Abstract
Study objective
The National Institutes of Health Stroke Scale (NIHSS) measures deficits caused by a stroke, but not all stroke signs are captured on the NIHSS. We determined the symptoms and stroke localization of patients with brain infarction and an NIHSS score of 0.
Methods
We studied all patients who presented with acute neurological symptoms to our stroke center from 2004–2008, had persistent symptoms at the time of evaluation in the emergency department, an NIHSS score of 0, and an infarct on diffusion weighted imaging (DWI). We characterized the symptoms, signs, lesion location, demographics and stroke etiologies.
Results
20 patients met inclusion criteria. Symptoms frequently experienced were headache, vertigo, and nausea. The posterior circulation was commonly infarcted in this group. Truncal ataxia was the most common neurological sign.
Conclusion
Ischemic stroke may cause symptoms that are associated with no deficits on the NIHSS score.
INTRODUCTION
The National Institutes of Health Stroke Scale (NIHSS) is a valid, reproducible scale that measures neurological deficits and is the most frequently used scoring system in stroke intervention trials.1,2 Physicians rely upon the NIHSS to evaluate patients with suspected acute stroke and to make decisions about acute treatment.1 The NIHSS correlates with infarct size, clinical severity, and long term outcome.3,4
However, not all signs caused by a stroke are captured as deficits on the NIHSS. The NIHSS is highly weighted toward deficits caused by anterior circulation strokes while deficits due to posterior circulation strokes receive fewer points.1,5,6 Within the anterior circulation, the scale underestimates the degree of right vs. left hemisphere injury.7,8 These studies indicate that the NIHSS performs unequally in the detection of stroke depending upon lesion location and it is therefore possible that some patients with persistent symptoms upon arrival to the emergency department (ED) and an NIHSS 0 still have an infarct.
In this study, we characterize the symptoms and the stroke location of patients who present to the ED with an acute cerebrovascular syndrome and an NIHSS 0; we hypothesize that such patients with persistent symptoms have more strokes in the posterior circulation causing deficits not detectable on the NIHSS.
METHODS
All patients with suspected acute cerebral ischemia admitted to our stroke service are initially evaluated by a NIHSS-certified member of our stroke team (vascular neurology fellow or stroke faculty member) in the ED. Unless contraindicated, all patients with suspected acute cerebral ischemia undergo multimodal MRI of the brain and MRA of the intracranial and extracranial vessels within 24 hrs of hospital presentation. The initial NIHSS examination is performed before the patient undergoes MRI. We studied all patients who presented to our stroke center from 2004–2008 with suspected acute cerebral ischemia and an NIHSS score of 0 at hospital presentation. The inclusion criteria for this study were: admission to the stroke service, NIHSS score of 0 while symptomatic, and MRI of the brain performed after initial NIHSS examination demonstrating an acute infarction. These patients were labeled “NIHSS 0 stroke”.
We collected symptoms, signs, lesion location, patient demographics, stroke etiologies, and early outcome at discharge. The symptoms were those that the patients described to the stroke team member at the time of evaluation in the ED and were abstracted from the initial consult note in the ED. The physical examination findings were abstracted from the initial consult note but in cases where the NIHSS was the only examination performed, additional signs on the neurological exam were collected from the attending note. The stroke attending at our institution performs a neurological examination while the patient is still in the ED or the next day when the patient is admitted to the hospital. The attending note was used if there were instances of conflicting findings. The retrospective chart review was approved by the institutional review board at the University of Texas-Houston (IRB protocol number HSC-MS-09-0348).
RESULTS
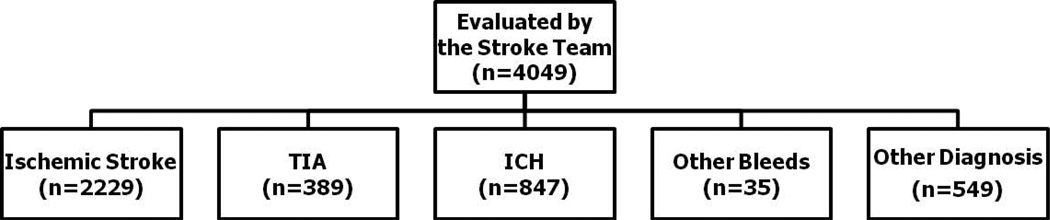
Figure 1 provides a flow chart of how many patients were seen in our ED in the study time frame by the stroke team and admitted to the stroke service. Twenty patients met inclusion criteria for this study. Out of the total population of 2,618 patients with acute cerebral ischemia that our stroke service admitted during the same time period, the “NIHSS 0 stroke” group represented 0.76%. We report the demographics, etiologies, symptoms, and outcome with respect to their discharge mRS or disposition (Table 1). The distributions of infarcts in the anterior and posterior circulation are presented in Table 2 and the physical exam findings of these patients are shown in Table 3.
Fig 1.
Stroke Patients Presenting to the ED from July 2004 to December 2008
Table 1.
Demographics, etiologies, and outcome of stroke patients with NIHSS 0
| Age, mean ± SD | 54.1 ± 15.4 n=20 |
| Gender, % female | 35.0% (7/20) |
| Ethnicity, % | |
| Black | 35.0% (7/20) |
| White | 50.0% (10/20) |
| Hispanic | 15.0% (3/20) |
| Asian | 0% (0/20) |
| Symptoms, % | |
| Headache | 45.0% (9/20) |
| Vertigo | 30.0% (6/20) |
| Nausea | 30.0% (6/20) |
| Ataxia | 25.0% (5/20) |
| Confusion | 20.0% (4/20) |
| Blurred vision | 20.0% (4/20) |
| Sensory disturbance | 15.0% (3/20) |
| Limb weakness | 10.0% (2/20) |
| Double vision | 5.0% (1/20) |
| Glucose, median (range) | 128 (83–289) n=20 |
| Treated with IV tPA | 5.0% (1/20) |
| h/o Afib | 6.3% (1/16) |
| h/o CAD | 16.7% (3/18) |
| h/o DM | 50.0% (9/18) |
| h/o HTN | 77.8% (14/18) |
| TOAST classification, % | |
| Cardioembolic | 30.0% (6/20) |
| Large vessel | 15.0% (3/20) |
| Small vessel | 20.0% (4/20) |
| Cryptogenic | 20.0% (4/20) |
| Other | 15.0% (3/20) |
| Disposition, % | |
| Home | 75.0% (15/20) |
| Inpatient rehab | 10.0% (2/20) |
| Skilled nursing | 10.0% (2/20) |
| Other | 5.0% (1/20) |
| mRS at Discharge | |
| 0 | 35.0% (7/20) |
| 1 | 30.0% (6/20) |
| 2 | 10.0% (2/20) |
| 3 | 15.0% (3/20) |
| 4 | 5.0% (1/20) |
| 5 | 5.0% (1/20) |
| 6 | 0% (0/20) |
| LOS, median (range) | 4.5 (2–10) n=20 |
Table 2.
Distribution of infarct location in patients with NIHSS 0
| ANTERIOR CIRCULATION | 42.1% (8/19) |
|---|---|
| frontal lobe | 0% (0/19) |
| temporal lobe | 5.3% (1/19) |
| parietal lobe | 5.3% (1/19) |
| insula | 5.3% (1/19) |
| multi-territory | 5.3% (1/19) |
| cortical & subcortical | 0% (0/19) |
| subcortical | 21.1% (4/19) |
| POSTERIOR CIRCULATION | 57.9% (11/19) |
| occipital lobe | 15.8% (3/19) |
| multi-territory | 0% (0/19) |
| cerebellum | 31.6% (6/19) |
| pons | 5.3% (1/19) |
| medulla | 5.3% (1/19) |
Table 3.
Neurological signs of patients with NIHSS 0
| Neurological Signs | % |
|---|---|
| Truncal Ataxia | 45 (9/20) |
| Agitated Confusion | 10 (2/20) |
| Normal Exam | 10 (2/20) |
| Nystagmus* | 5 (1/20) |
| Limb Weakness | 5 (1/20) |
| Memory impairment | 5 (1/20) |
| Horner’s syndrome | 5 (1/20) |
| Slow to respond | 5 (1/20) |
| Reduced visual acuity without field cut | 5 (1/20) |
| Tandem Gait Abnormality | 5 (1/20) |
not specified further in the medical chart
There was a higher percentage of posterior circulation compared to anterior circulation strokes. Headache, vertigo, and nausea occurred more frequently than motor and sensory symptoms (Table 1). The most common neurological finding was truncal ataxia (Table 3). There were two cases of agitated confusion, and single cases of memory impairment, Horner’s syndrome, etc. In two cases, no neurological deficits were found (right temporal infarct and a left pontine infarct) (Table 3).
Limitations
Our study is limited by small sample size, single center experience and the inter-rater variability in performing the NIHSS. In addition, we only studied admitted patients and therefore do not provide information on how many patients during the study period were discharged from the ED with an NIHSS of 0. Therefore, there is a possibility that patients with undetected posterior circulation strokes misdiagnosed as a peripheral vestibulopathy, for example, could have been missed by our methodology. However, the findings from this report support the idea that the NIHSS should not substitute for a thorough neurological examination, including a formal gait evaluation, when evaluating a patient for suspected stroke.
DISCUSSION
Patients with suspected acute cerebral ischemia presenting to the ED who have an NIHSS score of zero comprise a small but important population that has not been previously characterized. Our findings suggest that the NIHSS sometimes does not capture some ischemic strokes, more often within the posterior compared with the anterior circulation. There are several possible reasons why the NIHSS failed to detect strokes in our symptomatic patients. First, patients in the “NIHSS 0 stroke” group most commonly presented with nausea, vomiting, and headache, all of which are associated with posterior circulation ischemia (PCI).9 PCI occurred more frequently in this group and, depending upon infarct location, may not be associated with detectable deficits on the NIHSS. Second, midline lesions of the cerebellum cause truncal ataxia which is not part of the NIHSS and is not routinely tested in our center in the hyperacute setting. Truncal ataxia was the most common neurological finding in this patient population.
Our findings indicate that the NIHSS alone cannot be used to rule out a stroke in patients with acute persistent symptoms. The NIHSS does not substitute for a comprehensive neurological examination. Truncal ataxia, decreased visual acuity, Horner’s syndrome, and memory impairments are neurological deficits that were detected in this patient population and which the NIHSS does not capture. In addition, subtle limb weakness (4/5) in an upper motor neuron pattern (extensors of the arms or flexors of the legs) may not be observed on the motor component of the NIHSS. However, in two cases, no neurological deficits were found on a comprehensive neurological examination. Therefore, it should also be acknowledged that strokes may rarely cause symptoms not associated with deficits on a more thorough clinical evaluation. Even in the absence of exam findings, the pattern of symptoms in some patients should still prompt brain imaging with a focus on those posterior circulation strokes which have been missed by clinicians in the past, such as cerebellar strokes.9
The consequences of missing a stroke in a patient with NIHSS 0 are unknown. Fortunately, the median discharge mRS score was 1 and 75% of these patients were discharged home. However, the detection of stroke in these patients presents an opportunity to implement preventative measures. Thirty percent of the “NIHSS 0 stroke” group had cardioembolic etiologies which might prompt consideration for anticoagulation.
Acknowledgments
Funding and support:
This work was supported by the Howard Hughes Medical Institute, NIH training grant T32NS04712, and P50 NS044227.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster presented at the International Stroke Conference, San Antonio, Texas, February 2010.
There are no conflicts of interest.
REFERENCES
- 1.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 2.Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH stroke scale using video training. NINDS tpa stroke study group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]
- 3.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 4.Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: Lesion size by computed tomography. Stroke. 1989;20:871–875. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- 5.Libman RB, Kwiatkowski TG, Hansen MD, et al. Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc Dis. 2001;11:311–316. doi: 10.1159/000047659. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Toyoda K, Uehara T, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70:2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 7.Woo D, Broderick JP, Kothari RU, et al. Does the national institutes of health stroke scale favor left hemisphere strokes? NINDS t-pa stroke study group. Stroke. 1999;30:2355–2359. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- 8.Fink JN, Selim MH, Kumar S, et al. Is the association of national institutes of health stroke scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 9.Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–964. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]