Abstract
During spinal cord development, progenitors in the neural tube are arranged within spatial domains that generate specific cell types. The ependyma of the post-natal spinal cord seems to retain cells with properties of the primitive neural stem cells, some of which are able to react to injury with active proliferation. However, the functional complexity and organization of this stem cell niche in mammals remains poorly understood. Here, we combined immunohistochemistry for cell-specific markers with patch-clamp recordings to test the hypothesis that the ependyma of the neonatal rat spinal cord contains progenitor-like cells functionally segregated within specific domains. Cells on the lateral aspects of the ependyma combined morphological and molecular traits of ependymocytes and radial glia (RG) expressing S100β and vimentin, displayed passive membrane properties and were electrically coupled via Cx43. Cells contacting the ventral and dorsal poles expressed the neural stem cell markers nestin and/or vimentin, had the typical morphology of RG and appeared uncoupled displaying various combinations of K+ and Ca2+ voltage-gated currents. Although progenitor-like cells were mitotically active around the entire ependyma, the proliferative capacity seemed higher on lateral domains. Our findings represent the first evidence that the ependyma of the rat harbors progenitor-like cells with heterogeneous electrophysiological phenotypes organized in spatial domains. The manipulation of specific functional properties in the heterogeneous population of progenitor-like cells contacting the ependyma may in a future help to regulate their behavior and lineage potential, providing the cell types required for the endogenous repair of the injured spinal cord.
Keywords: progenitor cells, nestin, vimentin, patch-clamp, ependyma, spinal cord
Introduction
Neurogenesis in discrete niches of the adult mammalian brain represents a remarkable form of plasticity [1, 2]. Although the mature spinal cord in mammals is regarded as a non-neurogenic structure, the region surrounding the central canal (CC) shares key features with stem cell niches of the brain [3]. For example, cells lining the CC express markers of neural stem cells [4, 5] and keep the ability to proliferate [6, 7]. In addition, we recently reported that some CC-contacting cells in neonatal rats display molecular and functional features of immature neurons like those of adult neurogenic niches [8]. In response to spinal cord injury, ependymal cells proliferate and migrate to the lesion site where they differentiate into scar-forming astrocytes and myelinating oligodendrocytes [4, 9]. However, some studies in the normal and the diseased mammalian spinal cord suggest that ependymal cells may support neurogenesis [10–12].
Neural stem cell niches in the adult brain are formed by progenitors with heterogeneous properties that interact within a complex three dimensional organization [13]. During spinal cord development, progenitors in the neural tube are arranged within domains that generate specific cell types [14, 15]. Some of these progenitors are retained post-natally in low vertebrates. In turtles, for example, neurogenic progenitors are functionally clustered on the lateral aspects of the CC [16]. Little is known about the functional complexity and organization of the spinal cord ependymal region as a stem cell niche in mammals.
We speculated that as in low vertebrates, the ependyma of the mammalian spinal cord during early post-natal life -when developmental refinement of spinal circuits still occurs (e.g., myelination)- may contain progenitor-like cells functionally grouped within specific domains around the CC. To test this idea, we combined immunohistochemistry for molecular markers of neural stem cells and patch clamp recordings of CC-contacting cells in neonatal rats. Here we show that the cells lining the lateral aspects and those on the dorsal and ventral poles of the CC constitute a heterogenous population of progenitor-like cells with characterstic molecular and functional properties. Most of the cells on the lateral aspects of the ependyma had the morphology of typical ependimocytes, exhibited passive membrane properties and were electrically coupled via Cx43. In contrast, the cells contacting the poles of the CC had the morphology of radial glia (RG) with a single cilium, were uncoupled and displayed various combinations of K+ and Ca2+ voltage-gated currents. Although, progenitor-like cells were mitotically active around the ependyma, the proliferative capacity seemed higher on cells contacting the lateral aspects of the CC. Our findings show an unforeseen functional and molecular diversity of progenitors within the ependyma of the rat spinal cord segregated within specific spatial domains. It is tempting to speculate that like in the embryo, these domains represent a reservoir of progenitors with different functional roles and lineage potential.
Material and Methods
General
Neonatal rats (Sprague Dawley, P0–P5) were used. For some electrophysiological and immunohistochemical experiments P15-P21 and P40 rats were also used. All experimental procedures were performed in accordance with the ethical guidelines established by our local Committee for Animal Care.
Immunohistochemistry
Animals were anesthetized (50 mg/kg, i.p.; Pentobarbital) and fixed by intracardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). To identify the molecular phenotype of cells around the CC, the following primary antibodies were used (see supplementary table 1): anti-S100β, anti-vimentin, anti-nestin, anti-3CB2, anti-brain lipid binding protein (BLBP), anti-glial fibrillary acidic protein (GFAP), anti-connexin 43 (Cx43), anti-platelet derived growth factor receptor alpha (PDGFRα), anti-NG2, anti-pericentrin, anti-proliferating cell nuclear antigen (PCNA) and anti- phosphohistone H3 (pH3). Tissues were sectioned with a vibrating microtome (60–80 μm thick) and placed in PB with 0.5% bovine serum albumin (BSA) for 30 min and then incubated with primary antibodies in PB with 0.3% Triton X-100 (Sigma-Aldrich). Tissues were then incubated in secondary antibodies conjugated with different fluorophores or horseradish peroxidase. The horseradish peroxidase was revealed with 3,3-diaminobenzidine (DAB, Sigma-Aldrich). Nuclei were stained with Syto 64 (Invitrogen). For double-labelling using anti-3CB2 and anti-Nestin antibodies raised in mice, we applied a sequential immunofluorescence procedure using anti-mouse IgG1 Alexa 488 and anti-mouse IgM Cy3 (Jackson ImmunoResearch Laboratories). The number of PCNA and pH3 positive nuclei was counted in 60 μm thick sections chosen randomly (30 sections from 3 animals). Values are expressed as the mean ± SEM. Statistical significance was set at p< 0.05 and evaluated using the independent t-test. Control experiments were performed suppressing the primary antibodies. The confocal images were acquired with Fluoview 5 (Olympus VF300).
Slice preparation and electrophysiology
Rats anesthetized with isoflurane (Forane, Abbott) were decapitated and the cervical enlargement was dissected out. Transverse 300 μm thick slices were cut, placed in a chamber and superfused (1 ml min−1) with Ringer’s solution (in mM): NaCl, 124; KCl, 2.4; NaHCO3, 26; CaCl2, 2.4; MgSO4.6H2O, 1.3; HEPES, 1.25; KH2PO4, 1.2; and glucose, 10; saturated with 5% CO2 and 95% O2 to keep pH 7.4. In low Ca2+ Ringer’s solution CaCl2 was lowered to 0.2 mM whereas MgSO4 was increased to 4 mM. All experiments were performed at room temperature (22–24 °C). Cells were visualized with differential interference contrast (DIC) optics (Leica DM LFS) and patch-clamp whole-cell recordings obtained with electrodes filled with (in mM): K-gluconate, 122; Na2-ATP, 5; MgCl2, 2.5; CaCl2, 0.003; EGTA, 1; Mg-gluconate, 5.6;K-HEPES, 5; H-HEPES, 5; and biocytin, 10; pH 7.3 (5 to 10 MΩ). In some cases, Alexa 488 or 594 hydrazide (250–500 μM, Invitrogen) was added to the pipette solution. Current and voltage-clamp recordings were performed with a Multiclamp 700B and pClamp10 (Molecular Devices). Seal resistances were between 4 to 18 GΩ. The series resistance and whole-cell capacitance were not compensated. In voltage clamp mode, cells were held at −70 mV, and the resting membrane potential was estimated from the current-voltage relationship (at I=0). To subtract leak currents we used a P4 protocol by Clampex10 (Molecular Devices). Liquid junction potentials were determined and corrected off-line [17]. Values are expressed as the mean ± SEM. Statistical significance was set at p< 0.05 and evaluated using the independent t-test or the Wilcoxon matched-paired test. The activation and inactivation curves for K+ currents were determined as described elsewhere [8].
Morphological identification of the recorded cells
During whole-cell patch-clamp recordings cells were filled with a fluorophore and/or biocytin. In most cases, cells were first imaged in living slices with an FG7 frame grabber (Scion Instruments) using ImageJ (NIH). Then, slices were fixed by immersion in 4% paraformaldehyde in 0.1M PB for 12–24 h. Following PB rinsing, the slices were blocked with 0.5% BSA in PB (1 h) and then incubated in PB containing 0.3% Triton X-100 with the streptavidin-fluorophore complex.
Transmission electron microscopy
Anesthetized animals were fixed by intracardiac perfusion with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M PB, pH 7.4. Slices obtained with a vibrating microtome (100–200 μm thick) were washed in PB and post-fixed in 1% OsO4 in PB 0.1 M, dehydrated and epoxy-resin embedded.
Series of semithin sections were cut and stained with boraxic methylene blue (BMB). Ultrathin sections were obtained from trimmed blocks and mounted on one hole grids. To obtain frontal views of the apical processes of cells contacting the CC poles, we made alternating series of sections of different thickness. After reaching the level of the ependymal cells nuclei, several series of semithin and ultrathin sections were cut, mounted on one-hole grids and examined with a Jeol X 100 transmission electron microscope.
TEM immunohistochemistry
Sections were processed for revealing nestin using a secondary antibody conjugated with HRP and DAB as chromogen. The selected sections were postfixed for 1 h (1% OsO4 in PB 0.1 M), washed, dehydrated and epoxy-embedded in flat molds. Series of sections were processed as already described.
Results
Progenitor-like cells in the CC: molecular clues
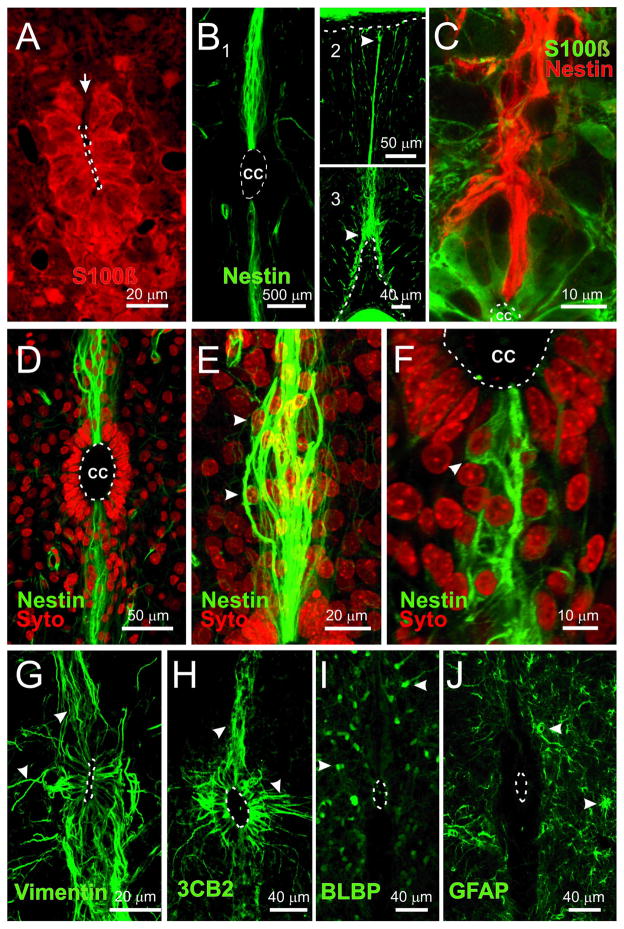
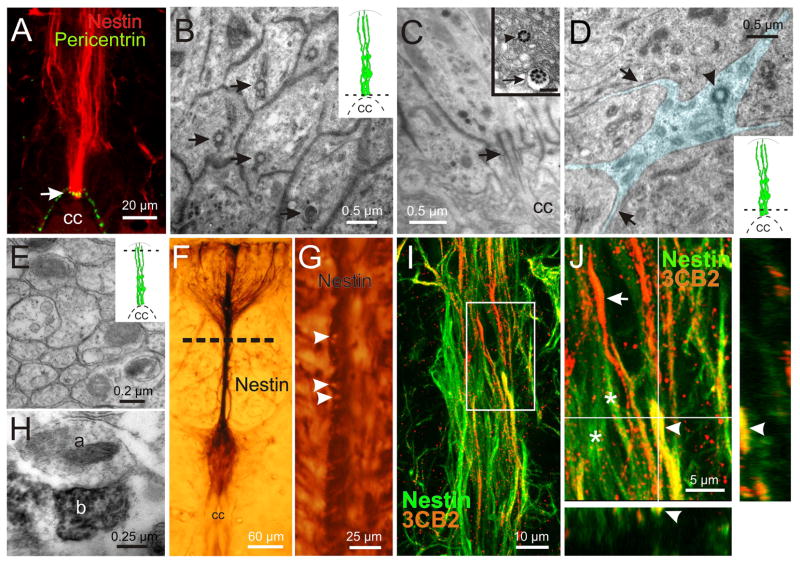
Some ependymal cells in adult mice express markers of neural precursors [3]. To identify potential progenitor-like cells and their spatial distribution within the ependymal region of the neonatal rat spinal cord, we analyzed the expression of molecules expressed in neural stem cells [18]. Most cells surrounding the CC expressed the ependymal cell marker S100β (Fig. 1A) but a narrow strip of tissue on the dorsal pole was unstained (Fig. 1A, arrow). Both the dorsal and ventral poles of the CC were contacted by closely packed nestin+ processes (Fig. 1B 1) that projected within the midline to reach the pia (Fig. 1B 2 and 3, arrowheads). Unlike the ventral pole, nestin+ fibers in the dorsal pole of the CC did not co-express S100β (Fig. 1C). As revealed by Syto 64, most cell bodies corresponding to nestin+ fibers located far from the CC (Fig. 1D and E, arrowheads) with only a few lying close to it (Fig 1F, arrowhead). We also found a faint expression of nestin in few short radial processes arising from the lateral aspects of the ependyma (data not shown). Vimentin and 3CB2 also expressed in cells that projected their distal processes to the midline. However, unlike nestin+ cells, vimentin+/3CB2+ cells were also abundant on the lateral aspects of the ependyma, giving rise to short processes projecting away from the CC (Fig. 1G and H, arrowheads). Although a subset of progenitors in the embryo and the adult brain, characteristically expresses GFAP and/or BLBP [19, 20], we did not find GFAP or BLBP expression in ependymal cells but outside the ependymal region (Fig. 1I and J). In the spinal cord of neonatal rats, BLBP+ cells had a morphology similar to migrating cells (Fig. 1I, arrowheads), whereas cells expressing GFAP showed the typical morphology of astrocytes (Fig. 1J, arrowheads). Most 3CB2+ cells on the lateral aspects of the CC also expressed S100β (Supplementary figure 1, arrowheads).
Figure 1. CC-contacting progenitor-like cells: molecular clues.
A, Most cells surrounding the CC expressed the ependymal cell marker S100β. However, a narrow portion on the dorsal pole of the ependyma was devoid of S100β immunoreactivity (arrow). B, Nestin immunoreactivity was observed mostly in cells with long radial processes in the dorsal and ventral midline that stretched from the CC lumen (1) to the pia on the dorsal (2, arrowhead) and ventral (3, arrowhead) aspects of the cord. C, Nestin+ fibers on the dorsal CC did not co-express S100β. D, Location of nuclei (Syto64) belonging to nestin reactive fibers in the dorsal and ventral midline. E, Nestin+ cells on the dorsal raphe had a bipolar shape with their cell bodies located at various distances from the CC (arrowheads). F, The cell bodies of nestin+ cells were also found close to the CC lumen (arrowhead). G–H, RG markers vimentin and 3CB2 expressed in cells on the lateral aspects and poles of the ependyma, with some fibers projecting away from the CC (arrowheads). I, BLBP is not expressed in the ependyma but in cells outside the ependymal region (arrowheads). J, GFAP+ cells were not detected in the ependymal layer, but cells with the morphology of astrocytes appeared in the spinal cord outside this region (arrowheads). A–D, F and J, single confocal optical planes. E, G–H, confocal Z-stack projection. Unless otherwise stated, in this and subsequent figures the dorsal pole of the CC is upward.
Electrophysiological properties of cells lining the CC
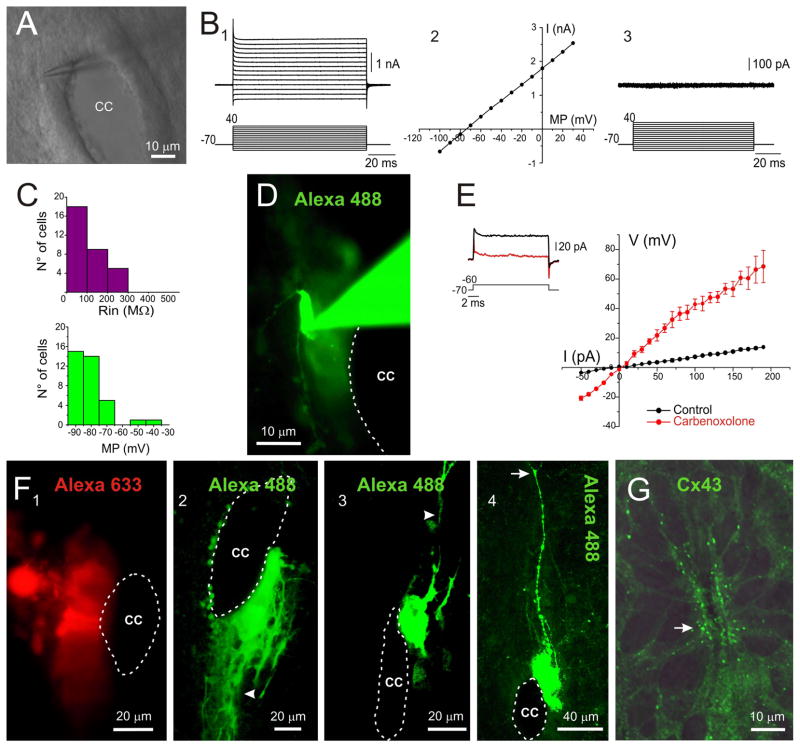
Progenitors in the embryo [21] and the adult brain [22–24] have characteristic electrophysiological signatures. To check whether progenitor-like cells in different regions of the ependyma have specialized functional properties, we made patch-clamp recordings of cells on the lateral aspects of the ependyma (Fig. 2A). We found cells (n= 36) with linear voltage-current relationships (Fig 2B), relatively low input resistances (123.63 ± 24.44 MΩ, n=34; Fig. 2B and C) and hyperpolarized resting membrane potentials (−84.35 ± 2.13 mV, n= 36; Fig. 2C). Morphological analysis revealed that recorded cells belonged to clusters of dye-coupled ependymocytes lining substantial portions of the CC (Fig. 2D and F). The low input resistance and dye coupling suggested coupling via gap junctions. In line with this interpretation, the gap junction decoupler carbenoxolone (100 μM) significantly increased their input resistance (from 103.8 ± 26.4 to 388.5 ± 189.6 MΩ, p< 0.05, Wilcoxon’s matched-pairs test, Fig. 2E). The size of the clusters ranged from large groups of cells covering the entire lateral aspect of the CC (Fig. 2F 1) to rather small cell conglomerates close to the ventral or dorsal poles (Fig. 2F 2–4). Interestingly, some of these clustered cells had processes entering the dorsal or ventral midline (Fig. 2F 2 and 3, arrowheads) and projecting towards the pia (Fig. 2F, 4 arrow). Occasionally, we recorded uncoupled cells on the lateral aspects of the CC with a process projecting toward the parenchyma (n= 4, data not shown). Because the electrophysiological data suggested the existence of gap junctions between clustered cells, we studied the molecular basis of electrical coupling. Although several connexin subtypes are expressed in the developing brain, Cx43 is the most abundant in the subventricular zone of the developing forebrain [25]. Immunohistochemistry for Cx43 revealed a high density of punctae on the lateral aspects of the CC (Fig. 2G, arrow). Notice however, the lack of Cx43 punctae on the dorsal and ventral poles of the ependyma. We conclude that on the lateral aspects of the CC, the non-neuronal cells have morphological and electrophysiological characteristics of ependymocytes and tanycytes [26] functionally associated by means of gap junctions.
Figure 2. Functional properties of cells lining the lateral CC: dye coupling and passive responses.
A, DIC image of the CC in a live slice. B, Responses of a cell recorded on the lateral CC to a series of voltage steps (1) that displayed a linear I/V relationship (2). This cell lacked voltage-gated currents as shown in the leak subtracted traces (3). C, Distribution of input resistances and membrane potentials of cells recorded on the lateral aspects of the CC. D, The cell recorded in B appeared dye-coupled with neighboring cells. E, Carbenoxolone (100 μM) increased the input resistance of cells in the lateral aspects of the CC (n=5). F, Clustered cells covered the whole lateral aspect of the CC (1) or formed rather small cell conglomerates on the ventral (2) or dorsal (3) halves of the lateral CC. Some clustered cells had processes that entered the dorsal or ventral midline (2 and 3, arrowhead), projecting toward the pia (4, arrow). G, Cx43 expression in the CC. Cx43 punctae concentrated on the lateral aspects close to the CC lumen (arrow). D, Conventional epifluorescence in a living slice. F1-4, Confocal Z-stack projections. G, single confocal optical plane.
Electrophysiological signature of midline progenitor-like cells
Because our data indicate that the cells contacting the poles of the CC have molecular phenotypes different from those on the lateral aspects, we speculated that they may also have different electrophysiological properties. Patch clamp recordings of CC-contacting cells projecting to the raphae revealed that these cells had hyperpolarized resting membrane potentials (−81.95 ± 2.31 mV, n= 47; Supplementary figure 2 A) and high input resistances compared with cells in the lateral aspects of the CC (361.22 ± 56.29, n=48, p< 0.05, independent t test; Supplementary figure 2 A). On the dorsal and ventral poles of the CC, cells had the typical morphology of RG (Figure 3A, F, Figure 4A) and appeared uncoupled (n= 71). Some cells had a relatively thick apical process (Supplementary figure 2 B 1, arrow) with many finger-like protrusions (Supplementary figure 2 B 2, arrowheads), and a thinner distal fiber projecting to the pia (Supplementary figure 2 B 1, arrowhead). However, other cells had smooth apical and distal processes (Supplementary figure 2 C, arrows). RG contacting the dorsal or ventral aspects of the CC had their cell bodies located at different distances from the CC lumen (Supplementary figure 2 D), resembling the morphology of RG during interkinetic nuclear migration [27]
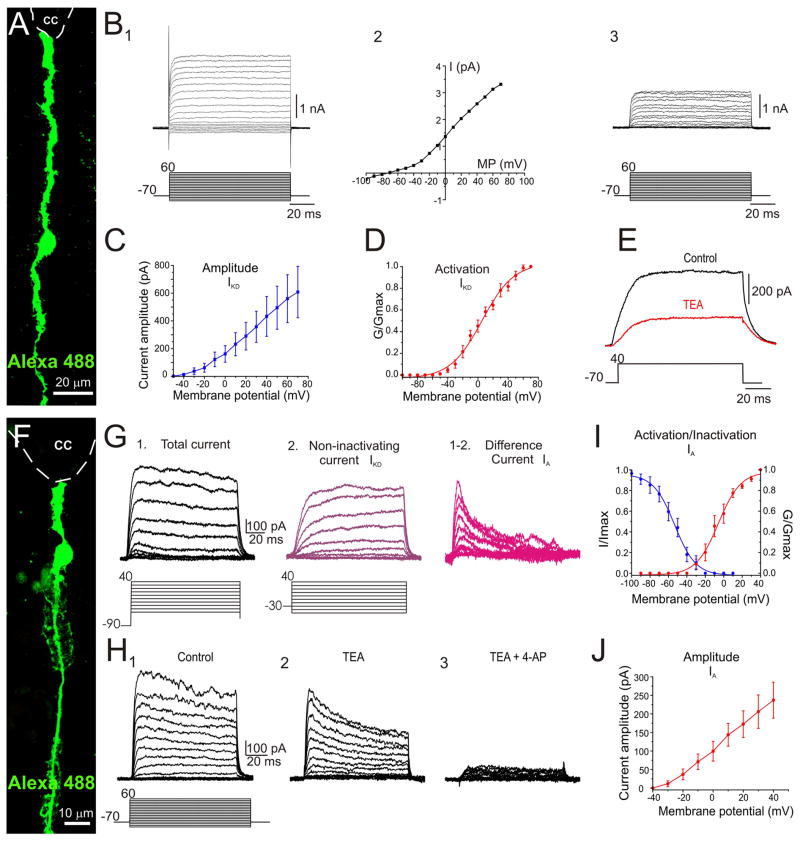
Figure 3. IKD and IA in midline RG.
A, Alexa 488 filled RG contacting the ventral pole of the CC. B, Raw (1) and leak-subtracted (3) currents in a RG in response to a series of voltage steps. An outward current with an activation threshold of −40 mV (2) was generated in response to depolarizing voltage steps. C and D, Current amplitude and activation curves for IKD, respectively. E, The current was blocked by TEA (10 mM). F, RG recorded in the ventral midline. G, Currents evoked in the cell shown in F by depolarizing voltage steps after a prepulse to −90 mV (1). Currents with a slower onset were evoked with the same protocol as in 1 but after a pre-pulse to −30 mV (2). By subtracting 1 and 2, we obtained a current with a fast onset and voltage-dependent inactivation. H, IKD was blocked by TEA (10 mM, 1 and 2) whereas the remaining current was blocked by the IA antagonist 4-AP (2 mM, 2 and 3). I and J, IA activation/inactivation and amplitude curves, respectively. A and F: Confocal Z-stack projection. I–J: data pooled from P1–P5 rats.
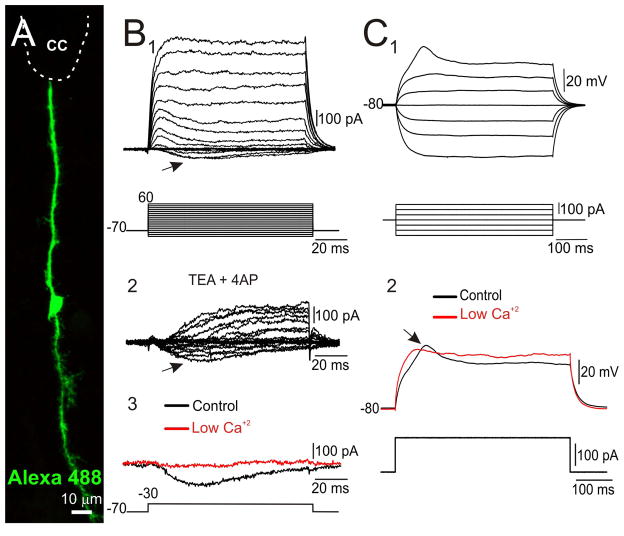
Figure 4. ICa in midline RG.
A, RG recorded and filled with Alexa 488. B, This cell displayed both outward and slow inward currents (1, arrow) in response to depolarizing voltage steps. The inward current remained in the presence of K+ channel antagonists (2, arrow, 4-AP 2 mM and TEA 10 mM) but was abolished in low Ca2+ Ringer solution (3), suggesting the involvement of voltage-gated Ca2+ channels. C, In current clamp mode, depolarizing current steps applied from a hyperpolarized membrane potential generated a slow spike (1, 2 arrow) that disappeared in low Ca2+ (2). A, Confocal Z-stack projection. .
RG lying within the midline had a complex repertoire of active properties with different types of outward and inward currents. In some RG (16 of 65, see supplementary table 2), depolarizing voltage steps produced an outward current (Fig. 3A, B 1) with minimal inactivation in response to sustained depolarization (Fig. 3B 1 and 3). This current had an activation threshold close to −40 mV with a Vh= 5.37 ± 1.77 mV (Fig. 3B 2, C, D) and was sensitive to 10 mM TEA (Fig. 3E; 3 out of 3 cells) suggesting the involvement of delayed rectifier K+ currents (IKD).
In other RG (25 of 65, see supplementary table 2), depolarizing voltage steps (from a holding potential of −90 mV) evoked outward currents that had both non-inactivating and inactivating components (Fig. 3F–H). To separate these components we applied the same stimulation protocol but from a holding potential of −30 mV (Fig. 3G 2). Under these conditions, we observed an outward current with a slower onset and no inactivation, suggesting the presence of IKD channels. By subtracting the delayed non-inactivating current (Fig. 3G 2) from the total current (Fig. 3G 1), we were able to separate an outward current with a fast onset and a prominent time-dependent inactivation (Fig. 3G 1–2), suggesting an A- type K+ current (IA, 28] In line with this interpretation, TEA (10 mM) blocked the non-inactivating component of the outward current (Fig. 3H 1 and 2, 10 out of 10 cells) but spared the inactivating current which was blocked by the selective A-type K+ channel blocker 4-AP (2 mM, Fig. 3H 3, 10 of 10 cells). IA activated transiently at membrane potentials of approximately −40 mV with a Vh= −5.79 ± 1.2 mV (Fig. 3I, J).
Besides displaying IKD and IA, another subgroup of RG characterized by generating voltage-gated inward currents (6 of 65, see supplementary table 2). The slow transient inward current required relatively modest depolarizations (threshold about −55 mV, Fig, 4 B 1) and remained in the presence of both TTX (1 μM, data not shown) and K+ channel antagonists (Fig. 4B 2). However, the inward current was abolished by 3 mM Mn2+ or in low Ca2+ Ringer’s solution (Fig. 4B 3, n= 7) suggesting the involvement of low voltage-activated Ca2+ currents (ICa). In current clamp mode, this inward current generated a slow low threshold spike (LTS, Fig. 4C1) that disappeared in low Ca2+ Ringer’s solution (Fig. 4C 2).
We also found RG that displayed IKD plus ICa without IA (10 of 65, data not show) and others that only had ICa (6 of 65, data not shown). Finally, we recorded few cells (2 of 65) displaying passive membrane responses similar to those of lateral ependymocytes. The electrophysiological phenotypes described above were equally found in the ventral or dorsal poles and in animals within the range of explored ages (P0–P5).
To identify the molecular phenotypes of recorded cells, we combined the labeling of recorded cells with immunohistochemistry for specific markers of progenitors. Some cells recorded on the poles of the CC expressed nestin (Supplementary figure 3 A 1–4), but others (15 of 18) did not react with nestin antibodies (Supplementary figure 3 B). Because the presence of IKD and IA is characteristic of oligodendrocyte progenitor cells [24], we speculated that nestin- cells may be early oligodendrocyte progenitors. To test this idea, we performed immunocytochemistry for oligodendrocyte progenitor markers such as the platelet derived growth factor receptor alpha (PDGFRα) and the chondroitin sulfate proteoglycan NG2. Although we found abundant PDGFRα and NG2 reactive cells both in the grey and white matter, CC-contacting cells were always negative (Supplementary figure 3 C, D).
Fine structure of midline CC-contacting cells
Progenitors in the embryo and post-natal neurogenic niches have characteristic fine structure features with pronounced apical-basal polarity [18]. A key component of this polarity is the presence in the apical pole of a centrosome, a structure that regulates the cell cycle and microtubule organization [29]. We found that pericentrin -a key component of the pericentriolar material- was expressed in the apical poles of nestin+ cells contacting the midline but not in their processes (Fig. 5A, arrow). The selective location of pericentrin in the apical poles of midline RG may reflect the presence of cilia. Because progenitors typically possess a single primary cilium [30], we made a careful analysis of the apical process of cells contacting the poles of the ependyma. Serial section studies revealed that the apical process of these cells bear a single cilium (Fig. 5B–D). Interestingly, some of the cilia exhibited a 9 + 0 microtubule organization (Fig. 5C, arrowhead in inset) whereas others had a 9+2 organization (Fig. 5C, arrow in inset; D, arrowhead). Some of the apical process of the midline RG exhibited irregular profiles with lateral projections and lamella (Fig. 5D). These images probably correspond to the abundant finger-like processes arising from the apical process of some midline RG stained intracellularly (see supplementary figure 2 B).
Figure 5. Molecular and ultrastructural characteristics of cells contacting the dorsal pole of the ependyma.
A, Nestin+ cells contained pericentrin+ granules in their apical processes (arrow). B, Electron micrograph of the apical processes of cells contacting the dorsal CC (inset). Each process had the insertion site of a single cilium (arrows). C, Longitudinal view of a cilium (arrow). Some cilia exhibited a 9 + 0 microtubule organization (inset, arrowhead) while others showed a 9+2 structure (inset, arrow). D, The apical process (shaded in light blue) of midline cells (inset) exhibited lateral projections and lamella (arrows). Note the insertion site of a cilium (arrowhead). The inset illustrates the level of TEM sections. E, Electron microscopy of cross sections at the level of the dorsal raphe (inset), showing bundles of circular or oval proceses. F, Light microscope image of nestin+ proceses in a coronal section. G, Image from a longitudinal section at the level of the dotted line shown in G. The nestin+ processes are intermingled with nestin- processes (arrowheads). H, The section level indicated in F as shown by TEM. A dense osmium-DAB precipitate (b) in a nestin+ fiber close to a non labeled process (a). I, Double-labeling of 3CB2 and nestin in the dorsal midline. J, High magnification of the boxed area in I showed the coexistence of nestin+ (asterisks), 3CB2+ (arrow) and nestin+/3CB2+ processes (arrowhead in main panel and orthogonal view). A, single confocal optical plane. I–J, Confocal Z-stack projection.
We extended our TEM analysis to the distal processes of dorsal RG (Fig. 5E, inset). Cross sections at the level of the dorsal raphe showed a compact population of circular/oval profiles with minor structural differences (Fig. 5E). Despite the similarities in fine structure, our patch clamp recordings suggested that RG in the midline actually represent a heterogeneous population. TEM immunohistochemistry showed that nestin+ processes running in the raphe (Fig. 5F) were intermingled with others lacking electrondense precipitate (Fig. 5G, H). In line with this, some processes in the midline region only expressed 3CB2 (Fig. 5I, arrow in J) whereas some were nestin+/3CB2- (Fig. 5I, asterisks in J) and others co-expressed both markers (Fig. 5I, arrowhead in J).
Proliferative potential of CC-contacting cells
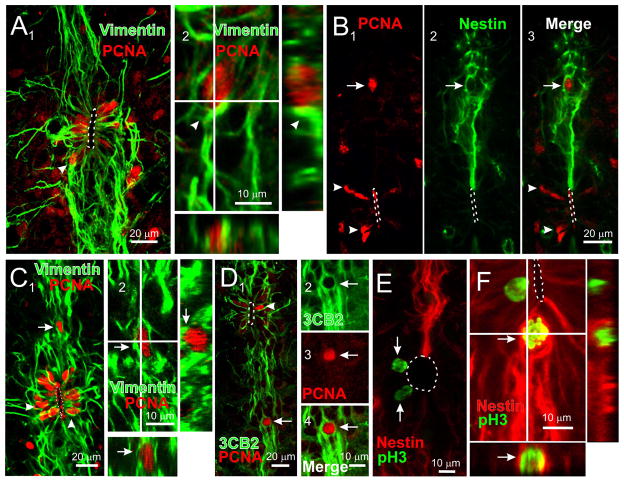
Because progenitor-like cells located in different portions of the ependyma have heterogenous functional and molecular properties, we explored whether they may have different proliferative activity by means of immunohistochemistry for endogenous cell cycle proteins such as the proliferating cell nuclear antigen (PCNA, expressed in all phases of the cell cycle) and the phosphohistone H3 (pH3, expressed mostly in M phase). We found abundant cells with PCNA+ nuclei on the lateral aspects of the CC (7.1 ± 0.79 cells/section, 30 sections, n=3 rats, Fig. 6A, Supplementary figure 4 A, B) that co-expressed vimentin (Fig. 6A, arrowhead; C, arrowheads) or 3CB2 (Fig. 6D, arrowhead). PCNA+ nuclei were also observed in the dorsal (Fig. 6B, arrow) or ventral (Fig. 6C, arrow) raphae at different distances from the CC lumen, but their number was significantly lower than in the lateral aspects of the CC (3.6 ± 0.68/section, 30 sections, n=3 rats, p < 0.05, independent t test; Supplementary figure 4 A, B). Many of the PCNA+ nuclei in the midline corresponded to cells that expressed RG markers such as nestin (Fig. 6B 1–3, arrows), vimentin (Fig. 6, C 1–2, arrows) or 3CB2 (Fig. 6D 1–4, arrows). The analysis of pH3 expression showed that mitotic cells appeared all around the CC but as expected from the PCNA immunostaining, were more abundant on the lateral aspects of the ependyma (0.96 ± 0.33 vs 0.131 ± 0.08 nuclei/section, 29 sections, n= 3 rats, p < 0.05, independent t test; Figs. 6E, arrows and Supplementary figure 4 B). pH3+ cells on the poles of the ependyma were nestin+ (Fig. 6F). In contrast with PCNA, pH3+/nestin+ nuclei in the midline were found only close to the CC lumen, suggesting that as during embryogenesis, cell division occurred close to the surface in contact with the cerebrospinal fluid. We conclude that there is proliferative activity all around the CC, but most cell divisions take place on the lateral domains of the ependyma.
Figure 6. Cell proliferation around the CC.
A, PCNA+ nuclei were abundant around vimentin+ cells on the lateral aspects of the CC (1). The PCNA+ nucleus pointed by the arrowhead in 1 corresponded to a vimentin+ cell (2). B, PCNA+ nucleus in the midline (1, arrow) corresponding to a nestin+ cell (2 and 3, arrows). Notice the presence of many PCNA+ nuclei close to the lumen on the lateral CC (arrowheads). C, Midline PCNA+ nucleus in a vimentin+ cell (1, arrow). Arrowheads indicated PCNA+ nucleus lining the CC. 2, Higher magnification and orthogonal images of the cell pointed in 1. D, A PCNA+ nucleus lying in the ventral midline (1 arrow) corresponded to a 3CB2+ cell (1–4, arrows). E, pH3+ nuclei on the lateral aspects of the CC (arrows). F, Some midline pH3+ nuclei close to the CC lumen on the ventral pole corresponded to nestin+ cells (arrow). A, C and D, confocal Z stack projections. B and E, confocal optical sections.
Collectively, our findings support the idea that the ependyma of neonatal rats contains progenitor-like cells with heterogeneous properties, organized in well defined spatial domains. It may be possible that as the animal develops, these progenitors disappear or change their properties and organization within spatial domains. To explore this possibility, we made patch clamp recordings in rats between P15–P21 (n= 6 cells) because during the first two postnatal weeks, spinal circuits mature quickly and rats acquire an adult pattern of locomotion [31]. We found that cells contacting the poles of the ependyma in older rats were still uncoupled with the morphological phenotype of RG and complex electrophysiological properties (Supplementary figure 5 A–D). Similar to neonatal animals, cells on the lateral aspects were extensively coupled and had passive electrical properties (Supplementary figure 5 E). As in mice [5], the expression of progenitor cell markers was also retained in the mature spinal cord of rats (P40), with nestin predominating on cells contacting the poles (Supplementary figure 5 F,G) whereas 3CB2 expressed on both the poles and lateral aspects of the ependyma (Supplementary figure 5 H). Finally, a substantial number of cells on the lateral aspects were positive for PCNA (Supplementary figure 5 I). Thus, progenitors in the ependyma of the mature rat spinal cord maintain the basic properties and compartmentalization observed in neonates.
Discussion
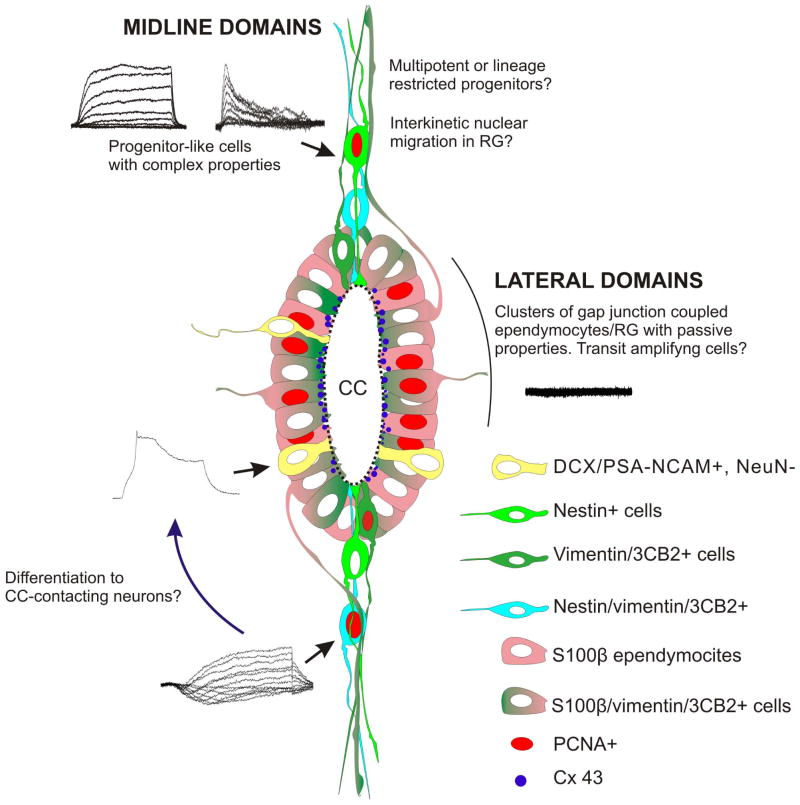
We show here that the ependyma of the rat spinal cord harbors progenitor-like cells organized in spatially defined domains (Fig. 7). Clusters of coupled cells within lateral domains combined molecular features of ependymocytes and RG [18]. Midline domains contained elements with typical characteristics of neural stem cells [32] and complex electrophysiological properties that may reflect different functional states or progenitor competence. The different proliferation potential between lateral and midline domains favors the idea they represent a functional compartmentalization of this spinal stem cell niche.
Figure 7. Cartoon depicting the ependyma as a stem cell niche organized in midline and lateral spatial domains.
The heterogeneous molecular and functional phenotypes are color coded or illustrated with representative data. Some of our working hypotheses brought about by our current findings are indicated with interrogation marks.
Structural and molecular clues of a spinal stem cell niche
Classical studies conceived the ependyma of the spinal cord as a layer of ephitelial cells [33]. Our findings in the rat and those by others in mice [4, 5, 34, 35] suggest that the region around the CC has a complex organization with heterogeneous progenitor-like cells. As in the brain [36], most cells lining the lateral aspects of the CC expressed the ependymal cell marker S100β, but some co-expressed the RG marker 3CB2 or vimentin and had a basal process projecting away from the CC suggesting a progenitor cell nature [37]. Indeed, many S100β+/3CB2+/vimentin+ cells expressed PCNA -thus being within the cell cycle- with few undergoing division as indicated by pH3 expression [38].
The expression of nestin -a marker of neuroepithelial cells and RG [37]- defined a second domain of heterogeneous cells contacting the poles of the CC, that may also express RG markers. In adult mice, nestin is expressed preferentially on cells contacting the dorsal pole of the CC [34]. The fact that cells contacting the poles of the CC in human neonates are also nestin+ [39] suggests this is an evolutionary preserved trait of early stages of post-natal development. Progenitors in adult neurogenic niches express GFAP in addition to nestin [40]. However, the ependyma in the rat lacked GFAP immunoreactivity, in contrast with GFAP-GFP transgenic mice which bear GFAP+ cells contacting the dorsal pole [35]. The discrepancy of our data with those in mice may be species specific or age related (neonatal versus adult). Interestingly, cells contacting the ventral but not the dorsal pole expressed the astrocyte and ependymal cell marker S100β, raising the possibility that midline domains may not be identical in their potential [37]. In fact, adult mice progenitors generating neurospheres presumably localize on the dorsal aspect of the CC [35]. Alternatively, nestin+/S100β+ cells on the ventral pole may be a transitional stage between RG and ependymal cells as described during brain development [36].
Vimentin+ and nestin+ cells contacting the poles of the ependyma had the morphological phenotype of RG with a pronounced apical-basal polarity as embryonic and adult neural stem cells [18]. Although their perikarya laid at various distances from the CC, their centrosomes were always located in apical endfeet. In addition, our EM study showed that some apical processes had a single cilium with a 9+0 organization, a structural specialization thought as a key determinant of neural stem cells [30]. The variety of midline cell morphologies resembled RG undergoing interkinetic nuclear migration during cortical development [27]. Indeed, the fact that pH3 nuclei belonging to nestin+ cells were always close to the CC lumen, whereas PCNA nuclei located at various distances, supports the possibility that as in the embryo [18], RG nuclei in the post-natal spinal cord move apically to divide.
Functional diversity: simple but working together or complex and working individually
Spinal ependymocytes in the neonatal rat had electrophysiological properties similar to those of progenitors during cortical development: low input resistances, passive responses, hyperpolarized resting membrane potentials and extensive gap junction coupling via Cx43 [21, 41]. These properties together with the expression of RG markers within cell clusters may indicate a lineage relationship from RG to ependymal cells as in the SVZ [36].
In contrast to cells on lateral domains, midline RG were not coupled and thus function as individual units. Unlike neurogenic RG in the developing cortex [21], RG in the post-natal spinal cord had complex electrophysiological phenotypes displaying various combinations of IKD, IA and/or ICa. The presence of IKD is a common feature among adult progenitors since it has been reported in hippocampal nestin+ type 2 cells [23] and GFAP+ cells in the SVZ [22]. Although IA was not found in the adult SVZ [22], progenitors from the embryonic [42] and neonatal [43] SVZ and human stem cells [44] express IA. The phenotype of midline RG with conspicuous IKD and IA is remarkably similar to that of oligodendrocyte progenitors [24], raising the possibility they are bipolar precursors committed to the oligodendrocyte lineage still negative for NG2 and PDGFRα [45].
The complex repertoire of K+ currents may regulate fundamental properties of ependymal progenitor-like cells. IKD channels are major regulators of cell proliferation [46–48] and IA channels are essential for proliferation of multipotent human neural stem cells [44]. Thus, K+ channels in midline RG may be part of epigenetic mechanisms that regulate proliferation. In addition, IA have been implied in the differentiation of oligodendrocyte precursors [49] and rat spinal cord astrocytes [50]. Thus, another possibility is that K+ currents participate in the transition from RG to post-mitotic spinal cells.
A minority of midline RG had ICa strong enough to sustain an LTS, a phenotype described in some floor plate cells [51]. Ca2+ electrogenesis plays a central role during development by regulating events from neural induction [52] to various aspects of neuronal differentiation [53]. For example, Ca2+ spikes are involved during early steps of differentiation of spinal neurons in Xenopus embryos [54] and newborn neurons in the adult hippocampus [55]. In rats, a sub-population of doublecortin+ CC-contacting neurons has a robust Ca2+ LTS [8]. RG displaying ICa could be precursors showing the first signs of differentiation into CC-contacting neurons [8].
Heterogeneous progenitors in two spatial domains
Neural progenitors in the developing and adult brain are heterogenous and regionally specified in terms of lineage potential [56, 57]. Based on the expression of various stem cell markers, it has been recently proposed that CC-contacting progenitors in mice are heterogeneous cells with different potentials [5]. We show here a new level of complexity demonstrating CC-contacting progenitors are also functionally heterogeneous and organized in spatial domains. The cells located in midline domains were almost quiescent exhibiting various molecular and structural features of neural stem cells [18]. It is not clear whether the molecular heterogeneity and complex electrophysiological phenotypes within midline domains represent different types of progenitors or various functional/developmental stages of a single precursor. Progenitor-like cells within lateral domains combined features of ependymocytes and RG functionally grouped in multicellular units by Cx43. Gap junction coupling has been shown to promote stem cell proliferation [58] and this may be a key factor determining the difference in proliferation capabilities between domains. The spatial profile of Cx43 expression and the clusters of coupled progenitors described here resemble those of turtles [16], suggesting a phylogenetically conserved functional organization. However, unlike their reptilian counterpart, clustered progenitors in the rat did not express BLBP, suggesting a non-neurogenic nature [4, 37].
Conclusions
The fate and potentiality of CC-contacting progenitors as development proceeds remains unclear. Although shortly after birth RG in the brain [59] and spinal cord [60] differentiate to astrocytes, progenitor-like cells remain in the adult spinal cord retaining the ability to react to injury [4, 5]. Ependyma-derived cells migrate away from the CC but unlike their counterparts in the ischemic brain [61], do not become neurons. Our current findings and the fact that some CC-contacting cells display molecular and functional properties of neuroblasts [8] supports the idea that the ependyma of the spinal cord has many elements of adult neurogenic niches. Although post-natal neurogenesis around the CC in mice has been reported [12], several studies suggest that under normal conditions this is just a latent capability of this stem cell niche [8, 35, 62]. The manipulation of specific functional properties in a heterogeneous population of CC-contacting progenitor-like cells may be useful to regulate their behavior and lineage potential, providing the cell types required to repair injured spinal circuits.
Supplementary Material
Supplementary figure 1. Co-expression of 3CB2 (1) and S100β (2) was observed in some cells on the lateral aspects of the CC (1–3, arrowheads). Single confocal optical planes.
Supplementary figure 2. Cells contacting the poles of the CC have RG morphology. A, Distribution of input resistances and resting membrane potentials in midline cells. B, Typical RG recorded in the dorsal pole of the CC, with a thick apical process reaching the lumen (1, arrow), bearing finger-like protrusions (2, arrowheads) and a thinner distal fiber projecting to the pia (1, arrowhead). C, Other RG showed smooth apical and distal processes (arrows). D, The perikarya of cells within the midline located at different distances from the CC lumen (1–4). All images are confocal Z-stack projections. In D the ventral pole of the CC is upward.
Supplementary figure 3. Molecular phenotypes of recorded midline RG. A, Biocytin-filled RG (1, arrow) in the ventral pole of the CC with apical and distal thin processes (arrowheads). This cell expressed nestin (2–4, arrows). B, Some midline RG did not react to nestin. The arrowhead points to a nearby nestin+ cell. C, Midline RG were negative for PDGFRα. However, a few PDGFRα+ cells were found close to midline but without a connection with the CC (arrowhead). D, The ependyma was devoid of NG2 immunoreactivity but abundant NG2+ cells outside the ependymal region occurred (arrowheads). A, Single confocal optical planes. B, C, Confocal Z- stack projections.
Supplementary figure 4. Differences in proliferative potential between the lateral and the midline domains of the CC. A, Schematic drawing showing the limits of the regions (area shaded in green: midline region, area shaded in red: lateral aspects of the CC) in which PCNA and pH3 nuclei were counted. B, Number of PCNA+ and pH3+ cells per section analyzed in the different domains surrounding the CC (mean ± SEM).
Supplementary figure 5. The ependyma of the mature rat spinal cord. A, Cell recorded in the dorsal pole of the CC in a P15 rat with a thick apical process reaching the lumen (arrow), a round perikarya (open arrowhead) and a thinner distal process projecting to the pia (arrowhead). B, response to a series of voltage steps of the cell shown in A. C, Cell in the dorsal pole of the CC in a P20 rat with a short process contacting the CC lumen (arrow) and a thin basal process (arrowhead). D, The cell bodies of some RG were also found apposed to the CC lumen in a P21 rat. E, Cell recorded on the lateral aspect appeared dye-coupled with neighboring cells in a P20 rat. Notice that clustered cells extended until the dorsal pole of the ependyma (arrowhead). The inset shows the electrical response of the cluster of cells in the main panel. F, Immunocytochemistry for nestin shows many positive cells with long radial processes in the dorsal and ventral poles in a P40 rat. G, Nestin+ cells maintained their cell bodies located at various distances from the CC lumen (arrowheads). H, 3CB2 immunoreactivity was observed both in the poles and lateral aspects of the ependyma. I, PCNA+ nuclei around the CC in a P40 rat. A, D-I, Confocal Z-stack projection. C, Conventional epifluorescence in a living slice.
Acknowledgments
The antibodies 40E-C developed by Dr. A. Álvarez-Buylla, 3CB2 developed by Dr. E.J. De La Rosa and rat-401 developed by S. Hockfield, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. The work described here was supported by Grant # FCE 2369 to N.M and FCE 2367 to G.G. from ANII; and Grant Number R01NS048255 from the National Institute of Neurological Disorders and Stroke to R.E.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. N.M. is a recipient of an ANII fellowship.
We thank G. Fabbiani and M.I. Rehermann for technical assistance. We thank the generous gift by Dr. J Sáez of the antibody against connexin 43; Dr. W. Stallcup of antibodies against NG2 and PDGFRα and Dr. S. Doxsey of the antibody against pericentrin.
Footnotes
Disclosure of potential conflicts of interests:
The authors declare no conflicts of interests.
Author’s contributions:
Nicolás Marichal: conception and design, collection and assembly of data, data analysis and interpretation, financial support, manuscript writing
Gabriela García: conception and design, collection of data, data analysis and interpretation, financial support.
Milka Radmilovich: collection of data, data analysis and interpretation
Omar Trujillo-Cenóz: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Raúl E. Russo: conception and design, collection of data, data analysis and interpretation, financial support, manuscript writing
References
- 1.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 2.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 3.Hugnot JP, Franzen R. The spinal cord ependymal region: a stem cell niche in the caudal central nervous system. Front Biosci. 2011;16:1044–1059. doi: 10.2741/3734. [DOI] [PubMed] [Google Scholar]
- 4.Meletis K, Barnabé-Heider F, Carlén M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:1494–1507. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petit A, Sanders AD, Kennedy TE, et al. Adult spinal cord radial glia display a unique progenitor phenotype. PLoS One. 2011;6:e24538. doi: 10.1371/journal.pone.0024538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 7.Horner PH, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marichal N, Garcia G, Radmilovich M, et al. Enigmatic central canal contacting cells: immature neurons in “standby mode”? J Neurosci. 2009;29:10010–10024. doi: 10.1523/JNEUROSCI.6183-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mothe AJ, Tator CH. Proliferation, migration, and diferentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord inury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Ke Y, Chi L, Xu R, et al. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24:1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danilov AI, Covacu R, Moe MC, et al. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J Neurosci. 2006;23:394–400. doi: 10.1111/j.1460-9568.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 12.Shechter R, Ziv Y, Schwartz M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells. 2007;25:2277–2282. doi: 10.1634/stemcells.2006-0705. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Buylla A, Kohwi M, Nguyen TM, et al. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- 14.Briscoe J, Pierani A, Jessell TM, et al. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 15.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 16.Russo RE, Reali C, Radmilovich M, et al. Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. J Neurosci. 2008;28:3298–3309. doi: 10.1523/JNEUROSCI.5736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry PH, Diamond JM. Junction potentials, electrode standard potentials, and other problems in interpreting electrical properties in membranes. J Membr Biol. 1970;3:93–122. doi: 10.1007/BF01868010. [DOI] [PubMed] [Google Scholar]
- 18.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 20.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noctor SC, Flint AC, Weissman TA, et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Bolteus AJ, Balkin DM, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- 23.Filippov V, Kronenberg G, Pivneva T, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 24.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadarajah B, Jones AM, Evans WH, Parnavelas JG, et al. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Noctor SC, Flint AC, Weissman TA, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 28.Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaval B, Doxsey SJ. Pericentrin in cellular function and disease. J Cell Biol. 2009;188:181–190. doi: 10.1083/jcb.200908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 31.Vinay L, Brocard F, Pflieger J-F, Simeoni-Alias J, Clarac F. Perinatal development of lumbar motoneurons and their inputs in the rat. Brain Res Bull. 2000;53:635–647. doi: 10.1016/s0361-9230(00)00397-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Peters A, Palay SL, Webster HdeF. The ependyma. In: Peters A, Palay SL, Webster HdeF, editors. The fine structure of the nervous system. Neurons and their supporting cells. Oxford: Oxford UP; 1991. pp. 312–327. [Google Scholar]
- 34.Hamilton LK, Truong MK, Bednarczyk MR, et al. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–1056. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin JC, Ackema KB, Ohayon D, et al. A mesenchymal-like ZEB1(+) niche harbors dorsal radial glial fibrillary acidic protein-positive stem cells in the spinal cord. Stem Cells. 2009;27:2722–2733. doi: 10.1002/stem.226. [DOI] [PubMed] [Google Scholar]
- 36.Spassky N, Merkle FT, Flames N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto L, Götz M. Radial glial cell heterogeneity--the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Eisch AJ, Mandyam CD. Adult neurogenesis: can analysis of cell cycle proteins move us “Beyond BrdU”? Curr Pharm Biotechnol. 2007;8:147–165. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- 39.Sakakibara A, Aoki E, Hashizume Y, et al. Distribution of nestin and other stem cell-related molecules in developing and diseased human spinal cord. Pathol Int. 2007;57:358–368. doi: 10.1111/j.1440-1827.2007.02108.x. [DOI] [PubMed] [Google Scholar]
- 40.Ma DK, Ming G-l, Gage FH, Song H, et al. Neurogenic niches in the adult mammalian brain. In: Gage FH, Kempermann G, Song H, editors. Adult neurogenesis. Cold Spring Harbor, NY: Cold Spring Harbor; 2008. pp. 207–226. [Google Scholar]
- 41.Bittman K, Owens DF, Kriegstein AR, et al. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DO, Rosenheimer JL, Kalil RE. Delayed rectifier and A-type potassium channels associated with Kv 2.1 and Kv 4. 3 expression in embryonic rat neural progenitor cells. PLoS One. 2008;3:e1604. doi: 10.1371/journal.pone.0001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart RR, Zigova T, Luskin MB. Potassium currents in precursor cells isolated from the anterior subventricular zone of the neonatal rat forebrain. J Neurophysiol. 1999;81:95–102. doi: 10.1152/jn.1999.81.1.95. [DOI] [PubMed] [Google Scholar]
- 44.Schaarschmidt G, Wegner F, Schwarz SC, et al. Characterization of voltage-gated potassium channels in human neural progenitor cells. PLoS One. 2009;4:e6168. doi: 10.1371/journal.pone.0006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 46.Ghiani CA, Yuan X, Eisen AM, et al. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells. J Neurosci. 1999;19:5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Chittajallu R, Chen Y, Wang H, et al. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sontheimer H, Trotter J, Schachner M, Kettenmann H, et al. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2:1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- 50.MacFarlane SN, Sontheimer H. Modulation of Kv1. 5 currents by Src tyrosine phosphorylation: potential role in the differentiation of astrocytes. J Neurosci. 2000;20:5245–5253. doi: 10.1523/JNEUROSCI.20-14-05245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frischknecht F, Randall AD. Voltage- and ligand-gated ion channels in floor plate neuroepithelia of the rat. Neuroscience. 1998;85:1135–1149. doi: 10.1016/s0306-4522(97)00641-6. [DOI] [PubMed] [Google Scholar]
- 52.Webb SE, Moreau M, Leclerc C, Miller AL, et al. Calcium transients and neural induction in vertebrates. Cell Calcium. 2005;37:375–385. doi: 10.1016/j.ceca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 56.Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 58.Elias LA, Kriegstein AR. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008;31:243–250. doi: 10.1016/j.tins.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noctor SC, Martínez-Cerdeño V, Ivic L, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 60.Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- 61.Carlén M, Meletis K, Göritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 62.Stoeckel ME, Uhl-Bronner S, Hugel S, et al. Cerebrospinal fluid-contacting neurons in the rat spinal cord, a gamma-aminobutyric acidergic system expressing the P2X2 subunit of purinergic receptors, PSA-NCAM, GAP-43 immunoreactivities: light and electron microscopic study. J Comp Neurol. 2003;457:159–174. doi: 10.1002/cne.10565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Co-expression of 3CB2 (1) and S100β (2) was observed in some cells on the lateral aspects of the CC (1–3, arrowheads). Single confocal optical planes.
Supplementary figure 2. Cells contacting the poles of the CC have RG morphology. A, Distribution of input resistances and resting membrane potentials in midline cells. B, Typical RG recorded in the dorsal pole of the CC, with a thick apical process reaching the lumen (1, arrow), bearing finger-like protrusions (2, arrowheads) and a thinner distal fiber projecting to the pia (1, arrowhead). C, Other RG showed smooth apical and distal processes (arrows). D, The perikarya of cells within the midline located at different distances from the CC lumen (1–4). All images are confocal Z-stack projections. In D the ventral pole of the CC is upward.
Supplementary figure 3. Molecular phenotypes of recorded midline RG. A, Biocytin-filled RG (1, arrow) in the ventral pole of the CC with apical and distal thin processes (arrowheads). This cell expressed nestin (2–4, arrows). B, Some midline RG did not react to nestin. The arrowhead points to a nearby nestin+ cell. C, Midline RG were negative for PDGFRα. However, a few PDGFRα+ cells were found close to midline but without a connection with the CC (arrowhead). D, The ependyma was devoid of NG2 immunoreactivity but abundant NG2+ cells outside the ependymal region occurred (arrowheads). A, Single confocal optical planes. B, C, Confocal Z- stack projections.
Supplementary figure 4. Differences in proliferative potential between the lateral and the midline domains of the CC. A, Schematic drawing showing the limits of the regions (area shaded in green: midline region, area shaded in red: lateral aspects of the CC) in which PCNA and pH3 nuclei were counted. B, Number of PCNA+ and pH3+ cells per section analyzed in the different domains surrounding the CC (mean ± SEM).
Supplementary figure 5. The ependyma of the mature rat spinal cord. A, Cell recorded in the dorsal pole of the CC in a P15 rat with a thick apical process reaching the lumen (arrow), a round perikarya (open arrowhead) and a thinner distal process projecting to the pia (arrowhead). B, response to a series of voltage steps of the cell shown in A. C, Cell in the dorsal pole of the CC in a P20 rat with a short process contacting the CC lumen (arrow) and a thin basal process (arrowhead). D, The cell bodies of some RG were also found apposed to the CC lumen in a P21 rat. E, Cell recorded on the lateral aspect appeared dye-coupled with neighboring cells in a P20 rat. Notice that clustered cells extended until the dorsal pole of the ependyma (arrowhead). The inset shows the electrical response of the cluster of cells in the main panel. F, Immunocytochemistry for nestin shows many positive cells with long radial processes in the dorsal and ventral poles in a P40 rat. G, Nestin+ cells maintained their cell bodies located at various distances from the CC lumen (arrowheads). H, 3CB2 immunoreactivity was observed both in the poles and lateral aspects of the ependyma. I, PCNA+ nuclei around the CC in a P40 rat. A, D-I, Confocal Z-stack projection. C, Conventional epifluorescence in a living slice.