Abstract
Sphingomyelin synthase (SMS) sits at the crossroads of sphingomyelin (SM), ceramide, diacylglycerol (DAG) metabolism. It utilizes ceramide and phosphatidylcholine as substrates to produce SM and DAG, thereby regulating lipid messengers which play a role in cell survival and apoptosis. There are two isoforms of the enzyme, SMS1 and SMS2. Both SMS1 and SMS2 contain two histidines and one aspartic acid which are evolutionary conserved within the lipid phosphate phosphatase superfamily. In this study, we systematically mutated these amino acids using site-directed mutagenesis and found that each point mutation abolished SMS activity without altering cellular distribution. We also explored the domains which are responsible for cellular distribution of both enzymes. Given their role as a potential regulator of diseases, these findings, coupled with homology modeling of SMS1 and SMS2, will be useful for drug development targeting SMS.
Keywords: Sphingomyelin synthase 1 and 2, Point mutagenesis, lipid phosphate phosphatase
1. Introduction
The de novo synthesis of SM requires the action of a serine palmitoyl-CoA transferase (SPT), 3-ketosphinganine reductase, ceramide synthase, dihydroceramide desaturase, and SMS [1]. SMS is the last enzyme for SM biosynthesis. It utilizes ceramide and PC as substrates to produce SM and DAG [1], and its activity thus directly influences SM, PC, and ceramide, as well as DAG levels. SMS activity directly links to plasma membrane structures and cell functions which may well have impact on disease development [2–7]. Many studies have indicated that SMS is located mainly in the cis-, medial-Golgi [8, 9], and plasma membranes [10–12]. There has been evidence of a form of SMS in the trans-Golgi network [13] and at the nuclear level [14]. In addition, SMS activity has been found in chromatin, and chromatin-associated SMS modifies the SM content [14–16]. Of the two mammalian SMS gene isoforms, SMS1 is located on cis-, medial-Golgi, while SMS2 is found in plasma membranes [17]. However, a recent report showed that SMS1 localized to the trans-Golgi network [18].
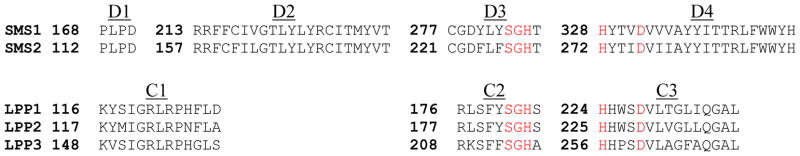
Hydrophobicity analysis has postulated that both SMS1 and SMS2 contain six membrane-spanning alpha helices connected by hydrophilic regions that would form extramembrane loops [16]. SMS1 and SMS2 contain four highly conserved sequence motifs, designated D1, D2, D3, and D4 (Fig. 1) [17]. Motifs D3 (C-G-D-X3-S-G-H-T) and D4 (H-Y-T-X-D-V-X3-Y-X6-F-X2-Y-H), containing conserved amino acids HHD (underlined), are similar to the C2 and C3 motifs in lipid phosphate phosphatase (LPPs) which form a catalytic triad mediating the nucleophilic attack on the lipid phosphate ester bond [17,19] (Fig. 1).
Fig. 1.
Alignment of conserved regions between SMS and LPPs. Evolutionary conserved domains (D1-D4) in human SMS1 and human SMS2 are aligned with conserved domains (C1-C3) in three human LPPs: LPP1, LPP2, and LPP3. Highlighted animo acids represent residues responsible for catalytic activity in LPPs.
In this study, using site directed mutagenesis, we systematically changed the HHD motif and found that each point mutation completely abolished SMS activity without altering cellular distribution. These results provide a molecular basis for designing SMS inhibitors. Furthermore, we analyzed the contribution of domains unique to each isoform on the differential localization pattern for SMS1 and SMS2.
2. Methods and Materials
2.1. Reagents
Bovine brain L-α-phosphatidylcholine (PC) and NBD-C6-ceramide were purchased from Sigma.
2.2. Plasmids
Expression vectors containing SMS1 (BC117782) and SMS2 (BC042899) were purchased from Open Biosystems. SMS1 and SMS2 were subcloned into pCMV-3xFlag14 (Sigma). All point mutations were introduced using the QuikChange Site-Directed Mutagenesis Kit (Strategene). N-terminal SAM domain deletion from SMS1 was performed by PCR amplification of base pairs 181-1239 using a 5′ primer containing an EcoR1 cutting site and a 3′ primer containing a BamH1 cutting site. N-terminal SAM addition to SMS2 was performed by ligation of a PCR amplicon of base pairs 1-184 from SMS1 (using a 3′ primer containing an Xba1 cutting site) to a PCR amplicon of base pairs 4 – 1095 from SMS2 (using a 5′ primer containing an Xba1 cutting site). All constructs were verified by DNA sequencing.
2.3. Cell Culture and Transfection
Hela and HEK-293 cells were grown in monolayers at 37°C in 5% CO2. All cells were cultured in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin and streptomycin, and 2mM glutamine. Plasmids were transfected using Lipofectamine 2000 (Invitrogen).
2.4. Imunoprecipitation and Immunoblotting
Cells were lysed in 200 mM NaCl, 50 mM Tris (pH 7.5), 1 mM EDTA, and 1% (v/v) protease inhibitor cocktail (Sigma). Cell debris were cleared by centrifugation at 8,200 g for 10 min. For immunoprecipitation, lysates were incubated with Flag antibody conjugated Nickel Affinity Gel (Sigma) for 2 hours. The gel was then washed in lysis buffer, bound proteins were eluted by additing SDS PAGE loading buffer. The eluted proteins were immunoblotted with an anti-Flag HRP (Sigma) (1:2000) for 1 hour. Following three washes, proteins were detected by the chemiluminescence method (Pierce).
2.5. Immunohistochemistry
Cells were grown, transfected with plasmid, and prepared for microscopy directly on an eight-well chamber slide (Nunc) for 48 hours. Subconfluent cells were washed three times with PBS and then fixed in 4% formaldehyde in PBS for 10 min. Cells were washed with PBS and permeablized with PBS containing 0.1% Triton X-100, washed, and then blocked in 3% BSA in PBS for 1 hour. Cells were then incubated with anti-Flag Cy3 1:250 (Sigma) and anti-panCadherin FITC 1:250 (Abcam) or anti-Flag Cy3 1:250 and anti-αMannosidase II 1:25 (USBiological) diluted in blocking buffer. Where applicable, cells were incubated with anti-rabbit FITC 1:1000 (Vector Labs). After three washes, TO-PRO 3 (Invitrogen), diluted 1:1000 in blocking buffer, was added to cells to stain the nuclei. Slides were mounted in VectaShield (Vector Labs) and analyzed on a confocal microscope (Bio-Rad Radiance 2000) using 488 nm, 543 nm, and 638 nm excitation and a 40 X objective lens. Images were processed with Photoshop (Adobe).
2.6. SMS1-, SMS2-Specific Activity Assay
Cells, transiently expressing Flag-tagged SMS1 or SMS2, were lysed in 200 mM NaCl, 50 mM Tris (pH 7.5), 1 mM EDTA, and 1% (v/v) protease inhibitor cocktail (Sigma). Cell debris were cleared by centrifugation at 8,200 g for 10 min. Immunoprecipitation was performed by incubating lysate with 30 μl of an anti-Flag M2 monoclonal antibody covalently bound to agarose beads (Sigma) for 2 hours. Immune complexes were washed three times with 50 mM Tris (pH 7.5), 5% sucrose, 1mM EDTA. SMS1 or SMS2 activity assay was then performed as previously described [2]. Briefly, 50 mM Tris–HCl (pH 7.4), 25 mM KCl, C6-NBD-ceramide (0.1 μg/μl), and phosphatidylcholine (0.01 μg/μl) was incubated with the immunoprecipitate at 37°C for 45 min. Lipids were extracted in chloroform: methanol (2:1), dried under N2 gas, and separated by thin layer chromatography (TLC) using Chloroform:MeOH:NH4OH (14:6:1).
3. Results and Discussion
Domains responsible for SMS activity
From ceramide and phosphatidylcholine, SMS generates SM, and DAG as a side product. In this process the phosphomonoester bond between phosphocholine and DAG of phosphatidylcholine must be broken, allowing the transfer of phosphocholine to ceramide. Due to similarities between SMS and LPPs, it has been speculated that they share a common catalytic mechanism, however, this concept has not been proven. Domains designated D3 and D4 from both SMS1 and SMS2 share key sequence homologies with the active site of lipid phosphate phosphatase (LPPs). Within the C2 and C3 domains of LPPs, two histidines and one aspartic acid form a catalytic triad mediating phosphomonoester hydrolysis [17, 19] (Fig. 1).
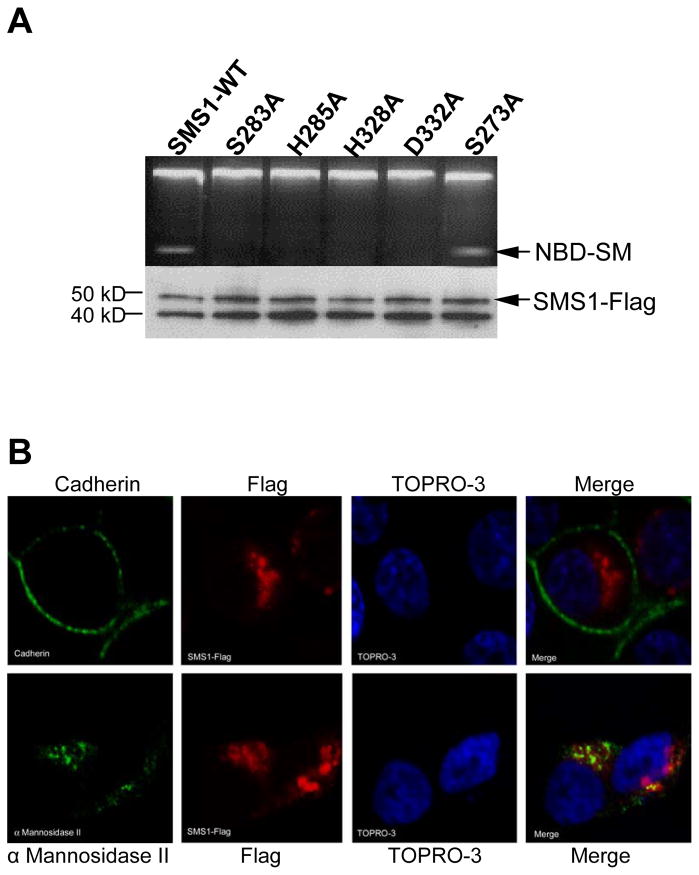
To demonstrate that the analogous His, His, Asp (HHD) triad in SMS1 is responsible for SMS activity, we utilized site-directed mutagenesis to replace each amino acid with alanine and prepared five unique SMS1 mutants (S273A, S283A, H285A, H328A, and D332A) along with WT SMS1 (Fig. 2A). In SMS1, H285 corresponds to the catalytic acid-base residue in LPPs [19]. H328 and D332, meanwhile, act coordinately to mediate a nucleophilic attack on the phosphomonoester bond. Serine 283 is evolutionarily conserved between animal SMSs [17] as well as within human LPPs [20]. This serine in LPPs has been implicated in hydrogen binding to the phosphate group [20]. Serine 273 mutant served as a control, since S273 is outside of D3, and therefore is not in the putative active site. In order to control for expression levels, we added a Flag tag at the C terminus of each construct. To accurately compare the specific activity of each mutant with wild type SMS1 without noise from endogenous SMS1 and SMS2 (both are expressed in all tissues which were tested), SMS activity assay was performed following Flag affinity immunopurification. As indicated in Figure 2A, SMS1-Flag (WT) and S273A have comparable specific SMS activity, but H285A, H328A, D332A, and S283A have no detectable activity. Therefore, each amino acid of the HHD triad in SMS1, as well as the conserved serine 283 is necessary for SMS1 activity.
Fig. 2.
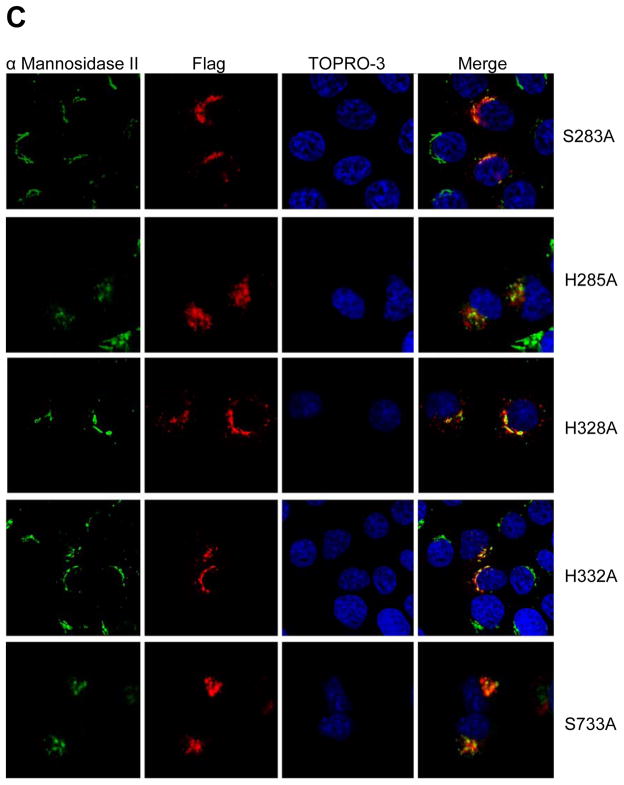
Point mutation of individual conserved residues within D3 and D4 abolishes SMS1 activity. (A) SMS activity was performed on wild type (WT) or mutant SMS1 following immunoprecipitation from Hela cells transiently expressing the respective enzyme. The protein level of each enzyme is shown in a western blot below. (B) Wild type SMS1 is a Golgi targeted protein. Both cadherin (top) and α Mannosidase II (bottom) are depicted in green, while SMS1-Flag is depicted in red. TOPRO-3, a DNA dye is in blue. (C) Each SMS1 mutant (S283A, H285A, H328A, H332A, S273A) exhibits a WT localization pattern. α Mannosidase II is depicted in green. Flag-tagged SMS1 and mutants are depicted in red. TOPRO-3 is in blue.
To rule out the possibility that any of these point mutations yielded an inactive enzyme secondary to causing a global change in protein structure, we confirmed that the subcellular localization of each mutant remained identical to the wild type enzyme. As expected, confocal analysis showed that SMS1-Flag (WT) are located on Golgi complex, since it was co-localized with α Mannosidase II, a well-known Golgi marker (Fig. 2B). There is no detectable signal on plasma membrane (Fig. 2B). This result confirmed a previous report [17]. Moreover, all mutants have a normal cellular distribution, compared with WT (Fig. 2B and 2C), indicating the normal enzyme topology is still maintained in the “dead” enzyme.
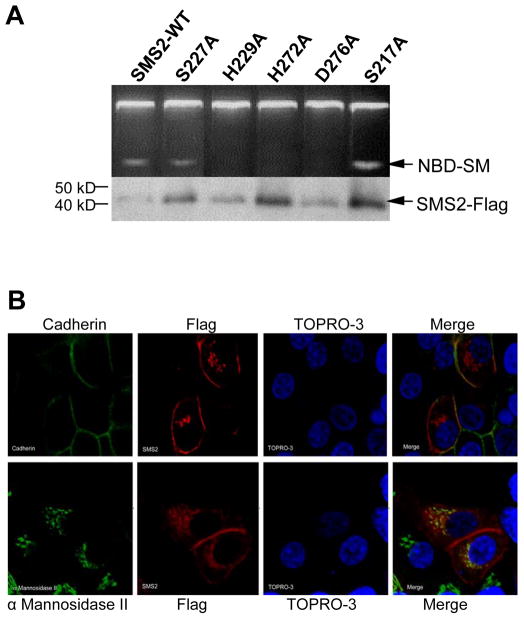
We also prepared Flag tagged wild type SMS2 as well as mutants analogous to those described above for SMS1 (Fig. 3). Similarly, individual mutations of the SMS2 HHD triad all abolished SMS2 activity as opposed to wild type SMS2 and the control (S217A) mutant (Fig. 3A). Interestingly, one difference between SMS1 and SMS2 is that SMS1-S283A has no activity, while SMS2-S227A retains approximately 30% of a wild type specific activity, suggesting that although both serines are located next to HHD motifs (Fig. 1A), they have a different impact on catalytic domain formation. It has been suggested that S181 in LPP1, analogous to S283 in SMS1 and S227 in SMS2, can form a hydrogen bond with the phosphate group while it is in the active site instead of directly participating in catalysis [19]. Therefore, it is not surprising that S227A is not completely necessary for SMS activity. There is likely a neighboring residue within the tertiary structure of SMS2 which is functionally equivalent to S227.
Fig. 3.
Point mutation of individual conserved residues within D3 and D4 abolishes SMS2 activity with the exception of S227. (A) SMS activity was performed on wild type (WT) or mutant SMS1 following immunoprecipitation from Hela cells transiently expressing the respective enzyme. The protein levels of each enzyme is shown below. (B) Wild type SMS2 localizes to both the plasma membrane and Golgi. Both cadherin (top) and α Mannosidase II (bottom) are depicted in green, while SMS2-Flag is depicted in red. TOPRO-3 is in blue. (C) Each SMS1 mutant (S2227A, H229A, H272A, H276A, S217A) exhibits a WT localization pattern. Cadherin is depicted in green. Flag-tagged SMS1 and mutants are depicted in red. TOPRO-3 is in blue.
As shown in Figure 3B, a portion of SMS2-Flag (WT) was located on plasma membrane where it co-localized with cadherin (a well-known plasma membrane marker), and a portion was found in the perinuclear region where it co-localized with Golgi marker α Mannosidase II. This result also confirmed a previous report [17]. Furthermore, all mutants have an identical cellular distribution as WT (Fig. 3B and 3C), suggesting that these point mutations only influenced SMS2 catalytic activity but not the enzyme topology.
So far, no experimental studies identifying the locale of an SMS active site have been reported. In this study, we utilized site-directed mutagenesis to elucidate the catalytic structure for both SMS1 and SMS2. SMS activity is closely related with four important lipids, ceramide, DAG, SM, and phosphatidylcholine, which are involved in plasma membrane formation, signal transduction, and lipoprotein metabolism. The catalytically inactive enzymes created by this study will be very useful in the future studies. Potentially, SMS is a therapeutic target for the treatment of diseases, such as cancer and atherosclerosis, and elucidating the amino acids that form an active site for SMS will provide a molecular basis for designing the drugs.
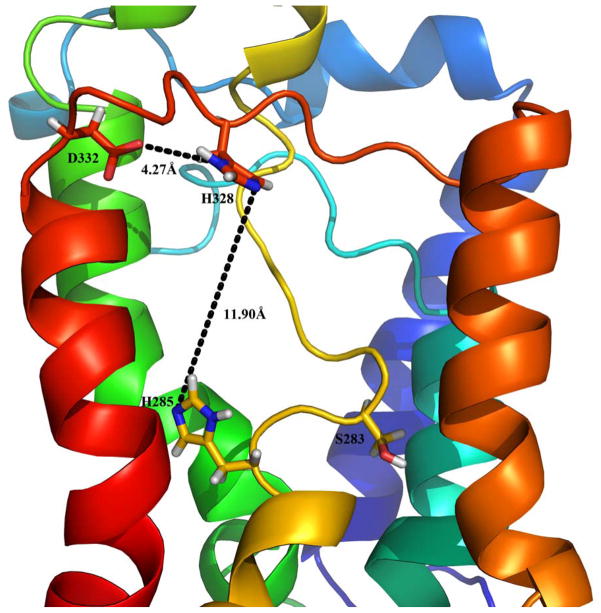
Based on the LPP structure [18] and through homology modeling, we have created a tertiary structure model of SMS1, which shows a favorable distance of 4.27 angstroms for the interaction between H328 and D332, as well as an 11.90 angstrom distance between H328 and H283 which can accommodate the phosphate group from phosphatidylcholine (Fig. 4). This model is also applicable to SMS2. From this model, we can clearly see that the replacement of any one of these three amino acids abolishes SMS1 or SMS2 activity in the cells.
Fig. 4.
A favorable conformation of active site of human SMS1. The three-dimensional structural model of human SMS1 was built through homology modeling. Two proteins, GlpG protein (PDB entry 2IC8) and PhoN protein (PDB entry 2AKC), with known structures, were used as templates. Multiple sequence alignments and construction of homology models were conducted by using the Modeler module in the Discovery Studio software (version 1.6, Accelrys Inc.). The conserved histidine 285, histidine 328, and histidine 332 along with the respective distances between residues are depicted.
Analysis of a domain unique to SMS1 on subcellular localization
SMS1 is a Golgi protein which has been co-localized with the trans Golgi marker sialyltransferase [17] (Fig. 2B). SMS2 on the other hand localizes to both the plasma membrane as well as the Golgi [17](Fig. 3B). There are no known or predicted targeting signals in either protein. Although the genes encoding these two isoforms are located on distinct chromosomes, SMS1 and SMS2 are 51.5% identical in protein sequence. Sequence-wise, the most striking difference between the two isoforms is that SMS1, but not SMS2 has an N-terminal Sterile Alpha Motif (SAM) domain. We explored the possibility that this SMS1 unique sequence may play a role in the differential subcellular localization of SMS1 and SMS2.
Truncation of 61 amino acids from the SMS1 SAM domain resulted in a mutant with an identical distribution pattern as the wild type enzyme (Fig. 5C). Furthermore, addition of these 61 amino acids to the N-terminus of SMS2 did not alter localization of the protein (Fig. 5D). This was surprising because trans Golgi resident proteins have been known to target to the Golgi through aggregation and subsequent exclusion from transport vesicles. SAM, conventionally thought of as a protein interaction domain, has also been shown to mediate homo-oligomerization [21].Although the results are not expected, we conclude that the SMS1 SAM domain is neither necessary nor sufficient for Golgi targeting of SMS. The domains, responsible to subcellular distribution of both enzymes, should be located within the region where SMS1 and SMS2 share the similarity. Furthermore, the SAM domain does not influence SMS activity. As shown in Fig. 5B, deletion of SAM from SMS1 or addition of SAM to SMS2 has no appreciable impact on SMS activity. Since SMS activity is potentially important in certain diseases, and SMS1 and SMS2 activity may have different impact on the process of disease development, to elucidate these domains could provide a molecular basis for studying isoform-specific properties for both SMS1 and SMS2. This aspect deserves further investigation. For an unknown reason SMS1-Flag western blot showed a doublet (Fig. 2A and Fig. 5B). The up band with correct molecular weight of SMS1-Flag was disappeared in truncated SMS1-Flag transfected cells (Fig. 5B).
Fig. 5.
Analysis of potentially important domains for the differential localization of SMS1 and SMS2. (A) Schematic of the Flag-tagged constructs used. SAM: Sterile Alpha Motif. TM: transmembrane domain. (B) SMS1ΔSAM-Flag, a mutant with truncation of the first 61 amino acids from SMS1, shows no defect in SMS activity compared with WT SMS1-Flag. Likewise, SAM-SMS2, a fusion protein comprised of the SAM domain from SMS1 (amino acids 1-61) and the entirety of SMS2, has unaltered SMS activity compared to WT-SMS2-Flag. The protein levels of each enzyme is shown at the bottom. (C) SMS1ΔSAM-Flag remains as a Golgi targeted protein. α Mannosidase II is depicted in green, while SMS1ΔSAM-Flag is depicted in red. TOPRO-3 is in blue. (D) SAM-SMS2-Flag exhibits an identical localization pattern compared to wild type SMS2. Cadherin is depicted in green, while SAM-SMS2-Flag is depicted in red. TOPRO-3 is in blue.
Acknowledgments
This work was supported by NIH HL-64735 and HL-69817.The authors thank Dr. Zhiguo Liu from the Shanghai Institute of Organic Chemistry for his help on the homology modeling of the human SMS1 structure.
Abbreviations used
- SM
sphingomyelin
- SMS
sphingomyelin synthase
- LPPs
lipid phosphate phosphatase
- SAM
Sterile Alpha Motif
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voelker DR, Kennedy EP. Cellular and enzymic synthesis of sphingomyelin. Biochemistry. 1982;21:2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake DA, Zhang Y, Kuo MS, Cao G, Jiang XC. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim Biophys Acta. 2007;1771:1186–1194. doi: 10.1016/j.bbalip.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding T, Li Z, Hailemariam TK, Mukherjee S, Maxfield FR, Wu MP, Jiang XC. SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J Lipid Res. 2008;49:376–385. doi: 10.1194/jlr.M700401-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 5.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the golgi. Biochem J. 2008 doi: 10.1042/BJ20071240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Separovic D, Semaan L, Tarca AL, Awad Maitah MY, Hanada K, Bielawski J, Villani M, Luberto C. Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp Cell Res. 2008;314:1860–1868. doi: 10.1016/j.yexcr.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Separovic D, Hanada K, Maitah MY, Nagy B, Hang I, Tainsky MA, Kraniak JM, Bielawski J. Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem Biophys Res Commun. 2007;358:196–202. doi: 10.1016/j.bbrc.2007.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeckel D, Karrenbauer A, Birk R, Schmidt RR, Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990;261:155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer AH, Clausen, van Meer G, Hauri HP. Localization of O-glycan initiation, sphingomyelin synthesis, and glucosylceramide synthesis in Vero cells with respect to the endoplasmic reticulum-Golgi intermediate compartment. J Biol Chem. 1994;269:4035–4041. [PubMed] [Google Scholar]
- 10.Malgat M, Maurice A, Baraud J. Sphingomyelin and ceramide-phosphoethanolamine synthesis by microsomes and plasma membranes from rat liver and brain. J Lipid Res. 1986;27:251–260. [PubMed] [Google Scholar]
- 11.Moreau P, Cassagne C. Phospholipid trafficking and membrane biogenesis. Biochim Biophys Acta. 1994;1197:257–290. doi: 10.1016/0304-4157(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Obradors MJ, Sillence D, Howitt S, Allan D. The subcellular sites of sphingomyelin synthesis in BHK cells. Biochim Biophys Acta. 1997;1359:1–12. doi: 10.1016/s0167-4889(97)00088-8. [DOI] [PubMed] [Google Scholar]
- 13.Allan D, Obradors MJ. Enzyme distributions in subcellular fractions of BHK cells infected with Semliki forest virus: evidence for a major fraction of sphingomyelin synthase in the trans-Golgi network. Biochim Biophys Acta. 1999;1450:277–287. doi: 10.1016/s0167-4889(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 14.Albi E, Magni MP. Sphingomyelin synthase in rat liver nuclear membrane and chromatin. FEBS Lett. 1999;460:369–372. doi: 10.1016/s0014-5793(99)01378-2. [DOI] [PubMed] [Google Scholar]
- 15.Albi E, Magni MP. Chromatin-associated sphingomyelin: metabolism in relation to cell function. Cell Biochem Funct. 2003;21:211–215. doi: 10.1002/cbf.1075. [DOI] [PubMed] [Google Scholar]
- 16.Albi E, Pieroni S, Magni MP, Sartori C. Chromatin sphingomyelin changes in cell proliferation and/or apoptosis induced by ciprofibrate. J Cell Physiol. 2003;196:354–361. doi: 10.1002/jcp.10314. [DOI] [PubMed] [Google Scholar]
- 17.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Mazière AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuwald AF. An unexpected structural relationship between integral membrane phosphatases and soluble haloperoxidases. Protein Sci. 1997;6:1764–1767. doi: 10.1002/pro.5560060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang QX, Pilquil CS, Dewald J, Berthiaume LG, Brindley DN. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its site of action. Biochem J. 2000;345:181–184. [PMC free article] [PubMed] [Google Scholar]
- 21.Thanos CD, Goodwill KE, Bowie JU. Oligermeric structure of the human EphB2 receptor SAM domain. Science. 1999;283:833–836. doi: 10.1126/science.283.5403.833. [DOI] [PubMed] [Google Scholar]