Abstract
The OMEGA/Mars Express hyperspectral imager identified gypsum at several sites on Mars in 2005. These minerals constitute a direct record of past aqueous activity and are important with regard to the search of extraterrestrial life. Gale Crater was chosen as Mars Science Laboratory Curiosity's landing site because it is rich in gypsum, as are some desert soils of the Cuatro Ciénegas Basin (CCB) (Chihuahuan Desert, Mexico). The gypsum of the CCB, which is overlain by minimal carbonate deposits, was the product of magmatic activity that occurred under the Tethys Sea. To examine this Mars analogue, we retrieved gypsum-rich soil samples from two contrasting sites with different humidity in the CCB. To characterize the site, we obtained nutrient data and analyzed the genes related to the N cycle (nifH, nirS, and nirK) and the bacterial community composition by using 16S rRNA clone libraries. As expected, the soil content for almost all measured forms of carbon, nitrogen, and phosphorus were higher at the more humid site than at the drier site. What was unexpected is the presence of a rich and divergent community at both sites, with higher taxonomic diversity at the humid site and almost no taxonomic overlap. Our results suggest that the gypsum-rich soils of the CCB host a unique microbial ecosystem that includes novel microbial assemblies. Key Words: Cuatro Ciénegas Basin—Gale Crater—Gypsum soil microbial diversity—Molecular ecology—Nitrogen cycle. Astrobiology 12, 699–709.
1. Introduction
Gypsum is a sedimentary evaporite rich in sulfates. The OMEGA/Mars Express hyperspectral imager identified gypsum (hydrated sulfates) at several sites on Mars in 2005 (Gendrin et al., 2005). These minerals constitute a direct record of past aqueous activity. Since water is considered one of the few prerequisites for the search for extraterrestrial life, evidence of past water activity has been taken into consideration for most landing sites on Mars. Gale Crater was chosen as the site where the Mars Science Laboratory Curiosity rover will land not only to search for evidence of an ancient aquatic environment but also to investigate the 5 km high central mount that resides on ancient layered sediments. The Cuatro Ciénegas Basin (CCB), in the Chihuahuan Desert of Mexico, is a valley surrounded by 3 km of the High Sierras with evident sediment layering that shows several fault systems and an ancient history of tectonics. We consider this Mexican oasis to be a Gale Crater analogue in part because it is an arid, gypsum-rich environment. However, the most unique feature of this site is that molecular data strongly suggest that the microbial mats and stromatolites in the CCB are direct descendants of ancient marine microorganisms that inhabited the western shores of Laurentia in the Panthalassa Ocean.
At the CCB, gypsum dune fields at Churince are extremely pure, and their δ34S values range from 14.6% to 15.9%, placing the main source of dissolved sulfate as evaporite strata of Middle Permian to Cretaceous ages (Szynkiewicz et al., 2010). The gypsum deposits of the CCB are the product of magmatic activity that occurred under the Tethys Sea. These evaporite deposits are unique in that they are overlain by minimal deposits of carbonate (Szynkiewicz et al., 2010). Besides magmatic activity, gypsum can arise from hydrothermal systems formed by way of meteorite impacts in water-bearing regions (Edwards et al., 2005). As mentioned earlier, Gale Crater on Mars is rich in gypsum, and it is very likely this mineral originated as a consequence of a large meteorite impact in an ancient lake or sea that resulted in an associated hydrothermal system (Newsom et al., 2001). This is not surprising, given that meteorite impact craters are possibly the most common geological landforms in the Solar System (Izawa et al., 2011). What was a surprise, however, was discovery of this indirect evidence of a wet Mars. If life once existed, or still exists, on Mars, evidence of its occurrence is likely to be found as mineralogical biosignatures. These include distinctive mineral surface structures or chemistry that arise when dissolution and crystal growth kinetics are influenced by metabolic by-products (Banfield et al., 2001). Raman spectrometry analyses of the microbiota in Haughton Crater (a meteorite impact formed in the Miocene) in the Canadian High Arctic indicate that microbial pigments in gypsum crystals can be used as a biosignature (Edwards et al., 2005).
Another relevant biosignature is the change of the isotopic ratio in the fundamental atoms, such as nitrogen (N), that make life possible on Earth. Nitrogen within the Solar System is not isotopically uniform in its proportions 15N/14N (Marti and Kerridge, 2010). Using this ratio, the source of N can be inferred. On Earth, N is a basic element for life, and its biogeochemical cycle is closely linked to the carbon (C), oxygen (O), and sulfur (S) cycle.
The N cycle can be summarized as an exchange of N between the atmosphere and the biosphere. The atmospheric N2, which comprises around 78% of the atmosphere, is inaccessible to most living organisms with the exception of a small number of nitrogen-fixing Archaea and Bacteria (Zehr and Kudela, 2011). Biological fixation is thought to have been the major source of N for the biosphere until that time when industrial-age fertilizers became widespread. This reaction is catalyzed by the enzyme nitrogenase (encoded by nif genes), which fixes N2 as biologically available ammonium 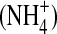 that breaks down to NH3 plus H+ such that NH3 is assimilated by life in general (Devol, 1991). In contrast, denitrification, the anaerobic reduction of nitrate
that breaks down to NH3 plus H+ such that NH3 is assimilated by life in general (Devol, 1991). In contrast, denitrification, the anaerobic reduction of nitrate  , nitrite
, nitrite  , and nitric oxide (NO) to nitrous oxide (N2O) or N2, is the major biological mechanism by which fixed N returns to the atmosphere from soil or water, thereby completing the N cycle. Nitrite reductase, which catalyzes the reduction of soluble nitrite into gaseous nitric oxide, is the key enzyme in the denitrification process, and it is encoded by the nirS and nirK genes that encode the cytochrome cd1 and copper nitrite reductases, respectively (Butler and Richardson, 2005). Consequently, these genes can effectively serve as functional markers for the identification of microorganisms that mediate nitrogen fixation and denitrification within any given community.
, and nitric oxide (NO) to nitrous oxide (N2O) or N2, is the major biological mechanism by which fixed N returns to the atmosphere from soil or water, thereby completing the N cycle. Nitrite reductase, which catalyzes the reduction of soluble nitrite into gaseous nitric oxide, is the key enzyme in the denitrification process, and it is encoded by the nirS and nirK genes that encode the cytochrome cd1 and copper nitrite reductases, respectively (Butler and Richardson, 2005). Consequently, these genes can effectively serve as functional markers for the identification of microorganisms that mediate nitrogen fixation and denitrification within any given community.
In the present study, we compared previous N-cycle and microbial ecology investigations at five arid Mars analog environments, including the Sonoran Desert of Arizona (Nagy et al., 2005), the Australian Desert (Cookson et al., 2006), the Colorado Plateau (Gundlapally and Garcia-Pichel, 2006), the Atacama Desert (Connon et al., 2007), and the McMurdo Dry Valleys of Antarctica (Pointing et al., 2009).
In the Australian Desert, the N cycle is governed by the limitation of C and N that arises from the patchy grass cover and from fluctuations in rainfall (Cookson et al., 2006). Similarly, in the deserts of North America, patches of mesquite (Prosopis) and Larrea represent areas of fertility within widespread arid soils (Schlesinger et al., 1996). This is also evident in the Atacama Desert, where the presence of water or vegetation increases the overall productivity and microbial diversity (Aguilera et al., 1999). The common observation in these systems is a low diversity with high ecological dominance of few taxa (in particular, Cyanobacteria with heterocysts). This community structure is probably the result of multiple stressors on the ecosystem, such as limited N and low water availability (Nagy et al., 2005; Gundlapally and Garcia-Pichel, 2006; Cary et al., 2010; Ríos et al., 2010).
The CCB, a gypsum-rich Mars analog arid environment, is located in the northern part of Mexico. In this basin, groundwater rises to the surface through the dissolution of limestone, which results in an “archipelago” of more than 300 pools surrounded by saline soils rich in calcium sulfates and extremely poor in nutrients (ultraoligotrophic), in particular phosphorous (P) (Elser et al., 2005). Vegetation cover and water availability in these soils depend on the degree of proximity to the aquatic systems and rainfall. Many investigations have addressed the microbial diversity of the aquatic systems of the CCB and concluded that these systems have a high microbial diversity and endemism (Souza et al., 2006; Desnues et al., 2008; Escalante et al., 2008; Breitbart et al., 2009; Cerritos et al., 2010). However, no such studies have addressed the soil microbial diversity.
To understand this Mars analogue, we investigated the nutrient content and availability and estimated the microbial diversity of two sites rich in gypsum within the Churince system in the CCB. Due to the extreme relevance of liquid water for life, we chose these sites because they represent two humidity levels within the same region. Moreover, given that desert soils have a scarce nutrient content and the N cycle is key to the overall nutrient availability, we explored the distribution and diversity of genes involved in the N cycle: nifH, nirK, and nirS.
2. Materials and Methods
2.1. Study site and sample collection
The CCB receives a mean annual precipitation of <150 mm, which falls mainly during the summer months. The dominant soil type is composed of gypsum (Word Reference Base for Soil Resources) and usually covered by halophilic and gypsophilic grassland. The two study sites are located within the Churince aquatic system on the western part of the CCB at 740 m above sea level. This system consists of a water spring and two desiccation lagoons that are connected by short shallow rivers (Fig. S1; Supplementary Data are available online at www.liebertonline.com/ast). Underground water exploitation has significantly decreased the volume of water within the system; consequently, soil moisture has been drastically reduced in the last 5 years (Souza et al., 2006; Cerritos et al., 2010).
During February 2007, samples were collected from 8×8 m plots at two sites separated by a distance of 1 km (Fig. S1). The first site (humid) is located on the border of a small river where the vegetation is dominated by the grass Sporobolus airoides, which covers 60% of the total plot area (Fig. S1). The second site (dry) is situated at a distance of 250 m from the main desiccation lagoon; we refer to this second site as “dry.” In contrast to the river site, the vegetation at the desiccation lagoon site predominantly consists of the gypsophilic grass Sesuvium erectum, which covers 10% of the total plot area (Fig. S1).
To characterize the overall nutrient availability at each site, we quantified total C, N, and P of 10 samples from each site. Samples were collected from the top 10 cm of the soil, and plant debris was removed by hand with the use of sterile gloves prior to sample storage and processing. For physicochemical characterization, 1 kg samples were stored in black plastic bags and refrigerated at 4°C until processed in the laboratory.
Given the negligible change in nutrient availability within each plot (as explained below), a composite soil sample (50 g) was assembled from each site and immediately stored in liquid N for DNA extraction.
2.2. Soil physicochemical characterization analyses
The 10 samples from each site were oven-dried at 75°C for soil moisture determination by the gravimetric method. Soil pH was measured in deionized water (soil:solution ratio, 1:2 w/v). Total organic and inorganic C forms were determined with a total carbon analyzer (UIC Mod. CM5012), whereas N and P forms were determined with a Bran-Luebbe Auto Analyzer III (Norderstedt, Germany) following standard procedures (Murphy and Riley, 1962; Huffman, 1977; Bremmer and Mulvaney, 1982). Inorganic N ( and
and  ) and inorganic dissolved phosphorous (IDP) were extracted as described by Murphy and Riley (1962).
) and inorganic dissolved phosphorous (IDP) were extracted as described by Murphy and Riley (1962).
Microbial C and N concentrations were determined from moist field samples by a chloroform fumigation extraction method that allows for assessment of the differences between the input of nutrients due to microbial processes during incubation (nonfumigated samples) versus the nutrient changes due to chemical reactions (Vance et al., 1987). Fumigated and nonfumigated samples were incubated during 24 h at 25°C and constant moisture. Microbial C was extracted from both fumigated and nonfumigated samples with 0.5 M K2SO4 and filtered through Whatman paper filter (type 42) to remove impurities (Brookes et al., 1985). The C concentration was measured in the C analyzer (see above). Microbial C was calculated by subtracting the extracted C in nonfumigated samples from that of fumigated samples and dividing it by a KEC value of 0.45 (Joergensen, 1996). Microbial N was extracted with the same procedure used for microbial C but was filtered through a Whatman No. 1 paper. The filtrate was acid digested and determined as total N by a macro-Kjeldahl method (Brookes et al., 1985). Microbial N was calculated in a similar manner as microbial C but divided by a KEN value of 0.54 (Joergensen and Mueller, 1996).
2.3. Statistical analyses of soil samples
Data from the 10 soil samples from each site were analyzed by using the normality Shapiro-Wilk test. If the data adhered to a normal distribution, differences in pH, soil moisture, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), dissolved organic carbon (DOC), dissolved organic nitrogen (DON), dissolved organic phosphorous (DOP),  , microbial C, and microbial N were evaluated by using t tests (Shapiro-Wilk test). If the data were not normally distributed, a Kruskal-Wallis test and a nonparametric ANOVA were applied. To determine the effect of the environmental parameters in relation to the nutrient characterization, a canonical correspondence analysis was performed on environmental parameters, which included pH, soil moisture, TOC, TN, TP, DOC, DON, DOP,
, microbial C, and microbial N were evaluated by using t tests (Shapiro-Wilk test). If the data were not normally distributed, a Kruskal-Wallis test and a nonparametric ANOVA were applied. To determine the effect of the environmental parameters in relation to the nutrient characterization, a canonical correspondence analysis was performed on environmental parameters, which included pH, soil moisture, TOC, TN, TP, DOC, DON, DOP,  , microbial C, and microbial N. The sample scores for the first two components were used to calculate the distance between each sample and the corresponding group (site) centroid (mean centroid distance). Average distances for each group were then used to estimate the variability within and between sampling sites. All the statistical analyses were performed with R statistical packages (R Development Core Team, 2011).
, microbial C, and microbial N. The sample scores for the first two components were used to calculate the distance between each sample and the corresponding group (site) centroid (mean centroid distance). Average distances for each group were then used to estimate the variability within and between sampling sites. All the statistical analyses were performed with R statistical packages (R Development Core Team, 2011).
2.4. Molecular analyses
Due to the high concentration of salts, polysaccharides, and secondary compounds present in the soil samples, total DNA was extracted by a combination of two methods. First, the bacterial fraction was isolated by using the fractionation centrifugation technique described in Holben et al. (1988). Second, the resulting pellet was weighed, and DNA was then purified with the Soil Master DNA Extraction Kit (EPICENTRE Biotechnology) according to the manufacturer's instructions. DNA was stored in the freezer at −20°C until further processing.
The PCR reactions were carried out in a final volume of 50 μL with use of 100–200 ng of DNA as template. The primer details and specific reaction conditions for each gene are shown in Tables S1 and S2 in the Supplementary Material. To amplify nifH, we used a modification of the nested PCR described in Zani et al. (2000). In the first round of amplification, we used the primers nifH3 and nifH4. In the second round of amplification, we used the primers nifH1 and nifH2. PCR reactions and thermocycling for the PCR were performed in the same way as in the first round of amplification, with direct use of 1 μL of the first-round PCR product as a template.
For every sample, four independent PCR reactions were performed for each gene to reduce PCR biases. For each sample and gene, the four reactions were pooled together and purified from a 2% agarose gel with the QIAquick Gel Extraction Kit (Qiagen) following the manufacturer's instructions. The purified fragments were cloned into the pCR2.1 vector according to the manufacturer's instructions (Invitrogen). Plasmid DNA containing inserts were isolated with the Montage Plasmid Miniprep Kit (Millipore) for subsequent sequencing. Inserts were sequenced with vector-based primer T7.
2.5. Sequence analyses
We checked for 16S rDNA chimeras with Chimera Check from the Ribosomal Database Project II, release 8.1. High-quality sequences were compared against the Greengenes database (DeSantis et al., 2006) to obtain their taxonomic rank. These sequences were aligned with ClustalX (Larkin et al., 2007), and the resulting alignment was manually edited. Rarefaction curves were constructed by using DOTUR (Schloss and Handelsman, 2005; DeSantis et al., 2006) with a sampling efficiency determined at 97% sequence identity. Distance matrices were constructed by the DNADIST program in PHYLIP with use of Jukes-Cantor distance. Relatedness between clone libraries from different soil samples was compared with ∫-LIBSHUFF (Singleton et al., 2001). The diversity estimates for each clone library were compared by calculating Renyi's profiles with the Biodiversity R (Kindt and Coe, 2005) and MEGAN (Oksanen et al., 2010) R packages.
We amplified fragments of approximately 400 bp from the clone libraries of nifH, nirK, and nirS genes. The identity of each gene was confirmed by comparing each sequence with the NCBI database by using the nucleotide BLAST algorithm. For each sequence, the best experimentally annotated hit was downloaded and incorporated into the alignments. Sequences were aligned with ClustalX and were manually edited and translated into amino acids with the Software BioEdit (Hall, 1999). We used the program Modeltest (Posada and Crandall, 1998) to determine the nucleotide substitution model with the best fit to the data. Tree reconstructions for each gene were performed with PAUP*4.0b8 (Swofford, 2003), with use of the neighbor-joining method with 1000 bootstrap replicates. Trees with the relative abundances of OTUs (operational taxonomic units defined at 95% of identity) were plotted with the interactive Tree of Life online program (Letunic and Bork, 2007). Finally, MOTHUR v.1.21.1 (Schloss et al., 2009) was used to assign sequences to OTUs based on a specific genetic similarity for each gene (16S rDNA 97%, nifH 97%, nirK 95%, and nirS 95%), and Venn diagrams were generated to estimate the shared richness between sites.
2.6. Sequence accession numbers
GenBank accession numbers for sequences obtained in this study are JN123055 to JN123353 (16S rRNA), JN122945 to JN122985 (nifH), JN122986 to JN123015 (nirK), and JN123016 to JN123054 (nirS).
3. Results
3.1. Soil physicochemical parameters
The C:N:P ratios for the dry and humid sites were 104:5:1 and 296:16:1, respectively. Since the Redfield ratio is 106:16:1 (Elser et al.,
2005), these ratios indicate that N is a more limiting nutrient at the dry site than at the humid site and that carbon is more abundant in the humid site. The physicochemical data confirm that the two sites differ in almost all measured parameters (Table 1). Soil samples from the two sites were very alkaline, but the sample from the dry site had a higher concentration of cations and anions. As expected, soil samples from the humid site were moister (27% soil moisture) than those from the other site (11%); this was expected since the humid site is closer to the river. The soil content for almost all measured forms of C, N, and P were also higher in the humid site, with the exception of IDP content, which was higher at the dry site. The  was null in the dry site.
was null in the dry site.
Table 1.
Soil Physicochemical Parameters (Mean±SE) of the Two Studied Sites Located within the Churince System in the Cuatro Ciénegas Basin, Mexico
| Dry | Humid | |
|---|---|---|
| Soil moisture (%)* | 11±0.6 | 27±1.0 |
| pH* | 8.8±0.04 | 8.5±0.04 |
| Cations (+) (cmol kg−1)* | 183±13 | 98±19 |
| Anions (−) (cmol kg−1)* | 16±2.3 | 5±0.6 |
| Total organic C (mg g−1)* | 3±0.2 | 15±1.5 |
| Total N (mg g−1)* | 0.11±0.01 | 0.80±0.04 |
| Total P (mg g−1)* | 0.024±0.003 | 0.05±0.003 |
| Dissolved organic C (μg g−1)* | 38±5 | 152±16 |
| Dissolved organic N (μg g−1)* | 2.1±0.2 | 2.4±0.3 |
| Dissolved organic P (μg g−1)* | 0.17±0.06 | 0.56±0.20 |
| Inorganic dissolved P (μg g−1)* | 0.012±0.02 | 0.003±0.001 |
Ammonium ( ) (μg g−1)* ) (μg g−1)*
|
0.43±0.03 | 0.68±0.06 |
Nitrate (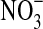 ) (μg g−1) ) (μg g−1) |
not detectable | 0.24±0.13 |
| Microbial C (μg g−1)* | 107±17 | 595±133 |
| Microbial N (μg g−1)* | 5.3±1.8 | 11.0±2.3 |
C, carbon; N, nitrogen; P, phosphorous.
Significant difference among sites (P<0.05).
Canonical correspondence analysis showed that 77% of the total variation could be explained by the first component (Fig. S2). The variables associated with this component were microbial C and DOC. The second component explained 9.8% of the total variation. The variable associated with this component was pH. As expected due to the differences in humidity, nutrient availability, and stoichiometric proportions, a clear separation between sampling sites was observed along the axis of the second component (Fig. S2).
3.2. Bacterial taxonomic composition
After the elimination of chimeras and low-quality sequences (84 sequences with short lengths, with undefined bases and chimeras produced during the PCR amplification), 516 sequences of 16S rRNA with a longitude of 600 bp were obtained and subsequently used in the diversity analyses.
In the harsher dry site, a total of 293 sequences were obtained that belong to 15 phyla and 40 classes. The more abundant bacterial phyla were as follows (Fig. 1): (1) Gammaproteobacteria of the family Moraxellaceae (19.1%), the family Pseudomonadaceae (3%), and the order Xanthomonadales (7.5%); (2) Bacteroidetes from the genus Algoriphagus (9.2%); (3) Actinobacteria of the order Acidimicrobiales (10.2%), Gemmatimonadetes (9.2%), and Acidobacteria (8.5%). Less abundant groups were the following: Chloroflexi (1.4%), Cyanobacteria (3%), Alphaproteobacteria of the genus Porphyrobacter (6%), Firmicutes of the class Bacilli (2.4%), and Planctomycetes (1%).
FIG. 1.
Taxonomic distribution of the 16S rRNA gene sequences obtained from the clone libraries of two studied sites: dry and humid, within the Churince system in the CCB, Mexico. Previously published clone libraries from biological soil crusts (BSCs) and soil: Sonoran Desert BSC (Nagy et al., 2005), Colorado Plateau BSC (Gundlapally and Garcia-Pichel, 2006), McMurdo Dry Valleys soil (Pointing et al., 2009), and Atacama Desert soil (Connon et al., 2007). Relative abundances are shown as percentages of the total number of clones retrieved from each library. Color images available online at www.liebertonline.com/ast
With the same sampling effort, a total of 223 sequences were obtained from the humid site, which represented 16 phyla and 36 classes. The most abundant phyla (Fig. 1) were Actinobacteria of the class Acidimicrobidae (18.4%), followed by Alphaproteobacteria (17.5%), Acidobacteria (14.8%), Chloroflexi (9.4%), Deltaproteobacteria (9%, 5.4% belong to the sulfur-reducing group), Gemmatimonadetes (4%), Cyanobacteria (3.6%), the Gammaproteobacteria of the family Halomonadaceae (1.3%) (common in alkaline soils), and Nitrospirae (0.45%) (related to ammonia oxidizers in soil).
As expected from the biogeochemical analyses, both sites were inhabited by significantly different microbial communities. The ∫-LIBSHUFF test and the similarity indices (Table 2) indicated that the relative abundance of taxonomic groups is variable among sites that show a different community structure. Furthermore, the rarefaction curves did not reach an asymptote at either of the sites (Fig. 2), which commonly occurs in highly diverse communities. The slope of the curve was greater for the humid site than for the dry site. Also, the nonparametric richness estimator Chao indicated that higher numbers of OTUs should be expected in the humid site (433 OTUs for the humid site and 289 OTUs for the dry site). This suggests that the more humid site has a higher taxonomic diversity. Accordingly, the Shannon index showed that diversity was significantly higher in the humid site than in the dry site (H=4.3 and 3.7, respectively). The Renyi's profiles also suggest that the humid site is more diverse than the dry site, both in terms of richness and evenness (Fig. S3).
Table 2.
Operational Taxonomic Unit (OTU) Similarity Indices Inferred from the 16S rRNA, nirH, nirS, and nirK Genes between the Two Studies' Sites Located within the Churince System in the Cuatro Ciénegas Basin, Mexico
| |
OTUs |
Similarity index |
Structure similarity coefficient |
|||
|---|---|---|---|---|---|---|
| Total | Shared (between sites) | Jaccard | Sorensen | Yue and Clayton's theta | Smith's theta | |
| 16S | 169 | 4 | 0.04 | 0.08 | 0.06 | 0.04 |
| nifH | 33 | 4 | 0.12 | 0.22 | 0.15 | 0.16 |
| nirK | 31 | 8 | 0.26 | 0.41 | 0.05 | 0.21 |
| nirS | 31 | 2 | 0.06 | 0.12 | 0.03 | 0.04 |
FIG. 2.
Rarefaction curves showing 95% confidence interval for the 16S rRNA gene clone libraries of the two studied sites: dry (DL) and humid (R) within the Churince system in the CCB, Mexico. OTUs were determined at 97% sequence identity.
3.3. Nitrogen fixing and denitrifying community
One hundred partial sequences of nifH were obtained for each site, which belonged to 25 different OTUs. A total of 19 OTUs were present in the humid site, whereas only 9 OTUs were present in the dry site and only three were shared between sites (Fig. 3). Out of the total OTUs identified, 12 were associated to Deltaproteobacteria, 7 to Alphaproteobacteria, 3 to Gammaproteobacteria, and 3 to Cyanobacteria. The most abundant OTU (17), present at both sites, was related to Rhizobiales. This observation, together with the absence of Leguminoseae in both sites, suggests the presence of nonsymbiotic Rhizobiales in the diazotrophic soil community.
FIG. 3.
Neighbor-joining tree for the nifH gene OTUs from the two studied sites: dry and humid within the Churince system in the CCB, Mexico. Gray circles indicate nodes with bootstrap support >0.5. Relative abundances are shown as gray bars for the dry site and as black bars for the humid site.
A total of 50 sequences of nirK were obtained for each site, from which 30 OTUs could be identified. The majority of the sequences could not be associated to known sequences in public databases (Fig. 4). A total of 24 and 13 OTUs were present in the humid and dry sites, respectively. From these, only seven OTUs were shared between sites (Fig. 4). For nirS, 50 sequences were obtained for each site. A total of 27 OTUs were recovered, 15 from the humid site, 14 from the dry site, and only 2 shared OTUs (Fig. 5). As in the case of nirK, most nirS sequences could not be associated to any known sequence reported in public databases (Fig. 5). The only sequence (OTU22) with a database hit was related to Pseudomonas. This OTU was present at both sites, being abundant at the dry site but rare at the humid site (Fig. 5). The other shared OTU was rare in both sites (OTU5).
FIG. 4.
Neighbor-joining tree for the nirK gene OTUs from the two studied sites: dry and humid, within the Churince system in the CCB, Mexico. Gray circles indicate nodes with bootstrap support >0.5. Relative abundances are shown as gray bars for the dry site and as black bars for the humid site.
FIG. 5.
Neighbor-joining tree for the nirS gene OTUs from the two studied sites: dry and humid, within the Churince system in the Cuatro Ciénegas Basin, Mexico. Gray circles indicate nodes with bootstrap support >0.5. Relative abundances are shown as gray bars for the dry site and as black bars for humid site.
4. Discussion
This is the first characterization of the gypsum-based soil community at the CCB. We consider this relevant since the soil is possibly a better analogue to actual dry Mars than the well-characterized aquatic communities from this oasis (Souza et al., 2012). As is the case with any first glimpse, this is much more a “natural history”-type description of the indirect response of the microbial community to the differences in humidity. We are aware that a well-rounded analyses of this complex soil is needed, and such studies are underway.
As revealed by the statistical analysis, the differences in the structure of the microbial community between sites can be attributed, besides to humidity, to differences in nutrient content (C) and pH among sites. Although the differences in pH are small, this might have an effect on biomass, microbial activity, and the community structure (Bååth and Anderson, 2003). Our results agree with other studies that have suggested that vegetation cover and water availability are the two most important factors that shape desert soil microbial communities (Aguilera et al., 1999; Cookson et al., 2006; Sponseller and Fisher, 2008). In this respect, the presence of denser vegetation at the humid site could explain the higher concentrations of C and N. The denser vegetation would also result in an increased input of organic matter into the soil, which would thus increase the organic acid content and lower the soil's pH. As a consequence of water availability, there is also a greater availability of organic nutrient forms, which would promote soil metabolism and a more diverse and even microbial community.
As expected from a richer community where nutrient cycling is more efficient, nutrient measurements indicate that DOC content was 4 times higher at the humid site. DOC is the principal source of C for heterotrophic microorganisms as observed in the humid site; this could promote higher heterotrophic microbial activity (Montaño et al.,
2007). In contrast, when the availability of DOC is low, autotrophic pathways, such as nitrification, would be favored, and  would accumulate (Vitousek, 2002). Nevertheless, even though the organic carbon is so different between sites, we found low concentrations of
would accumulate (Vitousek, 2002). Nevertheless, even though the organic carbon is so different between sites, we found low concentrations of 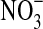 at both sites (undetectable in the dry site). This could be attributed to low temperatures (0–10°C) at the time of sampling, which would have inhibited soil metabolism and concomitantly lowered the rates of denitrification. Also at the surface of other deserts, low concentrations of
at both sites (undetectable in the dry site). This could be attributed to low temperatures (0–10°C) at the time of sampling, which would have inhibited soil metabolism and concomitantly lowered the rates of denitrification. Also at the surface of other deserts, low concentrations of 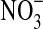 have been found (near to zero or undetectable), but large quantities are sequestered below a depth of 1 m because not all the available
have been found (near to zero or undetectable), but large quantities are sequestered below a depth of 1 m because not all the available 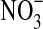 is consumed in the soil zone or returned to the atmosphere (Walvoord et al.,
2003).
is consumed in the soil zone or returned to the atmosphere (Walvoord et al.,
2003).
On the other hand, taxonomic and functional diversity at Churince's gypsum soil seems to be much higher than that reported in other studies of arid soil communities, including others Mars analogues (Schlesinger et al., 1996; Yeager et al., 2004; Nagy et al., 2005; Drees et al., 2006; Gundlapally and Garcia-Pichel, 2006; Dong et al., 2007; Pointing et al., 2009; Ríos et al., 2010). Even though this is the first study performed at the CCB in non-aquatic habitats, microbial community studies in the basin have demonstrated that the CCB encompasses diverse and unusual marine microorganisms (Souza et al., 2006; Desnues et al., 2008; Breitbart et al., 2009; Cerritos et al., 2011; see also several articles in this issue).
At first glimpse, the microbial taxonomic composition of the desert soil of the CCB is represented by common bacterial class and phyla, which have been previously encountered in other soils across the globe (Janssen, 2006; Fierer et al., 2007; Youssef and Elshahed, 2009). Nevertheless, at the finer taxonomic level of genotype (i.e., at OTU level), the sequences are diverse, which indicates a diverse soil community.
As an example, in most arid sites, dominant Cyanobacteria are the primary C producers and the primary N fixers (Nagy et al., 2005; Gundlapally and Garcia-Pichel, 2006). While at the CCB, we observed different kinds of phototrophs and potential N fixers besides Cyanobacteria; this includes ancient genera such as Chloroflexi and the Alphaproteobacteria Porphyrobacter (Fig. 1). Porphyrobacter is a genus that can fix C under aerobic conditions in the dark (Hiraishi and Imhoff, 2005), while Chloroflexi is a photosynthetic, anoxygenic, nonsulfur bacteria typically found in microbial mats in salty, shallow marine environments (Hanada and Pierson, 2006).
Additionally, the CCB uniqueness is also confirmed by the divergence of the studied N-cycle genes. In the present study, we obtained many nondescribed nifH sequences from a relatively small sample (Fig. 3), which suggests that the superficial soils of the CCB may be unusual when compared to biological soil crusts, where 80–90% of nifH sequences are from heterocystic Cyanobacteria (Yeager et al., 2004). We know that we cannot compare directly the superficial soil at the CCB with the well-typified biological soil crust composition; nevertheless, a higher proportion of Cyanobacteria would be expected for all the other desert studies. On the other hand, the dominance of Pseudomonas pseudoalcaligenes, Azospirillum brasilense, and Rhizobium sp. has been reported in association with roots of the drought-tolerant grass Lasiurus sindicus in the Thar Desert of Rajasthan, India (Chowdhury et al., 2009). This observation suggests that the grass species found in the CCB could be the source of the Rhizobiales and their associated nitrogenases. However, no symbiosis between halotolerant grasses and Rhizobiales has yet been reported.
In the case of nirK, one single OTU could be related to Nitrosomonas sp., a common group associated with denitrification processes, and another single OTU to Rhizobium etli, whereas the rest of the OTUs are highly divergent or have a high similarity with nondescribed taxa (Fig. 4). This uniqueness, however, can be the result of the low global sampling and the artifact of fewer deposited sequences from isolated strains. The same occurs with nirS, where our sequences show less than 80% identity with other nirS sequences from known denitrifying bacteria, which supports previous observations of a highly endemic microbiota within the CCB (Souza et al., 2006; Cerritos et al., 2011). This small sample suggests that a diverse denitrifying community is waiting to be discovered at the site. More in-depth studies are underway in the Churince system, which include a better sampling of soil and plant rhizosphere as well as metagenomics and transcriptomics of the lagoon sediments.
Another particular characteristic of this oasis soil is that, while previous studies have suggested an unequal distribution of denitrifying nirK or nirS genes (Jones and Hallin, 2010), which, in our study, showed different patterns, we observed a similar distribution of these denitrification genes, with one single group dominating the dry site while in the humid site there are more different groups with the potential to do this function. However, Braker et al. (2000) screened 46 clones from marine sediment samples of the North Pacific by using restriction fragment length polymorphism and found that the frequency of restriction patterns for nirK and nirS were also equally distributed. Our evidence, along with the molecular evidence from microbialite metagenomics (Bonilla-Rosso et al., 2012 in this issue; Peimbert et al., 2012 in this issue) and comparative genomics (Moreno-Letelier et al., 2012 in this issue), points toward an ancient marine ecology (Souza et al., 2006, 2012 in this issue).
5. Concluding Remarks and Perspectives
The present study of the soil microbial community gives us a glimpse of the microbial life of the CCB and highlights the need to avoid further desiccation of this unique and unusual oasis. In particular, the dry site in our investigation has a lower nutrient availability, lower species diversity, and lower functional diversity for the N cycle, which could make it more fragile to water loss. In a more recent survey of many soil sites in this gypsum-based ecosystem, we observed a fast degradation of the ecosystem as the loss of water is accelerated due to the over-exploitation of the aquifer (V. Souza and F. Garcia Oliva, personal communication). Given the extraordinary diversity and divergence of the site, this degradation alerts us to the loss of invaluable genetic resources and diversity. From the astrobiological perspective, the fact that this gypsum analogue to Gale Crater on Mars is so diverse taxonomically and functionally indicates that, on Earth, life not only has persisted in nearly all types of environments but has also transformed the limits of habitability with its metabolism. In the particular case of the CCB, the survival of ancient marine microorganisms in an isolated valley in the middle of the North American continent demonstrates the resilience of life on Earth. These microbial communities survived several catastrophes that led to global extinctions and marked the geological eras that are distinguished across the sedimentary layers of our planet.
Obviously, we do not expect such biological richness on Mars, given the nature of the martian atmosphere and its proportions of N and C relative to O. However, with the martian sea having left its imprint in the deposition of sediments over geological time in the central mount of Gale Crater, the Mars Science Laboratory will be able to trace the change in chemical stoichiometry as a biosignature with regard to martian eras. On the other hand, the great depth of impact craters, such as Gale Crater, may allow the breaching of local aquifers and thus in theory provide a source of water for lakes and hydrothermal systems (Newsom et al., 2001; Szynkiewicz et al., 2010). Nevertheless, if life exists or has existed in Gale Crater, it is very possible that it left a biosignature imprint in the gypsum crystals (Edwards et al., 2005). In this case, a firm understanding of an analogous region such as the CCB would be invaluable to the search for life on Mars.
Supplementary Material
Acknowledgments
We are grateful to Santiago Ramirez for his careful review of this manuscript; to Rodrigo González Chauvet, Ana E. Escalante, and Ana M. Noguez for the help during sample collection; to Rodrigo Velázquez-Durán and Maribel Nava-Mendoza for assisting with soil chemical analysis; to Laura Espinosa Asuar for help with molecular and sequencing work, and also to her and Erika Aguirre Planter for logistic support; to Heberto Ferreira and Alberto Valencia for assisting in data analyses. This study was funded by CONACyT 57507, CONACyT-Semarnat 2006-C01-23459, and WWF-Alianza Carlos Slim grants. N.E.L.-L. thanks the Instituto de Ecología UNAM, the Posgrado en Ciencias Biomédicas, and CONACyT (grant No 44810). V.S. and L.E.E. worked on this manuscript during their sabbatical leave, supported by grants by DGAPA (V.S. and L.E.E.) and UC-Mexus (L.E.E.).
Abbreviations
CCB, Cuatro Ciénegas Basin; DOC, dissolved organic carbon; DON, dissolved organic nitrogen; DOP, dissolved organic phosphorous; IDP, inorganic dissolved phosphorous; OTUs, operational taxonomic units; TN, total nitrogen; TOC, total organic carbon; TP, total phosphorus.
References
- Aguilera L.E. Gutierrez J.R. Meserve P.L. Variation in soil micro-organisms and nutrients underneath and outside the canopy of Adesmia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average rainfall. J Arid Environ. 1999;42:61–70. [Google Scholar]
- Bååth E. Anderson T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem. 2003;35:955–963. [Google Scholar]
- Banfield J.F. Moreau J.W. Chan C.S. Welch S.A. Little B. Mineralogical biosignatures and the search for life on Mars. Astrobiology. 2001;1:447–465. doi: 10.1089/153110701753593856. [DOI] [PubMed] [Google Scholar]
- Bonilla-Rosso G. Peimbert M. Alcaraz L.D. Hernández I. Eguiarte L.E. Olmedo-Alvarez G. Souza V. Comparative metagenomics of two microbial mats at Cuatro Ciénegas Basin II: community structure and composition in oligotrophic environments. Astrobiology. 2012;12:659–673. doi: 10.1089/ast.2011.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart M. Hoare A. Nitti A. Siefert J. Haynes M. Dinsdale E. Edwards R. Souza V. Rohwer F. Hollander D. Metagenomic and stable isotopic analyses of modern freshwater microbialites in Cuatro Ciénegas, Mexico. Environ Microbiol. 2009;11:16–34. doi: 10.1111/j.1462-2920.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Bremmer J.M. Mulvaney C.S. Nitrogen-total. In: Page A., editor; Miller R.H., editor; Keeney D.R., editor. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. American Society of Agronomy; Madison, WI: 1982. pp. 595–624. [Google Scholar]
- Braker G. Zhou J. Wu L. Devol A.H. Tiedje J.M. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific Northwest marine sediment communities. Appl Environ Microbiol. 2000;66:2096–2104. doi: 10.1128/aem.66.5.2096-2104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P. Landman A. Pruden G. Jenkinson D. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem. 1985;17:837–842. [Google Scholar]
- Butler C. Richardson D. The emerging molecular structure of the nitrogen cycle: an introduction to the proceedings of the 10th annual N-cycle meeting. Biochem Soc Trans. 2005;33:113–118. doi: 10.1042/BST0330113. [DOI] [PubMed] [Google Scholar]
- Cary S.C. McDonald I.R. Barrett J.E. Cowan D.A. On the rocks: the microbiology of Antarctic Dry Valley soils. Nat Rev Microbiol. 2010;8:129–138. doi: 10.1038/nrmicro2281. [DOI] [PubMed] [Google Scholar]
- Cerritos R. Eguiarte L.E. Avitia M. Siefert J. Travisano M. Rodríguez-Verdugo A. Souza V. Diversity of culturable thermo-resistant aquatic bacteria along an environmental gradient in Cuatro Ciénegas, Coahuila, México. Antonie van Leeuwenhoek. 2011;99:303–318. doi: 10.1007/s10482-010-9490-9. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.P. Schmid M. Hartmann A. Tripathi A.K. Diversity of 16S-rRNA and nifH genes derived from rhizosphere soil and roots of an endemic drought tolerant grass, Lasiurus sindicus. European Journal of Soil Biology. 2009;45:114–122. [Google Scholar]
- Connon S.A. Lester E.D. Shafaat H.S. Obenhuber D.C. Ponce A. Bacterial diversity in hyperarid Atacama Desert soils. J Geophys Res. 2007;112:1–9. [Google Scholar]
- Cookson W.R. Müller C. O'Brien P.A. Murphy D.V. Grierson P.F. Nitrogen dynamics in an Australian semiarid grassland soil. Ecology. 2006;87:2047–2057. doi: 10.1890/0012-9658(2006)87[2047:ndiaas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z. Hugenholtz P. Larsen N. Rojas M. Brodie E.L. Keller K. Huber T. Dalevi D. Hu P. Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnues C. Rodriguez-Brito B. Rayhawk S. Kelley S. Tran T. Haynes M. Liu H. Furlan M. Wegley L. Chau B. Ruan Y. Hall D. Angly F.E. Edwards R.A. Li L. Thurber R.V. Reid R.P. Siefert J. Souza V. Valentine D.L. Swan B.K. Breitbart M. Rohwer F. Biodiversity and biogeography of phages in modern stromatolites and thrombolites. Nature. 2008;452:340–343. doi: 10.1038/nature06735. [DOI] [PubMed] [Google Scholar]
- Devol A.H. Direct measurement of nitrogen gas fluxes from continental shelf sediments. Nature. 1991;349:319–321. [Google Scholar]
- Dong H. Rech J.A. Jiang H. Sun H. Buck B.J. Endolithic cyanobacteria in soil gypsum: occurrences in Atacama (Chile), Mojave (United States), and Al-Jafr Basin (Jordan) Deserts. J Geophys Res. 2007;112:1–11. [Google Scholar]
- Drees K.P. Neilson J.W. Betancourt J.L. Quade J. Henderson D.A. Pryor B.M. Maier R.M. Bacterial community structure in the hyperarid core of the Atacama Desert, Chile. Appl Environ Microbiol. 2006;72:7902–7908. doi: 10.1128/AEM.01305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H.G.M. Jorge-Villar S.E. Parnell J. Cockell C.S. Lee P. Spectroscopic analysis of cyanobacterial gypsum halotrophs and relevance for sulfate deposits on Mars. Analyst. 2005;130:917–923. doi: 10.1039/b503533c. [DOI] [PubMed] [Google Scholar]
- Elser J.J. Schampel J.H. Garcia-Pichel F. Wade B.D. Souza V. Eguiarte L. Escalante A. Farmer J.D. Effects of phosphorus enrichment and grazing snails on modern stromatolitic microbial communities. Freshw Biol. 2005;50:1808–1825. [Google Scholar]
- Escalante A.E. Eguiarte L.E. Espinosa-Asuar L. Forney L.J. Noguez A.M. Souza-Saldivar V. Diversity of aquatic prokaryotic communities in the Cuatro Cienegas Basin. FEMS Microbiol Ecol. 2008;65:50–60. doi: 10.1111/j.1574-6941.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- Fierer N. Bradford M.A. Jackson R.B. Toward an ecological classification of soil bacteria. Ecology. 2007;88:1354–1364. doi: 10.1890/05-1839. [DOI] [PubMed] [Google Scholar]
- Gendrin A. Mangold N. Bibring J.P. Langevin Y. Gondet B. Poulet F. Bonello G. Quantin C. Mustard J. Arvidson R. LeMouelic S. Sulfates in martian layered terrains: the OMEGA/Mars Express view. Science. 2005;307:1587–1591. doi: 10.1126/science.1109087. [DOI] [PubMed] [Google Scholar]
- Gundlapally S.R. Garcia-Pichel F. The community and phylogenetic diversity of biological soil crusts in the Colorado Plateau studied by molecular fingerprinting and intensive cultivation. Microb Ecol. 2006;52:345–357. doi: 10.1007/s00248-006-9011-6. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hallin S. Lindgren P.E. PCR detection of genes encoding nitrite reductase in denitrifying bacteria. Appl Environ Microbiol. 1999;65:1652–1657. doi: 10.1128/aem.65.4.1652-1657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada S. Pierson B. Stackebrandt E. The family Chloroflexaceae. In: Dworkin M., editor; Falkow S., editor; Rosenberg E., editor; Schleifer K.H., editor; The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community. 3rd. Springer; New York: 2006. pp. 815–842. [Google Scholar]
- Heffernan J.B. Sponseller R.A. Fisher S.G. Consequences of a biogeomorphic regime shift for the hyporheic zone of a Sonoran Desert stream. Freshw Biol. 2008;53:1954–1968. [Google Scholar]
- Hiraishi A. Genus Porphyrobacter. In: Imhoff J.F., editor; Brenner D.J., editor; Krieg N.R., editor; Staley J.T., editor. Bergey's Manual of Systematic Bacteriology, the Alpha-, Beta-, Delta- and Epsilonproteobacteria. Springer Science and Business Media Inc.; New York: 2005. pp. 275–279. [Google Scholar]
- Holben W.E. Jansson J.K. Chelm B.K. Tiedje J.M. DNA probe method for the detection of specific microorganisms in the soil bacterial community. Appl Environ Microbiol. 1988;54:703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman E.W.D. Performance of a new automatic carbon dioxide coulometer. Microchem J. 1977;22:567–573. [Google Scholar]
- Izawa M.R.M. Banerjee N.R. Osinski G.R. Flemming R.L. Parnell J. Cockell C.S. Weathering of post-impact hydrothermal deposits from the Haughton impact structure: implications for microbial colonization and biosignature preservation. Astrobiology. 2011;11:537–550. doi: 10.1089/ast.2011.0612. [DOI] [PubMed] [Google Scholar]
- Janssen P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol. 2006;72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joergensen R.G. The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem. 1996;28:25–31. [Google Scholar]
- Joergensen R.G. Mueller T. The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem. 1996;28:33–37. [Google Scholar]
- Jones C.M. Hallin S. Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J. 2010;4:633–641. doi: 10.1038/ismej.2009.152. [DOI] [PubMed] [Google Scholar]
- Kindt R. Coe R. Tree Diversity Analysis. A Manual and Software for Some Common Statistical Methods for Ecological and Biodiversity Studies. World Agroforestry Centre (ICRAF); Nairobi: 2005. [Google Scholar]
- Larkin M.A. Blackshields G. Brown N.P. Chenna R. McGettigan P.A. McWilliam H. Valentin F. Wallace I.M. Wilm A. Lopez R. Thompson J.D. Gibson T.J. Higgins D.G. Clustal W and CLustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Letunic I. Bork P. Interactive Tree Of Life (iTOL): an on line tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- Marti K. Kerridge J. Nitrogen in the Solar System. Science. 2010;328:1112–1113. doi: 10.1126/science.1189836. [DOI] [PubMed] [Google Scholar]
- Moreno-Letelier A. Olmedo G. Eguiarte L.E. Souza V. Divergence and phylogeny of Firmicutes from the Cuatro Ciénegas Basin, Mexico: a window to an ancient ocean. Astrobiology. 2012;12 doi: 10.1089/ast.2011.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño N.M. García-Oliva F. Jaramillo V.J. Dissolved organic carbon affects soil microbial activity and nitrogen dynamics in a Mexican tropical deciduous forest. Plant Soil. 2007;295:265–277. [Google Scholar]
- Murphy J. Riley J.P. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- Nagy M.L. Pérez A. Garcia-Pichel F. The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ) FEMS Microbiol Ecol. 2005;54:233–245. doi: 10.1016/j.femsec.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Newsom H.E. Hagerty J.J. Thorsos I.E. Location and sampling of aqueous and hydrothermal deposits in martian impact craters. Astrobiology. 2001;1:71–88. doi: 10.1089/153110701750137459. [DOI] [PubMed] [Google Scholar]
- Oksanen J. Blanchet F.G. Kindt R. Legendre P. O'Hara R.B. Simpson G.L. Solymos P. Stevens M.H.H. Wagner H. Vegan—Community Ecology Package. R package version 1.17-0. 2010. http://CRAN.R-project.org/package=vegan http://CRAN.R-project.org/package=vegan
- Peimbert M. Alcaraz L.D. Bonilla-Rosso G. Olmedo-Alvarez G. García-Oliva F. Segovia L. Eguiarte L.E. Souza V. Comparative metagenomics of two microbial mats at Cuatro Ciénegas Basin I: ancient lessons on how to cope with an environment under severe nutrient stress. Astrobiology. 2012;12:648–658. doi: 10.1089/ast.2011.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointing S.B. Chan Y. Lacap D.C. Lau M.C. Jurgens J. Farrell R.L. Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA. 2009;106:19964–19969. doi: 10.1073/pnas.0908274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2011. R: a language and environment for statistical computing. [Google Scholar]
- Ríos A. Valea S. Ascaso C. Kastovsky J. McKay C.P. Wierzchos J. Comparative analysis of the microbial communities inhabiting halite evaporites of the Atacama Desert. Int Microbiol. 2010;13:79–89. doi: 10.2436/20.1501.01.113. [DOI] [PubMed] [Google Scholar]
- Schlesinger W.H. Raikes J.A. Hartley A.E. Cross A.F. On the spatial pattern of soil nutrients in desert ecosystems. Ecology. 1996;77:364–374. [Google Scholar]
- Schloss P.D. Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D. Westcott S.L. Ryabin T. Hall J.R. Hartmann M. Hollister E.B. Lesniewski R.A. Oakley B.B. Parks D.H. Robinson C.J. Sahl J.W. Stres B. Thallinger G.G. Van Horn D.J. Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton D.R. Furlong M.A. Rathbun S.L. Whitman W.B. Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol. 2001;67:4374–4376. doi: 10.1128/AEM.67.9.4374-4376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V. Espinosa-Asuar L. Escalante A.E. Eguiarte L.E. Farmer J. Forney L. Lloret L. Rodríguez-Martínez J.M. Soberón X. Dirzo R. Elser J.J. An endangered oasis of aquatic microbial biodiversity in the Chihuahuan Desert. Proc Natl Acad Sci USA. 2006;103:6565–6570. doi: 10.1073/pnas.0601434103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V. Siefert J. Escalante A.E. Elser J. Eguiarte L.E. The Cuatro Ciénegas Basin in Coahuila, Mexico: an astrobiological Precambrian Park. Astrobiology. 2012;12:641–647. doi: 10.1089/ast.2011.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponseller R.A. Fisher S.G. The influence of drainage networks on patterns of soil respiration in a desert catchment. Ecology. 2008;89:1089–1090. doi: 10.1890/06-1933.1. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Phylogenetic Analysis Using Parsimony (*and other methods) ver. 4. Sinauer Associates; Sunderland, MA: 2003. PAUP*. [Google Scholar]
- Szynkiewicz A. Ewing R.C. Moore C.H. Glamoclija M. Bustos D. Pratt L.M. Origin of terrestrial gypsum dunes—implications for martian gypsum-rich dunes of Olympia Undae. Geomorphology. 2010;121:69–83. [Google Scholar]
- Throbäck I.N. Enwall K. Jarvis A. Hallin S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol. 2004;49:401–417. doi: 10.1016/j.femsec.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Vance E. Brookes P. Jenkinson D. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987;19:703–707. [Google Scholar]
- Vitousek P.M. Hawai'i as a Model System. Princeton University Press; Princeton, NJ: 2002. Nutrient Cycling and Limitation. [Google Scholar]
- Walvoord M.A. Phillips F.M. Stonestrom D.A. Evans R.D. Hartsough P.C. Newman B.D. Striegl R.G. A reservoir of nitrate beneath desert soils. Science. 2003;302:1021–1024. doi: 10.1126/science.1086435. [DOI] [PubMed] [Google Scholar]
- Wilmotte A. Van der Auwera G. De Wachter R. Structure of the 16 S ribosomal RNA of the thermophilic cyanobacterium chlorogloeopsis HTF (‘Mastigocladus laminosus HTF’) strain PCC7518, and phylogenetic analysis. FEBS Lett. 1993;317:96–100. doi: 10.1016/0014-5793(93)81499-p. [DOI] [PubMed] [Google Scholar]
- Yeager C.M. Kornosky J.L. Housman D.C. Grote E.E. Belnap J. Kuske C.R. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Appl Environ Microbiol. 2004;70:973–983. doi: 10.1128/AEM.70.2.973-983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef N.H. Elshahed M.S. Diversity rankings among bacterial lineages in soil. ISME J. 2009;3:305–313. doi: 10.1038/ismej.2008.106. [DOI] [PubMed] [Google Scholar]
- Zani S. Mellon M.T. Collier J.L. Zehr J.P. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl Environ Microbiol. 2000;66:3119–3124. doi: 10.1128/aem.66.7.3119-3124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J.P. Kudela R.M. Nitrogen cycle of the open ocean: from genes to ecosystems. Ann Rev Mar Sci. 2011;3:197–225. doi: 10.1146/annurev-marine-120709-142819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







