheimer’s Association, Madrid [Google Scholar]
- Ryu J, Kanapathipillai M, Lentzen G, Park CB 2008. Inhibition of β-amyloid peptide aggregation and neurotoxicity by α-d-mannosylglycerate, a natural extremolyte. Peptides 29: 578–584 [DOI] [PubMed] [Google Scholar]
- Saftig P, Peters C, von Figura K, Craessaerts K, Van Leuven F, De Strooper B 1996. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J Biol Chem 271: 27241–27244 [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. 2009. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73: 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AJ, Kim T-W, Tanzi R 1999. BACE maps to chromosome 11 and a BACE homolog, BACE2, reside in the obligate Down syndrome region of chromosome 21. Science 286: 1255a [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ 3rd, Kandel ER, Duff K, et al. 2004. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42: 23–36 [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC 2008. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci 105: 5585–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D 2002. Amyloid-β immunotherapy for Alzheimer’s disease: The end of the beginning. Nat Rev Neurosci 3: 824–828 [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. 1999. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature 400: 173–177 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. 1996. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 2: 864–870 [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM 2009. The role of RAGE in amyloid-β peptide-mediated pathology in Alzheimer’s disease. Curr Opin Investig Drugs 10: 672–680 [PubMed] [Google Scholar]
- Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, Gao H, Higgins MA, May PC, Ryan TP 2003. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J Biol Chem 278: 46107–46116 [DOI] [PubMed] [Google Scholar]
- Seiffert D, Bradley JD, Rominger CM, Rominger DH, Yang F, Meredith JE Jr, Wang Q, Roach AH, Thompson LA, Spitz SM, et al. 2000. Presenilin-1 and -2 are molecular targets for γ-secretase inhibitors. J Biol Chem 275: 34086–34091 [DOI] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T, Esselmann H, Paul S, Schafer MK, Berezovska O, et al. 2009. γ-Secretase heterogeneity in the Aph1 subunit: Relevance for Alzheimer’s disease. Science 324: 639–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. 1992. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature 359: 325–327 [DOI] [PubMed] [Google Scholar]
- Seubert P, Barbour R, Khan K, Motter R, Tang P, Kholodenko D, Kling K, Schenk D, Johnson-Wood K, Schroeter S, et al. 2008. Antibody capture of soluble Aβ does not reduce cortical Aβ amyloidosis in the PDAPP mouse. Neurodegener Dis 5: 65–71 [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. 2008. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, Ness D, May PC 2005. Safety, tolerability, and changes in amyloid β concentrations after administration of a γ-secretase inhibitor in volunteers. Clin Neuropharmacol 28: 126–132 [DOI] [PubMed] [Google Scholar]
- Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, Zoulnouni P, Galvin JE, Holtzman DM, Knopman DS, et al. 2006. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 66: 602–604 [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC 2007. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-β after inhibition of γ-secretase. Clin Neuropharmacol 30: 317–325 [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T 2004. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-β derivatives. J Neurosci 24: 6277–6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri R 2008. Boom in the development of non-peptidic b-secretase (BACE1) inhibitors for the treatment of Alzheimer’s Disease. Med Res Rev 29: 295–338 [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, et al. 1999. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 402: 537–540 [DOI] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E 1997. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc Natl Acad Sci 94: 4109–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT 2005. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 25: 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, Deng A, Irizarry MC, Andersen OM, Willnow TE, Hyman BT 2006. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and β-secretase β-site APP-cleaving enzyme. J Neurosci 26: 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel SJ 2009. Progress toward the development of a viable BACE-1 inhibitor. Drug Devl Res 70: 101–110 [Google Scholar]
- Steiner H, Duff K, Capell A, Romig H, Grim MG, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, et al. 1999. A loss of function mutation of presenilin-2 interferes with amyloid β-peptide production and notch signaling. J Biol Chem 274: 28669–28673 [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ 1997. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 48: 626–632 [DOI] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y 2009. γ-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J Neurosci 29: 13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, et al. 2005. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 30: 1204–1215 [DOI] [PubMed] [Google Scholar]
- Thomas T, Nadackal TG, Thomas K 2001. Aspirin and non-steroidal anti-inflammatory drugs inhibit amyloid-β aggregation. Neuroreport 12: 3263–3267 [DOI] [PubMed] [Google Scholar]
- Thompson LA, Bronson JJ, Zusi FC 2005. Progress in the discovery of BACE inhibitors. Curr Pharm Des 11: 3383–3404 [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Asano S, Suwa Y, Morita T, Kataoka K, Mori H, Endo N 1994. Rifampicin prevents the aggregation and neurotoxicity of amyloid β protein in vitro. Biochem Biophys Res Commun 204: 76–83 [DOI] [PubMed] [Google Scholar]
- Tong G, Castaneda L, Wang J-S, Sverdlov A, Huang S-P, Slemmnon R, Gu H, Wong O, Li H, Berman RM, et al. 2010. A study to evaluate the effects of single oral doses of BMS-708163 in the cerebrospinal fluid of healthy young men. In Alzheimer’s and Demential, Proceedings of International Conference on Alzheimer’s Disease, S143, O143-p107-107 Alzheimer’s Associaiton, Honolulu, HI [Google Scholar]
- Tournoy J, Bossuyt X, Snellinx A, Regent M, Garmyn M, Serneels L, Saftig P, Craessaerts K, De Strooper B, Hartmann D 2004. Partial loss of presenilins causes seborrheic keratosis and autoimmune disease in mice. Hum Mol Genet 13: 1321–1331 [DOI] [PubMed] [Google Scholar]
- Townsend M, Cleary JP, Mehta T, Hofmeister J, Lesne S, O’Hare E, Walsh DM, Selkoe DJ 2006a. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-β oligomers. Ann Neurology 60: 668–676 [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ 2006b. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol 572: 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevko V, Xu F, Previti ML, Van Nostrand WE, Cribbs DH 2007. Experimental investigation of antibody-mediated clearance mechanisms of amyloid-β in CNS of Tg-SwDI transgenic mice. J Neurosci 27: 13376–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. 1999. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735–741 [DOI] [PubMed] [Google Scholar]
- Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M, et al. 2009. Long-term follow-up of patients immunized with AN1792: Reduced functional decline in antibody responders. Curr Alzheimer Res 6: 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ 2007. A β oligomers—A decade of discovery. J Neurochem 101: 1172–1184 [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, et al. 2001. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414: 212–216 [DOI] [PubMed] [Google Scholar]
- Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, Tsaparikos K, Jani JP, Hosea N, Sands M, et al. 2010. Evaluation of selective γ-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther 9: 1618–1628 [DOI] [PubMed] [Google Scholar]
- Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ 2000. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurology 48: 567–579 [PubMed] [Google Scholar]
- Wiesehan K, Stohr J, Nagel-Steger L, van Groen T, Riesner D, Willbold D 2008. Inhibition of cytotoxicity and amyloid fibril formation by a d-amino acid peptide that specifically binds to Alzheimer’s disease amyloid peptide. Protein Eng Des Sel 21: 241–246 [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D 2003. Intracranially administered anti-Aβ antibodies reduce β-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci 23: 3745–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D 2004. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflamm 1: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA 2008. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: A randomised phase II trial. Lancet Neurol 7: 483–493 [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Gharkholonarehe N, Van Nostrand WE, Davis J, Vitek MP, Colton CA 2009. Amyloid reduction by amyloid-β vaccination also reduces mouse tau pathology and protects from neuron loss in two mouse models of Alzheimer’s disease. J Neurosci 29: 7957–7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C 2006. Control of peripheral nerve myelination by the β-secretase BACE1. Science 314: 664–666 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398: 513–517 [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, et al. 2004. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 279: 12876–12882 [DOI] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N 2005. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J Biol Chem 280: 23009–23017 [DOI] [PubMed] [Google Scholar]
- Wood SJ, MacKenzie L, Maleeff B, Hurle MR, Wetzel R 1996. Selective inhibition of Aβ fibril formation. J Biol Chem 271: 4086–4092 [DOI] [PubMed] [Google Scholar]
- Woodard-Grice AV, McBrayer AC, Wakefield JK, Zhuo Y, Bellis SL 2008. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of α4β1 integrins. J Biol Chem 283: 26364–26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X 2009. Gamma-secretase catalyzes sequential cleavages of the AβPP transmembrane domain. J Alzheimer’s Dis 16: 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, Terasaki T, Hashimoto T, Iwatsubo T 2009. A β immunotherapy: Intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J Neurosci 29: 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, et al. 1999. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 402: 533–537 [DOI] [PubMed] [Google Scholar]
- Yan R, Munzner JB, Shuck ME, Bienkowski MJ 2001. BACE2 functions as an alternative α-secretase in cells. J Biol Chem 276: 34019–34027 [DOI] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, et al. 2003. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 9: 3–4 [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, et al. 2005. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280: 5892–5901 [DOI] [PubMed] [Google Scholar]
- Yates PA, Sirisriro R, Villemagne VL, Farquharson S, Masters CL, Rowe CC, AIBL Research Group 2011. Cerebral microhemorrhage and brain β-amyloid in aging and Alzheimer disease. Neurology 77: 48–54 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W 2003. A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and β-amyloid plaques in a mouse model of Alzheimer’s disease. Neurobiol Dis 14: 365–379 [DOI] [PubMed] [Google Scholar]
- Zhao B, Yu M, Neitzel M, Marugg J, Jagodzinski J, Lee M, Hu K, Schenk D, Yednock T, Basi G 2008. Identification of γ-secretase inhibitor potency determinants on presenilin. J Biol Chem 283: 2927–2938 [DOI] [PubMed] [Google Scholar]
- Ziora Z, Kimura T, Kiso Y 2006. Small-sized BACE1 inhibitors. Drugs Future 31: 53–63 [Google Scholar]