Abstract
An Ugi one-pot three-component 4-center reaction was coupled with a subsequent acid mediated cyclodehydration step to furnish a multitude of unique scaffolds having in common an embedded or attached benzimidazole and often a ring system formed through lactamization. Using combinations of tethered Ugi inputs typically via tethered acid-ketone inputs and supporting reagents containing masked internal nucleophiles, such scaffolds were produced in good to excellent yields in an operationally friendly manner.
Keywords: post-condensation, Ugi reaction, benzimidazole, lactams, one-pot
1. INTRODUCTION
The Ugi multi-component reaction (MCR) and several closely related isocyanide based MCRs1 are useful synthetic tools that are frequently applied in the drug design and development process.2 The fact that the Ugi reaction utilizes four diversity reagents (acid, aldehyde, amine and isocyanide) in one-pot3 makes it ideal for the high-throughput construction of chemical libraries.4 Indeed, a key features associated with such condensations is the process of tethering the diversity reagents in various combinations to generate new heterocyclic chemotypes.5 In this context, the most commonly and successfully used tethered combination comprises an acid and ketone/aldehyde input6 resulting in the formation of lactams of varying ring sizes which have widespread utility in disease modifying small molecules.7 While exploring the potential of Ugi post-condensations, we have previously synthesized a number of novel scaffolds: benzodiazepines,8 benzimidazoles,9 ketopiperazines,10 imidazoline-γ-lactams,11 hydantoins12 and quinazolines13 to name a few.
Herein, we report a post-condensation intramolecular-Ugi strategy that enables production of nitrogen-enriched polycyclic scaffolds endowed with benzimidazoles, lactams of various ring sizes, dihydroquinazolines and other moieties, coalesced within a single constrained molecular architecture. The range and average values of molecular weights (MW), polar surface areas (PSA) and clogP for all target molecules depicted in this article are as follows: [MW 255 to 399, av. 340], [PSA 40 to 63 Å2, av. 47Å2], [clogP 1.1 to 5.1, av. 4.0] and suggesting potential for high oral bioavailability and consequently interest from the file enhancement community. Indeed, there is a plethora of literature invoking the pharmacological relevance of benzimidazole scaffold.14 Encouragingly, many of these compounds have been accepted by the Molecular Libraries Small Molecule Repository (MLSMR) for interrogation of targets of interest nation-wide.
2. RESULTS AND DISCUSSION
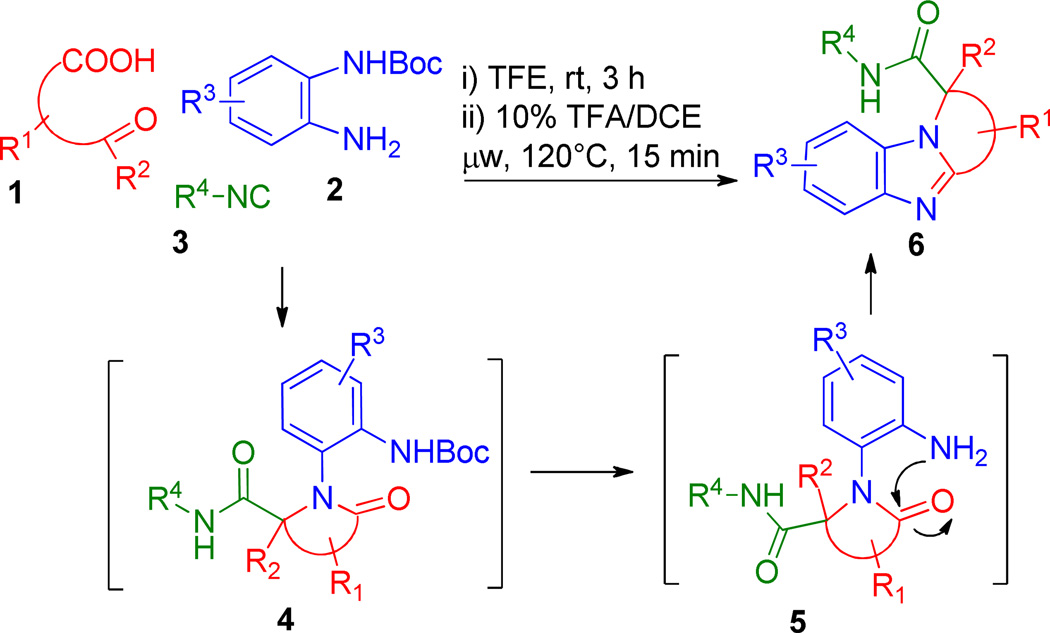
First studies were conducted employing the bi-functional reagent levulinic acid 1{1} in combination with N-Boc-1,2-phenylenediamine 2{1} and pentyl-isocyanide 3{1} using trifluoroethanol (TFE) as solvent to enhance yields of the condensation product (Scheme 1).15
Scheme 1.
Synthesis of α-quaternized benzimidazole-carboxamides
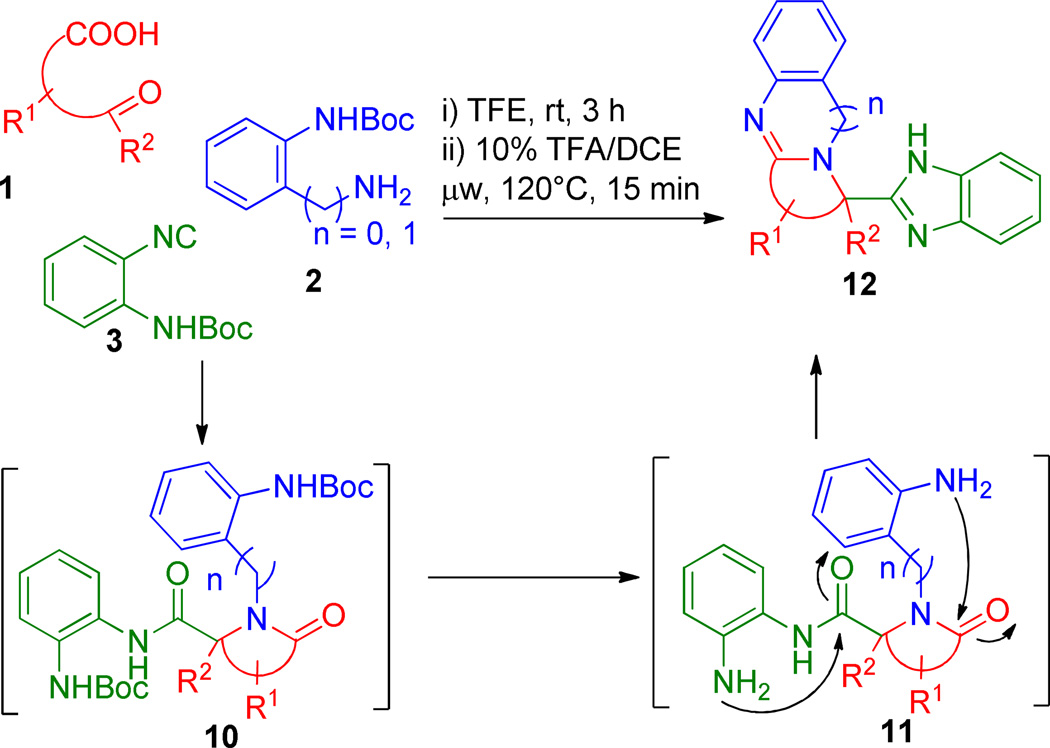
After monitoring the Ugi reaction by LCMS, the reaction was found to be complete in 3 h at room temperature affording 4{1,1,1} (72% isolated yield). The Ugi product was subsequently dissolved in a 10% solution of trifluoroacetic acid (TFA) in dichloroethane (DCE) and exposed to microwave irradiation at 120°C for 15 minutes. As such, these conditions promoted an amino-cyclodehydration to render the tricyclic scaffold 6{1,1,1} containing a valuable α-quaternary methyl group (70% isolated yield, 50 % for two steps), Scheme 1.
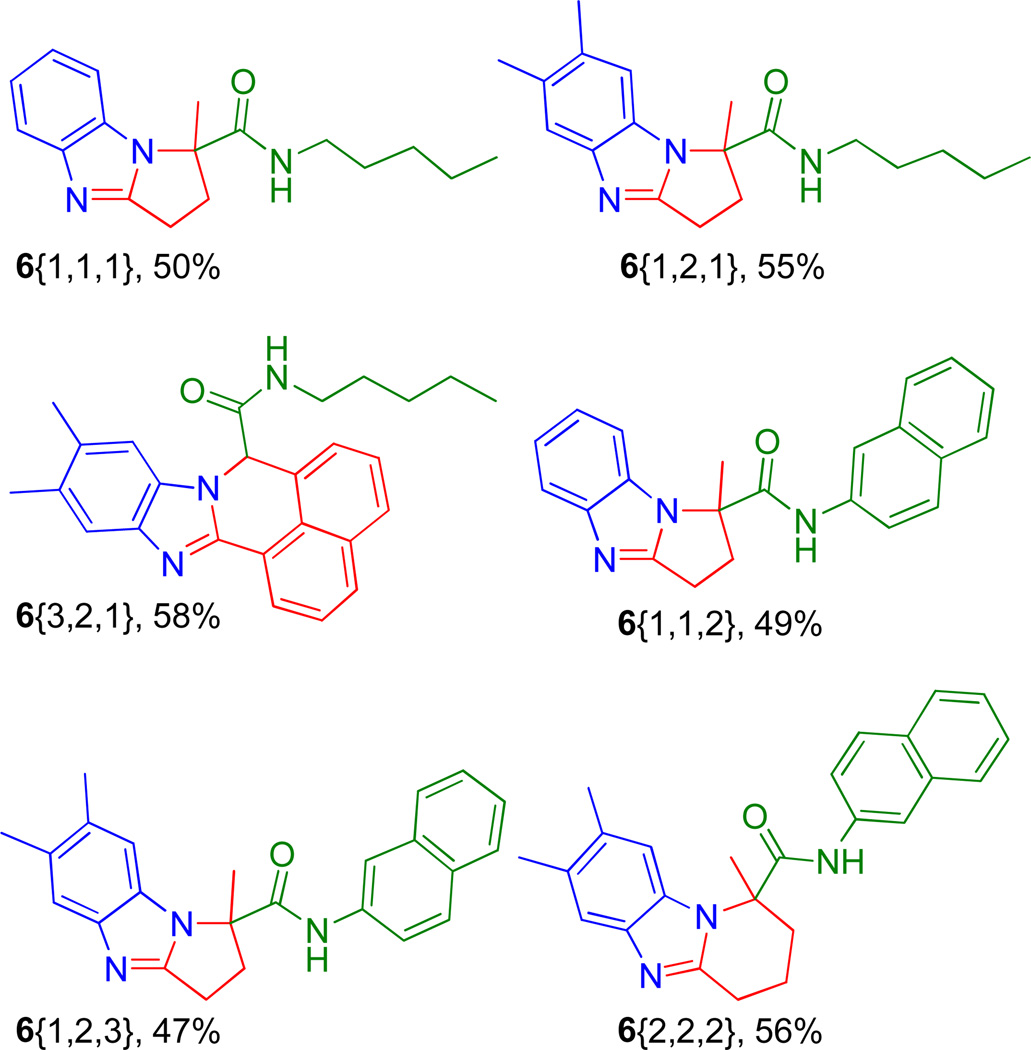
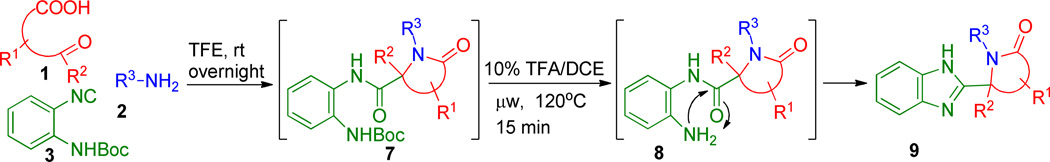
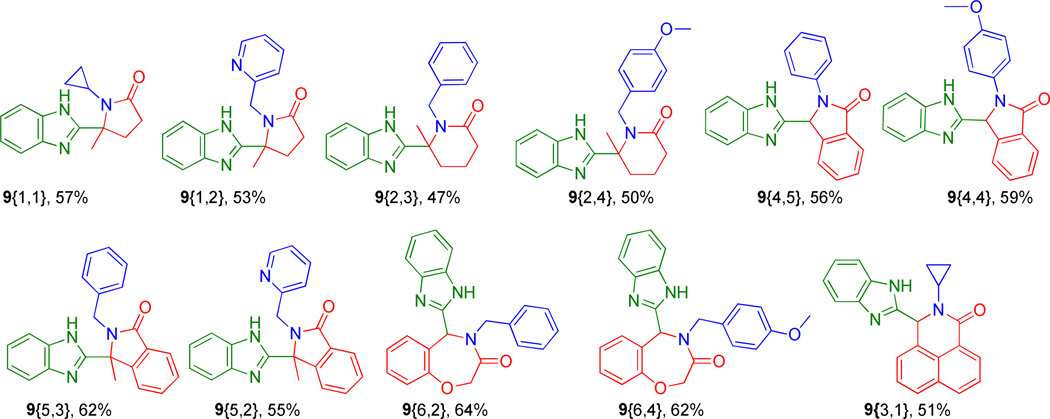
Heating the reaction mixture for shorter times or at lower temperatures resulted in only partial product formation with the amine 5{1,1,1} being the major product. With this robust protocol inhand, six congeners (6{1,1,1} through 6{2,2,2}) were produced in up to 58% yields for the two overall steps, Figure 1. The comparable yields demonstrate the generic nature of the reaction with no apparent preference for aldehyde-acids over keto-acids or selected tethers under these expedited conditions. These findings prompted us to apply the procedure to aid formation of the chemically more complex scaffolds of generic formulae 9, Scheme 2. Thus, isocyanide 3 (2-(N-Boc-amino)-phenyl-isocyanide) was prepared from N-Boc-1,2-phenylenediamine using standard methodology16 and treated with tethered acids (1{1} to 1{7}) and various amines (2{1} to 2{6}) to form the Ugi adducts of generic structure 7. Exposure of the adducts 7 to the conditions used to promote cyclization in Scheme 2, successfully afforded a range of bis-heterocyclic scaffolds 9 in overall isolated yields of up to 64% (over two steps), Figure 3.
Figure 1.
Products 6{1,1,1} through 6{2,2,2}
Scheme 2.
Generic scheme for the synthesis of polycyclic scaffolds 9
Figure 3.
Products 9{1,1} through 9{3,1}
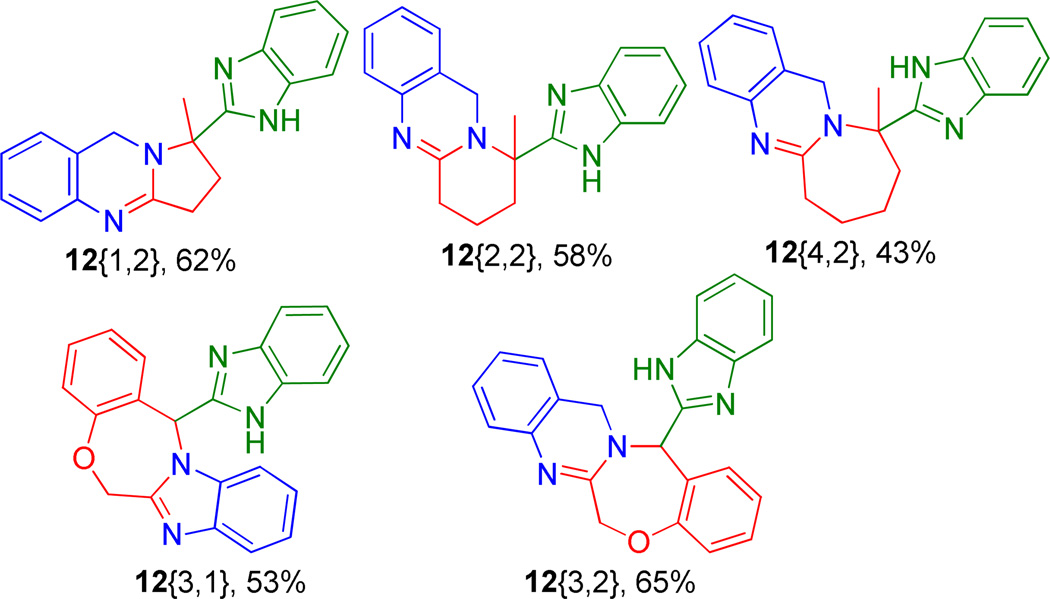
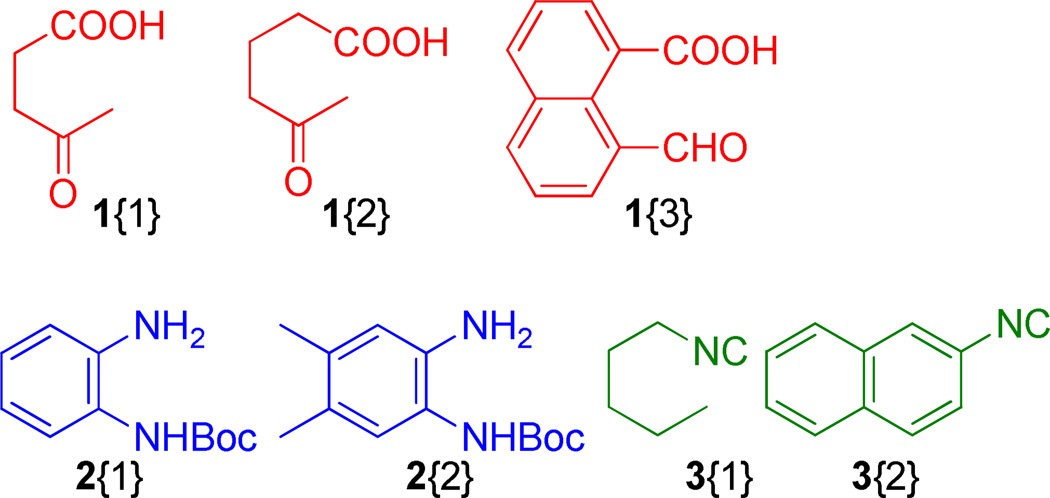
The developed process was then employed with amines carrying a second masked internal nucleophile (2{1} and 2{2}), isocyanide 3 and tethered bifunctional reagents, 1{1} through 1{4} (Scheme 3). As expected, intramolecular Ugi reactions performed well and gratifyingly subsequent acid treatment of the Ugi adducts (10) and microwave irradiation afforded products 12 in excellent yields in a mere two synthetic operations, Figure 5.
Scheme 3.
Synthesis of polycyclic scaffolds 12
Figure 5.
Products 12{1,2} through 12{3,2}
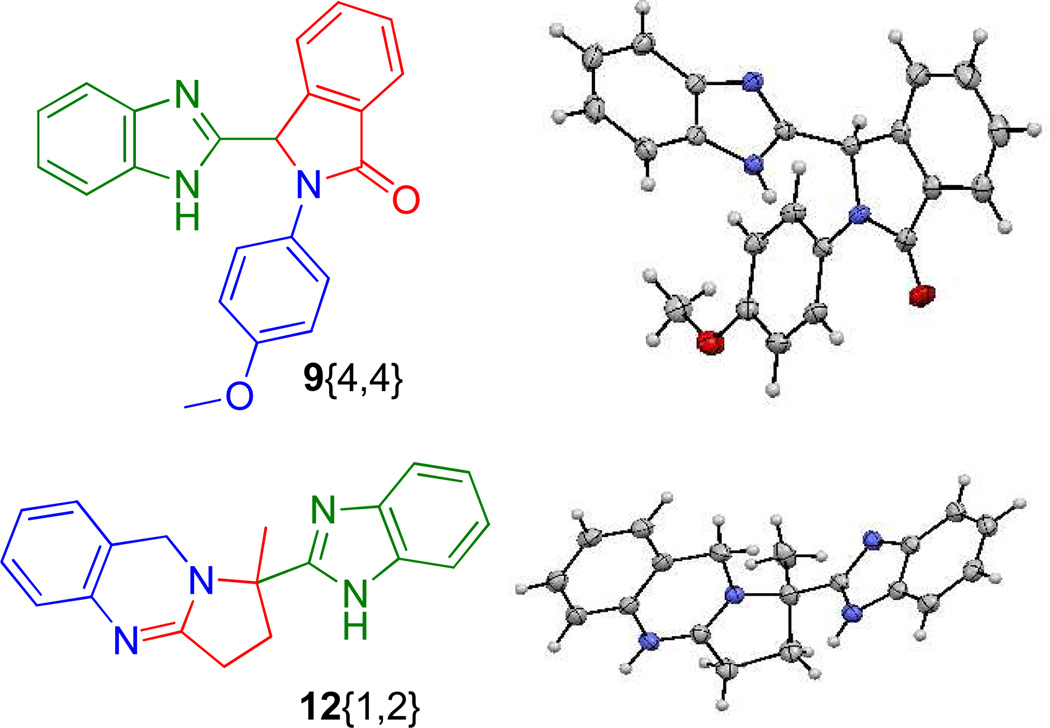
Considering the medicinal potential of quinazolines embedded with benzimidazoles, scaffolds of generic structure 12 could be particularly interesting since the calculated values for bioavailabilty criteria fall well within the ideal ranges. Definitive structural elucidation of products 9{4,4} and 12{1,2} was confirmed by X-ray crystallographic analysis, Figure 7.
Figure 7.
X-rays structures of compound 9{4,4} and 12{1,2}
3. CONCLUSIONS
A range of tethered keto-acids or aldehyde acids were successfully employed in intramolecular ring forming Ugi reactions, followed by either one or two consecutive amino-cyclodehydrations to afford a range of unique scaffolds with excellent physicochemical properties in a general one pot, two step protocol. These concise routes coupled with attractive final products represent an excellent file enhancement opportunity delivering potential libraries of high ‘skeletal diversity’ with ‘lead-like’ properties.
4. EXPERIMENTAL PROCEDURES
General procedure for the preparation of benzotetrazolodiazepinones 6
Using 8 mL microwave vial equipped with a magnetic stirring bar, a solution of 0.25 mmol of keto- or formyl acid (1) and 0.25 mmol of N-Boc-diamine (2) in 0.7 mL of trifluoroethanol (TFE) was stirred for a few minutes at room temperature followed by the addition of isocyanide (3) (0.25 mmol). The progress of the reaction was monitored using LCMS and completion of the Ugi reaction was observed after 3 h stirring. The reaction mixture was concentrated using a nitrogen flush and ~2 mL of 10% (v/v) trifluoroacetic acid (TFA) in dichloroethane (DCE) were added. The reaction was heated in a CEM microwave at 120°C for 15 minutes. After cooling the reaction mixture to room temperature, it was directly loaded on a CombiFlash Rf system (silica gel) and using a gradient of ethyl acetate/hexane (0 to 100%) followed by methanol/ethyl acetate (0 to 20%) the product 6 was purified.
General Procedure for Compounds 9
The same procedure as for compounds 6, was used for this series of compounds with the following exceptions: reactions were conducted at 0.5 mmol scale in 3 mL of solvent (TFE) and after the deprotection/cyclization stage, purification was done on a CombiFlash Rf system (silica gel) using a gradient of ethyl acetate/hexane (0 to 100%).
General Procedure for Compounds 12
Exactly the same procedure (as for 9) was employed for the preparation and purification of compounds 12.
Supplementary Material
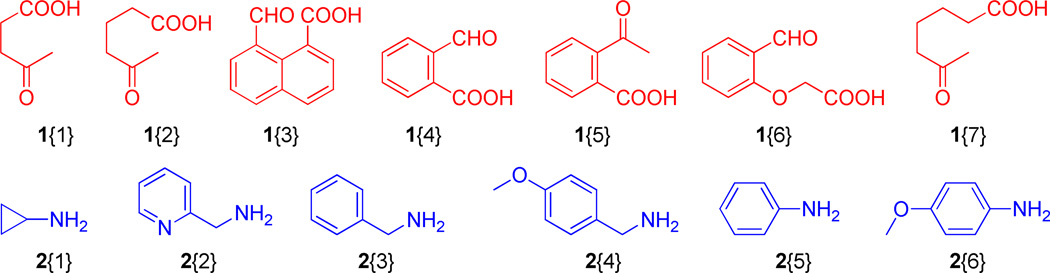
Figure 2.
Employed diversity reagents, 1, 2 and 3.
Figure 4.
Employed diversity reagents, 1 and 2
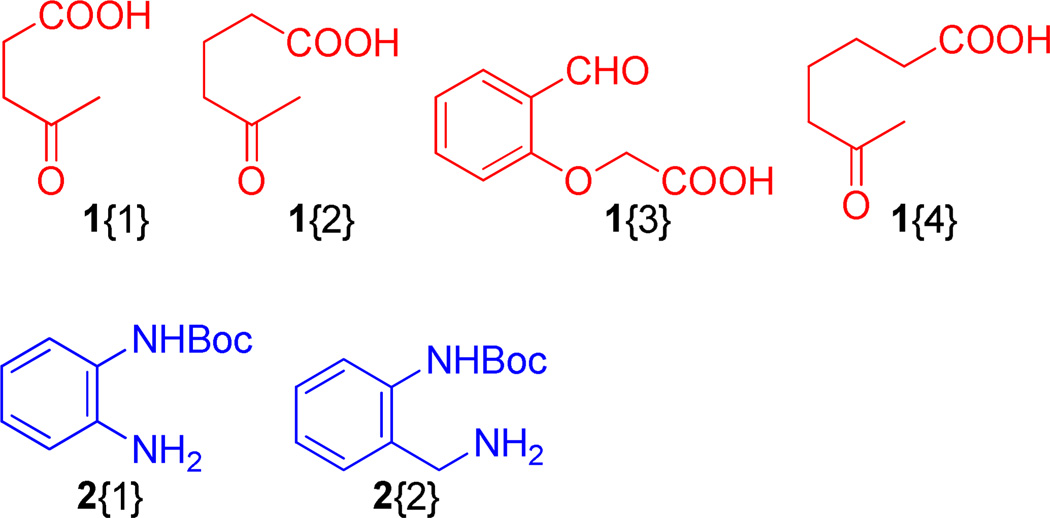
Figure 6.
Employed diversity reagents, 1 and 2
Acknowledgments
The authors are thankful to Gary S. Nichol for X-rays analysis and Nicole Schechter for proofreading.
Funding Sources
This work was supported by the National Institutes of Health (P41GM086190).
ABBREVIATIONS
- MCR
multi-component reaction
- TFE
trifluoroethanol
- TFA
trifluoroacetic acid
- MLSMR
molecular libraries small molecule repository
- BOC
tertiary butoxy carbonyl
- DCE
dichloromethane.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supporting information including all experimental procedures, CIF files for compounds 9{4,4} and 12{1,2} and characterization data for all the compounds are available free of charge via the internet at http://pubs.acs.org.
The Authors declare no competing financial interest.
REFERENCES
- 1.(a) Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; (b) Dömling A, Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (c) Hulme C, Gore V. “Multi-component reactions: emerging chemistry in drug discovery” ‘From Xylocain to Crixivan’. Curr. Med. Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]; (d) Cuny G, Bois-Choussy M, Zhu J. Palladium- and copper-catalyzed synthesis of medium- and large-sized ring-fused dihydroazaphenanthrenes and 1,4-benzodiazepine-2,5-diones. Control of reaction pathway by metal-switching. J. Am. Chem. Soc. 2004;126:14475–14484. doi: 10.1021/ja047472o. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dolle RE, La Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2008. J. Comb. Chem. 2009;11:739–190. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]; (b) Breslin JH, Miskowski AT, Rafferty MB, Coutinho VS, Palmer MJ, Wallace HN, Schneider RC, Kimball SE, Zhang S, Li J, Colburn WR, Stone JD, Martinez PR, He W. Rationale, design, and synthesis of novel phenyl imidazoles as opioid receptor agonists for gastrointestinal disorders. J. Med. Chem. 2004;47:5009–5020. doi: 10.1021/jm030548r. [DOI] [PubMed] [Google Scholar]; (c) Huang Y, Wolf S, Bista M, Meireles L, Camacho C, Holak AT. Dömling, A1,4-Thienodiazepine-2,5-diones via MCR(I): synthesis, virtual space and p53-Mdm2 activity. Chem. Biol. Drug Des. 2010;76:116–129. doi: 10.1111/j.1747-0285.2010.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Ugi I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. 1962;1:8–21. [Google Scholar]; (b) Kappe CO, Dallinger D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006;5:51–63. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]; (c) Basso A, Banfi L, Guanti G, Riva R. One-pot synthesis of α-acyloxyaminoamides via nitrones as imine surrogates in the Ugi MCR. Tetrahedron Lett. 2005;46:8003–8006. [Google Scholar]; (d) Mossetti R, Pirali T, Seggiorato D, Tron GC. Imides: forgotten players in the Ugi reaction. One-pot multicomponent synthesis of quinazolinones. Chem. Commun. 2011;47:6966–6968. doi: 10.1039/c1cc12067k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hatanaka M, Nitta H, Ishimaru T. The synthesis of the 1-carbopenem antibiotic (±)-ps-5 and its 6-epi analogue. Tetrahedron Lett. 1984;25:2387–2390. [Google Scholar]; (b) Pirrung M, Sarma KD. Beta-Lactam Synthesis by Ugi Reaction of beta-Ketoacids in Aqueous Solution. SynLett. 2004;8:1425–1427. [Google Scholar]; (c) Basso A, Banfi L, Riva R, Guanti G. A Novel Highly Selective Chiral Auxiliary for the Asymmetric Synthesis of L- and D-α-Amino Acid Derivatives via a Multicomponent Ugi Reaction. J. Org. Chem. 2005;70:575–579. doi: 10.1021/jo048389m. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mironov MA, Ivantsova MN, Mokrushin VS. Ugi reaction in aqueous solutions: a simple protocol for libraries production. Mol. Divers. 2003;6:193–197. doi: 10.1023/b:modi.0000006758.61294.57. [DOI] [PubMed] [Google Scholar]; (b) Basso A, Banfi L, Riva R, Guanti G. U-4C-3CR versus U-5C-4CR and stereochemical outcomes using suitable bicyclic β-amino acid derivatives as bifunctional components in the Ugi reaction. Tetrahedron Lett. 2004;45:587–590. [Google Scholar]; (c) Hanusch-Kompa C, Ugi I. Multi-component reactions 13: Synthesis of γ-lactams as part of a multiring system via Ugi-4-centre-3-component reaction. Tetrahedron Lett. 1998;39:2725–2728. [Google Scholar]
- 6.(a) Zhang J, Jacobson A, Rusche JR, Herlihy W. Unique structures generated by Ugi 3CC reactions using bifunctional starting materials containing aldehyde and carboxylic acid. J. Org. Chem. 1999;64:1074–1076. doi: 10.1021/jo982192a. [DOI] [PubMed] [Google Scholar]; (b) Krelaus R, Westermann B. Preparation of peptide-like bicyclic lactams via a sequential Ugi reaction—olefin metathesis approach. Tetrahedron Lett. 2004;45:5987–5990. [Google Scholar]; (c) Westermann B, Diedrichs N, Krelaus R, Walter A, Gedrath I. Diastereoselective Synthesis of Homologous Bicyclic Lactams - Potential Building Blocks for Peptide Mimics. Tetrahedron Lett. 2004;45:5983–5986. [Google Scholar]; (d) Ilyin AP, Trifilenkov AS, Kurashvili ID, Krasavin M, Ivachtchenko AV. One-step construction of peptidomimetic 5- carbamoyl-4-sulfonyl-2-piperazinones. J. Comb. Chem. 2005;7:360–363. doi: 10.1021/cc0500147. [DOI] [PubMed] [Google Scholar]
- 7.(a) Samanen JM, Ali FE, Barton LS, Bondinell WE, Burgess JL, Callahan JF, Calvo RR, Chen W, Chen L, Erhard K, Feuerstein G, Heys R, Hwang SM, Jakas DR, Keenan RM, Ku TW, Kwon C, Lee CP, Miller WH, Newlander KA, Nichols A, Parker M, Peishoff CE, Rhodes G, Ross S, Shu A, Simpson R, Takata D, Yellin TO, Uzsinskas I, Venslavsky JW, Yuan CK, Huffman WF. Potent, selective, orally active 3-oxo-1,4-benzodiazepine GPIIb/IIIa integrin antagonists. J. Med. Chem. 1996;39:4867–4870. doi: 10.1021/jm960558a. [DOI] [PubMed] [Google Scholar]; (b) Chibale K. Economic drug discovery and rational medicinal chemistry for tropical diseases. Pure. Appl. Chem. 2005;77:1957–1964. [Google Scholar]; (c) Musonda CC, Gut J, Rosenthal JP, Yardley V, Carvalho de Souza CR, Chibale K. Application of multicomponent reactions to antimalarial drug discovery. Part 2: New antiplasmodial and antitrypanosomal 4-aminoquinoline gamma- and delta-lactams via a 'catch and release' protocol. Bioorg. Med. Chem. 2006;14:5605–5615. doi: 10.1016/j.bmc.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Hulme C, Peng J, Tang SY, Burns CJ, Morize I, Labaudiniere R. Improved procedure for the solution phase preparation of 1,4- benzodiazepine-2,5-dione libraries via Armstrong's convertible isonitrile and the Ugi reaction. J. Org. Chem. 1998;63:8021–8023. [Google Scholar]
- 9.(a) Xu Z, Shaw AY, Dietrich J, Cappelli AP, Nichol G, Hulme C. Facile, novel two-step syntheses of benzimidazoles, bis-benzimidazoles, and bis-benzimidazole-dihydroquinoxalines. Mol. Divers. 2012;16:73–79. doi: 10.1007/s11030-011-9354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tempest P, Ma L, Thomas S, Hua Z, Kelly MG, Hulme C. Two-step solution-phase synthesis of novel benzimidazoles utilizing a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2001;42:4959–4962. [Google Scholar]; (c) Hulme C, Ma L, Romano J, Morrissette M. Remarkable 3-step-1-pot Solution Phase Synthesis of Imidazolines via a UDC Strategy. Tetrahedron Lett. 1999;40:7925–7928. [Google Scholar]
- 10.(a) Hulme C, Peng J, Louridas B, Menard P, Krolikowski P, Kumar NV. Applications of N-BOC-diamines for the solution phase synthesis of ketopiperazine libraries utilizing a Ugi/De- BOC/Cyclization (UDC) strategy. Tetrahedron Lett. 1998;39:8047–8050. [Google Scholar]; (b) Hulme C, Peng J, Morton G, Salvino J, Herpin T, Labaudiniere R. Novel safety-catch linker and its application with a Ugi/De-BOC/Cyclization (UDC) strategy to access carboxylic acids, 1,4-benzodiazepines, diketopiperazines, ketopiperazines and dihydroquinoxalinones. Tetrahedron Lett. 1998;39:7227–7230. [Google Scholar]
- 11.Hulme C, Ma L, Cherrier MP, Romano JJ, Morton G, Duquenne C, Salvino J, Labaudiniere R. Novel applications of convertible isonitriles for the synthesis of mono and bicyclic g-lactams via a UDC strategy. Tetrahedron Lett. 2000;41:1883–1887. [Google Scholar]
- 12.Hulme C, Ma L, Romano JJ, Morton G, Tang SY, Cherrier MP, Choi S, Salvino J, Labaudiniere R. Novel applications of carbon dioxide/MeOH for the synthesis of hydantoins and cyclic ureas via the Ugi reaction. Tetrahedron Lett. 2000;41:1889–1893. [Google Scholar]
- 13.Dietrich J, Kaiser C, Meurice N, Hulme C. Concise two-step solution phase syntheses of four novel dihydroquinazoline scaffolds. Tetrahedron Lett. 2010;51:3951–3955. doi: 10.1016/j.tetlet.2010.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Wenlock MC, Austin RP, Barton P, Davis AM, Leeson PD. A comparison of physicochemical property profiles of development and marketed oral drugs. J. Med. Chem. 2003;46:1250–1256. doi: 10.1021/jm021053p. [DOI] [PubMed] [Google Scholar]; (b) Velík J, Baliharová V, Fink-Gremmels J, Bull S, Lamka J, Skálová L. Benzimidazole drugs and modulation of biotransformation enzymes. Res Vet Sci. 2004;76:95–108. doi: 10.1016/j.rvsc.2003.08.005. [DOI] [PubMed] [Google Scholar]; (c) Merino G, Jonker GM, Wagenaar E, Pulido MM, Molina JA, Alvarez IA, Schinkel HA. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2. Drug. Metab. Dispos. 2005;33:614–618. doi: 10.1124/dmd.104.003319. [DOI] [PubMed] [Google Scholar]
- 15.(a) Nenajdenko VG, Gulevich AV, Balenkova ES. The Ugi reaction with 2- substituted cyclic imines. Synthesis of substituted proline and homoproline derivatives. Tetrahedron. 2006;62:5922–5930. [Google Scholar]; (b) Short MK, Mjalli MMA. A solid-phase combinatorial method for the synthesis of novel 5- and 6-membered ring lactams. Tetrahedron Lett. 1997;38:359–362. [Google Scholar]
- 16.Ito Y, Ohnishi A, Ohsaki H, Murakami M. A Preparative Method for o- Diisocyanoarenes. Synthesis. 1988;9:714–715. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.