Abstract
We previously demonstrated that prenatal alcohol exposure results in brain defects at different embryonic stages. This study is aimed at characterizing the influence of prenatal alcohol exposure on the levels of several neurotransmitters at early embryonic stage 13 (E13). Pregnant C57BL/6 mice were exposed to either a 25% ethanol derived calorie diet (ALC) or pair-fed (PF) liquid diet from E7 to E13. At E13, fetal brains were collected from dams of the ALC and PF groups. Liquid chromatography/tandem mass spectrometry (LC-MS) was then used to evaluate neurotransmitter levels. This approach involved the use of an LC column in conjunction with multiple-reaction monitoring mass spectrometry. Quantitative analyses of catecholamines, idolamine, and amino acid neurotransmitters revealed significant reductions in the levels of dopamine (p=0.004), norepinephrine (p=0.0009), epinephrine (p=0.0002), serotonin (p=0.004), and GABA (p=0.002) in the ALC group compared to the PF group. However, there was no significant change in the levels of glutamate in E13 fetal brains. These findings demonstrate that prenatal alcohol exposure reduces the concentrations of some catecholamines, idolamine, and amino acid neurotransmitters in E13 fetal brains. This study suggests that alterations of selective neurotransmitters may be the cause of abnormalities in brain function and behavior found in fetal alcohol spectrum disorders.
Keywords: fetal alcohol exposure, serotonin, dopamine, norepinephrine, GABA, LC-MS
Introduction
Alcohol abuse during pregnancy can cause neurodevelopmental disorder in the fetus, or partial syndromes primarily affecting the central nervous system (CNS). Fetal alcohol syndrome has been characterized by distinctive, multifaceted clinical pictures [for review see (Mattson and Riley, 2000, Streissguth and Martin, 1983)]. Clinical and experimental evidence shows that alcohol exposure causes various disruptions in the proliferation and migration of neuronal and glial cells during development (Clarren et al., 1978, Miller, 1992). It also impedes growth of the CNS in rats (Barron et al., 1988, Bauer-Moffett and Altman, 1975, Bauer-Moffett and Altman, 1977, Bonthius and West, 1990, Kornguth et al., 1979, Samson and Diaz, 1981, Sulik et al., 1981) and mice (Sari, 2009, Sari et al., 2009, Sari and Gozes, 2006, Sari and Zhou, 2004). A recent study demonstrated alteration of the amniotic fluid proteome of alcohol-treated prenatally fetuses compared to control groups (Datta et al., 2008).
The neurotoxic effects of ethanol may also cause disruptions in the development of neurotransmitter systems, such as the serotonin, dopamine, gamma-aminobutyric acid (GABA), glutamate and norepinephrine systems at prenatal or postnatal stages in rodents and non-human primates (Druse et al., 1991, Maier et al., 1996, Miller, 2006, Sari and Gozes, 2006, Sari et al., 2001, Sari and Zhou, 2004, Tajuddin and Druse, 1999, Zhou et al., 2002). Serotonin is one of the first neurotransmitter systems to develop during ontogeny; it forms before the genesis of most other transmitter neurons. It has also been proposed that serotonin is a signaling chemical for neuronal development [for a review see (Lauder, 1990)], and that during development serotonin acts to signal maturation of the CNS (Lauder, 1993, Whitaker-Azmitia et al., 1996). Moreover, serotonin has been shown to induce neurogenesis and neuronal differentiation (Buznikov et al., 2001, Lauder, 1993, Whitaker-Azmitia et al., 1996).
Prenatal alcohol exposure lowers serotonin uptake in rats at a mid-gestation stage (Druse et al., 1991, Tajuddin and Druse, 1999, Druse et al., 2004). We previously found that prenatal alcohol exposure from embryonic day 7 (E7) to E15–18 reduces the number of serotonin neurons and the density of serotonin-immunoreactive fibers in several fetal brain regions (Sari and Gozes, 2006, Sari et al., 2001, Sari and Zhou, 2004, Zhou et al., 2002, Zhou et al., 2005). HPLC assays from a previous study demonstrated that prenatal alcohol exposure reduces the levels of dopamine and serotonin while increasing the levels of GABA in E20 fetal brains (Maier et al., 1996). However, a number of other studies examining the effects of prenatal alcohol exposure on the concentration of individual neurotransmitters have reported variant results that show increases, decreases, or no change in the level of each specific neurotransmitter.
In this study we used C57BL/6 mice in a paradigm similar to our previous alcohol liquid diet experiments to examine whether prenatal alcohol exposure affects biogenic amines and amino acid neurotransmitters in E13 fetal brains prenatally exposed to alcohol. We chose an earlier stage of brain development, i.e. E7, as the starting point for alcohol treatment and E13 as the treatment endpoint. This time period covers neural tube fusion through 5–HT neuronal differentiation and early fiber projection in the developing forebrain. The levels of several types of neurotransmitters (serotonin, dopamine, glutamate, GABA, norepinephrine and epinephrine) were quantitatively determined using our established liquid chromatography/mass-spectrometry (LC-MS) technique, employing chromatographic separation in conjunction with multiple-reaction monitoring MS/MS experiments performed on a triple quadrupole mass spectrometer (Rojkovicova et al., 2008). This approach was highly sensitive to and selective for the determination of neurotransmitters levels in E13 fetal brains from control and prenatally alcohol-treated groups.
Experimental procedures
Materials
Internal standards for dopamine, epinephrine, norepinephrine, GABA, glutamic acid, serotonin, salsolinol, and 3, 4-dihydroxybenzylamine, were purchased from Sigma-Aldrich (St Louis, MO). The solvents used for the LC separations and protein precipitations were purchased from EMD Chemicals (San Diego, CA). Formic acid (98% assay, p.a.) was purchased from Fluka Scientific (Steinheim, Germany). The SPE C18 (25 mg) cartridges were purchased from Varian (Walnut Creek, CA). A Milli-Q water purifying system (Millipore Corp, Bedford, MA) was utilized to generate 18.2-MΏ deionized water.
Animals
C57BL/6 mice were used in this study. They were housed at the Indiana University Laboratory Animal Research Center (LARC) in a vivarium with a controlled climate (temperature 22�C, 30% humidity) and a 12:12 reverse light-dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University Bloomington and were in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals.
Breeding and treatment procedure
Female mice were placed into male home cages for 2 hours; immediately afterward, females were checked for a sperm plug by vaginal smear—when a female tested positive, this point in time was designated E0. Weight-matched pregnant females were assigned on E7 to the following groups: (1) Ethanol liquid diet group (ALC, n=7) fed with chocolate sustacal (supplemented with vitamins and minerals) liquid diet 25% (4.49%, v/v) ethanol derived calories (EDC), (2) Pair-fed control group (PF, pair-fed to ethanol-fed group, n=4) fed with a maltose-dextrin solution isocaloric to the dose of ethanol used. ALC groups were adjusted to a pair-fed liquid diet on E6 as previously performed in our laboratory [for review see (Sari, 2009, Sari and Gozes, 2006)]. On E13, animals were sacrificed, and the fetal brains were removed and frozen until analysis. Only one fetal brain from each dam was selected randomly for analyses.
The fortified liquid diet contained 237 ml of chocolate-flavored sustacal (Mead Johnson), 1.44 g of Vitamin Diet Fortification Mixture and 1.2 g of Salt Mixture XIV. The ethanol diet contained 15.3 ml (4.49% v/v; 25% EDC) of 95% ethanol, the fortified sustacal formula, and water that was added to make 320 ml of diet with 1 cal/ml (ethanol). For the isocaloric control diet, 20.2 Maltose Dextrin was added to the fortified sustacal formula and water was then added to bring it to 1 cal/ml (Middaugh and Boggan, 1995, Middaugh et al., 1988). One day before treatment, the ALC dams and the PF dams were adapted to the liquid diet. The dams were weighed, the volume of liquid diet consumed during the previous 24 h was recorded from 30-ml graduated screw-cap tubes, and freshly prepared diet was provided. The ALC subjects had unlimited access to the EDC liquid diet each day to match the drinking of PF subjects. We chose PF control rather than Chow control because the PF control allows us to control the amount of calories given to the ALC group. Importantly, we have not found any differing nutritional effects between Chow and PF groups in previous studies from our laboratory [for review see ref. (Sari and Gozes, 2006)].
Maternal Blood Alcohol Levels
Maternal blood alcohol levels were tested in a separate group of C57Bl/6 dams with the 25% (4.49%, v/v) ethanol derived calorie (EDC) diet as described previously (Sari, 2009, Zhou et al., 2003). Briefly, pregnant mice were given the same feeding protocol as the other experimental dams (EDC diet provided on E7 at 900 h in a reverse dark cycle), and two 50-µl tail blood samples were obtained (at 1100) and 1300 h) on E8 and E11. The blood samples were collected in heparinized capillary tubes and centrifuged, then 5-µl plasma samples were analyzed for alcohol concentrations using the Analox Alcohol Analyzer, calibrated with a 100 mg/dl ethanol standard. The average peaks of blood alcohol concentrations (BACs) obtained in the 25% EDC group were ~40 mg/dl on E8 and ~55 mg/dl on E11. As stated in recent study (Sari et al., 2009), although the present study focuses on the E13 stage, we have previously reported that E14 plasma BAC was about 142.7 ± 39.5 mg/dl (Sari and Zhou, 2004, Zhou et al., 2005, Zhou et al., 2004). Thus, it is possible that the BAC might be also increased at the E13 stage.
Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS)
Preparation of Standards and Calibration Curves
Standard stock solutions were prepared in a 0.1% formic acid solution to assist dissolution and minimize oxidation of labile samples. These solutions were stored at −20°C until use. The standards utilized for construction of calibration curves were diluted to a proper concentration with the same previous solution. Standard curves were prepared by adding different amounts of analytes to a 0.1% formic acid solution to produce a range of concentrations of 4–100 pg/µl, 7–200 pg/µl, 3–60 pg/µl, 7–2300 pg/ µl, 100–6000 pg/µl, and 3–25 ng/µl for dopamine, norepinephrine, serotonin, epinephrine, GABA, and glutamate, respectively. A range of concentrations, 0.05–20 pg/µl, was used for salsolinol. A constant amount of internal standard (3, 4-dihydroxybenzylamine, DHB) was added to all standard solutions to produce a fixed concentration (500 pg/µl). The standard curves were generated through least-square linear regression.
Sample Preparation for LC/MS
The brain samples were weighed and homogenized with 0.4 ml of a solution of 1% formic acid prepared in cold (−20°C) acetonitrile using Model 398 tissue Tearor (Biospec Products, INC., Bartlesville, OK). The internal standard (DHB) was then added (35-µl aliquot corresponding to 500 pg/µl final concentration). Next, the sample was incubated in the freezer (−20°C) for 15 min prior to centrifugation at 16,000 rpm for 20 min at 4°C. The supernatant was then collected and dried in a vacuum concentrator. Next, the dried samples were re-suspended in 0.1 ml of 15% acetonitrile and loaded onto pre-conditioned Varian C18, 25 mg cartridges. This treatment was necessary to ensure the complete depletion of peptides and proteins. The flow-through liquid was collected with a 0.8 ml column wash of 15% acetonitrile. The final extract was dried and re-suspended in 35 µl of mobile phase.
LC-MS/MS
A Dionex Ultimate 3000 LC pump (Sunnyvale, CA) was used, consisting of an isocratic pump, temperature-regulated autosampler, and a column cooling compartment. A Nucleodex β-OH column (200 × 4 mm, 5µm particle size, Macherey-Nagel Inc., Easton, PA) with cyclodextrin chiral stationary phase was used for the chiral separations. The mobile phase consisted of 25 mM ammonium formate (pH 3.8) and 10% acetonitrile, at a flow rate of 0.5 ml/min. A 20-µl aliquot of the re-suspended sample solution was injected on the column using a full-loop injection mode. The column eluent was coupled to a triple-quadrupole/linear ion-trap QTrap 4000 Mass Spectrometer (Applied Biosystems, Framingham MA) that was run in the multiple-reaction-monitoring mode (MRM) where a precursor ion is fragmented in the second quadrupole (Q2), and the resulting fragments are detected in the third quadrupole (Q3). The turbo ion-spray ionization source was heated to 450°C due to the high LC flow rate, and had a nebulization gas pressure of 50 psi and a vaporization gas flow of 55 psi. The capillary voltage was held at 4800 V, while the entrance potential was set to 30 V. The [M+H]+ precursor ions were used for epinephrine (m/z 184), norepinephrine (m/z 170), GABA (m/z 180), glutamate (m/z 148) and salsolinol (m/z 180). The [M+H-NH3]+ parent ions were used for serotonin (m/z 160), dopamine (m/z 137), and DHBA (m/z 123). The product ions selected for the MRM scans were the most abundant product ions for each of the precursor ions, including m/z 107 for epinephrine, m/z 107 for norepinephrine, m/z 117 for GABA, m/z 84 for glutamate, m/z 117 for salsolinol, m/z 115 for serotonin, m/z 91 for dopamine, and m/z 77 for DHBA (ISTD). For quantification, the peak area ratios of analytes to the ISTD were calculated as a function of the concentration of the analytes. Calibration curves were generated using standard solutions that were treated in a manner identical to the tissue samples, securing compensation for sample loss encountered during preparation. This method demonstrated a linear dynamic range extending over four orders of magnitude (5 pg – 50 ng) with a correlation coefficient value (R2) between 0.98 and 0.99 (Rojkovicova et al., 2008). The limits of quantification of GABA, dopamine, glutamate, and salsolinol were 0.1 ng/ml, 5 ng/ml, 40 ng/ml and 0.05 ng/ml, respectively, while that of serotonin, epinephrine and norepinephrine was 2 ng/ml.
Statistical analyses
Data were statistically analyzed using an unpaired student t-test. This type of statistical analysis was chosen to account for the unbalanced number of samples utilized in this study as well as the limited number of samples used for each group. Bonferroni method was used to adjust for the multiple comparison issue (i.e. alpha value = 0.007), originating from the fact that we were testing for more than one neurotransmitter between the two groups. Accordingly, the mean of the two groups is considered to be statistically significant only if p < 0.007. When working with biological samples, any observed variation might be intrinsic to the phenomenon that distinct members of a population differ greatly (biochemical individuality). Consequently, SEM signifies an estimate of the standard deviation of the sampling distribution of means, based on the data from one or more random samples. SEM accounts then for the number of real samples, implicating their biodiversity in the evaluation process. Therefore, the range of values throughout this study was expressed as a standard error of mean (SEM) value.
Results
In this study we used a triple quadrupole mass spectrometer in conjunction with a chiral chromatographic column to determine the effects of prenatal alcohol exposure on the levels of biogenic amines including catecholamines (dopamine, norepinephrine, and epinephrine) and indolamine (serotonin). We have also quantitatively determined the levels of amino acid neurotransmitters such as GABA and glutamate.
Catecholamines: Dopamine, norepinephrine, and epinephrine
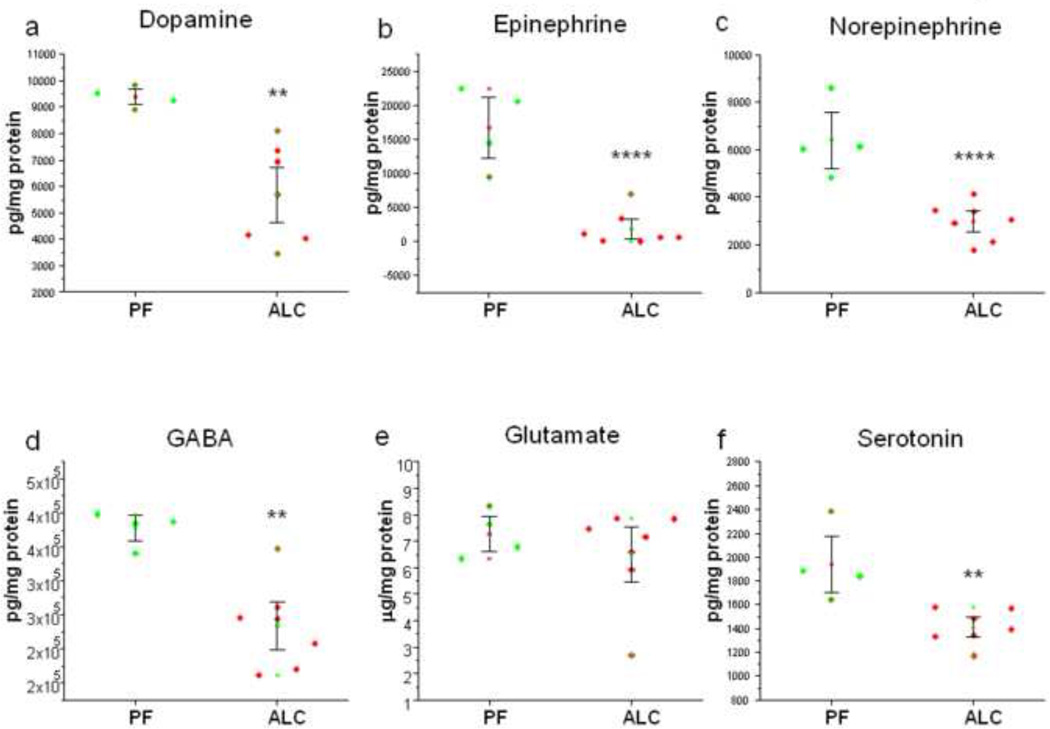
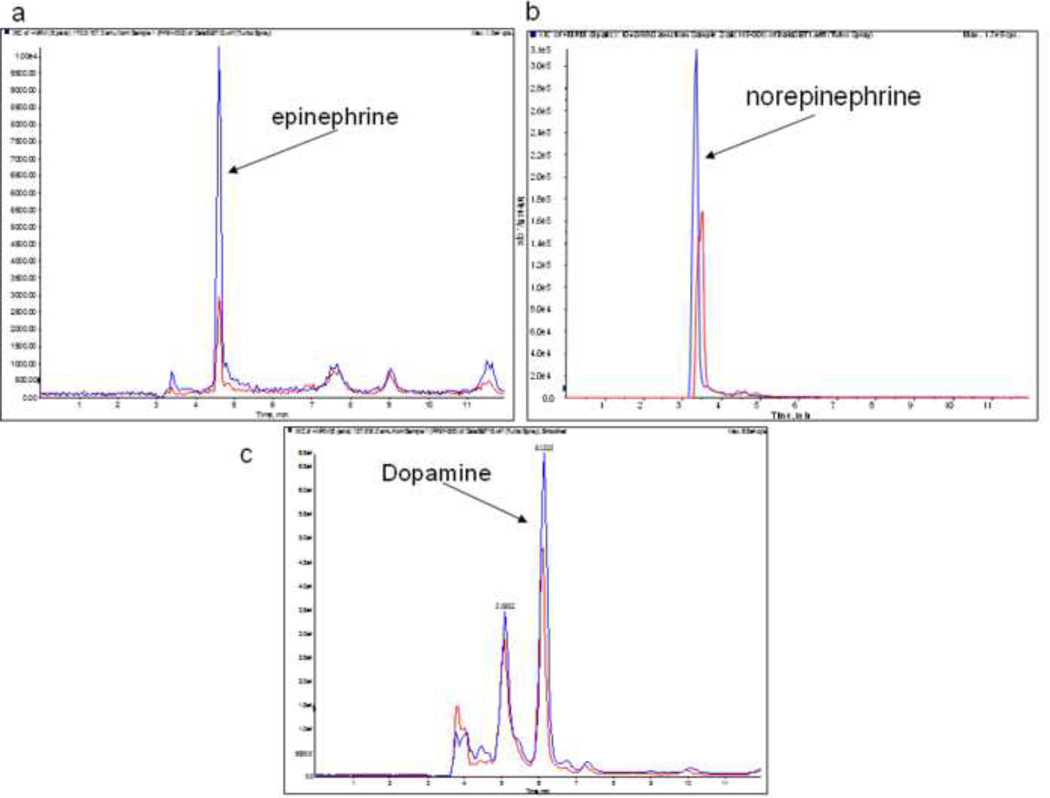
Quantitative analyses of catecholamines in E13 fetal brains revealed a statistically significant (p=0.004) decrease in the concentration of dopamine in the ALC group (5.7 ± 0.7 ng/mg protein) compared to the PF group (9.4 ± 0.2 ng/mg protein; Figure 1a). A dramatically significant (p=0.0002) decrease in the concentration of epinephrine was found in the ALC group (1.8 ± 1.0 ng/mg protein) as compared to the PF group (16.8 ± 3.0 ng/mg protein; Figure 1b). In addition, a significant (p=0.0009) decrease in the concentration of norepinephrine was observed in the case of the ALC group (3.0 ± 0.3 ng/mg protein) as compared to the PF group (6.4 ± 0.8 ng/mg protein; Figure 1c). The MRM traces for epinephrine, norepinephrine, and dopamine demonstrating differences in the levels of these catecholamines in both the PF group (high peaks) and the ALC group (lower peaks) are shown in Figures 2a, 2b, and 2c, respectively.
Figure 1.
Dot-plots illustrating the changes in the levels of dopamine (a), epinephrine (b), norepinephrine (c), GABA (d), glutamate (e) and serotonin (f) as a result of prenatal alcohol exposure. Asterisks depict the statistically significance differences.
Figure 2.
LC/MSMS chromatograms of catecholamines: dopamine (a), epinephrine (b) and norepinephrine (c) depicting significant reduction in the levels of these neurotransmitters in E13 fetal brains of alcohol-treated prenatally mice. In all cases, high signal (blue) is associated with PF control group and low signal (red) is associated with ALC group.
We have also quantitatively analyzed the levels of salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline) which is a dopaminergic tetrahydroisoquinoline neurotoxin. Salsolinol is a condensation product of the alcohol metabolite, acetaldehyde, and dopamine. However, results did not demonstrate any significant difference in the concentrations of salsolinol (p=0.1).
Amino acids: GABA and Glutamate
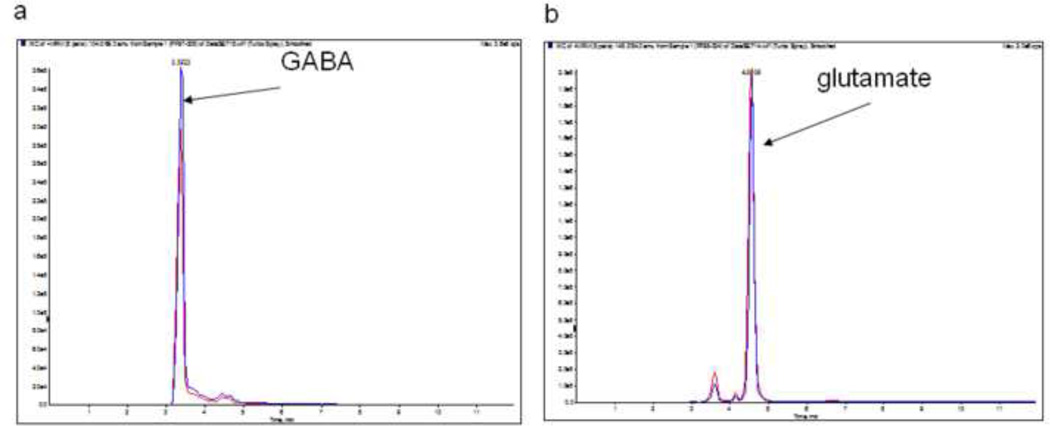
Quantitative analyses of amino acid neurotransmitters in E13 fetal brains of both groups revealed a significant (p=0.002) decrease in the levels of GABA in the ALC group (0.23 ± 0.02 µg/mg protein) compared to the PF group (0.38 ± 0.01 ng/mg protein; Figure 1d). However, there was no significant difference in the levels of glutamate between ALC and PF E13 fetal brains (p=0.4) (Figure 2e). The MRM traces for GABA (Figure 3a) show different levels of this amino acid in the PF group (high peaks) and the ALC group (lower peaks). However, no difference was found in the MRM traces for glutamate between the ALC and PF groups (Figure 3b).
Figure 3.
LC/MSMS chromatograms of amino acids: GABA (a) and glutamate (b) depicting significant reduction in the levels of GABA in E13 fetal brain of alcohol-treated prenatally mice. High signal (blue) is associated with PF control group and low signal (red) is associated with ALC group. However, no significance difference in the level of glutamate was found between ALC and PF groups.
Indolamine: Serotonin
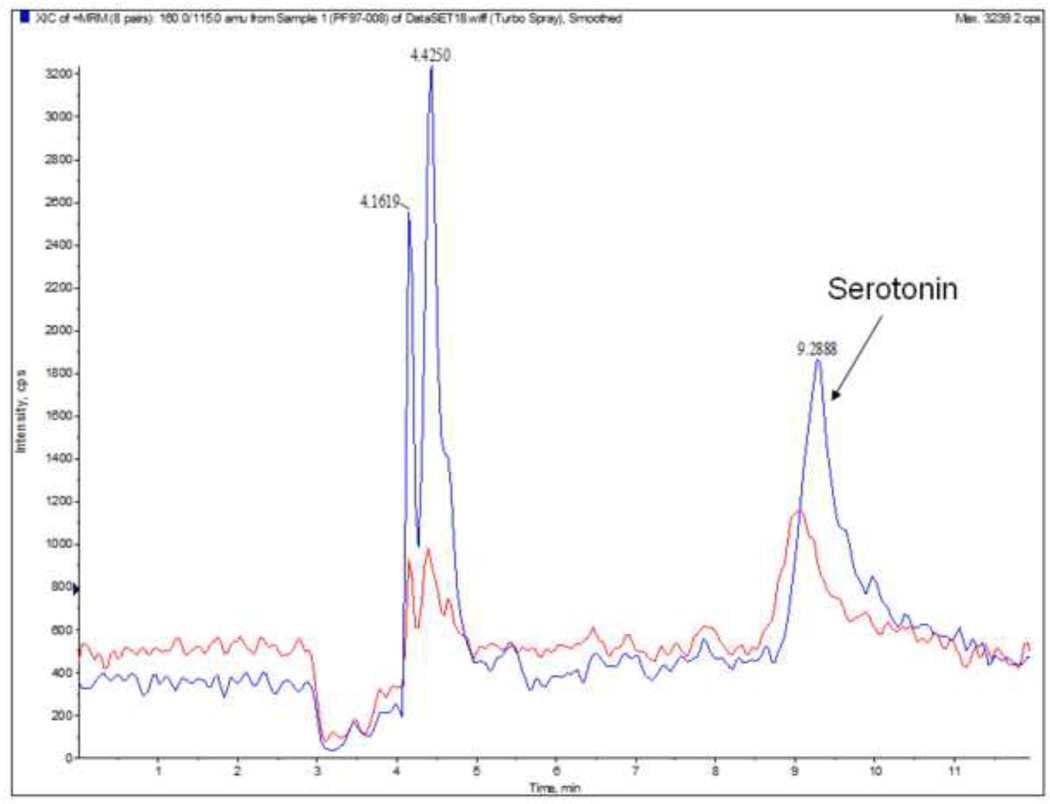
Quantitative analysis of indolamine in E13 fetal brains revealed a significant (p=0.004) decrease in the concentration of serotonin in the ALC group (1.4 ± 0.06 ng/mg protein) compared to the PF group (1.9 ± 0.2 ng/mg protein; Figure 1f). The MRM traces for serotonin (Figure 4) show differences in the level of this indolamine between the PF group (higher peaks) and the ALC group (lower peaks).
Figure 4.
LC/MSMS chromatograms show the levels of serotonin in E13 fetal brain as a result of prenatal alcohol exposure. Significant down-regulation of the level of serotonin was found in the ALC group compared to PF group. Upper trace is that of PF group (blue) and lower trace is that of ALC group (red).
Discussion
Our results show alterations in concentration of several neurotransmitters in prenatally alcohol-treated E13 fetal brains. The concentrations of dopamine, norepinephrine, epinephrine, serotonin, and GABA were significantly reduced in the ALC group compared to the PF control group. However, there was not a statistically significant change in the levels of glutamate or salsolinol between the two groups.
We have previously shown that moderate prenatal alcohol exposure induced brain defects at different embryonic stages [for a review see (Sari and Gozes, 2006)]. Here, we further quantify the levels of several neurotransmitters underlying ethanol-induced teratogenesis at E13. E13 has been suggested to be an early embryonic stage during which changes in neurotransmitter presence, in protein expression, and other factors occur as a consequence of prenatal alcohol exposure (Sari and Gozes, 2006). The E13 stage holds several interests in our ongoing research projects in the field of FAE. At this age, most of the developmental regulatory proteins are highly expressed and altered as a consequence of alcohol exposure (Sari and Gozes, 2006, Zhou et al., 2002, Sari et al., 2001). At E13, the neural tube has undergone five major divisions to form a fetal brain composed of telencephalon, diencephalon, midbrain, hindbrain and spinal cord. Moreover, at this embryonic stage, the serotonergic neurotransmitter system has formed and initiated its differentiation. Our previous studies reported that prenatal alcohol exposure impedes early development of serotonin neurons (Sari and Gozes, 2006, Sari et al., 2001, Zhou et al., 2002).
The deficits in the levels of serotonin in E13 fetal brains prenatally treated with alcohol are in accordance with our previous reports. Using an immunocytochemistry technique, we demonstrated that prenatal alcohol exposure disrupts the development of serotonergic neurons by decreasing the number of neurons and retarding their migration at E11, E13, E15, and E18 (Sari and Gozes, 2006, Sari et al., 2001, Zhou et al., 2002). In addition, the length of rostral raphe nucleus, where serotonin resides, and the diameter of serotonin-immunoreactive medial forebrain bundle, which the majority of serotonin neurons course through, are both reduced in ALC groups (Sari et al., 2001). The present study demonstrates that prenatal alcohol exposure induces a reduction in the level of serotonin as early as E13 when serotonin neurons have been born and started to differentiate in the raphe nuclei. Serotonin itself is known to play a key role in the normal development and maturation of serotonin neurons and their serotonergic and non-serotonergic targets (Lauder et al., 1986, Lauder et al., 1983, Lauder and Krebs, 1978, Whitaker-Azmitia and Azmitia, 1986, Whitaker-Azmitia et al., 1987). A deficiency in serotonin in the fetal brain caused by prenatal alcohol exposure at the appropriate age in the development of the serotonin system, could contribute to the abnormalities in CNS development. We have shown that serotonin neurons were less developed at mid-stages of development (Sari et al., 2001). Others have also reported that the number of serotonin neurons is lower in fetal brain after prenatal alcohol exposure (Druse et al., 1991, Druse et al., 2004, Tajuddin and Druse, 1999). In this present study, we used LC-MS assay to determine the levels of serotonin quantitatively. The LC-MS assay provides high detection sensitivity (Zhang et al., 2008) and allows us to determine the levels of serotonin in picograms per milligram of total protein or tissue. The reduction in serotonin concentration in E13 fetal brains exposed to alcohol is indeed in accordance with the findings from our laboratory and others using immunocytochemistry to count the number of serotonin neurons in mouse and rat models (Druse et al., 1991, Druse et al., 2004, Sari and Gozes, 2006, Sari et al., 2001, Sari and Zhou, 2004, Tajuddin and Druse, 1999).
The quantitative analyses in this study also show a decrease in the concentration of dopamine in E13 fetal brains that were prenatally exposed to alcohol. Similarly, HPLC assays of prenatal bingelike alcohol exposure from E1-E20 showed reduced levels of dopamine in E20 fetal brain (Maier et al., 1996). Although this study tested a different paradigm of alcohol exposure, the outcomes were comparable to our present finding using a liquid diet of alcohol exposure from E7 to E13. Importantly, early decreases in the level of dopamine may support other findings in rat models. Prenatal alcohol exposure markedly affected postnatal development of the dopaminergic system (Druse et al., 1990). In addition, another study used intragastric intubation methods to investigate the effects of prenatal alcohol exposure from E8–20 on the dopaminergic system at an adult age (Shen et al., 1999); this study demonstrated that alcohol exposure induced a long-lasting reduction in the spontaneous activity of dopaminergic neurons which might be correlated to a chronic deficit in dopamine.
We were also interested in investigating the levels of salsolinol, which is one of the dopaminergic tetrahydroisoquinoline neurotoxins. We asked whether prenatal alcohol exposure alters the overall concentration of salsolinol. The local concentration of this isoquinoline derivative increases in the adult rat brain during ethanol intoxication (Collins and Kahn, 1982, Myers et al., 1985). This is assumed to result from the Pictet-Spengler condensation reaction between dopamine and acetaldehyde, a product of ethanol metabolism (Collins and Kahn, 1982). Urinary concentrations of salsolinol were found to be significantly higher in chronic alcoholics than in nonalcoholic control subjects (Adachi et al., 1986, Collins et al., 1979). Moreover, alcoholics exhibit significantly elevated plasma levels of salsolinol sulfate (Baum et al., 1995). We did not observe any significant changes in the level of salsolinol in our fetal alcohol exposure paradigm. This might be because prenatal alcohol exposure significantly reduces the concentration of dopamine in the fetal brain. Lower levels of dopamine in the ALC group may have prevented increases in the level of salsolinol.
Moreover, prenatal alcohol exposure causes dramatic reductions in the levels of both norepinephrine and epinephrine. Epinephrine was dramatically reduced compared to the other neurotransmitters in ALC groups. Although there are no studies investigating epinephrine in a fetal alcohol exposure model, several studies have investigated the effects of prenatal alcohol exposure on norepinephrine levels. For example, in offspring prenatally exposed to ethanol, the concentration of norepinephrine was found to be lower in the brain overall, specifically in the hypothalamus, striatum, and septal areas (Detering et al., 1980a, Detering et al., 1980b, Detering et al., 1981, Rudeen and Weinberg, 1993, Shoemaker et al., 1983). Another study has shown a significant decrease in norepinephrine reuptake sites in the dorsomedial hypothalamus nucleus and anteroventral thalamic nucleus of offspring that were prenatally exposed to ethanol (Gillespie et al., 1997). Based on these findings and our findings, we suggest that prenatal alcohol exposure induces a long-lasting deficit in norepinephrine and possibly epinephrine.
In the case of amino acid neurotransmitters, quantitative analyses fetal brains demonstrated a deficit in GABA in the ALC group relative to the PF group. A deficit in GABA levels is consistent with a recent study demonstrating a decrease in the density of GABA-immunoreactivity in somatosensory and motor cortices of macaque brains that were exposed prenatally to alcohol (Miller, 2006). Our findings suggest that the deficit in GABA is long lasting. In addition, moderate ethanol consumption during gestation can produce long-lasting alterations in neuromodulatory influences on GABAA receptor-mediated inhibitory neurotransmission in adult offspring (Allan et al., 1998). Moreover, it is unclear why prenatal alcohol exposure did not alter the level of glutamate. It is warranted to examine the level of glutamate at later embryonic stages.
We conclude here that prenatal alcohol exposure causes deficits in catecholamines, indolamine, and the amino acid neurotransmitter GABA. However, there was no difference in glutamate levels in the alcohol-treated group versus the control group. Our current findings report the use of LC-MS in determining the concentrations of neurotransmitters in fetal brains exposed prenatally to alcohol for the first time. The LC-MS method is considered as an accurate assay for the determination of the levels of chemicals including neurotransmitters.
Acknowledgement
We would like to thank NIH-NIAAA for their full support in this project, grant number R21AA016115 (YS). This work was also supported by the metabolomic and cytomic initiative (METACyt) funded by a Lilly endowment. The authors would like to thank Dr. Min Zhang for her contribution to the statistical analyses. The authors would like to thank Faye Caylor for her administrative assistance. The authors would like finally to thank Verity Johnson for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi J, Mizoi Y, Fukunaga T, Kogame M, Ninomiya I, Naito T. Effect of acetaldehyde on urinary salsolinol in healthy man after ethanol intake. Alcohol. 1986;3:215–220. doi: 10.1016/0741-8329(86)90047-9. [DOI] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Barron S, Gagnon WA, Mattson SN, Kotch LE, Meyer LS, Riley EP. The effects of prenatal alcohol exposure on odor associative learning in rats. Neurotoxicol Teratol. 1988;10:333–339. doi: 10.1016/0892-0362(88)90036-0. [DOI] [PubMed] [Google Scholar]
- Bauer-moffett C, Altman J. Ethanol-induced reductions in cerebellar growth of infant rats. Exp Neurol. 1975;48:378–382. doi: 10.1016/0014-4886(75)90164-8. [DOI] [PubMed] [Google Scholar]
- Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain Res. 1977;119:249–268. doi: 10.1016/0006-8993(77)90310-9. [DOI] [PubMed] [Google Scholar]
- Baum SS, Hill R, Rommelspacher H. Norharman-induced changes of extracellular concentrations of dopamine in the nucleus accumbens of rats. Life Sci. 1995;56:1715–1720. doi: 10.1016/0024-3205(95)98578-4. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Collins MA, Kahn AJ. Attraction to ethanol solutions in mice: induction by a tetrahydroisoquinoline derivative of L-DOPA. Subst Alcohol Actions Misuse. 1982;3:299–302. [PubMed] [Google Scholar]
- Collins MA, Nijm WP, Borge GF, Teas G, Goldfarb C. Dopamine-related tetrahydroisoquinolines: significant urinary excretion by alcoholics after alcohol consumption. Science. 1979;206:1184–1186. doi: 10.1126/science.505002. [DOI] [PubMed] [Google Scholar]
- Datta S, Turner D, Singh R, Ruest LB, Pierce WM, Jr, Knudsen TB. Fetal alcohol syndrome (FAS) in C57BL/6 mice detected through proteomics screening of the amniotic fluid. Birth Defects Res A Clin Mol Teratol. 2008;82:177–186. doi: 10.1002/bdra.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detering N, Collins R, Hawkins RL, Ozand PT, Karahasan AM. The effects of ethanol on developing catecholamine neurons. Adv Exp Med Biol. 1980a;132:721–727. doi: 10.1007/978-1-4757-1419-7_75. [DOI] [PubMed] [Google Scholar]
- Detering N, Collins RM, Jr, Hawkins RL, Ozand PT, Karahasan A. Comparative effects of ethanol and malnutrition on the development of catecholamine neurons: changes in norepinephrine turnover. J Neurochem. 1980b;34:1788–1791. doi: 10.1111/j.1471-4159.1980.tb11280.x. [DOI] [PubMed] [Google Scholar]
- Detering N, Collins RM, Jr, Hawkins RL, Ozand PT, Karahasan A. Comparative effects of ethanol and malnutrition on the development of catecholamine neurons: a long-lasting effect in the hypothalamus. J Neurochem. 1981;36:2094–2096. doi: 10.1111/j.1471-4159.1981.tb10841.x. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin N, Kuo A, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. J Neurosci Res. 1990;27:233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, Le PT. The serotonin-1A agonist ipsapirone prevents ethanol-associated death of total rhombencephalic neurons and prevents the reduction of fetal serotonin neurons. Brain Res Dev Brain Res. 2004;150:79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gillespie RA, Eriksen J, Hao HL, Druse MJ. Effects of maternal ethanol consumption and buspirone treatment on dopamine and norepinephrine reuptake sites and D1 receptors in offspring. Alcohol Clin Exp Res. 1997;21:452–459. doi: 10.1111/j.1530-0277.1997.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Kornguth SE, Rutledge JJ, Sunderland E, Siegel F, Carlson I, Smollens J, Juhl U, Young B. Impeded cerebellar development and reduced serum thyroxine levels associated with fetal alcohol intoxication. Brain Res. 1979;177:347–360. doi: 10.1016/0006-8993(79)90785-6. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Wallace JA, Wilkie MB, Dinome A, Krebs H. Roles for serotonin in neurogenesis. Monogr Neural Sci. 1983;9:3–10. doi: 10.1159/000406871. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WJ, West JR. Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacol Biochem Behav. 1996;55:521–529. doi: 10.1016/s0091-3057(96)00282-1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res. 2000;24:226–231. [PubMed] [Google Scholar]
- Middaugh LD, Boggan WO. Perinatal maternal ethanol effects on pregnant mice and on offspring viability and growth: influences of exposure time and weaning diet. Alcohol Clin Exp Res. 1995;19:1351–1358. doi: 10.1111/j.1530-0277.1995.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Randall CL, Favara JP. Prenatal ethanol exposure in C57 mice: effects on pregnancy and offspring development. Neurotoxicol Teratol. 1988;10:175–180. doi: 10.1016/0892-0362(88)90082-7. [DOI] [PubMed] [Google Scholar]
- Miller MW. Development of the Central Nervous System: Effects of alcohol and opiates. New York: Alan R Liss press; 1992. The effects of prenatal exposure to ethanol on cell proliferation and neuronal migration; pp. 47–69. [Google Scholar]
- Miller MW. Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: critical timing of exposure. Neuroscience. 2006;138:97–107. doi: 10.1016/j.neuroscience.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Myers WD, Mackenzie L, Ng KT, Singer G, Smythe GA, Duncan MW. Salsolinol and dopamine in rat medial basal hypothalamus after chronic ethanol exposure. Life Sci. 1985;36:309–314. doi: 10.1016/0024-3205(85)90115-8. [DOI] [PubMed] [Google Scholar]
- Rojkovicova T, Mechref Y, Starkey JA, Wu G, Bell RL, Mcbride WJ, Novotny MV. Quantitative chiral analysis of salsolinol in different brain regions of rats genetically predisposed to alcoholism. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863:206–214. doi: 10.1016/j.jchromb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Rudeen PK, Weinberg J. Prenatal ethanol exposure: changes in regional brain catecholamine content following stress. J Neurochem. 1993;61:1907–1915. doi: 10.1111/j.1471-4159.1993.tb09833.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Diaz J. Altered development of brain by neonatal ethanol exposure: zinc levels during and after exposure. Alcohol Clin Exp Res. 1981;5:563–569. doi: 10.1111/j.1530-0277.1981.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Sari Y. Activity-dependent neuroprotective protein-derived peptide NAP, preventing alcoholinduced apoptosis in fetal brain of C57BL/6 mouse. Neuroscience. 2009;158:1426–1435. doi: 10.1016/j.neuroscience.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Chiba T, Yamada M, Rebec GV, Aiso S. A novel peptide, colivelin, prevents alcohol-induced apoptosis in fetal brain of C57BL/6 mice: signaling pathway investigations. Neuroscience. 2009;164:1653–1664. doi: 10.1016/j.neuroscience.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected, in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Brain Res Rev. 2006;52:107–118. doi: 10.1016/j.brainresrev.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sari Y, Powrozek T, Zhou FC. Alcohol deters the outgrowth of serotonergic neurons at midgestation. J Biomed Sci. 2001;8:119–125. doi: 10.1007/BF02255980. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Shen RY, Hannigan JH, Kapatos G. Prenatal ethanol reduces the activity of adult midbrain dopamine neurons. Alcohol Clin Exp Res. 1999;23:1801–1807. [PubMed] [Google Scholar]
- Shoemaker WJ, Baetge G, Azad R, Sapin V, Bloom FE. Effect of prenatal alcohol exposure on amine and peptide neurotransmitter systems. Monogr Neural Sci. 1983;9:130–139. doi: 10.1159/000406885. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Martin JC. The Pathogensis of Alcoholism. New York: Plenum Publishing Corp; 1983. Prenatal effects of alcohol abuse in humans and laboratory animals; pp. 539–589. [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. In utero ethanol exposure decreased the density of serotonin neurons. Maternal ipsapirone treatment exerted a protective effect. Brain Res Dev Brain Res. 1999;117:91–97. doi: 10.1016/s0165-3806(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Autoregulation of fetal serotonergic neuronal development: role of high affinity serotonin receptors. Neurosci Lett. 1986;67:307–312. doi: 10.1016/0304-3940(86)90327-7. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Lauder JM, Shemmer A, Azmitia EC. Postnatal changes in serotonin receptors following prenatal alterations in serotonin levels: further evidence for functional fetal serotonin receptors. Brain Res. 1987;430:285–289. doi: 10.1016/0165-3806(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Zhang MY, Kagan N, Sung ML, Zaleska MM, Monaghan M. Sensitive and selective liquid chromatography/tandem mass spectrometry methods for quantitative analysis of 1-methyl-4-phenyl pyridinium (MPP(+)) in mouse striatal tissue. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;874:51–56. doi: 10.1016/j.jchromb.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Li TK, Goodlett C, Azmitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotox Res. 2002;4:337–342. doi: 10.1080/10298420290030532. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA, Spong CY. A neuroprotective peptide antagonizes fetal alcohol exposure-compromised brain growth. J Mol Neurosci. 2004;24:189–199. doi: 10.1385/JMN:24:2:189. [DOI] [PubMed] [Google Scholar]