Abstract
Objective
It is unknown whether measurement site of visceral adipose tissue (VAT) influences the relationship between VAT and associated health risk in youth and if so, whether ethnic differences exist in this relationship. We examined the influence of the measurement site of VAT on the relationships between VAT and metabolic syndrome (MetS) in African-American (AA) and American-White (AW) youth.
Subjects
Healthy AA (n = 54) and AW (n = 54) children and adolescents (age: 8–18 yr; BMI: 15.3–42.5 kg/m2).
Measurements
VAT mass was derived using a series of five transverse images measured by magnetic resonance imaging, extending from 5 cm below to 15 cm above L4-L5. MetS was defined using a modified IDF criteria.
Results
In AA, VAT measure at 5 cm above L4-L5 (R2 = 0.93) was most strongly (p < 0.05) correlated with VAT mass and was a significantly (p < 0.05) stronger correlate as compared to L4-L5 (R2 = 0.84). In AW, VAT measures at 5 cm (R2 = 0.93) and 10 cm (R2 = 0.93) above L4-L5 were most strongly (p < 0.05) correlated with VAT mass; however, these were not stronger correlates as compared to L4-L5 (R2 = 0.91). In AW, all VAT measures were significantly (p < 0.05) associated with an increased odds ratio (OR) for prevalent MetS, wherein the VAT mass [OR = 5.32(1.9–15.0)] and VAT at L4-L5 [OR = 5.99(1.9–18.4)] were most strongly associated with MetS. In contrast, only VAT at 10 cm above L4-L5 [OR = 4.39 (1.1–18.1)] was significantly (p < 0.05) associated with MetS in AA.
Conclusion
In AA and AW youth, the measurement site for VAT has impact on the estimation of total VAT and the magnitude of the association with obesity-related health risks.
Keywords: adolescents, insulin resistance, magnetic resonance imaging, metabolic syndrome, race, visceral adipose tissue
A growing body of evidence suggests that visceral adipose tissue (VAT) is an independent correlate of obesity-related morbidities in children and adolescents (1–5). Currently, multiple-image protocols using computed tomography (CT) or magnetic resonance imaging (MRI) are considered the criterion methods to measure VAT. However, because of cost, accessibility, labor-intensive imaging analyses, and radiation exposure in the case of CT, the vast majority of studies employ a single image at L4-L5 (e.g., between the fourth and fifth lumbar vertebrae) as a surrogate for total VAT (6).
Recently, several studies in adults questioned whether a single image obtained at L4-L5 was the most optimal surrogate measure for total VAT and obesity-related health risks. Kuk et al. (7) reported that VAT area (cm2) in the upper abdominal region (e.g., L1-L2) is more strongly associated with the total VAT volume and metabolic syndrome (MetS) than VAT measured at L4-L5 in Caucasian men. Similarly, Shen et al. observed that VAT area (cm2) measured at 5 cm above L4-L5 in women and 10 cm above L4-L5 in men are stronger correlates of total VAT volume (8) and metabolic risk factors (9) than VAT measured at L4-L5. Currently, it is unknown whether similar variations in VAT distribution exist across the abdomen in African-American (AA) and American-White (AW) children and adolescents. Moreover, whether measurement site influences the relationship between VAT and associated health risk in youth is unknown. Therefore, we examined the relationships between abdominal AT area (cm2, a single image) at different measurement sites, abdominal AT mass (kg, a series of multiple images) and metabolic markers such as insulin resistance and MetS in healthy AA and AW children and adolescents with wide variation in adiposity.
Methods
Subjects
Subjects consisted of healthy AA (n = 54) and AW (n = 54) adolescents who participated in ongoing research studies examining ethnic differences in childhood insulin resistance, metabolism and body composition. Study inclusion required subjects to have had abdominal AT assessed by a multiple-image MRI protocol (10). The subjects varied in age (8–18 yr) and body mass index (BMI, 15.3–42.5 kg/m2). Study participants were recruited via newspaper advertisements in the greater Pittsburgh area, flyers posted in city public transportation, posters placed on campus, and the Weight Management and Wellness Center at Children’s Hospital of Pittsburgh. The investigation was approved by the University of Pittsburgh Institutional Review Board. Parental informed consent and child assent were obtained from all participants before participation. No subjects were taking medications known to affect glucose metabolism or body composition (e.g., hormone therapy). All participants underwent physical examination and routine hematological and biochemical tests at the Pediatric Clinical and Translational Research Center (PCTRC) at Children’s Hospital of Pittsburgh. Pubertal development was assessed according to Tanner criteria (breast development in females, genital development in males, and pubic hair in both) and was confirmed by measurement of plasma testosterone in males, estradiol in females, and dehydroepiandrosterone sulfate in both. Ethnic background was verified by self-identification in three generations of both sides of the parents and those of mixed ethnicity were excluded during the screening procedure.
Anthropometric measurements and total adiposity
Body weight was measured on a balanced scale to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm using a stadiometer. BMI was calculated as weight (kg) divided by the square of height (m2). Waist circumference was obtained either at the midpoint (n = 91) between the lowest rib and the iliac crest or at umbilicus (n = 17). Total body fat (%) was assessed by dual-energy X-ray absorptiometry as reported previously (11).
Measurement of abdominal adipose tissue by MRI
Abdominal AT distribution was measured with a 1.5-T (General Electric Medical Systems, Milwaukee, WI, USA; n = 81) or 3.0-T MR scanners (Siemens, Magnetom TIM Trio; n = 27) at the University of Pittsburgh Magnetic Resonance Research Center using an established protocol (10). Using L4-L5 as the point of origin, five axial images (10 mm thickness) were acquired one every 50 mm over the span of 200 mm, extending from 5 cm below to 15 cm above L4-L5 (e.g., L4-L5−5 cm, L4-L5, L4-L5+5 cm, L4-L5+10 cm, L4-L5+15 cm) as shown previously (10,12). The 1.5-T images were obtained using T1-weighted spin-echo sequence (210-ms repetition time and 17-ms echo time) with a 48 × 36 field of view and a 256 × 256 matrix, during which time the subjects were asked to hold their breath for 26-s to minimize the respiratory motion artifacts (10,12). The 3.0-T images were obtained using T1-weighted spin-echo sequence (700-ms repetition time and 5.5-ms echo time) with a 48 × 36 field of view and a 320 × 240 matrix during a 20-s breath hold. The intra-observer variability in the analyses of 1.5 and 3.0 T MR images in two subjects was 3.9% for VAT and 2.1% for ASAT.
Once acquired, the MRI data were transferred electronically to a stand-alone computer for analysis using specially designed image analysis software (Tomovision, Montreal, Canada), the procedures for which are fully described and illustrated elsewhere (10,12). The volume (cm3) of VAT and abdominal subcutaneous AT (ASAT) for each image was calculated by multiplying the VAT or ASAT tissue area (cm2) by the slice thickness (10 mm). The volume of VAT and ASAT for the space (40 mm) between two consecutive images was calculated by using a mathematical algorithm given elsewhere (13). VAT and ASAT volume were converted to mass units (kg) by multiplying the volumes by the assumed constant density for fat (0.92 kg/L) (14).
Oral glucose tolerance test
After a 10–12-h overnight fast, a 2-h oral glucose tolerance test (OGTT) (1.75 g/kg, max 75 g) was performed in overweight youth (e.g., BMI ≥ 85th percentile, 39 AA and 32 AW) (15) only. Blood samples were obtained at −15, 0, 15, 30, 60, 90 and 120 min for determination of glucose and insulin levels. Areas under the glucose and insulin curves (AUC) were determined using a trapezoid model (16). Insulin sensitivity was calculated using the whole-body insulin sensitivity index (WBISI = 10000/√ [(fasting glucose × fasting insulin) × (mean glucose× mean insulin during OGTT)]) (17), which has been shown to be a valid estimate of euglycemic clamp-derived insulin sensitivity in adults (17) and children (18).
Biochemical measurements
Plasma glucose was measured by the glucose oxidase method using an YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin was measured by radioimmunoassay (RIA) (LINCO Research Inc. St. Charles, MO) which is 100% specific for human insulin with less than 0.2% cross-reactivity with human proinsulin and no cross-reactivity with C-peptide or insulin-like growth factor. The intra-assay and inter-assay coefficients of variation are 2.9–9.4% and 5.5–8.5%, respectively. Fasting plasma lipids were determined in the Nutrition Laboratory of the University of Pittsburgh certified by the National Heart, Lung, and Blood Institute standardization program. Blood pressure (BP) was obtained during one of the overnight PCTRC admissions to participate in the clamp studies not reported here. BP was measured when the subjects were resting in the supine position with an automated sphygmomanometer every 10 min between 06:00 and 07:00 a.m. after an overnight stay at the PCTRC. The average value of seven measurements was used in the current analysis.
MetS was defined according to the criteria established by the International Diabetes Federation (IDF) (19). Subjects were classified as having MetS if they had abdominal obesity (waist circumference ≥90th percentile for those aged 10 to <16 yr; ≥94 cm for boys and ≥80 cm for girls aged ≥16 yr) and two or more of the following risk factors: (i) fasting plasma glucose ≥100 mg/dL, (ii) triglycerides ≥150 mg/dL, (iii) high-density lipoprotein (HDL) cholesterol ≤40 mg/dL for those aged 10 to <16 yr; <40 mg/dL in boys and <50 mg/dL in girls aged ≥16 yr, and (iv) systolic BP ≥130 or diastolic BP ≥85 mmHg.
Statistical analyses
Ethnic differences in the baseline characteristics were assessed using independent t -tests. Pearson’s correlations were performed to determine the interrelationships between abdominal AT at various measurements with abdominal AT volume and metabolic variables. For the metabolic variables, correlations were adjusted for age, tanner stage, sex and race. The strength of the correlations was compared by using the Hotelling method (20). The association between abdominal AT and the MetS was assessed using logistic regression. To create the most parsimonious model, age, sex and tanner stage were only included if significantly associated with the MetS. All statistical procedures were performed using SAS v9.1.
Results
The subject characteristics are shown in Table 1. Despite similar total body fat (%), waist circumference and ASAT, AA had lower (p = 0.06) VAT mass (kg) than their AW peers. In addition, AA had significantly (p < 0.05) lower cholesterol, triglyceride, low-density lipoprotein (LDL) and VLDL levels than AW.
Table 1.
Subject characteristics
| African-Americans (29 M + 25 F)
|
American-Whites (30 M + 24 F)
|
p | |||
|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | ||
| Age (yr) | 14.0 ± 0.3 | 8.3–18.1 | 12.9 ± 0.3 | 8.6–17.9 | 0.023 |
| Tanner stage, I | n = 4 | n = 17 | |||
| II–V | n = 50 | n = 37 | |||
| BMI (kg/m2) | 29.1 ± 1.0 | 15.3–42.4 | 26.2 ± 1.1 | 15.5–42.5 | 0.060 |
| BMI Percentile | 88.6 ± 19.5 | 26.9–99.8 | 87.2 ± 20.4 | 20.1–99.8 | NS |
| Body fat (%) | 34.8 ± 1.5 | 10.3–51.2 | 34.5 ± 1.3 | 12.3–49.9 | NS |
| Waist circumference (cm) | 88.8 ± 2.6 | 58.0–131.2 | 85.2 ± 2.9 | 55.5–126.3 | NS |
| VAT at L4-L5 (cm2) | 38.6 ± 4.0 | 7.9–175.5 | 47.2 ± 4.2 | 5.9–110.1 | NS |
| ASAT at L4-L5 (cm2) | 313.9 ± 27.1 | 35.1–709.6 | 269.4 ± 27.6 | 42.0–799.1 | NS |
| VAT (kg) | 0.72 ± 0.07 | 0.16–2.56 | 0.92 ± 0.09 | 0.14–2.47 | 0.064 |
| ASAT (kg) | 4.62 ± 0.41 | 0.62–10.69 | 4.07 ± 0.44 | 0.61–11.78 | NS |
| Fasting Glucose (mg/dL) | 90.5 ± 0.9 | 79.0–104.0 | 94.1 ± 0.9 | 82.0–114.0 | 0.005 |
| Fasting Insulin (μU/mL) | 19.3 ± 1.7 | 7.1–66.4 | 21.3 ± 3.2 | 4.7–155.5 | NS |
| WBISI* | 2.68 ± 0.2 | 0.67–6.02 | 2.29 ± 0.2 | 0.42–5.71 | NS |
| Systolic BP (mmHg) | 112.0 ± 1.8 | 84.6–139.4 | 108.5 ± 1.4 | 77.3–139.9 | NS |
| Diastolic BP (mmHg) | 58.5 ± 1.0 | 40.6–74.1 | 57.5 ± 0.9 | 40.6–68.0 | NS |
| Cholesterol (mg/dL) | 143.1 ± 3.9 | 79.0–254.0 | 159.7 ± 4.0 | 118.0–244.0 | 0.003 |
| Triglycerides (mg/dL) | 71.9 ± 5.1 | 30.0–255.0 | 102.8 ± 8.7 | 31.0–416.0 | 0.003 |
| HDL (mg/dL) | 46.4 ± 1.4 | 31.4–80.0 | 46.5 ± 1.7 | 27.8–83.5 | NS |
| LDL (mg/dL) | 83.0 ± 3.3 | 34.0–149.0 | 92.9 ± 3.7 | 41.9–170.6 | 0.049 |
| VLDL (mg/dL) | 14.4 ± 1.0 | 6.0–51.0 | 19.4 ± 1.3 | 6.2–51.4 | 0.003 |
AA, African-American; AW, American-White; VAT, visceral adipose tissue; ASAT, abdominal subcutaneous adipose tissue. Mean ± standard error of the mean.
WBISI, whole body insulin sensitivity index (39 AAs and 32 AWs).
Abdominal AT distribution across the abdomen
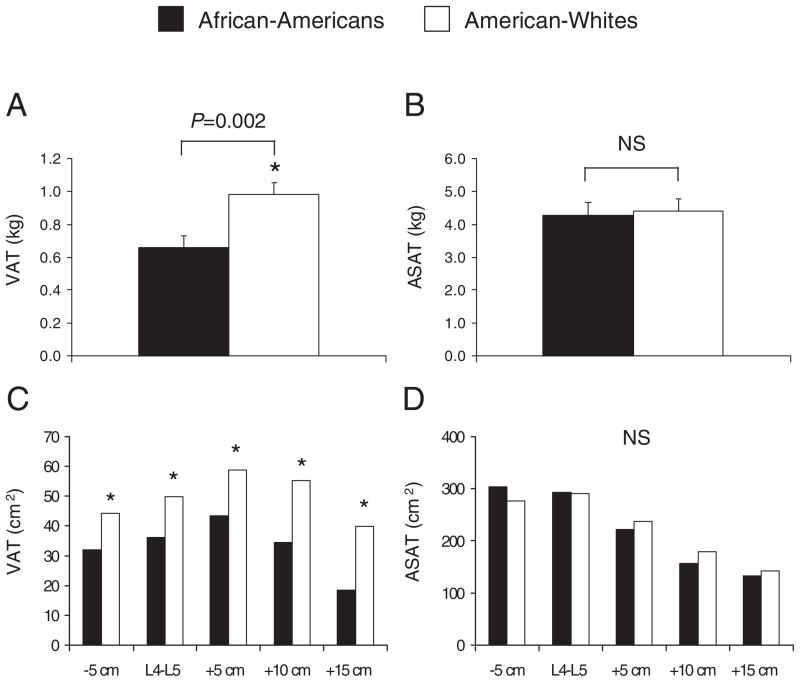
Because AA youth were older than AW youth, and age is well known to influence VAT, we show the age-adjusted VAT and ASAT values in Fig. 1. After adjusting for age, AA had less total VAT mass (Fig. 1A, 0.98 vs. 0.66 kg, p < 0.05) and VAT areas at any given landmark than AW (Fig. 1C, p < 0.05). In general, there was less VAT in the lower abdominal region (e.g., L4-L5−5 cm, L4-L5) by comparison to the mid-to upper abdominal region (e.g., L4-L5+5 cm, L4-L5+10 cm) in both AA and AW adolescents. Opposite to the pattern observed in VAT, ASAT area (cm2) was most abundant in the lower abdominal region (e.g., L4-L5−5 cm and L4-L5), and lowest in the mid- to upper abdominal region (e.g., L4-L5+5 cm to +15) independent of race (Fig. 1D). At any given measurement site, ASAT did not differ (p > 0.1) between AA and AW.
Fig. 1.
Age-adjusted VAT (visceral adipose tissue) and ASAT (abdominal subcutaneous adipose tissue) areas (cm2) across the abdomen in African-American and American-White adolescents. *p < 0.05. NS, not significant.
Relationships between regional abdominal AT area (cm2) and abdominal AT mass (kg)
The inter-relationships between abdominal AT mass and areas at different measurement sites are shown in Table 2. In both AA and AW adolescents, VAT and ASAT areas were significantly (p < 0.01) correlated with the respective AT mass. In AA, VAT measure at 5 cm above L4-L5 (R2 = 0.93) was most strongly (p < 0.05) correlated with VAT mass, and was a significantly stronger correlate as compared to L4-L5 (R2 = 0.84). In AW, VAT measures at 5 cm (R2 = 0.93) and 10 cm (R2 = 0.93) above L4-L5 were most strongly (p < 0.05) correlated with VAT mass; however, these were not significantly stronger correlates as compared to L4-L5 (R2 = 0.91).
Table 2.
Relationships between abdominal AT area (cm2) at different levels of the abdomen and abdominal AT mass (kg)
| Measurement location | VAT (kg)
|
ASAT (kg)
|
||||||
|---|---|---|---|---|---|---|---|---|
| African-Americans
|
American-Whites
|
African-Americans
|
American-Whites
|
|||||
| R2 | SEE | R2 | SEE | R2 | SEE | R2 | SEE | |
| +15 cm | 0.72a | 0.30 | 0.81a | 0.36 | 0.93a | 0.12 | 0.98a | 0.17 |
| +10 cm | 0.85b | 0.18 | 0.93b | 0.26 | 0.98b | 0.09 | 0.99b | 0.12 |
| +5 cm | 0.93c | 0.19 | 0.93b | 0.18 | 0.98b | 0.08 | 0.99b | 0.10 |
| L4-L5 | 0.84b | 0.21 | 0.91b | 0.27 | 0.96c | 0.09 | 0.99b | 0.09 |
| −5 cm | 0.60a | 0.34 | 0.76a | 0.43 | 0.93a | 0.20 | 0.94c | 0.19 |
ASAT, abdominal subcutaneous adipose tissue; SEE, standard error of the estimate; VAT, visceral adipose tissue.
All correlations are significant at p < 0.01. Significant differences in the correlation coefficients within a given column (p < 0.05), as determined using the Hotelling method, are depicted by different superscript letters.
With respect to ASAT, measures 5 and 10 cm above L4-L5 were most strongly associated with the total ASAT mass (R2 = 0.98) in AA. In AW, ASAT measures at L4-L5, 5 cm above and 10 cm above were all similarly associated with the total ASAT mass (R2 = 0.99).
Relationships between regional abdominal AT area (cm2) and mass (kg), and waist circumference
The correlations between abdominal AT area and mass, and waist circumference are shown in Table 3. Regardless of measurement site, the associations between VAT or ASAT and waist circumference were significant in both AA and AW (p < 0.05). In both AA and AW, WC was most strongly related to VAT mass, and then measures at 5 and 10 cm above L4-L5. For ASAT, waist circumference was also most strongly associated with the total ASAT mass, but was similarly correlated with all the ASAT areas.
Table 3.
Correlations (r) between abdominal AT mass (kg) and area (cm2), waist circumference and metabolic variables
| African-Americans
|
American-Whites
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kg | +15 cm | +10 cm | +5 cm | L4-L5 | −5 cm | kg | +15 cm | +10 cm | +5 cm | L4-L5 | −5 cm | |
| VAT | ||||||||||||
| Waist | 0.86a | 0.65b | 0.77b | 0.83a,c | 0.75b,c | 0.71b | 0.90a | 0.75b,d | 0.87a,c | 0.85c,d | 0.81b,c | 0.73b |
| Triglycerides | 0.27a | (0.22) | 0.27a | 0.29a | (0.17) | (0.25) | 0.31a | (0.16) | 0.33a | 0.31a | 0.29a | (0.22) |
| HDL | −0.31a | (0.25) | −0.30a | −0.32a | (0.20) | −0.33a | −0.36a | −0.28a | −0.35a | −0.32a | −0.32a | −0.32a |
| VLDL | 0.27a | (0.22) | 0.27a | 0.29a | (0.17) | (0.25) | 0.38a | 0.31a | 0.37a | 0.36a | 0.32a | 0.33a |
| Fasting insulin | 0.52a | 0.49a,b | 0.52a,b | 0.52a,b | 0.39b | 0.42a,b | 0.46a,b | 0.38a,c | 0.42a,c | 0.51a | 0.39c | 0.30c |
| Insulin AUC * | 0.36a | 0.36a | 0.44a | 0.38a | (0.17) | (0.17) | 0.42a | (0.14) | (0.28) | 0.50a | 0.55a | 0.37a |
| Insulin at 120 min* | 0.36a | 0.33a | 0.44a | 0.39a | (0.17) | (0.17) | (0.17) | (−0.10) | (0.08) | (0.24) | 0.37 | (0.11) |
| WBISI* | −0.38a | −0.38a | −0.40a | −0.39a | (−0.23) | (−0.28) | −0.72a | −0.52b | −0.57b | −0.70a,b | −0.74a,b | −0.65a,b |
| ASAT | ||||||||||||
| Waist | 0.95a | 0.94a,c,d | 0.94a,c,d | 0.93b,d | 0.93b,d | 0.91b | 0.93a,d | 0.91a,b | 0.91b | 0.91b | 0.92a | 0.89b |
| HDL | −0.36a | −0.31a | −0.34a | −0.35a | −0.36a | −0.39a | −0.32a | −0.32a | −0.34a | −0.33a | −0.31a | (−0.26) |
| Fasting insulin | 0.66a | 0.67a | 0.65a,b | 0.68a | 0.64a | 0.56b | 0.41a | 0.35b | 0.38a,b | 0.40a,b | 0.43a | 0.41a,b |
| Insulin AUC* | 0.56a | 0.52a | 0.52a | 0.56a | 0.57a | 0.49a | (0.36) | (0.25) | (0.31) | (0.32) | (0.35) | 0.44 |
| Insulin at 120 min* | 0.51a | 0.50a | 0.50a | 0.52a | 0.51a | 0.45a | (0.17) | (0.17) | (0.12) | (0.13) | (0.16) | (0.30) |
| WBISI* | −0.58a | −0.53a | −0.55a | −0.62a | −0.57a | −0.50a | −0.59a | −0.59a | −0.57a | −0.58a | −0.57a | −0.59a |
ASAT, abdominal subcutaneous adipose tissue; AUC, areas under the glucose and insulin curves; HDL, high-density lipoprotein; VAT, visceral adipose tissue; WBISI, whole-body insulin sensitivity index.
Correlations are adjusted for tanner stage and sex. Correlation coefficients shown in brackets are not significant at p > 0.05Different superscripts denote significant differences in the significant correlation coefficients as determined using the Hotelling method (p < 0.05) Metabolic variables not listed (systolic and diastolic BP, fasting glucose, glucose AUC, glucose @ 120 min cholesterol, and LDL) were generally not significantly correlated with VAT or ASAT (p > 0.01)
Overweight only (BMI ≥85th percentile, 39 AA and 32 AW).
Relationships between regional abdominal AT area (cm2) and mass (kg), and metabolic risks
The correlations between abdominal AT area and mass, and metabolic risks for AA and AW are shown in Table 3. In general, VAT measure at L4-L5 was generally more strongly associated with metabolic markers in AW as compared to AA. In AW, there were subtle differences in the association between VAT, wherein measures at L4-L5, 5 cm above and 10 cm above were comparable in their association as compared to the total mass, but there was no measure that was consistently better than any other in predicting health risk. In AA, VAT measures at 5 and 10 cm above L4-L5 were generally more strongly associated with metabolic markers as compared to L4-L5. ASAT was generally more strongly associated with metabolic markers in AA as compared to AW. There was little variation in the strength of the associations observed between ASAT and metabolic risk. Overall, the pattern of association was similar when correlation analyses were performed in overweight youth only (data not shown).
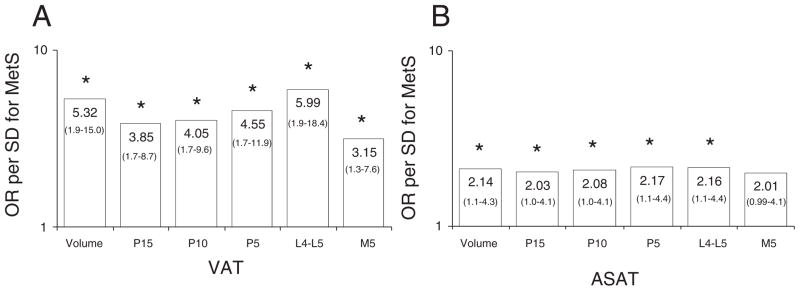
In the study sample, IDF-MetS was present in 4% (n = 2) in AA and 17% (n = 9) in AW. In AW, all VAT measures were significantly associated with an increased OR for prevalent MetS, wherein the VAT volume and VAT at L4-L5 were most strongly associated with MetS (Fig. 2A). In AA, only VAT at 10 cm above L4-L5 [OR = 4.39(1.1–18.1)] was significantly associated with MetS (data not shown). For ASAT, all measures of ASAT were associated with MetS, except 5 cm below L4-L5 in AW, wherein the strength of the association was similar across the measures (Fig. 2B). In AA, no measure of ASAT was significantly associated with MetS (data not shown).
Fig. 2.
Odds ratio (OR) and confidence interval for the prevalence of the metabolic syndrome (MetS) according to measurement location for VAT (visceral adipose tissue, Panel A) and abdominal SAT (subcutaneous adipose tissue, Panel B) in American-White adolescents on a log scale. *p < 0.05.
Discussion
The primary finding of this study was that the measurement site for VAT has significant influence on the relationships with VAT and obesity-related metabolic risk factors in youth. It appears that this observation is greater in AA than in AW youth, but there is still variability between the different landmarks in both races. In AA youth, VAT area at 5 cm above L4-L5 is a better proxy measure of total VAT than VAT area measured at the traditional L4-L5, whereas in AW youth, VAT areas at L4-L5, and 5 and 10 cm above L4-L5 were all similarly correlated with total VAT. Similarly, in AA, VAT measures in the upper abdominal region (e.g., L4-L5 +5 cm and +10 cm) had stronger associations with lipid profiles and markers of insulin resistance than VAT at L4-L5, whereas in AW, VAT measures at L4-L5, and 5 and 10 cm had similar associations with health risks. These findings suggest that the measurement site for VAT has impact on the prediction of total VAT and the magnitude of the association with health risks in AA and AW youth.
In adults, previous studies (7,9) have shown that VAT areas in the upper abdominal region are better surrogate measures of total VAT volume as compared to VAT measure at L4-L5. Kuk et al. (7) report that although all VAT areas, between L5-S1 and T10-T11, are significantly correlated with total VAT volume, VAT areas at L1-L2 and L2-L3 are better surrogates of total VAT as compared to VAT area at L4-L5 in Caucasian men. Similarly, Shen et al. (9) demonstrated in a sample of men and women with mixed ethnicity that 10 cm above L4-L5 in men, and 5 cm above L4-L5 in women is the most optimal surrogate for total VAT after adjusting for confounding factors (e.g., age, race, menopause status). In the present study, we demonstrate that the strongest correlation between VAT areas and total VAT was observed at 5 cm above L4-L5 in AA youth, while L4-L5, and 5 and 10 cm above L4-L5 were all similarly correlated with total VAT in AW youth. In agreement with a previous observation in adults (7), the relationships between ASAT areas and total ASAT were generally similar throughout the abdomen in both AA and AW youth.
In this study, we found ethnic differences in the relationship between VAT areas at different locations and health risk factors. In AA youth, VAT measures at 5 cm and 10 cm above L4-L5 were more likely to be significantly correlated with lipids and markers of insulin resistance than VAT at L4-L5. In AW, VAT areas at 5 cm or 10 cm above L4-L5 were similarly associated with health outcomes compared to VAT measure at L4-L5. These findings suggest that the measurement sites that are most predictive of total VAT are also most strongly associated with metabolic risk factors in both AA and AW youth.
Our finding that, regardless of measurement site, AA have significantly lower levels of VAT than their AW peers extends the previous findings in the pediatric body composition studies (21–23) and is similar to a previous finding in adults (24). In a large sample of AA and AW adults (n = 820), Demerath et al. (24) reported significantly higher VAT in white men as compared to black men, independent of measurement site. In this study (24), significant gender-specific patterning of VAT was observed such that men tend to carry more VAT in the middle to upper abdomen (e.g., 5 cm or 10 cm above L4-L5), whereas women tend to deposit more VAT in the lower abdomen (e.g., L4-L5). Because of a small sample size, we were unable to examine the influence of gender in the VAT patterning and its relationships with obesity-related health risks in our study.
In agreement with observations in Caucasian adults (7), all VAT measures, independent of measurement site, were significantly associated with MetS in AW youth. Further, VAT was more strongly associated with MetS as compared to ASAT in AW youth. However, the lack of relationships between VAT and MetS in AA youth in our study may be because of the small number of AA who presented with MetS (n = 2). It appears that the manifestation of MetS differed between AA and AW. In our study, the prevalence of hypertriglyceridemia was lower in AAs (4.6%) as compared to AWs (14.8%), whereas hypertension was more common in AAs (6.5%) as compared to AWs (1.9%) despite comparable levels of abdominal obesity. Currently, whether race-specific cut-offs would improve the clinical utility of MetS is unclear and warrants further investigation.
Limitations of this study warrant mention. This sample was a convenience sample that may not be representative of youth from these ethnic groups. In this study, we did not employ contiguous MRI scans to assess abdominal AT distribution, but used a well-accepted abdominal MRI protocol to quantify VAT area and volume (10). Consequently, we are unable to examine these associations using specific anatomical landmarks (e.g., L2-L3, L3-L4). Further, the relatively low number of AA youth with MetS may have limited our ability to observe the true influence of measurement site on the association with risk. However, that we observed a similar pattern with the other risk factors lends support to our observations. The difference in waist circumference measurement site is an additional limitation. Lastly, because of the small number of participants we were unable to study the effect of gender and age on the relationship between VAT measurement sites and metabolic risk.
In conclusion, the measurement site for VAT has significant influence on the relationships with total VAT and obesity-related metabolic risk factors in AA and AW. In AA, VAT measures in the upper abdominal region (e.g., L4-L5 +5 cm and +10 cm) were more strongly associated with lipid profiles and markers of insulin resistance than VAT at L4-L5. In AW, VAT measures at L4-L5, and 5 and 10 cm displayed similar correlations with health risk.
Our findings suggest that future studies need to consider the influence of measurement site of abdominal AT when assessing abdominal obesity and associated health risk in the pediatric age group.
Acknowledgments
This research was funded by grants 7-08-JF-27 (SL, American Diabetes Association Junior Faculty Award), R01-HD-27503 (SA), K24-HD-01357 (SA) and UL1 RR024153 CTSA (SA). The authors express their gratitude to the study participants, and to Nancy Guerra, Sabrina Kadri, Allison Prince, Resa Stauffer, Katie McDowell and PCTRC staff for their assistance.
References
- 1.Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007;30:2091–2097. doi: 10.2337/dc07-0203. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatrics. 2006;149:809–816. doi: 10.1016/j.jpeds.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goran MI, Lane C, Toledo-Corral C, Weigens-berg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med. 2008;162:453–461. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Janssen I. Human Body Composition. 2. Champaign: Human Kinetics; 2004. Computed tomography and magnetic resonance imaging. [Google Scholar]
- 7.Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen W, Punyanitya M, Chen J, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (Lond) 2007;31:763–769. doi: 10.1038/sj.ijo.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445–2455. doi: 10.1152/jappl.1996.81.6.2445. [DOI] [PubMed] [Google Scholar]
- 11.Danadian K, Balasekaran G, Lewy V, Meza MP, Robertson R, Arslanian SA. Insulin sensitivity in African-American children with and without family history of type 2 diabetes. Diabetes Care. 1999;22:1325–1329. doi: 10.2337/diacare.22.8.1325. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol. 2004;97:948–954. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- 13.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection. Report of the Task Group on Reference Man: a Report. Oxford, UK: Pergamon; 1975. Task Group on Reference Man. [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 16.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 19.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 20.Hotelling H. The selection of variants for use in prediction with some comments on the general problem of nuisance parameters. Ann Math Stat. 1940;11:271–283. [Google Scholar]
- 21.Goran MI, Nagy TR, Treuth MS, et al. Visceral fat in white and African American prepubertal children. Am J Clin Nutr. 1997;65:1703–1708. doi: 10.1093/ajcn/65.6.1703. [DOI] [PubMed] [Google Scholar]
- 22.Owens S, Gutin B, Barbeau P, et al. Visceral adipose tissue and markers of the insulin resistance syndrome in obese black and white teenagers. Obes Res. 2000;8:287–293. doi: 10.1038/oby.2000.34. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 24.Demerath EW, Sun SS, Rogers N, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]