Abstract
The advent of organic synthesis and the understanding of the molecule as they occurred in the nineteenth century and were refined in the twentieth century constitute two of the most profound scientific developments of all time. These discoveries set in motion a revolution that shaped the landscape of the molecular sciences and changed the world. Organic synthesis played a major role in this revolution through its ability to construct the molecules of the living world and others like them whose primary element is carbon. Although the early beginnings of organic synthesis came about serendipitously, organic chemists quickly recognized its potential and moved decisively to advance and exploit it in myriad ways for the benefit of mankind. Indeed, from the early days of the synthesis of urea and the construction of the first carbon-carbon bond, the art of organic synthesis improved to impressively high levels of sophistication. Through its practice, today chemists can synthesize organic molecules—natural and designed—of all types of structural motifs and for all intents and purposes. The endeavor of constructing natural products—the organic molecules of nature—is justly called both a creative art and an exact science. Often called simply total synthesis, the replication of nature’s molecules in the laboratory reflects and symbolizes the state of the art of synthesis in general. In the last few decades a surge in total synthesis endeavors around the world led to a remarkable collection of achievements that covers a wide ranging landscape of molecular complexity and diversity. In this article, we present highlights of some of our contributions in the field of total synthesis of natural products of biological and medicinal importance. For perspective, we also provide a listing of selected examples of additional natural products synthesized in other laboratories around the world over the last few years.
1. Introduction
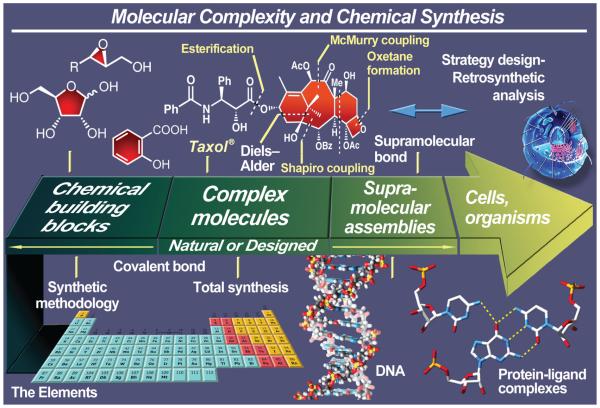
Understanding nature at the molecular level is one of the most fundamental problems preoccupying science today. Elucidating and synthesizing nature’s molecules provide invaluable insights into the inner structure of the world and how it works. When it comes to molecular architecture—its design and synthesis, nature is the undisputed master artisan.1 Man’s fascination with the structure of matter has taken us from Plato’s regular polyhedra to Kekulé’s hexagons of benzene and Watson and Crick’s double helix of our genetic material.2 We traveled from the atom to the molecule and then the supramolecular assembly. Chemical synthesis, the twin sister of structural elucidation, has also followed a similarly dramatic path in its development; from the extraction of metals from their minerals that goes back to ancient times to the preparation of urea and, more recently, the total synthesis3-7 of nature’s most complex and intriguing molecules8—its biopolymers and secondary metabolites, the field has continued to blossom. The two disciplines, structural elucidation and chemical synthesis, are often dependent on each other. In many ways these two areas are synergistic in their efforts to understand nature at its core, its material constitution, and the properties of its constituents as individual molecules and combinations thereof (Figure 1).
Figure 1.
The arrow of molecular complexity and the art and science of chemical synthesis.
To our senses, the beauty of nature is in its mountains and valleys, its rivers and oceans, its skies and stars and, most dramatically, in its living creatures. While most people are only able to admire nature’s macroscopic grandeur, chemists are privileged to be able to peek at its microscopic landscape and experience the beauty of its architectural creations at the molecular level (Figure 1). Nature’s molecules, particularly those made of the elements of carbon, hydrogen, oxygen, nitrogen, and sulfur, and often a few additional elements such as halogens, boron, and metals, are little miracles to the eyes of the synthetic chemist. They are fascinating and inspiring, often defying our imagination and daring our synthetic acumen with their imposing and challenging structures. Of the myriad naturally occurring substances, the secondary metabolites, collectively called natural products, are the most admired and sought-after targets by total synthesis connoisseurs. They pose intellectually stimulating puzzles to the mind and challenge the experimental prowess of the practitioner. Such molecules come in all sorts of shapes and forms. As a result, to those experienced in these matters, they are objects of art, some to be appreciated more than others, some to be feared more than others. Their beauty and wonder come not only from their intricate atomic composition and bond connectivities, but also from their individual complexity and collective diversity (Figure 1). Their size, although often impressive, does not always inspire the same intrigue and fascination as that which we perceive in their complexity and beauty. The practitioners of total synthesis, therefore, often find themselves in a position of having to choose from a large pool of natural products of various characteristics. The frequently asked question of “how do you choose a target for total synthesis” is, therefore, not surprising. There are several criteria to consider in answering this inquiry.
The first criterion has to do with beauty, not unlike that used to select a potential partner in human relationships. The first impression of what the structure of the molecule looks like, which after all is its face and body, is very important. This generally results in eliciting a closer examination, and if that more than cursory inspection confirms the worthiness sensed from the first glance, then one may proceed to the next phase of the selection process. Structural beauty may be found in rings and junctions, unusual bond connectivities and functional groups, individual atoms, and the molecule as a whole. It is the façade and the entire body of the molecule that attracts, and there is a scale, more or less arbitrary, by which the individual observer can define his or her fascination with molecular architecture. Then comes the personality and the heart of the molecule, its properties, of which the biological activities are the most interesting and potentially useful. Is the molecule likely to cure cancer or Alzheimer’s disease? Is it an inhibitor of a certain enzymatic machinery or a blocker of some signal transduction pathway that is likely to assist in making important biological discoveries or cure diseases? Most importantly, can we conceive of ways to design and synthesize analogues of the substance by tweaking its structure in the hope of enhancing its potency and selectivity as a biological tool or medicinal agent? Depending on the answers to these questions, we can, again, use our personal scale to judge how important the molecule is to biology and medicine. Not so far away from the actual biological properties of the molecule are its mechanism of action and biosynthesis. Here, a fascinating hypothesis or observation would add considerably to the value of the target as an opportunity for significant research. Provided the naturally occurring substance is of potential use in biology and medicine, its natural abundance or scarcity becomes important. Often, an exceedingly scarce substance may offer the key to a drug discovery and development program if only it could be made in abundance. In such a case, the molecule receives high marks as a target for synthesis for the urgency and potential benefits can be of major importance and, as such, may overshadow other considerations. It is of course worth noting that, while novelty may be objective, beauty is subjective—in the eye of the beholder.
Once a molecule scores well under such scrutiny, then comes the issue of the opportunity to discover and invent new chemistry such as new synthetic methods, technologies, and strategies. In order to make an evaluation of a projected program directed toward a particular molecular target as an opportunity for discovering new reactivity, the practitioner has to penetrate beneath the surface and start the process of analyzing the target, first retrosynthetically,9a and then synthetically (Figure 1).3-9 It is during this process that the scrutiny of existing synthetic technologies begins; and it is here that new ideas can be generated in response to the challenges posed by the structural motifs of the molecule. Some will deliberately seek new methods in order to derive more discoveries and inventions from the project, while others will prefer to use combinations of known methods, but in unprecedented and novel ways: in other words, devise new synthetic strategies in the process of constructing the molecule. Others, still, will prefer a blend of the two approaches. Challenging synthetic targets frequently provides the ultimate test for the condition of the state of the art of synthesis. Thus, total synthesis endeavors provide opportunities to test and demonstrate the applicability of emerging synthetic methods and technologies in term of their generality, scope, and practicality. The demonstration of the usefulness and value of such innovations in academic laboratories encourages industry to adopt them as tools and processes for their discovery efforts and manufacturing. Asymmetric catalysis, palladium-catalyzed carbon–carbon bond forming cross couplings, and the olefin metathesis reactions are three recent examples of this phenomenon. When limitations are encountered for the existing tools, the molecule enforces the dictum of inventing a new method for accomplishing the task previously considered impossible. And, not to be forgotten is serendipity, the invisible but always watching “goddess of luck” that ushers in unexpected discoveries. To fully exploit such occurrences the well-prepared mind converts the unexpected observation into a rational scientific approach, one based on mechanistic considerations that may lead to new fundamental knowledge. Stockpiling fundamental knowledge is prudent and wise for it is such knowledge that is eventually translated into new inventions and benefits for mankind.
Among the purposes of contemporary total synthesis must be included its original one, the confirmation of the target’s assigned molecular structure. For even in these days of ultra-sensitive and precise instrumentation mistakes are still being made, primarily because of human error, which becomes more likely as the structures become more complex and the isolated quantities smaller.10
In short, the selection of a target molecule is closely linked with the purpose of total synthesis. These objectives are to understand how individual molecules behave, how they interact with other molecules, and how to use the encountered chemical knowledge to advance the art of making new molecules for all intents and purposes. As described above, the criteria used in this selection are based on beauty and logic,3-9a,b the aesthetic impulses coming from the artistic resonances between man and molecule, while the rationale has more to do with intellectual aspects as well as justification of the endeavor in terms of deliverable benefits to society. To be sure, few endeavors, if any, within chemistry are as celebrated as the creative pursuit of nature’s molecules in the laboratory.8 As a consequence, total synthesis is, and will remain for some time, an enviable discipline for its appeal and delivery of benefits in terms of intellectual and experimental challenge, discovery, invention, and personal satisfaction.
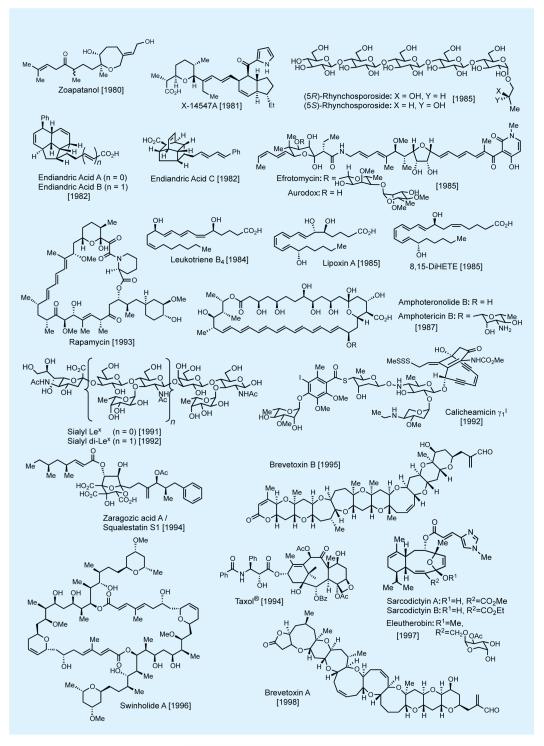
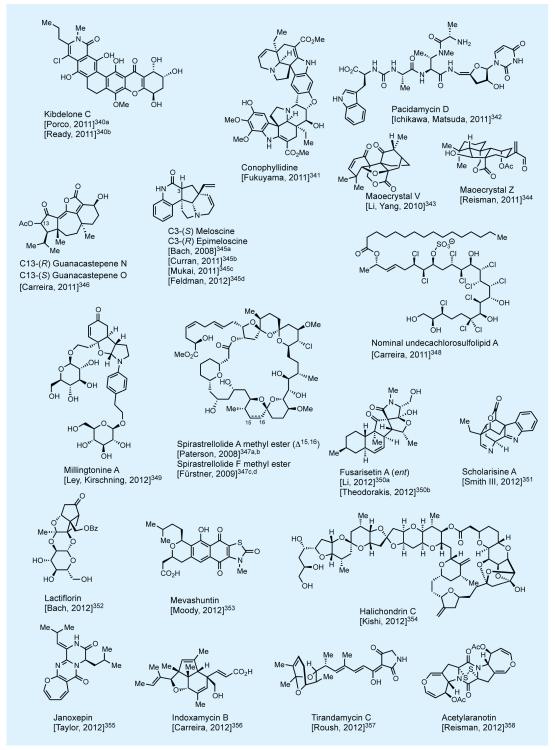
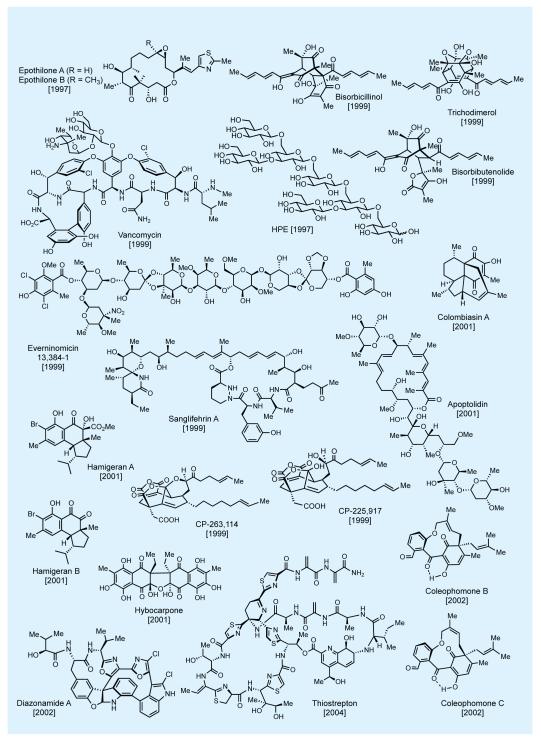
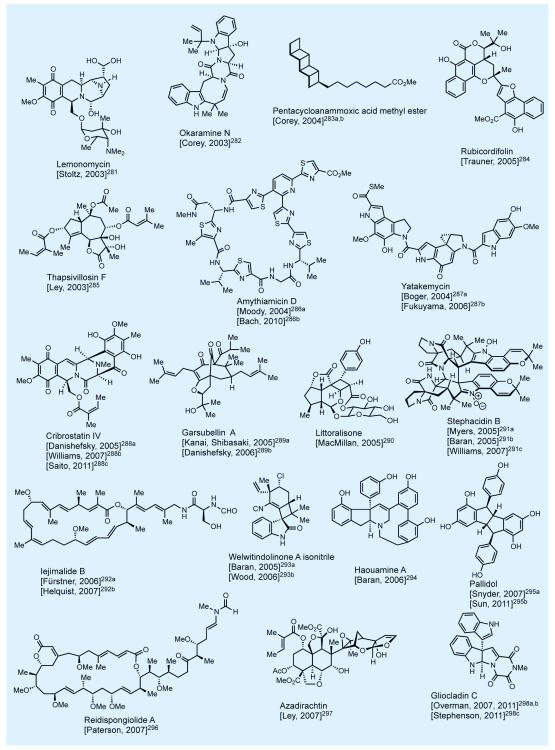
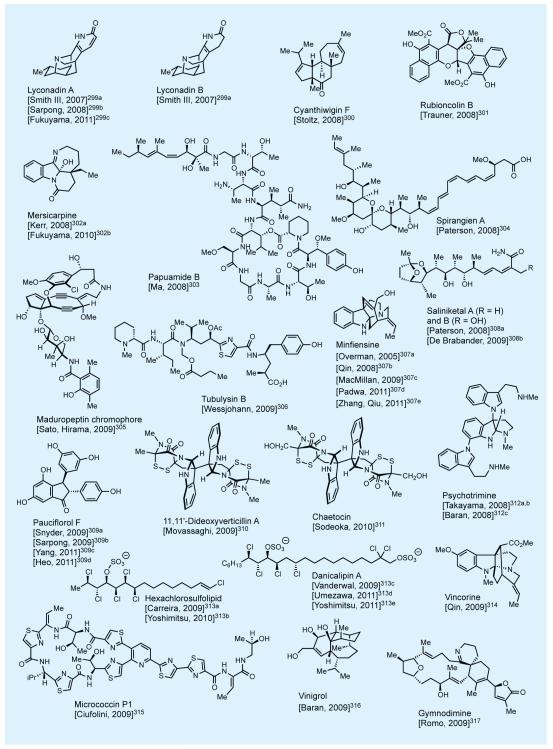
In practicing the art of total synthesis, and in addition to the specific merits of each such endeavor, the practitioner should also aim at extending its reach into new domains of molecular complexity and diversity. In that mission we have as our best ‘ally’, as well as ‘foe’, the master of the art—nature itself. Nature’s molecular wealth is slowly being unraveled in front of our eyes, much to our awe and delight, and it is ours to pursue and exploit as a platform for discovery. Figures 2-4 shows some of nature’s most intriguing molecules that attracted our attention over the last few decades.7,8,10-12 This elite collection includes novel carbocyclic and heterocyclic, unique and sensitive functionalities, and a varying number and form of stereogenic elements. At the time of their selection as synthetic targets, these molecules had yet to succumb to synthesis—and some even looked impossible to synthesize. Most of these total syntheses enjoyed the recognition as accomplishments that broke new ground, with some establishing new fields of investigations for us and others to develop and enrich. A number of these total syntheses represented an expansion of the boundaries of the science in terms of molecular complexity and landscape. Their accomplishment required motivation and resilience by the students involved in the campaigns leading to the final conquest. One thing is certain: courage and excitement accompanied the beginning of each project but stamina and perseverance held the secret to reaching the final destination, with a bit of luck added to the mix.
Figure 2.
Selected natural products synthesized in the author’s laboratories (selected references given in the text).
Figure 4.
Selected natural products synthesized in the author’s laboratories (selected references given in the text).
To experience the exhilaration of the process of synthesizing the target molecule and learning about its chemical character as we proceeded toward our final destination in each campaign was added the gratification of the rich bounty that we collected on the way. Examples of this bounty included a plethora of new synthetic methods and technologies, novel strategies for rapid construction of complex structural motifs,6,7,11,12 and a diverse array of designed, biologically active analogues of the targeted natural products.13,14
Highlighted below are the strategies employed in some of the most memorable total syntheses completed in our laboratories. To appreciate fully the essence of the following vignettes, one should keep in mind in glancing over the synthetic strategies both the logical and the aesthetic, for it is the combination of the two that makes the whole of the endeavor so appealing and rewarding. Remembering that total synthesis is being called both a precise science and a fine art, comparisons of synthesis with art are most appropriate. The following quote from John Pentland Mahaffy (1839-1919), an Anglo-Irish polymath, speaks to the meaning of genius as in music, but also in synthesis:
“The first and most superficial answer is that genius is original, that it strikes out new ideas, new solutions of problems, new lines of research, while the average man can only learn what others have already discovered for him. But a deeper and more careful inquiry reveals to us that absolutely new ideas are of the very rarest occurrence; almost the whole work of human genius consists in assimilating what others have thought, in combining what others have imagined separate, in recasting the form of their thought, and so producing what seems a perfectly new thing, and yet is only the old under a new aspect. No instance of this is more signal than that of a great composer in music. The gift of original melody, as it is called, is rare and precious. The possessor of it is justly considered a genius. But no melody could possibly speak to us except a combination of perfectly well known elements. The only originality is in their assimilation and reproduction.”15
Beyond the art of total synthesis and reproduction of nature’s molecules lies the almost infinite landscape of molecules of biological and medicinal importance that are often inspired and synthesized through the developed technologies.
2. Highlights of Contributions from the Author’s Laboratories
2.1. Endiandric Acids
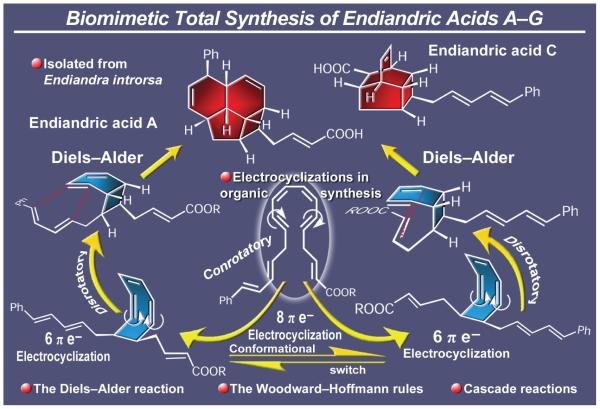
One of the most memorable total syntheses performed in our laboratories and reported in 1982 was that of the endiandric acids (Figure 5).16 What was special about this synthesis was that it proceeded with a rather spectacular show of synthetic ‘fireworks’ that had been postulated by David St. C. Black and his collaborators as a possible biosynthetic route to these fascinating natural products.17,18 Found within the tree Endiandra introrsa, endemic to Australia, the endiandric acids exhibited no optical activity despite their eight stereogenic centers, a rare phenomenon indeed for naturally occurring substances. This observation was in line with a non-enzymatic formation of these compounds from a prochiral precursor and through a spontaneous chemical cascade, an intriguing hypothesis that was too beautiful to ignore.17,18 Upon devising a synthetic route to this precursor—a polyunsaturated open-chain system containing a centrally located trans,cis,cis,trans-conjugated tetraene system strategically flanked by a trans,trans-conjugated diene moiety on one side two carbons away, and a trans-α,β-unsaturated methyl ester on the other side, also two carbons away—we were able to test this hypothesis. Upon generating this rather labile biomimetic precursor, it was gratifying to observe the formation of the two architecturally different endiandric acids, A and C, each with their four newly generated rings and eight stereogenic centers cast stereospecifically. The geometry and positions of the double bonds within the polyunsaturated precursor, with the carboxylate group on one end and the phenyl moiety on the other, were translated faithfully into the precise structures of the final products by allowing the intervening cascade sequence—a conrotatory 8π electrocyclization, a disrotatory 6π electrocyclization and a 6π [4+2] cycloaddition—to proceed according to plan and deliver these natural products as racemates, their naturally occurring forms. This splendid example of biomimetic total synthesis, which also demonstrates the power of cascade sequences and electrocyclic reactions in chemical synthesis, was not the first, for it was preceded by the classic syntheses of the alkaloid tropinone19 in 1917 by Sir Robert Robinson and the steroid progesterone20 by William S. Johnson (1913-1995) in 1971. Neither would this be the last however, for the beauty and logic of cascade and biomimetic strategies have continued to fascinate us and others since then, leading to a fashionable, systematic and productive theme in total synthesis.21 We shall return to this topic below with more highlights of such total syntheses featuring other types of cascade reactions.22
Figure 5.
Highlights of the biomimetic total synthesis of endiandric acids (1982).
2.2. Efrotomycin
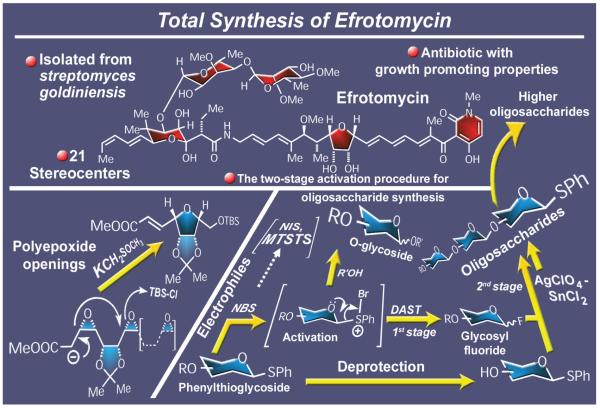
In the beginning of the 1980s we became enamored with the structure of efrotomycin (Figure 6),23 the flagship member of a family of antibiotics that also includes aurodox24 (a close relative that lacks the terminal carbohydrate units of efrotomycin). We were particularly intrigued and challenged by the all-cis tetrasubstituted tetrahydrofuran ring of the molecule whose entire size and complexity (encompassing 21 stereocenters and seven sites of geometrical isomerism) elicited both awe and admiration. The opportunity offered by efrotmycin, originally isolated from Nocardia lactamdurans, was well exploited: not only was its total synthesis accomplished by a highly convergent strategy,25 but the endeavor also resulted in certain methodological advances that still resonate today. One of them was the original use of a diepoxide opening cascade to construct the unusual tetrahydrofuran segment of the molecule.26 Another was the development of the chemistry of phenylthioglycosides as glycosyl donors, either directly upon activation with N-bromosuccinimide27 or indirectly through their glycosyl fluoride descendants in a process we coined the two-stage activation procedure.28 It was rewarding to realize later how influential both of these discoveries were; in addition to many follow-up applications in our laboratories,29 they served as the basis for further developments in other laboratories within the fields of polyepoxide-based cascade reactions and polysaccharide assembly, respectively. Indeed, one of the most practical and popular glycosidation methods today involves N-iodosuccinimide,30 another electrophilic reagent and a mere sibling of N-bromosuccinimide, as an activator of aryl thioglycosides, while polyepoxide openings have been extensively applied in the construction of complex macrolide31 and polyether molecular frameworks.32
Figure 6.
Highlights of the total synthesis of efrotomycin (1984).
2.3. Amphotericin B
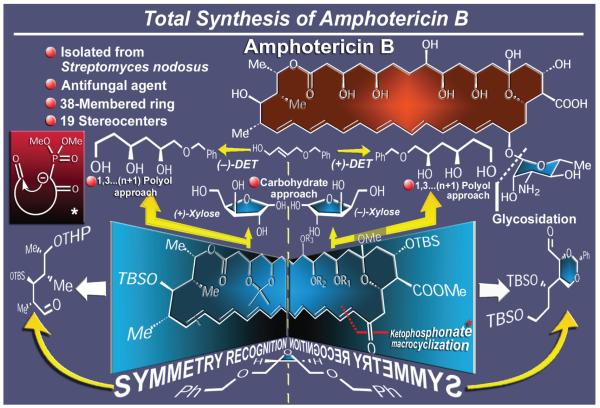
Another antibiotic that stood defiant in the 1980s was amphotericin B (Figure 7),33 the prominent member of the polyene macrolide antibiotic class and a long time, clinically used antifungal agent. Isolated from Streptomyces nodosus and boasting a 38-membered ring that includes an all-trans conjugated heptaene system and nineteen stereogenic centers, this defiant giant of a molecule stimulated our synthetic impulses and commanded our respect, for it presented several challenges, not least of which were its unusually large macrocycle, numerous hydroxyl-bearing stereocenters, and β-glycoside bond that joined the aminosugar moiety to the mainframe of its structure. Accomplished in 1987,34 our total synthesis of amphotericin B featured a number of new, at the time, synthetic strategies and tactics. Following retrosynthetic analysis, the defined building blocks were constructed enantioselectively through two distinct strategies. One route started from the two enantiomers of xylose as the source of chirality, while the other commenced from a prochiral allylic alcohol and introduced the chirality through a Sharpless asymmetric epoxidation reaction. The problem of the macrocycle was solved by a remarkable intramolecular ketophosphonate–aldehyde reaction that led to a beautifully reddish-orange polyenone intermediate. This advanced intermediate was then reduced stereoselectively to the required hydroxy compound in preparation for the obligatory glycosidation, a process that was subsequently accomplished in a stereoselective manner employing a trichloroacetimidate carbohydrate donor equipped with an azide moiety as the amino group surrogate. It was pleasing to note that our work in the 1980s on this important molecule34 proved inspiring, if not enabling for a number of more recent investigations.35,36
Figure 7.
Highlights of the total synthesis of amphotericin B (1987).
2.4. Calicheamicin γ1I
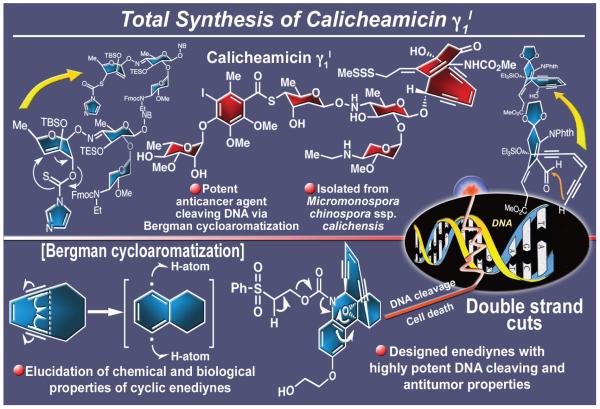
One of the most intriguing natural products to be isolated in the last few decades is calicheamicin γ1I (Figure 8),37 a beautifully crafted molecule whose architectural splendor is surpassed only by its fascinating mode of biological action. Isolated from Micromonosporα chinospora ssp. calichensis and standing as the most prominent member of a growing class of compounds collectively known as the enediyne antitumor antibiotics,38 calicheamicin γ1I exerts its phenomenal anticancer activity by inflicting double strand cuts to DNA. This genetic material damage mechanism disables the replication machinery of the cell. Although highly toxic, it was tamed by scientists at the then American Home Products (subsequently Wyeth and now Pfizer) through conjugation to an antibody to a degree that allowed its introduction as a clinical agent against acute myeloid leukemia (AML) under the trade name of Mylotarg® (the drug was withdrawn in 2010 due to hepatotoxicity).
Figure 8.
Highlights of the total synthesis of calicheamicin γ1I (1992).
The daunting molecular architecture of calicheamicin γ1I was intriguing not only due to its startling novelty when unraveled in the late 1980s, but also because of its unimaginable structural connectivity, severe strain, and much feared instability. In the end, the appeal was too much to resist and so, in 1987, we embarked on a campaign to synthesize it in the laboratory, a mission that we were not so sure we could accomplish. In addition to the arduous road that would surely lie ahead, the entire enterprise was a gamble due to the lack of error-proof evidence, such as X-ray crystallographic analysis, to support the molecule’s structural assignment. As matters transpired, we were able to reach calicheamicin γ1I by total synthesis and confirm its assigned structure in 1992, five years after the initiation of the campaign.39 The total synthesis of calicheamicin γ1I was an extraordinary adventure, one filled with formidable barricades but also with new discoveries and inventions. To be sure, it represented a significant milestone in our systematic journey toward building higher molecular complexity and diversity.
Calicheamicin’s structure boasted seven rings and 19 stereocenters, an iodine residue on its fully substituted aromatic ring, an unusual NH-O glycoside bond, a 2,5-dideoxy aminosugar, and a demonic 10-membered conjugated enediyne system with a bridging cyclohexenone moiety carrying a carbamate functionality, as well as an olefinic side-chain ending in a methyl-capped trisulfide moiety. When woven together by nature, these components resulted in an incredible molecular machine intended by its producing microorganism to kill another, not unlike a modern man-made weapon. The conquest of this diabolical chemical killer was accomplished through a carefully orchestrated strategy that combined convergence with risky, but novel, techniques to construct its oligosaccharide domain and enediyne core. Most notable among these maneuvers were an intramolecular [3+2] nitrile oxide cycloaddition, an intramolecular acetylide-aldehyde reaction to forge the 10-membered enediyne ring, a novel [3,3] sigmatropic rearrangement to construct the sulfur-containing carbohydrate unit,40 a stereocontrolled Mitsunobu-type reaction to implant the unusual NH-O functionality, and a crucial glycosidation-based coupling reaction featuring the powerful trichloroacetimidate-based glycosidation technology to join the oligosaccharide domain to the enediyne fragment that forged the entire ring framework of the target molecule. Along the way we were delighted and fascinated in equal measure by the many twists, turns, and facets of the synthesis and the technologies developed during its course. These aspects of the project included the unusual resolution41 of the aromatic fragment of calicheamicin γ1I and the use of the Ramberg-Bäcklund reaction for the preparation of enediynes.42
Most importantly, calicheamicin γ1I gifted us with the opportunity to explore the chemistry and biology of the enediynes as a tunable class of compounds that could be used to cleave DNA and kill tumor cells. Thus, an array of such systems was designed, synthesized, and studied.43 These systems ranged from simple, 10-membered, conjugated enediyne hydrocarbons to highly sophisticated enediynes equipped with DNA-binding domains and activating devices such as those inspired by the structure and mechanism of action of dynemicin A,44 another naturally occurring member of the enediyne family. These investigations provided the foundation for further developments to occur within a field that continues to fascinate to this day, as evidenced from continued research activities toward the synthesis of newer members of the enediyne class as well as of novel designed enediynes and related compounds.43 All in all, the total synthesis of calicheamicin γ1I that started as a questionable, if not impossible dream ended up as one of the most memorable and rewarding campaigns in total synthesis that we ever experienced in our laboratories.45 An equally elegant total synthesis of calicheamicin γ1I was reported from the Danishefsky laboratories in 1994.46
2.5. Rapamycin
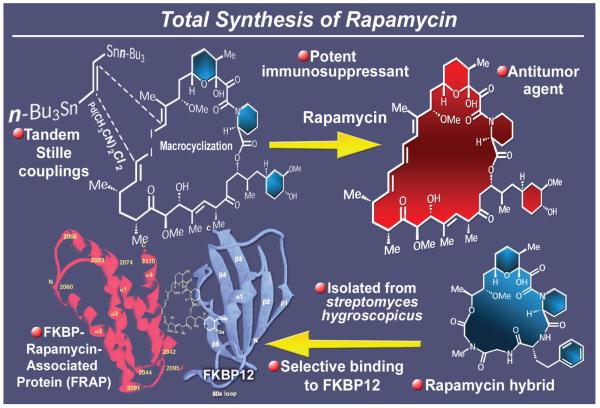
Despite its known and fascinating structure, rapamycin (Figure 9),47 isolated from Streptomyces hygroscopicus, remained on the sidelines of synthesis until its biological properties and mechanism of action as an immunosuppressant became known.48 This information grouped it together with cyclosporine and FK506, two pivotal natural products that impacted decisively the advent of organ transplantation. Captivated by the impressive biological profile and unique molecular architecture of rapamycin, we set out to construct it in the laboratory in the early 1990s, and by 1993 had developed the first total synthesis route to this fascinating target molecule.49 A most memorable and influential maneuver in this total synthesis was the double-Stille stitching cyclization that knitted together a vinyl di-stannane, carrying a two-carbon ethane unit, with an open-chain unprotected di-vinyliodide precursor, thus forming the 28-membered macrocyclic system of the molecule. It was significant, and not without considerable satisfaction, that we watched this synthetic technology become a paradigm that inspired further developments in the field of macrocycle construction. Another interesting side investigation within this project was the design and synthesis of a truncated version of rapamycin which, not possessing rapamycin’s FRAP-binding domain, refrained from binding to that target and, therefore, lacked immunosuppressive activity.50 The information thus obtained provided further support for the proposed mechanism of action for this clinically important compound (which involves cooperative binding to FKBP12 and FRAP). Finally, we note with contentment that subsequent to our total synthesis of rapamycin a number of its derivatives were developed as clinical agents, namely Torisel® (Pfizer) and Afinitor® (Novartis) for cancer chemotherapy and Rapamune® (Pfizer) for immunosuppression in organ transplantation patients. Since our synthesis, a number of other elegant total syntheses of rapamycin have been reported.51
Figure 9.
Highlights of the total synthesis of rapamycin (1993).
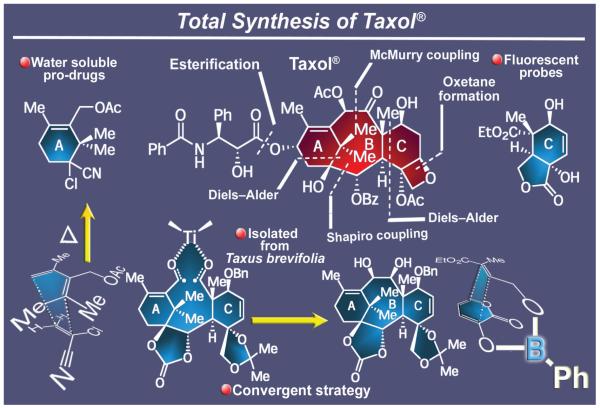
2.6. Taxol®
A formidable synthetic challenge of the latter part of the twentieth century was that posed by the much celebrated molecule of Taxol® (Figure 10). Isolated from the Pacific yew tree, Taxus brevifolia, and structurally characterized in 1971,52 Taxol® was then hailed as a highly promising anticancer agent, but its low abundance in nature was expected to cause serious supply shortages in the event of its approval as a drug. In 1994, and after a thrilling chase, our group reported the total synthesis53,54 of Taxol®, followed closely by a publication from the Holton group.55 In our total synthesis,53,54 the two flanking rings of the main framework of Taxol®, A and C, were cast through Diels-Alder reactions, each of which is memorable for its own reasons. The first reaction, leading to ring A, is remarkable for its high yield and faithful obedience to the rules, exclusively furnishing the product with the desired regiochemistry despite the extreme steric congestion associated with its formation. The second reaction, leading to ring C, is notable for the way it was forced to proceed in the desired regiochemical sense through a boron tethering device that appropriately oriented the two reaction partners in space. Subsequent steps in this highly convergent synthesis included two other important carbon-carbon bond forming processes, namely the Shapiro reaction (rarely used in total synthesis) and the McMurry coupling reaction, the latter employed as the key process to form Taxol®’s most strained and challenging domain, ring B. In addition to the total synthesis of the targeted molecule, the Taxol® campaign led to a number of synthetic technology discoveries56 and new biological insights.56,57 These include: (a) the regioselective opening of cyclic carbonates to hydroxy esters; (b) the design, synthesis, and biological investigation of several water-soluble taxoids and other Taxol® prodrugs and analogues;57a (c) a fascinating self-assembling designed Taxol® that formed helices in aqueous solution;57c and (d) a number of Taxol®-based fluorescent probes for tubulin imaging studies.58 The availability of such molecules through chemical synthesis enabled conformational and tubulin binding investigations and led to insights into nanostructures with remarkable physical and biological properties. Together, Taxol® and its synthetic sibling Taxotere® became the best-selling anticancer drugs in the world at the dawn of the twenty first century, saving and extending many lives around the world. Despite its inability to compete with a semisynthetic approach to Taxol®, this total synthesis and those that followed53-55,59 served chemical synthesis well, not only because of the methodological and biological advances that they enabled, but also because they provided a measure of the state-of-the art of total synthesis and a symbol of its power and sharp edge at the time.45b,60
Figure 10.
Highlights of the total synthesis of Taxol® (1994).
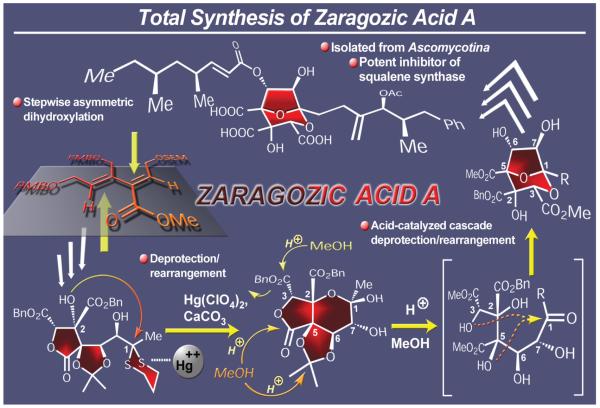
2.7. Zaragozic Acids
The zaragozic acids, also known as squalestatins, comprise a family of natural products with unusual structural motifs and important biological properties (Figure 11).61 Isolated from certain species of fungi, these molecules exhibit potent cholesterol lowering activity through squalene synthase inhibition, antifungal properties, and ras-farnesyl transferase activity, the latter making them potential anticancer agents. Their molecular architectures include a highly oxygenated bicyclic core carrying three carboxyl moieties and two aliphatic chains. Our convergent total synthesis of zaragozic acid A, reported in 1994,62 employed a conjugated diene system as an early precursor that was assembled through a Stille coupling reaction. This prochiral precursor was then converted enantioselectively through two consecutive dihydroxylations and further elaboration to a dithiane-containing polyoxygenated species which was deprotected and concurrently rearranged to a more advanced intermediate. The latter underwent an impressive, acid-catalyzed cascade rearrangement to generate the desired zaragozic acid core from which the final product, zaragozic acid A, emerged through a series of manipulations that culminated in the attachment of the required side chains and final deprotection. This synthesis demonstrated the power of a number of new synthetic technologies available at the time, including palladium-catalyzed cross coupling reactions, asymmetric dihydroxylation, and cascade reactions. At about the same time,63a-c a number of other elegant total syntheses of zaragozic acids were also published.63d-m,64
Figure 11.
Highlights of the total synthesis of zaragozic acid A (1994).
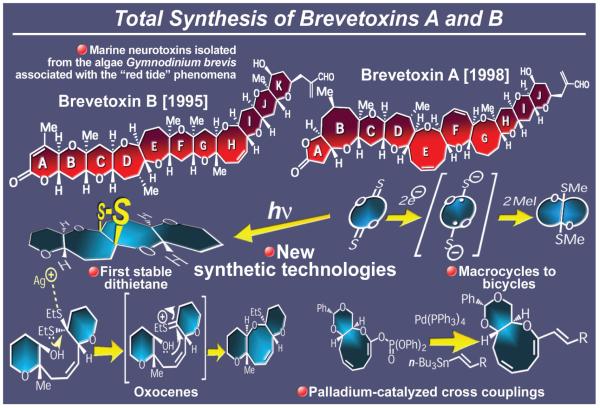
2.8. Brevetoxins A and B
The stunningly beautiful and aesthetically pleasing structures of brevetoxins B65 and A66 (Figure 12) were gifted to us by the dinoflagellate Karenia brevis (previously known as Gymnodinium breve), dense blooms of which are responsible for the “red tide” phenomena. These highly potent neurotoxins, the main toxic principles associated with the red tides, offered us unimaginable challenges in the 1980s and 1990s that turned into exciting adventures and opportunities for discovery and invention. Indeed, the synthetic odyssey67 that was initiated in 1983 toward the total synthesis of brevetoxin B was distinguished not only by the stunning beauty and complexity of the molecular architecture of the targeted molecule, but also by its duration, 12 years in all. Most importantly, this campaign stands out as a highly enlightening and enriching experience in terms of new synthetic technologies and overall impact on the art and science of total synthesis.63,67-76 With regard to synthetic strategy, brevetoxin B proved its intransigence over and over again, forcing us back to the drawing board several times. This process necessitated the invention and development of numerous new synthetic methods for construction of cyclic ethers and carbon-carbon bonds that were applied to finally reach the target. Among the most important of these technologies were the following: (a) the regio- and stereocontrolled intramolecular epoxide openings by an internal hydroxyl group for the synthesis of tetrahydropyrans directed by a neighboring olefinic bond;70,71 (b) the silver-promoted hydroxy dithioketal ring closure for the construction of oxocene systems;72 (c) the bridging of macrodithionolactones to form bicyclic systems induced by electron donating reagents such as sodium naphthalenide;73 (d) the reductive hydroxyl ketone cyclization induced by silicon reagents to form oxepane systems;74 (e) the photolytic bridging of dithionoesters to form, upon extrusion of sulfur, oxepanes;75 (f) the facile addition of nucleophiles to thionolactones and elaboration of the products as a means to convert lactones to cyclic ethers;76 and (g) the deoxygenation of lactones to cyclic ethers through radical-based reduction of the corresponding thionolactones.76b
Figure 12.
Highlights of the total synthesis of brevetoxins A and B (1983-1998).
These useful synthetic methods were supplemented with several other synthetic technologies that emerged from the brevetoxin A campaign77,78 (Figure 12) and related polyether projects. A powerful method was developed for the functionalization of lactones79 and lactams80 to a variety of useful intermediates through palladium-catalyzed carbon-carbon bond forming reactions between the corresponding keteneacetal phosphates and a number of organometallic reagents such as vinylstannanes and zinc reagents. Accordingly, a variety of strained, medium-sized cyclic ethers,79 including the 9-membered ring of brevetoxin A, were constituted, and an asymmetric synthesis of cyclic amino acids was developed.80
The olefin metathesis reaction did not escape our attention as a possible means to construct cyclic ethers, following its timely arrival in the mid-1990s. As a result of these early investigations, a number of olefin metathesis-based strategies were designed and executed, leading to the assembly of cyclic polyether arrays directly from olefinic esters.81a These synthetic technologies were successfully applied to the construction of two of maitotoxin’s (a highly complex sibling of the brevetoxins) structural domains.81b Another rewarding experience derived from the brevetoxin project was the opportunity to synthesize the first stable 1,2-dithietane ring system73c (see Figure 12), whose X-ray crystallographic analysis revealed its theoretically interesting structural parameters. Finally, in addition to our total synthesis of hemibrevetoxin B,82 a truncated version of brevetoxin B lacking four of the eleven fused rings of the natural product was designed and synthesized employing the developed technology.83 Biological investigations with this and other brevetoxin-like molecules shed light on the mechanism of action of this intriguing neurotoxin.84 It is with much pleasure that we note the continued appeal of these fascinating structures and the ever-increasing bounty that endeavors in this field bring to their still many suitors. Indeed, a whole new field of investigation was founded on those early expeditions toward the two beautiful sister molecules brevetoxins A and B.32,45b,67,85,86
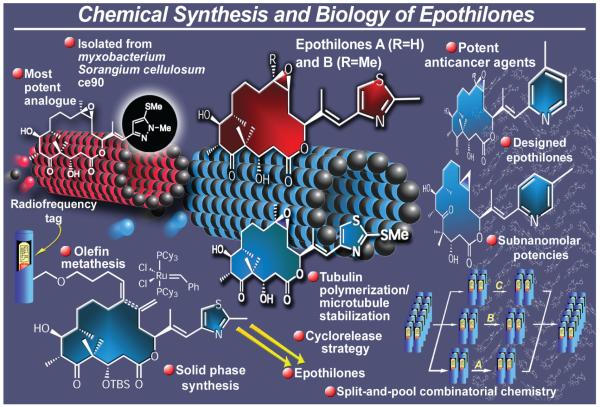
2.9. Epothilones A and B
Hailed to be superior to Taxol® as potential anticancer drugs because of their higher potency and ability to kill Taxol®-resistant tumor cells, epothilones A and B (Figure 13) took the scientific community by storm in the mid-1990s.87 Isolated from the myxobacterium Sorangium cellulosum So ce90,88 their appeal was not only due to their novel molecular architectures and potential in cancer chemotherapy, but also because of the opportunity they presented for new discoveries and inventions in chemistry.
Figure 13.
Highlights of the total synthesis of epothilones (1996-2006).
Our most significant contributions in the epothilone field included enabling technologies for both chemical synthesis and chemical biology. The group also exploited the opportunity to demonstrate, for the first time, the power of solid phase chemistry and radiofrequency encoded combinatorial synthesis89 in complex molecule synthesis and compound library construction. Thus, we first developed the total synthesis of epothilones A and B in solution by two different strategies, the first employing an olefin metathesis-based approach to the macrocyclic framework,90 and the second a macrolactonization-based91 strategy to the same structural motif. These accomplishments were followed by a solid phase total synthesis of epothilone A through an olefin metathesis-based strategy involving a cyclorelease step.92 The so-emerged synthetic technology was then applied, in conjunction with the previously developed IRORI technology,89 to the combinatorial synthesis of epothilone libraries for biological screening purposes.89,93 These tests included tubulin polymerization, cytotoxicity assays, and, in certain cases, animal studies. The combinatorial chemistry developed during this program represents the first case where analogue libraries of complex natural products were synthesized by solid phase total synthesis and a classic example of the use of IRORI radiofrequency-based technology, which was later widely applied in combinatorial chemistry,94 both in academia and industry. The combined solution and solid phase synthetic studies, coupled with biological screening, led to the discovery of a number of powerful epothilones, including a highly potent series of pyridine-,95a cyclopropyl-95b,c and thiomethyl-containing analogues.95d-f Besides ours, several other groups contributed decisively to the emergence of the epothilone field.96 Notable among them were the Danishefsky group, whose total synthesis of epothilones was reported just before ours,97 and the Schinzer group, whose synthesis appeared shortly after ours.98 Collectively, these investigations led to a comprehensive structure-activity relationship picture in the epothilone field and set the stage for follow-up studies96 in academic and industrial laboratories that led to major advances in the search for new chemotherapeutic agents. Indeed, we note the introduction of a number of epothilone drug candidates, including one from our own collection, into clinical trials as anticancer agents.99 One of these, ixabepilone (Ixempra®, Bristol-Myers Squibb), was approved as a clinical agent for breast cancer chemotherapy in 2007.
2.10. Eleutherobin and Sarcodictyins A and B
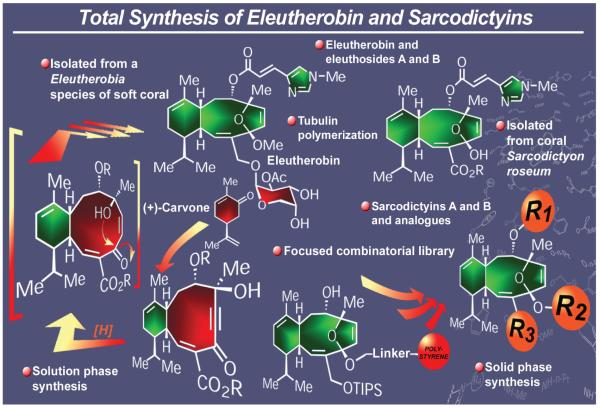
Eleutherobin100 and sarcodictyins A and B101 are marine natural products endowed with novel molecular architectures and important biological properties (Figure 14). Their main structural difference lies in the fact that the eleutherobin molecule includes a carbohydrate tail, whereas the sarcodictyins are capped as methyl esters. All three molecules exhibit potent cytotoxic properties exerted through a mechanism that involves tubulin polymerization. Coupled with their scarcity, their biological activities prompted us to pursue their syntheses, aimed at rendering them readily available for further biological investigations. In 1997, we reported first the total synthesis of eleutherobin,102 and later the total synthesis of its siblings eleuthosides A and B103 and sarcodictyins A and B.104 The general synthetic strategy toward these molecules employed the naturally occurring and readily available (+)-carvone as starting material and involved an acetylide-aldehyde cyclization to afford the 10-membered ring of the molecule, a selective reduction of the acetylenic unit to a cis olefinic bond, lactol formation to generate the tetrasubstituted furanoid system, and attachment of the side chains. Adaptation of the synthetic sequence to solid phase chemistry allowed the preparation of a focused library of over 60 analogues of these natural products.105 Chemical biology studies with these analogues led to the discovery of several compounds possessing equal or superior potencies to the natural products against tumor cells, underscoring the enabling nature of total synthesis for biological investigations and drug discovery efforts.105 These endeavors also demonstrated the power of solid phase synthesis in the construction of designed compound libraries for biological screening purposes.106
Figure 14.
Highlights of the total synthesis of eleutherobin and sarcodictyins A and B (1997).
2.11. Vancomycin
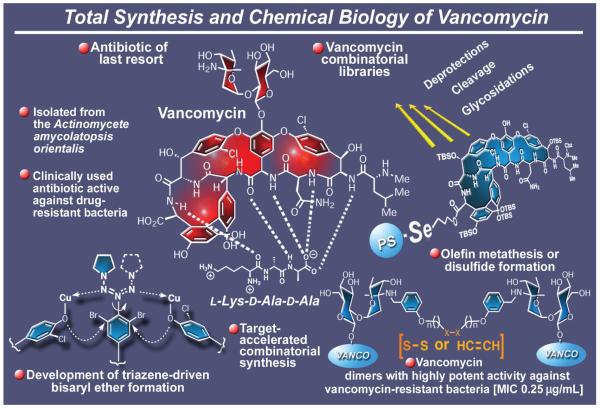
The vancomycin-producing organism (Figure 15) Actinomycete amycolatopsis orientalis spared little imagination in designing a demonically complex molecular architecture that was to tantalize and defy synthetic chemists for decades after its structural elucidation in 1956.107 Indeed, it was because of its daunting structure, featuring three atropisomerism sites and three macrocyclic systems, that this glycopeptide antibiotic, often called the antibiotic of last resort, and its aglycon resisted total synthesis until the late 1990s.108-110 Our total synthesis of the aglycon of vancomycin was accomplished in 1998,109 while the total synthesis of vancomycin itself had to wait one more year before it would succumb to our advances.111 Happily, the accomplished total synthesis of vancomycin was accompanied by the development of a number of new synthetic technologies and strategies.112 Among them are a new method for the construction of macrocyclic bis-aryl ethers that provided the means by which the two macrocycles of the target molecule were assembled112a,b and an improved version of asymmetric synthesis of biphenyl-type systems, one of which is embedded within the vancomycin structure.112d Gratifyingly, the triazene-driven, arylether-forming method utilized to construct the bis-aryl ether domains of vancomycin has been proven to be of general utility and applicable to both cyclic and acyclic systems as well as cyclic thioethers.112a,b In addition to the total synthesis of the imposing vancomycin, this campaign also led to enabling technologies for solid phase synthesis and combinatorial chemistry. Of special interest was a new selenium-based linker that was developed in order to allow carboxylic acids, amines, and alcohols to be attached onto a solid support for chemical elaboration and eventual release.113a This useful technology proved instrumental in loading vancomycin onto a polystyrene-type resin, degrading it to its aglycon for reconstruction purposes, and, finally, constructing and liberating arrays of vancomycin analogues for biological screening.113b A second combinatorial strategy based on dynamic chemistry was developed for the synthesis of vancomycin dimers.114 Termed target-accelerated combinatorial synthesis because it was performed in the presence of vancomycin’s biological target (the peptide segment L-Lys-D-Ala-D-Ala), this strategy led, through either olefin metathesis or disulfide bond formation, to the rapid identification of a number of remarkably potent antibacterial agents effective against methicillin- and vancomycin-resistant bacteria.114 With the fall of vancomycin,115 another domain of nature’s molecular diversity had been conquered; it was time to move on to new territories, of which they were many at the time.
Figure 15.
Highlights of the total synthesis of vancomycin (1998-2002).
2.12. Sanglifehrin A
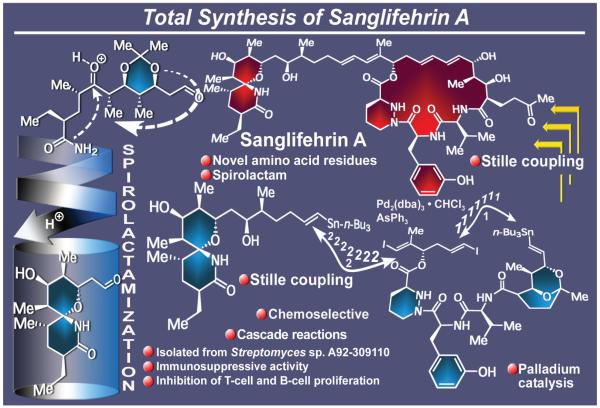
Sanglifehrin A (Figure 16), a novel naturally occurring substance isolated from Streptomyces sp. A92-309110 in the 1990s by a Novartis team of scientists,116 constituted an unusually appealing synthetic target not only because of its unprecedented molecular architecture, but also due to its striking immunosuppressive properties.116 Featuring a novel spirolactam moiety linked with a long chain to a 22-membered macrocycle domain, the molecule of sanglifehrin included two conjugated diene systems that stood out as strategic sites for disconnection in the retrosynthetic analysis of the molecule. The challenging structure of sanglifehrin A was reached by total synthesis in 1999 through a convergent route that was distinguished by the swiftness of the construction of its two domains, namely the spirolactam system as a vinylstannane, and the macrocycle precursor as a bis(vinyliodide).117 Also remarkable was the selective manner by which the macrocycle was formed through an intramolecular Stille coupling reaction and then connected to the spirolactam system through a second, now intermolecular, Stille coupling to form the entire framework of the molecule. From the latter, sanglifehrin A finally emerged upon rupture of the internal ketal that served, up to that point, as a loyal guardian for the desired functionality. This total synthesis serves as a reminder of the remarkable power of the palladium-catalyzed carbon-carbon bond forming reactions, a group of processes that revolutionized the field of chemical synthesis beginning in the 1970s and 1980s,118 early examples of which are our total syntheses of the lipoxins and related eicosanoids.119,120
Figure 16.
Highlights of the total synthesis of sanglifehrin A (1999).
2.13. Bisorbicillinoids
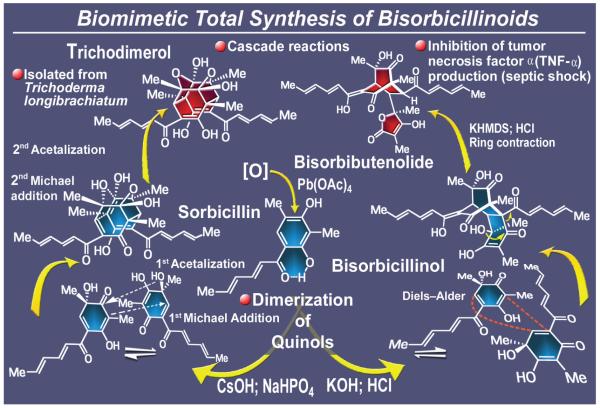
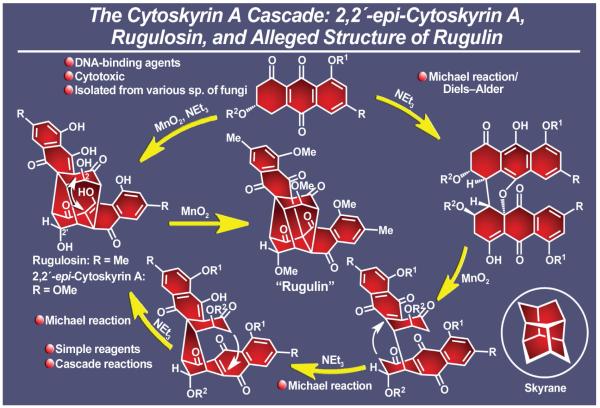
The bisorbicillinoids (Figure 17) appeared on the scene from different directions, at first camouflaging themselves behind substantially different structural motifs, but soon revealing their isomerism and common origins through subtle structural clues. Most striking among them are trichodimerol (isolated from Trichoderma longibrachiatum),121 bisorbibutenolide,122 and bisorbicillinol123 (the latter two isolated from the fermentation of Trichoderma sp. USF-2690). These naturally occurring substances are characterized by novel and divergent molecular structures and possess biological activities ranging from inhibition of tumor necrosis factor a (TNF-a) production to antioxidant properties. Closer inspection of their molecular architectures led to a hypothesis for their biosynthesis and implicated sorbicillin, another naturally occurring substance, as their possible biogenetic precursor. According to this idea, initial enzymatic oxidation of sorbicillin at a specific site would produce a reactive species whose chemical reactivity would offer it the option of entering into distinctly different cascade reactions, leading, through dimerization, to individual bisorbicillinoids. Our proposal for a plausible biosynthetic pathway for the generation of trichodimerol was followed by a swift execution of its biomimetic synthesis, which also led, upon slight but crucial modification of reaction conditions, to the construction of its sibling, bisorbicillinol, and thence to that of bisorbibutenolide.124 This total synthesis of trichodimerol commenced with sorbicillin and featured an impressive double Michael reaction/acetalization sequence between an equilibrated mixture of two isomeric quinols unleashed under certain basic conditions.124,125 Equally impressive was the diversion of this quinol mixture toward the bisorbicillinol structure under a different set of conditions. Base-induced rearrangement of bisorbicillinol then led directly to bisorbibutenolide through contraction of a 6-membered ring to a 5-membered ring. This chemistry amounts to a remarkable series of biomimetic cascade reactions toward a family of complex natural products and, along with the endiandric acid cascade16 discussed earlier, constitutes some of our most impressive signature contributions to biomimetic total synthesis, an approach that we came to appreciate and apply whenever possible.21 A few more examples of this cascade-based strategy to molecular complexity will be highlighted below.126
Figure 17.
Highlights of the total synthesis of bisorbicillinoids trichodimerol, bisorbibutenolide and bisorbicillinol (1999).
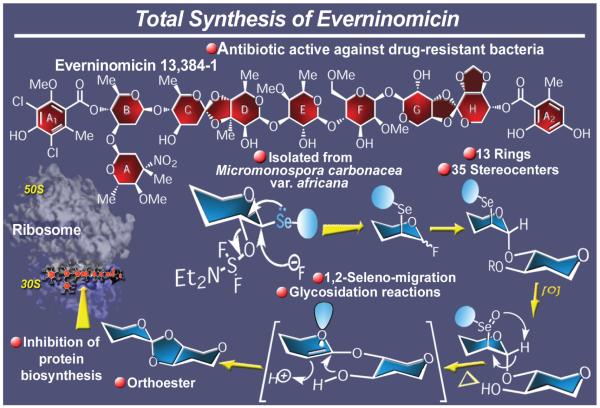
2.14. Everninomicin 13,384-1
As one of the most exciting and promising oligosaccharide antibiotics (isolated from Micromonospora carbonacea var. africana),127 everninomicin 13,384-1 (Figure 18) presented to us a highly attractive synthetic target. Its appeal was considerably enhanced by its novel and challenging molecular architecture that included no less than thirteen rings and 35 stereocenters. Particularly daring within everninomicin’s structure were the two orthoester linkages, not only due to their chemical sensitivity, but also because of their specific stereochemical arrangement. The molecule of everninomicin also boasted several other synthetically challenging structural motifs, including multiple 2-deoxy glycoside bonds and two 1,1-disaccharide bridges linking rings F and G, and their corresponding stereochemical requirements. In 1999, we reported the total synthesis128d-j of everninomicin 13,384-1, a structure that arguably may be considered as one of the most, if not the most, complex oligosaccharide-based molecules to be synthesized in the laboratory. Rewardingly, the total synthesis of everninomicin 13,384-1 was marked with several new synthetic technologies and strategies, notable among them were the selenium-based methods for stereoselectively assembling orthoester groupings and constructing 2-deoxy glycoside bonds.29b,128a-c,129
Figure 18.
Highlights of the total synthesis of everninomicin 13,384-1 (1999).
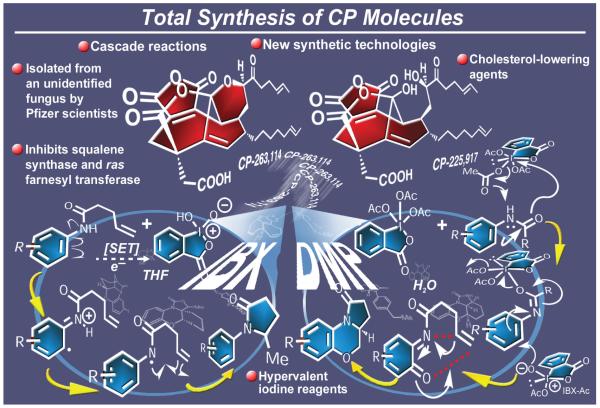
2.15. CP Molecules
The CP molecules (Figure 19), originally isolated from an unidentified fungus and later also named phomoidrides, took the chemistry community by surprise when they first made their appearance,130 causing feelings of awe and disbelief with regard to their molecular connectivities. Their unprecedented and stunningly beautiful molecular structures reported in the mid-1990s and posing as daunting synthetic challenges elicited an avalanche of research activities around the world directed toward their synthesis. In addition to their architectural appeal and because of their inhibitory activities against squalene synthase (therefore acting as cholesterol-lowering agents) and farnesyl transferase (providing potential leads for drug discovery in cancer chemotherapy), these molecules presented opportunities for biological discoveries as well as potential medical applications. After a relentless campaign,131a,b our group reported the first total synthesis of the CP molecules in 1999,131c-g an accomplishment that was followed shortly thereafter by the determination of their hitherto unknown absolute stereochemistry131d and three other total syntheses of the CP molecules from the Shair, Fukuyama, and Danishefsky groups.132
Figure 19.
Highlights of the total synthesis of CP molecules (1999).
The CP molecule labyrinth, as we coined this project, turned out to be an amazingly complex campaign from which emerged an aesthetically pleasing strategy, surpassed only by the harvest of new synthetic technologies collected along the way to the target molecules. Indeed, a plethora of cascade sequences and a series of new reactions were discovered or invented. Among them are those leading to the maleic anhydride motif of these molecules,133 the one-pot DMP (Dess-Martin periodinane)-induced conversion of 1,4-diols to the γ-hydroxy lactone system,134 and the several DMP-initiated processes leading, from aryl amides, to a variety of novel molecular architectures.135 A second, equally impressive set of reactions based on the special reactivity of IBX, another hypervalent iodine reagent, was discovered and developed during this program. These reactions included radical-based ring closures to form novel heterocycles, the introduction of unsaturation adjacent to carbonyl groups,136 benzylic oxidations,137 and a number of mild deprotection procedures.138 Furthermore, the CP project led to the development of a mild and effective procedure for the synthesis of sterically hindered diazoketones from carboxylic acid mesylates,139 a new method for the one-carbon homologation of aldehydes,140 and several DMP-based technologies for the preparation and redeployment of reactive species such as p-quinones and o-azaquinones.141 Additionally, solution and solid phase methods enabling the conversion of α-sulfonated ketones to a wide range of heterocyclic compounds and useful synthetic building blocks were developed.142 It is important to note here that, although the initial discovery of the DMP-induced cascade leading to polycyclic systems was serendipitous (underscoring this mode of discovery during total synthesis endeavors), it was logic and mechanistic rationale that led to the invention and understanding of the majority of these new chemical processes. Overall, the CP molecules program was one of the richest of our experiences in terms of new synthetic strategies and synthetic technologies, serving in that respect as a paradigm of how endeavors in total synthesis should be carried out, especially when the objective is to advance the art of chemical synthesis for its own sake.143
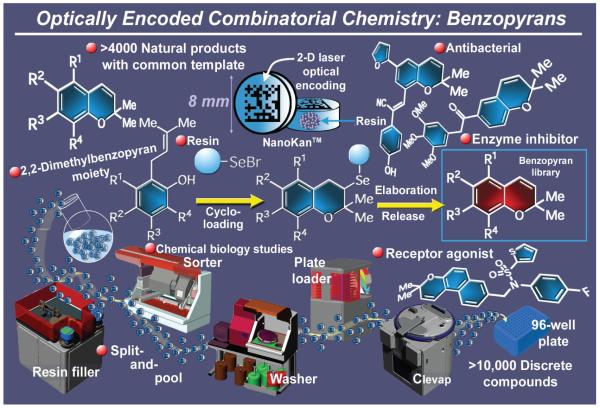
2.16. Benzopyrans
With thousands of members, the benzopyran class of natural products (Figure 20) must be one of nature’s favorite molecular types. This family of compounds boasts both a large membership and an impressively diverse range of biological activities. It was, therefore, attractive and of considerable practical interest to consider a total synthesis of these privileged structures en masse that would lead not only to a number of such natural products, but also to other biologically relevant compounds. As an integral part of this program, we also set as our goal the development of new technologies for preparing the intended compound libraries expeditiously, and in sufficient quantities and quality, for multiple biological assays. Specifically, our intention was to synthesize a benzopyran library consisting of approximately 10,000 compounds and with 1-4 mg per compound of relatively high purity. For this task we needed both automation and enabling synthetic technologies. While the automation problem had already been solved with the aforementioned IRORI technology,89 utilizing optical encoding operating NanoKans™ in conjunction with a sophisticated robotic system,144 the problem of the required chemistry remained to be addressed. It was to this end that we developed a new resin and a robust, yet synthetically fertile, linker in order to exploit the benefits of solid phase chemistry.145 Thus, starting with polystyrene, we synthesized an arylselenenyl bromide resin which rapidly absorbs suitably substituted ortho-prenyl phenols, forming solid benzopyran scaffolds via a cyclo-loading process. A highly branching scheme was then implemented to elaborate these resin conjugates into a wide range of modified scaffolds, from which the designed benzopyran compounds were released by exposure to hydrogen peroxide in a process that was accompanied with concomitant introduction of a double bond within the pyran ring.146 The latter functionality was then exploited to expand the library through epoxidation followed by nucleophilic opening of the resulting epoxides, leading to a second generation library enjoying a wide range of molecular diversity. Application of the powerful split-and-pool strategy for combinatorial synthesis in conjuction with IRORI’s optically encoded NanoKans™ and a multi-station robotic system allowed completion of the designed library in short order. Biological screening of this compound library revealed a number of leads and optimized compounds with antibacterial147 and antitumor properties,148 among others. One of the most impressive discoveries of our collaborative investigations in this area was the identification of a potent ligand for FXR, an important nuclear receptor involved in cholesterol processing, which allowed crystallization and X-ray structural elucidation of this important protein as a complex with the ligand.149 As part of a separate study involving screening against the hypoxia-inducing factor (HIF) pathway, a number of leads were identified.150 Originally inspired by natural products chemistry, and as the first application of optically-encoded combinatorial synthesis of drug-like, small organic molecules, this new technology for combinatorial chemistry represented the state of the art in the field at the time.
Figure 20.
Highlights of the solid phase combinatorial synthesis of benzopyran natural and designed molecules (2000).
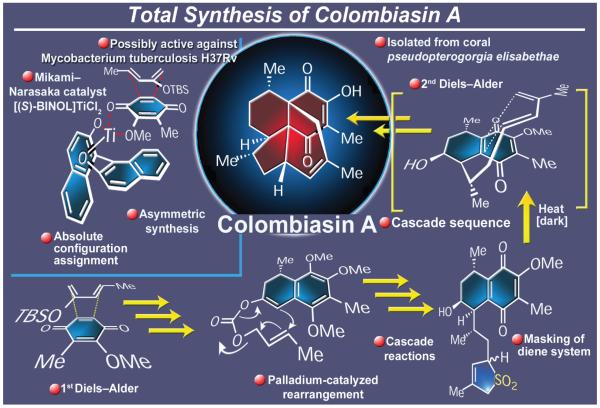
2.17. Colombiasin A
Isolated from the gorgonian coral Pseudopterogorgia elizabethae and fully characterized in 2000,151 colombiasin A (Figure 21) was attractive as a synthetic target not only because of its action against the H37Rv strain of tuberculosis bacteria, but also for its unique and challenging molecular architecture. The striking structural motifs of this tetracyclic diterpene include six stereogenic carbons, two of which are adjacent quaternary centers, and a periphery decorated with four methyl substituents, two carbonyl groups, two olefinic bonds, and one hydroxyl moiety. Moreover, and despite the scholarly spectroscopic studies which led to the elucidation of the colombiane skeleton, the absolute stereochemistry of the natural product remained unknown at the time. In view of these conditions, a total synthesis was clearly in order. In 2001, we reported the first such synthesis of this novel molecular architecture.152 Our biosynthesis-inspired strategy included two Diels-Alder reactions which served to append the three rings onto the central quinone core of the molecule. Thus, starting from a prochiral precursor, the first Diels-Alder reaction was induced to proceed in an asymmetric fashion through the use of the impressive Mikami–Narasaka catalyst. This operation was followed by a subsequent novel, palladium-catalyzed allylation process that involved two highly substituted olefinic bonds. This sequence of reactions set the stage for the second, now intramolecular, Diels-Alder reaction, which was preceded by a cheletropic elimination of sulfur dioxide from a 5-membered heterocycle that generated the required diene system. In this notable cascade reaction, the complete colombiasin framework was forged as a single endo adduct, a construction that included the installation of the two adjacent quaternary carbons of the molecule. The substrate stereochemical control achieved throughout the synthesis orchestrated by the lone chiral center initially cast by the first asymmetric Diels-Alder reaction is remarkable. As a consequence of these investigations the absolute structural assignment of colombiasin A was also accomplished.152 It was gratifying to read about the subsequently completed total syntheses of this natural product as well.153a-d
Figure 21.
Highlights of the total synthesis of colombiasin A (2001).
2.18. Hybocarpone
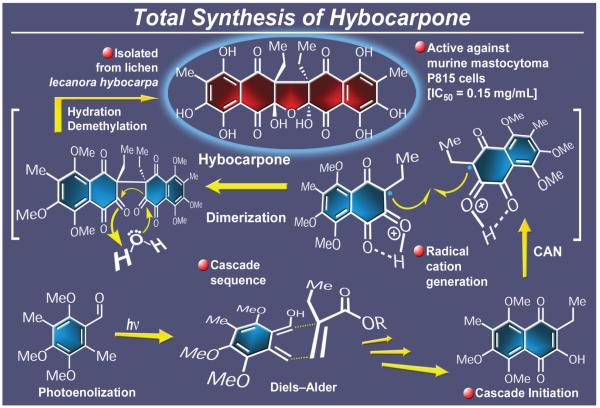
Hybocarpone (Figure 22), a unique, cytotoxic natural product, was isolated from mycobiant cultures originating from the lichen Lecanora hybocarpa.154 Posturing with an aesthetically pleasing C2-symmetric structure, this naturally occurring molecule suggested to us a biosynthetic hypothesis whereby a precursor naphtharazin unit dimerizes to afford a polycarbonyl framework, whose hydration would presumably furnish the final structure. Our postulate was confirmed by experiment and led, in 2001, to an expedient total synthesis of hybocarpone,155 but not without a painstaking campaign. After a search for the “magic” single electron transfer (SET) reagent, it was found that cerium ammonium nitrate (CAN) performed the best, leading, upon basic work-up, to the coveted crowded dimeric structure through combination of two monomeric naphtharazin units. Once formed, this dimer was pleasantly observed to undergo first hydration and then equilibration to a protected form of the natural product. Finally, hybocarpone emerged from this last intermediate upon demethylation with BBr3. This SET-induced dimerization cascade sequence is noteworthy, for it achieved, concurrently, the casting of hybocarpone’s highly hindered, central carbon–carbon bond and the forging of all four of its stereogenic centers in a stereoselective manner.
Figure 22.
Highlights of the total synthesis of hybocarpone (2001).
From the steps employed to prepare the monomeric hybocarpone precursor, the most impressive was the generation of a fleeting hydroxy-o-quinodimethane species from an aromatic aldehyde by ultraviolent irradiation. This reactive diene was swiftly captured with methyl-2-ethylacrylate through a Diels-Alder reaction to form the requisite bicyclic system. This type of reaction, although previously known in its embryonic form, was developed as part of this campaign to an advanced and highly efficient synthetic technology operating in both its intermolecular and intramolecular versions to deliver valuable intermediates and new chemical entities. As such it proved instrumental not only in the hybocarpone total synthesis just outlined, but also in the total synthesis of several members of the hamigeran family of marine natural products,156 impressive substances in their own right by virtue of their molecular architectures and antiviral properties. Thus, in this highly rewarding program, one finds a blend of biosynthesis-inspired cascade reaction strategies, development of new synthetic technologies, and synthesis of several designed molecules for biological investigation purposes.157
2.19. Apoptolidin
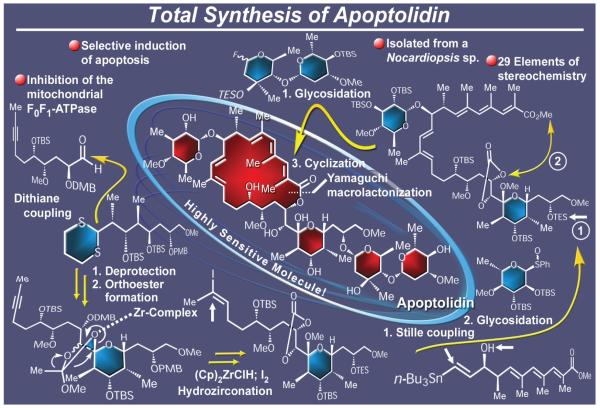
The ever-expanding macrolide class of natural products boasts some of the most impressive molecular architectures and medicinally useful agents of our times. Although numerous methods have been developed over the past few decades for their chemical synthesis, one recent isolate from Nocardiopsis sp., apoptolidin (Figure 23),158 stood out in 1997 as an exceptionally attractive synthetic challenge. Apoptolidin was so-named for its ability to selectively induce apoptosis in oncogenic rat glia cells, a property that elevated it to the status of a highly promising clinical candidate for cancer chemotherapy. Its daunting molecular architecture impressively displays no less than 30 stereogenic elements (25 stereocenters and five geometrical sites), a highly unsaturated 20-membered macrocyclic framework, and four carbohydrate units. Heightening the synthetic challenge was apoptolidin’s troublesome chemical sensitivity. Conditions arising from prolonged dissolution at ambient temperature, chromatographic manipulation, or exposure to either mild acid or mild base are sufficient cause for its transformation and destruction. Such instability dictated special considerations not only for the final stages of the total synthesis, but also for the manipulations of potentially labile intermediates carrying apoptolidin’s conjugated systems, glycoside bonds, and reactive functionalities en route. Indeed, it was after a relentless campaign full of twists and turns, frustrations, and exhilarations that we completed, in 2001, the first total synthesis of apoptolidin.159 Our successful strategy featured a Stille coupling reaction and a Yamaguchi macrolactonization to assemble the molecule’s macrocyclic core structure, and a dithiane technology-based carbon-carbon bond forming process to append a fragment appropriately functionalized for elaboration to the final C-ring glycoside domain. Other noteworthy features of this total synthesis were the subtle adjustments of reaction conditions that were necessary in order to evade the noted idiosyncrasies of the target molecule toward certain reagents and conditions, and the specific protecting group ensembles that were properly designed and installed at key points in order to allow selective deprotections along the way and in the end. Not to be forgotten was the collapse of an anomeric orthoester group to the corresponding methyl glycoside during a critical hydrozirconation step, for this event was completely unexpected and unusual. The remarkably convergent strategy employed for the total synthesis of apoptolidin from five building blocks of roughly equal molecular complexity allowed the generation of several simpler analogues whose biological evaluation established informative structure-activity relationships (SARs) within this structural type.159d It was pleasing to see the subsequent accomplishments of the total synthesis of apoptolidin.160
Figure 23.
Highlights of the total synthesis of apoptolidin (2001).
2.20. Coleophomones B and C
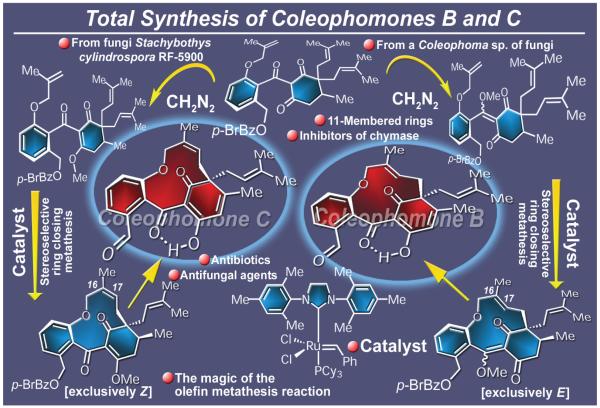
Isolated from certain species of fungi (e.g. Stachybothys cylindrospora),161 coleophomones B and C (Figure 24) exhibit impressive biological properties, including chymase inhibition as well as antifungal and antibacterial activities. Their highly strained and rigid molecular frameworks, characterized by an 11-membered ring carrying a Z-(coleophomone C) or an E-(coleophomone B) olefinic bond and a unique polycarbonyl network, presented an unusual synthetic challenge and an opportunity to test the limits of the metathesis reaction. Indeed, all tried known methods failed to form the congested macrocyclic framework of the coleophomones until an olefin ring closing metathesis-based strategy was designed and applied. The total synthesis thus developed involved an initially convergent route to obtain an advanced intermediate, followed by divergence to allow stereospecific access to both coleophomone C and coleophomone B.162 Requiring the use of the second generation Grubbs catalyst, this total synthesis extended the reach of the olefin metathesis reaction in terms of the complex and challenging molecular diversity that could be reached, and opened the way to analogue construction for biological investigations within the coleophomone class. This is but one of a number of applications of this venerable reaction in total synthesis emanating from our laboratories in recent years, a trend that is also reflected in the works of other investigators around the world.163
Figure 24.
Highlights of the total synthesis of coleophomones B and C (2002).
2.21. Diazonamide A
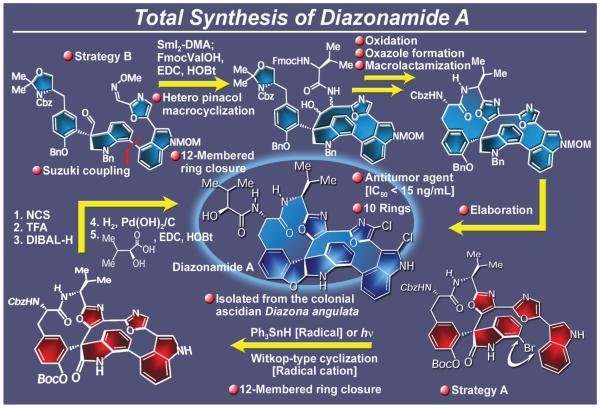
From the myriad of marine natural products isolated thus far, only a few stood out as defiant as diazonamide A164 (Figure 25), whose diabolically complex molecular architecture had to be revised165 to an even more complex and synthetically puzzling array of rings before it would be finally conquered. Isolated from the colonial ascidian Diazona angulata in the early 1990s, this marine natural product possesses potent antitumor activity164 and tantalized and defied the synthetic chemistry community for at least a decade before it was constructed in the laboratory by total synthesis.166 The first two total syntheses of the true structure of diazonamide A emerged from our laboratories in 2002167 and 2003.168 The first one167 (Strategy A, Figure 25) employed a photoinduced ring closure, a novel process that was elegantly applied in the molecule’s structural revision by Harran.165 Our second total synthesis (Strategy B, Figure 25)168 featured a cascade involving: a) a hetero-pinacol reaction induced by samarium diiodide complexed to N,N-dimethylacetamide; b) in situ N-O bond cleavage to generate an amino alcohol; and c) a peptide coupling that created the molecule’s most intransigent ring (that defined by ten atoms) and concurrently installed the required elements for the casting of the second oxazole ring onto the newly-formed macrocycle. In addition to the two distinctly different total syntheses that proved beyond doubt the revised structure of diazonamide A, the diazonamide campaign in our laboratories was marked with a rich bounty of new synthetic technologies. Most prominent among them were a series of new designed reactions of Burgess-type reagents which provided entries into several new chemical entities of medicinal interest, particularly of the sulfamide169 and sulfamidate types.170 These studies also allowed us to carry out chemical biology and structure-activity relationship (SAR) studies through analogue construction.166c,d A number of other elegant syntheses of diazonamide A have been described.171
Figure 25.
Highlights of the total synthesis of diazonamide A (2002-2003).
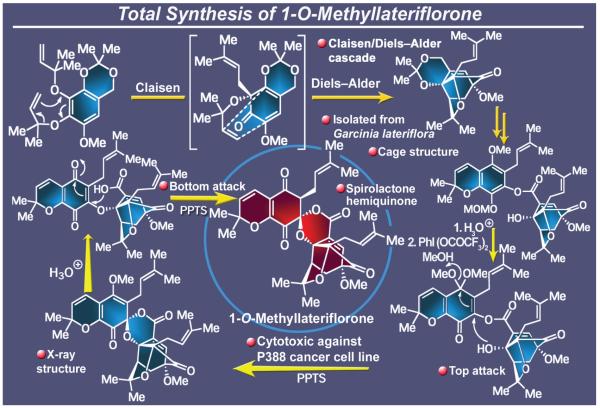
2.22. 1-O-Methyllateriflorone
Among the many secondary metabolides isolated from the Garcinia species, lateriflorone (Figure 26),172 isolated from the stem bark of Garcinia lateriflora Bl (Guttiferae), is architecturally the most complex and attractive. The reason for the distinction lies in the fact that, in addition to the signature motif of this class of natural products (the cage-like 4-oxatricyclo [4.3.1.0]decan-2-one framework), lateriflorone contains a spiroxalactone moiety whose construction presents an additional hurdle within the overall synthetic challenge. The molecule’s intriguing structural connectivity coupled with its potent antitumor activity elevated lateriflorone to a status of considerable interest as a synthetic target. Our biomimetic strategy toward this 1-O-methyllateriflorone first called upon a tandem sequence of reactions that would deploy, starting from a prochiral substrate, a Claisen rearrangement in order to set the stage for an intramolecular Diels-Alder reaction that was destined to cast the molecule’s cage-type structural unit.173 Following accomplishment of this task, the spiroxalactone motif was secured after an initial ester-based union of the two domains of the molecule through a delicate series of steps that included activation of a phenolic substrate with phenyliodonium bis-trifluoroacetate in methanol, an oxidative process that left a superfluous methoxy group within the product. This undesired moiety was concurrently removed under the acidic conditions subsequently employed to induce the formation of the required spirocyclization. The final steps leading to the methyl derivative of lateriflorone were not unlike a walk on a tight rope over the abyss, and yet they were delicately (and nervously!) carried out with success. Thus, aqueous acid conditions were required to hydrolyze the remaining methyl enol ether in a process that, distressfully, ruptured the prefabricated and obligatory ester bond, leaving the entire scaffold literally hanging by a thread, a mere oxygen atom bridge. Delightfully, the spirolactone structural motif was reconstructed in a subsequent move that entailed the mildest of acid conditions and strictly excluded moisture. This remarkable pathway, avoiding as it did the various landmines lurking in the wings, cast all the stereocenters of the lateriflorone scaffold in their correct configuration. With this region of molecular diversity landscape conquered, new pastures of molecular complexity were sought as opportunities for discovery and invention.
Figure 26.
Highlights of the total synthesis of 1-O-methyllateriflorone (2003-2004).
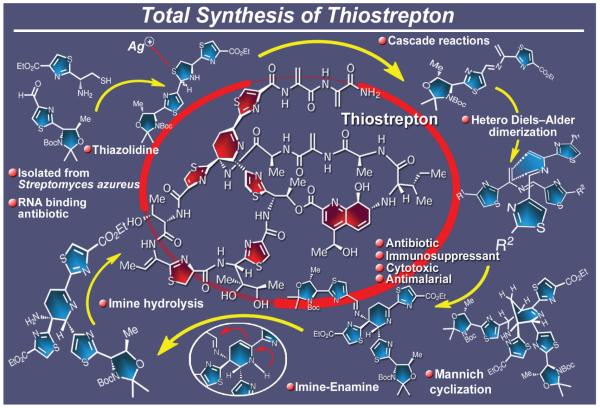
2.23. Thiostrepton
Thiostrepton, a naturally occurring antibiotic used in veterinary medicine, belongs to the thiopeptide class of natural products. Its action against bacteria is exerted through binding to the ribosome leading to inhibition of bacterial protein biosynthesis. It’s impressive biological actions include, in addition to antibacterial properties, cytotoxic, antimalarial, and immunosuppressive activities. Originally isolated from Streptomyces azureus, and subsequently from Streptomyces hawaiiensis and Streptomyces laurentii,174 thiostrepton enjoys flagship status within its class. As such, and because of its important biological and medicinal properties and challenging structure, it became a prime synthetic target in our laboratory. Figure 27 highlights our total synthesis175 of thiostrepton, which was based partly on its reported biosynthesis.176 Decorated with a number of novel and sensitive structural motifs that included a dehydropiperidine, a thiazoline, and several thiazole and dehydroanaline moieties as well as numerous elements of stereochemistry, thiostrepton posed a formidable synthetic task. Crucial to the accomplishment of this task was the biomimetic hetero Diels-Alder dimerization of an azadiene system generated from a thiazolidine precursor through the action of silver. Fine-tuning of this reaction led to an efficient synthesis of the dehydropiperidine domain of the molecule that served as the scaffold onto which thiostrepton was crafted. The target molecule was reached through a convergent strategy, but not before several thorny obstacles were overcome. Among them were the unexpected side reactions of the dehydroalanine structural motifs, including their propensity to undergo reduction under palladium-catalysis conditions, fragmentation in basic media, and conjugate addition in the presence of nucleophiles. These difficulties were evaded by masking these reactive moieties with phenylselenenyl groups, a tactic that allowed the intermediates to pass through the treacherous landscape of reactions until the second to last step, at which time the desired dehydroalanine moieties were generated through oxidation and syn-elimination of the selenium masking groups. The last step, involving fluoride-induced desilylation of all silicon-protected hydroxyl groups, had the benefit of also generating the desired olefinic bond conjugated to the thiazoline moiety in its correct geometrical configuration.
Figure 27.
Highlights of the total synthesis of thiostrepton (2004).
Proving itself to be highly rewarding in terms of synthetic strategies and tactics, the thiostrepton campaign also served as a prelude to the total synthesis of other members of the thiopeptide class of antibiotics, such as siomycin177 and antibiotics GE2270A,178,179 GE2270T178 and GE 2270C,178 as well as amythiamicins A-C.180 Amythiamicin D was first synthesized in 2004 by the Moody group.181
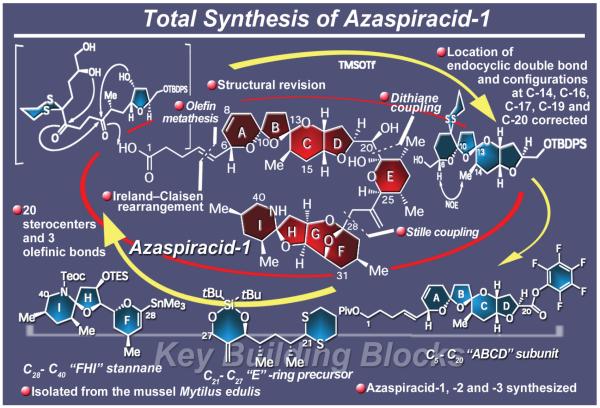
2.24. Azaspiracid-1
Few campaigns provided as much drama as that directed toward the total synthesis of the mussel toxin azaspiracid-1 (Figure 28), a substance isolated in minute quantities from the mussel Mytilus edulis.182 Causing periodic food poisoning in humans, this marine neurotoxin was first isolated in the 1990s, and a structure was assigned to it in 1998 on the basis of NMR spectroscopic and mass spectrometric evidence alone, as it was not possible to obtain a crystalline sample for X-ray crystallographic analysis. Its fascinating molecular architecture featuring nine rings and 19 stereogenic centers was characterized by three spiroketals, a spiroaminal, and a lactol, among several other reactive structural motifs. Coupled with this unique molecular framework was azaspiracid’s natural scarcity and need for larger quantities in order to be used as a tool for the device of suitable tests to detect and measure the toxin. These considerations dictated a total synthesis, a task that we undertook in the early part of the year 2000. Three years later we completed the first total synthesis of the originally proposed structure of azaspiracid-1, only to discover that it was wrongly assigned.183 Instead of ending the campaign, this unfortunate circumstance strengthened our resolve to demystify the newly precipitated conundrum. The heightened campaign now required the determination of the molecule’s true structure as well as its total synthesis.
Figure 28.
Highlights of the total synthesis and structural revision of azaspiracid-1, -2, and -3 (2004-2006).
It proved to take one more year for us to reach this dual goal, through a climactic chemical detective adventure. The revised structure of azaspiracid-1 differed considerably from the original one in that the configurations of seven stereocenters had to be inverted and a double bond had to be shifted by one position.184 Most importantly, the total synthesis of azaspiracid-1 substantially enriched its world supply and opened the way for analytical method development and biological investigations to determine the toxin’s mode of action. The convergent strategy employed for the total synthesis of azaspiracid-1 required three key building blocks, which had to be constructed in both of their enantiomeric forms –for it was not known at the outset which one was needed, and their joining together through a dithiane-based reaction and a Stille-type coupling to form the entire carbon backbone of the target molecule. Further manipulation of the carefully installed functionalities led to synthetic azaspiracid-1, which proved identical to the naturally occurring substance, thus ending the second phase of the campaign. This signaled the beginning of a third phase of the project that dealt with further analytical, biological, and chemical investigations of azaspiracid-1 and its siblings. These investigations culminated in second and improved generation total syntheses of azaspiracid-1, -2, and -3,185 and several insights regarding the chemical biology and mode of action of this class of marine neurotoxins.186 The total synthesis of ent-azaspiracid-1 has also been achieved.187
The fascinating chases of azaspiracid-1 and diazonamide A by chemical synthesis, resulting, as they did, in the revision of their originally assigned structures, provide classic examples of how total synthesis endeavors contribute, still, to the science of structural elucidation of natural products.10
2.25. 2,2|-epi-Cytoskyrin A, Rugulosin, and the Alleged Structure of Rugulin
2,2|-epi-Cytoskyrin A, cytoskyrin A,188 rugulosin,189 and rugulin190 are naturally occurring substances with striking molecular architectures (Figure 29). The latter in particular possesses a unique cage-like structural motif made of twelve carbon atoms arranged in a twisted hexagonal cylinder whose walls feature four fused 5-membered rings. These intriguing compounds belong to a large family of natural products comprised of a variety of structures featuring modified bisanthraquinone systems of differing degrees of complexity. Isolated from certain species of fungi and lichen, these compounds possess an array of biological properties, including cytotoxic, antibiotic, and anti-HIV activities. The appeal of the structural and biological features of these molecules prompted us to undertake their total synthesis utilizing a series of cascade reactions that, in fact, may be involved in their biosynthesis.189,191c During the years 2005–2007, we reported our investigations that culminated in expedient syntheses191 of several natural and designed members of this class, including 2,2|-epi-cytoskyrin, rugulosin, and the alleged structure of rugulin. These syntheses demonstrated the power of cascade reactions in total synthesis and proved that rugulin’s originally assigned structure was incorrect.191c The success of the employed cascade sequences clearly demonstrate the impact of such strategies on the art of total synthesis in terms of efficiency and elegance. It is also striking that, in this instance, these cascade reactions were initiated and sustained with the simplest of reagents (e.g. MnO2, Et3N, CSA) and conditions (thermal, 25–45 °C).
Figure 29.
Highlights of the total synthesis of 2,2|-epi-cytoskyrin A, rugulosin and alleged structure of rugulin (2005-2007).
2.26. Abyssomycin C and atrop-Abyssomycin C
Abyssomycin C and its subsequently discovered sibling, atrop-abyssomycin C, are isomeric forms of a newly discovered antibiotic found in the marine actinomycete strain Verrocosispora AB18-032 (Figure 30).192 The originally isolated abyssomycin C inhibited the growth of methycillin-resistant Staphylococcus aureus (MRSA) at MIC = 4 μg mL−1 and vancomycin-resistant S. aureus (VRSA) at MIC = 13 μg mL−1. Its mechanism of action was shown to involve inhibition of 4-amino-4-deoxychorismate (ADC) synthase or lyase, two essential enzymes for the conversion of chorismate to p-aminobenzoic acid (PABA) in bacteria, giving the distinction to abyssomycin C as the first natural product to exhibit this activity.192 The lure of abyssomycin C’s novel structural motifs and its important biological activity prompted us and several other groups to embark on its total synthesis. In addition to rendering the originally isolated abyssomycin C readily available, our total synthesis,193reported in 2006, unearthed atrop-abyssomycin C, which proved to be an even more potent antibiotic than its originally discovered isomer. Interestingly, atrop-abyssomycin C was later isolated as a naturally occurring substance.194 The synthetic strategies and tactics employed in this adventurous campaign enriched the art of synthesis in ways that included improvement in the Diels-Alder reaction (Lewis acid-templated), expansion of the scope of the ring closing metathesis reaction, insights into the phenomenon of atropisomerism in natural products chemistry, and mechanistic understanding of the chemical reactivity of the unusual structural motifs found in the molecular architectures of the abyssomycins. In addition, this program provided, through chemical biology studies, a number of structure-activity relationships (SARs) within this structural class.193 That total synthesis sometimes leads to a natural product prior to its isolation from nature was once again highlighted in this program, as it was in the endiandric acid campaign conducted earlier in our laboratories.16 This scenario most likely will be encountered again in future endeavors of this kind. We note with pleasure the elegant contributions in the abyssomycin field by Sorensen195 and others.196
Figure 30.
Highlights of the total synthesis of abyssomycin C and atrop-abyssomycin C (2006-2007).
2.27. Marinomycins A–C
Reported in 2006, marinomycins A–C are marine-derived natural products isolated from an actinomycete Marinispora strain that possess striking molecular structures and biological properties (Figure 31).197 The most abundant of the three, marinomycin A features a C2-symmetric 44-membered macrodiolide structure with extended all-trans conjugated systems, while its siblings, marinomycins B and C, are geometrical isomers at one or both of the olefinic sites adjacent to the aromatic rings, respectively. Marinomycin A is converted to marinomycins B and C under the influence of light. Most importantly, this compound exhibits potent antibacterial, antifungal, and cytotoxic activities. Its scarcity and unusual molecular architecture coupled with its important biological properties made it and its siblings, marinomycins B and C, attractive synthetic targets. In 2007, we reported the total synthesis of marinomycins A–C and their monomeric versions, monomarinomycin A and isomonomarinomycin A.198 The total synthesis featured a ketophosphonate-aldehyde coupling and a hydroxyl-directed carbonyl reduction to generate the C-12 to C-27 fragment, which was coupled with the C-1 to C-11 fragment through a Mitsunobu esterification [①] (which was accompanied by configurational inversion of the participating hydroxyl group). These operations were followed by hydroboration [②] and Suzuki coupling [③] to afford the required monomeric unit of the molecule. Reiteration of these processes ([④], [⑤], [⑥]) ended with the challenging macrocyclization from which emerged, through an intramolecular Suzuki coupling and deprotection, marinomycin A. It is rather interesting to note that, despite its C2-symmetric nature, marinomycin A resisted a direct dimerization approach (through Suzuki, Stille, and Heck reactions), forcing us to employ a stepwise strategy. All direct dimerization attempts employing the Suzuki reaction led to a monomeric macrolide species which, upon deprotection with TBAF, furnished a mixture at C-25 and C-27 monomeric lactones, named monomarinomycin A and isomonomarinomycin A.198,199 Both monomeric marinomycins were found to be devoid of the biological properties of the naturally occurring marinomycin A, underscoring the strict structural requirements of the latter for biological activity. The synthetic challenge posed by marinomycins A-C provided us with the opportunity to explore and compare the scope of a number of popular carbon-carbon bond forming reactions such as the Suzuki, Stille, and Heck cross coupling reactions118 as well as the olefin metathesis reaction.163 In this particular instance it was the Suzuki reaction that proved the most valuable, and that success was realized only when a stepwise synthetic strategy was adopted.
Figure 31.
Highlights of the total synthesis of marinomycins A, B and C (2006-2007).
2.28. Platensimycin
Platensimycin was reported in 2006200 by a Merck group who discovered it from a strain of Streptomyces platensis by means of a new screening technique specifically tailored to detect inhibitors of the FabH and FabF condensing enzymes of the fatty acid synthesis pathway in bacteria (Figure 32). Acting through inhibition at FabF, a mechanism not shared by any clinically used antibiotic, platensimycin was hailed initially as a highly promising compound for development as a potential antibacterial agent, particularly since it showed no cross resistance and was found to be active against a broad range of Gram-positive bacteria. The excitement generated by the potential of platensimycin as a new antibiotic, coupled with its novel molecular structure, proved irresistible to many groups of synthetic organic chemists around the world, including ours. Among the several syntheses of this molecule from our laboratories,201,202 highlighted here is the asymmetric version of our original approach which utilized a spiro bis-enone aldehyde as a key intermediate obtained through an enantioselective rhodium-catalyzed cycloisomerization of a prochiral enyne bis-enone.201c,f This enantiopure compound was converted to the tetracyclic ketolide core of the target molecule through a samarium diiodide-induced ring closure followed by an acid-facilitated cyclic ether formation. The latter was elaborated to the required carboxylic acid through a sequence involving alkylation and olefin cross metathesis. This fragment was then coupled to the polar domain of the molecule featuring an aromatic amine through amide bond formation. This and the other strategies developed in this research program served admirably well to prepare several other natural and designed members of the platensimycin family for biological studies,203 which established useful structure-activity relationships (SARs) within this structural type. Among the most interesting and potent platensimycin analogues synthesized in this campaign were adamantaplatensimycin203a and carbaplatensimycin,203b both of which exhibited comparable activities to the natural product despite the lack of the ether oxygen atom in their polycyclic core. In addition to these chemical biology studies, this program provided the opportunity to discover and develop several synthetic methods, among which the rhodium-catalyzed asymmetric cycloisomerization of enynes201d,e proved to be the most useful. Numerous other syntheses of platensimycin have been achieved.202,204
Figure 32.
Highlights of the total synthesis of platensimycin (2007-2010).
2.29. Kinamycins C, F and J
Boasting the unique diazofluorene structural motif, the kinamycins discovery in 1970205 challenged structural and synthetic organic chemists alike for decades before yielding to their advances (Figure 33). Their appeal derives not only from their novel tetracyclic structures but also from their potent biological activities as antibiotics and antitumor agents. Our enantioselective and convergent total synthesis of kinamycins, as reported in 2007,206 included a number of interesting strategies and tactics such as an Ullmann coupling of an aryl bromide and a vinyl iodide under improved conditions that required Pd2(dba)3 (cat.), CuI (cat.) and Cu metal, an organocatalytic benzoin-type ring closure to forge the 5-membered ring of the molecule, a samarium diiodide-induced deoxygenation, and a stereoselective selenium dioxide mediated hydroxylation to afford an advanced fluorenone intermediate. Elaboration of the latter through its tosyl hydrazone led to kinamycin C. The efficiency and flexibility of the developed route allowed the synthesis of other members of this class of compounds, including kinamycins F and J. A number of other syntheses of members of the kinamycin family have also been reported.207
Figure 33.
Highlights of the total synthesis of kinamycin C, F and J (2007).
2.30. Uncialamycin
Isolated from a marine species related to Streptomyces cyanogenus, uncialamycin was reported in 2005 and assigned a structure that placed it in the enediyne class (Figure 34).208 The extremely limited amounts isolated from nature, however, prevented its full structural elucidation and biological evaluation. The latter was as important as the former, for initial results indicated highly potent DNA cleavage and phenomenal antibacterial properties against, for example, Staphylococcus aureus (MIC = 0.0000064 μg mL |1), Burkholderia cepacia (MIC = 0.001 μg mL |1), and Escherichia coli (MIC = 0.002 μg mL |1).208 Lured by the opportunities presented by uncialamycin for chemical synthesis and chemical biology studies, we initiated a program directed at its total synthesis, full structural characterization, and biological investigation. In 2007, we reported the first total synthesis and relative configuration assignment of racemic uncialamycin.209 In 2008, we completed an enantioselective total synthesis of the molecule that delivered its naturally occurring antipode in multimilligram quantities that allowed its extensive biological investigation.210 The enantioselective total synthesis of uncialamycin featured a catalytic asymmetric Noyori reduction to install the first chiral center, an acetylide-aldehyde ring closure to forge the 10-membered enediyne ring, and a Hauser annulation to complete the anthraquinone system of the molecule. This chemistry was reminiscent of our previous enediyne model studies in the dynemicin area38,43i,j and the total synthesis of dynemicin itself by the groups of Myers211 and Danishefsky.212 The ample supplies of synthetic uncialamycin allowed us to demonstrate its Bergman cycloaromatization-based mechanism of action, its ability to inflict double strand DNA cuts, and its potent antibacterial and cytotoxic activities.210 Based on the latter results, uncialamycin was selected for further study by the National Cancer Institute (NCI, USA) in their 60 human tumor cell line panel, an evaluation that revealed its lethal and selective potencies against melanoma (M14, LC50 = 9 nM; compare Taxol®: LC50 = 80 μM, epothilone B: LC50 = 80 μM), breast cancer (MDA-MB-468, LC50 = 8 nM), lung cancer (NCI-H226, LC50 = 20 nM), and central nervous system cancer (SF-295, LC50 = 6 nM) cells.210 The uncialamycin campaign demonstrated several important aspects of the art of total synthesis, including its value in structural elucidation and biological investigation of scarce natural products. The latter endeavors are becoming increasingly more common as isolation chemists spread their nets wider and deeper into the oceans to discover exotic and rare bioactive molecules.
Figure 34.
Highlights of the total synthesis, structural elucidation, and biological evaluation of uncialamycin (2007-2010).
2.31. Biyouyanagins A and B
Biyouyanagins A213 and B,214 isolated from Hypericum chinense, provided fascination and intrigue because of their novel molecular structures and important biological properties, including anti-HIV activity and lipopolysaccharide (LPS)-induced cytokine production inihibition (Figure 35). First to be reported from nature was biyouyanagin A, whose originally assigned all-cis stereochemical arrangements around the cyclobutane ring seemed unorthodox, if not impossible. Our attempts to synthesize it through a biomimetic [2+2] photocycloaddition reaction from its perceived components, a zingiberene and hyperolactone C, led to both its total synthesis and structural revision to a cis, trans, cis, trans configuration around the cyclobutane system.215 Equally satisfying to the photoinduced fusion of the two domains of the molecule into its final form were the cascade-based constructions of the various isomeric zingiberenes and hyperolactones employed in this synthesis and the subsequent studies. Following our total synthesis and structural revision of biyouyanagin A,215 its sibling, biyouyanagin B, was reported as a natural product isolated from the same plant species. The originally assigned structure of this new biyouyanagin had the same overall pentacyclic framework and functionalities as our revised biyouyanagin A structure, but the opposite configurations at C-4, C-22, and C-24.216 However, our synthetic investigations proved that the new structure was also in error and revealed its true structural identity as that shown in Figure 35.216 In addition to the chemical synthesis and structural elucidation advances made in this program, the modular synthetic strategy developed toward the biyouyanagin molecular scaffold allowed us to make significant contributions to the chemical biology of these molecules.217 Thus, a series of designed biyouyanagin analogues were designed, synthesized, and evaluated in a number of biological assays leading to the discovery of promising anti-HIV and anti-arenavirus agents, as well as a number of selective inhibitors of lipopolysaccharide (LPS)-induced cytokine production. These biological studies underscored, once again, the importance of natural products as lead compounds for chemical biology and drug discovery research efforts, and of total synthesis as a tool for implementing such studies.218
Figure 35.
Highlights of the total synthesis and structural revision of biyouyanagins A and B (2007-2011).
2.32. Platencin
A relative of platensimycin, platencin (Figure 36) was isolated by Merck scientists from Streptomyces platensis MA7339219 and exhibited the same mechanism of action and comparable in vitro and in vivo antibiotic activites against Gram-positive bacteria, including methycillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant S. aureus (VRSA),219 to those of its earlier isolated sibling.200 The two antibiotics contain the same aromatic moiety but differ in their ketolide domain, with platencin possessing a wholly carbocyclic core comprised of three rings in contrast to platensimycin whose core structure includes a tetracyclic framework with an oxygen atom knitted within as an ether moiety. In view of the potential importance of platencin to medicine and its significant structural differences from platensimycin, this newly discovered antibiotic was deemed a worthy synthetic target. In 2008, we reported the first asymmetric total synthesis of platencin in its natural enantiomeric form.220 Notable highlights of this synthesis include a Rawal catalytic asymmetric Diels-Alder reaction, a gold-catalyzed cyclization, a homoallylic radical rearrangement to form the [2.2.2] carbocyclic system, an intramolecular Aldol reaction, and an amide bond-forming coupling reaction that joined together the two domains of the molecule. Several other syntheses of platencin have been published subsequently.221
Figure 36.
Highlights of the total synthesis of platencin (2008-2010).
2.33. Cortistatin A
Cortistatin A is a member of a new marine-derived family of natural products with novel molecular structures and exceptional biological properties (Figure 37).222 Isolated from the sponge Corticium simplex, this natural product boasts an unusual heptacyclic framework featuring the unique abeo-9(10-19)-androstane-type steroidal system with substituents on rings A and E, the latter carrying an isoquinoline structural motif.222 Its biological activities include selective antiproliferative properties against human umbilical vein endothelial cells (HUVECs, IC50 = 1.8 nM) with a selectivity index of more than 3000 in comparison to its action against normal human dermal fibroblast (NHDF) and several other tumor cells (KB3-1, K562, Neuro2A). The exciting biological profile and promising potential for medical applications of cortistatin A and its congeners was only tempered by their natural scarcity, which prevented full biological investigations and structure-activity relationship (SAR) studies. Urgently fulfilling the criteria of an attractive synthetic target, cortistatin A became the object of our investigations aiming at its total synthesis and biological evaluation. Carried out in our Singapore laboratories (ICES, A*STAR) in association with D. Y.-K. Chen, the total synthesis of cortistatin A featured a number of cascade reactions, most notable of which was a sequence involving a 1,4-addition of an alkoxide to an enone followed by dehydration of the resulting aldol to afford the molecule’s oxabicyclo system.223 Other worthy reactions employed in this endeavor were a palladium-catalyzed Suzuki-Miyaura coupling to attach the isoquinoline fragment onto the main domain and the Hajos-Parrish reaction224 that provided the starting bicyclic building block225 for the construction of the ABCDE steroidal framework of the molecule.
Figure 37.
Highlights of the total synthesis of cortistatin A (2008-2009).
In addition to the synthesis of cortistatin A, this campaign also delivered cortistatin J and an array of analogues that served well in establishing a number of structure-activity relationships.223b Important insights gained from these chemical biology studies were the recognition that considerably simpler structures resembling the parent structure are capable of exhibiting comparable activities and potencies to those of the natural cortistatins.223b Significantly, studies in collaboration with an Amgen team revealed clues about the mechanism of action of the cortistatins and the nature of their biological receptor.226 We were pleased to see the many other important contributions to the cortistatin field from various other laboratories.227-230
2.34. Hopeanol and Hopeahainol A
Isolated from the plants Hopea exalata and Hopea hainanensis, hopeanol was reported as a cytotoxic agent.231 Its sibling, hopeahainol A, was also discovered in the latter plant species and found to exhibit acetylcholinesterase inhibitory properties, establishing it as a lead compound for discovery programs in the field of Alzheimer’s disease.232 Possessing novel molecular architectures, hopeanol and hopeahainol A belong to the polyphenol class of natural products (Figure 38). Their unusual structural motifs and similarities, together with their reported biological profiles, prompted us to embark on their total synthesis, a task that was accomplished in our Singapore laboratory (ICES, A*STAR) in collaboration with D. Y.-K. Chen in 2009233 and reported in full detail in 2010.234 The enantioselective synthesis of these molecules involved a number of interesting intramolecular reactions and cascade sequences, including: a) an intramolecular Friedel-Crafts type reaction to set the C7b quaternary center; b) a Grob-type fragmentation to rupture a γ-lactone and generate the required olefinic bond in the growing molecule; c) epoxidation of the newly generated olefinic bond followed by a second intramolecular Friedel-Crafts reaction to forge the 7-membered ring; d) a cascade reaction that involved global demethylation and oxidation to the p-quinodimethane structural motif, leading to hopeahainol A; and e) base-induced rearrangement of hopeahainol A to hopeanol that involved ring opening methanolysis of the γ-lactone and ring closure to form the complete ring framework of the latter. The successful conversion of hopeahainol A to hopeanol allowed us to propose a hypothesis for the biosynthesis of the latter natural product from the former, whereas our inability to carry out the reverse transformation cast doubts over the previously proposed biosynthesis.232 In addition, our synthetic endeavors rendered both natural products and some of their isomers readily available for biological studies. These studies confirmed234 the reported acetylcholinesterase inhibitory activity of natural hopeahainol A, but failed to confirm the reported cytotoxic activities of hopeanol.231 Overall, this project contributed to our growing knowledge of the polyphenols and other resveratrol-related secondary metabolites235 and enriched our repertoire of cascade reactions.21,236
Figure 38.
Highlights of the total synthesis of polyphenols hopeanol and hopeahainol A (2009).
2.35. Bisanthraquinone Antibiotic BE-43472B
Isolated from certain species of Streptomyces,237 bisanthraquinone antibiotic BE-43472B (Figure 39) exhibited antitumor237 and antibiotic properties,238 including bactericidal activity against clinical bacterial isolates of the Gram-positive pathogens methycillin-susceptible Staphylococcus aureus (MSSA), methycillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VRE), and tetracyclic-resistant S. aureus (TRSA).238 The appeal of its unique molecular architecture coupled with its promising antibacterial properties led us to initiate a program directed toward its total synthesis. In 2009,239 we reported the first total synthesis and absolute configuration of antibiotic BE-43472B featuring a number of striking reactions. One of the most impressive features of this enantioselective synthesis239,240 is the cascade sequence initiated by a thermally-facilitated intermolecular Diels-Alder process that led, upon two additional ring closures, to the formation of the entire octacyclic framework of the target molecule. Another important finding of the endeavor was and the photochemically-induced α,β-epoxyketone rearrangement to afford the molecule’s conjugated ketoenol structural motif. Demonstrating the power of cascade reactions in the synthesis of complex molecules,21 the developed strategy also emphasized the importance of the use of light and heat as means of achieving green chemistry in chemical synthesis. The synthetic technologies developed also delivered, in addition to the natural antibiotic BE-43472B, its enantiomer (that, interestingly, exhibited comparable antibacterial properties) and a number of analogues for biological evaluation. The latter investigations240 led to a set of useful structure-activity relationships (SARs), opening the way for further chemical biology and medicinal chemistry efforts.
Figure 39.
Highlights of the total synthesis of bisanthraquinone antibiotic BE-43472B (2009–2010).
2.36. Sporolide B
Sporolide B241 is thought to be one of two chemical metabolites of the yet to be isolated natural product named presporolide (a 9-membered ring enediyne whose spontaneous Bergman cycloaromatization is expected to give sporolide B and its p-chlororegioisomer, Figure 40).241 The unprecedented molecular architecture of this substance (isolated from the marine Actinomycetes Salinospora tropica) and the mystique surrounding its origin prompted us to undertake the ambitious task of its total synthesis. In 2009, we reported the total synthesis of sporolide B242 and in the following year we published the full details of our investigation.243 These included the synthesis of 9-epi-sporolide B and the exploration of the hetero [4+2] cycloaddition reaction of o-quinones with indene-type dienophile partners. Central to the synthetic strategy employed in this construction are a) the ruthenium-catalyzed [2+2+2] cycloaddition reaction between a propargylic alcohol and a chloroacetylenic cyclopentene to fashion the chloroindene domain, and b) the intramolecular, thermally-induced [4+2] cycloaddition process to forge, concurrently, the [1,4]-dioxane and the 13-membered macrocycle structural motifs of the molecule. The exquisite performance of the intramolecular [4+2] cycloaddition process in selectively forming the desired product became possible only after inversion of the C-9 substituent in the precursor, a condition that was reversed in a subsequent step through a 1,3-directed reduction of a carbonyl group to a hydroxyl moiety with the correct configuration. This rewarding campaign enriched our knowledge of o-quinones as partners in [4+2] cycloadditions and demonstrated the power of this reaction in complex molecule construction. It also expanded the scope of the ruthenium-catalyzed [2+2+2] cycloadditions of chloroacetylenic substrates and highlighted their importance in reaching molecular complexity of previously unknown type.
Figure 40.
Highlights of the total synthesis of sporolide B and 9-epi-sporolide B (2009–2010).
2.37. Vannusals A and B
One of the most puzzling structural problems we had the opportunity to solve in our group through total synthesis was that of the vannusals. Isolated from the marine ciliate Euplotes vannus, vannusals A and B were reported in 1999 (Figure 41).244 Their unique hexacyclic molecular architectures, displaying 13 asymmetric centers, provided a formidable synthetic challenge that lured us into a most adventurous campaign directed toward their total synthesis. Indeed, the undertaken synthetic endeavor led to a successful synthesis of the originally assigned vannusal B structure, but only to prove that it was incorrect.245 This initially disappointing event precipitated a new campaign directed at the equally challenging task of determining the true structures of vannusals A and B. It was after considerable effort, and not before synthesizing eight vannusal isomers (of the 213 = 8192 possible stereoisomers of the originally assigned structures), that we finally triumphed over this structural conundrum.246 To be sure, it was because of this unexpected additional structural challenge that, in the end, the vannusal project proved so enriching and rewarding, for it was full of adventure and intrigue not to mention serendipity, defeat, triumph, and excitement.
Figure 41.
Highlights of the total synthesis and structural revision of vannusals A and B (2009-2010).
Chemical highlights of the vannusal total synthesis endeavor include: a) admirable and well-serving convergency as expressed in the lithium-mediated coupling of the AB domain (as a vinyl lithium species) with the DE domain (as an aldehyde); b) a samarium diiodide-mediated SN2|-type cyclization to forge the final ring (C) of the molecule’s polycyclic framework; and c) a number of stereoselective reactions that transformed the synthetic strategy into a highly flexible route delivering an array of vannusal stereoisomers. In addition to the prowess of the art of total synthesis, credit for the successful conclusion of this project should be given to the students for their astuteness to employ enantiopure AB fragment in combination with racemic DE fragment, a particularly fortunate event in this case, for it eventually led to the realization that the required DE domain was the antipode of that embedded in the originally assigned structure.244 This recognition was indeed the key to solving the puzzle of the true structures of the vannusals, which turned out to require inversion at eight stereogenic centers of the originally assigned structure. In concluding this vignette, we should mention that the vannusal endeavor also included the development of a rhodium enolate-based catalytic asymmetric reaction to form carbon-carbon bonds,247 the expansion of the scope of samarium diiodide-based reactions in chemical synthesis,248 the opportunity to test and demonstrate the power of computational methods to predict structures,249 and the rendering of these scarce marine natural products and their analogues readily available for biological investigations.
2.38. Hirsutellone B
Isolated from a species of fungi, hirsutellone B and its congeners exhibited promising properties against Mycobacterium tuberculosis, the causative pathogen of tuberculosis (Figure 42).250 Its novel molecular architecture includes a fused [6,5,6]-tricyclic core, a γ-lactam, and a 13-membered p-cyclophane structural motif that possesses an ether linkage, a ketone group, and a tertiary hydroxyl moiety. All in all, the hirsutellone B molecule includes five rings and 10 stereogenic centers and presents a formidable and thorny synthetic problem. Its major synthetic challenge derives from its rather strained p-cyclophane ring. Its total synthesis251 featured an aesthetically pleasing diethylaluminium chloride-induced cascade sequence that started from a polyunsaturated epoxide and proceeded through a regio- and stereoselective epoxide opening and an intramolecular Diels-Alder reaction to generate the molecule’s tricyclic core. Equally satisfying was a transannular Ramberg-Bäcklund reaction that formed the macrocyclic structural motif from a macrocyclic sulfone one size larger than the rather intransigent 13-membered p-cyclophane system of the target molecule. The developed synthetic route was also diverted from a key intermediate to deliver, through a number of bioinspired steps, hirsutellones A and C,252 two of hirsutellone B’s siblings as well as a number of analogues for biological investigations. A second total synthesis of hirsutellone B was recently reported.253
Figure 42.
Highlights of the total synthesis of hirsutellone B (2009).
2.39. Aspidophytine and Haplophytine
Haplophytine is the active insecticidal principle of the wild flower Haplophyton cimicidum, valued for eons in Central America for its beauty and insecticidal properties (Figure 43). Isolated in 1952254 and structurally elucidated in 1973,255 this highly complex and challenging natural product resisted the advances of synthetic organic chemists for almost four decades256 before it was conquered in 2009 by two groups, those of Fukuyama–Tokuyama257 and ours (Nicolaou-Chen).258 In contrast, multiple syntheses of its smaller relative (and one that represents its entire right wing where the crowded bridging C-C bond is replaced with a hydrogen atom), aspidophytine, have been reported during the decade ending in 2008,259 beginning with Corey’s unsurpassed cascade synthesis in 1999.259a The last of these total syntheses of aspidophytine,259f emanating from our laboratories, also served to prepare the ground for our eventual total synthesis of the long sought-after haplophytine.258
Figure 43.
Highlights of the total synthesis of haplophytine (2009).
This strategy toward aspidophytine entailed rapid annulation of the D ring onto the indole structural motif and a radical-based cyclization to forge the E ring of the molecule, allowing for sufficient flexibility to accommodate the more complex haplophytine intermediates that were eventually grown into the target molecule. The final assembly of haplophytine was achieved in an enantioselective manner and involved construction of the left wing of the molecule with an indoline domain already installed, followed by conversion of the latter to the requisite indole boronic acid derivative that allowed the incorporation of the D ring through a palladium-catalyzed cross coupling reaction and sequential forging of the remaining rings through a radical cyclization and an oxidative lactonization. Relying heavily on powerful synthetic strategies and technologies, the total synthesis of haplophytine reflects and symbolizes the sharp state of the art of chemical synthesis as it stands today.
2.40. Epicoccin G
Epicoccin G is a member of a large family of natural products characterized by their conspicuous diketopiperazine structural motif which carries either a bis-methylthio moiety or an epidithio system (Figure 44). Indeed, it is this unique structural domain that distinguishes this class of compounds from any other and endows them with an array of biological activities which include antiviral and cytotoxic properties. Isolated from the endophytic fungus Epicoccum nigrum, epicoccin G exhibits anti-HIV activity in infected C8166 cells with an IC50 value of 13.5 μM.260 Its C2-symmetric architecture displays, in addition to the central bis-methylthio diketopiperazine system, two flanking bicyclic frameworks, each carrying a 1,4-hydroxyketone moiety onto the outer, 6-membered carbocyclic ring. Amenable to a bis-directional synthetic strategy, the structure of epicoccin G pointed to a concise approach that utilized N-boc-tyrosine as a starting material, and employed a stepwise dimerization process to construct a C2-symmetric bis-diene diketopiperazine intermediate that served as the key template onto which the required functionalities were crafted regio- and stereoselectively. These delicate operations were carried out through a series of reactions— some of the cascade type—that are notable for their aesthetic appeal and overall efficiency. These reactions included sulfenylation followed by sodium borohydride reduction of the resulting polysulfide species and in situ methylation, singlet oxygen bis-[4+2] cycloaddition to furnish the two plannedendoperoxide units, and regioselective Kornblum-DeLaMare rearrangement261 to afford the corresponding bis-hydroxy enone, whose smooth hydrogenation in the presence of palladium hydroxide catalyst gave the targeted natural product epicoccin G.262 Employing the same bis-diene intermediate through a slightly modified sequence, 8,8′-epi-ent-rostratin B,263 a relative to epicoccin G featuring an epidithiodiketopiperazine structural motif, was also synthesized,262 demonstrating the scope of the developed synthetic strategies and technologies. Among the latter, the improved sulfenylation procedure264 involving in situ generation of reactive sulfur species by reacting S8 and NaHMDS followed by addition of the diketopiperazine substrate, and the rarely used Kornblum-DeLaMare rearrangement,261 are particularly notable. Furthermore, this endeavor led to an array of rather exotic compounds carrying the special epidithioketopiperazine and endoperoxide moieties that may reveal interesting chemical and biological properties.
Figure 44.
Highlights of the total synthesis of dithioketopiperazines epicoccin G and 8,8|-epi-ent-rostratin (2011).
The above examples from our laboratories provide a demonstration of the newly acquired power of total synthesis to reach higher levels of molecular complexity and a wider range of structural diversity. Of particular significance in most of these projects was the broadening of the total synthesis endeavor to include in its scope the discovery and development of new synthetic strategies and technologies as well as the design, synthesis, and biological investigation of novel analogues of the target molecules. Figure 45 summarizes the inspirational features of nature’s molecules for total synthesis and the discoveries and inventions often derived from such challenging but rewarding endeavors. Not to be forgotten or underestimated are the unique educational and training opportunities provided to students through such demanding research programs.
Figure 45.
Advancing the art and science of total synthesis through inspirations from nature (1976–2001).
3. Contributions from Other Laboratories
The enterprise of the total synthesis of natural products and their derivatives of biological and medical significance has seen a major surge that resulted in impressive strides in the last few decades. These developments occurred primarily because of the abundance of enabling new synthetic methods and strategies that emerged during this period, including asymmetric catalysis, metal catalysis, the olefin metathesis reactions, organocatalysis, and cascade sequences, among others. The achievements in total synthesis in recent decades from the many laboratories practicing the art around the world are far too many to present here in any detail due to space limitations. It suffices to say, however, collectively these accomplishments provide a testament to the remarkable level of sophistication of the art of total synthesis, its ability to replicate nature’s molecules in the laboratory, and it’s potential to make important contributions to biology and medicine.
Figures 46-50 depicts a collection of selected natural products synthesized, together with the principal investigators from whose laboratories these remarkable syntheses emanated265-358 since 1999, when we last reviewed the art and science of total synthesis.6 In that review,6 we provided collections of some of the most intriguing molecules constructed in the laboratory through total synthesis from 1828 when Friedrich Wöhler synthesized urea to the end of the twentieth century. As a new generation of synthetic organic chemists takes over the trajectory of the art and science of total synthesis, we will, for sure, witness new leaps forward in terms of efficiency, practicality, and biomedical applications.
Figure 46.
Selected natural products synthesized in various laboratories since 2000.
Figure 50.
Selected natural products synthesized in various laboratories since 2000.
4. Conclusion and Future Perspectives
As highlighted above, the state of the art of total synthesis continues its ascent through innovative approaches and support from new methodological and conceptual advances. These improvements are manifested in higher efficiencies, shorter processes, greener chemistry, elegant strategies and new synthetic technologies. In addition, and most significantly, the science of total synthesis has moved beyond the molecule and into the domain of its biological function and potential in medicine. Thus, endeavors in total synthesis are often the prelude of further investigations directed toward the design, synthesis, and biological evaluation of analogues. Frequently, such studies lead to useful biological tools and drug candidates, thereby resulting in much added value to the endeavor. Another recent phenomenon within the realm of total synthesis is the increase in interdisciplinary collaborations of its practitioners with biologists and scientists from the pharmaceutical and biotechnology industries. These efforts should strengthen and accelerate translational research, which is destined to yield new biomedical breakthroughs and cures for disease. Indeed, the road from nature to the laboratories of chemistry and biology and into the clinic has seen many glorious achievements in the past.1,14 With the newer trends in total synthesis, this road can only get smoother and more effective in delivering benefits to society.
Figure 3.
Selected natural products synthesized in the author’s laboratories (selected references given in the text).
Figure 47.
Selected natural products synthesized in various laboratories since 2000.
Figure 48.
Selected natural products synthesized in various laboratories since 2000.
Figure 49.
Selected natural products synthesized in various laboratories since 2000.
Acknowledgements
It is with great pleasure that we acknowledge the extraordinary contributions of our co-workers, whose names are found in the cited papers. We also wish to thank David J. Edmonds and James J. Crawford for their assistance in the preparation of the manuscript. The work described in this article was primarily supported over the years by grants from the National Institutes of Health (USA), the National Science Foundation, The Skaggs Institute for Chemical Biology, and A*STAR Singapore. C. R. H. H. thanks the NSF for a Graduate Research Fellowship and C. N. thanks the Alexander von Humboldt Foundation for a Feodor Lynen Research-Fellowship. We also thank our many colleagues and friends from the pharmaceutical and biotechnology sectors for their generous financial support that made the work described herein possible.
Author Biosketches
K. C. Nicolaou, born in Cyprus and educated in UK and USA, is Chairman of the Department of Chemistry at The Scripps Research Institute where he holds the Darlene Shiley Chair in Chemistry and Aline W. & L. S. Skaggs Professorship in Chemical Biology, and is Distinguished Professor of Chemistry at the University of California, San Diego. The impact of his work in chemistry, biology and medicine flows from his contributions to chemical synthesis as described in over 700 publications. His dedication to chemical education is reflected in his Classics in Total Synthesis book series and Molecules that Changed the World, and his training of hundreds of graduate students and postdoctoral fellows. He is a Member of the National Academy of Sciences (USA), the German Academy of Sciences Leopoldina, and the American Philosophical Society, and a Corresponding Member of the Academy of Athens.
Christopher R. H. Hale, born in Minneapolis, MN, USA, received his Bachelor of Science degree summa cum laude in chemistry from the University of Minnesota in 2009, where he began studying organic chemistry under the tutelage of Professor Thomas R. Hoye. He then began his Ph.D. studies under the supervision of Professor K. C. Nicolaou at The Scripps Research Institute as a National Science Foundation Predoctoral Fellow. His research interests include natural product total synthesis and the development of enabling synthetic methodology.
Christian Nilewski, born in Essen, Germany, in 1982, began Chemistry studies in 2001 at the University of Dortmund and moved to the Westfälische Wilhelms-Universität Münster in 2003. After completing a diploma thesis under the supervision of Prof. Dr. Gerhard Erker, he received his diploma in chemistry with distinction in 2006. He then joined the research group of Prof. Dr. Erick M. Carreira at the ETH Zürich for Ph.D. studies and obtained his degree in 2011. Currently, he is carrying out postdoctoral research in the group of Prof. Dr. K. C. Nicolaou as a Feodor Lynen Research-Fellow of the Alexander von Humboldt-Foundation.
Heraklidia A. Ioannidou, was born in Limassol, Cyprus, in 1985. She received her B.Sc. in Chemistry from the University of Cyprus in 2007, where she also completed her Ph.D. under the supervision of Dr. P. A. Koutentis in 2011 on the chemistry of sulfur and/or nitrogen heterocycles. In March 2012, she joined Professor K. C. Nicolaou’s group where she has continued to pursue her research interests in total synthesis of natural products.
References
- 1.Nicolaou KC, Montangon T. Molecules That Changed the World. Wiley-VCH Publishers; Weinheim: 2008. p. 366. [Google Scholar]
- 2.a) Rocke AJ. Image and Reality: Kekulé, Kopp, and the Scientific Imagination. The University of Chicago Press; Chicago: 2010. p. 375. [Google Scholar]; b) Levere TH. Transforming Matter. Johns Hopkins Univesity Press; 2001. p. 215. [Google Scholar]
- 3.Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. VCH Publishers; Weinheim, Germany; 1996. p. 798. [Google Scholar]
- 4.Nicolaou KC, Snyder SA. Classics in Total Synthesis II. Wiley-VCH Publishers; Weinheim, Germany: 2003. p. 636. [Google Scholar]
- 5.Nicolaou KC, Chen JS. Classics in Total Synthesis III. Wiley-VCH Publishers; Weinheim, Germany: 2011. p. 746. [Google Scholar]
- 6.Nicolaou KC, Vourloumis D, Winssinger N, Baran PS. Angew. Chem. Int. Ed. 2000;39:44–122. [PubMed] [Google Scholar]
- 7.Nicolaou KC, Snyder SA. Proc. Natl. Acad. Sci. USA. 2004;101:11929–11936. doi: 10.1073/pnas.0403799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall N. Chem. Commun. 2003:661–664. [PubMed] [Google Scholar]
- 9.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. John Wiley & Sons; New York: 1989. p. 436. For other books from the Corey group, see: Corey EJ, Czakó B, Kürti L. Molecules and Medicine. Wiley Publishers; Weinheim: 2008. p. 254. Corey EJ, Kürti L. Enantioselective Chemical Synthesis. Direct Publishing Group; LLC: 2010. p. 336.
- 10.Nicolaou KC, Snyder SA. Angew. Chem. Int. Ed. 2005;44:1012–1044. doi: 10.1002/anie.200460864. Corrigendum: Angew. Chem. Int. Ed. 2005, 44, 2050. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaou KC. Tetrahedron. 2003;59:6683–6738. [Google Scholar]
- 12.Nicolaou KC. J. Org. Chem. 2005;70:7007–7027. doi: 10.1021/jo050963m. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou KC. J. Med. Chem. 2005;48:5613–5638. doi: 10.1021/jm050524f. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaou KC, Chen JS, Dalby SM. Bioorg. Med. Chem. 2009;17:2290–2303. doi: 10.1016/j.bmc.2008.10.089. Symposium-in-Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahaffy JP. What Have the Greeks Done for Modern Civilisation. G. P. Putnam’s Sons; New York: 1910. p. 263. [Google Scholar]
- 16.Nicolaou KC, Petasis NA, Zipkin RE, Uenishi J. J. Am. Chem. Soc. 1982;104:5555–5557. [Google Scholar]; Nicolaou KC, Petasis NA, Uenishi J, Zipkin RE. J. Am. Chem. Soc. 1982;104:5557–5558. [Google Scholar]; Nicolaou KC, Zipkin RE, Petasis NA. J. Am. Chem. Soc. 1982;104:5558–5560. [Google Scholar]; Nicolaou KC, Petasis NA, Zipkin RE. J. Am. Chem. Soc. 1982;104:5560–5562. [Google Scholar]; Nicolaou KC, Petasis NA. In: Strategies and Tactics of Organic Synthesis. Academic Press. Lindberg T, editor. New York: 1984. pp. 155–173. ch. 6. [Google Scholar]
- 17.Bandaranayake WM, Banfield JE, Black D. St. C., Fallon GD, Gatehouse BM. 1980:162–163. J. Chem. Soc., Chem. Commun. [Google Scholar]
- 18.Bandaranayake WM, Banfield JE, Black D. St. C. J. Chem. Soc., Chem. Commun. 1980:902–903. [Google Scholar]
- 19.Robinson R. J. Chem. Soc. 1917;111:762–768. [Google Scholar]
- 20.a) Johnson WS, Gravestock MB, McCarry BE. J. Am. Chem. Soc. 1971;93:4332–4334. doi: 10.1021/ja00746a062. [DOI] [PubMed] [Google Scholar]; b) Gravestock MB, Johnson WS, McCarry BE, Parry RJ, Ratcliffe BE. J. Am. Chem. Soc. 1978;100:4274–4282. [Google Scholar]
- 21.For reviews on cascade reactions from these laboratories, see: Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551–561. doi: 10.1039/b209440c. Nicolaou KC, Snyder SA. L’Actualite Chimique. 2003:83–88. Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. Nicolaou KC, Chen JS. Chem. Soc. Rev. 2009;38:2993–3009. doi: 10.1039/b903290h.
- 22.For another total synthesis of endiandric acid A, see: May SA, Grieco PA, Lee HH. Synlett. 1997:493–494.
- 23.Dewey RS, Arison BH, Hannah J, Shih DH, Albers-Schonberg GJ. J. Antibiot. 1985;38:1691–1698. doi: 10.7164/antibiotics.38.1691. [DOI] [PubMed] [Google Scholar]
- 24.a) Maehr H, Leach M, Williams TH, Blount JF. Can. J. Chem. 1980;58:501–526. [Google Scholar]; b) Maehr H, Blount JF, Leach M, Stempel A, Buchi G. Helv. Chim. Acta. 1972;55:3054–3056. doi: 10.1002/hlca.19720550838. [DOI] [PubMed] [Google Scholar]; c) Maehr H, Blount JF, Evans RH, Leach M, Westley JW, Williams TH, Stempel A, Buchi G. Helv. Chim. Acta. 1972;55:3051–3054. doi: 10.1002/hlca.19720550837. [DOI] [PubMed] [Google Scholar]; d) Maehr H, Leach M, Yarmchuk L, Stempel A. J. Am. Chem. Soc. 1973;95:8449–8450. doi: 10.1021/ja00806a043. [DOI] [PubMed] [Google Scholar]; e) Maehr H, Leach M, Williams TH, Benz W, Blount JF. J. Am. Chem. Soc. 1973;95:8448–8449. doi: 10.1021/ja00806a042. [DOI] [PubMed] [Google Scholar]
- 25.a) Dolle RE, Nicolaou KC. J. Am. Chem. Soc. 1985;107:1691–1694. [Google Scholar]; b) Dolle RE, Nicolaou KC. J. Am. Chem. Soc. 1985;107:1695–1698. [Google Scholar]
- 26.Dolle RE, Nicolaou KC. J. Chem. Soc., Chem. Commun. 1985:1016–1018. [Google Scholar]
- 27.Nicolaou KC, Seitz SP, Papahatjis DP. J. Am. Chem. Soc. 1983;105:2430–2434. [Google Scholar]
- 28.a) Nicolaou KC, Dolle RE, Chucholowski A, Randall JL. J. Chem. Soc., Chem. Commun. 1984:1153–1154. [Google Scholar]; b) Nicolaou KC, Chucholowski A, Dolle RE, Randall JL. J. Chem. Soc., Chem. Commun. 1984:1155–1156. [Google Scholar]
- 29.Nicolaou KC, Winssinger N, Pastor J, DeRoose F. J. Am. Chem. Soc. 1997;119:449–450. Review: Nicolaou KC, Mitchell HJ. Angew. Chem. Int. Ed. 2001;40:1576–1624.
- 30.Zhang Z, Ollmann IR, Ye X-S, Wischnat R, Baasov T, Wong C-H. J. Am. Chem. Soc. 1999;121:734–753. [Google Scholar]
- 31.a) Still WC, Romero AG. J. Am. Chem. Soc. 1986;108:2105–2106. [Google Scholar]; b) Schreiber SL, Sammakia T, Hulin B, Schulte G. J. Am. Chem. Soc. 1986;108:2106–2107. [Google Scholar]
- 32.For selected examples, see: Nicolaou KC, Frederick MO, Aversa RJ. Angew. Chem. Int. Ed. 2008;47:7182–7225. doi: 10.1002/anie.200801696. and references cited therein.
- 33.Vandeputte J, Wachtel JL, Stiller ET. Antibiot. Ann. 1956:587–591. [PubMed] [Google Scholar]
- 34.a) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1987;109:2205–2508. [Google Scholar]; b) Nicolaou KC, Daines RA, Chakraborty TK. J. Am. Chem. Soc. 1987;109:2208–2210. [Google Scholar]; c) Nicolaou KC, Chakraborty TK, Daines RA, Ogawa Y. J. Chem. Soc., Chem. Commun. 1987:686–689. [Google Scholar]; d) Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J. Am. Chem. Soc. 1987;109:2821–2822. [Google Scholar]; e) Nicolaou KC, Chakraborty TK, Ogawa Y, Daines RA, Simpkins NS, Furst GT. J. Am. Chem. Soc. 1988;110:4660–4672. [Google Scholar]; f) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4672–4685. [Google Scholar]; g) Nicolaou KC, Daines RA, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4685–4696. [Google Scholar]; h) Nicolaou KC, Daines RA, Ogawa Y, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4696–4705. [Google Scholar]; i) Nicolaou KC, Ogilvie WW. ChemTracts. 1990;3:327–349. [Google Scholar]
- 35.a) Paquet V, Zumbuehl A, Carreira EM. Bioconjugate Chem. 2006;17:1460–1463. doi: 10.1021/bc060205i. [DOI] [PubMed] [Google Scholar]; b) Zumbuehl A, Stano P, Sohrmann M, Peter M, Walde P, Carreira EM. Org. Biomol. Chem. 2007;5:1339–1342. doi: 10.1039/b701953j. [DOI] [PubMed] [Google Scholar]; c) Szpilman AM, Cereghetti DM, Wurtz NR, Manthorpe JM, Carreira EM. Angew. Chem. Int. Ed. 2008;47:4335–4338. doi: 10.1002/anie.200800589. [DOI] [PubMed] [Google Scholar]; d) Szpilman AM, Carreira EM. Angew. Chem. Int. Ed. 2008;47:4339–4342. doi: 10.1002/anie.200800590. [DOI] [PubMed] [Google Scholar]; e) Paquet V, Volmer AA, Carreira EM. Chem. Eur. J. 2008;14:2465–2481. doi: 10.1002/chem.200701237. [DOI] [PubMed] [Google Scholar]; f) Zumbuehl A, Stano P, Sohrmann M, Dietiker R, Peterand M, Carreira EM. ChemBioChem. 2009;10:1617–1620. doi: 10.1002/cbic.200900096. [DOI] [PubMed] [Google Scholar]; g) Szpilman AM, Cereghetti DM, Manthorpe JM, Wurtz NR, Carreira EM. Chem. Eur. J. 2009;15:7117–7128. doi: 10.1002/chem.200900231. [DOI] [PubMed] [Google Scholar]; h) Volmer AA, Carreira EM. Chem. Eur. J. 2010;11:778–781. doi: 10.1002/cbic.201000080. [DOI] [PubMed] [Google Scholar]; i) Croatt MP, Carreira EM. Org. Lett. 2011;13:1390–1393. doi: 10.1021/ol2000765. [DOI] [PubMed] [Google Scholar]; j) Palacios DS, Anderson TM, Burke MD. J. Am. Chem. Soc. 2007;129:13804–13805. doi: 10.1021/ja075739o. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Lee SJ, Gray KC, Panek JS, Burke MD. J. Am. Chem. Soc. 2008;130:466–468. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Palacios DS, Dailey I, Siebert DM, Wilcock BC, Burke MD. Proc. Natl. Acad. Sci. USA. 2011;108:6733–6738. doi: 10.1073/pnas.1015023108. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Cereghetti DM, Carreira EM. Synthesis. 2006:914–942. For a review on the chemistry and biology of amphotericin B, see: [Google Scholar]
- 36.For a total synthesis of 19-dehydroamphoteronolide B, see: Kennedy RM, Abiko A, Takemasa T, Okumoto H, Masamune S. Tetrahedron Lett. 1988;29:451–454.
- 37.a) Lee MD, Dunne TS, Siegel MM, Chang CC, Morton GO, Borders DB. J. Am. Chem. Soc. 1987;109:3464–3466. [Google Scholar]; b) Lee MD, Dunne TS, Chang CC, Ellestad GA, Siegel MM, Morton GO, McGahren WJ, Borders DB. J. Am. Chem. Soc. 1987;109:3466–3468. [Google Scholar]; c) Lee MD, Dunne TS, Chang CC, Siegel MM, Morton GO, Ellestad GA, McGahren WJ, Borders DB. J. Am. Chem. Soc. 1992;114:985–997. [Google Scholar]
- 38.For selected reviews on enediynes, see: Nicolaou KC, Dai W-M. Angew. Chem. Int. Ed. Engl. 1991;30:1387–1416. Nicolaou KC, Smith AL, Yue EW. Proc. Natl. Acad. Sci. USA. 1993;90:5881–5888. doi: 10.1073/pnas.90.13.5881.
- 39.a) Smith AL, Hwang C-K, Pitsinos E, Scarlato GR, Nicolaou KC. J. Am. Chem. Soc. 1992;114:3134–3136. [Google Scholar]; b) Nicolaou KC, Hummel CW, Pitsinos EN, Nakada M, Smith AL, Shibayama K, Saimoto H. J. Am. Chem. Soc. 1992;114:10082–10084. [Google Scholar]; c) Groneberg RD, Miyazaki T, Stylianides NA, Schulze TJ, Stahl W, Schreiner EP, Suzuki T, Iwabuchi Y, Smith AL, Nicolaou KC. J. Am. Chem. Soc. 1993;115:7593–7611. [Google Scholar]; d) Smith AL, Pitsinos E, Hwang C-K, Mizuno Y, Saimoto H, Scarlato GR, Suzuki T, Nicolaou KC. J. Am. Chem. Soc. 1993;115:7612–7624. [Google Scholar]; e) Nicolaou KC, Hummel CW, Nakada M, Shibayama K, Pitsinos EN, Saimoto H, Mizuno Y, Baldenius K-U, Smith AL. J. Am. Chem. Soc. 1993;115:7625–7635. [Google Scholar]
- 40.Nicolaou KC, Groneberg RD. J. Am. Chem. Soc. 1990;112:4085–4086. [Google Scholar]
- 41.Nicolaou KC, Ebata T, Stylianides NA, Groneberg RD, Carroll PJ. Angew. Chem. Int. Ed. Engl. 1988;27:1097–1099. [Google Scholar]
- 42.Nicolaou KC, Zuccarello G, Ogawa Y, Schweiger EJ, Kumazawa T. J. Am. Chem. Soc. 1988;110:4866–4868. [Google Scholar]
- 43.a) Nicolaou KC, Ogawa Y, Zuccarello G, Kataoka H. J. Am. Chem. Soc. 1988;110:7247–7248. [Google Scholar]; b) Nicolaou KC, Hwang C-K, Smith AL, Wendeborn SV. J. Am. Chem. Soc. 1990;112:7416–7418. [Google Scholar]; c) Nicolaou KC, Maligres P, Shin J, de Leon E, Rideout D. J. Am. Chem. Soc. 1990;112:7825–7826. [Google Scholar]; d) Nicolaou KC, Skokotas G, Furuya S, Suemune H, Nicolaou DC. Angew. Chem. Int. Ed. Engl. 1990;29:1064–1067. [Google Scholar]; e) Nicolaou KC, Wendeborn S, Maligres P, Isshiki K, Zein N, Ellestad G. Angew. Chem. Int. Ed. Engl. 1991;30:418–420. [Google Scholar]; f) Nicolaou KC, Schreiner EP, Stahl W. Angew. Chem. Int. Ed. Engl. 1991;30:585–588. [Google Scholar]; g) Nicolaou KC, Zuccarello G, Reimer C, Estevez VA, Dai W-M. J. Am. Chem. Soc. 1992;114:7360–7371. [Google Scholar]; h) Nicolaou KC, Liu A, Zeng Z, McComb S. J. Am. Chem. Soc. 1992;114:9279–9282. [Google Scholar]; i) Nicolaou KC, Sorensen E, Discordia R, Hwang C-K, Minto RE, Bharucha KN, Bergman RG. Angew. Chem. Int. Ed. Engl. 1992;31:1044–1046. [Google Scholar]; j) Nicolaou KC, Dai W-M, Tsay S-C, Estevez VA, Wrasidlo W. Science. 1992;256:1172–1178. doi: 10.1126/science.256.5060.1172. [DOI] [PubMed] [Google Scholar]; k) Nicolaou KC, Dai W-M. J. Am. Chem. Soc. 1992;114:8908–8921. [Google Scholar]; l) Nicolaou KC, Maligres P, Suzuki T, Wendeborn SV, Dai W-M, Chadha R. J. Am. Chem. Soc. 1992;114:8890–8907. [Google Scholar]; m) Nicolaou KC, Dai W-M, Hong YP, Tsay S-C, Baldridge KK, Siegel JS. J. Am. Chem. Soc. 1993;115:7944–7953. [Google Scholar]; n) Nicolaou KC, Pitsinos EN, Theodorakis EA, Saimoto H, Wrasidlo W. Chem. Biol. 1994;1:57–66. doi: 10.1016/1074-5521(94)90041-8. [DOI] [PubMed] [Google Scholar]; o) Nicolaou KC, Li T, Nakada M, Hummel CW, Hiatt A, Wrasidlo W. Angew. Chem. Int. Ed. Engl. 1994;33:183–186. [Google Scholar]
- 44.a) Konishi M, Ohkuma H, Matsumoto K, Tsuno T, Kamei H, Miyaki T, Oki T, Kawaguchi H, VanDuyne GD, Clardy J. J. Antibiot. 1989;42:1449–1452. doi: 10.7164/antibiotics.42.1449. [DOI] [PubMed] [Google Scholar]; b) Konishi M, Ohkuma H, Tsuno T, Oki T, VanDuyne GD, Clardy J. J. Am. Chem. Soc. 1990;112:3715–3716. [Google Scholar]
- 45.a) Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1993;32:1377–1385. [Google Scholar]; b) Nicolaou KC, Hale CRH, Nilewski C. Chem. Rec. doi: 10.1002/tcr.201200005. in press. [DOI] [PubMed] [Google Scholar]
- 46.a) Aiyar J, Hitchcock SA, Denhart DJ, Liu KK-C, Danishefsky SJ, Crothers DM. Angew. Chem. Int. Ed. Engl. 1994;33:855–858. [Google Scholar]; b) Hitchcock SA, Boyer SH, Chu-Moyer MY, Olson SH, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1994;33:858–862. [Google Scholar]
- 47.a) Vezina C, Kudelski A, Sehgal SN. J. Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]; b) Sehgal SN, Baker H, Vezina C. J. Antibiot. 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 48.a) Rosen MK, Schreiber SL. Angew. Chem. Int. Ed. Engl. 1992;31:384–400. [Google Scholar]; b) Snyder SH, Sabatini DM. Nature Medicine. 1995;1:32–37. doi: 10.1038/nm0195-32. [DOI] [PubMed] [Google Scholar]
- 49.a) Piscopio AD, Minowa N, Chakraborty TK, Koide K, Bertinato P, Nicolaou KC. J. Chem. Soc., Chem. Commun. 1993:617–618. [Google Scholar]; b) Nicolaou KC, Bertinato P, Piscopio AD, Chakraborty TK, Minowa N. J. Chem. Soc., Chem. Commun. 1993:619–622. [Google Scholar]; c) Nicolaou KC, Chakraborty TK, Piscopio AD, Minowa N, Bertinato P. J. Am. Chem. Soc. 1993;115:4419–4420. [Google Scholar]; d) Nicolaou KC, Piscopio AD, Bertinato P, Chakraborty TK, Minowa N, Koide K. Chem. Eur. J. 1995;1:318–322. [Google Scholar]
- 50.Chakraborty TK, Weber HP, Nicolaou KC. Chem. Biol. 1995;2:157–164. doi: 10.1016/1074-5521(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 51.a) Romo D, Meyer SD, Johnson DD, Schreiber SL. J. Am. Chem. Soc. 1993;115:7906–7907. [Google Scholar]; b) Hayward CM, Yohannes D, Danishefsky SJ. J. Am. Chem. Soc. 1993;115:9345–9346. [Google Scholar]; c) Smith AB, III, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1995;117:5407–5408. [Google Scholar]; d) Smith AB, III, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1997;119:947–961. [Google Scholar]; e) Smith AB, III, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1997;119:962–973. [Google Scholar]; f) Maddess ML, Tackett MN, Watanabe H, Brennan PE, Spilling CD, Scott JS, Osborn DP, Ley SV. Angew. Chem. Int. Ed. 2007;46:591–597. doi: 10.1002/anie.200604053. [DOI] [PubMed] [Google Scholar]; g) Ley SV, Tackett MN, Maddess ML, Anderson JC, Brennan PE, Cappi MW, Heer JP, Helgen C, Kori M, Kouklovsky C, Marsden SP, Norman J, Osborn DP, Palomero MA, Pavey JBJ, Pinel C, Robinson LA, Schnaubelt J, Scott JS, Spilling CD, Watanabe H, Wesson KE, Willis MC. Chem. Eur. J. 2009;15:2874–2914. doi: 10.1002/chem.200801656. [DOI] [PubMed] [Google Scholar]
- 52.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 53.Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud JB, Couladouros EA, Paulvannan K, Sorensen EJ. Nature. 1994;367:630–634. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]
- 54.a) Nicolaou KC, Nantermet PG, Ueno H, Guy RK, Couladouros EA, Sorensen EJ. J. Am. Chem. Soc. 1995;117:624–633. [Google Scholar]; b) Nicolaou KC, Liu J-J, Yang Z, Ueno H, Sorensen EJ, Claiborne CF, Guy RK, Hwang C-K, Nakada M, Nantermet PG. J. Am. Chem. Soc. 1995;117:634–644. [Google Scholar]; c) Nicolaou KC, Yang Z, Liu J-J, Nantermet PG, Claiborne CF, Renaud J, Guy RK, Shibayama K. J. Am. Chem. Soc. 1995;117:645–652. [Google Scholar]; d) Nicolaou KC, Ueno H, Liu J-J, Nantermet PG, Yang Z, Renaud J, Paulvannan K, Chadha R. J. Am. Chem. Soc. 1995;117:653–659. [Google Scholar]
- 55.a) Holton RA, Somoza C, Kim HB, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH. J. Am. Chem. Soc. 1994;116:1597–1598. [Google Scholar]; b) Holton RA, Kim HB, Somoza C, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH. J. Am. Chem. Soc. 1994;116:1599–1600. [Google Scholar]
- 56.a) Nicolaou KC, Guy RK. New Perspectives in Drug Design Symposium, Turnberry, Scotland. Academic Press; London: 1995. pp. 69–87. ch. 5. [Google Scholar]; b) Nicolaou KC, Guy RK, Potier P. Sci. Am. 1996 Jun;:84–88. doi: 10.1038/scientificamerican0696-94. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Dai W-M, Guy RK. Angew. Chem. Int. Ed. Engl. 1994;33:15–44. [Google Scholar]; d) Nicolaou KC, Claiborne CF, Paulvannan K, Postema MHD, Guy RK. Chem. Eur. J. 1997;3:399–409. [Google Scholar]
- 57.a) Nicolaou KC, Reimer C, Kerr M, Rideout D, Wrasidlo W. Nature. 1993;364:464–466. doi: 10.1038/364464a0. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Couladouros EA, Nantermet PG, Renaud J, Guy RK, Wrasidlo W. Angew. Chem. Int. Ed. Engl. 1994;33:1581–1583. [Google Scholar]; c) Nicolaou KC, Guy RK, Pitsinos EC, Wrasidlo W. Angew. Chem. Int. Ed. Engl. 1994;33:1583–1587. [Google Scholar]; d) Nicolaou KC, Claiborne CF, Nantermet PG, Couladouros EA, Sorensen EJ. J. Am. Chem. Soc. 1994;116:1591–1592. [Google Scholar]; e) Gomez-Paloma L, Guy RK, Wrasidlo W, Nicolaou KC. Chem. Biol. 1994;1:107–112. doi: 10.1016/1074-5521(94)90048-5. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Renaud J, Nantermet PG, Couladouros EA, Guy RK, Wrasidlo W. J. Am. Chem. Soc. 1995;117:2409–2420. [Google Scholar]
- 58.Guy RK, Scott ZA, Sloboda RS, Nicolaou KC. Chem. Biol. 1996;3:1021–1031. doi: 10.1016/s1074-5521(96)90168-4. [DOI] [PubMed] [Google Scholar]
- 59.Masters JJ, Link JT, Snyder LB, Young WB, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1995;34:1723–1726. Danishefsky SJ, Masters JJ, Young WB, Link JT, Snyder LB, Magee TV, Jung DK, Isaacs RCA, Bornmann WG, Alaimo CA, Coburn CA, Di Grandi MJ. J. Am. Chem. Soc. 1996;118:2843–2859. Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Houze JB, Krauss NE, Lee D, Marquess DG, McGrane PL, Meng W, Natchus MG, Shuker AJ, Sutton JC, Taylor RE. J. Am. Chem. Soc. 1997;119:2757–2758. Mukaiyama Chem. Eur. J. 1999;5:121–161. Mukaiyama T, Shiina I, Iwadare H, Sakoh H, Tani Y, Haseqawa M, Saitoh K. Proc. Jpn. Acad. 1997;73B:95–100. Morihira K, Hara R, Kawahara S, Nishimori T, Nakamura N, Kusama H, Kuwajima I. J. Am. Chem. Soc. 1998;120:12980–12981. Kusama H, Hara R, Kawahara S, Nishimori T, Kashima H, Nakamura N, Morihira K, Kuwajima I. J. Am. Chem. Soc. 2000;122:3811–3820. For a formal total synthesis of Taxol®, see: Doi T, Fuse S, Miyamoto S, Nakai K, Sasuga D, Takahashi T. Chem. Asian J. 2006;1:370–383. doi: 10.1002/asia.200600156.
- 60.Nicolaou KC, Guy RK. Angew. Chem. Int. Ed. Engl. 1995;34:2079–2090. [Google Scholar]
- 61.a) Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, VanMiddlesworth FL, Hensens OD, Liesch JM, Zink DL, Wilson KE, Onishi J, Milligan JA, Bills G, Kaplan L, Nallin-Omstead M, Jenkins RG, Huang L, Meinz MS, Quinn L, Burg RW, Kong YL, Mochales S, Mojena M, Martin I, Pelaez F, Diez MT, Alberts AW. Proc. Natl. Acad. Sci. USA. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hensens OD, Dufresne C, Liesch JM, Zink DL, Reamer RA, VanMiddlesworth F. Tetrahedron Lett. 1993;34:399–402. [Google Scholar]; c) Dawson MJ, Farthing JE, Marshall PS,, Middleton RF, O’Neill MJ, Shuttleworth A, Stylli C, Tait RM, Taylor PM, Wildman HG, Buss AD, Langley D, Hayes MV. J. Antibiot. 1992;45:639–647. doi: 10.7164/antibiotics.45.639. [DOI] [PubMed] [Google Scholar]; d) Sidebottom PJ, Highcock RM, Lane SJ, Procopiou PA, Watson NS. J. Antibiot. 1992;45:648–658. doi: 10.7164/antibiotics.45.648. [DOI] [PubMed] [Google Scholar]; e) Hasumi K, Tachikawa K, Sakai K, Murdkawa S, Yoshikawa N, Kumazawa S, Endo A. J. Antibiot. 1993;46:689–691. doi: 10.7164/antibiotics.46.689. [DOI] [PubMed] [Google Scholar]
- 62.a) Nicolaou KC, Yue EW, Naniwa Y, De Riccardis F, Nadin A, Leresche JE, La Greca S, Yang Z. Angew. Chem. Int. Ed. 1994;33:2184–2187. [Google Scholar]; b) Nicolaou KC, Nadin A, Leresche JE, La Greca S, Tsuri T, Yue EW, Yang Z. Angew. Chem. Int. Ed. 1994;33:2187–2190. [Google Scholar]; c) Nicolaou KC, Nadin A, Leresche JE, Yue EW, La Greca S. Angew. Chem. Int. Ed. 1994;33:2190–2191. [Google Scholar]; d) Nicolaou KC, Yue EW, La Greca S, Nadin A, Yang Z, Leresche JE, Tsuri T, Naniwa Y, De Riccardis F. Chem. Eur. J. 1995;1:467–494. [Google Scholar]
- 63.Carreira EM, Du Bois J. J. Am. Chem. Soc. 1994;116:10825–10826. Carreira EM, Du Bois J. J. Am. Chem. Soc. 1995;117:8106–8125. Evans DA, Barrow JC, Leighton JL, Robichaud AJ, Sefkow MJ. J. Am. Chem. Soc. 1994;116:12111–12112. Sato H, Nakamura S, Watanabe N, Hashimoto S. Synlett. 1997:451–454. Armstrong A, Jones LH, Barsanti PA. Tetrahedron Lett. 1998;39:3337–3340. Tomooka K, Kikuchi M, Igawa K, Suzuki M, Keong PH, Nakai T. Angew. Chem. Int. Ed. 2000;39:4502–4505. doi: 10.1002/1521-3773(20001215)39:24<4502::aid-anie4502>3.0.co;2-k. Armstrong A, Barsanti PA, Jones LH, Ahmed G. J. Org. Chem. 2000;65:7020–7032. doi: 10.1021/jo000700m. Nakamura S, Hirata Y, Kurosaki T, Anada M, Kataoka O, Kitagaki S, Hashimoto S. Angew. Chem. Int. Ed. 2003;42:5351–5355. doi: 10.1002/anie.200352629. Nakamura S, Sato H, Hirata Y, Watanabe N, Hashimoto S. Tetrahedron. 2005;61:11078–11106. Hirata Y, Nakamura S, Watanabe N, Kataoka O, Kurosaki T, Anada M, Kitagaki S, Shiro M, Hashimoto S. Chem. Eur. J. 2006;12:8898–8925. doi: 10.1002/chem.200601212. Nicewicz DA, Satterfield AD, Schmitt DC, Johnson JS. J. Am. Chem. Soc. 2008;130:17281–17283. doi: 10.1021/ja808347q. Wang Y, Metz P. Chem. Eur. J. 2011;17:3335–3337. doi: 10.1002/chem.201003399. For a formal total synthesis of zaragozic acid C, see: Bunte JO, Cuzzupe AN, Daly AM, Rizzacasa MA. Angew. Chem. Int. Ed. 2006;45:6376–6380. doi: 10.1002/anie.200602507.
- 64.For a review on zaragozic acids, see: Nadin A, Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1996;35:1623–1656.
- 65.Lin YY, Risk M, Ray SM, VanEngen D, Clardy J, Golik J, James JC, Nakanishi K. J. Am. Chem. Soc. 1981;103:6773–6776. [Google Scholar]
- 66.Shimizu Y, Chou HN, Bando H, Van Duyne G, Clardy J. J. Am. Chem. Soc. 1986;108:514–515. doi: 10.1021/ja00263a031. [DOI] [PubMed] [Google Scholar]
- 67.Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1996;35:589–607. [Google Scholar]
- 68.a) Nicolaou KC, Theodorakis E, Rutjes FPJT, Tiebes J, Sato M, Untersteller E, Xiao X-Y. J. Am. Chem. Soc. 1995;117:1171–1172. [Google Scholar]; b) Nicolaou KC, Rutjes JT, Theodorakis E, Tiebes J, Sato M, Untersteller E. J. Am. Chem. Soc. 1995;117:1173–1174. [Google Scholar]; c) Nicolaou KC, Hwang C-K, Duggan ME, Nugiel DA, Abe Y, Bal Reddy K, DeFrees SA, Reddy DR, Awartani RA, Conley SR, Rutjes FPJT, Theodorakis EA. J. Am. Chem. Soc. 1995;117:10227–10238. [Google Scholar]; d) Nicolaou KC, Theodorakis EA, Rutjes FPJT, Sato M, Tiebes J, Xiao X-Y, Hwang C-K, Duggan ME, Yang Z, Couladouros EA, Sato F, Shin J, He H-M, Bleckman T. J. Am. Chem. Soc. 1995;117:10239–10251. [Google Scholar]; e) Nicolaou KC, Rutjes FPJT, Theodorakis EA, Tiebes J, Sato M, Untersteller E. J. Am. Chem. Soc. 1995;117:10252–10263. [Google Scholar]
- 69.a) Nicolaou KC, Duggan ME, Hwang C-K. J. Am. Chem. Soc. 1989;111:6666–6675. [Google Scholar]; b) Nicolaou KC, Duggan ME, Hwang C-K. J. Am. Chem. Soc. 1989;111:6676–6682. [Google Scholar]; c) Nicolaou KC, Hwang C-K, Duggan ME. J. Am. Chem. Soc. 1989;111:6682–6690. [Google Scholar]; d) Nicolaou KC, Nugiel DA, Couladouros E, Hwang C-K. Tetrahedron. 1990;46:4517–4552. [Google Scholar]
- 70.Nicolaou KC, Duggan ME, Hwang C-K, Somers PK. J. Chem. Soc., Chem. Commun. 1985:1359–1362. [Google Scholar]
- 71.Nicolaou KC, Prasad CVC, Somers PK, Hwang C-K. J. Am. Chem. Soc. 1989;111:5330–5334. [Google Scholar]
- 72.a) Nicolaou KC, Duggan ME, Hwang C-K. J. Am. Chem. Soc. 1986;108:2468–2469. doi: 10.1021/ja00269a067. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Prasad CVC, Hwang C-K, Duggan ME, Veale CA. J. Am. Chem. Soc. 1989;111:5321–5330. [Google Scholar]
- 73.a) Nicolaou KC, Hwang CK, Duggan ME, Bal Reddy K, Marron BE, McGarry DG. J. Am. Chem. Soc. 1986;108:6800–6802. [Google Scholar]; b) Nicolaou KC, Hwang C-K, Marron BE, DeFrees SA, Couladouros EA, Abe Y, Carrol PJ, Snyder J. J. Am. Chem. Soc. 1990;112:3040–3054. [Google Scholar]; c) Nicolaou KC, Hwang C-K, Duggan ME, Carroll PJ. J. Am. Chem. Soc. 1986;109:3801–3802. [Google Scholar]
- 74.Nicolaou KC, Hwang C-K, Nugiel DA. J. Am. Chem. Soc. 1989;111:4136–4137. [Google Scholar]
- 75.Nicolaou KC, Hwang C-K, Nugiel DA. Angew. Chem. Int. Ed. Engl. 1988;27:1362–1364. [Google Scholar]
- 76.a) Nicolaou KC, McGarry DG, Veale CA, Somers PK. J. Am. Chem. Soc. 1987;109:2504–2506. [Google Scholar]; b) Nicolaou KC, McGarry DG, Somers PK, Kim BH, Ogilvie WW, Yiannikouros G, Prasad CVC, Veale CA, Hark RR. J. Am. Chem. Soc. 1990;112:6263–6276. [Google Scholar]
- 77.a) Nicolaou KC, McGarry DG, Somers PK. J. Am. Chem. Soc. 1990;112:3696–3697. [Google Scholar]; b) Nicolaou KC, Prasad CVC, Ogilvie WW. J. Am. Chem. Soc. 1990;112:4988–4989. [Google Scholar]; c) Nicolaou KC, Veale CA, Hwang C-K, Hutchinson J, Prasad CVC, Ogilvie WW. Angew. Chem. Int. Ed. Engl. 1991;30:299–303. [Google Scholar]; d) Nicolaou KC, Yang Z, Ouellette M, Shi G-Q, Gärtner P, Gunzner JL, Agrios C, Huber R, Huang DH. J. Am. Chem. Soc. 1997;119:8105–8106. [Google Scholar]
- 78.a) Nicolaou KC, Yang Z, Shi G-Q, Gunzner JL, Agrios KA, Gärtner P. Nature. 1998;392:264–269. doi: 10.1038/32623. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Bunnage ME, McGarry DG, Shi S, Somers PK, Wallace PA, Chu X-J, Agrios KA, Gunzner JL, Yang Z. Chem. Eur. J. 1999;5:599–617. [Google Scholar]; c) Nicolaou KC, Wallace PA, Shi S, Ouellette MA, Bunnage ME, Gunzner JL, Agrios KA, Shi G-Q, Yang Z. Chem. Eur. J. 1999;5:618–627. [Google Scholar]; d) Nicolaou KC, Shi Q-G, Gunzner JL, Gärtner P, Wallace PA, Ouellette MA, Shi S, Bunnage ME, Agrios KA, Veale CA, Hwang C-K, Hutchinson J, Prasad CVC, Ogilvie WW, Yang Z. Chem. Eur. J. 1999;5:628–645. [Google Scholar]; e) Nicolaou KC, Gunzner JL, Shi G-Q, Agrios KA, Gärtner P, Yang Z. Chem. Eur. J. 1999;5:646–658. [Google Scholar]
- 79.Nicolaou KC, Shi G-Q, Gunzner JL, Gärtner P, Yang Z. J. Am. Chem. Soc. 1997;119:5467–5468. [Google Scholar]
- 80.Nicolaou KC, Shi G-Q, Namoto K, Bernal F. Chem. Commun. 1998:1757–1758. [Google Scholar]
- 81.a) Nicolaou KC, Postema MHD, Claiborne CF. J. Am. Chem. Soc. 1996;118:1565–1566. [Google Scholar]; b) Nicolaou KC, Postema MHD, Yue EW, Nadin A. J. Am. Chem. Soc. 1996;118:10335–10336. [Google Scholar]
- 82.a) Nicolaou KC, Reddy KR, Skokotas G, Sato F, Xiao X-Y. J. Am. Chem. Soc. 1992;114:7935–7936. [Google Scholar]; b) Nicolaou KC, Reddy KR, Skokotas G, Sato F, Xiao X-Y, Hwang C-K. J. Am. Chem. Soc. 1993;115:3558–3575. [Google Scholar]
- 83.Nicolaou KC, Tiebes J, Theodorakis EA, Rutjes FPJT, Koide K, Sato M, Untersteller E. J. Am. Chem. Soc. 1994;116:9371–9372. [Google Scholar]
- 84.Gawley RE, Rein KS, Jeglitsche G, Adams DJ, Theodorakis EA, Tiebes J, Nicolaou KC, Baden DG. Chem. Biol. 1995;2:533–541. doi: 10.1016/1074-5521(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 85.Nicolaou KC, Aversa RJ. Isr. J. Chem. 2011;51:359–377. doi: 10.1002/ijch.201100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Another total synthesis of brevetoxin A has recently been disclosed by the Crimmins group: Crimmins MT, McDougall PJ, Emmitte KA. Org. Lett. 2005;7:4033–4036. doi: 10.1021/ol051543m. Crimmins MT, Zuccarello JL, Cleary PA, Parrish JD. Org. Lett. 2006;8:159–162. doi: 10.1021/ol0526625. Crimmins MT, McDougall PJ, Ellis JM. Org. Lett. 2006;8:4079–4082. doi: 10.1021/ol0615782. Crimmins MT, Zuccarello JL, Ellis JM, McDougall PJ, Haile PA, Parrish JD, Emmitte KA. Org. Lett. 2009;11:489–492. doi: 10.1021/ol802710u. Crimmins MT, Ellis JM, Emmitte KA, Haile PA, McDougall PJ, Parrish JD, Zuccarello JL. Chem. Eur. J. 2009;15:9223–9234. doi: 10.1002/chem.200900776. Crimmins MT, Zucarello JL, McDougall PJ, Ellis JM. Chem. Eur. J. 2009;15:9235–9244. doi: 10.1002/chem.200900777. For other total syntheses of brevetoxin B, see: Matsuo G, Kawamura K, Hori N, Matsukara H, Nakata T. J. Am. Chem. Soc. 2004;126:14374–14376. doi: 10.1021/ja0449269. Kadota I, Takamura H, Nishii H, Yamamoto Y. J. Am. Chem. Soc. 2005;127:9246–9250. doi: 10.1021/ja051171c.
- 87.a) Höfle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Angew. Chem. Int. Ed. Engl. 1996;35:1567–1569. [Google Scholar]; b) Bollag DM. Exp. Opin. Invest. Drugs. 1997;6:867–873. doi: 10.1517/13543784.6.7.867. [DOI] [PubMed] [Google Scholar]
- 88.a) Ger. Pat. DE-B 4138042. 1993; b) Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 89.Nicolaou KC, Xiao X-Y, Parandoosh Z, Senyei A, Nova MP. Angew. Chem. Int. Ed. Engl. 1995;34:2289–2291. [Google Scholar]
- 90.a) Nicolaou KC, He Y, Vourloumis D, Vallberg H, Yang Z. Angew. Chem. Int. Ed. Engl. 1996;35:2399–2401. [Google Scholar]; b) Nicolaou KC, He Y, Vourloumis D, Vallberg H, Yang Z. Angew. Chem. Int. Ed. Engl. 1997;36:166–168. [Google Scholar]; c) Nicolaou KC, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z, Trujillo JI. J. Am. Chem. Soc. 1997;119:7960–7973. [Google Scholar]; d) Nicolaou KC, King NP, He Y. Alkene Metathesis in Organic Synthesis, vol. ed. A. Fürstner, in Topics in Organometallic Chemistry. Vol. 1. Springer-Verlag; New York: 1998. pp. 73–104. [Google Scholar]
- 91.a) Nicolaou KC, Sarabia F, Ninkovic S, Yang Z. Angew. Chem. Int. Ed. Engl. 1997;36:525–527. [Google Scholar]; b) Nicolaou KC, Ninkovic S, Sarabia F, Vourloumis D, He Y, Vallberg H, Finlay MRV, Yang Z. J. Am. Chem. Soc. 1997;119:7974–7991. [Google Scholar]
- 92.Nicolaou KC, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature. 1997;387:268–272. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]
- 93.Nicolaou KC, Vourloumis D, Li T, Pastor J, Winssinger N, He Y, Ninkovic S, Sarabia F, Vallberg H, Roschangar F, King NP, Finlay MRV, Giannakakou P, Verdier-Pinard P, Hamel E. Angew. Chem. Int. Ed. Engl. 1997;36:2097–2103. [Google Scholar]
- 94.Nicolaou KC, Hanko R, Hartwig W, editors. Handbook of Combinatorial Chemistry. 1 & 2. Wiley-VCH; Weinheim, Germany: 2001. p. 1112. [Google Scholar]
- 95.a) Nicolaou KC, Scarpelli R, Bollbuck B, Werschkun B, Pereira MMA, Wartmann M, Altmann K-H, Zaharevitz D, Gussio R, Giannakakou P. Chem. Biol. 2000;7:593–599. doi: 10.1016/s1074-5521(00)00006-5. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Namoto K, Ritzén A, Ulven T, Shoji M, Li J, D’Amico G, Liotta D, French CT, Wartmann M, Altmann K-H, Giannakakou P. J. Am. Chem. Soc. 2001;123:9313–9323. doi: 10.1021/ja011338b. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Namoto K, Li J, Ritzén A, Ulven T, Shoji M, Zaharevitz D, Gussio R, Sackett DL, Ward RD, Hensler A, Fojo T, Giannakakou P. ChemBioChem. 2001;2:69–75. doi: 10.1002/1439-7633(20010105)2:1<69::AID-CBIC69>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Ritzén A, Namoto K, Buey RM, Fernando Díaz J, Andreu JM, Wartmann M, Altmann K-H, O’Brate A, Giannakakou P. Tetrahedron. 2002;58 [Google Scholar]; e) Nicolaou KC, He Y, Roschangar F, King NP, Vourloumis D, Li T. Angew. Chem. Int. Ed. 1998;37:84–87. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Pratt BA, Arseniyadis S, Wartmann M, O’Brate A, Giannakakou P. ChemMedChem. 2006;1:41–44. doi: 10.1002/cmdc.200500056. [DOI] [PubMed] [Google Scholar]
- 96.For selected reviews, see: Altmann K-H, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. ChemMedChem. 2007;2:396–423. doi: 10.1002/cmdc.200600206. Altmann K-H, Höfle G, Müller R, Mulzer J, Prantz K. In: The Epothilones: A Outstanding Family of Anti-Tumor Agents; Progress in the Chemistry of Natural Products. Kinghorn AD, Falk H, Kobayashi J, editors. Vol. 90. Springer; New York: 2009. p. 260. Watkins EB, Chittiboyina AG, Jung J-C, Avery MA. Curr. Pharm. Design. 2005;11:1615–1653. doi: 10.2174/1381612053764742. Altmann K-H. Org. Biomol. Chem. 2004;2:2137–2152. doi: 10.1039/b405839a. Nicolaou KC, Ritzén A, Namoto K. Chem. Commun. 2001:1523–1535. doi: 10.1039/b104949f. Nicolaou KC, Hepworth D, King NP, Finlay MRV. Pure Appl. Chem. 1999;71:989–997. Harris CR, Danishefsky SJ. J. Org. Chem. 1999;64:8434–8456. Nicolaou KC, Roschangar F, Vourloumis D. Angew. Chem. Int. Ed. 1998;37:2014–2045. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2.
- 97.a) Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen EJ, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1996;35:2801–2803. [Google Scholar]; Su D-S, Meng D, Bertinato P, Balog A, Sorensen EJ, Danishefsky SJ, Zheng Y-H, Chou T-C, He L, Horwitz SB. Angew. Chem. Int. Ed. Engl. 1997;36:757–759. [Google Scholar]
- 98.a) Schinzer D, Limberg A, Böhm OM. Chem. Eur. J. 1996;2:1477–1482. [Google Scholar]; b) Schinzer D, Limberg A, Bauer A, Böhm OM, Cordes M. Angew. Chem. Int. Ed. Engl. 1997;36:523–524. [Google Scholar]
- 99.a) Altmann K-H, Cachoux F, Feyen F, Gertsch J, Kuzniewski CN, Wartmann M. Chimia. 2010;64:8–13. doi: 10.2533/chimia.2010.8. [DOI] [PubMed] [Google Scholar]; b) Okuno S, Maples WJ, Mahoney MR, Fitch T, Stewart J, Fracasso PM, Kraut M, Ettinger DS, Dawkins F, Erlichman C. J. Clin. Oncol. 2005;23:3069–3073. doi: 10.1200/JCO.2005.00.372. [DOI] [PubMed] [Google Scholar]; c) Altmann K-H. Curr. Pharm. Design. 2005;11:1595–1614. doi: 10.2174/1381612053764715. [DOI] [PubMed] [Google Scholar]; d) Rivkin A, Chou T-C, Danishefsky SJ. Angew. Chem. Int. Ed. 2005;44:2838–2850. doi: 10.1002/anie.200461751. [DOI] [PubMed] [Google Scholar]; e) Kolman A. Curr. Opin. Invest. Drugs. 2004;5:1292–1297. [PubMed] [Google Scholar]; f) Kolman A. Curr. Opin. Invest. Drugs. 2004;5:657–667. [PubMed] [Google Scholar]; g) Galmarini CM, Dumontet C. iDrugs. 2003;6:1182–1187. [PubMed] [Google Scholar]; h) Biswas K, Lin H, Njardarson JT, Chappell MD, Chou T-C, Guan Y, Tong WP, He L, Horwitz SB, Danishefsky SJ. J. Am. Chem. Soc. 2002;124:9825–9832. doi: 10.1021/ja0262333. [DOI] [PubMed] [Google Scholar]
- 100.Lindel T, Jensen PR, Fenical W, Long BH, Casazza AM, Carboni J, Fairchild CR. J. Am. Chem. Soc. 1997;119:8744–8745. [Google Scholar]
- 101.a) D’Ambrosia M, Guerrierov A, Pietra F. Helv. Chim. Acta. 1987;70:2019–2027. [Google Scholar]; b) D’Ambrosia M, Guerriero A, Pietra F. Helv. Chim. Acta. 1988;71:964–976. [Google Scholar]
- 102.Nicolaou KC, van Delft F, Ohshima T, Vourloumis D, Xu J, Hosokawa S, Pfefferkorn J, Kim S, Li T. Angew. Chem. Int. Ed. Engl. 1997;36:2520–2524. [Google Scholar]
- 103.Nicolaou KC, Ohshima T, Hosokawa S, van Delft F, Vourloumis D, Xu J, Pfefferkorn J, Kim S. J. Am. Chem. Soc. 1998;120:8674–8680. [Google Scholar]
- 104.a) Nicolaou KC, Xu J-Y, Kim S, Ohshima T, Hosokawa S, Pfefferkorn J. J. Am. Chem. Soc. 1997;119:11353–11354. [Google Scholar]; b) Nicolaou KC, Kim S, Pfefferkorn J, Xu J-Y, Ohshima T, Hosokawa S, Vourloumis D, Li T. Angew. Chem. Int. Ed. 1998;37:1418–1421. doi: 10.1002/(SICI)1521-3773(19980605)37:10<1418::AID-ANIE1418>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Xu J, Kim S, Pfefferkorn J, Ohshima T, Vourloumis D, Hosokawa S. J. Am. Chem. Soc. 1998;120:8661–8673. [Google Scholar]
- 105.Nicolaou KC, Winssinger N, Vourloumis D, Ohshima T, Kim S, Pfefferkorn J, Xu JY, Li T. J. Am. Chem. Soc. 1998;120:10814–10826. [Google Scholar]
- 106.For a total synthesis of eleutherobin by the Danishefsky group, see: Chen XT, Zhou BS, Bhattacharya SK, Gutteridge CE, Pettus TRR, Danishefsky SJ. Angew. Chem. Int. Ed. 1998;37:789–792. doi: 10.1002/(SICI)1521-3773(19980403)37:6<789::AID-ANIE789>3.0.CO;2-3. Chen XT, Bhattacharya SK, Zhou BS, Gutteridge CE, Pettus TRR, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:6563–6579. For a formal total synthesis of eleutherobin, see: Castoldi D, Caggiano L, Panigada L, Sharon O, Costa AM, Gennari C. Angew. Chem. Int. Ed. 2005;44:588–591. doi: 10.1002/anie.200461767. Castoldi D, Caggiano L, Panigada L, Sharon O, Costa AM, Gennari C. Chem. Eur. J. 2006;12:51–62. doi: 10.1002/chem.200500749.
- 107.McCormick MH, Stark WM, Pittenger GE, Pittenger RC, McGuire JM. Antibiot. Annu. 1955-1956:606–611. [PubMed] [Google Scholar]
- 108.a) Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew. Chem. Int. Ed. 1998;37:2700–2704. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; b) Evans DA, Dinsmore CJ, Watson PS, Wood MR, Richardson TI, Trotter BW, Katz JL. Angew. Chem. Int. Ed. 1998;37:2704–2708. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2704::AID-ANIE2704>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 109.a) Nicolaou KC, Natarajan S, Li H, Jain NF, Hughes R, Solomon ME, Ramanjulu JM, Boddy CNC, Takayanagi M. Angew. Chem. Int. Ed. 1998;37:2708–2714. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2708::AID-ANIE2708>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Jain NF, Natarajan S, Hughes R, Solomon ME, Li H, Ramanjulu JM, Takayanagi M, Koumbis AE, Bando T. Angew. Chem. Int. Ed. 1998;37:2714–2716. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2714::AID-ANIE2714>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Takayanagi M, Jain NF, Natarajan S, Koumbis AE, Bando T, Ramanjulu JM. Angew. Chem. Int. Ed. 1998;37:2717–2719. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2717::AID-ANIE2717>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 110.a) Boger DL, Miyazaki S, Kim SH, Wu JH, Loiseleur O, Castle SL. J. Am. Chem. Soc. 1999;121:3226–3227. [Google Scholar]; b) Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. J. Am. Chem. Soc. 1999;121:10004–10011. [Google Scholar]
- 111.a) Nicolaou KC, Mitchell HJ, Jain NF, Winssinger N, Hughes R, Bando T. Angew. Chem. Int. Ed. 1999;38:240–245. [Google Scholar]; b) Nicolaou KC, Li H, Boddy CNC, Ramanjulu JM, Yue TY, Natarajan S, Chu X-J, Bräse S, Rübsam R. Chem. Eur. J. 1999;5:2584–2601. [Google Scholar]; c) Nicolaou KC, Boddy CNC, Li H, Koumbis AE, Hughes R, Natarajan S, Jain NF, Ramanjulu JM, Bräse S. Chem. Eur. J. 1999;5:2602–2621. [Google Scholar]; d) Nicolaou KC, Koumbis AE, Takayanagi M, Natarajan S, Jain NF, Bando T, Li H, Hughes R. Chem. Eur. J. 1999;5:2622–2647. [Google Scholar]; e) Nicolaou KC, Mitchell HJ, Jain NF, Hughes R, Winssinger N, Natarajan S, Koumbis AE. Chem. Eur. J. 1999;5:2648–2667. [Google Scholar]
- 112.a) Nicolaou KC, Boddy CN, Natarajan S, Yue T-Y, Li H, Bräse S, Ramanjulu JM. J. Am. Chem. Soc. 1997;119:3421–3422. [Google Scholar]; b) Nicolaou KC, Chu X-J, Ramanjulu JM, Natarajan S, Bräse S, Rübsam F, Boddy CNC. Angew. Chem. Int. Ed. Engl. 1997;36:1539–1540. [Google Scholar]; c) Nicolaou KC, Ramanjulu JM, Natarajan S, Bräse S, Li H, Boddy CNC, Rübsam F. Chem. Commun. 1997:1899–1900. [Google Scholar]; d) Nicolaou KC, Boddy CNC. J. Am. Chem. Soc. 2002;124:10451–10455. doi: 10.1021/ja020736r. [DOI] [PubMed] [Google Scholar]
- 113.a) Nicolaou KC, Winssinger N, Hughes R, Smethurst C, Cho SY. Angew. Chem. Int. Ed. 2000;39:1084–1088. doi: 10.1002/(sici)1521-3773(20000317)39:6<1084::aid-anie1084>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Cho SY, Hughes R, Winssinger N, Smethurst C, Labischinski H, Endermann R. Chem. Eur. J. 2001;7:3798–3823. doi: 10.1002/1521-3765(20010903)7:17<3798::aid-chem3798>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 114.a) Nicolaou KC, Hughes R, Cho S-Y, Winssinger N, Smethurst C, Labischinski H, Endermann R. Angew. Chem. Int. Ed. 2000;39:3823–3828. doi: 10.1002/1521-3773(20001103)39:21<3823::AID-ANIE3823>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Hughes R, Cho SY, Winssinger N, Labischinski H, Endermann R. Chem. Eur. J. 2001;7:3824–3843. doi: 10.1002/1521-3765(20010903)7:17<3824::aid-chem3824>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 115.a) Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew. Chem. Int. Ed. 1999;38:2096–2152. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Boddy CNC. Sci. Am. 2001;284:46–53. doi: 10.1038/scientificamerican0501-54. [DOI] [PubMed] [Google Scholar]
- 116.a) Int. Pat. WO-A 9702285A/970123. 1997; b) Sanglier J-J, Quesniaux V, Fehr T, Hofmann H, Mahnke M, Memmert K, Schuler W, Zenke G, Gschwind L, Mauer C, Schilling W. J. Antibiot. 1999;52:466–473. doi: 10.7164/antibiotics.52.466. [DOI] [PubMed] [Google Scholar]; c) Fehr T, Kallen J, Oberer L, Sanglier J-J, Schilling W, Antibiot J. 1999;52:474–479. doi: 10.7164/antibiotics.52.474. [DOI] [PubMed] [Google Scholar]
- 117.a) Nicolaou KC, Ohshima T, Murphy F, Barluenga S, Xu J. Chem. Commun. 1999:809–910. [PubMed] [Google Scholar]; b) Nicolaou KC, Xu J, Murphy F, Barluenga S, Baudoin O, Wei H-X, Gray D, Ohshima T. Angew. Chem. Int. Ed. 1999;38:2447–2451. [PubMed] [Google Scholar]; c) Nicolaou KC, Murphy F, Barluenga S, Ohshima T, Wei H, Xu J, Gray DLF, Baudoin O. J. Am. Chem. Soc. 2000;122:3830–3838. [Google Scholar]
- 118.For a review on palladium-catalyzed cross coupling reactions in total synthesis, see: Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368.
- 119.a) Nicolaou KC, Veale CA, Webber SE, Katerinopoulos H. J. Am. Chem. Soc. 1985;107:7515–7518. [Google Scholar]; b) Nicolaou KC, Marron BE, Veale CA, Webber SE, Serhan CN. J. Org. Chem. 1989;54:5527–5535. [Google Scholar]; c) Nicolaou KC, Webber SE. J. Chem. Soc., Chem. Commun. 1985:297–298. [Google Scholar]; d) Nicolaou KC, Webber SE. Synthesis. 1986:453–461. [Google Scholar]; e) Nicolaou KC, Ramphal JY, Petasis NA, Serhan CN. Angew. Chem. Int. Ed. Engl. 1991;30:1100–1116. [Google Scholar]
- 120.For another total synthesis of sanglifehrin A, see: Duan MS, Paquette LA. Angew. Chem. Int. Ed. 2001;40:3632–3636. doi: 10.1002/1521-3773(20011001)40:19<3632::aid-anie3632>3.0.co;2-5. Paquette LA, Duan MS, Konetzki I, Kempmann C. J. Am. Chem. Soc. 2002;124:4257–4270. doi: 10.1021/ja020091v.
- 121.Andrade R, Ayer WA, Mebe PP. Can. J. Chem. 1992;70:2526–2535. [Google Scholar]
- 122.Abe N, Murata T, Hirota A. Biosci., Biotechnol., Biochem. 1998;62:2120–2126. doi: 10.1271/bbb.62.2120. [DOI] [PubMed] [Google Scholar]
- 123.Abe N, Murata T, Hirota A. Biosci., Biotechnol., Biochem. 1998;62:661–666. doi: 10.1271/bbb.62.661. [DOI] [PubMed] [Google Scholar]
- 124.a) Nicolaou KC, Simonsen KB, Vassilikogiannakis G, Baran PS, Vidali V, Pitsinos EN, Couladouros EA. Angew. Chem. Int. Ed. 1999;38:3555–3559. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; b) Barnes-Seeman D, Corey EJ. Org. Lett. 1999;1:1503–1504. doi: 10.1021/ol991070h. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Vassilikogiannakis G, Simonsen KB, Baran PS, Zhong Y-L, Vidali VP, Pitsinos EN, Couladouros EA. J. Am. Chem. Soc. 2000;122:3071–3079. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 125.Nicolaou KC, Jautelat R, Vassilikogiannakis G, Baran PS, Simonsen K. Chem. Eur. J. 1999;5:3651–3665. [Google Scholar]
- 126.For another total synthesis of the bisorbicillinoids, see: Hong R, Chen YG, Deng L. Angew. Chem. Int. Ed. 2005;44:3478–3481. doi: 10.1002/anie.200500480.
- 127.a) Ganguly AK, Pramanik B, Chan TC, Sarre O, Liu Y-T, Morton J, Girijavallabhan VM. Heterocycles. 1989;28:83–88. [Google Scholar]; b) Int. Pat. WO 87/02366. 1987; c) Int. Pat. EP 0538 011A1. 1992; d) Ganguly AK, McCormick JL, Jinping L, Saksena AK, Das PR, Pradip R, Chan TM. Bioorg. Med. Chem. Lett. 1999;9:1209–1212. doi: 10.1016/s0960-894x(99)00163-8. [DOI] [PubMed] [Google Scholar]
- 128.a) Nicolaou KC, van Delft FL, Conley SR, Mitchell HJ, Jin Z, Rodriguez RM. J. Am. Chem. Soc. 1997;119:9057–9058. [Google Scholar]; b) Nicolaou KC, Mitchell HJ, van Delft FL, Rübsam F, Rodriguez RM. Angew. Chem. Int. Ed. 1998;37:1871–1874. [Google Scholar]; c) Nicolaou KC, Rodriguez RM, Mitchell HJ, van Delft FL. Angew. Chem. Int. Ed. 1998;37:1874–1876. [Google Scholar]; d) Nicolaou KC, Mitchell HJ, Suzuki H, Rodríguez RM, Baudoin O, Fylaktakidou KC. Angew. Chem. Int. Ed. 1999;38:3334–3339. [PubMed] [Google Scholar]; e) Nicolaou KC, Rodríguez RM, Fylaktakidou KC, Suzuki H, Mitchell HJ. Angew. Chem. Int. Ed. 1999;38:3340–3345. doi: 10.1002/(sici)1521-3773(19991115)38:22<3340::aid-anie3340>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Mitchell HJ, Rodríguez RM, Fylaktakidou KC, Suzuki H. Angew. Chem. Int. Ed. 1999;38:3345–3350. doi: 10.1002/(sici)1521-3773(19991115)38:22<3345::aid-anie3345>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; g) Nicolaou KC, Rodríguez RM, Mitchell HJ, Suzuki H, Fylaktakidou KC, Baudoin O, van Delft FL. Chem. Eur. J. 2000;6:3095–3115. doi: 10.1002/1521-3765(20000901)6:17<3095::aid-chem3095>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; h) Nicolaou KC, Mitchell HJ, Fylaktakidou KC, Rodríguez RM, Suzuki H. Chem. Eur. J. 2000;6:3116–3148. doi: 10.1002/1521-3765(20000901)6:17<3116::aid-chem3116>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]; i) Nicolaou KC, Mitchell HJ, Rodríguez RM, Fylaktakidou KC, Suzuki H, Conley SR. Chem. Eur. J. 2000;6:3149–3165. doi: 10.1002/1521-3765(20000901)6:17<3149::aid-chem3149>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]; j) Nicolaou KC, Fylaktakidou KC, Mitchell HJ, van Delft FL, Rodríguez RM, Conley SR, Jin Z. Chem. Eur. J. 2000;6:3166–3185. doi: 10.1002/1521-3765(20000901)6:17<3166::aid-chem3166>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 129.Nicolaou KC, Mitchell HJ, Snyder SA. In: Carbohydrate-based Drug Discovery. Wong C-H, editor. Vol. 1. Wiley-VCH; Weinheim: 2003. pp. 215–252. ch. 8. [Google Scholar]
- 130.a) Stinson S. Chem. Eng. News. 1995;73:29. [Google Scholar]; b) Dabrah TT, Harwood HJ, Huang LH, Jankovich ND, Kaneko T, Li J-C, Lindsey S, Moshier PM, Subashi TA, Therien M, Watts PC. J. Antibiot. 1997;50:1–7. doi: 10.7164/antibiotics.50.1. [DOI] [PubMed] [Google Scholar]; c) Dabrah TT, Kaneko T, Massefski W, Whipple EB. J. Am. Chem. Soc. 1997;119:1594–1598. [Google Scholar]
- 131.a) Nicolaou KC, Härter MW, Boulton L, Jandeleit B. Angew. Chem. Int. Ed. Engl. 1997;36:1194–1196. [Google Scholar]; b) Nicolaou KC, Postema MHD, Miller ND, Yang G. Angew. Chem. Int. Ed. Engl. 1997;36:2821–2823. [Google Scholar]; c) Nicolaou KC, Baran PS, Zhong Y-L, Choi H-S, Yoon WH, He Y, Fong KC. Angew. Chem. Int. Ed. 1999;38:1669–1675. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1669::AID-ANIE1669>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Baran PS, Zhong Y-L, Fong KC, He Y, Yoon WH, Choi H-S. Angew. Chem. Int. Ed. 1999;38:1676–1678. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1676::AID-ANIE1676>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; e) Nicolaou KC, Jung J, Yoon WH, Fong KC, Choi H-S, He Y, Zhong Y-L, Baran PS. J. Am. Chem. Soc. 2002;124:2183–2189. doi: 10.1021/ja012010l. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Baran PS, Zhong Y-L, Fong KC, Choi H-S. J. Am. Chem. Soc. 2002;124:2190–2201. doi: 10.1021/ja012011d. [DOI] [PubMed] [Google Scholar]; g) Nicolaou KC, Zhong Y-L, Baran PS, Jung J, Choi H-S, Yoon WH. J. Am. Chem. Soc. 2002;124:2202–2211. doi: 10.1021/ja0120126. [DOI] [PubMed] [Google Scholar]; h) Nicolaou KC, Jung J-K, Yoon WH, He Y, Zhong Y-L, Baran PS. Angew. Chem. Int. Ed. 2000;39:1829–1832. doi: 10.1002/(sici)1521-3773(20000515)39:10<1829::aid-anie1829>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 132.a) Chen C, Layton ME, Shair MD. J. Am. Chem. Soc. 1998;120:10784–10785. [Google Scholar]; b) Waizumi N, Itoh T, Fukuyama T. J. Am. Chem. Soc. 2000;122:7825–7826. [Google Scholar]; c) Chen C, Layton ME, Sheehan SM, Shair MD. J. Am. Chem. Soc. 2000;122:7424–7425. [Google Scholar]; d) Tan Q, Danishefsky SJ. Angew. Chem. Int. Ed. 2000;39:4509–4511. [PubMed] [Google Scholar]
- 133.Nicolaou KC, Baran PS, Jautelat R, He Y, Fong KC, Choi H-S, Yoon WH, Zhong Y-L. Angew. Chem. Int. Ed. 1999;38:549–552. doi: 10.1002/(SICI)1521-3773(19990215)38:4<549::AID-ANIE549>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 134.Nicolaou KC, He Y, Fong KC, Yoon WH, Choi HS, Zhong YL, Baran PS. Org. Lett. 1999;1:63–66. doi: 10.1021/ol990551y. [DOI] [PubMed] [Google Scholar]
- 135.a) Nicolaou KC, Zhong Y-L, Baran PS. Angew. Chem. Int. Ed. 2000;39:622–625. [PubMed] [Google Scholar]; b) Nicolaou KC, Zhong Y-L, Baran PS. Angew. Chem. Int. Ed. 2000;39:625–628. doi: 10.1002/(sici)1521-3773(20000204)39:3<625::aid-anie625>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 136.Nicolaou KC, Zhong Y-L, Baran PS. J. Am. Chem. Soc. 2000;122:7596–7597. [Google Scholar]
- 137.Nicolaou KC, Baran PS, Zhong Y-L. J. Am. Chem. Soc. 2001;123:3183–3185. doi: 10.1021/ja004218x. [DOI] [PubMed] [Google Scholar]
- 138.Nicolaou KC, Mathison CJN, Montagnon T. J. Am. Chem. Soc. 2004;126:5192–5201. doi: 10.1021/ja0400382. [DOI] [PubMed] [Google Scholar]
- 139.Nicolaou KC, Baran PS, Zhong Y-L, Choi H-S, Fong KC, He Y, Yoon WH. Org. Lett. 1999;1:883–886. doi: 10.1021/ol990790l. [DOI] [PubMed] [Google Scholar]
- 140.Nicolaou KC, Vassilikogiannakis G, Kranich R, Baran PS, Zhong Y-L, Natarajan S. Org. Lett. 2000;2:1895–1898. doi: 10.1021/ol000102u. [DOI] [PubMed] [Google Scholar]
- 141.Nicolaou KC, Sugita K, Baran PS, Zhong Y-L. Angew. Chem. Int. Ed. 2001;40:207–210. doi: 10.1002/1521-3773(20010105)40:1<207::AID-ANIE207>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 142.Nicolaou KC, Montagnon T, Ulven T, Baran PS, Zhong Y-L, Sarabia F. J. Am. Chem. Soc. 2002;124:5718–5728. doi: 10.1021/ja012146j. [DOI] [PubMed] [Google Scholar]
- 143.For a review of the CP molecules synthetic campaign, see: Nicolaou KC, Baran PS. Angew. Chem. Int. Ed. 2002;41:2678–2720. doi: 10.1002/1521-3773(20020802)41:15<2678::AID-ANIE2678>3.0.CO;2-V.
- 144.Xiao X.-y., Zhao C, Potash H, Nova MP. Angew. Chem. Int. Ed. Engl. 1997;36:780–782. [Google Scholar]
- 145.Nicolaou KC, Pastor J, Barluenga S, Winssinger N. Chem. Commun. 1998;1947:1948. [Google Scholar]
- 146.a) Nicolaou KC, Pfefferkorn JA, Cao G-Q. Angew. Chem. Int. Ed. 2000;39:734–739. [PubMed] [Google Scholar]; b) Nicolaou KC, Pfefferkorn JA, Cao G-Q. Angew. Chem. Int. Ed. 2000;39:739–743. [PubMed] [Google Scholar]; c) Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao G-Q, Barluenga S, Mitchell HJ. J. Am. Chem. Soc. 2000;122:9939–9953. [Google Scholar]; d) Nicolaou KC, Pfefferkorn JA, Mitchell HJ, Roecker AJ, Barluenga S, Cao G-Q, Affleck RL, Lillig JE. J. Am. Chem. Soc. 2000;122:9954–9967. [Google Scholar]; e) Nicolaou KC, Pfefferkorn JA, Barluenga S, Mitchell HJ, Roecker AJ, Cao G-Q. J. Am. Chem. Soc. 2000;122:9968–9976. [Google Scholar]
- 147.Nicolaou KC, Roecker AJ, Barluenga SB, Pfefferkorn JA, Cao G-Q. ChemBioChem. 2001;2:460–465. doi: 10.1002/1439-7633(20010601)2:6<460::AID-CBIC460>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 148.Nicolaou KC, Pfefferkorn JA, Schuler F, Roecker AJ, Cao G-Q, Casida JE. Chem. Biol. 2000;7:979–992. doi: 10.1016/s1074-5521(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 149.a) Nicolaou KC, Evans RM, Roecker AJ, Hughes R, Downes M, Pfefferkorn JA. Org. Biomol. Chem. 2003;1:908–920. [PubMed] [Google Scholar]; b) Downes M, Verdecia M, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer J-L, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JGA, Noel JP, Nicolaou KC, Evans RM. Mol. Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
- 151.Rodríguez AD, Ramírez C. Org. Lett. 2000;2:507–510. doi: 10.1021/ol991362i. [DOI] [PubMed] [Google Scholar]
- 152.a) Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Angew. Chem. Int. Ed. 2001;40:2482–2486. doi: 10.1002/1521-3773(20010702)40:13<2482::AID-ANIE2482>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Chem. Eur. J. 2001;7:5359–5371. doi: 10.1002/1521-3765(20011217)7:24<5359::aid-chem5359>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 153.a) Kim AI, Rychnovsky SD. Angew. Chem. Int. Ed. 2003;42:1267–1270. doi: 10.1002/anie.200390325. [DOI] [PubMed] [Google Scholar]; b) Harrowven DC, Pascoe DD, Demurtas D, Bourne HO. Angew. Chem. Int. Ed. 2005;44:1221–1222. doi: 10.1002/anie.200462268. [DOI] [PubMed] [Google Scholar]; c) Boezio AA, Jarvo ER, Lawrence BM, Jacobsen EN. Angew. Chem. Int. Ed. 2005;44:6046–6050. doi: 10.1002/anie.200502178. [DOI] [PubMed] [Google Scholar]; d) Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485–2490. doi: 10.1021/ja056877l. [DOI] [PubMed] [Google Scholar]
- 154.Ernst-Russel M, Elix J, Chai C, Willis A, Hamada N, Nash T., III Tetrahedron Lett. 1999;40:6321–6334. [Google Scholar]
- 155.a) Nicolaou KC, Gray D. Angew. Chem. Int. Ed. 2001;40:761–763. [PubMed] [Google Scholar]; b) Nicolaou KC, Gray DLF. J. Am. Chem. Soc. 2004;126:607–612. doi: 10.1021/ja030497n. [DOI] [PubMed] [Google Scholar]
- 156.Nicolaou KC, Gray D, Tae J. Angew. Chem. Int. Ed. 2001;40:3675–3678. doi: 10.1002/1521-3773(20011001)40:19<3675::aid-anie3675>3.0.co;2-g. Nicolaou KC, Gray D, Tae J. Angew. Chem. Int. Ed. 2001;40:3679–3683. doi: 10.1002/1521-3773(20011001)40:19<3679::aid-anie3679>3.0.co;2-t. Nicolaou KC, Gray DLF, Tae J. J. Am. Chem. Soc. 2004;126:613–627. doi: 10.1021/ja030498f. For subsequent syntheses of hamigeran B, see: Mukherjee H, Nolan NT, Scott SC, Stoltz BM. Org. Lett. 2011;13:825–827. doi: 10.1021/ol102669z. and references cited therein.
- 157.For formal total syntheses of hybocarpone, see: Wu K-L, Cohen EPMT, Huang Y, Pettus TRR. Synlett. 2009:1273–1276. Chai CLL, Elix JA, Moore FKE. J. Org. Chem. 2006;71:992–1001. doi: 10.1021/jo0519561.
- 158.Kim JW, Adachi H, Shin-Ya K, Hayakawa Y, Seto H. J. Antibiot. 1997;50:628–630. doi: 10.7164/antibiotics.50.628. [DOI] [PubMed] [Google Scholar]
- 159.a) Nicolaou KC, Li Y, Fylaktakidou KC, Mitchell HJ, Wei H-X, Weyershausen B. Angew. Chem. Int. Ed. 2001;40:3849–3854. [PubMed] [Google Scholar]; b) Nicolaou KC, Li Y, Fylaktakidou KC, Mitchell HJ, Sugita K. Angew. Chem. Int. Ed. 2001;40:3854–3857. doi: 10.1002/1521-3773(20011015)40:20<3854::AID-ANIE3854>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Fylaktakidou KC, Monenschein H, Li Y, Weyershausen B, Mitchell HJ, Wei H, Guntupalli P, Hepworth D, Sugita K. J. Am. Chem. Soc. 2003;125:15433–15442. doi: 10.1021/ja0304953. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Li Y, Sugita K, Monenschein H, Guntupalli P, Mitchell HJ, Fylaktakidou KC, Vourloumis D, Giannakakou P, O’Brate A. J. Am. Chem. Soc. 2003;125:15443–15454. doi: 10.1021/ja030496v. [DOI] [PubMed] [Google Scholar]
- 160.a) Wehlan H, Dauber M, Fernaud MTM, Schuppan J, Keiper S, Mahrwald R, Garcia M-EJ, Koert U. Chem. Eur. J. 2006;12:7378–7397. doi: 10.1002/chem.200600462. [DOI] [PubMed] [Google Scholar]; b) Crimmins M, Christie HS, Long A, Chaudhary K. Org. Lett. 2009;11:831–824. doi: 10.1021/ol802829n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jap. Pat. 10101666. 1998
- 162.a) Nicolaou KC, Vassilikogiannakis G, Montagnon T. Angew. Chem. Int. Ed. 2002;41:3276–3281. doi: 10.1002/1521-3773(20020902)41:17<3276::AID-ANIE3276>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Montangon T, Vassilikogiannakis G. Chem. Commun. 2002:2478–2479. [Google Scholar]; c) Nicolaou KC, Montagnon T, Vassilikogiannakakou G, Mathison CJN. J. Am. Chem. Soc. 2005;127:8872–8888. doi: 10.1021/ja0509984. [DOI] [PubMed] [Google Scholar]
- 163.For a review of metathesis in total synthesis, see: Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4490–4527. doi: 10.1002/anie.200500369.
- 164.Lindquist N, Fenical W, Van Duyne GD, Clardy J. J. Am. Chem. Soc. 1991;113:2303–2304. [Google Scholar]
- 165.a) Li J, Jeong S, Esser L, Harran PG. Angew. Chem. Int. Ed. 2001;40:4765–4769. doi: 10.1002/1521-3773(20011217)40:24<4765::aid-anie4765>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; b) Li J, Burgett AWG, Esser L, Amezcua C, Harran PG. Angew. Chem. Int. Ed. 2001;40:4770–4773. doi: 10.1002/1521-3773(20011217)40:24<4770::aid-anie4770>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 166.For synthetic approaches to diazonamide A from these laboratories, see: Nicolaou KC, Snyder SA, Simonsen KB, Koumbis KE. Angew. Chem. Int. Ed. 2000;39:3473–3478. doi: 10.1002/1521-3773(20001002)39:19<3473::aid-anie3473>3.0.co;2-e. Nicolaou KC, Huang X, Giuseppone N, Bheema Rao P, Bella M, Reddy MV, Snyder SA. Angew. Chem. Int. Ed. 2001;40:4705–4709. doi: 10.1002/1521-3773(20011217)40:24<4705::aid-anie4705>3.0.co;2-d. Nicolaou KC, Snyder SA, Huang X, Simonsen KB, Koumbis AE, Bigot A. J. Am. Chem. Soc. 2004;126:10162–10173. doi: 10.1021/ja040090y. Nicolaou KC, Snyder SA, Giuseppone N, Huang X, Bella M, Reddy MV, Bheema Rao P, Koumbis AE, Giannakakou P, O’Brate A. J. Am. Chem. Soc. 2004;126:10174–10182. doi: 10.1021/ja040091q.
- 167.a) Nicolaou KC, Bella M, Chen DY-K, Huang X, Ling T, Snyder SA. Angew. Chem. Int. Ed. 2002;41:3495–3499. doi: 10.1002/1521-3773(20020916)41:18<3495::AID-ANIE3495>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Chen DY-K, Huang X, Ling T, Bella M, Snyder SA. J. Am. Chem. Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i. [DOI] [PubMed] [Google Scholar]
- 168.a) Nicolaou KC, Rao PB, Hao J, Reddy MV, Rassias G, Huang X, Chen DY-K, Snyder SA. Angew. Chem. Int. Ed. 2003;42:1753–1758. doi: 10.1002/anie.200351112. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Hao J, Reddy MV, Bheema Rao P, Rassias G, Snyder SA, Huang X, Chen DY-K, Brenzovich WE, Giuseppone N, Giannakakou P, O’Brate A. J. Am. Chem. Soc. 2004;126:12897–12906. doi: 10.1021/ja040093a. [DOI] [PubMed] [Google Scholar]
- 169.Nicolaou KC, Snyder SA, Longbottom DA, Nalbandian AZ, Huang X. Chem. Eur. J. 2004;10:5581–5606. doi: 10.1002/chem.200400503. [DOI] [PubMed] [Google Scholar]
- 170.Nicolaou KC, Snyder SA, Nalbandian AZ, Longbottom DA. J. Am. Chem. Soc. 2004;126:6234–6235. doi: 10.1021/ja049293c. [DOI] [PubMed] [Google Scholar]
- 171.a) Burgett WG, Li Q, Wei Q, Harran PG. Angew. Chem. Int. Ed. 2003;42:4961–4966. doi: 10.1002/anie.200352577. [DOI] [PubMed] [Google Scholar]; b) Cheung C-M, Goldberg FW, Magnus P, Russell CJ, Turnbull R, Lynch V. J. Am. Chem. Soc. 2007;129:12320–12327. doi: 10.1021/ja0744448. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mai C-K, Sammons MF, Sammakia T. Angew. Chem. Int. Ed. 2010;49:2397–2400. doi: 10.1002/anie.200906318. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Knowles RR, Carpenter J, Blakely SB, Kayano A, Mangion IK, Sinz CJ, MacMillan DWC. Chem. Sci. 2011;2:308–311. doi: 10.1039/C0SC00577K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kosela S, Cao S-G, Wu X-H, Vitaal JJ, Sukri T, Masdianto, Goh S-H, Sim K-Y. Tetrahedron Lett. 1999;40:157–160. [Google Scholar]
- 173.a) Nicolaou KC, Sasmal PK, Xu H, Namoto K, Ritzén A. Angew. Chem. Int. Ed. 2003;42:4225–4229. doi: 10.1002/anie.200351805. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Sasmal PK, Xu H. J. Am. Chem. Soc. 2004;126:5493–5501. doi: 10.1021/ja040037+. [DOI] [PubMed] [Google Scholar]
- 174.a) Pagano JF, Weinstein MJ, Stout HA, Donovick R. Antibiot. Annu. 1955-1956:554–559. [PubMed] [Google Scholar]; b) Vandeputte J, Dutcher JD. Antibiot. Annu. 1955-1956:560–561. [PubMed] [Google Scholar]; c) Steinberg BA, Jambor WP, Suydam LO. Antibiot. Annu. 1955-1956:562–565. [PubMed] [Google Scholar]
- 175.a) Nicolaou KC, Safina BS, Zak M, Estrada AA, Lee SH. Angew. Chem. Int. Ed. 2004;43:5087–5092. doi: 10.1002/anie.200461340. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Zak M, Safina BS, Lee SH, Estrada AA. Angew. Chem. Int. Ed. 2004;43:5092–5097. doi: 10.1002/anie.200461341. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Safina BS, Zak M, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J. Am. Chem. Soc. 2005;127:11159–11175. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176–11183. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 176.a) Mocek U, Knaggs AR, Tsuchiya R, Nguyen T, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7557–7568. [Google Scholar]; b) Mocek U, Zeng Z, O’Hagan D, Zhou P, Fan L-DG, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7992–8001. [Google Scholar]
- 177.a) Mori T, Higashibayashi S, Goto T, Kohno M, Satouchi Y, Shinko K, Suzuki K, Suzuki S, Tohmiya H, Hashimoto K, Nakata M. Tetrahedron Lett. 2007;48:1331–1335. [Google Scholar]; b) Mori T, Higashibayashi S, Goto T, Kohno M, Satouchi Y, Shinko K, Suzuki K, Suzuki S, Tohmiya H, Hashimoto K, Nakata M. Chem. Asian J. 2008;3:984–1012. doi: 10.1002/asia.200800032. [DOI] [PubMed] [Google Scholar]; c) Mori T, Higashibayashi S, Goto T, Kohno M, Satouchi Y, Shinko K, Suzuki K, Suzuki S, Tohmiya H, Hashimoto K, Nakata M. Chem. Asian J. 2008;3:1013–1025. doi: 10.1002/asia.200800033. [DOI] [PubMed] [Google Scholar]
- 178.a) Nicolaou KC, Zou B, Dethe D-H, Li DB, Chen DY-K. Angew. Chem. Int. Ed. 2006;45:7786–7792. doi: 10.1002/anie.200602798. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Dethe D-H, Leung GYC, Zou B, Chen DY-K. Chem. Asian J. 2008;3:413–429. doi: 10.1002/asia.200700361. [DOI] [PubMed] [Google Scholar]
- 179.a) Müller HM, Delgado O, Bach T. Angew. Chem. Int. Ed. 2007;46:4771–4774. doi: 10.1002/anie.200700684. [DOI] [PubMed] [Google Scholar]; b) Delgado O, Müller HM, Bach T. Chem. Eur. J. 2008;14:2322–2339. doi: 10.1002/chem.200701823. [DOI] [PubMed] [Google Scholar]
- 180.Nicolaou KC, Dethe DH, Chen DY-K. Chem. Commun. 2008:2632–2634. doi: 10.1039/b805069b. [DOI] [PubMed] [Google Scholar]
- 181.a) Hughes RA, Thompson SP, Alcaraz L, Moody CJ. Chem. Commun. 2004:946–948. doi: 10.1039/b401580k. [DOI] [PubMed] [Google Scholar]; b) Hughes RA, Thompson SP, Alcaraz L, Moody CJ. J. Am. Chem. Soc. 2005;127:15644–15651. doi: 10.1021/ja0547937. [DOI] [PubMed] [Google Scholar]
- 182.Satake M, Ofuji K, Naoki H, James KJ, Fruey A, McMahon T, Silke J, Yasumoto T. J. Am. Chem. Soc. 1998;120:9967–9968. [Google Scholar]
- 183.a) Nicolaou KC, Pihko PM, Diedrichs N, Zou N, Bernal F. Angew. Chem. Int. Ed. 2001;40:1262–1265. [PubMed] [Google Scholar]; b) Nicolaou KC, Qian W, Bernal F, Uesaka N, Pihko PM, Hinrichs J. Angew. Chem. Int. Ed. 2001;40:4068–4071. doi: 10.1002/1521-3773(20011105)40:21<4068::AID-ANIE4068>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Li Y, Uesaka N, Koftis TV, Vyskocil S, Ling T, Govindasamy M, Qian W, Bernal F, Chen DY-K. Angew. Chem. Int. Ed. 2003;42:3643–3648. doi: 10.1002/anie.200351825. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Chen DY-K, Li Y, Qian W, Ling T, Vyskocil S, Koftis TV, Govindasamy M, Uesaka N. Angew. Chem. Int. Ed. 2003;42:3649–3653. doi: 10.1002/anie.200351826. [DOI] [PubMed] [Google Scholar]
- 184.a) Nicolaou KC, Vyskocil S, Koftis TV, Yamada YMA, Ling T, Chen DY-K, Tang W, Petrovic G, Frederick MO, Li Y, Satake M. Angew. Chem. Int. Ed. 2004;43:4312–4318. doi: 10.1002/anie.200460695. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Ling T, Yamada YMA, Tang W, Frederick MO. Angew. Chem. Int. Ed. 2004;43:4318–4324. doi: 10.1002/anie.200460696. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Pihko PM, Bernal F, Frederick MO, Qian W, Uesaka N, Diedrichs N, Hinrichs J, Koftis TV, Loizidou E, Petrovic G, Rodriquez M, Sarlah D, Zou N. J. Am. Chem. Soc. 2006;128:2244–2257. doi: 10.1021/ja0547477. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Chen DY-K, Li Y, Uesaka N, Petrovic G, Koftis TV, Bernal F, Frederick MO, Govindasamy M, Ling T, Pihko PM, Tang W, Vyskocil S. J. Am. Chem. Soc. 2006;128:2258–2267. doi: 10.1021/ja054748z. [DOI] [PubMed] [Google Scholar]; e) Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Tang W, Frederick MO, Chen DY-K, Li Y, Ling T, Yamada YMA. J. Am. Chem. Soc. 2006;128:2859–2872. doi: 10.1021/ja054750q. [DOI] [PubMed] [Google Scholar]
- 185.a) Nicolaou KC, Frederick MO, Petrovic G, Cole KP, Loizidou EZ. Angew. Chem. Int. Ed. 2006;45:2609–2615. doi: 10.1002/anie.200600295. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Frederick MO, Loizidou EZ, Petrovic G, Cole KP, Koftis TV, Yamada YMA. Chem. Asian J. 2006;1-2:245–263. doi: 10.1002/asia.200600059. [DOI] [PubMed] [Google Scholar]
- 186.a) Ito E, Frederick MO, Koftis TV, Tang W, Petrovic G, Ling T, Nicolaou KC. Harmful Algae. 2006;5:586–591. [Google Scholar]; b) Alfonso A, Vieytes MR, Ofuji K, Satake M, Nicolaou KC, Frederick MO, Botana LM. Biochem. Biophys. Res. Commun. 2006;346:1091–1099. doi: 10.1016/j.bbrc.2006.06.019. [DOI] [PubMed] [Google Scholar]; c) Vilariño N, Nicolaou KC, Frederick MO, Cagide E, Ares IM, Louzao MC, Vieytes MR, Botana LM. Chem. Res. Toxicol. 2006;19:1459–1466. doi: 10.1021/tx060131z. [DOI] [PubMed] [Google Scholar]; d) Vale C, Nicolaou KC, Frederick MO, Gomez-Limia B, Alfonso A, Vieytes MR, Botana LM. J. Med. Chem. 2007;50:356–363. doi: 10.1021/jm061063g. [DOI] [PubMed] [Google Scholar]; e) Vilariño N, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. Biochem. Pharm. 2007;74:327–335. doi: 10.1016/j.bcp.2007.04.004. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Guduru R, Sun Y-P, Banerji B, Chen DY-K. Angew. Chem. Int. Ed. 2007;46:5896–5900. doi: 10.1002/anie.200702243. [DOI] [PubMed] [Google Scholar]; g) Vale C, Wandscheer C, Nicolaou KC, Frederick MO, Alfonso C, Alfonso A, Vieytes MR, Botana LM. J. Neurosci. Res. 2008;86:2952–2962. doi: 10.1002/jnr.21731. [DOI] [PubMed] [Google Scholar]; h) Frederick MO, Marin SD, Janda KD, Nicolaou KC, Dickerson TJ. ChemBioChem. 2009;10:1625–1629. doi: 10.1002/cbic.200900201. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Vale C, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. Toxicol. Sci. 2010;113:158–168. doi: 10.1093/toxsci/kfp246. Correction: Toxicol. Sci. 2010, 116, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.a) Evans DA, Kværnø L, Mulder JA, Raymer B, Dunn TB, Beauchemin A, Olhava EJ, Juhl M, Kagechika K. Angew. Chem. Int. Ed. 2007;46:4693–4697. doi: 10.1002/anie.200701515. [DOI] [PubMed] [Google Scholar]; b) Evans DA, Dunn TB, Kværnø L, Beauchemin A, Raymer B, Olhava EJ, Mulder JA, Juhl M, Kagechika K, Favor DA. Angew. Chem. Int. Ed. 2007;46:4698–4703. doi: 10.1002/anie.200701520. [DOI] [PubMed] [Google Scholar]; c) Evans DA, Kværnø L, Dunn TB, Beauchemin A, Raymer B, Mulder JA, Olhava EJ, Juhl M, Kagechika K, Favor DA. J. Am. Chem. Soc. 2008;130:16295–16309. doi: 10.1021/ja804659n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Augusta A, Ohashi K, Shibuya H. Chem. Pharm. Bull. 2006;54:579–582. doi: 10.1248/cpb.54.579. [DOI] [PubMed] [Google Scholar]
- 189.a) Ogihara Y, Kobayashi N, Shibata S. Tetrahedron Lett. 1968;9:1881–1886. [Google Scholar]; b) Takeda N, Seo S, Ogihara Y, Sankawa U, Iitaka I, Kitagawa I, Shibata S. Tetrahedron. 1973;29:3703–3719. [Google Scholar]; c) Shibata S. Pure Appl. Chem. 1973;33:109–128. doi: 10.1351/pac197333010109. [DOI] [PubMed] [Google Scholar]; d) Seo S, Sankawa U, Ogihara Y, Iitaka Y, Shibata S. Tetrahedron. 1973;29:3721–3726. [Google Scholar]; e) Yang D-M, Sankawa U, Ebizuka Y, Shibata S. Tetrahedron. 1976;32:333–335. [Google Scholar]
- 190.Sedmera P, Podojil M, Vokoun J, Betina V, Nemec P. Folia Microbiol. 1978;23:64–67. doi: 10.1007/BF02876598. [DOI] [PubMed] [Google Scholar]
- 191.a) Nicolaou KC, Papageorgiou CD, Piper JL, Chadha RK. Angew. Chem. Int. Ed. 2005;44:5846–5851. doi: 10.1002/anie.200502011. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Lim YH, Papageorgiou CD, Piper JL. Angew. Chem. Int. Ed. 2005;44:7917–7921. doi: 10.1002/anie.200503678. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Lim YH, Piper JL, Papageorgiou CD. J. Am. Chem. Soc. 2007;129:4001–4013. doi: 10.1021/ja0685708. [DOI] [PubMed] [Google Scholar]
- 192.a) Riedlinger J, Reicke A, Zähner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Süssmuth RD, Fiedler H-P. J. Antibiot. 2004;57:271–279. doi: 10.7164/antibiotics.57.271. [DOI] [PubMed] [Google Scholar]; b) Bister B, Bischoff D, Ströbele M, Riedlinger J, Riecke A, Wolter F, Bull AT, Zähner H, Fiedler H-P, Süssmuth RD. Angew. Chem. Int. Ed. 2004;43:2574–2576. doi: 10.1002/anie.200353160. [DOI] [PubMed] [Google Scholar]
- 193.a) Nicolaou KC, Harrison ST. Angew. Chem. Int. Ed. 2006;45:3256–3260. doi: 10.1002/anie.200601116. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Harrison ST. J. Am. Chem. Soc. 2007;127:429–440. doi: 10.1021/ja067083p. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Harrison ST, Chen JS. Synthesis. 2009:33–42. doi: 10.1055/s-0028-1083259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.a) Keller S, Nicholson G, Drahl C, Sorensen EJ, Fiedler H-P, Süssmuth RD, Antibiot J. 2007;60:391–394. doi: 10.1038/ja.2007.54. [DOI] [PubMed] [Google Scholar]; b) Keller S, Schadt HS, Ortel I, Süssmuth RD. Angew. Chem. Int. Ed. 2007;46:8284–8286. doi: 10.1002/anie.200701836. [DOI] [PubMed] [Google Scholar]
- 195.Zapf CW, Harrison BA, Drahl C, Sorensen EJ. Angew. Chem. Int. Ed. 2005;44:6533–6537. doi: 10.1002/anie.200502119. [DOI] [PubMed] [Google Scholar]
- 196.a) Snider BB, Zou Y. Org. Lett. 2005;7:4939–4941. doi: 10.1021/ol0518941. [DOI] [PubMed] [Google Scholar]; b) Rath J-P, Eipert M, Kinast S, Maier ME. Synlett. 2005:314–318. [Google Scholar]; c) Rath J-P, Kinast S, Maier ME. Org. Lett. 2005;7:3089–3092. doi: 10.1021/ol0511068. [DOI] [PubMed] [Google Scholar]; d) Couladouros EA, Bouzas EA, Magos AD. Tetrahedron. 2006;62:5272–5279. [Google Scholar]
- 197.a) Jensen PR, Mincer TJ, Williams PG, Fenical W. Antonie van Leeuwenhoek. 2005;87:43–48. doi: 10.1007/s10482-004-6540-1. [DOI] [PubMed] [Google Scholar]; b) Kwon HC, Kauffman CA, Jensen PR, Fenical W. J. Am. Chem. Soc. 2006;128:1622–1632. doi: 10.1021/ja0558948. [DOI] [PubMed] [Google Scholar]
- 198.a) Nicolaou KC, Nold AL, Milburn RR, Schindler CS. Angew. Chem. Int. Ed. 2006;45:6527–6532. doi: 10.1002/anie.200601867. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Nold AL, Milburn RR, Schindler CS, Cole KP, Yamaguchi J. J. Am. Chem. Soc. 2007;129:1760–1768. doi: 10.1021/ja068053p. [DOI] [PubMed] [Google Scholar]
- 199.Monomarinomycin A has also been synthesized by Cossy and coworkers: Amans D, Bareielle L, Bellosta V, Cossy J. J. Org. Chem. 2009;74:7665–7674. doi: 10.1021/jo900945x.
- 200.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio Á, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 201.a) Nicolaou KC, Li A, Edmonds DJ. Angew. Chem. Int. Ed. 2006;45:7086–7090. doi: 10.1002/anie.200603892. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew. Chem. Int. Ed. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Tang Y, Wang J. Chem. Commun. 2007:1922–1923. doi: 10.1039/b704589a. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Li A, Ellery SP, Edmonds DJ. Angew. Chem. Int. Ed. 2009;48:6293–6295. doi: 10.1002/anie.200901992. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Nicolaou KC, Li A, Edmonds DJ, Tria GS, Ellery SP. J. Am. Chem. Soc. 2009;131:16905–16918. doi: 10.1021/ja9068003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.For recent reviews on the synthesis of platensimycin, platencin, and other antibiotics, see: Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Angew. Chem. Int. Ed. 2009;48:660–719. doi: 10.1002/anie.200801695. Tiefenbacher K, Mulzer J. Angew. Chem. Int. Ed. 2008;47:2548–2555. doi: 10.1002/anie.200705303.
- 203.a) Nicolaou KC, Lister T, Denton RM, Montero A, Edmonds DJ. Angew. Chem. Int. Ed. 2007;46:4712–4714. doi: 10.1002/anie.200701548. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Tang Y, Wang J, Stepan AF, Li A, Montero A. J. Am. Chem. Soc. 2007;129:14850–14851. doi: 10.1021/ja076126e. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Stepan AF, Lister T, Li A, Montero A, Tria GS, Turner CI, Tang Y, Wang J, Denton RM, Edmonds DJ. J. Am. Chem. Soc. 2008;130:13110–13119. doi: 10.1021/ja8044376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.For other syntheses of platensimycin, see: Zou Y, Chen C-H, Taylor CD, Foxman BM, Snider BB. Org. Lett. 2007;9:1825–1828. doi: 10.1021/ol070563g. Kaliappan KP, Ravikumar V. Org. Lett. 2007;9:2417–2419. doi: 10.1021/ol070848t. Li P, Payette JN, Yamamoto H. J. Am. Chem. Soc. 2007;129:9534–9535. doi: 10.1021/ja073547n. Ghosh AK, Xi K. Org. Lett. 2007;9:4013–4016. doi: 10.1021/ol701783z. Tiefenbacher K, Mulzer J. Angew. Chem. Int. Ed. 2007;46:8074–8075. doi: 10.1002/anie.200702852. Lalic G, Corey EJ. Org. Lett. 2007;9:4921–4923. doi: 10.1021/ol702323s. Heretsch P, Giannis A. Synthesis. 2007:2614–2616. Kim CH, Jang KP, Choi SY, Chung YK, Lee E. Angew. Chem. Int. Ed. 2008;47:4009–4011. doi: 10.1002/anie.200800568. Matsuo J, Takeuchi K, Ishibashi H. Org. Lett. 2008;10:4049–4052. doi: 10.1021/ol801584r. Ghosh AK, Xi K. J. Org. Chem. 2009;74:1163–1170. doi: 10.1021/jo802261f. Yun SY, Zheng J-C, Lee D. J. Am. Chem. Soc. 2009;131:8413–8415. doi: 10.1021/ja903526g. McGrath NA, Bartlett ES, Sittihan S, Njardarson JT. Angew. Chem. Int. Ed. 2009;48:8543–8546. doi: 10.1002/anie.200903347. Tiefenbacher K, Tröndlin L, Mulzer J, Pfaltz A. Tetrahedron. 2010;66:6508–6513. Xing S, Pan W, Liu C, Ren J, Wang Z. Angew. Chem. Int. Ed. 2010;49:3215–3218. doi: 10.1002/anie.201000563. Eey ST-C, Lear MJ. Org. Lett. 2010;12:5510–5513. doi: 10.1021/ol102390t. Magnus P, Rivera H, Lynch V. Org. Lett. 2010;12:5677–5679. doi: 10.1021/ol102557k. Beaulieu M-A, Sabot C, Achache N, Guérard KC, Canesi S. Chem. Eur. J. 2010;16:11224–11228. doi: 10.1002/chem.201001813. Hirai S, Nakada M. Tetrahedron. 2011;67:518–530. Oblak EZ, Wright DL. Org. Lett. 2011;13:2263–2265. doi: 10.1021/ol2005775. Ueda Y, Iwahashi K, Iguchi K, Ito H. Synthesis. 2011;1532:1536.
- 205.Ito S, Matsuya T, Omura S, Otani M, Nakagawa A. J. Antibiot. 1970;23:315–316. doi: 10.7164/antibiotics.23.315. [DOI] [PubMed] [Google Scholar]
- 206.Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J. Am. Chem. Soc. 2007;129:10356–10357. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]
- 207.a) Lei X, Porco JA., Jr. J. Am. Chem. Soc. 2006;128:14790–14791. doi: 10.1021/ja066621v. [DOI] [PubMed] [Google Scholar]; b) Kumamoto T, Kitani Y, Tsuchiya H, Yamaguchi K, Seki H, Ishikawa T. Tetrahedron. 2007;63:5189–5199. [Google Scholar]; c) Woo CM, Lu L, Gholap SL, Smith DR, Herzon SB. J. Am. Chem. Soc. 2010;132:2540–2541. doi: 10.1021/ja910769j. [DOI] [PubMed] [Google Scholar]
- 208.Davies J, Wang H, Taylor T, Warabi K, Huang X-H, Anderson RJ. Org. Lett. 2005;7:5233–5236. doi: 10.1021/ol052081f. [DOI] [PubMed] [Google Scholar]
- 209.Nicolaou KC, Zhang H, Chen JS, Crawford JJ, Pasunoori L. Angew. Chem. Int. Ed. 2007;46:4704–4707. doi: 10.1002/anie.200700917. [DOI] [PubMed] [Google Scholar]
- 210.Nicolaou KC, Chen JS, Zhang H, Montero A. Angew. Chem. Int. Ed. 2008;47:185–189. doi: 10.1002/anie.200704577. [DOI] [PubMed] [Google Scholar]
- 211.Myers AG, Tom NJ, Fraley ME, Cohen SB, Madar DJ. J. Am. Chem. Soc. 1997;119:6072–6094. [Google Scholar]
- 212.a) Shair MD, Yoon T, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1995;34:1721–1723. [Google Scholar]; b) Shair MD, Yoon TY, Mosny KK, Chou TC, Danishefsky SJ. J. Am. Chem. Soc. 1996;118:9509–9525. [Google Scholar]
- 213.Tanaka N, Okasaka M, Ishimaru Y, Takaishi Y, Sato M, Okamoto M, Oshikawa T, Ahmed SU, Consentino LM, Lee KH. Org. Lett. 2005;7:2997–2999. doi: 10.1021/ol050960w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tanaka N, Kashiwada Y, Kim SY, Hashida W, Sekiya M, Takaishi Y. J. Nat. Prod. 2009;72:1447–1452. doi: 10.1021/np900109y. [DOI] [PubMed] [Google Scholar]
- 215.a) Nicolaou KC, Sarlah D, Shaw DM. Angew. Chem. Int. Ed. 2007;46:4708–4711. doi: 10.1002/anie.200701552. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Wu TR, Sarlah D, Shaw DM, Rowcliffe E, Burton DR. J. Am. Chem. Soc. 2008;130:11114–11121. doi: 10.1021/ja802805c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nicolaou KC, Sanchini S, Wu TR, Sarlah D. Chem. Eur. J. 2010;16:7678–7682. doi: 10.1002/chem.201001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nicolaou KC, Sanchini S, Sarlah D, Lu G, Wu TR, Nomura DK, Cravatt BF, Cubitt B, de la Torre JC, Hessell AJ, Burton DR. Proc. Natl. Acad. Sci. USA. 2011;108:6715–6720. doi: 10.1073/pnas.1015258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.For a second total synthesis of biyouyanagin A, see: Du C, Li L, Li Y, Xie Z. Angew. Chem. Int. Ed. 2009;48:7853–7856. doi: 10.1002/anie.200902908.
- 219.a) Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti S, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully D, Singh SB. Proc. Natl. Acad. Sci. USA. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Teresa Diez M, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Angew. Chem. Int. Ed. 2007;46:4684–4688. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- 220.a) Nicolaou KC, Tria GS, Edmonds DJ. Angew. Chem. Int. Ed. 2008;47:1780–1783. doi: 10.1002/anie.200800066. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Tria GS, Edmonds DJ, Kar M. J. Am. Chem. Soc. 2009;131:15909–15917. doi: 10.1021/ja906801g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hayashida J, Rawal VH. Angew. Chem. Int. Ed. 2008;47:4373–4376. doi: 10.1002/anie.200800756. Tiefenbacher K, Mulzer J. Angew. Chem. Int. Ed. 2008;47:6199–6200. doi: 10.1002/anie.200801441. Yun SY, Zheng J-C, Lee D. Angew. Chem. Int. Ed. 2008;47:6201–6203. doi: 10.1002/anie.200801587. Waalboer DCJ, Schaapman MC, Van Delft FL, Rutjes FPJT. Angew. Chem. Int. Ed. 2008;47:6576–6578. doi: 10.1002/anie.200802912. Nicolaou KC, Toh Q-Y, Chen DY-K. J. Am. Chem. Soc. 2008;130:11292–11293. doi: 10.1021/ja804588r. Correction: J. Am. Chem. Soc. 2008, 130, 14016. Austin KAB, Banwell MG, Willis AC. Org. Lett. 2008;10:4465–4468. doi: 10.1021/ol801647h. Tiefenbacher K, Mulzer J. J. Org. Chem. 2009;74:2937–2941. doi: 10.1021/jo9001855. Varseev GN, Maier ME. Angew. Chem. Int. Ed. 2009;48:3685–3688. doi: 10.1002/anie.200900447. Ghosh AK, Xi K. Angew. Chem. Int. Ed. 2009;48:5372–5375. doi: 10.1002/anie.200902338. Hirai S, Nakada M. Tetrahedron Lett. 2010;51:5076–5079. Singh K, Sahu BC, Bansal V, Mobin SM. Org. Biomol. Chem. 2010;8:4472–4481. doi: 10.1039/c004316h. Li P, Yamamoto H. Chem. Commun. 2010;46:6294–6295. doi: 10.1039/c0cc01619e. Hirai S, Nakada M. Tetrahedron. 2011;67:518–530. Yoshimitsu T, Nojima S, Hashimoto M, Tanaka T. Org. Lett. 2011;13:3698–3701. doi: 10.1021/ol2013439. For selected reviews on platencin and platensimycin, see: Harsh P, O’Doherty GA. ChemTracts. 2009;22:31–40. Palanichamy K, Kaliappan KP. Chem. Asian J. 2010;5:668–703. doi: 10.1002/asia.200900423. Lu X, You Q. Curr. Med. Chem. 2010;17:1139–1155. doi: 10.2174/092986710790827852. also see ref [202].
- 222.a) Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. J. Am. Chem. Soc. 2006;128:3148–3149. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]; b) Watanabe Y, Aoki S, Tanabe D, Setiawan A, Kobayashi M. Tetrahedron. 2007;63:4074–4079. [Google Scholar]; c) Aoki S, Watanabe Y, Tanabe D, Setiawan A, Arai M, Kobayashi M. Tetrahedron Lett. 2007;48:4485–4488. [Google Scholar]
- 223.a) Nicolaou KC, Sun Y-P, Peng X-S, Polet D, Chen DY-K. Angew. Chem. Int. Ed. 2008;47:7310–7313. doi: 10.1002/anie.200803550. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Peng X-S, Sun Y-P, Polet D, Zou B, Lim CS, Chen DY-K. J. Am. Chem. Soc. 2009;131:10587–10597. doi: 10.1021/ja902939t. [DOI] [PubMed] [Google Scholar]
- 224.Olton Z, Hajos G, Parrish R. Org. Synth. 1990;7:363. [Google Scholar]
- 225.Isaacs RCA, Di Grandi MJ, Danishefsky SJ. J. Org. Chem. 1993;58:3938–3941. [Google Scholar]
- 226.Cee VJ, Chen DY-K, Lee MR, Nicolaou KC. Angew. Chem. Int. Ed. 2009;48:8952–8957. doi: 10.1002/anie.200904778. [DOI] [PubMed] [Google Scholar]
- 227.a) Aoki S, Watanabe Y, Tanabe D, Arai M, Suna H, Miyamoto K, Tsujibo H, Tsujikawa K, Yamamoto H, Kobayashi M. Bioorg. Med. Chem. 2007;15:6758–6762. doi: 10.1016/j.bmc.2007.08.017. [DOI] [PubMed] [Google Scholar]; b) Sato Y, Kamiyama H, Usui T, Saito T, Osada H, Kuwahara S, Kiyota H. Biosci., Biotechnol., Biochem. 2008;72:2992–2997. doi: 10.1271/bbb.80562. [DOI] [PubMed] [Google Scholar]; c) Kotoku N, Sumii Y, Hayashi T, Kobayashi M. Tetrahedron Lett. 2008;49:7078–7081. [Google Scholar]; d) Lee HM, Nieto-Oberhuber C, Shair MD. J. Am. Chem. Soc. 2008;130:16864–16866. doi: 10.1021/ja8071918. [DOI] [PubMed] [Google Scholar]; f) Craft DT, Gung BW. Tetrahedron Lett. 2008;49:5931–5934. doi: 10.1016/j.tetlet.2008.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Dai M, Danishefsky SJ. Tetrahedron Lett. 2008;49:6610–6612. doi: 10.1016/j.tetlet.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Dai MJ, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613–6616. doi: 10.1016/j.tetlet.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Yamashita S, Iso K, Hirama M. Org. Lett. 2008;10:3413–3415. doi: 10.1021/ol8012099. [DOI] [PubMed] [Google Scholar]; j) Simmons EM, Hardin AR, Guo X, Sarpong R. Angew. Chem. Int. Ed. 2008;47:6650–6653. doi: 10.1002/anie.200802203. [DOI] [PubMed] [Google Scholar]; k) Yamashita S, Kitajima K, Iso K, Hirama M. Tetrahedron Lett. 2009;50:3277–3279. [Google Scholar]; l) Dai MJ, Danishefsky SJ. Heterocycles. 2009;77:157–161. [Google Scholar]; m) Liu LZ, Gao YX, Che C, Wu N, Wang DZ, Li CC, Yang Z. Chem. Commun. 2009:662–664. doi: 10.1039/b817376a. [DOI] [PubMed] [Google Scholar]; n) Magnus P, Littich R. Org. Lett. 2009;11:3938–3941. doi: 10.1021/ol901537n. [DOI] [PubMed] [Google Scholar]; o) Frie JL, Jeffrey CS, Sorensen EJ. Org. Lett. 2009;11:5394–5397. doi: 10.1021/ol902168g. [DOI] [PMC free article] [PubMed] [Google Scholar]; p) Baumgartner C, Ma S, Liu Q, Stoltz BM. Org. Biomol. Chem. 2010;8:2915–2917. doi: 10.1039/c004275g. [DOI] [PubMed] [Google Scholar]; q) Simmons EM, Hardin-Narayan AR, Guo XL, Sarpong R. Tetrahedron. 2010;66:4696–4700. doi: 10.1016/j.tet.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; r) Flyer AN, Si C, Myers AG. Nat. Chem. 2010;2:886–892. doi: 10.1038/nchem.794. [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Yu F, Li G, Gao P, Gong H, Liu Y, Wu Y, Cheng B, Zhai H. Org. Lett. 2010;12:5135–5137. doi: 10.1021/ol102058f. [DOI] [PubMed] [Google Scholar]; t) Fang L, Chen Y, Huang J, Liu L, Quan J, Li C-C, Yang Z. J. Org. Chem. 2011;76:2479–2487. doi: 10.1021/jo102202t. [DOI] [PubMed] [Google Scholar]; u) Yamashita S, Iso K, Kitajima K, Himuro M, Hirama M. J. Org. Chem. 2011;76:2408–2425. doi: 10.1021/jo2002616. [DOI] [PubMed] [Google Scholar]
- 228.For reviews and perspectives, see: Nising CF, Bräse S. Angew. Chem. Int. Ed. 2008;47:9389–9391. doi: 10.1002/anie.200803720. Chen DY-K, Tseng CC. Org. Biomol. Chem. 2010;8:2900–2911. doi: 10.1039/c003935g. Narayan ARH, Simmons EM, Sarpong R. Eur. J. Org. Chem. 2010;3553:3567. Shi Y, Tian WS. Chin. J. Org. Chem. 2010;30:515–527.
- 229.a) Shenvi RA, Guerrero CA, Shi J, Li C-C, Baran PS. J. Am. Chem. Soc. 2008;130:7241–7243. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shi J, Shigehisa H, Guerrero CA, Shenvi RA, Li C-C, Baran PS. Angew. Chem. Int. Ed. 2009;48:4328–4331. doi: 10.1002/anie.200901116. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shi J, Manolikakes G, Yeh C-H, Guerrero CA, Shenvi RA, Shigehisa H, Baran PS. J. Am. Chem. Soc. 2011;133:8014–8027. doi: 10.1021/ja202103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.a) Kurti L, Czako B, Corey EJ. Org. Lett. 2008;10:5247–5250. doi: 10.1021/ol802328n. [DOI] [PubMed] [Google Scholar]; b) Czako B, Kurti L, Mammoto A, Ingber DE, Corey EJ. J. Am. Chem. Soc. 2009;131:9014–9019. doi: 10.1021/ja902601e. [DOI] [PubMed] [Google Scholar]
- 231.Ge HM, Xu C, Wang XT, Huang B, Tan RX. Eur. J. Org. Chem. 2006:5551–5554. [Google Scholar]
- 232.Ge HM, Zhu CH, Shi DH, Zhang LD, Xie DQ, Yang J, Ng SW, Tan RX. Chem. Eur. J. 2008;14:376–381. doi: 10.1002/chem.200700960. [DOI] [PubMed] [Google Scholar]
- 233.Nicolaou KC, Wu TR, Kang Q, Chen DY-K. Angew. Chem. Int. Ed. 2009;48:3440–3443. doi: 10.1002/anie.200900438. [DOI] [PubMed] [Google Scholar]
- 234.Nicolaou KC, Kang Q, Wu TR, Lim CS, Chen DY-K. J. Am. Chem. Soc. 2010;132:7540–7548. doi: 10.1021/ja102623j. [DOI] [PubMed] [Google Scholar]
- 235.a) Snyder SA, Gollner A, Chiriac MI. Nature. 2011;474:461–466. doi: 10.1038/nature10197. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Snyder SA, ElSohly AM, Kontes F. Nat. Prod. Rep. 2011;28:897–924. doi: 10.1039/c1np00001b. [DOI] [PubMed] [Google Scholar]; c) Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 236.For another total synthesis of hopeanol and hopeahainol A, see: Snyder SA, Thomas SB, Mayer AC, Breazzano SP. Angew. Chem. Int. Ed. 2012:51. doi: 10.1002/anie.201107730. DOI: 10.1002/anie.201107730.
- 237.Jap. Pat. JP 08143569. 1996
- 238.a) Socha AM, Garcia D, Sheffer R, Rowley DC. J. Nat. Prod. 2006;69:1070–1073. doi: 10.1021/np050449b. [DOI] [PubMed] [Google Scholar]; b) Socha AM, LaPlante KL, Rowley DC. Bioorg. Med. Chem. 2006;14:8446–8454. doi: 10.1016/j.bmc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 239.Nicolaou KC, Lim YH, Becker J. Angew. Chem. Int. Ed. 2009;48:3444–3448. doi: 10.1002/anie.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nicolaou KC, Becker J, Lim YH, Lemire A, Neubauer T, Montero A. J. Am. Chem. Soc. 2009;131:14812–14826. doi: 10.1021/ja9073694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Buchana GO, Williams PG, Feling RH, Kauffman CA, Jensen PR, Fenical W. Org. Lett. 2005;7:2731–2734. doi: 10.1021/ol050901i. [DOI] [PubMed] [Google Scholar]
- 242.Nicolaou KC, Tang Y, Wang J. Angew. Chem. Int. Ed. 2009;48:3449–3453. doi: 10.1002/anie.200900264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nicolaou KC, Wang J, Tang Y, Botta L. J. Am. Chem. Soc. 2010;132:11350–11363. doi: 10.1021/ja1048994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Guella G, Dini F, Pietra F. Angew. Chem. Int. Ed. 1999;38:1134–1136. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1134::AID-ANIE1134>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 245.Nicolaou KC, Zhang H, Ortiz A, Dagneau P. Angew. Chem. Int. Ed. 2008;47:8605–8610. doi: 10.1002/anie.200804228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.a) Nicolaou KC, Zhang H, Ortiz A. Angew. Chem. Int. Ed. 2009;48:5642–5647. doi: 10.1002/anie.200902028. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nicolaou KC, Ortiz A, Zhang H. Angew. Chem. Int. Ed. 2009;48:5648–5652. doi: 10.1002/anie.200902029. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nicolaou KC, Ortiz A, Zhang H, Dagneau P, Lanver A, Jennings MP, Arseniyadis S, Faraoni R, Lizos DE. J. Am. Chem. Soc. 2010;132:7138–7152. doi: 10.1021/ja100740t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nicolaou KC, Ortiz A, Zhang H, Guella G. J. Am. Chem. Soc. 2010;132:7153–7176. doi: 10.1021/ja100742b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew. Chem. Int. Ed. 2005;44:3874–3897. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]
- 248.For selected reviews on the use of samarium diiodide in chemical synthesis, see: Edmonds DJ, Johnston D, Procter DJ. Chem. Rev. 2004;104:3371–3401. doi: 10.1021/cr030017a. Nicolaou KC, Ellery SP, Chen JS. Angew. Chem. Int. Ed. 2009;48:7140–7165. doi: 10.1002/anie.200902151.
- 249.Saielli G, Nicolaou KC, Ortiz A, Zhang H, Bagno A. J. Am. Chem. Soc. 2011;133:6072–6077. doi: 10.1021/ja201108a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.a) Isaka M, Rugseree N, Maithip P, Kongsaeree P, Prabpai S, Thebtaranonth Y. Tetrahedron. 2005;61:5577–5583. [Google Scholar]; b) Isaka M, Prathumpai W, Wongsa P, Tanticharoen M. Org. Lett. 2006;8:2815–2817. doi: 10.1021/ol060926x. [DOI] [PubMed] [Google Scholar]
- 251.Nicolaou KC, Sarlah D, Wu TR, Zhan W. Angew. Chem. Int. Ed. 2009;48:6870–6874. doi: 10.1002/anie.200903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nicolaou KC, Sun Y-P, Sarlah D, Zhan W, Wu TR. Org. Lett. 2011;13:5708–5710. doi: 10.1021/ol202239u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Uchiro H, Kato R, Arai Y, Hasegawa M, Kobayakawa Y. Org. Lett. 2011;13:6268–6271. doi: 10.1021/ol202748e. [DOI] [PubMed] [Google Scholar]
- 254.a) Rogers EF, Snyder HR, Fisher RF. J. Am. Chem. Soc. 1952;74:1987–1989. [Google Scholar]; b) Snyder HR, Fisher RF, Walker JF, Els HE, Nussberger GA. J. Am. Chem. Soc. 1954;76:2819–2825. [Google Scholar]; c) Snyder HR, Fisher RF, Walker JF, Els HE, Nussberger GA. J. Am. Chem. Soc. 1954;76:4601–4605. [Google Scholar]; d) Snyder HR, Strohmayer HF, Mooney RA. J. Am. Chem. Soc. 1958;80:3708–3710. [Google Scholar]
- 255.a) Rae ID, Rosenberger M, Szabo AG, Willis CR, Yates P, Zacharias DE, Jeffrey GA, Douglas B, Kirkpatrick JL, Weisbach JA. J. Am. Chem. Soc. 1967;89:3061–3062. [Google Scholar]; b) Zacharias DE. Acta Crystallogr. Sect. B. 1970;26:1455–1464. [Google Scholar]; c) Yates P, MacLachlan FN, Rae ID, Rosenberger M, Szabo AG, Willis CR, Cava MP, Behforouz M, Lakshmikanthan MV, Zeiger W. J. Am. Chem. Soc. 1973;95:7842–7850. [Google Scholar]
- 256.For synthetic studies toward haplophytine, see: P Yates, Schwartz DA. Can. J. Chem. 1983;61:509–518. Schwartz DA, Yates P. Can. J. Chem. 1983;61:1126–1131. Rege PD, Tian Y, Corey EJ. Org. Lett. 2006;8:3117–3120. doi: 10.1021/ol061319c.
- 257.Ueda H, Satoh H, Matsumoto K, Sugimoto K, Fukuyama T, Tokuyama H. Angew. Chem. Int. Ed. 2009;48:7600–7603. doi: 10.1002/anie.200902192. [DOI] [PubMed] [Google Scholar]
- 258.Nicolaou KC, Dalby SM, Li S, Suzuki T, Chen DY-K. Angew. Chem. Int. Ed. 2009;48:7616–7620. doi: 10.1002/anie.200904588. For a formal total synthesis, see: Tian W, Chennamaneni LR, Suzuki T, Chen DY-K. Eur. J. Org. Chem. 2011;1027:1031.
- 259.For syntheses of aspidophytine, see: He F, Bo Y, Altom JD, Corey EJ. J. Am. Chem. Soc. 1999;121:6771–6772. Sumi S, Matsumoto K, Tokuyama H, Fukuyama T. Org. Lett. 2003;5:1891–1893. doi: 10.1021/ol034445e. Sumi S, Matsumoto K, Tokuyama H, Fukuyama T. Tetrahedron. 2003;59:8571–8587. Mejía-Oneto JM, Padwa A. Org. Lett. 2006;8:3275–3278. doi: 10.1021/ol061137i. Marino JP, Cao G. Tetrahedron Lett. 2006;47:7711–7713. Nicolaou KC, Dalby SM, Majumder U. J. Am. Chem. Soc. 2008;130:14942–14943. doi: 10.1021/ja806176w.
- 260.a) Guo H, Sun B, Gao H, Chen X, Liu S, Yao X, Liu X, Che Y. J. Nat. Prod. 2009;72:2115–2119. doi: 10.1021/np900654a. Correction: J. Nat. Prod. 2010, 73, 796. [DOI] [PubMed] [Google Scholar]; b) Wang J-M, Ding G-Z, Fang L, Dai J-G, Yu S-S, Wang Y-H, Chen X-G, Ma S-G, Qu J, Xu S, Du D. J. Nat. Prod. 2010;73:1240–1249. doi: 10.1021/np1000895. [DOI] [PubMed] [Google Scholar]
- 261.a) Kornblum N, DeLaMare HE. J. Am. Chem. Soc. 1951;73:880–881. [Google Scholar]; b) Staben ST, Linghu X, Toste FD. J. Am. Chem. Soc. 2006;128:12658–12659. doi: 10.1021/ja065464x. [DOI] [PubMed] [Google Scholar]
- 262.Nicolaou KC, Totokotsopoulos S, Giguère D, Sun Y-P, Sarlah D. J. Am. Chem. Soc. 2011;133:8150–8153. doi: 10.1021/ja2032635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tan RX, Jensen PR, Williams PG, Fenical W. J. Nat. Prod. 2004;67:1374–1382. doi: 10.1021/np049920b. [DOI] [PubMed] [Google Scholar]
- 264.Nicolaou KC, Giguère D, Totokotsopoulos S, Sun Y-P. Angew. Chem. Int. Ed. 2012;51:728–732. doi: 10.1002/anie.201107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Glabrescol: Morimoto Y, Iwai T, Kinoshita T. J. Am. Chem. Soc. 2000;122:7124–7125. Xiong Z, Corey EJ. J. Am. Chem. Soc. 2000;122:9328–9329. For a synthesis of the originally proposed structure of glabrescol, see: Hioki H, Kanehara C, Ohnishi Y, Umemori Y, Sakai H, Yoshio S, Matsushita M, Kodama M. Angew. Chem. Int. Ed. 2000;39:2552–2554.
- 266.Salicylihalamide A: Wu Y, Esser L, De Brabander JK. Angew. Chem. Int. Ed. 2000;39:4308–4310. doi: 10.1002/1521-3773(20001201)39:23<4308::AID-ANIE4308>3.0.CO;2-4. Fürstner A, Dierkes T, Thiel OR, Blanda G. Chem. Eur. J. 2001;7:5286–5298. doi: 10.1002/1521-3765(20011217)7:24<5286::aid-chem5286>3.0.co;2-g. Snider BB, Song F. Org. Lett. 2001;3:1817–1820. doi: 10.1021/ol015822v. Smith AB, III, Zheng J. Tetrahedron. 2002;58:6455–6471.
- 267.Tetracycline: Tatsuta K, Yoshimoto T, Gunji H, Okado Y, Takahashi M. Chem. Lett. 2000:646–647. Charest MG, Siegel DR, Myers AG. J. Am. Chem. Soc. 2005;127:8292–8293. doi: 10.1021/ja052151d.
- 268.Callipeltoside A: Trost BM, Dirat O, Gunzner JL. Angew. Chem. Int. Ed. 2002;41:841–843. doi: 10.1002/1521-3773(20020301)41:5<841::aid-anie841>3.0.co;2-2. Evans DA, Hu E, Burch JD, Jaeschke G. J. Am. Chem. Soc. 2002;124:5654–5655. doi: 10.1021/ja026235n. Paterson I, Davies RDM, Heimann AC, Marquez R, Meyer A. Org. Lett. 2003;5:4477–4480. doi: 10.1021/ol0357853. Huang H, Panek JS. Org. Lett. 2004;6:4383–4385. doi: 10.1021/ol0480325. Hoye TR, Danielson ME, May AE, Zhao H. J. Org. Chem. 2010;75:7052–7060. doi: 10.1021/jo101598y.
- 269.Ramoplanin A2: Jiang W, Wanner J, Lee RJ, Bounaud P-Y, Boger DL. J. Am. Chem. Soc. 2002;124:5288–5290. doi: 10.1021/ja020237q.
- 270.FR182877: Vosburg DA, Vanderwal CD, Sorensen EJ. J. Am. Chem. Soc. 2002;124:4552–4553. doi: 10.1021/ja025885o. Evans DA, Starr JT. Angew. Chem. Int. Ed. 2002;41:1787–1790. doi: 10.1002/1521-3773(20020517)41:10<1787::aid-anie1787>3.0.co;2-v. Tanaka N, Suzuki T, Matsumura T, Hosoya Y, Nakada M. Angew. Chem. Int. Ed. 2009;48:2580–2583. doi: 10.1002/anie.200900097.
- 271.Strychnofoline: Lerchner A, Carreira EM. J. Am. Chem. Soc. 2002;124:14826–14827. doi: 10.1021/ja027906k.
- 272.Pectenotoxin-4: Evans DA, Rajapakse HA, Stenkamp D. Angew. Chem. Int. Ed. 2002;41:4569–4573. doi: 10.1002/1521-3773(20021202)41:23<4569::AID-ANIE4569>3.0.CO;2-V. Evans DA, Rajapakse HA, Chiu A, Stenkamp D. Angew. Chem. Int. Ed. 2002;41:4573–4576. doi: 10.1002/1521-3773(20021202)41:23<4573::AID-ANIE4573>3.0.CO;2-S.
- 273.Microcin SF608: Valls N, Vallribera M, Lopéz-Canet M, Bonjoch J. J. Org. Chem. 2002;67:4945–4950. doi: 10.1021/jo025709y. Diethelm S, Schindler CS, Carreira EM. Org. Lett. 2010;12:3950–3953. doi: 10.1021/ol1017189.
- 274.Siphonarin B: Paterson I, Chen DY-K, Franklin AS. Org. Lett. 2002;4:391–394. doi: 10.1021/ol017082w. Beye GE, Ward DE. J. Am. Chem. Soc. 2010;132:7210–7215. doi: 10.1021/ja102356j.
- 275.Vinblastine: Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2002;124:2137–2139. doi: 10.1021/ja0177049. Ishikawa H, Colby DA, Boger DL. J. Am. Chem. Soc. 2008;130:420–421. doi: 10.1021/ja078192m.
- 276.Plicamine: Baxendale IR, Ley SV, Piutti C. Angew. Chem. Int. Ed. 2002;41:2194–2197. doi: 10.1002/1521-3773(20020617)41:12<2194::aid-anie2194>3.0.co;2-4. Baxendale IR, Ley SV, Nessi M, Piutti C. Tetrahedron. 2002;58:6285–6304.
- 277.Quadrigemine C and Psycholeine: Lebsack AD, Link JT, Overman LE, Stearns BA. J. Am. Chem. Soc. 2002;124:9008–9009. doi: 10.1021/ja0267425.
- 278.Longithorone A: Layton ME, Morales CA, Shair MD. J. Am. Chem. Soc. 2002;124:773–775. doi: 10.1021/ja016585u.
- 279.Dragmacidin D: Gang NK, Sarpong R, Stoltz BM. J. Am. Chem. Soc. 2002;124:13179–13184. doi: 10.1021/ja027822b. Mandal D, Yamaguchi AD, Yamaguchi J, Itami K. J. Am. Chem. Soc. 2011;133:19660–19663. doi: 10.1021/ja209945x.
- 280.Asparagamine A: Brüggemann M, McDonald AI, Overman LE, Rosen MD, Schwink L, Scott JP. J. Am. Chem. Soc. 2003;125:15284–15285. doi: 10.1021/ja0388820.
- 281.Lemonomycin: Ashley ER, Cruz EG, Stoltz BM. J. Am. Chem. Soc. 2003;125:15000–15001. doi: 10.1021/ja039223q.
- 282.Okaramine N: Baran PS, Guerrero CA, Corey EJ. J. Am. Chem. Soc. 2003;125:5628–5629. doi: 10.1021/ja034491+.
- 283.Pentacycloanammoxic acid methyl ester: Mascitti V, Corey EJ. J. Am. Chem. Soc. 2004;126:15664–15665. doi: 10.1021/ja044089a. Mascitti V, Corey EJ. J. Am. Chem. Soc. 2006;128:3118–3119. doi: 10.1021/ja058370g.
- 284.Rubicordifolin: Lumb J-P, Trauner D. J. Am. Chem. Soc. 2005;127:2870–2871. doi: 10.1021/ja042375g.
- 285.Thapsivillosin F: Oliver SF, Högenauer K, Simic O, Antonello A, Smith MD, Ley SV. Angew. Chem. Int. Ed. 2003;42:5996–6000. doi: 10.1002/anie.200353140.
- 286.Amythiamicin D: Hughes RA, Thompson SP, Alcaraz L, Moody CJ. Chem. Commun. 2004:946–948. doi: 10.1039/b401580k. Ammer C, Bach T. Chem. Eur. J. 2010;16:14083–14093. doi: 10.1002/chem.201002144.
- 287.Yatakemycin: Tichenor MS, Kastrinsky DB, Boger DL. J. Am. Chem. Soc. 2004;126:8396–8398. doi: 10.1021/ja0472735. Okano K, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2006;128:7136–7137. doi: 10.1021/ja0619455.
- 288.Cribrostatin IV: Chan C, Heid R, Zheng S, Guo J, Zhou B, Furuuchi T, Danishefsky SJ. J. Am. Chem. Soc. 2005;127:4596–4598. doi: 10.1021/ja050203t. Vincent G, Williams RM. Angew. Chem. Int. Ed. 2007;46:1517–1520. doi: 10.1002/anie.200604126. Yokoya M, Ito H, Saito N. Tetrahedron. 2011;67:9185–9192.
- 289.Garsubellin A: Kuramochi A, Usuda H, Yamatsugu K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2005;127:14200–14201. doi: 10.1021/ja055301t. Siegel DR, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:1048–1049. doi: 10.1021/ja057418n.
- 290.Littoralisone: Mangion IK, MacMillan DWC. J. Am. Chem. Soc. 2005;127:3696–3697. doi: 10.1021/ja050064f.
- 291.Stephacidin B: Herzon SB, Myers AG. J. Am. Chem. Soc. 2005;127:5342–5344. doi: 10.1021/ja0510616. Baran PS, Guerrero CA. Angew. Chem. Int. Ed. 2005;44:3892–3895. doi: 10.1002/anie.200500655. Artman GD, III, Grubbs AW, Williams RM. J. Am. Chem. Soc. 2007;129:6336–6342. doi: 10.1021/ja070259i.
- 292.Iejimalide B: Fürstner A, Nevado C, Tremblay M, Chevrier C, Teplý F, Aïssa C, Waser M. Angew. Chem. Int. Ed. 2006;45:5837–5842. doi: 10.1002/anie.200601860. Schweitzer D, Kane JJ, Strand D, McHenry P, Tenniswood M, Helquist P. Org. Lett. 2007;9:4619–4622. doi: 10.1021/ol702129w.
- 293.Welwitindolinone A isonitrile: Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J. Am. Chem. Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s.
- 294.Haouamine A: Baran PS, Burns NZ. J. Am. Chem. Soc. 2006;128:3908–3909. doi: 10.1021/ja0602997.
- 295.Pallidol: Snyder SA, Zografos AL, Lin Y. Angew. Chem. Int. Ed. 2007;46:8186–8191. doi: 10.1002/anie.200703333. Zhong C, Zhu J, Chang J, Sun X. Tetrahedron Lett. 2011;52:2815–2817.
- 296.Reidispongiolide A: Paterson I, Ashton K, Britton R, Cecere G, Chouraqui G, Florence GJ, Stafford J. Angew. Chem. Int. Ed. 2007;46:6167–6171. doi: 10.1002/anie.200702178.
- 297.Azadirachtin: Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV. Angew. Chem. Int. Ed. 2007;46:7629–7632. doi: 10.1002/anie.200703027.
- 298.Gliocladin C: Overman LE, Shin Y. Org. Lett. 2007;9:339–341. doi: 10.1021/ol062801y. DeLorbe JE, Jabri SY, Mennen SM, Overman LE, Zhang F-L. J. Am. Chem. Soc. 2011;133:6549–6552. doi: 10.1021/ja201789v. Furst L, Narayanam JMR, Stephenson CRJ. Angew. Chem. Int. Ed. 2011;50:9655–9659. doi: 10.1002/anie.201103145.
- 299.Lyconadin A and B: Beshore DC, Smith AB., III J. Am. Chem. Soc. 2007;129:4148–4149. doi: 10.1021/ja070336+. Bisai A, West SP, Sarpong R. J. Am. Chem. Soc. 2008;130:7222–7223. doi: 10.1021/ja8028069. Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J. Am. Chem. Soc. 2011;133:418–419. doi: 10.1021/ja109516f.
- 300.Cyanthiwigin F: Enquist JA, Jr., Stoltz BM. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046.
- 301.Rubioncolin B: Lumb J-P, Choong KC, Trauner D. J. Am. Chem. Soc. 2008;130:9230–9231. doi: 10.1021/ja803498r.
- 302.Mersicarpine: Magolan J, Carson CA, Kerr MA. Org. Lett. 2008;10:1437–1440. doi: 10.1021/ol800259s. Nakajima R, Ogino T, Yokoshima S, Fukuyama T. J. Am. Chem. Soc. 2010;132:1236–1237. doi: 10.1021/ja9103233.
- 303.Papuamide B: Xie W, Ding D, Zi W, Li G, Ma D. Angew. Chem. Int. Ed. 2008;47:2844–2848. doi: 10.1002/anie.200705557.
- 304.Spirangien A: Paterson I, Findlay AD, Noti C. Chem. Commun. 2008:6408–6410. doi: 10.1039/b816229h.
- 305.Maduropeptin chromophore: Komano K, Shimamura S, Norizuki Y, Zhao D, Kabuto C, Sato I, Hirama M. J. Am. Chem. Soc. 2009;131:12072–12073. doi: 10.1021/ja905397p.
- 306.Tubulysin B: Pando O, Drner S, Preusentanz R, Denkert A, Porzel A, Richter W, Wessjohann L. Org. Lett. 2009;11:5567–5569. doi: 10.1021/ol902320w.
- 307.Minfiensine: Dounay AB, Overman LE, Wrobleski AD. J. Am. Chem. Soc. 2005;127:10186–10187. doi: 10.1021/ja0533895. Shen L, Zhang M, Wu Y, Qin Y. Angew. Chem. Int. Ed. 2008;47:3618–3621. doi: 10.1002/anie.200800566. Jones SB, Simmons B, MacMillan DWC. J. Am. Chem. Soc. 2009;131:13606–13607. doi: 10.1021/ja906472m. Li G, Padwa A. Org. Lett. 2011;13:3767–3769. doi: 10.1021/ol201320v. Liu P, Wang J, Zhang J, Qiu FG. Org. Lett. 2011;13:6426–6428. doi: 10.1021/ol2027224.
- 308.Saliniketal A and B: Paterson I, Razzak M, Anderson EA. Org. Lett. 2008;10:3295–3298. doi: 10.1021/ol801148d. Liu J, De Brabander JK. J. Am. Chem. Soc. 2009;131:12562–12563. doi: 10.1021/ja9061757.
- 309.Pauciflorol F: Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos AL. J. Am. Chem. Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. Jeffrey JL, Sarpong R. Org. Lett. 2009;11:5450–5453. doi: 10.1021/ol902141z. Yang Y, Philips D, Pan S. J. Org. Chem. 2011;76:1902–1905. doi: 10.1021/jo102298p. Lee BH, Choi YL, Shin S, Heo J-N. J. Org. Chem. 2011;76:6611–6618. doi: 10.1021/jo2009164.
- 310.11,11′−Dideoxyverticillin A: Kim J, Ashenhurst JA, Movassaghi M. Science. 2009;324:238–241. doi: 10.1126/science.1170777.
- 311.Chaetocin: Iwasa E, Hamashima Y, Fujishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. J. Am. Chem. Soc. 2010;132:4078–4079. doi: 10.1021/ja101280p.
- 312.Psychotrimine: Matsuda Y, Kitajima M, Takayama H. Org. Lett. 2008;10:125–128. doi: 10.1021/ol702637r. Takahashi N, Ito T, Matsuda Y, Kogure N, Kitajima M, Takayama H. Chem. Commun. 2010;46:2501–2503. doi: 10.1039/b923918a. Newhouse T, Baran PS. J. Am. Chem. Soc. 2008;130:10886–10887. doi: 10.1021/ja8042307.
- 313.Hexachlorosulfolipid: Nilewski C, Geisser RW, Carreira EM. Nature. 2009;457:573–576. doi: 10.1038/nature07734. Yoshimitsu T, Fukumoto N, Nakatani R, Kojima N, Tanaka T. J. Org. Chem. 2010;75:5425–5437. doi: 10.1021/jo100534d. Danicalipin A: Bedke DK, Shibuya GM, Pereira A, Gerwick WH, Haines TH, Vanderwal CD. J. Am. Chem. Soc. 2009;131:7570–7572. doi: 10.1021/ja902138w. Umezawa T, Shibata M, Kaneko K, Okino T, Matsuda F. Org. Lett. 2011;13:904–907. doi: 10.1021/ol102882a. Yoshimitsu T, Nakatani R, Kobayashi A, Tanaka T. Org. Lett. 2011;13:908–911. doi: 10.1021/ol1029518.
- 314.Vincorine: Zhang M, Huang X, Shen L, Qin Y. J. Am. Chem. Soc. 2009;131:6013–6020. doi: 10.1021/ja901219v.
- 315.Micrococcin P1: Lefranc D, Ciufolini MA. Angew. Chem. Int. Ed. 2009;48:4198–4201. doi: 10.1002/anie.200900621.
- 316.Vinigrol: Maimone TJ, Shi J, Ashida S, Baran PS. J. Am. Chem. Soc. 2009;131:17066–17067. doi: 10.1021/ja908194b.
- 317.Gymnodimine: Kong K, Romo D, Lee C. Angew. Chem. Int. Ed. 2009;48:7402–7405. doi: 10.1002/anie.200903432.
- 318.Nominal banyaside B: Schindler CS, Bertschi L, Carreira EM. Angew. Chem. Int. Ed. 2010;49:9229–9232. doi: 10.1002/anie.201004047.
- 319.Omphadiol: Liu G, Romo D. Angew. Chem. Int. Ed. 2011;50:7537–7540. doi: 10.1002/anie.201102289.
- 320.Chloptosin: Yu S-M, Hong W-X, Wu Y, Zhong C-L, Yao Z-J. Org. Lett. 2010;12:1124–1127. doi: 10.1021/ol100135a. Oelke AJ, France DJ, Hofmann T, Wuitschik G, Ley SV. Angew. Chem. Int. Ed. 2010;49:6139–6142. doi: 10.1002/anie.201002880.
- 321.Aplykurodinone-1: Zhang Y, Danishefsky SJ. J. Am. Chem. Soc. 2010;132:9567–9569. doi: 10.1021/ja1035495.
- 322.Leuconicine A and B: Sirasani G, Andrade RB. Org. Lett. 2011;13:4736–4737. doi: 10.1021/ol202056w.
- 323.Schindilactone A: Xiao Q, Ren W-W, Chen Z-X, Sun T-W, Li Y, Ye Q-D, Gong J-X, Meng F-K, You L, Liu Y-F, Zhao M-Z, Xu L-M, Shan Z-H, Shi Y, Tang Y-F, Chen J-H, Yang Z. Angew. Chem. Int. Ed. 2011;50:7373–7377. doi: 10.1002/anie.201103088.
- 324.Aplyviolene: Schnermann MJ, Overman LE. J. Am. Chem. Soc. 2011;133:16425–16427. doi: 10.1021/ja208018s.
- 325.Psychotetramine: Foo K, Newhouse T, Mori I, Takayama H, Baran PS. Angew. Chem. Int. Ed. 2011;50:2716–2719. doi: 10.1002/anie.201008048.
- 326.Lomaiviticin aglycon: Herzon SB, Lu L, Woo CM, Gholap SL. J. Am. Chem. Soc. 2011;133:7260–7263. doi: 10.1021/ja200034b.
- 327.Palau’amine: Seiple IB, Su S, Young IS, Lewis CA, Yamaguchi J, Baran PS. Angew. Chem. Int. Ed. 2010;49:1095–1098. doi: 10.1002/anie.200907112.
- 328.Gelsemoxonine: Shimokawa J, Harada T, Yokoshima S, Fukuyama T. J. Am. Chem. Soc. 2011;133:17634–17637. doi: 10.1021/ja208617c.
- 329.Pactamycin: Hanessian S, Vakiti RR, Dorich S, Banerjee S, Lecomte F, DelValle JR, Zhang J, Deschênes-Simard B. Angew. Chem. Int. Ed. 2011;50:3497–3500. doi: 10.1002/anie.201008079.
- 330.Rippertenol: Snyder SA, Wespe DA, von Hof JM. J. Am. Chem. Soc. 2011;133:8850–8853. doi: 10.1021/ja202859f.
- 331.Trigonoliimine C: Qi X, Bao H, Tambar UK. J. Am. Chem. Soc. 2011;133:10050–10053. doi: 10.1021/ja203960b. Han S, Movassaghi M. J. Am. Chem. Soc. 2011;133:10768–10771. doi: 10.1021/ja204597k.
- 332.Aspidophylline A: Zu L, Boal BW, Garg NK. J. Am. Chem. Soc. 2011;133:8877–8879. doi: 10.1021/ja203227q.
- 333.Piperarborenine B and D: Gutekunst WR, Baran PS. J. Am. Chem. Soc. 2011;133:19076–19079. doi: 10.1021/ja209205x.
- 334.Salvileucalin B: Levin S, Nani RR, Reisman SE. J. Am. Chem. Soc. 2011;133:774–776. doi: 10.1021/ja110192b.
- 335.Lithospermic acid: S J. O’Malley, Tan KL, Watzke A, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2005;127:13496–13497. doi: 10.1021/ja052680h. Wang D-H, Yu J-Q. J. Am. Chem. Soc. 2011;133:5767–5769. doi: 10.1021/ja2010225.
- 336.Fastigiatine: Liau BB, Shair MD. J. Am. Chem. Soc. 2011;132:9594–9595. doi: 10.1021/ja104575h.
- 337.Naseseazine B: Kim J, Movassaghi M. J. Am. Chem. Soc. 2011;133:14940–14943. doi: 10.1021/ja206743v.
- 338.Daphmanidin E: Weiss ME, Carreira EM. Angew. Chem. Int. Ed. 2011;50:11501–11505. doi: 10.1002/anie.201104681.
- 339.Streptorubin B: Hu DX, Clift MD, Lazarski KE, Thomson RJ. J. Am. Chem. Soc. 2011;133:1799–1804. doi: 10.1021/ja109165f.
- 340.Kibdelone C: Sloman DL, Bacon JW, Porco JA., Jr J. Am. Chem. Soc. 2011;133:9952–9955. doi: 10.1021/ja203642n. Butler JR, Wang C, Bian J, Ready JM. J. Am. Chem. Soc. 2011;133:9956–9959. doi: 10.1021/ja204040k.
- 341.Conophyllidine: Hanya Y, Tokuyama H, Fukuyama T. Angew. Chem. Int. Ed. 2011;50:4884–4887. doi: 10.1002/anie.201100981.
- 342.Pacidamycin D: Okamoto K, Sakagami S, Feng F, Togame H, Takemoto H, Ichikawa S, Matsuda A. Org. Lett. 2011;13:5240–5243. doi: 10.1021/ol202124b.
- 343.Maoecrystal V: Gong J, Lin G, Sun W, Li C-C, Yang Z. J. Am. Chem. Soc. 2010;132:16745–16746. doi: 10.1021/ja108907x.
- 344.Maoecrystal Z: Cha JY, Yeoman JTS, Reisman SE. J. Am. Chem. Soc. 2011;133:14964–14967. doi: 10.1021/ja2073356.
- 345.Meloscine and Epimeloscine: Selig P, Bach T. Angew. Chem. Int. Ed. 2008;47:5082–5084. doi: 10.1002/anie.200800693. Zhang H, Curran DP. J. Am. Chem. Soc. 2011;133:10376–10378. doi: 10.1021/ja2042854. Hayashi Y, Inagaki F, Mukai C. Org. Lett. 2011;13:1778–1780. doi: 10.1021/ol200311y. Feldman KS, Antoline JF. Org. Lett. 2012;14:934–937. doi: 10.1021/ol203463n.
- 346.C13-(R) guanacastepene N and C13-(S) guanacastepene O: Gampe CM, Carreira EM. Angew. Chem. Int. Ed. 2011;50:2962–2965. doi: 10.1002/anie.201007644.
- 347.Spirastrellolide A Methyl Ester: Paterson I, Anderson EA, Dalby SM, Lim JH, Genovino J, Maltas P, Moessner C. Angew. Chem. Int. Ed. 2008;47:3016–3020. doi: 10.1002/anie.200705565. Paterson I, Anderson EA, Dalby SM, Lim JH, Genovino J, Maltas P, Moessner C. Angew. Chem. Int. Ed. 2008;47:3021–3025. doi: 10.1002/anie.200705566. Spirastrellolide F Methyl Ester: G W. O’Neil, Ceccon J, Benson S, Collin M-P, Fasching B, Fürstner A. Angew. Chem. Int. Ed. 2009;48:9940–9945. doi: 10.1002/anie.200906121. Benson S, Collin M-P, O’Neil GW, Ceccon J, Fasching B, Fenster DB, Godbout C, Radkowski K, Goddard R, Fürstner A. Angew. Chem. Int. Ed. 2009;48:9946–9950. doi: 10.1002/anie.200906122.
- 348.Nominal undecachlorosulfolipid A: Nilewski C, Deprez NR, Fessard TC, Li DB, Geisser RW, Carreira EM. Angew. Chem. Int. Ed. 2011;50:7940–7943. doi: 10.1002/anie.201102521.
- 349.Millingtonine A: Wegner J, Ley SV, Kirschning A, Hansen A-L, Garcia JM, Baxendale IR. Org. Lett. 2012;14:696–699. doi: 10.1021/ol203158p.
- 350.Fusarisetin A: Deng J, Zhu B, Lu Z, Yu H, Li A. J. Am. Chem. Soc. 2012;134:920–923. doi: 10.1021/ja211444m. Xu J, Caro-Diaz EJE, Trzoss L, Theodorakis EA. J. Am. Chem. Soc. 2012;134:5072–5075. doi: 10.1021/ja300807e.
- 351.Scholarisine A: Adams GL, Carroll PJ, Smith AB., III J. Am. Chem. Soc. 2012;134:4037–4040. doi: 10.1021/ja211840k.
- 352.Lactiflorin: Lu P, Bach T. Angew. Chem. Int. Ed. 2012;51:1261–1264. doi: 10.1002/anie.201106889.
- 353.Mevashuntin: Nawrat CC, Moody CJ. Org. Lett. 2012;14:1484–1487. doi: 10.1021/ol300221e.
- 354.Halichondrin C: Yamamoto A, Ueda A, Brémond P, Tiseni PS, Kishi Y. J. Am. Chem. Soc. 2012;134:893–896. doi: 10.1021/ja2108307.
- 355.Janoxepin: Doveston RG, Steendam R, Jones S, Taylor RJK. Org. Lett. 2012;14:1122–1125. doi: 10.1021/ol300039x.
- 356.Indoxamycin B: Jeker OF, Carreira EM. Angew. Chem. Int. Ed. 2012;51:3474–3477. doi: 10.1002/anie.201109175.
- 357.Tirandamycin C: Chen M, Roush WR. Org. Lett. 2012;14:426–428. doi: 10.1021/ol203161u.
- 358.Acetylaranotin: Codelli JA, Puchlopek ALA, Reisman SE. J. Am. Chem. Soc. 2012;134:1930–1933. doi: 10.1021/ja209354e.