Abstract
Tumor associated carbohydrate antigens (TACAs) are being actively studied as targets for anti-tumor vaccine development. One serious challenge was the low immunogenecity of these antigens. Herein, we report the results on using the tobacco mosaic virus (TMV) capsid as a promising carrier of a weakly immunogenic TACA, the monomeric Tn antigen. The copper(I) catalyzed azide-alkyne cycloaddition reaction was highly efficient in covalently linking Tn onto the TMV capsid without resorting to a large excess of the Tn antigen. The location of Tn attachment turned out to be important. Tn introduced at the N terminus of TMV was immunosilent, while that attached to tyrosine 139 elicited strong immune responses. Both Tn specific IgG and IgM antibodies were generated as determined by enzyme linked immunosorbent assay and a glycan microarray screening study. The production of high titers of IgG antibodies suggested that the TMV platform contained the requisite epitopes for helper T cells and was able to induce antibody isotype switching. The antibodies exhibited strong reactivities towards Tn antigen displayed in its native environment, i.e., cancer cell surface, thus highlighting the potential of TMV as a promising TACA carrier.
Introduction
Anti-cancer vaccines and immunotherapy are attractive approaches for cancer therapy. Recent FDA approval of Provenge has lent credence to the idea that an effective immune response induced against tumor associated antigens can benefit cancer treatment. Tumor cells are often characterized with aberrant glycosylation resulting in high levels of tumor associated carbohydrate antigens (TACAs) expressed on cell surfaces (1–3). For example, while the Tn antigen (GalNAc-α-O-Ser/Thr) (4, 5) is rarely found in normal tissues, it is expressed on the surface of a variety of cancer cells including breast (6), colon (7) and prostate cancer (8). The Springer group reported that more than 90% primary invasive ductal breast cancer tissues expressed Tn and the high level of Tn correlated significantly with shortened disease-free interval and increased tumor metastasis in patients (9). Histochemical studies by Kawaguchi and coworkers showed that the atypical mucin 1 bearing the Tn antigen was involved in aggressive growth and lymphatic metastasis of primary breast cancers (10). These observations strongly suggest the key role of Tn antigen in cancer development. Thus, Tn antigens, and TACAs in general, present excellent targets for the development of carbohydrate based anti-cancer vaccines (11, 12).
One serious challenge in the development of carbohydrate based anti-cancer vaccines is that TACAs are only weakly immunogenic because they are T-cell independent B cell antigens. Upon binding with TACAs through B cell receptors, B cells can secret antibodies against TACAs. However, these antibodies are the low affinity IgM type, which typically only last for a short period of time. To elicit a long lasting immuno-memory, high affinity IgG antibodies need to be generated, which requires the co-activation of helper T (Th) cells to potentiate the maturation of B cells and to induce antibody isotype switching from IgM to IgG (13). Thus, an immunogenic carrier containing the requisite epitopes for Th cells is required for an effective TACA based vaccine construct.
Due to the importance of the Tn antigen, many innovative studies have been performed to link Tn antigens with Th epitopes for anti-cancer vaccine studies. Livingston and coworkers conjugated Tn with a highly immunogenic carrier protein, keyhole limpet hemocyanine (KLH) (14). However, the Tn-KLH conjugate failed to elicit a humoral immune response presumably due to the weak immunogenicity of Tn. To overcome this obstacle, a Tn cluster composed of three consecutive Tn antigens was synthesized and subsequently linked to KLH (14, 15). With the increasing size of Tn antigen clusters, strong antibody responses were obtained.
Instead of employing a whole protein such as KLH to introduce Th epitopes, Lo-man and coworkers used a single Th epitope derived from a poliovirus (PV) (16). They designed a fully synthetic multiple antigen glycopeptide (MAG) construct connecting the PV peptide and Tn antigen. Consistent with the results from the Livingston group, Tn trimer cluster conjugated to MAG-PV gave higher immune responses (16–18). The Andreana group demonstrated that zwitterionic polysaccharide could also provide the necessary Th help to boost the immune response to covalently linked Tn antigen (19). Other novel constructs include the self-adjuvanting multi-component assemblies from the Dumy (20, 21) and Boons groups (22), the Calix[4]arene platform (23), dendrimers (24) as well as co-conjugation of a xenoantigen such as rhamnose to boost the antibody response to Tn (25).
Besides the requirement of Th epitopes, the manner TACAs are displayed in the vaccine construct can be crucial. B cells are known to be sensitive to the display pattern of B cell antigens (26, 27). While antigens displayed randomly often weakly activate B cells, the same antigens presented in a highly organized manner can elicit potent antibody responses (28). This is attributed to the effective crosslinking of B cell receptors by the highly patterned antigens, thus inducing B cell maturation and isotype switching. The widely used carrier such as KLH is amorphous, which renders it impossible to control the display pattern of antigen. In addition, the inherent glycans on native KLH (29) may compete with the desired TACA antigen for anti-carbohydrate immune responses (30).
Plant viruses or virus like particles (VLPs) are becoming attractive platforms for displaying B cell epitopes (31, 32). VLPs often contain multiple copies of Th epitopes, which can facilitate the potent activation of Th cells. Furthermore, due to the simplicity of their genome, viruses use repetitive protein units to construct their capsids, which lead to highly organized structures on the surface. This can provide a highly ordered platform to display antigens, which in turn can potentially break B cell tolerance and generate strong immune responses (28, 33–37).
Given the aforementioned potential of VLPs, we become interested in examining a VLP, the tobacco mosaic virus (TMV), as the carrier for Tn antigen to elicit anti-Tn immune responses. The capsid of TMV is composed of 2130 copies of identical coat proteins, which self assemble into a 300 nm long rod (38). Non-infectious to humans, TMV has been utilized to present peptide antigens through genetic engineering (39). Fitchen and coworkers reported that a B-epitope peptide from murine zona pellucid inserted into TMV could break the immuno-tolerance and induce antigen specific antibodies (40, 41). Exogenous peptide antigens have been introduced to the N-terminus of the TMV coat protein (42, 43), which self-assembled into stable virion and led to a high anti-peptide immune response. These results suggest TMV contains the requisite Th epitopes for Th activation. TMV particles are stable in various harsh conditions, such as temperatures up to 60 °C, a wide range of pH values (2–10) and organic solvents (ethanol, methanol and dimethyl sulfoxide), thus allowing chemical derivatizations without affecting the integrity of the particles (44). Furthermore, kilogram quantities of TMV virion can be produced from infected leaves, which suggest the strong potential of TMV for large scale clinical studies or production.
Herein we report our results on engineering of TMV as a carrier for the monomeric Tn antigen and evaluation of the immunological effects of various TMV-Tn constructs. Our results demonstrated that the immuno-tolerance of Tn can be broken when delivered by the TMV carrier and the location of TMV modification is crucial for high immune responses.
Experimental Procedures
1. General experimental procedures and methods
All chemicals were purchased from VWR and used as received. Dialysis tubing of 300,000 molecular weight cutoff (MWCO) was purchased from Spectrum Laboratories. TEM analyses were carried out by depositing 20 μL aliquots of each sample at a concentration of 0.1 mg/mL onto 100-mesh carbon-coated copper grids for 2 min. The grids were then stained with 20 μL of 2% uranyl acetate and viewed with a Hitachi H-8000 TEM electron microscope. For MALDI-TOF MS analysis, each sample was prepared by mixing the viral sample (0.1 mg/mL, 1μL) with 9 μL of matrix solution (saturated sinapic acid in 70% acetonitrile, 0.1% trifluoroacetic acid). Using a Zip Tip, 1 μL of the mixture was spotted on a MALDI plate, air-dried and analyzed by MALDI-TOF mass spectrometry (Bruker Daltonics Ultraflex ITOF/TOF).
2. General procedures for mouse immunization
Pathogen-free C57BL/6 female mice age 6–10 weeks were obtained from Charles River and maintained in the University Laboratory Animal Resources facility of Michigan State University. All animal care procedures and experimental protocols have been approved by the Institutional Animal Care and Use Committee of Michigan State University. Groups of five C57BL/6 mice were injected subcutaneously under the scruff on day 0 with various TMV constructs as emulsions in complete Freund’s adjuvant (0.1 mL, Fisher) or TiterMax Gold adjuvant (Sigma-Aldrich) for the TMV-Tyr-Tn studies. Boosters were given subcutaneously under the scruff on days 14 and 28 with the constructs as emulsions in incomplete Freund’s adjuvant (0.1 mL) or TiterMax Gold adjuvant. Blood (~ 0.2 mL/mouse) was collected from both groups of mice on days 0, 7 and 35. Sera from each group of mice were isolated and pooled. Human lymphoma Jurkat cells (kindly provided by Profs. Barbara Kaplan and Norbert Kaminski, Michigan State University) were cultured in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, minimal essential medium nonessential amino acid, 100 units/mL each of penicillin G and streptomycin (all from Invitrogen).
3. General procedures for ELISA and competitive ELISA
A 96-well microtiter plate was first coated with a solution of Neutravidin in PBS buffer (8 μg/mL) and then incubated overnight at 4 °C. The plate was then washed four times with PBS/0.5% Tween-20 (PBST), followed by the addition of 1% (w/v) BSA in PBS to each well and incubation at room temperature for one hour. The plate was washed again with PBST and a solution of biotinylated Tn 2 (5 μg/mL) in 0.1% BSA in PBS was added. The plate was incubated for one hour at 37 °C. The plate was then washed and mouse sera were added in a 1:3 serial dilution in 0.1% BSA/PBS. The plate was incubated for two hours at 37 °C and washed. A 1:2000 diluted horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG+IgM, IgG or IgM antibody (Jackson ImmunoResearch Laboratory IgG+IgM catalog #115-035-068, IgM #115-035-075, IgG #115-035-071) in 0.1% BSA/PBS was added to each well respectively. The plate was incubated for one hour at 37 °C, washed and a solution of 3,3′,5,5′-tetramethylbenzidine (TMB) was added. Color was allowed to develop for 20 minutes and then a solution of 0.5 M H2SO4 was added to quench the reaction. The optical density was then measured at 450 nm. Each experiment was repeated at least four times and the average of the quadruplicate was used to calculate the titer. Errors of each measurement were typically within 10%. Antibody titers were defined as the maximum folds of dilution resulting in 0.1 OD unit higher than the average absorbance after greater than one hundred thousand folds of dilution.
For competitive ELISA, the same procedure was adapted with the only exception that 0.1 M GalNAc was added to the serum prior to addition of the mouse sera to the ELISA plate.
Results and Discussion
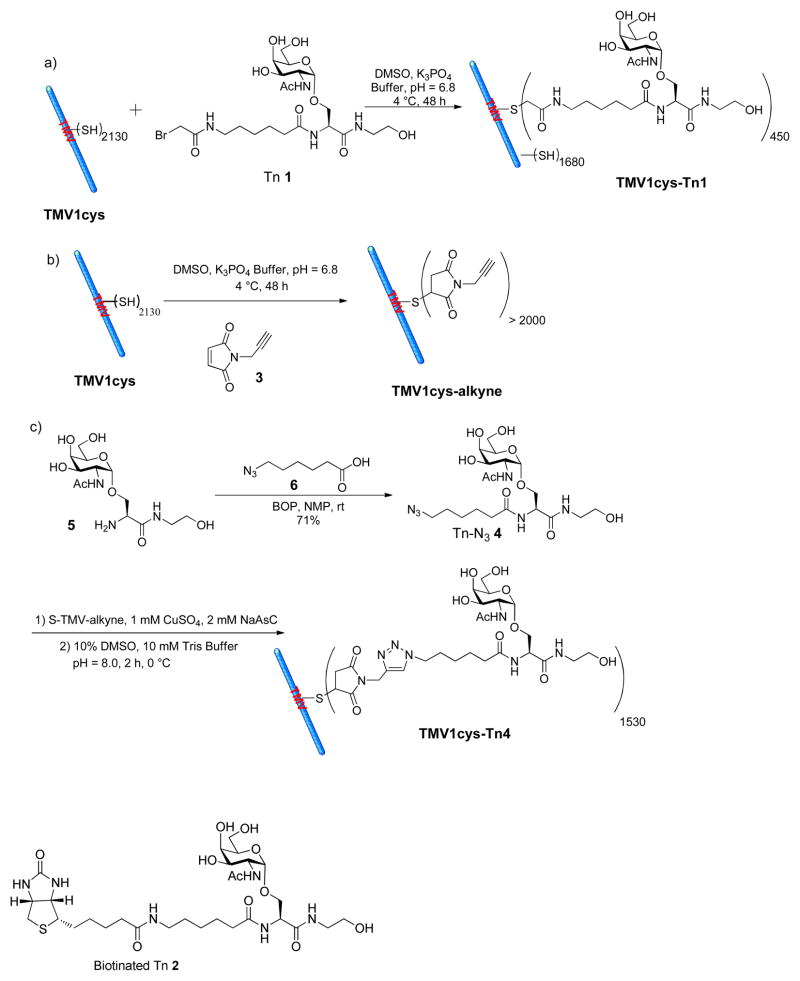
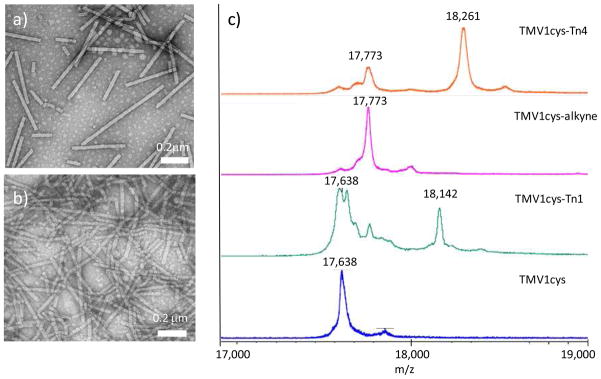
As the Tn antigen cannot be directly inserted into the TMV capsid, a reactive functional group needs to be available on TMV for bioconjugation. Although there are no lysine or cysteine residues exposed on the exterior surface of native TMV (39), X-ray structure of TMV revealed that the N-terminus, the C-terminus, and a loop between amino acid residues 55 and 60 of TMV were surface exposed (45). Through mutagenesis, surface accessible cysteine or lysine residues have been introduced to the N-terminus of TMV coat protein to produce stable virions, which could be subsequently functionalized (39, 44, 46–48). In our study, a cysteine mutant of TMV (TMV1cys) (46, 48), which contains a cysteine residue inserted between Ser 2 and Tyr 3 of the TMV coat protein, was used for the conjugation with the Tn antigen. The bromoacetamide Tn derivative 1 (Tn 1) (34) was conjugated to TMV1cys through a SN2 displacement reaction with the Cys 3 residue (Scheme 1a). Due to the low reactivity of bromoacetamide towards thiols, an excess of Tn 1 (200 eq. per subunit) was utilized. The resulting TMV1cys-Tn1 was analyzed by transmission electron microscopy (TEM), which verified that the capsid remained intact (Figure 1a). MALDI-TOF MS analysis of TMV1cys-Tn1 showed a 1:4 ion intensity ratio of the peaks corresponding to the monomeric units of TMV1cys-Tn1 and TMV1cys (Figure 1b). Assuming similar ionization efficiencies of the Tn functionalized and unmodified monomers of coat proteins, we concluded that 20% of the capsid subunits were derivatized with Tn 1. This suggested approximately 450 Tn antigens were coupled onto TMV1cys out of the 2130 possible Cys 3 sites.
Scheme 1.
Synthesis of a) TMV1cys-Tn1, b) TMV1cys-alkyne, and c) TMV1cys-Tn4.
Figure 1.
TEM images of a) TMV1cys-Tn1 and b) TMV1cys-Tn4 confirming that TMV was still intact after Tn conjugation. c) MALDI-TOF MS spectra of the monomer units of TMV1cys, TMV1cys-Tn1, TMV1cys-alkyne, and TMV1cys-Tn4. The ion intensity ratio of the peaks corresponding to Tn functionalized monomer of TMV1cys-Tn1 (m/z = 18,142) and the underivatized monomer (m/z = 17,638) was 1: 4, suggesting approximately 450 of the possible 2330 Cys 3 sites were derivatized. Analogously, it was determined that on average 1530 Tn antigens were attached on each TMV1cys-Tn4 capsid.
With TMV1cys-Tn1 in hand, immunological evaluations were carried out in C57BL/6 mice. Each mouse received a subcutaneous injection of TMV1cys-Tn1 (equivalent to 4 μg of Tn) together with the complete Freund’s adjuvant. On days 14 and 28 after the initial injection, the same amounts of TMV1cys-Tn1 in the presence of incomplete Freund’s adjuvant were given to the mice to boost the immune responses. Mice in the control group underwent the identical procedure except that native TMV was used to replace TMV1cys-Tn1. On day 35, blood was collected from each mouse and anti-Tn antibody titers from the sera were analyzed by Enzyme-Linked Immunosorbent Assay (ELISA) against biotinated Tn 2 immobilized on the ELISA plate. Negligible amounts of IgG or IgM antibodies were found in all sera at a 1600-fold serum dilution (denoted as 1/1600 dilution). As only about 20% of the TMV subunits in TMV1cys-Tn1 were functionalized with Tn, it was possible that the lack of immune responses resulted from the low Tn density on TMV1cys. Since the reactivity of bromoacetamide towards thiol was low, it was difficult to improve the Tn loading level by increasing the amount of Tn 1 for bioconjugation. This led us to examine alternative bioconjugation chemistry.
In the past decade, the copper(I) catalyzed azide-alkyne cycloaddition reaction (CuAAC) has become a powerful technique for biomacromolecule functionalization (49, 50). The biocompatible reaction conditions, simple purification procedures, wide functional group tolerance, and high conjugation yield render the CuAAC reaction a good tool for vaccine construction.
In order to take advantage of the CuAAC reaction, an alkyne group was introduced to TMV1cys by coupling with a maleimide containing bifunctional linker 3 (Scheme 1b). Only 5 eq. of maleimide alkyne 3 was needed to generate TMV1cys-alkyne in near-quantitative yield as verified by MALDI-TOF MS (Figure 1c). The azido bearing Tn 4 was prepared in 71% yield by amidation of glycosyl amino acid 5 (34) with azido carboxylic acid 6 (Scheme 1c). Unlike the maleimide and bromoacetamide Tn derivatives that were side reaction prone (34), Tn-N3 4 was very stable and was much easier to be purified. The Tn-N3 4 was conjugated to TMV1cys-alkyne under the standard CuAAC reaction condition in the presence of CuSO4 and sodium ascorbate (NaAsC), with the structural integrity of the resulting TMV1cys-Tn4 proven by TEM (Figure 1b). The high reaction efficiencies were confirmed by MALDI-TOF MS (Figure 1c) with about 1530 Tn antigens displayed on one TMV capsid based on intensity analysis of the corresponding MS peaks. It should be noted that only 16 eq. of Tn-N3 4 per subunit protein was needed to achieve the observed 70% conjugation efficiency. This result highlights the power of CuAAC reaction for the site-directed functionalization of TMV.
The immunological properties of TMV1cys-Tn4 were evaluated in mice at two doses (1 μg and 4 μg Tn per mouse) with the Freund’s adjuvant. To our disappointment, sera from both groups showed negligible amounts of IgG or IgM antibodies at 1/1600 dilution. Although peptide antigens inserted at the N-terminus of TMV could elicit strong immune responses (43), our results suggested that even with high loading of Tn, little immune responses were generated against Tn linked close to the N- terminus of TMV. This is unlikely due to the disruption of Th cell epitope since Cys 3 was inserted close to the N-terminus (between Ser 2 and Tyr 3). Although the exact reason for the low immune response is not clear, one possible explanation is that Tn displayed at the N terminus of TMV was not recognized by B cell receptors. This consideration led us to explore other locations for Tn conjugation.
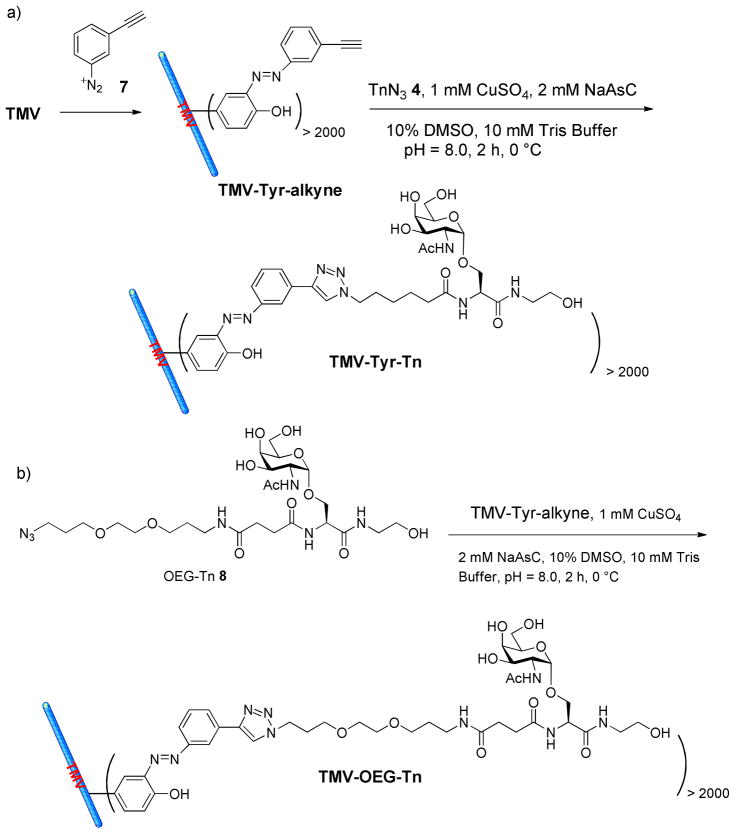
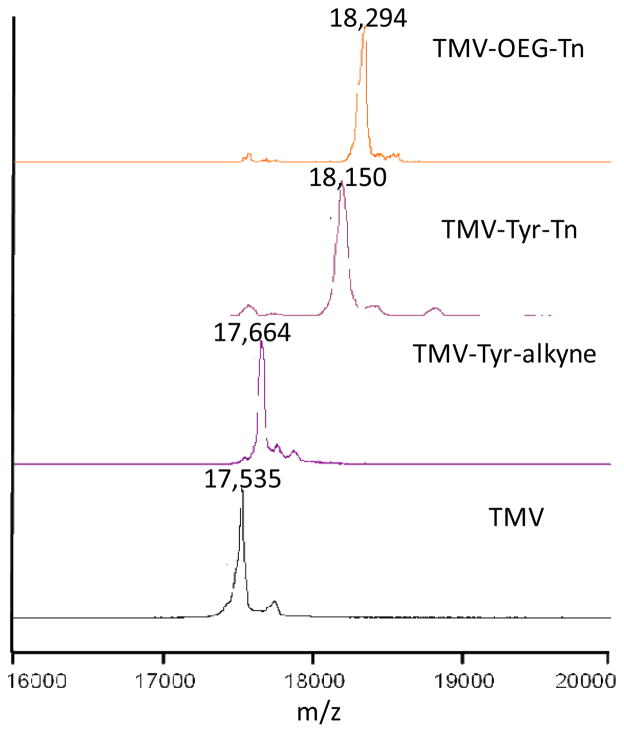
Recently, Francis and others described that Tyr 139 of TMV could be modified to install various functionalities by diazonium coupling (51, 52), and this chemistry was examined for our vaccine construction. Tyr 139 was functionalized by treatment of TMV with the diazonium alkyne 7 to give TMV-Tyr-alkyne (Scheme 2a). Close to complete modification was achieved as evident from MALDI-TOF MS analysis, with the m/z ratio of the capsid shifting completely from 17,535 to 17,664 (Figure 2). TMV-Tyr-alkyne was then coupled to Tn-N3 4 via the CuAAC reaction. Even with just 5 eq. of Tn-N3 4, more than 2000 copies of Tn antigens were successfully coupled to TMV surface as demonstrated by MALDI-TOF analysis of TMV-Tyr-Tn (Figure 2). TMV-Tyr-Tn was further characterized by TEM and UV-vis spectroscopy confirming the integrity of the virion (Figure S1).
Scheme 2.
Synthesis of a) TMV-Tyr-Tn, and b) TMV-OEG-Tn.
Figure 2.
MALDI-TOF MS spectra of the monomer units of TMV, TMV-Tyr-alkyne, TMV-Tyr-Tn, and TMV-OEG-Tn. The near complete MS shifts following each derivatization step suggested the high conversion in TMV functionalization.
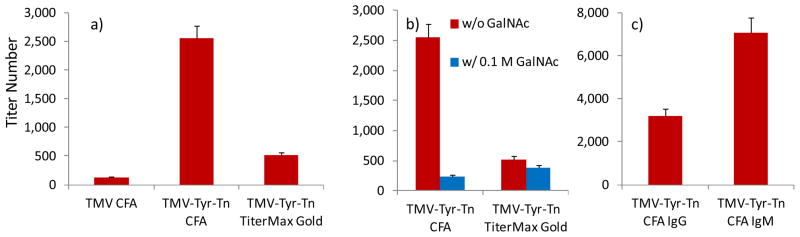
The immunological responses against the TMV-Tyr-Tn construct were evaluated in C57BL/6 mice, by administering TMV-Tyr-Tn (20 μg Tn per mouse) subcutaneously with the Freund’s adjuvant (CFA) followed by booster injections on days 14 and 28. Mice in the control group underwent the identical procedure using native TMV. Excitingly, significant amounts of anti-Tn antibodies were induced by TMV-Tyr-Tn on day 35 as compared to the control group (Figure 3a). Adjuvants can play important roles in directing immune responses. To test the effect of adjuvants, another common adjuvant TiterMax Gold was evaluated. The total antibody (IgG + IgM) titer in the Freund’s adjuvant group (titer 2,550) was five times higher than that from the TiterMax Gold group (titer 520), suggesting that the Freund’s adjuvant was superior.
Figure 3.
a) ELISA titers of anti-Tn IgG + IgM antibodies for control group TMV, and TMV-Tyr-Tn (20 μg Tn) with either CFA or TiterMax Gold adjuvant; b) ELISA titers of anti-Tn IgG + IgM antibodies generated by TMV-Tyr-Tn with either CFA or TiterMax Gold in the absence and presence of 0.1 M GalNAc; c) ELISA titers of anti-Tn IgG and IgM antibodies.
The triazole ring was believed to be immunogenic due to its aromatic and rigid structure. The unwanted anti-triazole linker response may suppress the immuno-responses against the desired epitopes. Since our ELISA was performed against Tn 2 lacking the triazole, the observed antibody titers were not against the triazole linker. Our results demonstrate that in the presence of the triazole, antibody responses could still be generated against the weak carbohydrate antigen Tn.
In order to confirm the carbohydrate recognition specificity of the antibodies, a competitive ELISA procedure was developed by adding free GalNAc to the serum prior to serum incubation with the ELISA plate. As GalNAc is part of the Tn antigen, the free GalNAc should bind with Tn specific antibodies, thus reducing the amount of antibodies available to bind with the plate. Indeed, addition of 0.1 M GalNAc for competitive ELISA significantly reduced anti-Tn antibody titers (Figure 3b). The inhibitory effect was more pronounced for the Freund’s adjuvant group than the TiterMax Gold group.
For an effective vaccine, the generation of IgG is important. Subtyping the antibodies from the Freund’s adjuvant group demonstrated that both IgG and IgM antibodies were produced with higher IgM titers than IgG (Figure 3c). The generation of IgG antibodies indicated that the TMV platform could stimulate activation of Th cells and isotype switching of antibodies. The strong humoral responses induced by TMV-Tyr-Tn as compared to the ineffective TMV1cys-Tn construct highlighted the importance of the position where the antigen was introduced (53). Tyr 139 allowed better access of the reagent to functionalize the viral capsid, which may also be more accessible for recognition by B cells.
A close examination of TMV structure revealed that Tyr 139 is located in a shallow groove on the external surface of TMV (Figure 4) (52). It is possible that the Tn antigen could be partially buried in the groove. A longer linker between Tn and TMV could potentially extend the Tn antigen out of the groove and enhance B cell recognition. To test this, Tn antigen derivative 8 with an oligoethylene oxide (OEG) linker was conjugated to TMV-Tyr-alkyne through the CuAAC reaction (Scheme 2b). MALDI-TOF confirmed that close to complete alkyne functionalization and CuAAC reaction were achieved (Figure 2).
Figure 4.
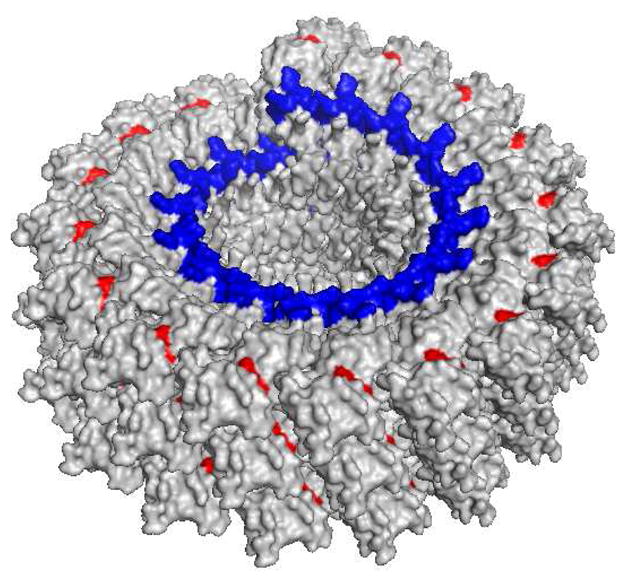
Cross section of a TMV capsid. The red colored residue is Tyr 139, residing in a shallow groove. The genomic RNA is marked in blue.
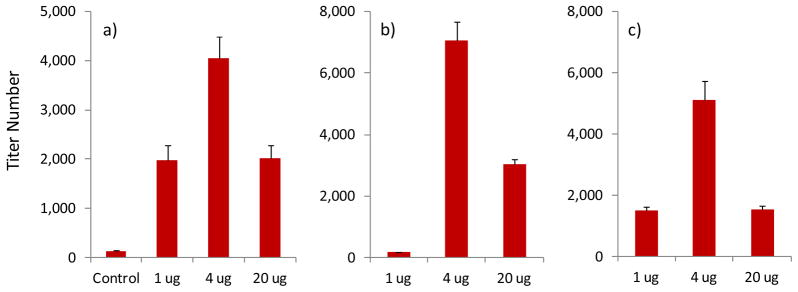
The immune responses to TMV-OEG-Tn were evaluated following the previously established prime and boost protocol with the Freund’s adjuvant. To investigate the effect of vaccine dose on immune response, three groups of five mice received vaccine constructs corresponding to 1 μg, 4 μg, and 20 μg Tn antigen respectively. As shown in Figure 5, all three groups generated significant antibodies compared to the control group immunized with TMV only. The 4 μg group led to the highest antibody titers, and high IgG titers were produced in the 4 μg and 20 μg groups (Figure 5b,c). The antibody responses to the 20 μg group were comparable to those elicited by the TMV-Tyr-Tn construct, indicating the length of the linker between Tn and TMV did not have a significant role.
Figure 5.
Anti-Tn ELISA titers of a) IgG + IgM for TMV control group and TMV-OEG-Tn groups corresponding to 1 μg, 4 μg, and 20 μg Tn respectively; b) IgG and c) IgM of TMV-OEG-Tn groups corresponding to 1 μg, 4 μg, and 20 μg Tn respectively.
To further analyze the specificity of the induced antibodies, the sera from the 4 μg and 20 μg groups were screened at a dilution of 1:100 against a carbohydrate microarray, which is a powerful high throughput method to rapidly establish carbohydrate binding specificities (54–56). The microarray used in this study contained 329 array components, including a variety of O-linked glycopeptides, N-linked glycopeptides, glycolipids, and glycoproteins. After the array was incubated with sera and the unbound antibodies were washed off the slide, serum antibody binding to individual array components was detected using fluorescently labeled secondary antibodies (54).
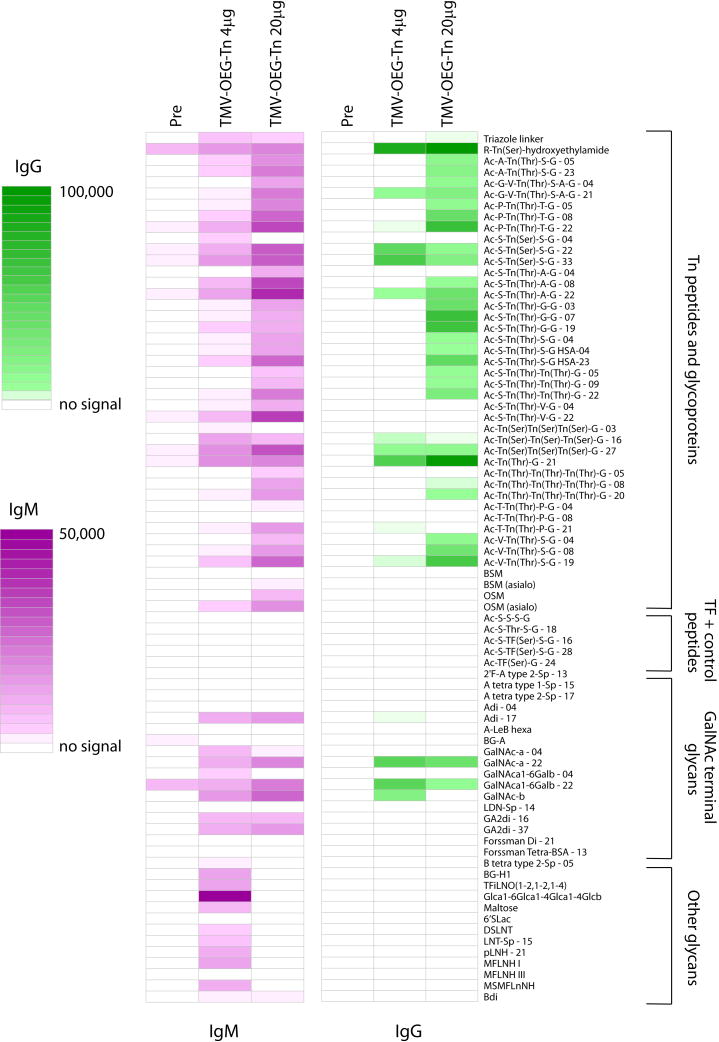
The 4 μg and 20 μg groups both produced significant IgM and IgG responses relative to the pre-vaccination sera (see Figure 6. For the full list of glyco-conjugate structures on the microarray, see Table S1 in supporting information). The induced IgM and IgG bound to a variety of Tn peptides, along with some reactivity with a subset of other GalNAc-terminal glycans. Neither IgM nor IgG reacted with non-glycosylated peptides or TF-containing peptides, which possess an internal GalNAc (for example, see compound Ac-S-TF(Ser)-S-G), demonstrating that a non-reducing terminal GalNAc is critical for recognition. In general, induced IgG antibodies had better specificity than IgM antibodies. For example, the induced IgM antibodies reacted with the triazole linker and the GA2 disaccharide (GA2di, see Table S1 for structure), while the IgG antibodies did not show any appreciable binding to either of these array components.
Figure 6.
Microarray profiles comparing pre- and post-immune sera from mice immunized with TMV-OEG-Tn. Colored boxes correspond to the average fluorescence signal from duplicate experiments. IgM data is shown in magenta, and IgG data is shown in green. Glycans are attached to albumin prior to printing on the array surface. The numbers after the glycan abbreviation correspond to the average number of glycans per molecule of albumin [for example, Ac-A-Tn(Thr)-S-G-05 is a neoglycoprotein with an average of 5 Tn peptides per molecule of albumin]. Selected array results are shown and full results can be found in supporting information.
While the overall patterns from the 4 and 20 μg groups were similar, the vaccine dose affected the antibody responses with the 20 μg group generally having higher levels of induced antibodies to various Tn antigen containing array components (Figure 6). The antibodies from the 20 μg group showed better reactivities towards Tn peptides with a GalNAc linked to a threonine. Of note, the 20 μg group also showed less cross-reactivity to glycans that are not part of a Tn peptide. While this was true for both IgM and IgG responses, the effect was more pronounced for IgMs. This is possibly because with higher antigen dose, the immune response generated was more focused towards the desired antigen.
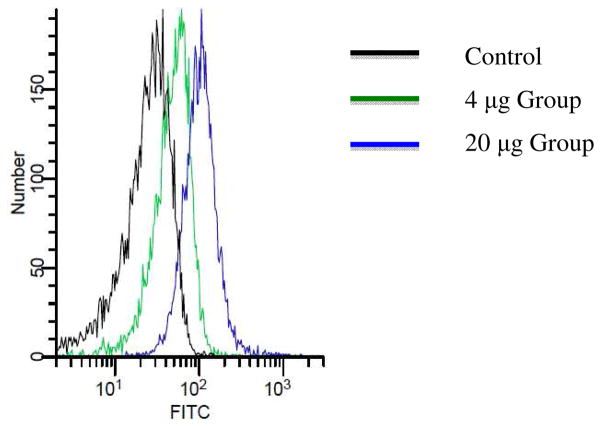
As both ELISA and microarray studies measure antibody binding against synthetic Tn antigens, it would be important to test antibody recognition of Tn antigens present in the native environment, i.e., cancer cell surface. It is known that Tn antigen is expressed on human leukemia Jurkat cells (57, 58). Upon incubation of Jurkat cells with the post-immune sera, a significant increase in binding to Jurkat cells was found (Figure 7), suggesting the antibodies were able to recognize Tn antigens displayed in its native configurations on tumor cell surface. Although the serum from the 4 μg group gave high titers of antibodies against Tn as determined by ELISA, the 20 μg group showed stronger reactivity with cancer cells presumably due to the higher affinities and/or broader reactivities towards Tn containing peptides by antibodies from the 20 μg group.
Figure 7.
Fluorescence activated cell sorting analysis of IgG antibodies present in post-immune sera binding with Jurkat cells.
In conclusion, we have demonstrated that TMV is a promising platform to deliver TACAs. The location where antigen was immobilized had great influence on the ease of viral capsid functionalization as well as outcome of the immune responses. Even though Tn is a very weakly immunogenic tumor associated carbohydrate antigen, when placed at strategic sites, strong humoral immune responses could be induced. High titers of IgG antibodies were elicited by the TMV-Tyr-Tn and TMV-OEG-Tn constructs, which recognized human tumor cells. Further studies are in progress to optimize this new platform for the development of TACA based anti-cancer vaccine.
Supplementary Material
Acknowledgments
The authors are indebted to Professor J. Culver (University of Maryland) for providing the mutant virus TMV1cys. We would like to thank the National Cancer Institute for generous financial support of our work (XH, R01CA149451-01A1), partial financial support from NSF (QW, CHE-0748690), the Alfred P. Sloan Scholarship (QW), and the Camille Dreyfus Teacher Scholar Award (QW). This work was supported in part by the intramural research program of the NIH, NCI.
Abbreviations
- CFA
Freund’s adjuvant
- CuAAC
the copper(I) catalyzed azide-alkyne cycloaddition reaction
- ELISA
enzyme-linked immunosorbent assay
- KLH
keyhole limpet hemocyanine
- MAG
multiple antigen glycopeptide
- MALDI-TOF
matrix assisted laser desorption ionization – time of flight
- MWCO
molecular weight cutoff
- NaAsC
sodium ascorbate
- PV
poliovirus
- TACAs
tumor associated carbohydrate antigens
- Th
helper T cells
- TMV
tobacco mosaic virus
- TMV1cys
a cysteine mutant of TMV
- VLPs
virus like particles
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information: Information on purification, characterization and functionalization TMV, various TMV mutants and TMV conjugates. Procedures for glycan microarray analysis and fluorescence activated cell sorting. This information is available free of charge via the Internet at http://pubs.acs.org/.
Contributor Information
Qian Wang, Email: wang263@mailbox.sc.edu.
Xuefei Huang, Email: xuefei@chemistry.msu.edu.
References
- 1.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 2.Danishefsky SJ, Allen JR. From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chem Int Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 4.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 5.Springer GF, Desai PR, Spencer BD, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced breast carcinoma. Cancer Detect Prev. 1995;19:374–380. [PubMed] [Google Scholar]
- 6.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010;12:204–216. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 8.Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther. 2009;8:971–979. doi: 10.1158/1535-7163.MCT-08-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang BL, Springer GF, Carlstedt SC. Quantitative computerized image analysis of Tn and T (Thomsen–Friedenreich) epitopes in prognostication of human breast carcinoma. J Histochem Cytochem. 1997;45:1393–1400. doi: 10.1177/002215549704501007. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi T, Takazawa H, Imai S, Morimoto J, Watanabe T, Kanno M, Igarashi S. Expression of vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast Cancer cells in relation to lymphatic metastasis: is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res Treat. 2006;98:31–43. doi: 10.1007/s10549-005-9115-6. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z, Huang X. Recent development in carbohydrate based anticancer vaccines. J Carbohydr Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freire T, Bay S, Vichier-Guerre S, Lo-Man R, Leclerc C. Carbohydrate antigens: synthesis aspects and immunological applications in cancer. Mini-Rev Med Chem. 2006;6:1357–1373. doi: 10.2174/138955706778992996. [DOI] [PubMed] [Google Scholar]
- 13.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. Freeman; New York: 2000. pp. 461–464. [Google Scholar]
- 14.Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ, Livingston PO. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, Scher HI. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 16.Lo-Man R, Bay S, Vichier-Guerre S, Deriaud E, Cantacuzene D, Leclerc C. A fully synthetic immunogen carrying a carcinoma-associated carbohydrate for active specific immunotherapy. Cancer Res. 1999;59:1520–1524. [PubMed] [Google Scholar]
- 17.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A Fully Synthetic Therapeutic Vaccine Candidate Targeting Carcinoma-Associated Tn Carbohydrate Antigen Induces Tumor-Specific Antibodies in Nonhuman Primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 18.Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a Tri-Tn glycotope. J Immunol. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 19.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 20.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B Cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem. 2008;3:737–741. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 22.Ingale S, Wolfert MA, Buskas T, Boons G-J. Increasing the Antigenicity of Synthetic Tumor-Associated Carbohydrate Antigens by Targeting Toll-Like Receptors. ChemBiochem. 2009;10:455–463. doi: 10.1002/cbic.200800596. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geraci C, Consoli GML, Galante E, Bousquet E, Pappalardo M, Spadaro A. Calix[4]arene decorated with four Tn antigen glycomimetic units and P3CS immunoadjuvant: synthesis, characterization, and anticancer immunological evaluation. Bioconjugate Chem. 2008;19:751–758. doi: 10.1021/bc700411w. [DOI] [PubMed] [Google Scholar]
- 24.Vepřek P, Hajdúch M, Džubák P, Kuklík R, Poláková J, Bezouška K. Comblike dendrimers containing Tn antigen modulate natural killing and induce the production of Tn specific antibodies. J Med Chem. 2006;49:6400–6407. doi: 10.1021/jm050741g. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar S, Lombardo SA, Herner DN, Talan RS, Wall KA, Sucheck SJ. Synthesis of a single molecule L-rhamnose- containing three component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies. J Am Chem Soc. 2010;132:17236–17246. doi: 10.1021/ja107029z. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 28.Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard MC, Simard S, Lecours K, Bolduc M, Pare C, Willems B, Shoukry N, Tessier P, Lacasse P, Lamarre A, Lapointe R, Lopez Macias C, Leclerc D. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: evidence for the critical function of multimerization. Virology. 2007;363:59–68. doi: 10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Geyer H, Wuhrer M, Kurokawa T, Geyer R. Characterization of keyhole limpet hemocyanin (KLH) glycans sharing a carbohydrate epitope with Schistosoma mansoni glycoconjugates. Micron. 2004;35:105–106. doi: 10.1016/j.micron.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Oyelaran O, Gildersleeve JC. Evaluation of human antibody responses to keyhole limpet hemocyanin (KLH) on a carbohydrate microarray. Proteomics - Clin Appl. 2010;4:285–294. doi: 10.1002/prca.200900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chackerian B. Virus-like particles-flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 32.Jennings GT, Bachmann MF. Designing recombinant vaccines with viral properties: a rational approach to more effective vaccines. Curr Mol Med. 2007;7:143–155. doi: 10.2174/156652407780059140. [DOI] [PubMed] [Google Scholar]
- 33.Cornuz J, Zwahlen S, Jungi Walter F, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann Martin F, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miermont A, Barnhill H, Strable E, Lu X, Wall KA, Wang Q, Finn MG, Huang X. Cowpea mosaic virus capsid: a promising carrier for the development of carbohydrate based antitumor vaccines. Chem Eur J. 2008;14:4939–4947. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBiochem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 36.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006;24:6321–31. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 37.Brennan FR, Jones TD, Hamilton WDO. Cowpea mosaic virus as a vaccine carrier of heterologous antigens. Mol Biotechnol. 2001;17:15–26. doi: 10.1385/MB:17:1:15. [DOI] [PubMed] [Google Scholar]
- 38.Culver JN. Tobacco mosaic virus assembly and disassembly: determinants in pathogenicity and resistance. Annu Rev Phytopathol. 2002;40:287–308. doi: 10.1146/annurev.phyto.40.120301.102400. [DOI] [PubMed] [Google Scholar]
- 39.McCormick AA, Palmer KE. Genetically engineered tobacco mosaic virus as nanoparticle vaccines. Expert Rev Vaccines. 2006;7:33–41. doi: 10.1586/14760584.7.1.33. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 40.Koo M, Bendahmane M, Lettieri GA, Paoletti AD, Lane TE, Fitchen JH, Buchmeier MJ, Beachy RN. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci, USA. 1999;96:7774–7779. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitchen J, Beachy RN, Hein MB. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine. 1995;13:1051–1057. doi: 10.1016/0264-410x(95)00075-c. [DOI] [PubMed] [Google Scholar]
- 42.McCormick AA, Corbo TA, Wykoff-Clary S, Nguyen LV, Smith ML, Palmer KE, Pogue GP. TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models. Vaccine. 2006;24:6414–6423. doi: 10.1016/j.vaccine.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 43.McCormick AA, Corbo TA, Wykoff-Clary S, Palmer KE, Pogue GP. Chemical conjugate TMV-peptide bivalent fusion vaccines improve cellular immunity and tumor protection. Bioconjugate Chem. 2006;17:1330–1338. doi: 10.1021/bc060124m. [DOI] [PubMed] [Google Scholar]
- 44.Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, Nguyen LV, Palmer KE. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;348:475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Namba K, Stubbs G. Structure of tobacco mosaic virus at 3.6Å resolution: implications for assembly. Science. 1986;231:1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- 46.Lewis CL, Lin Y, Yang C, Manocchi AK, Yuet KP, Doyle PS, Yi H. Microfluidic fabrication of hydrogel microparticles containing functionalized viral nanotemplates. Langmuir. 2010;26:13436–13441. doi: 10.1021/la102446n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi H, Nisar S, Lee SY, Powers MA, Bentley WE, Payne GF, Ghodssi R, Rubloff GW, Harris MT, Culver JN. Patterned assembly of genetically modified viral nanotemplates via nucleic acid hybridization. Nano Lett. 2005;5:1931–1936. doi: 10.1021/nl051254r. [DOI] [PubMed] [Google Scholar]
- 48.Lee LA, Nguyen HG, Wang Q. Altering the landscape of viruses and bionanoparticles. Org Biomol Chem. 2011;9:6189–6195. doi: 10.1039/c1ob05700f. [DOI] [PubMed] [Google Scholar]
- 49.Gupta SS, Kuzelka J, Singh P, Lewis WG, Manchester M, Finn MG. Accelerated bioorthogonal conjugation: a practical method for the ligation of diverse functional molecules to a polyvalent virus scaffold. Bioconjugate Chem. 2005;16:1572–1579. doi: 10.1021/bc050147l. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 51.Schlick TL, Ding Z, Kovacs EW, Francis MB. Dual-surface modification of the tobacco mosaic virus. J Am Chem Soc. 2005;127:3718–3723. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- 52.Bruckman MA, Kaur G, Lee A, Xie F, Sepulveda J, Breitenkamp R, Zhang X, Joralemon M, Russell TP, Emrick T, Wang Q. Surface modification of tobacco mosaic virus with “Click” chemistry. Chembiochem. 2008;9:519–523. doi: 10.1002/cbic.200700559. [DOI] [PubMed] [Google Scholar]
- 53.Schödel F, Moriarty AM, Peterson DL, Zheng JA, Hughes JL, Will H, Leturcq DJ, McGee JS, Milich DR. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Viology. 1992;66:106–114. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oyelaran O, Gildersleeve JC. Glycan arrays: recent advances and future challenges. Curr Opin Chem Biol. 2009;13:406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 56.Feizi T, Chai W. Oligosaccharide microarrays to decipher the glyco code. Nat Rev Mol Cell Biol. 2004;5:582–588. doi: 10.1038/nrm1428. [DOI] [PubMed] [Google Scholar]
- 57.Inoue M, Nakada H, Tanaka N, Yamashina I. Tn antigen is expressed on leukosialin from T-lymphoid cells. Cancer Res. 1994;54:85–88. [PubMed] [Google Scholar]
- 58.Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I. Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun. 1991;179:762–767. doi: 10.1016/0006-291x(91)91882-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.