Abstract
The Cuatro Ciénegas Basin (CCB) is a rare oasis in the Chihuahuan Desert in the state of Coahuila, Mexico. It has a biological endemism similar to that of the Galapagos Islands, and its spring-fed ecosystems have very low nutrient content (nitrogen or phosphorous) and are dominated by diverse microbialites. Thus, it has proven to be a distinctive opportunity for the field of astrobiology, as the CCB can be seen as a proxy for an earlier time in Earth's history, in particular the late Precambrian, the biological frontier when prokaryotic life yielded at least partial dominance to eukaryotes and multicellular life. It is a kind of ecological time machine that provides abundant opportunities for collaborative investigations by geochemists, geologists, ecologists, and population biologists in the study of the evolutionary processes that structured Earth-based life, especially in the microbial realm. The CCB is an object of investigation for the identification of biosignatures of past and present biota that can be used in our search for extraterrestrial life. In this review, we summarize CCB research efforts that began with microbial ecology and population biology projects and have since been expanded into broader efforts that involve biogeochemistry, comparative genomics, and assessments of biosignatures. We also propose that, in the future, the CCB is sanctioned as a “Precambrian Park” for astrobiology. Key Words: Microbial mats—Stromatolites—Early Earth—Extremophilic microorganisms—Microbial ecology. Astrobiology 12, 641–647.
1. Introduction
The Cuatro Ciénegas Basin (hereafter the CCB) is a rare place. Located in the center of the Chihuahuan Desert in the Mexican state of Coahuila, the CCB is a small, intermontane, butterfly-shaped valley with a basin floor about 740 m above sea level and surrounded by mountains rising higher than 2500 m (Meyer, 1973). The CCB receives less than 200 mm of precipitation per year but has many groundwater-fed streams, ponds, rivers, and lakes. Elemental composition at the CCB is anomalous with regard to other similar environments (Elser et al., 2005a, 2005b). In the CCB, the N:P ratio can range from very low P (157:1) to very low N (1.8:1), while a ratio of N:P 16:1 is considered as the adequate proportion (stoichiometric balance) for life in the ocean (Redfield, 1934). These unusual and extreme stoichiometric ratios at the CCB generate nutrimental stresses that dictate population dynamics perhaps more akin to those of earlier times in Earth's history rather than more conventional extant environments. Additionally, the extreme oligotrophy has likely contributed to a biological endemism at the CCB similar to that of the Galapagos (Stein et al., 2000). The site is distinctive in that it is dominated by diverse microbial communities such as microbialites (Minckley, 1969), entities that were common and indeed hallmarks of the Precambrian. The CCB qualifies as an environment so unique that it has been designated as an “Area de Protección de Flora y Fauna” by the Mexican federal government. The area is administered by the Mexican SEMARNAT (Secretaría del Medio Ambiente y Recursos Naturales); it is a high-priority site for conservation by the Nature Conservancy, the World Wildlife Fund, and UNESCO, and it has been listed as a Wetland of International Importance by Ramsar.
For the past 10 years, we have been studying the CCB as a living laboratory for understanding evolutionary processes involving microbes. Emerging from this work is a realization that the CCB might be viewed as a living model of earlier ecosystems (Souza et al., 2006, 2008; Desnues et al., 2008; Breitbart et al., 2009), a place so poor in nutrients that the food web is dominated by microbes. We see the CCB as a reasonable proxy for an earlier time in Earth's history, the late Precambrian, the biological frontier when microbial life was giving way to the dominance of more complex eukaryotic organisms. As a “Precambrian Park,” a kind of modern ecological time machine, CCB study requires multidisciplinary collaboration. As such, the CCB has the potential to help bridge the evolutionary divides in our understanding of the biosignatures of Earth's microbial communities and those that might be, or have been, possible on nearest neighbor Solar System bodies. The CCB bridges this gap by scaling local evolutionary consequences recorded in the genomic, biogeochemical, and geological context to a broader astrobiological framework. More recently, we have increased the resolution of this framework by the formulation of experimental regimes that target key hypotheses of interest.
2. The CCB as a Model for Preservation of Past, Local Ecosystem Events: Endemism and a Relict Marine Signal
Reviewed extensively by Minckley (1969) and Wilson and Pit (2010), mountain building and subsequent desertification of northern Mexico and the southwestern United States was significant in creating ecosystems that led to the evolution and isolation of desert-adapted taxa along the entire North American continent. From the late Triassic to the early Cretaceous, all of northern Mexico was covered by seawater, and it is likely that the Laramide Uplift [70–50 Ma before present (BP); one of two major mountain-building events in North America] initiated a progressive regression of the Western Interior Seaway, which began to regress in the late Cretaceous due to the uplift caused by the Laramide Orogeny (Minckley, 1969; Ferrusquía-Villafranca, 1998). The final regression of the sea occurred during the late Paleocene, and the complete isolation of the CCB from the Gulf of Mexico happened with the uplift of the Sierra Madre Oriental in the mid Eocene (Ferrusquía-Villafranca, 1998) with the close of the Western Interior Seaway. There is still debate (Wilson and Pit, 2010) as to whether the Laramide event or the more recent Neogene Uplift (15–2 Ma BP) was responsible for the severe deformation that produced the land surfaces now evident in the modern mountain ranges of North America. However, the majority of paleontological evidence supports the progressive desertification of the Chihuahuan Desert (thereby the CCB) by 15 Ma BP. Hence, the region has largely been geologically and climatically stable since that time (Axelrod, 1979; Wilson and Pit, 2010). With regard to the CCB specifically, there is palynological evidence that during the late Quaternary (2.5 Ma BP) the area experienced climatic changes that include the migration of woodland-type vegetation upslope as the climate warmed (Meyer, 1973; Metcalfe et al., 2000; Minckley and Jackson, 2008). However, it should be noted that, despite this climatic intrusion, there is an apparent lack of change in the basin floor biota; this has been explained by a consistently high soil salinity (Minckley and Jackson, 2008) that provided persistent desert refugia (Elias et al., 1995).
The historical biogeographic reconstruction of the CCB details a pattern of considerable antiquity and constancy. By comparison of various phylogenetic and genomic records, we have exposed phylogenetic patterns that corroborate that geological history and are surprising in the degree to which they retain a record of that history. Here, we describe these biological findings.
The CCB has a level of macrofauna endemism equal to that of the Galapagos and the highest in North America (Stein et al., 2000). Rather than islands of land surrounded by water, the CCB is an oasis where islands of water are surrounded by desert, all contained in a mountain-ringed basin. These biogeographic barriers have likely been the driver for speciation and diversification of the over 70 endemic species, including diatoms (Winsborough et al., 2009), snails (Johnson, 2005), fish (Carson and Dowling, 2006; Tobler and Carson, 2010), and more recently bacteria (Escalante et al., 2009; Cerritos et al., 2011). It is intriguing to postulate that endemism is present in the microbiota of the CCB, but an isolate of Bacillus, B. coahuilensis, appears to have adaptive elements unique to the CCB in its genome (Alcaraz et al., 2008).
Our recent studies of the microbialite microbiota also revealed an anomalous marine signal that is best explained by the geological history of the CCB, which suggests a genomic imprint of the ancient sea that inhabited the area (Souza et al., 2006; Desnues et al., 2008; Breitbart et al., 2009). Our phylogenomic analyses indicated that CCB microbes align most closely with extant marine taxa and not with taxa from other inland saline waters. This association crosses a number of lines of descent in the microbial population, which suggests that the CCB is indeed a “lost world” where a relict genetic signature persists across a majority of individual lineages in the extant population (Souza et al., 2006, 2008; Alcaraz et al., 2008, 2010; Cerritos et al., 2008, 2011; Desnues et al., 2008; Breitbart et al., 2009; Domínguez-Escobar et al., 2011; Moreno-Letelier et al., 2011). This marine signal is corroborated in studies of other inhabitants of the valley, including the diatoms (Winsborough et al., 2009) and snails (Johnson, 2005).
This marine signature is an unexpected, but somewhat reasonable, observation given that the mountain-building process of the CCB and subsequent desertification most likely resulted in conditions that provided refugia for the marine biota that then adapted and, in some cases, diversified in the changing arid environment in which it was sequestered. The biological data suggest a continual presence of water in this oasis in northern Mexico, first as a sea and then as subterranean hydrologic systems. Evaporites (mineral sediments that result from evaporation) of different ages indicate that aquatic environments have always been present at the CCB (Minckley and Jackson, 2008; Szynkiewicz et al., 2010; Wilson and Pit, 2010). Consistent with our research efforts and those of others, it might appear that, over time, the chemistry of the ancient subterranean continental waters changed from sodium chloride to calcium, magnesium, sulfate, and carbonate ions that dominate the current chemistry (Minckley and Cole, 1968; Forti et al., 2003). While these ions dominate the waters in the CCB, they are not chemically uniform across the basin (Tobler and Carson, 2010). Measurements of water chemistry that cover both sides of the valley indicate that there are regions in the valley that are dominated by different minerals. For instance, some are richer in sulfates, while others are rich in carbonates (Minckley and Cole, 1968; Tobler and Carson, 2010). Consistent with this, complex hard-surfaced microbialites, including the spectacular stromatolite reefs of Pozas Azules (PA in Fig. 1), are found in some locales, while soft, less-developed mats can be found in other locations (Minckley and Cole, 1968). This provides an interesting template for comparative studies.
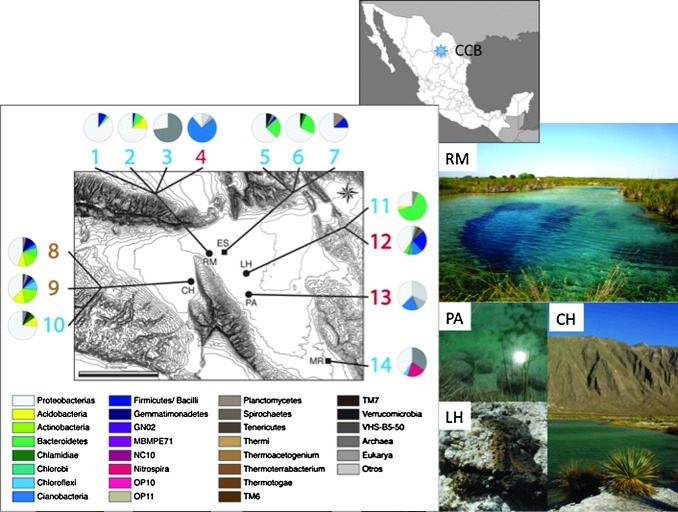
FIG. 1.
Cuatro Ciénegas Coahuila microbial diversity at the phylum level in different sites within the valley. The taxonomy was obtained using environmental DNA and 16S rDNA gene as marker. The numbers refer to different samples within the region. CH (Churince), RM (Río Mesquites, a site with desiccation pools and a river with oncolites), ES (Escobedo), LH (Los Hundidos region, with the Poza Roja), PA (Pozas Azules ranch, with the sites Poza Azul II and Green Mat), MR (a mine shaft where a sample was obtained 600 m below the surface). The map of Mexico shows the CCB site in the state of Coahuila. The photographs correspond to CCB sites.
3. The CCB as a Model for Understanding Evolutionary Trajectory: Diversity and Population Genetics
Beyond the intriguing marine ancestry of microbial communities at the CCB, we have also observed, surprisingly, that dominance by one or two species has never been found in any of our microbial diversity analyses (Souza et al., 2006; Desnues et al., 2008; Escalante et al., 2008; Cerritos et al., 2011). Instead, we have always observed even distributions, with a long tail of rare lineages, geographic differentiation (Fig. 1) (Escalante et al., 2008; Cerritos et al., 2011), and local endemicity. This is unusual in extreme environments where dominance is the rule (Aguilera et al., 1999; Cookson et al., 2006; Pointing et al., 2009). The exceptional microbial community structure found at the CCB could be either the product of many recent adaptive radiation events where migrants diversified in the new environment (Galapagos Islands model) or of a long history of coexistence of ancient marine lineages that have adapted locally to the new continental conditions (the “lost world, time capsule” model). To help answer this question, we focused on the microbial group Firmicutes, a taxonomic group that has several endemic taxa at the CCB and for which several whole genome sequences have been produced (Alcaraz et al., 2008, 2010; Moreno-Letelier et al., 2011). We reconstructed the phylogenetic relationships of Firmicutes, focusing on Bacillus that use a concatenated data set of 17 housekeeping genes shared among all taxa (Moreno-Letelier et al., 2011). Our results show that, while Bacillus species in marine environments form a monophyletic group, Bacillus from the CCB does not. Each species from the CCB had a marine sister species and divergence times that, in most cases, dated back to the Proterozoic. Thus, the diversity of Firmicutes in the CCB is not the product of a recent radiation (at least not at this taxonomic level) but instead is the product of the isolation of ancient lineages followed by diversification, at the population level, and posterior adaptation to the local conditions. Hence, the endemicity and evenness observed in all our samples appears to be the result of a very long history of local adaptation and coexistence.
We have additional evidence that supports the ancient origin and recent diversification of CCB lineages. Using genetic information from those taxa, we have inferred recent clonal expansions for many new lineages in all the groups of planktonic microbes that have been studied at the CCB (Cerritos et al., 2008; Cerritos et al., 2011; Avitia et al., personal communication; Rebollar et al., 2012). This clonal model is confirmed by comparative genomics of several CCB Bacillus species where the clonal frame is interrupted by very few adaptive events of horizontal gene transfer. Examples of these are (i) genes related to photorodopsins (Alcaraz et al., 2008), (ii) genes that allow Bacillus coahuilensis to constitutively produce sulfolipids instead of phospholipids (Alcaraz et al., 2008), (iii) genes for phosphonate utilization (Alcaraz et al., 2010), and (iv) genes for phosphorus intake (Moreno-Letelier et al., 2011).
While we have long hypothesized environmental conditions and geological history as drivers of species diversification at the CCB, new data on temporal and spatial heterogeneity are helping to provide answers to the enduring question: Why so many species? Heterogeneity can be understood not only at spatial scales but also at temporal scales by the imposition of dramatic seasonal changes that exert strong selective pressures on microbial populations. Heterogeneous patterns among drainage systems within the area are seasonal (Carson and Dowling, 2006), and diurnal fluctuations may maintain high overall levels of diversity. We conducted one study focused on the role of seasonal fluctuations in the diversity of Pseudomonas populations. We obtained isolates from four different time points (winter and summer) over the course of two years and analyzed the genetic diversity by fingerprinting and 16S rDNA sequences. The data show significant seasonality in lineage composition, which suggests ecological mechanisms of recurrence that are likely driven by environmental conditions associated with temperature (Rodríguez-Verdugo, 2008).
Given the differences in environmental structuring between the water column and the microbialites, diversification processes in each habitat may also be different (Saxer et al., 2009). We hypothesize that, in the water column, direct interference competition should be very weak because the water column is extremely dilute (average of 3000 colonies per milliliter). In contrast, in the microbialites, spatial heterogeneity allows coexistence of competing taxa by diversification of the niche.
As part of our research on the processes of diversification in those structured environments, we produced four metagenomes of CCB microbialites. Metagenomic data show that the microbialites at the CCB are not only highly divergent from each other but also very diverse taxonomically and functionally (Breitbart et al., 2009; Peimbert et al., 2012; Bonilla-Rosso et al., personal communications). This type of diverse gene composition is not common in the modern world (Rusch et al., 2007). It is possible that during the Precambrian most local patches of life in the shallow ocean were associated with microbialites; and if the CCB model is true, each of those complex microbial communities were, metabolically speaking, complete. In other words, the complete biogeochemical cycles happened at a local scale. In the modern world this is seldom true, as the biosphere is so interconnected that different parts of each cycle occur in different places, which makes the biogeochemical cycles global.
In the microbialite reef from Poza Azul II (in the Pozas Azules ranch in the southeastern part of the valley), we found that systems involved in photosynthesis and energy production are overrepresented, while in an oncolite (round microbialite) of Río Mesquites (RM in Fig. 1), genes for sulfur and nitrogen are overrepresented (Breitbart et al., 2009). Both communities are from stable environments with extremely low nutrient contents (biomass C:N:P ratios of 900:150:1), and as expected, they also had an overrepresentation of high-affinity phosphorus acquisition genes and genes to make exopolysaccharides that are important in export of excess fixed carbon and ultimately maintain the structured community of a hard microbialite (Breitbart et al., 2009). We also sequenced two microbialites from contrasting environments, a nitrogen-poor, constant environment pool (“Green Mat,” with biomass C:N:P of 55:1.8:1) as well as an extremely low P, fluctuating desiccation pond (Poza Roja; biomass C:N:P ratio of 15820:157:1). Not surprisingly, we found that their metagenomes are totally different taxonomically, even though both have a very similar proportion of reads within carbohydrate central metabolism (Peimbert et al., 2012). Poza Roja is dominated by heterotrophs, particularly 24 different types of novel Pseudomonas. In contrast, the Green Mat is to date the most diverse site at the CCB with at least 160 species at 97% identity with a high representation of Cyanobacteria, the marine noncultivated phyla GN02 and OP11, as well as Chloroflexi and Chlorobi, which indicates an important primary productivity component in this mat. Unexpectedly, even though Poza Roja is the most nutrient-deprived site that we know, the genes associated with phosphorus metabolism are not overrepresented. Thus, this extreme oligotrophic mat is not using the same strategies to deal with the lack of phosphorus as ocean microbiota or even the other microbialites from the CCB (Peimbert et al., 2012). The presence of several genes for antibiotic production and resistance suggests that Pseudomonas spp. from this site are under strong pressure to escape antagonistic and competitive interactions, probably following the “Red Queen” model (Venditti et al., 2010; Wilson and Sherman, 2010), where species arise constantly by reproductive isolation and rare stochastic events, which allows them to follow a constantly changing and challenging environment (Yoshida et al., 2007). The convergence of high endemic microbial biodiversity and severe in situ P limitations have led us to propose a hypothesis that connects the two (Souza et al., 2008). We suggest that P limitation sets the stage for development of endemic microbiota not only by imposing a strong selection pressure but also by inhibiting mechanisms of horizontal gene transfer that would otherwise homogenize diverging lineages. This hypothesis suggests that P limitation reduces horizontal gene transfer via three mechanisms: (i) low cell densities due to low P supply mean reduced cell-cell contact and therefore low rates of direct conjugation, (ii) P limitation reduces viral replication and thus reduces transformation, and (iii) P limitation results in scavenging of P from dissolved organic compounds (such as free DNA), reducing transformation. Experiments to test these ideas are being conducted.
4. The CCB as a Model for Understanding Nutrient Limitation Effects on Food Web Evolution
As a result of its long history of weathering, low accumulation of organic matter, and an abundance of calcium carbonate especially in the eastern lobe, CCB waters have very low concentrations of key nutrients, especially phosphorus (P; Elser et al., 2005a). As discussed above, this imposes severe P limitation, which results in biomass atomic C:P ratios that can be as high as 15,000 in the valley microbial communities (Elser et al., 2005a; Peimbert et al., 2012). Indeed, the laminated microbialites of the CCB appear to be very sensitive to the supply of phosphate; enrichment with P causes large increases in the abundance of eukaryotic diatoms (Elser et al., 2005a), which are generally present at low abundances in unfertilized CCB communities. It is well known that severe P limitation causes cyanobacteria and algae to develop high C:P and N:P ratios in biomass (Sterner and Elser, 2002) and that this stoichiometrically imbalanced biomass provides poor-quality food for consumers. Based on this, Elser et al. (2005b; Elser and Hamilton, 2007) tested for a possible role of poor food quality in constraining consumer dynamics at the CCB (and thus on ancient Earth), to the extent that the CCB's stromatolite-based food webs are analogous to a world on the brink of the Cambrian explosion.
In an enrichment experiment in which oncoid microbialites from Río Mesquites and their associated snail populations were used, PO4 was added to stream water, reducing the C:P ratio of the superficial microbial biomass. Over intermediate ranges of P enrichment and microbial C:P, snail performance was stimulated; but for high levels of P enrichment and low C:P, snails grew more slowly and had high mortality (Elser et al., 2005a, 2005b; Elser and Hamilton, 2007). This led Elser and Hamilton (2007) to suggest that consumers at the CCB live on a “stoichiometric knife edge” and are generally constrained by low dietary P content when microbes are P-limited but suffering from negative effects of excess dietary P in artificially enriched conditions. Given the analogue role of CCB microbialites for Precambrian communities, Elser and Hamilton (2007) proposed that such stoichiometric effects may have contributed to patterns of early animal evolution, with diversification stimulated by early events of P mobilization from continents 600 Ma BP. More work is needed in an astrobiological context to further explore the utility of the CCB's stromatolite-based food webs as a test bed for ideas about key events in early Earth history.
5. The CCB as a Model Precambrian Park
Our work at the CCB continues to indicate that, perhaps by sheer geological circumstance, this small valley in northeastern Mexico has maintained its ancient marine ancestry as an extended outcome from its desertification and the geochemical consequences that promote endemism. It serves as a biological repository for biosignatures of past, current, and future events in biological evolution. As such a model system, the CCB has unprecedented value for future discovery and warrants a high priority for preservation that will provide venues for conscientious and responsible manipulation of experimental regimes. We hope that we have demonstrated its value in testing hypotheses about the diversity of species that live on our planet and, by extension, on other planets and moons.
The CCB provides a proxy, albeit an imperfect one, to an earlier time in Earth's history when microbes dominated and many of the key metabolic strategies upon which Earth's biosphere still depends first evolved. The presence of diverse microbialites is the most prominent feature that links the CCB to strategies that were common in Precambrian ecology. The microbialites and their natural predators and other macrofauna provide a proxy for the vibrant dynamics that were a hallmark of the late Precambrian: a time in Earth's history when evolving complex macrofauna would displace microbial constructions such as stromatolites as the visually dominating biology on Earth's surface.
Moreover, with the Mars Science Laboratory mission coming soon, the CCB pure gypsum and its extensive evaporates also represent a model for Gale Crater on Mars, and an opportunity exists to characterize early wetter conditions in the Noachian epoch, when intensive volcanism in an ancient ocean on Mars originated similar deposits rich in sulfur and poor in phosphorites.
However, the CCB is not without its 21st-century woes. The basin's environmental integrity is under threat due to local irrigation practices that divert water from the CCB to alfalfa farms in the region, which has led us to argue for the creation of a Precambrian Park that encompasses the entire valley and its watershed and aquifer (Souza et al., 2007). What is certain is that, by securing the future of the CCB, we could preserve opportunities for inspiration and discovery and models of how life evolves on Earth and, potentially, elsewhere.
Acknowledgments
This project was supported by grants 0023459-SEMARNAT/CONACYT, 57507 SEP CONACyT, and WWF-Alianza Carlos Slim to V.S. and was written during sabbatical leaves of V.S. and L.E.E., who had a grant from DGAPA (V.S. and L.E.E.) and UC-Mexus (L.E.E.). J.S. was funded by NASA Astrobiology NNA08CN78A, Exobiology Grant NNX08AP64G, and National Science Foundation (NSF) grant DEB-0949570. J.J.E. was also funded by NASA Astrobiology NNA08CN78A and NSF grant DEB-0950179. We especially thank PRONATURA Noreste for all the support and logistics, as well as the APFF of Cuatro Ciénegas for all the technical and logistic help, as well as Laura Espinosa-Asuar, Rodrigo Gonzalez Chauvet, Santiago Ramírez, Manuel Rosas, Eria Rebollar, and Erika Aguirre-Planter for technical help and logistic support.
Author Disclosure Statement
All the authors are academics; none of them work for the US or Mexican government.
Abbreviations
BP, before present; CCB, Cuatro Ciénegas Basin.
References
- Aguilera L.E. Gutierrez J.R. Meserve P. Variation in soil micro-organisms and nutrients underneath and outside the canopy of Adesmia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average rainfall. J Arid Environ. 1999;42:61–70. [Google Scholar]
- Alcaraz L.D. Olmedo G. Bonilla G. Cerritos R. Hernandez G. Cruz A. Ramirez E. Putonti C. Jimenez B. Martinez E. López V. Arvizu J.L. Ayala F. Razo F. Caballero J. Siefert J. Eguiarte L. Vielle J. Martinez O. Souza V. Herrera-Estrella A. Herrera-Estrella L. The genome of Bacillus coahuilensis reveals adaptations essential for survival in the relic of an ancient marine environment. Proc Natl Acad Sci USA. 2008;105:5803–5808. doi: 10.1073/pnas.0800981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz L.D. Moreno-Hagelsieb G. Souza V. Herrera-Estrella L. Olmedo G. Understanding the evolutionary relationships and major traits of Bacillus through comparative genomics. BMC Genomics. 2010;11:332. doi: 10.1186/1471-2164-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D.I. Age and origin of Sonoran Desert vegetation. Occasional Papers of the California Academy of Sciences. 1979;132:1–74. [Google Scholar]
- Bonilla-Rosso G. Peimbert M. Alcaraz L.D. Hernández I. Eguiarte L.E. Olmedo-Alvarez G. Souza V. Comparative metagenomics of two microbial mats at Cuatro Ciénegas Basin II: community structure and composition in oligotrophic environments. Astrobiology. 2012;12:659–673. doi: 10.1089/ast.2011.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart M. Hoare A. Nitti A. Siefert J. Haynes M. Dinsdale E. Edwards R. Souza V. Rohwer F. Hollander D. Metagenomic and stable isotopic analyses of modern freshwater microbialites in Cuatro Cienegas, Mexico. Environ Microbiol. 2009;11:16–34. doi: 10.1111/j.1462-2920.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Carson E. Dowling T. Influence of hydrogeographic history and hybridization on the distribution of genetic variation in the pupfishes Cyprinodon atrorus and C. bifasciatus. Mol Ecol. 2006;15:667–679. doi: 10.1111/j.1365-294X.2005.02763.x. [DOI] [PubMed] [Google Scholar]
- Cerritos R. Vinuesa P. Eguiarte L.E. Herrera-Estrella L. Alcaraz-Peraza L.D. Arvizu-Gomez J.L. Olmedo G. Ramirez E. Siefert J.L. Souza V. Bacillus coahuilensis sp. nov., a moderately halophilic species from a desiccation lagoon in the Cuatro Cienegas Valley in Coahuila, Mexico. Int J Syst Evol Microbiol. 2008;58:919–923. doi: 10.1099/ijs.0.64959-0. [DOI] [PubMed] [Google Scholar]
- Cerritos R. Eguiarte L.E. Avitia M. Siefert J.L. Travisano M. Rodríguez-Verdugo A. Souza V. Diversity of culturable thermo-resistant aquatic bacteria along an environmental gradient in Cuatro Cienegas, Coahuila, México. Antonie van Leeuwenhoek. 2011;99:303–318. doi: 10.1007/s10482-010-9490-9. [DOI] [PubMed] [Google Scholar]
- Cookson W.R. Müller C. O'Brien P.A. Murphy D.V. Grierson P.F. Nitrogen dynamics in an Australian semiarid grassland soil. Ecology. 2006;87:2047–2057. doi: 10.1890/0012-9658(2006)87[2047:ndiaas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Desnues C.M. Rodriguez-Brito B. Rayhawk S. Kelley S. Tran T. Haynes M. Liu H. Furlan M. Wegley L. Chau B. Ruan Y. Hall D. Angly F.E. Edwards R.A. Li L. Vega-Thurber R. Reid P. Siefert J. Souza V. Valentine D.L. Swan B.K. Breitbart M. Rowher F. Diversity and evolution of viruses in modern stromatolites and thrombolites. Nature. 2008;452:340–343. doi: 10.1038/nature06735. [DOI] [PubMed] [Google Scholar]
- Domínguez-Escobar J. Beltrán Y. Bergman B. Díez B. Ininbergs K. Souza V. Falcon L. Phylogenetic and molecular clock inferences of cyanobacterial strains within Rivulariaceae from distant environments. FEMS Microbiol Lett. 2011;316:90–99. doi: 10.1111/j.1574-6968.2010.02195.x. [DOI] [PubMed] [Google Scholar]
- Elias S.A. Van Devender T.R. De Baca R. Insect fossil evidence of late glacial and Holocene environments in the Bolson de Mapimi, Chihuahuan Desert, Mexico: comparisons with the paleobotanical record. Palaios. 1995;10:454–464. [Google Scholar]
- Elser J.J. Hamilton A. Stoichiometry and the new biology: the future is now. PLoS Biol. 2007;5:e181. doi: 10.1371/journal.pbio.0050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elser J.J. Schampel J.H. Garcia-Pichel F. Wade B.D. Souza V. Eguiarte L. Escalante A. Farmer J.D. Effects of phosphorus enrichment and grazing snails on modern stromatolitic microbial communities. Freshw Biol. 2005a;50:1808–1825. [Google Scholar]
- Elser J.J. Schampel J.H. Kyle M. Watts J. Carson E. Dowling T. Tang C. Roopnarine P. Effects of PO4 enrichment of microbial communities on hydrobiid snails in an ecosystem with living stromatolites. Freshw Biol. 2005b;50:1826–1835. [Google Scholar]
- Escalante A.E. Eguiarte L.E. Espinosa-Asuar L. Forney L.J. Noguez A.M. Souza V. Diversity of aquatic prokaryotic communities in the Cuatro Cienegas basin. FEMS Microbiol Ecol. 2008;65:50–60. doi: 10.1111/j.1574-6941.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- Escalante A.E. Caballero-Mellado J. Martinez-Aguilar L. Rodriguez-Verdugo A. Gonzalez-Gonzalez A. Toribio-Jimenez J. Souza V. Pseudomonas cuatrocienegasensis sp. nov., isolated from an evaporating lagoon in the Cuatro Cienegas valley in Coahuila, Mexico. Int J Syst Evol Microbiol. 2009;59:1416–1420. doi: 10.1099/ijs.0.006189-0. [DOI] [PubMed] [Google Scholar]
- Ferrusquía-Villafranca I. Bye R. Lot A. Geología de México: una sinopsis. In: Ramamoorthy T.P., editor; Diversidad biológica de México: orígenes y distribución. Instituto de Biologia UNAM; México D.F.: 1998. pp. 3–108. [Google Scholar]
- Forti P. Giulivo I. Piccini L. Tedeschi R. The karst aquifer feeding the Cuatro Cienegas pools (Coahuila, Mexico): its vulnerability and safeguard. Proceedings of the First International Workshop on Aquifer Vulnerability and Risk; México: Salamanca; 2003. pp. 287–299. [Google Scholar]
- Johnson S.G. Age, phylogeography and population structure of the microendemic banded spring snail, Mexipyrgus churinceanus. Mol Ecol. 2005;14:2299–2318. doi: 10.1111/j.1365-294x.2005.02580.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe S.E. O'Hara S.L. Caballero M. Davies S.J. Records of Pleistocene–Holocene climatic change in Mexico—a review. Quat Sci Rev. 2000;19:699–721. [Google Scholar]
- Meyer E.R. Late Quaternary paleoecology of the Cuatro Cienegas basin, Coahuila, Mexico. Ecology. 1973;54:982–985. [Google Scholar]
- Minckley T. Jackson S. Ecological stability in a changing world? Reassessment of the paleo-environmental history of Cuatrociénegas, Mexico. J Biogeogr. 2008;35:188–190. [Google Scholar]
- Minckley W.L. Environments of the Bolson of Cuatro Cienegas. Coahuila, Mexico: Texas Western Press, The University of Texas, El Paso; 1969. [Google Scholar]
- Minckley W.L. Cole G.A. Preliminary limnological information on waters of the Cuatro Ciénegas Basin, Coahuila, México. Southwest Nat. 1968;13:421–431. [Google Scholar]
- Moreno-Letelier A. Olmedo G. Eguiarte L.E. Martínez-Castilla L. Souza V. Parallel evolution and horizontal gene transfer of the pst operon in Firmicutes from oligotrophic environments. Int J Evol Biol. 20112011 doi: 10.4061/2011/781642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peimbert M. Alcaraz L.D. Bonilla-Rosso G. Olmedo-Alvarez G. García-Oliva F. Segovia L. Eguiarte L.E. Souza V. Comparative metagenomics of two microbial mats at Cuatro Ciénegas Basin I: ancient lessons on how to cope with an environment under severe nutrient stress. Astrobiology. 2012;12:648–658. doi: 10.1089/ast.2011.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointing S.B. Chan Y. Lacap D.C. Lau M.C. Jurgens J.A. Farrell R.L. Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA. 2009;106:19964–19969. doi: 10.1073/pnas.0908274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollar E.A. Avitia M. Eguiarte L.E. González-González A. Mora L. Bonilla-Rosso G. Souza V. Water-sediment niche differentiation in ancient marine lineages of Exiguobacterium endemic to the Cuatro Cienegas Basin. Environ Microbiol. 2012 doi: 10.1111/j.1462-2920.2012.02784.x. [DOI] [PubMed] [Google Scholar]
- Redfield A.C. On the proportions of organic derivations in sea water and their relation to the composition of plankton. In: Daniel R.J., editor. James Johnstone Memorial Volume. University Press of Liverpool; Liverpool: 1934. pp. 177–192. [Google Scholar]
- Rodríguez-Verdugo A. Thesis, School of Sciences, UNAM; México D.F.: 2008. Variación estacional en la diversidad de Pseudomonas asociadas a un sistema acuático fluctuante. [Google Scholar]
- Rusch D.B. Halpern A.L. Sutton G. Heidelberg K.B. Williamson S. Yooseph S. Wu D. Eisen J.A. Hoffman J.M. Remington K. Beeson K. Tran B. Smith H. Baden-Tillson H. Stewart C. Thorpe J. Freeman J. Andrews-Pfannkoch C. Venter J.E. Li K. Kravitz S. Heidelberg J.F. Utterback T. Rogers Y.H. Falcon L.I. Souza V. Bonilla-Rosso G. Eguiarte L.E. Karl D.M. Sathyendranath S. Platt T. Bermingham E. Gallardo V. Tamayo-Castillo G. Ferrari M.R. Strausberg R.L. Nealson K. Friedman R. Frazier M. Venter J.C. The Sorcerer II Global Ocean Sampling expedition: northwest Atlantic through eastern tropical Pacific. PLoS Biol. 2007;5:e77. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxer G. Doebeli M. Travisano M. Spatial structure leads to ecological breakdown and loss of diversity. Proc Biol Sci. 2009;276:2065–2070. doi: 10.1098/rspb.2008.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V. Espinosa-Asuar L. Escalante A.E. Eguiarte L.E. Farmer J. Forney L. Lloret L. Rodriguez-Martinez J.M. Soberon X. Dirzo R. Elser J.J. An endangered oasis of aquatic microbial biodiversity in the Chihuahuan desert. Proc Natl Acad Sci USA. 2006;103:6565–6570. doi: 10.1073/pnas.0601434103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V. Falcón L.I. Elser J.J. Eguiarte L.E. Protecting a window into the ancient Earth: towards a Precambrian park at Cuatro Cienegas, Mexico. The Citizen's Page, Evolutionary Ecology Research. 2007. http://www.evolutionary-ecology.com/citizen/citizen.html http://www.evolutionary-ecology.com/citizen/citizen.html
- Souza V. Eguiarte L.E. Siefert J. Elser J.J. Microbial endemism: does phosphorus limitation enhance speciation? Nat Rev Microbiol. 2008;6:559–564. doi: 10.1038/nrmicro1917. [DOI] [PubMed] [Google Scholar]
- Stein B.A. Kutner L.S. Adams J.S. Precious Heritage: The Status of Biodiversity in the United States. Oxford University Press; Oxford: 2000. [Google Scholar]
- Sterner R.W. Elser J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to Biosphere. Princeton University Press; Princeton, NJ: 2002. [Google Scholar]
- Szynkiewicz A. Ewing R.C. Moore C.H. Glamoclija M. Bustos D. Pratt L.M. Origin of terrestrial gypsum dunes—implications for martian gypsum-rich dunes of Olympia Undae. Geomorphology. 2010;121:69–83. [Google Scholar]
- Tobler M. Carson E.W. Environmental variation, hybridization, and phenotypic diversification in Cuatro Ciénegas pupfishes. J Evol Biol. 2010;23:1475–1489. doi: 10.1111/j.1420-9101.2010.02014.x. [DOI] [PubMed] [Google Scholar]
- Venditti C. Meade A. Pagel M. Phylogenies reveal new interpretation of speciation and the Red Queen. Nature. 2010;463:349–352. doi: 10.1038/nature08630. [DOI] [PubMed] [Google Scholar]
- Wilson C.G. Sherman P.W. Anciently asexual bdelloid rotifers escape lethal fungal parasites by drying up and blowing away. Science. 2010;327:574–576. doi: 10.1126/science.1179252. [DOI] [PubMed] [Google Scholar]
- Wilson J.S. Pit J.P. Illuminating the lack of consensus among descriptions of earth history data in the North American deserts: a resource for biologists. Progress in Physical Geography. 2010;34 doi: 10.1177/0309133310363991. [DOI] [Google Scholar]
- Winsborough B.M. Theriot E. Czarnecki D.B. Diatoms on a continental “island”: Lazarus species, marine disjuncts and other endemic diatoms of the Cuatro Cienegas basin, Coahuila, Mexico. Nova Hedwigia, Suppl. 2009;135:257–274. [Google Scholar]
- Yoshida T. Ellner S.P. Jones L.E. Bohannan B.J.M. Lenski R.E. Hairston N.G., Jr. Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol. 2007;5:e235. doi: 10.1371/journal.pbio.0050235. [DOI] [PMC free article] [PubMed] [Google Scholar]