Abstract
This is the second of 2 articles that discuss the biology and pathophysiology of wound healing, reviewing the role that growth factors play in this process and describing the current methods for growth factor delivery into the wound bed.
Keywords: acute wound healing, drug delivery, growth factors, wound healing
The first part of this article published in the July issue discussed the biology of acute and chronic wound healing and covered modern approaches to wound bed preparation and infection control. Thorough wound bed preparation can, in some cases, be sufficient to induce proper cellular responses and healing of complicated wounds. Often, however, because such wounds may become chronic, specific additional therapies may be necessary. Since the 1980s, the application of growth factors to the chronic wound bed has been considered as one such “specific” therapy. Currently, the only Food and Drug Administration (FDA)–approved formulation of this type for treatment of chronic wounds is becaplermin (Regranex; Healthpoint Biotherapeutics, Fort Worth, Texas), containing recombinant human platelet-derived growth factor (PDGF). Several other growth factors are currently under investigation as treatment modalities in wound care. This article reviews the current state of knowledge regarding the utility and mechanisms of action for growth factor–dependent wound healing therapeutic approaches. In addition, the methods that can be used for delivery of growth factors into the chronic and acute wound bed are discussed.
PLATELET-DERIVED GROWTH FACTOR FAMILY
Platelet-derived growth factor (Figure 1, Table 1) is one of the first factors produced in response to injury and induces cellular responses throughout all phases of the repair process. Platelet-derived growth factor is multifunctional and is released by platelets, macrophages, keratinocytes, fibroblasts, and endothelial cells.1 Currently, 4 PDGF isoforms are known, including PDGF-A, PDGF-B, PDGF-C, and PDGF-D. Platelet-derived growth factors A and B can form both heterodimers and homodimers and are secreted in their active form, whereas PDGF-C and -D are homodimer-forming species and require extracellular activation.2 Platelet-derived growth factor family members exert their activity on cells after binding to a complementary family of receptor tyrosine kinases—PDGFR-αα, PDGFR-αβ, and PDGF-ββ. Ligand binding followed by homodimerization or heterodimerization of PDGFR and their phosphorylation triggers several signaling pathways, including phospholipase Cγ pathway, phosphatidylinositol-3-kinase (PI3K), and a number of mitogen-activated protein kinase (MAPK) pathways.3,4 Activation of these pathways by PDGF generally leads to enhanced cellular migration and proliferation and causes increased vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) production. Importantly, enhanced growth factor receptor expression and extracellular matrix (ECM) (fibronectin, hyaluronic acid) production ensue.1,5 It should be noted that, under certain conditions, PDGF-α can have a negative impact on cell motility, while retaining its promitogenic effect.3,6 Platelet-derived growth factor receptors α and β are normally expressed by several cell types at low levels; however, in response to injury and in the presence of exogenous growth factors, such as epidermal growth factor (EGF) and PDGF-AB, receptor expression is markedly up-regulated. This up-regulation of PDGFR and its phosphorylation appear to be necessary for timely healing, whereas uncontrolled PDGF signaling may play a role in hypertrophic scarring.1,6,7
Figure 1. SIMPLIFIED REPRESENTATION OF PDGF SIGNALING CASCADE.
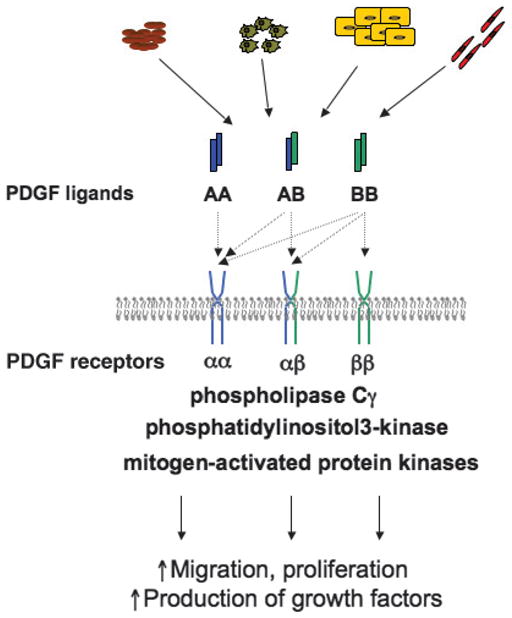
Several cell types—platelets, macrophages, keratinocytes, and endothelial cells—produce ligands—PDGF-AA, PDGF-AB, and PDGF-BB during wound healing. These ligands interact with 1 or several of 3 types of PDGF receptors: PDGFR-αα, PDGFR-αβ, and PDGFR-ββ. PDGF-AA can activate only PDGFR-αα; PDGF-AB can interact with both PDGFR-αα and PDGFR-αβ, whereas PDGF-BB activates all 3 receptor kinds. This specificity of ligand-receptor interactions is shown with dotted arrows. Receptor-ligand interactions, receptor dimerization, and phosphorylation lead to activation of phospholipase Cγ, phosphatidy-linositol-3-kinase, and mitogen-activated kinase pathways, which in turn induce increased cellular migration, proliferation, and growth factor production.
Table 1.
ROLE OF GROWTH FACTORS IN CELLULAR RESPONSES TO INJURY AND THEIR CLINICAL USE
| Growth Factor | Sources | Target Cells | Clinical Use |
|---|---|---|---|
| PDGF | Platelets, macrophages, endothelial cells | Neutrophils, macrophages, smooth muscle cells, endothelial cells, fibroblasts | PDGF-BB is FDA approved for treatment of diabetic ulcers |
| FGF-1 | Macrophages, endothelial cells | Endothelial cells, fibroblasts, smooth muscle cells, keratinocytes | In clinical trials for spinal cord injuries, burns25,182 |
| FGF-2 | Fibroblasts, endothelial cells, inflammatory cells | Endothelial cells, fibroblasts, and keratinocytes | In clinical trials for chronic wounds4 |
| FGF-7 | Fibroblasts | Endothelial cells, keratinocytes | FDA approved for treatment of oral mucositis14; in clinical trials for treatment of venous ulcers26 |
| VEGF-A | Fibroblasts, keratinocytes, smooth muscle cells, macrophages, platelets | Endothelial cells, keratinocytes | In clinical trials for limb ischemia, coronary ischemia183,184 |
| EGF | Platelets, macrophages, endothelial cells, smooth muscle cells (HB-EGF), keratinocytes | Keratinocytes, fibroblasts, endothelial cells | Early clinical trials, not approved53 |
| TGF-β1-β3 | Platelets, macrophages, fibroblasts, keratinocytes | Monocytes, macrophages, neutrophils, keratinocytes, fibroblasts | TGF-β3 is in clinical trials for pressure ulcers185; avotermin (rh-TGF-β3) is in trials for treatment of excessive scarring186 |
Abbreviations: EGF, epidermal growth factor; FDA, Food and Drug Administration; FGF, fibroblast growth factor; HB, heparin-binding; PDGF, platelet-derived growth factor; rh, recombinant human; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
The levels of PDGF and PDGF receptor expression have been shown to be low in diabetic and aged mice that have a delayed response to injury.1 Similarly, PDGF levels are depressed within nonhealing human ulcers,8 perhaps because of both underproduction and/or excessive protease-mediated degradation. Therefore, the delivery of exogenous PDGF was investigated and found to be beneficial for those patients with chronic wounds. As a result, in 1997, the FDA granted approval of becaplermin containing PDGF-BB for the treatment of diabetic ulcers.9 Despite this approval, the outcomes of treating diabetic ulcers with this PDGF-containing preparation have been found to be less than convincing or inconclusive. Moreover, it has been demonstrated that excessive use of this ointment may lead to increased risk of cancer.10 It may be that nonresponsiveness to PDGF treatment, observed in some patients, is due to low expression levels of PDGF receptors by cells residing within chronic wounds,11 or it could be caused by rapid degradation of the growth factor by proteolytic enzymes within the chronic wound bed.12 Also confounding the use of PDGF is the fact that epithelial cells within chronic wounds lack PDGF receptors. Moreover, the complexity and persistence of the chronic wound bed suggest that delivery of a single entity as a corrective therapeutic may not be sufficient. A combination and/or patient-specific approach to chronic wound treatment might be required to enable optimal healing and wound closure.13,14 Therefore, improved outcomes of PDGF and other growth factor–based therapies will undoubtedly come with better understanding of cellular and molecular abnormalities occurring in chronic wounds and/or with development of better drug delivery methods, which are discussed in the following section.
FIBROBLAST GROWTH FACTOR
The fibroblast growth factor (FGF) (Figure 2, Table 2) family includes 23 members. Fibroblast growth factors 1, 2, 7, 10, and 22 are expressed upon dermal injury.4 The biology of these paracrine growth factors has recently been reviewed.15 After their liberation from the ECM, FGF ligands bind and activate FGF receptors (FGFRs) in a heparan sulfate proteoglycan (HSPG)–dependent manner. Receptor-ligand interaction induces receptor dimerization and transphosphorylation, leading to activation of downstream signaling including Ras/MAPK and PI3K/Akt pathways.15
Figure 2. FGF SIGNALING AND WOUND HEALING.
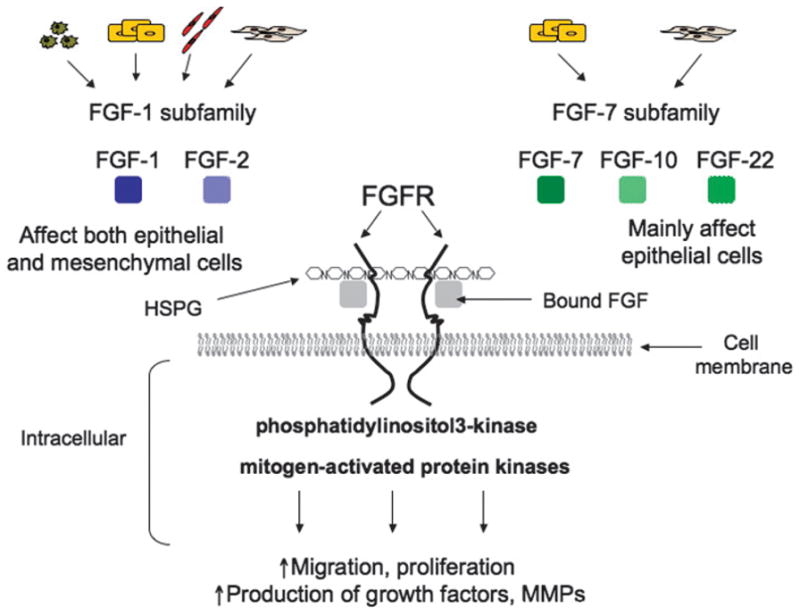
Five FGF family members are expressed upon dermal injury. Fibroblast growth factors 1 and 2 belong to FGF-1 subfamily and are produced by macrophages, keratinocytes, endothelial cells, and fibroblasts to stimulate both epithelial and mesenchymal cells. Members of FGF-7 subfamily (FGF-2, FGF-10, and FGF-22) mainly affect epithelial cells. Interactions between FGF ligands and FGF receptor lead to receptor dimerization and are enhanced by heparan sulfate proteoglycan (HSPG). Receptor-ligand interactions lead to activation of phosphatidylinositol-3-kinase and mitogen-activated kinase pathways and induce an increase in cell migration, proliferation, and production of growth factors and matrix remodeling enzymes, including MMPs.
Table 2.
GENE DELIVERY INTO THE WOUND BED
| DNA Carriers | Delivery Mode | Growth Factors Tested | Outcome |
|---|---|---|---|
| Plasmids | “Gene gun” | PDGF | Increased wound tensile strength164 |
| EGF | Increased epithelialization, granulation tissue165 | ||
| Injection via microneedles | EGF | Increase in transgene expression, effects on wound healing are not reported167 | |
| Electroporation | FGF-7 | Increased wound epithelialization169 | |
| VEGF | Improved skin flap healing170 | ||
| Liposomal formulations | FGF-1 | Accelerated wound closure171 | |
| IGF/KGF | Increased collagen deposition, VEGF production, improved healing173 | ||
| Viral delivery | |||
| Adenoviruses | Topical application | VEGF | Improved angiogenesis and wound healing174 |
| “Gene gun” | Erb3 + topical EGF | Improved epithelialization64 | |
| Injection via microneedles | VEGF | No effect on wound healing168 | |
| Adeno-associated viruses | Intradermal injection | VEGF | Increased angiogenesis and rate of wound healing176,177 |
| Retroviruses | Ex vivo cell transfection followed by transplantation into animals | PDGF | Increase epithelial thickness and angiogeneis187 |
Abbreviations: EGF, epidermal growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor.
Fibroblast growth factors 1 and 2, also known as acidic and basic FGF, respectively, are produced by inflammatory cells, vascular endothelial cells, fibroblasts, and keratinocytes. They play roles in re-epithelialization, angiogenesis, and granulation tissue formation.1,16 Fibroblast growth factor 2 also stimulates production of ECM- and matrix-degrading enzymes, thus contributing to matrix synthesis and remodeling, which is critical for normal wound healing.17 Fibroblast growth factors 7, 10, and 22 are expressed by fibroblasts and proliferating keratinocytes.18 These factors are mitogenic and motogenic for keratinocytes and induce enzymes important for nucleotide synthesis, as well as production of matrix metalloproteinases (MMPs).19 In addition to their direct role in wound healing, FGF-7 and FGF-10 stimulate production of transforming growth factor α (TGF-α) and other ErbB ligands by dermal keratinocytes, thus contributing to epithelialization.19 Fibroblast growth factor 7 subfamily members also have cytoprotective effects and up-regulate reactive oxygen species–protective (antioxidant) enzymes, such as peroxiredoxin VI, as well as reduce the levels of inflammatory mediators induced by the injury.15,18 Finally, FGF-7 has been shown to increase production of VEGF, MMP-9, and urokinase plasminogen activator (uPA) by several tumor types possibly contributing to cancer-induced angiogenesis.20,21 More work will be required to reveal whether FGF-7 can indirectly contribute to angiogenesis during repair of normal tissue.
It is generally accepted that FGF-FGFR–mediated signaling is impaired in chronic wounds.4 Clinically, both a decrease in FGF production and increase in its sequestration by an inhibitory heparan sulfate present in chronic wound fluid have been observed.22,23 In animal models of delayed wound healing (diabetic and aged animals), abnormal expression of FGF-1, FGF-2, and FGF-7 and diminished expression of FGFRs have been reported, and exogenous FGFs have been successfully used to improve tissue repair.24,25 These observations led to development of a number of clinical trials. Fibroblast growth factors 1 and 2 have been used for treatment of chronic wounds and burns, with only modest improvements in healing rates being observed.4,26 Fibroblast growth factor 7, which currently is FDA approved for treatment of oral mucositis,15 was shown to enhance the repair of venous ulcers in a phase 2A clinical trial,27 but failed to increase the percentage of wounds fully healed within the 20 weeks of the study.28 This failure has been attributed to insufficient retention of the growth factors within the wound bed, which could be significantly improved using advanced delivery methods such as growth factor–containing biodegradable dressings described in the following section.
VASCULAR ENDOTHELIAL GROWTH FACTOR
The VEGF family (Figure 3, Table 1) includes 6 members—placental growth factor (PLGF), VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E. Vascular endothelial growth factors are heparin-binding glycoproteins and exert their functions after binding to several cell-surface tyrosine kinase receptors VEGFR1, VEGFR2, and VEGFR3, with VEGFR-1 and VEGFR-2 mainly mediating angiogenesis and VEGFR-3 important for lymphangiogenesis.29 Novel VEGF receptors known as neuropilins may also be involved in wound-healing angiogenesis.30
Figure 3. VEGF, VEGF RECEPTORS, AND THE CELLULAR RESPONSES TO INJURY.
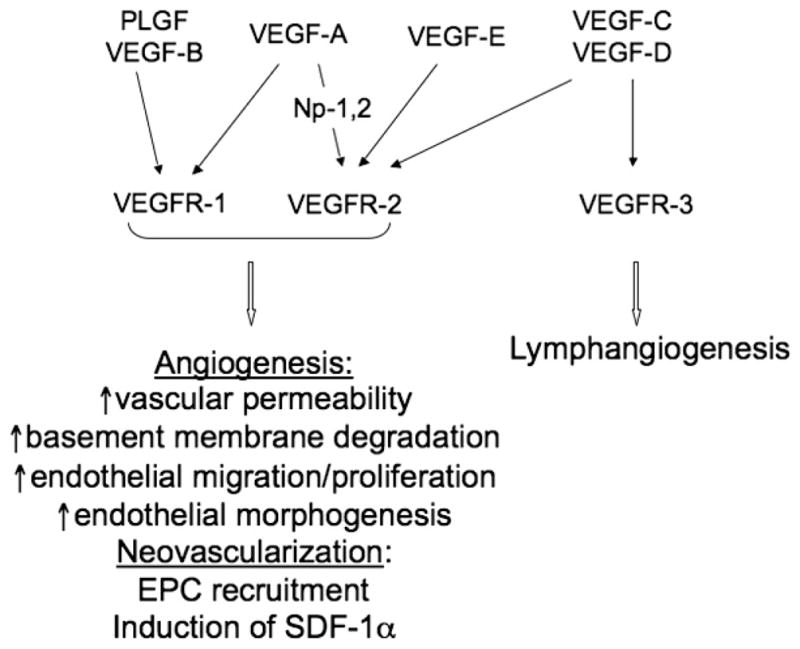
Cellular effects of the members of VEGF family are mediated by 3 VEGF receptors—VEGFR-1, VEGFR-2, and VEGFR-3. Activation of VEGFR-1 and VEGFR-2 mainly induces wound-healing angiogenesis and neovascularization, whereas VEGFR-3 is important for lymphangiogenesis. Ligand-receptor interactions and their specificities are shown with arrows. Note the interaction between VEGF-A and VEGF-R2 is enhanced in the presence of coreceptors neuropilins 1 and 2 (Np-1,2).
Although expression of VEGF family members in normal skin is negligible, in response to injury-induced hypoxia their production is markedly up-regulated. In addition to hypoxia, several growth factors, including TGF-β1, FGF-2, and PDGF-BB, are important inducers of VEGF.4,31
During wound healing, platelets, macrophages, fibroblasts, and keratinocytes secrete VEGF where it acts in a paracrine manner on endothelial cells, inducing and supporting wound angiogenesis.1 Vascular endothelial growth factor receptors 1 and 2 activation by VEGF triggers multiple events required for successful angiogenesis during injury repair. These include an increase in vascular permeability; degradation of the basement membrane by uPA and tissue-type plasminogen activators, MMP-1 and MMP-2; endothelial migration mediated by αvβ3, αvβ5, α1β1, and α2β1 integrin receptors and their ligands32,33; and proliferation of vascular cells within the wound bed.31 Vascular endothelial growth factor together with PLGF take part in mobilization of VEGFR-2–expressing endothelial progenitor cells (EPC) into the circulation.34 The mechanisms of VEGF/PLGF-mediated EPC homing to the wound site, however, remain unknown.
Other effects of VEGF family members include monocyte migration and activation35 and production of MMPs by smooth muscle cells, inducing their migration and proliferation during hypoxia,36–38 fibroblast proliferation and formation of scars,39 and keratinocyte motility required for wound re-epithelialization.31
In a similar manner to other growth factors, such as FGF-2, VEGF family members, particularly VEGF-A and VEGF-B, exist in an ECM-bound state.40–42 Vascular endothelial growth factor binding to tenascin-X both localizes and enhances VEGF stimulatory effects. Interestingly, tenascin-X,42 as well as tenascin-X–derived fragments,43 has proangiogenic properties, which may prove instrumental as enhancers of wound healing.
A number of studies performed with chronic wounds of different origin have shown both an increase in VEGF mRNA but a paradoxical decrease in VEGF protein levels because of augmented proteolytic activity observed within the wound bed.44 Additional disruption of VEGF signaling in chronic wounds may come from an increase in soluble VEGFR-1 observed in venous ulcers.45 Importantly, exogenous VEGF has been successfully used in animal studies46 and proposed for use in treatment of chronic wounds in humans. Recombinant human VEGF was well tolerated in a clinical phase 1 trial in patients with chronic diabetic foot ulcers but its outcomes as a therapeutic remain to be evaluated/established.47 However, results of the Phase II clinical trial were negative. The instability of VEGF in the protease-rich wound environment could be one of the reasons for its inefficiency. In turn, the use of biologically active and protease-resistant VEGF isoforms could potentially overcome this problem.48 Nonetheless, it should be recognized that VEGF, which was initially identified as a vascular permeability factor, has been shown to induce uncontrolled growth of nonfunctional vessels.49 Therefore, this growth factor alone may not be sufficient (or appropriate) for the formation of stable blood vessels necessary to successfully repair injury sites or chronic wound beds. If VEGF were combined with other enhancers, however, wound healing might be improved.
EPIDERMAL GROWTH FACTOR FAMILY
The EGF family (Figure 4, Table 1) includes several members, 4 of which—EGF, heparin-binding EGF-like growth factor (HB-EGF), epiregulin), and TGF-α—have been implicated in wound healing.1,50 Epidermal growth factor family members are synthesized in a membrane-bound form and require activation by MMPs or ADAMs (a disintegrin and metalloproteinase). In an autocrine, paracrine, or juxtacrine fashion, EGF family members bind and activate the ErbB (ErbB1-4) family of receptor tyrosine kinases. Ligand binding leads to homodimer and heterodimer formation, autophosphorylation of cytoplasmic tyrosine residues, and activation of intracellular signaling pathways, including Ras/MAPK, PLCγ/PKC, PI3K/Akt, and STAT.1,4,51
Figure 4. EGF SIGNALING AND WOUND-HEALING RESPONSES.
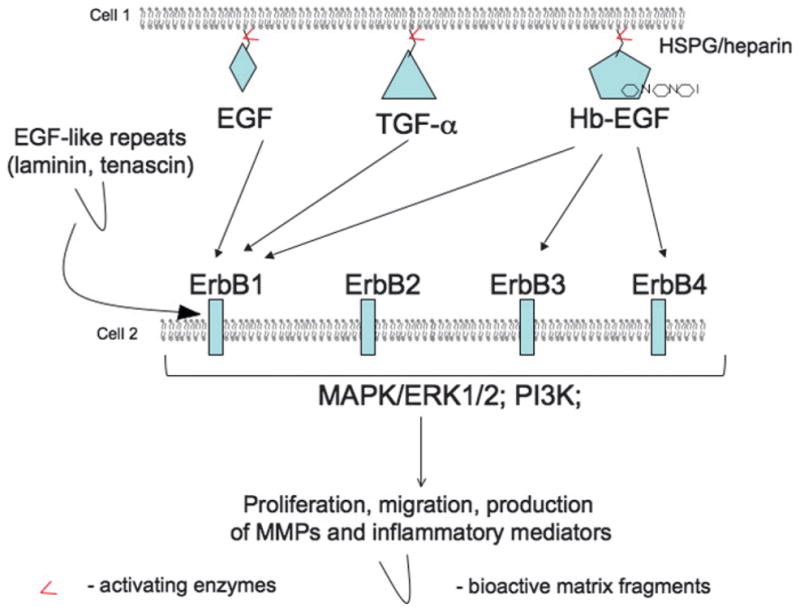
Members of the EGF family are synthesized in a membrane-bound form. Their release from a membrane and activation is performed by matrix metalloproteinases of MMP and ADAM families. Activated epidermal growth factors (EGFs)—EGF, transforming growth factor α (TGF-α), and heparin-binding EGF (HB-EGF)—interact with 1 or more Erb receptors (arrows). Note that Erb2 receptor does not bind EGF ligands and acts as a dimer-forming partner or a coreceptor. Ligand-receptor interactions initiate several signaling pathways, including mitogen-activated protein kinase pathways (MAPK), Erk1/2, and phosphatidylinositol-3-kinase leading to increase in post-injury migration, proliferation, and production of MMPs and inflammatory mediators. Interestingly, Erb receptors can be activated by matrix fragments that contain EGF-like repeats.
Transforming growth factor α is produced by platelets, macrophages, lymphocytes, and keratinocytes. Cleavage and activation of TGF-α are carried out by ADAM17. Cellular effects of TGF-α are mediated by the ErbB1 receptor present on keratinocytes, endothelial cells, and fibroblasts.52 In keratinocytes, TGF-α acts in both paracrine and autocrine manner to stimulate mainly migration and, to some extent, proliferation.53 The effects of TGF-α on fibroblasts and endothelial cells are virtually identical to those of EGF.54 As such, TGF-α–deficient mice have no defects in formation of granulation tissue and only slight abnormalities in epithelialization.1
Epidermal growth factor is secreted by platelets, macrophages, fibroblasts, and bone marrow–derived mesenchymal stem cells.4,55 It is synthesized in an inactive membrane-bound form and has to be cleaved by ADAM10 and possibly other yet unknown proteases to exert its functions.52,56 Epidermal growth factor is a potent stimulator of epithelialization, angiogenesis, fibroblast proliferation, and survival.54 Cellular effects of EGF are mediated by the ErbB1 receptor. In keratinocytes, ErbB1 activation by EGF requires the presence of urokinase receptor and leads to an increase in cell proliferation, migration, and laminin (γ2 chain) deposition via activation of MAPK/ERK1/2 pathway.57 In endothelial cells, EGF (as well as HB-EGF) signals via PI3K and MAPK pathways to induce migration and vascular tube formation, but not proliferation.58 Finally, EGF effects on fibroblasts are mediated by PI3K-, Rac-, and ERK-dependent signaling pathways,59 which leads to an increase in MMP production and cell proliferation.
Heparin-binding EGF-like growth factor is another EGF family member that is important for wound healing.1 It is produced by macrophages, hematopoietic cells, endothelial cells, vascular smooth muscle cells, and keratinocytes. Expression of HB-EGF is regulated in a tissue-specific manner; in keratinocytes, it is induced by injury and stress and is mediated by p38 MAPK, PKC, Ras, and ERK.60 Activation of membrane-bound HB-EGF is achieved by metalloproteinases, such as MMP-3 and ADAM family members, particularly ADAM 9 and 17 as well as by cellular stress.61,62 Moreover, it has been shown that exogenous enzymes, particularly collagenase derived from Clostridium histolyticum, can also activate HB-EGF, possibly making it available to cells residing within the wound bed.63
Activated HB-EGF (also known as soluble HB-EGF) directly interacts with ErbB1, ErbB3, and ErbB4 and is a potent stimulator of keratinocyte migration and epithelialization.4,64,65 Heparin-binding EGF-like growth factor also activates PI3K, MAPK, and endothelial nitric oxide synthase in endothelial cells and promotes angiogenesis.58
An important distinction between HB-EGF and other family members described here (EGF and TGF-α) is its high affinity for heparin and HSPG. This binding modulates the activity of HB-EGF, and (at least in smooth muscle cells and Chinese hamster ovary cells) the interactions of HB-EGF with HSPGs are required for optimal receptor-ligand interactions and enhanced activity of EGF receptor.66,67 At present, it is not known whether HB-EGF–HB-EGF receptor interactions are dependent on the presence of heparin-like species or HSPGs and whether this association plays a pivotal role in regulating keratinocyte or endothelial cell function during the cellular responses to injury and wound healing.
Although activation of ErbB receptors generally occurs after specific ligand binding, some ErbB receptor functions are EGF-ligand independent. It has been shown that in cancer cells these receptors are activated after interactions with G protein–coupled receptors and integrins. Similarly, during wound healing, ERbB1 receptor–mediated keratinocyte responses may be independent of EGF-ErbB interactions.57,68 Furthermore, EGF-like repeats of ECM molecules tenascin C and laminin 332, both involved in repair processes, can bind and activate EGF receptors and stimulate fibroblast proliferation.69
The role of EGF family members in wound healing is not limited to direct effects on keratinocytes, fibroblasts, and endothelial cells. Many of these factors are potent inducers of inflammatory mediators and their receptors. For instance, TGF-α induces expression of several toll-like receptors (TLR5 and TLR9) and enhances TLR responses to their cognate ligands (bacterial flagellin and unmethylated bacterial DNA sequences), thus leading to an increase in production of antimicrobial peptides and the proinflammatory interleukin 8.53,70 Production of another important inflammatory mediator, nitric oxide produced by nitric oxide synthase, is also regulated by EGF and HB-EGF.53,71 Moreover, it has been shown in vitro that EGF and HB-EGF induce keratinocyte VEGF and fibroblast FGF-2 production.72,73 In summary, EGF family members are essential for all aspects of wound healing: They are important modulators of inflammatory responses, directly and indirectly stimulate re-epithelialization, and contribute to angiogenesis and granulation tissue formation. As such, EGFs are critical for normal injury and repair processes.
In chronic wounds, inadequate levels of EGF and EGFR have been observed.74 Because of this, exogenous EGF has been used in clinical trials for treatment of nonhealing wounds. Unfortunately, EGF did not lead to significant improvement of healing rates, perhaps because of MMP-mediated EGF degradation within the “hostile” chronic wound environment.75 Other reasons for the failure of exogenous EGF to improve injury repair include possible instability or inadequate expression of its receptors found in persistent wounds.74
TGF-β FAMILY
The TGF-β superfamily (Figure 5, Table 1) members play multiple regulatory roles in modulating wound healing responses16 and scarring.76 Although this family includes more than 30 members in mammals,77 so far only TGF-β1-3, bone morphogenetic proteins (BMPs), and the activins have been implicated in wound healing and therefore are discussed in detail.4,78
Figure 5. SCHEMATIC REPRESENTATION OF TGF-A–DEPENDENT SIGNAL TRANSDUCTION.
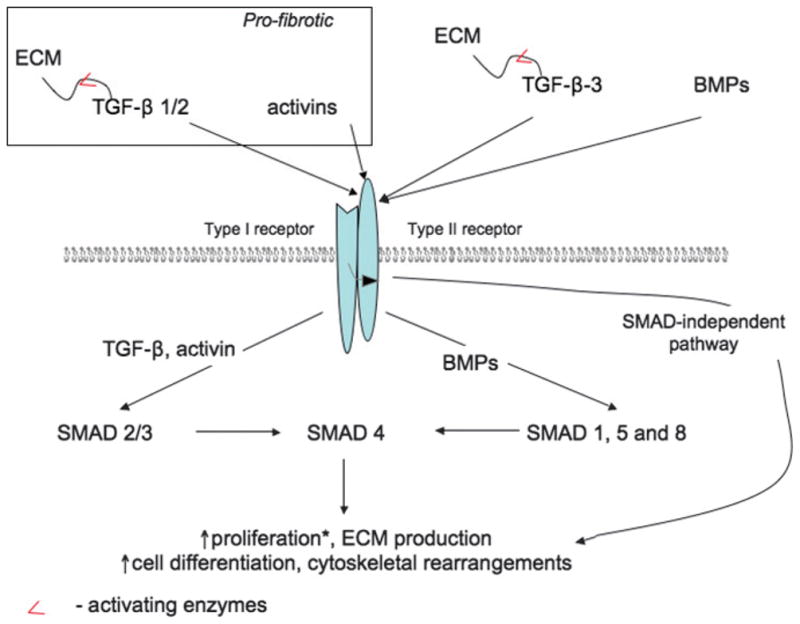
The members of the TGF-β family (TGF-β1, TGF-β2, TGF-β3, bone morphogenetic proteins [BMP], and activins) bind and activate TGF-β receptor type II, which then recruit (curved arrow) and transphosphorylate receptor type I. Following injury and during wound healing, ligand-receptor interactions lead to the activation of canonical (SMAD-mediated) or noncanonical pathways, which in turn induce changes in cell proliferation, matrix production, cell differentiation, and cytoskeletal rearrangements. The effects of TGF-β on cell proliferation (*) are cell type and growth factor concentration dependent.
Transforming growth factors β1, β12, and β13—the “first-discovered members” of the TGF-β family—are produced by a variety of cell types including macrophages, platelets, keratinocytes, and fibroblasts. With the exception of TGF-β1 that is produced by platelets in its active form, all TGF-β family members are generated in an inactive precursor form complex with latent TGF-binding proteins linked to ECM components. Activation of TGF-β is achieved by MMP-2, MMP-9, thrombospondin 1, and integrin αvβ6 together with membrane-type MMP.79 Typically, active TGF-β binds serine/threonine kinase receptor TβRII, which recruits and phosphorylates a related TβRI. After activation, the receptors trigger canonical SMAD (Sma and Mad–related proteins)–mediated and noncanonical signaling pathways leading to cytoskeletal rearrangements, induction of cell motility, and activation of transcriptional machinery.80
Transforming growth factors β1, β2, and β3 have overlapping but distinct functions during wound healing. All 3 are important for recruitment of the inflammatory cells and fibroblasts to the wound bed and facilitation of keratinocyte migration. Transforming growth factors β1 and β2 are prominent inducers of fibroblast-myofibroblast differentiation, ECM deposition, contraction, and scar formation, whereas TGF-β3 has been shown to inhibit scarring.4 The effects of TGF-β1 on cells depend on its concentration: Low levels of TGF-β1 stimulate en-dothelial proliferation and migration, and at high concentrations, it enhances matrix production.79
Bone morphogenetic proteins 1, 2, 4, 6, and 7 have been detected in normal skin, where they are involved in the maintenance of the stem cell niche within the hair follicles and regulate matrix assembly.79,81 Although BMPs (BMP-6, in particular) seem to be involved in keratinocyte differentiation, their role during the wound-healing process remains uncertain.4
Activins βA and βB have been implicated in wound healing. They are expressed by fibroblasts, endothelial cells, and keratinocytes and act in a paracrine manner, inducing keratinocyte differentiation and leading to an increase in matrix deposition by fibroblasts.78,82 Moreover, activins play a prominent role during fibrosis and are involved in formation of hypertrophic scars and keloids.83 Therefore, antiactivin and anti–TGF-β1-β2 therapies could be used to treat fibrotic wound-healing complications.
In chronic wounds, decreased levels of TGF-β ligands have been reported, suggesting that addition of exogenous TGF-βs may be beneficial for injury repair.84 However, numerous clinical trials using this growth factor to treat nonhealing wounds have failed.4 This apparent discrepancy can be due to several reasons. First, TGF-β signaling is extremely complex, and its effects are cell type and growth factor concentration dependent. Second, the TGF-β receptor density on cells residing within chronic wounds is decreased, affecting their ability to adequately respond to this growth factor.
The use of TGF-β–related proteins as wound healing therapeutics should be approached with great caution as they are implicated in the development of hypertrophic scars and keloids.76 Anti–TGF-β therapies, such as neutralizing antibodies,85 are now under investigation for treatment of these distressing conditions. More work needs to be done to fully reveal the potential of pro– and anti–TGF-β therapies.
Clearly, the growth factors discussed in this section of the review are indispensable for the normal wound-healing process. Therefore, many of them have been proposed, and one (PDGF) is currently used to treat both chronic and acute wounds and burns, although with limited success. Several considerations mitigate against the effective and safe use of these growth factors in treatment of wounds.
First, most of the current therapies do not take into consideration the fact that normally growth factors act in a concerted manner working both together and in sequence to regulate the repair process. Therefore, although use of a single growth factor is the standard practice at present, it is probably not the best option. Second, during wound healing in vivo, protein growth factors often interact with nonproteinaceous soluble mediators of repair such as bioactive lipids.86 In fact, the lipid molecules act synergistically with growth factors, enhancing their activity and promoting in vivo wound healing.87,88 Therefore, therapies combining growth factors and other bio-active, particularly lipid moieties, could be beneficial. Finally, the effectiveness of a growth factor depends on its availability to the cells within the wound bed. Methods for its improvement and ways for delivery of the growth factors into the wound bed are discussed in the following section.
DELIVERY OF GROWTH FACTORS INTO THE CHRONIC WOUND BED: CURRENT APPROACHES AND FUTURE PERSPECTIVES
Growth factors are critically important for coordinating cell-cell and cell-matrix interactions during normal injury repair. Insufficient bioavailability of growth factors, because of diminished synthesis and/or excessive degradation, is a hallmark of chronic wounds. Therefore, exogenous growth factors delivered to non-healing wounds may facilitate cellular responses and lead to timely wound closure.12 Despite a number of promising studies in animal models showing acceleration of healing upon addition of a variety of growth factors, their clinical use remains limited because methods for their delivery need further development. As mentioned, the only growth factor approved for clinical use is recombinant human PDGF-BB becaplermin, which is supplied in a gel form with sodium carboxymethylcellulose serving as a vehicle. The ointment is normally applied once daily, and the wound is dressed after the application. This straightforward preparation of PDGF-BB is relatively effective in the treatment of both chronic and acute wounds.14,89 However, as discussed, a large number of patients do not respond to the treatment, in part because of rapid degradation of the growth factor in proteolytic wound environment resulting in insufficient concentration of the PDGF in the chronic wound bed.12
The main purpose of a drug delivery system for wound healing would be to protect the labile growth factor from a protease-rich wound environment, extend its presence/activity at the site of injury, minimize its systemic absorption, and, if possible, prevent immune responses. Several types of delivery systems fulfilling these requirements have been reported including proteinaceous ECM-derived vehicles and carbohydrate-based and synthetic matrices. Many of these matrices are biodegradable or biocompatible and are already safely used/FDA approved for other applications,90 whereas others are under investigation. These delivery systems are discussed in detail.
DELIVERY OF PROTEINS TO THE WOUND BED
Protein-Based Delivery Systems
Collagen (Figure 6A). Collagens are multifunctional ECM proteins constituting roughly 30% of the protein mass in the human body.91 Collagens maintain tissue structural integrity and function as signaling molecules via the interactions with their cellular receptors, particularly integrins. These triple helical proteins (Figure 6A) always contain collagenous glycine-X-Y sequences, where X is often proline and Y is 4-hydroxyproline. Frequently, collagen molecules also include noncollagenous domains that are important for intermolecular interactions and signaling functions of these proteins.92
Figure 6. CHEMICAL STRUCTURES OF PROTEINACEOUS MATRICES USED FOR DRUG DELIVERY AND WOUND HEALING.
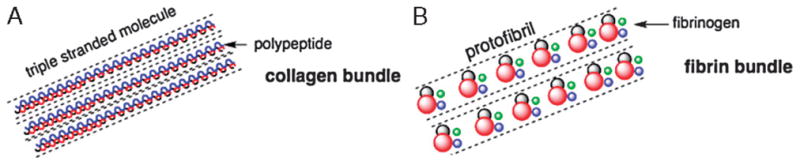
(A) Schematic representation of the chemical structure of collagen. The molecule is composed of helices containing collagenous glycine-X-Y sequences (blue and red lines). These helices then coil again into a triple helix (bundle) stabilized by hydrogen bonds (dotted lines). (B) Schematic representation of a fibrin molecule. A fibrin bundle is formed after cleavage of the fibrinogen by thrombin and is composed of fibrinogen monomers containing 1 E domain (red) and 2 D domains (green and blue). The bundle is stabilized by plasma transglutaminase (gray).
Collagens are classified into fibrillar and network-forming collagens; in addition, a number of collagens with interrupted triple helices have been described.93 Cutaneous fibrillar collagens include collagen types I, III, and V, whereas network-forming family members are collagen types IV and VII (located in the anchoring fibrils). Type I collagen is the base for the majority of wound healing products94; some dressings obtained from natural matrices contain other collagens as well.95
Collagens can be easily obtained in large (milligram-gram) quantities from bovine, porcine, and human sources. Furthermore, even nonhuman collagens do not induce excessive inflammatory reactions, have relatively low antigenicity, and can support the growth of a variety of cell types, such as fibroblasts, keratinocytes, and endothelial cells.96 Consequently, collagen-based materials are often used in tissue engineering and are under investigation for a variety of applications, such as wound dressings.96 The latter can be classified in the following ways: collagen sponges produced from natural lyophilized matrices (OASIS Wound Matrix; Healthpoint Biotherapeutics), skin substitutes containing dermal and/or epidermal cells (Apligraf; Organogenesis, Canton, Massachusetts), and collagen-based matrices with synthetic backings (Integra; LifeSciences, Plainsboro, New Jersey). Collagens can be also combined with oxidized regenerated cellulose (Promogran; Systagenix, Quincy, Massachusetts). Collagen-based dressings are particularly suitable for treatment of chronic wounds, as they have been shown to effectively control wound exudate, inactivate proteases, and can protect exogenously added growth factors from degradation.97,98
The use of collagen-based materials for growth factor delivery was proposed more than 20 years ago.99 Loading of the collagen scaffolds with growth factors can be achieved by a number of different methods (Figure 7).
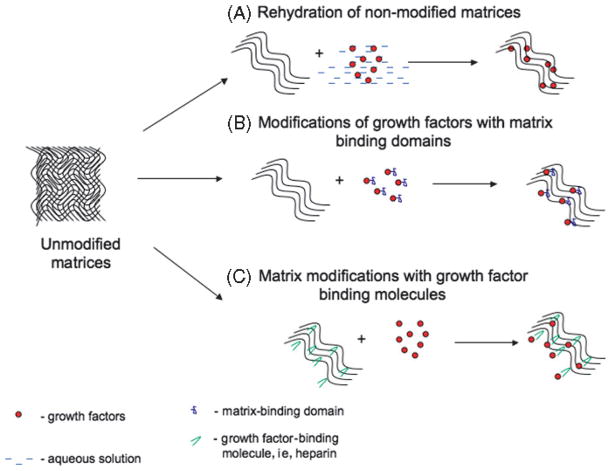
Figure 7. METHODS OF GROWTH FACTOR INCORPORATION INTO PROTEINACEOUS MATRIX-BASED BIOMATERIALS.
Incorporation of growth factors into proteinaceous matrices (scaffolds) can be achieved via several mechanisms. (a) Rehydration of nonmodified matrices involves soaking of dry matrices in aqueous solutions of growth factors. (b) Growth factors can be genetically modified by addition of matrix-binding moieties (ie, collagen-binding domain of fibronectin) and then combined with unmodified scaffolds. On the other hand, (c) matrix itself can be altered with growth factor–binding molecules, such as heparin, thus increasing its affinity for growth factors.
Simple sponge rehydration with a solution of biologically active molecules is the easiest way to load the scaffold.100–102 In 1998, Pandit et al100 used a solution of FGF-1 applied onto collagen sponges implanted in dorsal wounds in a rabbit model. There was a significant but moderate improvement in the rates of healing in wounds treated with collagen–FGF-1 combination compared with collagen alone. However, it remained unclear whether incorporation of FGF-1 into the collagen sponge improved the growth factor delivery because FGF-1 alone was not used in this study.100 Two similar studies were performed in Japan using EGF and FGF-2 applied to spongy collagen matrices.101,103 In both cases, growth factors incorporated into the collagen matrices were more efficient in prevention of wound contraction and promoting epithelialization as compared with vehicle alone.
In another study,102 collagen rehydration was performed using solutions of several radioactively labeled growth factors, including FGF-2, PDGF, HB-EGF, and VEGF. The matrices then were implanted into dorsal subcutaneous pockets in mice. This study revealed significant differences in growth factor release kinetics. Although 50% of FGF-2 remained within the scaffold for over 10 days, PDGF, and especially VEGF, demonstrated burst release. Only 40% of incorporated PDGF stayed intact at day 3 after implantation, and more than 90% of VEGF and HB-EGF were released by this time. The effects of such collagen–growth factor complexes on wound healing were not studied.102 However, these results suggest that simple collagen soaking could potentially be an acceptable way for loading of specific growth factors, such as FGF-2, into collagen matrices. Different approaches might be necessary for other active molecules, such as HB-EGF and VEGF.
One way to increase collagen-growth factor affinity is to incorporate heparin-like moieties into a collagen scaffold.104 This is particularly effective for integration of heparin-binding growth factors, such as members of the FGF, VEGF, and EGF families.15,29,52 Vascular endothelial growth factor loading into heparinized collagen increased retention of this growth factor within the matrix up to 48 hours.104 This is in contrast to almost immediate release of VEGF that was simply added to a dry collagen sponge.102 Importantly, in both cases, incorporation of growth factors into the collagen matrices provided protection against proteolytic degradation and preserved the activity of the growth factor.102,105,106 Similarly, growth factors can be cross-linked directly to the collagen matrix.105 These studies suggest that heparinized collagen scaffolds or sponges to which growth factors have been cross-linked could be used to deliver these bioactive molecules into the wound bed.
Derivatizing growth factors with affinity tags have also been tested in efforts aimed at enhancing wound-healing dynamics and injury responses. For example, Stompro et al99 used biotinylated growth factors and/or matrices cross-linked with avidin molecules and took advantage of high-affinity biotin-avidin interactions. The authors used biotinylated EGF that was attached to biotinylated collagen in the presence of tetravalent avidin. In vitro keratinocyte growth assays performed in this study demonstrated that this modified growth factor retained its activity and therefore could be available to the cells. The effectiveness of this method for delivery of EGF to wounds is not known.
More recently, growth factors have been modified by the addition of collagen-binding domains (CBDs) of different origins.107 For example, EGF fused with CBD derived from fibronectin (FNCBD-EGF) was able to bind to collagens I, II, III, and IV with high affinity and increased the growth rates of keratinocytes on modified matrices.107 Moreover, when tested in vivo using animal models of impaired wound healing, FNCBD-EGF combined with collagen sponges improved wound epithelialization. This work was followed by a number of studies utilizing another collagen-binding stretch of amino acids (TKKTLRT) to modify PDGF-BB and FGF-2.108,109 This modification of growth factors improved their binding to collagen scaffolds and allowed for increased scaffold vascularization and wound healing,108,109 suggesting that CBD-containing growth factors could successfully be delivered into the wound bed.
Unfortunately, none of the methods described are currently used to routinely deliver growth factors to wounds in patients. One of the concerns raised here is the necessity of using animal-based products. These products may pose a risk of disease transmission and are prone to variability in chemical composition dependent on their origin. In order to eliminate these issues, artificial biomimetic collagen-like substrates could be used.93 The synthesis of these molecules is possible because collagen-like (Gly-X-Y)n peptides have a unique ability to self-assemble. Proteinaceous fragments with length and thermal stability resembling naturally occurring collagens have been synthesized using helicogenic ProYaaGly (Yaa = hydroxyproline or proline) strands linked by disulfide bonds.110 To the authors’ knowledge, no research has been performed in order to evaluate whether these artificial collagen mimetics could be used to deliver growth factors to the wound bed. These studies would have to address a number of important questions. For instance, it is presently unknown whether the artificial collagen-like materials would induce immune response. Moreover, it remains to be determined whether these molecules can be degraded with naturally occurring proteases. Finally, it would be necessary to develop means by which the growth factors and/or other biologically active molecules could be incorporated into these artificial biomaterials.
Fibrin
Fibrin (Figure 6B) is a fibrous protein arising from fibrinogen (produced in the liver) after its cleavage by thrombin and forms complex 3-dimensional gel networks stabilized by plasma transglutaminase. It is a major component of the natural wound provisional matrix formed immediately after injury as part of the blood-clotting cascade and plays a key role in initial events of healing. Fibrin gels, structurally similar to native fibrin, can be formed in vitro from purified blood plasma containing fibrinogen and thrombin in the presence of calcium chloride111 and are used for a number of biomedical applications. Fibrin-based formulations are currently FDA approved for use as hemostatic agents, as sealants for colostomy closures, and for skin graft attachment.112 In addition, these biocompatible and biodegradable matrices whose configuration can be modified by varying the polymerization conditions (pH, salt, and fibrinogen concentration, etc) have a great potential as vehicles for drug delivery.
Several groups have used fibrin gels or scaffolds to deliver growth factors, either alone or in combination with cells into the cutaneous wound bed. In an early study by Pandit et al,113 the authors used fibrin scaffold impregnated into polytetra-fluoroethylene to deliver FGF-1 to full-thickness dorsal wounds in normal rabbits. An increase in angiogenic and fibroblastic responses to injury was observed in the presence of fibrin-incorporated FGF-1, but not in response to topical free FGF-1.113 In another study, fibrin was used to deliver FGF-2 to normal or ischemic wounds in rats.114 At day 10 after injury, a moderate increase in the tensile strength of the ischemic wound was observed. However, the same FGF-2–containing fibrin gel preparation had a negative effect on normal wound healing.114 It is known that the healing of ischemic wounds is significantly delayed as compared with normal wounds.115 Therefore, it is likely that timing for delivery of growth factor to normal and ischemic wounds might be different.
Relatively simple modifications of fibrin-based delivery systems can regulate the growth factor release rates,116 so that they could be adjusted to supply the growth factors to wounds of different etiologies and at different stages of the healing process. For example, blending fibrin-based delivery systems with naturally occurring polysaccharides, such as glycosaminoglycans, has been demonstrated to be effective in altering rate/extent of growth factor release. In vitro addition of heparin to a fibrin solution before polymerization decreased the initial burst release of FGF-2, extending the growth factor retention for up to 7 days.116 Increase in thrombin concentration had a similar effect. In this case, FGF-2 remained in the scaffold for more than 14 days. As a result, in a mouse model of hind-limb ischemia, fibrin gel preparation containing 100 mg/mL fibrinogen, 500 IU/mL thrombin, 200 mg heparin, and 25 mg FGF-2 increased blood vessel density by more than 2-fold.116 These data demonstrate that prolonged release of proangiogenic growth factors from heparin-containing fibrin scaffolds may be beneficial for cutaneous wound healing via stimulation of wound-healing angiogenesis.
Modification of the growth factor itself is another method to increase the retention of the biologically active molecule within the scaffold (Figure 7). In the study published by Geer et al,117 FGF-10 was modified with a fibrin-binding site of α2-plasmin inhibitor, allowing for an interaction between the FGF-10 and fibrin matrix in the presence of factor XIII. In vivo release of active FGF-10 was achieved after fibrin degradation by the cells residing within the wound. This system allowed for a 7-day-long presence of the growth factor in the wound and a significant increase in epithelialization of full-thickness wounds that had been created in human bioengineered skin transplanted into athymic mice.117
Because of the diversity of growth factors and other signaling molecules involved in the process of normal wound healing, delivery of a single growth factor to a wound bed may not be sufficient or adequate to significantly promote wound healing. Therefore, there have been attempts to deliver multiple growth factors using fibrin-based delivery systems. For example, using the chicken embryo chorioallantoic membrane model of neovascularization, it has been shown that fibrin gels could be used to deliver FGF-2 and VEGF simultaneously.118 To the authors’ knowledge, no studies have been performed to determine whether fibrin-based systems can be used to deliver multiple growth factors to the wound bed. Such scaffolds, however, have been successfully used to deliver a combination of a growth factor and cells to excisional wounds in athymic mice.119 In this research, human keratinocytes and EGF were suspended in a fibrin matrix and sprayed onto the wound surface. This scaffold preparation extended the presence of EGF within the wound for 3 days. In addition, it increased the rates of wound epithelialization as compared with fibrin alone or fibrin matrices containing either EGF or keratinocytes.119
More recently, fibrin gels were used to deliver fibroblasts and PDGF-BB into excisional wounds in rabbits.120 Cells and growth factor were both embedded into fibrinogen before mixing with thrombin and applied to wounds in the form of dressing. The treatment significantly increased formation of granulation tissue and its incorporation into the fibrin sealant and enhanced epithelialization. The authors have tested 4 formulations containing different concentrations of fibrinogen and thrombin. The scaffolds containing a 5-fold excess of thrombin-to-fibrinogen were the most effective120 when compared with delivery systems with other thrombin and fibrinogen ratios. This study confirmed that fibrin is a promising scaffold for delivery of growth factors and cells into surgical wounds.
The studies previously described119,120 utilized fibrin matrices to deliver either epidermal or dermal cells into the wound bed. A vast majority of acute, chronic wounds, and burns, however, affect both the epidermal and dermal layers of the skin. Therefore, the engineering of skin equivalents containing both components is necessary. It has been shown that fibrin gels can be successfully used for the development of such products.121 For example, fibroblasts and keratinocytes were isolated from human subjects, expanded in 2-dimensional cultures, and resuspended separately within human plasma, in the presence of CaCl2. After gel polymerization, the dermal and epidermal layers were overlaid and cultured for 7 days to achieve construct stabilization and differentiation of dermal and epidermal layers. These composites were transplanted into dorsal excisions made in athymic mice. By 4 weeks after transplantation, skin equivalents were integrated into the host tissues with concurrent healing of excisional wounds. Moreover, it has been shown that a fibrin-based dermal substitute has higher chances of revascularization compared with its collagen-based counterpart,122 suggesting that the former has better potential in clinical applications. Recently, work from the authors’ group has demonstrated that silk-based microfluidics and MEMS-based nanofabrication may prove extremely useful for the creation of prevascularized living skin equivalents possessing patient-derived cells, complete with a preexisting vasculature, dermal compartment, and epithelial covering derived from patient progenitor cells. This, in turn, should prove extremely beneficial for individualized applications, regardless of wound type.123
Overall, the work discussed in this section113,114,116,117,119,121,122 opens the possibility of creation of completely autologous skin substitutes with the capability to stimulate angiogenic response within the host tissues via both cellular components and addition of exogenous growth factors. At present, it remains unknown whether introduction of cultured endothelial cells contained in fibrin skin substitutes would further improve artificial skin survival. Therefore, additional research aimed at optimization of the scaffold and cellular/growth factor constituents is needed to make them available for clinical use.
In summary, methodologies for loading of growth factors into proteinaceous matrices can be classified as (Figure 7) (a) simple soaking of dry matrices with the solutions of growth factors,102 (b) modifications of both matrix and growth factors allowing for better interactions between the two,99 (c) growth factor modifications with ECM-binding motifs,107 and (d) matrix modification using naturally occurring molecules such as heparin.104 To the authors’ knowledge, no single study has compared the effectiveness of these approaches. Therefore, further research is required to estimate the best strategy with which the best release kinetics and efficacy of growth factor delivery can be achieved. Also, all systems using ECM to deliver growth factors to cutaneous wounds have a significant disadvantage—a requirement for a secondary dressing. Incorporation of the matrices onto an adhesive and use of dressings for growth factor delivery could potentially solve this problem. Another option is the use of photo–cross-linkable matrices that would adhere to the wound bed upon exposure to light of specific wavelength.124,125
POLYSACCHARIDE-BASED MATRICES FOR GROWTH FACTOR DELIVERY
Carboxymethyl Cellulose
Carboxymethyl cellulose (CMC) (Figure 8A) is a derivative of the common plant polysaccharide, cellulose. In CMC, hydroxyl groups of the 2-glucopyranose residues are substituted by carboxymethyl groups.126 This substitution makes CMC soluble in water and is useful for a wide variety of applications in the pharmaceutical industry. For instance, CMC is a major component of several wound-healing products, including Solosite gel (Smith & Nephew, St Petersburg, Florida)63 and Aquacel Hydrofiber dressing (ConvaTec, Skillman, New Jersey).127 In addition, CMC serves as an excipient and carrier in the PDGF-BB–containing ointment becaplermin (Regranex).128 This CMC-based formulation is not ideal as it is characterized by fast bolus release and requires repeated application.129 Nonetheless, Regranex remains the only growth factor preparation approved by the FDA for treatment of diabetic wounds.9
Figure 8. POLYSACCHARIDE-BASE MATRICES USED FOR GROWTH FACTOR DELIVERY.
(A) Carboxymethyl cellulose (CMC) is a polymer composed of 2-glucopyranose residues (cellulose) with hydroxyl groups substituted by carboxymethyl groups. The CMC monomers are connected with β-(1-4)-glycosidic bonds. The structure of CMC sodium salt is shown. (B) Chitosan polymers consisting of N-acetyl-D-glucosamine and β-(1,4)-linked d-glucosamine. (C) Alginate sodium salt polymers containing 1-4 linked β-D-mannuronic acid and α-L-glucuronic acid residues.
Experimentally, CMC has been successfully used to deliver FGF-2 to the wound bed.130 The growth factor was suspended in CMC and applied at 1, 10, or 100 μg/cm2 every third day and improved the rates of closure in infected wounds in rats. Other growth factors that have been delivered in CMC include TGF-β family members, such as BMP-2 used for bone repair.131 The authors’ laboratory has used CMC to deliver ECM-derived peptides into the excisional wounds in cyclophosphamide-treated mice. A 3% solution of CMC in saline was used as a carrier and demonstrated a significant improvement of epithelialization and granulation tissue formation after daily application of the peptides compared with vehicle alone. Thus, the authors’ work and studies published by others130,131 suggest that CMC is a useful vehicle for both small and large protein delivery to the wound bed. These studies also indicate that, in some cases, CMC provides sufficient protection from degradation, allowing for retention of protein activity.
Chitosan
Chitosan (Figure 8B) is a polysaccharide consisting of N-acetyl-D-glucosamine and β-(1,4)-linked D-glucosamine.132 It is produced by deacetylation of chitin—a major structural material of invertebrate exoskeleton and fungal cell wall.133 Chitosans can be chemically modified and used in the form of films, hydrogels, fibers, and dressings.133 The relative availability of chitosan, as well as its biological characteristics, including superior hemostatic ability134 and antimicrobial and wound-healing properties,135,136 and recent FDA approval134 make it an attractive biomaterial.
In wound-healing research, chitosan preparations have been used to deliver a number of growth factors (eg, FGF-2 and EGF) to wounds of different origin.
Most of the studies compare the healing rates of untreated wounds, wounds treated with chitosan, and those treated with chitosan impregnated with growth factors. Despite considerable increase in growth factor retention within the wound in the presence of chitosan, very few studies have demonstrated significant improvement of healing by growth factor–containing matrices compared with matrices alone.137,138 For example, Hong et al139 applied recombinant human EGF (rhEGF) contained in chitosan films daily onto excisional wounds in normal pigs. Addition of the growth factor to the dressing did not significantly improve the rates of epithelialization but accelerated granulation tissue formation when compared with control.139 Similarly, chitosan impregnated with FGF-2–containing gelatin microparticles failed to significantly improve healing of pressure ulcers in aged mice.138 Conversely, FGF-2 incorporated into photo–cross-linkable chitosan matrices was able to stimulate wound healing in diabetic mice.124,125 Here the growth factor was mixed into the aqueous solution of chitosan modified with azide and lactose moieties, applied to a wound and exposed to a low dose of UV light, leading to the formation of a hydrogel inside the wound. Gradual degradation of the matrix allowed for sustained presence (up to 14 days) of the FGF-2 inside the wound and induced an enhancement of wound-healing rates. In addition to its effectiveness, this preparation of chitosan has an important advantage: It does not require a secondary dressing after application and remains in the wound for a long time. These studies demonstrate that chitosan is an effective prohealing wound dressing. Chemical modification of chitosan matrices and/or simultaneous delivery of several growth factors may be necessary to make them appropriate vehicles for growth factor delivery.
Alginate
Alginate (Figure 8C) is a linear polysaccharide derived from several species of brown marine algae. It is composed of 1 to 4 linked β-D-mannuronic acid and α-L-glucuronic acid that, depending on the source, can form homopolymers or hetero-polymers.140 In the presence of Ca2+ ion alginates form gels. Since the 1970s, alginate gels have been used as excipients in the pharmaceutical industry and later for management of acute and chronic wounds.141–143 Gel biocompatibility and relatively mild gelation conditions make alginate gels promising vehicles for growth factor and cell delivery.141 Weak growth factor–alginate interactions, however, can lead to uncontrolled rapid release rates.144 Increased growth factor retention within the alginate matrices can be achieved by covalent cross-linking use of heparins bound via ethylenediamine chemistry. Such modifications both allow for sustained FGF-2 release from the matrix while increasing implant vascularization in vivo.145 Similar techniques have also been used to create and deliver proangiogenic hybrids composed of laminin and elastin-derived peptides to a dermal ulcer in a rabbit.146 Significant increases in wound epithelialization and granulation tissue formation were achieved. These studies, as well as work describing pro–wound-healing properties of alginate, suggest that these dressings may be promising vehicles for delivery of growth factors into the wound bed.
SYNTHETIC POLYMERS AND THEIR USE FOR GROWTH FACTOR DELIVERY
Naturally occurring matrix-derived delivery systems can protect growth factors from hostile wound environments and are bio-compatible and biodegradable. Moreover, some natural matrices such as collagen may contain ECM-bound growth factors that attract cells and enhance cell adhesion, growth, and motility.96 Despite these beneficial features, natural scaffolds can pose a risk of disease transmission, are hard to produce in large quantities, may be expensive (animal-derived collagens and fibrins), and could induce allergic reaction (chitosan). The use of synthetic polymers can help to overcome these problems. Next, the authors review the most prominent synthetic carriers that can be used to deliver growth factors into the wound bed.
Poly(α-hydroxyacids-poly(lactic acid), Poly(glycolic acid), and Poly(lactide-co-glycolide)
Poly(lactic acids) (PLA) and poly(glycolic acids) (PGA) (Figure 9A and B) are synthesized as a result of ring-opening polymerization of cyclic lactic and glycolic acid dimers, respectively.96 Poly(lactic acids) and PGA can be copolymerized, creating poly(lactide-co-glycolic acids) (PLGA) (Figure 9C). In addition, the PLA, PGA, and PLGA can be further modified to have tailored degradation rates, hydrophilicity/hydrophobicity, and scaffold shape. These biocompatible and biodegradable polymers have been FDA approved for a number of biomedical applications and have been proposed for use in drug delivery.147 Poly(lactide-co-glycolic acid) is a component of 2 commercially available skin substitutes—Dermagraft and TransCyte. Dermagraft contains PLGA with embedded allogenic fibroblasts that secrete growth factors and ECM components. This dressing serves both as a source of biologically active molecules and living cells beneficial for chronic wound healing. TransCyte, on the other hand, is used as nonliving skin replacement for treatment of burns. It does not contain living cells but cell-derived factors and ECM.141,148
Figure 9. SYNTHETIC POLYMERS USED FOR DRUG DELIVERY.
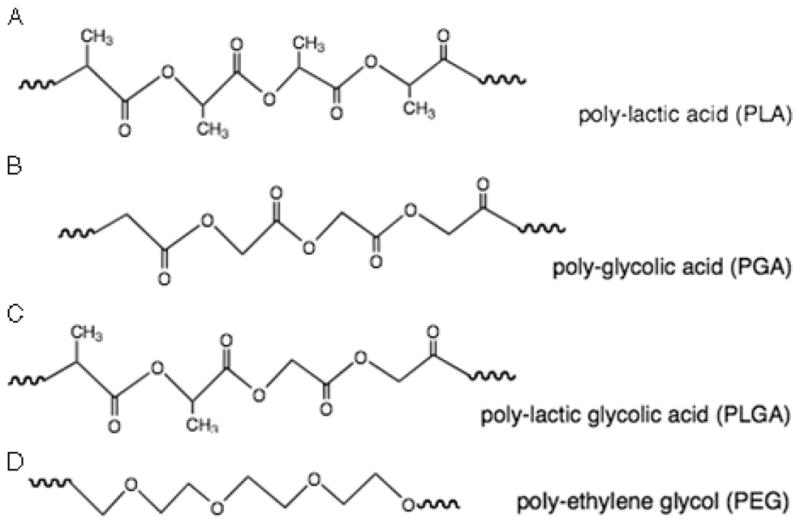
(A) Chemical structure of poly-lactic acid. (B) Chemical structure of poly-glycolic acid. (C) Chemical structure of a copolymer of poly(lactic acid) and poly(glycolic acid), and poly(lactide-co-glycolic acid). (D) Chemical structure of polyethylene glycol.
Poly-(α-hydroxyacids) (PHA) have also been tested as carriers for a number of growth factors, as well as their combinations.149 Several methods can be used to incorporate the bioactive molecules into PHA.148 The most commonly used approach is foaming of growth factor–scaffold mixture with high-pressure gas (CO2) in the presence of porogen, usually sodium chloride. A rapid pressure drop in the system leads to formation of bubbles and a consequent arrangement of scaffold material around the porogen particles.150 Subsequent dialysis removes the porogen, creating porous growth factor–containing scaffold. This procedure performed in mild conditions allows for nondestructive incorporation of a single growth factor151 or multiple growth factor combinations.149 The latter strategy was used to incorporate and deliver proangiogenic VEGF and PDGF-BB to ischemic hind limbs in nonobese diabetic mice.149 In this study, the scaffold was fabricated by mixing PDGF-containing PLG microspheres with lyophilized VEGF followed by high-pressure gas foaming. This ensured controlled distribution of the growth factors within the scaffold, with VEGF largely present on the surface of the scaffold and PDGF located within the microspheres dispersed throughout the polymer. Incorporation of this scaffold into the ischemic areas led to sustained delivery of both growth factors and a significant increase in density of stable blood vessels.149 This was in contrast to sustained delivery of these individual growth factors separately, which did not induce the formation of mature blood vessels.
Although this delivery system was not tested in a model of cutaneous wound healing, it has the potential to increase PDGF/VEGF concentrations inside the wound, improve angiogenesis, and increase the rates of wound healing. Alternatively, FDA-approved PHA-containing wound dressings (Dermagraft and TransCyte) that currently incorporate growth factors and matrix components synthesized by cultured allogenic cells could be modified to carry recombinant bioactive molecules. In principle, this would not only eliminate the risk of disease transmission, but also allow for better control of the amount, delivery, and nature of integration for active biomolecules.
Polyethylene Glycol
Polyethylene glycol (PEG) (Figure 9D) is synthesized by polymerization of ethylene oxide that can be initiated by methanol or water.152 Further polymerization and covalent cross-linking of functionalized PEG can be achieved by several methods including chain-growth, step-growth, and mixed step-chain growth polymerization.153 Chain-growth polymerization requires the presence of free radicals, can leave behind potentially dangerous unreacted monomers, and often is performed in harsh conditions. On the other hand, step-growth polymerization is known to produce more uniform polymers and can be performed in a relatively mild setting. High water solubility, biocompatibility, and versatility of PEGs make them attractive materials for delivery of biologically active molecules. Several methods can be used to load growth factors into PEG scaffolds. The first approach includes entrapment of the active molecules within the prepared gel by simply soaking the gels in concentrated solutions of the drug of interest.153 This method provides little control over the amount of the drug loaded into and released from a scaffold and therefore is not very appropriate for use in biological systems. Second, scaffolds can be loaded during the PEG cross-linking process.154,155 In this case, bioactive molecules are added to a modified scaffold, such as acryloyl-PEG-N-hydroxysuccinimide (PEG-NHS), in the presence of cross-linking buffer.155 This technique was used to prepare a substrate for growth of vascular smooth muscle cells: PEG-NHS scaffold was linked to TGF-β1 and several ECM fragments. In turn, this process allows for better cellular attachment and enhancement of matrix production without an increase in cell proliferation.154 Unfortunately, this TGF-β1 incorporation technique did not achieve significant release of the growth factor to culture media. Therefore, hydroxysuccinimide-mediated cross-linking of bioactive molecules may not be suitable for drug delivery to the wound bed. In contrast, cross-linking of thiol-bearing growth factor to vinyl sulfone–functionalized PEG (VSF-PEG) allowed for cell and protease-dependent release of growth factor and might be more appropriate for this application. Zisch et al155 used this strategy to tether VEGF with an additional c-terminal cysteine (VEGF-cys) to VSF-PEG. These cross-linking reactions were performed in the presence of short peptides bearing MMP-2 cleavage sites and cysteine residues flanking cell-adhesive amino acid sequences (RGDSP).155 Such cross-linking conditions not only preserve the proangiogenic activity of VEGF, but also permit its release from the matrix (either in the presence of exogenous or cell-derived ECM-remodeling enzymes in vitro). In vivo, 14 days after subcutaneous implantation in rats, VEGF-PEG conjugates were replaced with a highly cellular and vascularized tissue,155 suggesting that this growth factor incorporation technology allows for sustained release of VEGF from the scaffold. Vinyl sulfone–functionalized PEG has also been used to deliver VEGF and TGF-β1 combinations.156,157 In this case, sequential release of the bioactive molecules can be achieved when one of them is covalently conjugated to the scaffold, and the other is incorporated via a simple soaking. The possibility of whether PEG scaffolds or their modifications can be used for drug delivery to a wound bed was never explored. Yet, it has been demonstrated that covalent linkage of the PEG molecule to the N-terminus of an rhEGF using monomethoxy PEG-butyraldehyde derivatives increased the stability of the growth factor inside a wound.158 Moreover, it has been shown that PEG in combination with PLGA can be a promising vehicle for delivery of stem cells to the injury site.159 Additional studies will be needed to evaluate whether PEGs can serve as functionalized scaffolding capable of delivering growth factors to the wound beds with defined release kinetics.
GENE DELIVERY
The often-discussed approaches used to deliver protein therapeutics into wounded tissue cannot protect the protein from proteolytic degradation. The problem of protein instability could be eliminated if the resident cells could produce the protein in situ. This can be achieved by supplying relevant genetic material directly to the resident cells—a technique called gene therapy (Table 2). This relatively new approach has been used in clinical trials for treatment of congenital blindness,160 ischemic conditions,161 and certain types of cancer162 and could be used for treatment of chronic wounds. Cutaneous diseases, particularly chronic wounds, are attractive targets for gene therapy. To date, several methods of gene delivery to the wound bed have been described, including nonviral (plasmid DNA) and virus-mediated delivery.163 Here, the authors briefly describe several of these methods.
Delivery of Plasmid DNA
A variety of plasmid DNA delivery methods have been proposed (Table 2). Simple methods, including direct topical applications or injections of DNA into the site of interest, have proved inefficient and may not be appropriate for the delivery of genes encoding for growth factors or other proteins that accelerate wound healing.163
More effective methods that have been considered for DNA delivery take advantage of technologies that use physical force to propel cDNA-coated gold particles into the skin or other tissues with a “particle-bombardment device” or “gene gun.”163–166 In addition, DNA can be directly injected into the wound bed–associated cells using microneedles.167 This technique allowed for high levels of growth factor expression within the wound, but no stimulation of angiogenesis or epithelialization was achieved.168 The failure of growth factors delivered using the microneedles to enhance cellular responses to injury suggests that these methods may require modifications, perhaps allowing for a more precise cellular targeting regimen.
Electroporation, which is commonly used to transfect cells in culture, can be similarly used in vivo to deliver growth factor–encoding DNA into the wound bed.169,170 This method was used to deliver FGF-7 encoding plasmids into the wounds in rats, leading to improvement of both the rates and quality of wound healing as determined by epithelial thickness and by the number of inflammatory cells present.169 Further work will be required before electroporation-based methodologies will become a viable gene therapy option for the treatment of chronic wounds in human patients.
Liposomal formulations can also be used to transport negatively charged DNA into living cells. Several groups have demonstrated that cationic liposomal complexes containing DNA constructs encoding growth factors (FGF-1 and IGF/KGF [keratinocyte growth factor] combination) when delivered to incisional wounds or burns can stimulate wound healing.171–173 Optimal combination of growth factor genes, as well as determination of optimal composition of liposomal delivery vehicles, will ensure the effectiveness of this delivery mode.
In summary, among several methods for nonviral gene delivery to the wound bed, electroporation and liposomal administration appear most promising. To our knowledge, neither of them is currently used in clinical practice. Virus-mediated gene delivery methods, on the other hand, are currently undergoing clinical trials.
Viral Gene Delivery
This method of gene administration uses the natural ability of viruses to introduce genetic material into the host cells. Typically, 3 types of viruses are used, including adenoviruses, adeno-associated viruses (AAVs), and retroviruses.163 Double-stranded DNA adenoviruses are most commonly used in gene therapy and can achieve 95% infection efficiency.161 Adenoviruses can infect both dividing and nondividing cells and therefore could be used to carry growth factor–encoding genes to both chronic and acute wounds. Because of their inability to integrate foreign genes into the host genome, adenoviruses can induce only a temporary increase in expression of the gene of interest. This property makes them exceptionally suitable for delivery of growth factor–encoding genes considering the fear that excessive or permanent expression of these potent molecules could be detrimental and possibly lead to neoplastic growth. Adenoviruses can be used to administer genes encoding growth factor receptors and their ligands. Okwueze at al64 recently reported successful transfection of Erb3 receptor into a porcine wound using adenovirus delivered with a gene gun. This procedure alone did not significantly improve healing, but the additional topical application of Erb3 ligands, particularly HB-EGF, led to significant improvement of re-epithelialization.65 Growth factor ligand gene delivery can also be performed using adenoviral vectors. Vascular endothelial growth factor–encoding genes have been administered into excisional wounds in streptozotocin-induced diabetic mice.174 This treatment promoted early responses to injury, improving granulation tissue formation, and increased wound-healing rates at days 3 to 5, but not on day 13 after wounding. The absence of long-term stimulation of wound healing could possibly be explained by the transient nature of foreign gene expression that is achieved after adenoviral delivery. Importantly, no adverse effects of adenoviral gene delivery were observed. This is in contrast to an earlier study,175 where wound epithelialization was impaired after intradermal injection of Ad-LacZ. It is possible that this particular mode of vector delivery—intradermal injection—but not topical application, is detrimental for wound healing. Another possible reason why adverse effects were seen in one, but not the other study, is the different animal model used. It is possible that even minor virus-induced inflammatory responses may be damaging for healing wounds made in one versus another tissue location, for example, the ischemic ear versus dorsal wounds. Clearly, more thorough investigation and better understanding of potential risks linked to adenovirus-mediated gene delivery will be required before this approach will gain acceptance and be adopted for clinical use.
Adeno-associated viruses are also commonly used for gene administration in wound-healing studies. These vectors can deliver genes to either dividing or nondividing cells and can induce transient or more permanent gene expression, depending on the degree of host genome integration. Furthermore, AAVs induce limited immune response and have been used to deliver VEGF transgenes in several types of animal wound-healing models.176,177
Retroviruses are another viral group used for gene delivery. Despite low transfection efficiency combined with relatively high particle instability, these viruses account for more than 30% of all gene therapy clinical trials. The use of retroviruses as vectors for growth factor–encoding DNA delivery to the chronic wound bed is limited because of an inability of these viruses to infect nondividing cells.163
Both AAVs and retroviruses can be also used ex vivo, where autologous or donor cells are transfected, while grown in culture and then transplanted to a host. For example, this approach was used to genetically modify human keratinocytes to express human PDGF-AA, which were then transplanted into the wounds in athymic mice. This treatment greatly improved skin graft survival and increased the number of infiltrating host cells.178
Finally, viral vectors bearing DNA encoding a growth factor can be immobilized on a matrix and then introduced into the wound bed. This technology was used with PDGF-B, FGF-2, or VEGF encoding adenoviruses, which were immobilized on a collagen matrix.179,180 This approach allowed for extended (at least 28 days) expression of the transgene inside the wound bed, production of PDGF-B mRNA, and enhanced epithelialization/granulation tissue formation and angiogenesis, suggesting increased protein production. In contrast to delivery of Ad-PDGF-B in an aqueous formulation, no hyperplasia was observed in tissues surrounding the wound upon the exposure to virus embedded in collagen scaffold, and no vectors were disseminated beyond the lymph nodes located near the wound.180
It should be mentioned that delivery of growth factors— encoding genes using viral or nonviral systems—should be approached with caution as the exact localization of the transgene, the extent, localization, and durability of gene expression by the cells may be hard to control. This is especially important because many growth factors used to promote wound healing are also implicated in cancer.181 Therefore, future work should focus on both identification of wound healing–specific target genes and better methods for drug delivery allowing for localized and controlled gene expression.
SUMMARY
In recent years, considerable progress has been made in understanding the molecular mechanisms controlling normal wound healing and those mediators that impair repair. In turn, these insights have offered opportunities leading to the development of enhanced wound-healing therapies. Although proteases and inflammatory mediators have been suggested as molecular “obstacles” or impediments to wound healing, it is now clear that their action can be avoided by adding protease inhibitors to growth factor–containing formulations or the use of recombinant truncated proteins lacking proteinase-binding sites.176 With advances in clinicians’ understanding of the biology of gene expression, it will become possible to develop gene therapy approaches that allow for expression of relevant genes on demand at the site of injury. Even though this approach poses certain risks linked to an excessive gene expression, having a better understanding of the mechanisms controlling gene expression may help to overcome this problem. For instance, drug-responsive and/or cell-type specific promoters and in vitro cell transfections before grafting could improve the control over the production of growth factors within the wounds.177,178 Finally, recent progress in the field of material science has made possible the development of better scaffolds/vehicles for both protein and gene delivery into the wound bed.
As scientists and clinicians continue working on both improvement and further testing of existing delivery modalities, this will surely lead to both improvement of existing and creation of novel therapeutics for chronic and acute wound sufferers.
Footnotes
Drs Demidova-Rice and Hamblin have disclosed they have no financial relationships related to this article. Dr Herman has disclosed that he is/was a recipient of grant/research funding from the National Institutes of Health, and Wound Care Partners, LLC; is/was a consultant/advisor to Healthpoint Biotherapeutics, Inc, and Nell One, Inc; was a consultant/advisor to Healthpro Bioventures and Amach Partners; and is a stock shareholder in Wound Care Partners, LLC.
References
- 1.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 2.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Z, Sasaoka T, Fujimori T, et al. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem. 2005;280:9375–89. doi: 10.1074/jbc.M413081200. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133–46. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–57. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 7.Gope ML, Gope R. Tyrosine phosphorylation of EGF-R and PDGF-R proteins during acute cutaneous wound healing process in mice. Wound Repair Regen. 2009;17:71–9. doi: 10.1111/j.1524-475X.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 8.Pierce GF, Tarpley JE, Tseng J, et al. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96(3):1336–50. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leahy PJ, Lawrence WT. Biologic enhancement of wound healing. Clin Plast Surg. 2007;34:659–71. doi: 10.1016/j.cps.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Vasquez R, Marien BJ, Gram C, Goodwin DG, Menzoian JO, Raffetto JD. Proliferative capacity of venous ulcer wound fibroblasts in the presence of platelet-derived growth factor. Vasc Endovascular Surg. 2004;38:355–60. doi: 10.1177/153857440403800408. [DOI] [PubMed] [Google Scholar]
- 11.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 12.Tomic-Canic M, Ayello EA, Stojadinovic O, Golinko MS, Brem H. Using gene transcription patterns (bar coding scans) to guide wound debridement and healing. Adv Skin Wound Care. 2008;21:487–92. doi: 10.1097/01.ASW.0000323563.59885.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinchliffe RJ, Valk GD, Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24(Suppl 1):S119–44. doi: 10.1002/dmrr.825. [DOI] [PubMed] [Google Scholar]
- 14.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Bian H, Qi S, et al. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin Exp Dermatol. 2008;33:176–82. doi: 10.1111/j.1365-2230.2007.02573.x. [DOI] [PubMed] [Google Scholar]
- 17.Braun S, auf dem Keller U, Steiling H, Werner S. Fibroblast growth factors in epithelial repair and cytoprotection. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):753–57. doi: 10.1098/rstb.2004.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.auf demKeller U, Krampert M, Kümin A, Braun S, Werner S. Keratinocyte growth factor: effects on keratinocytes and mechanisms of action. Eur J Cell Biol. 2004;83:607–12. doi: 10.1078/0171-9335-00389. [DOI] [PubMed] [Google Scholar]
- 19.Niu J, Chang Z, Peng B, et al. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem. 2007;282:6001–11. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
- 20.Narita K, Fujii T, Ishiwata T, et al. Keratinocyte growth factor induces vascular endothelial growth factor-A expression in colorectal cancer cells. Int J Oncol. 2009;34:355–60. [PubMed] [Google Scholar]
- 21.Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219:688–91. doi: 10.1097/00000658-199406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landau Z, David M, Aviezer D, Yayon A. Heparin-like inhibitory activity to fibroblast growth factor-2 in wound fluids of patients with chronic skin ulcers and its modulation during wound healing. Wound Repair Regen. 2001;9:323–8. doi: 10.1046/j.1524-475x.2001.00323.x. [DOI] [PubMed] [Google Scholar]
- 23.Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol. 1994;103(4):469–73. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- 24.Komi-Kuramochi A, Kawano M, Oda Y, et al. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J Endocrinol. 2005;186:273–89. doi: 10.1677/joe.1.06055. [DOI] [PubMed] [Google Scholar]
- 25.Ma B, Cheng DS, Xia ZF, et al. Randomized, multicenter, double-blind, and placebo-controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair Regen. 2007;15:795–9. doi: 10.1111/j.1524-475X.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 26.Robson MC, Phillips TJ, Falanga V, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001;9:347–52. doi: 10.1046/j.1524-475x.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Hess CT. Clinical Trial News. Adv Skin Wound Care. 2003;16:336. [Google Scholar]
- 28.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 29.Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg. 2009;96:1484–91. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- 30.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94(25):13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuma K, Takagi H, Otani A, Honda Y. Hypoxia and vascular endothelial growth factor stimulate angiogenic integrin expression in bovine retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1998;39:1028–35. [PubMed] [Google Scholar]
- 33.Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95:244–51. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 34.Barleon B, Sozzani S, Zhou D, Weich H, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 35.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–40. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 36.Grosskreutz CL, Anand-Apte B, Duplaa C, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–36. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 37.Parenti A, Brogelli L, Filippi S, Donnini S, Ledda F. Effect of hypoxia and endothelial loss on vascular smooth muscle cell responsiveness to VEGF-A: role of flt-1/VEGF-receptor-1. Cardiovasc Res. 2002;55:201–12. doi: 10.1016/s0008-6363(02)00326-7. [DOI] [PubMed] [Google Scholar]
- 38.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–90. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikuta T, Ariga H, Matsumoto K. Extracellular matrix tenascin-X in combination with vascular endothelial growth factor B enhances endothelial cell proliferation. Genes Cells. 2000;5:913–27. doi: 10.1046/j.1365-2443.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- 40.Wijelath ES, Rahman S, Namekata M, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor–binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–60. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishitsuka T, Ikuta T, Ariga H, Matsumoto K. Serum tenascin-X strongly binds to vascular endothelial growth factor. Biol Pharm Bull. 2009;32:1004–11. doi: 10.1248/bpb.32.1004. [DOI] [PubMed] [Google Scholar]
- 42.Demidova-Rice TN, Geevarghese A, Herman IM. Bioactive peptides derived from vascular endothelial cell extracellular matrices promote microvascular morphogenesis and wound healing in vitro. Wound Repair Regen. 2011;19:59–70. doi: 10.1111/j.1524-475X.2010.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115(1):12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 44.Eming SA, Lauer G, Cole M, et al. Increased levels of the soluble variant of the vascular endothelial growth factor receptor VEGFR-1 are associated with a poor prognosis in wound healing. J Invest Dermatol. 2004;123:799–802. doi: 10.1111/j.0022-202X.2004.23310.x. [DOI] [PubMed] [Google Scholar]
- 45.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanft JR, Pollak RA, Barbul A, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care. 2008;17(1):30–2. 34–7. doi: 10.12968/jowc.2008.17.1.27917. [DOI] [PubMed] [Google Scholar]
- 47.Mineur P, Colige AC, Deroanne CF, et al. Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. J Cell Biol. 2007;179:1261–73. doi: 10.1083/jcb.200703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senger D, Galli S, Dvorak A, Perruzzi C, Harvey V, Dvorak H. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 49.Draper BK, Komurasaki T, Davidson MK, Nanney LB. Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J Cell Biochem. 2003;89:1126–37. doi: 10.1002/jcb.10584. [DOI] [PubMed] [Google Scholar]
- 50.Shao H, Yi XM, Wells A. Epidermal growth factor protects fibroblasts from apoptosis via PI3 kinase and Rac signaling pathways. Wound Repair Regen. 2008;16:551–8. doi: 10.1111/j.1524-475X.2008.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 52.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–74. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 53.Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346–52. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahin U, Weskamp G, Kelly K, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of 6 EGFR ligands. J Cell Biol. 2004;164(5):769–79. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Alessio S, Gerasi L, Blasi F. uPAR-deficient mouse keratinocytes fail to produce EGFR-dependent laminin-5, affecting migration in vivo and in vitro. J Cell Sci. 2008;121(Pt 23):3922–32. doi: 10.1242/jcs.037549. [DOI] [PubMed] [Google Scholar]
- 57.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–63. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 58.Kajanne R, Miettinen P, Mehlem A, et al. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J Cell Physiol. 2007;212(2):489–97. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- 59.Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y. Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. J Invest Dermatol. 2008;128:717–27. doi: 10.1038/sj.jid.5701069. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–7. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 61.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 62.Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005;4:e8. [PMC free article] [PubMed] [Google Scholar]
- 63.Shirakata Y, Kimura R, Nanba D, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118(Pt 11):2363–70. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 64.Okwueze MI, Cardwell NL, Pollins AC, Nanney LB. Modulation of porcine wound repair with a transfected ErbB3 gene and relevant EGF-like ligands. J Invest Dermatol. 2007;127:1030–41. doi: 10.1038/sj.jid.5700637. [DOI] [PubMed] [Google Scholar]
- 65.Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–50. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higashiyama S, Abraham JA, Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 1993;122:933–40. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aviezer D, Yayon A. Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc Natl Acad Sci U S A. 1994;91:12173–7. doi: 10.1073/pnas.91.25.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–84. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–8. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 70.Miller LS, Sorensen OE, Liu PT, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174:6137–43. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- 71.Boissel JP, Ohly D, Bros M, Godtel-Armbrust U, Forstermann U, Frank S. The neuronal nitric oxide synthase is upregulated in mouse skin repair and in response to epidermal growth factor in human HaCaT keratinocytes. J Invest Dermatol. 2004;123:132–9. doi: 10.1111/j.0022-202X.2004.22731.x. [DOI] [PubMed] [Google Scholar]
- 72.Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hashimoto K. So-called biological dressing effects of cultured epidermal sheets are mediated by the production of EGF family, TGF-beta, and VEGF. J Dermatol Sci. 2003;32:209–15. doi: 10.1016/s0923-1811(03)00103-8. [DOI] [PubMed] [Google Scholar]
- 73.Liu J, Rich CB, Buczek-Thomas JA, Nugent MA, Panchenko MP, Foster JA. Heparin-binding EGF-like growth factor regulates elastin and FGF-2 expression in pulmonary fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1106–15. doi: 10.1152/ajplung.00180.2003. [DOI] [PubMed] [Google Scholar]
- 74.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411–420. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 75.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing–past, present and future perspectives. Surgeon. 2008;6:172–7. doi: 10.1016/s1479-666x(08)80114-x. [DOI] [PubMed] [Google Scholar]
- 76.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-beta. Expert Rev Mol Med. 2003;5:1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 77.Attisano L, Wrana JL. Signal Transduction by the TGF-beta Superfamily. Science. 2002;296:1646–7. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 78.McDowall M, Edwards NM, Jahoda CA, Hynd PI. The role of activins and follistatins in skin and hair follicle development and function. Cytokine Growth Factor Rev. 2008;19:415–26. doi: 10.1016/j.cytogfr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 79.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 80.Moustakas A, Heldin CH. Dynamic control of TGF-beta signaling and its links to the cytoskeleton. FEBS Lett. 2008;582:2051–65. doi: 10.1016/j.febslet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 81.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLean CA, Cleland H, Moncrieff NJ, Barton RJ, de Kretser DM, Phillips DJ. Temporal expression of activin in acute burn wounds—from inflammatory cells to fibroblasts. Burns. 2008;34:50–5. doi: 10.1016/j.burns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Mukhopadhyay A, Chan SY, Lim IJ, Phillips DJ, Phan TT. The role of the activin system in keloid pathogenesis. Am J Physiol Cell Physiol. 2007;292:C1331–8. doi: 10.1152/ajpcell.00373.2006. [DOI] [PubMed] [Google Scholar]
- 84.Cowin AJ, Hatzirodos N, Holding CA, et al. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J Invest Dermatol. 2001;117:1282–9. doi: 10.1046/j.0022-202x.2001.01501.x. [DOI] [PubMed] [Google Scholar]
- 85.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 86.Watterson KR, Lanning DA, Diegelmann RF, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15:607–16. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 87.Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation, and G-protein signaling. J Cell Sci. 2001;114(Pt 15):2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 88.Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007;48(1):53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Cohen MA, Eaglstein WH. Recombinant human platelet-derived growth factor gel speeds healing of acute full-thickness punch biopsy wounds. J Am Acad Dermatol. 2001;45:857–62. doi: 10.1067/mjd.2001.117721. [DOI] [PubMed] [Google Scholar]
- 90.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 91.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies, and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Gelse K, Poschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55:1595–611. doi: 10.1016/j.addr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Lindberg K, Badylak SF. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns. 2001;27:254–66. doi: 10.1016/s0305-4179(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 96.Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–6. doi: 10.1203/PDR.0b013e31816c5bc3. [DOI] [PubMed] [Google Scholar]
- 97.Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34:1544–56. doi: 10.1016/s1357-2725(02)00054-7. [DOI] [PubMed] [Google Scholar]
- 98.Kakagia DD, Kazakos KJ, Xarchas KC, et al. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications. 2007;21:387–91. doi: 10.1016/j.jdiacomp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Stompro BE, Hansbrough JF, Boyce ST. Attachment of peptide growth factors to implantable collagen. J Surg Res. 1989;46:413–21. doi: 10.1016/0022-4804(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 100.Pandit A, Ashar R, Feldman D, Thompson A. Investigation of acidic fibroblast growth factor delivered through a collagen scaffold for the treatment of full-thickness skin defects in a rabbit model. Plast Reconstr Surg. 1998;101:766–75. doi: 10.1097/00006534-199803000-00028. [DOI] [PubMed] [Google Scholar]
- 101.Ono I, Tateshita T, Inoue M. Effects of a collagen matrix containing basic fibroblast growth factor on wound contraction. J Biomed Mater Res. 1999;48:621–30. doi: 10.1002/(sici)1097-4636(1999)48:5<621::aid-jbm5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 102.Kanematsu A, Yamamoto S, Ozeki M, et al. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 103.Inoue M, Ono I, Tateshita T, Kuroyanagi Y, Shioya N. Effect of a collagen matrix containing epidermal growth factor on wound contraction. Wound Repair Regen. 1998;6:213–22. doi: 10.1046/j.1524-475x.1998.60307.x. [DOI] [PubMed] [Google Scholar]
- 104.Yao C, Roderfeld M, Rath T, Roeb E, Bernhagen J, Steffens G. The impact of proteinase-induced matrix degradation on the release of VEGF from heparinized collagen matrices. Biomaterials. 2006;27:1608–16. doi: 10.1016/j.biomaterials.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 105.Yao C, Markowicz M, Pallua N, Noah EM, Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29:66–74. doi: 10.1016/j.biomaterials.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 106.Sun B, Chen B, Zhao Y, et al. Crosslinking heparin to collagen scaffolds for the delivery of human platelet-derived growth factor. J Biomed Mater Res B Appl Biomater. 2009;91:366–72. doi: 10.1002/jbm.b.31411. [DOI] [PubMed] [Google Scholar]
- 107.Ishikawa T, Terai H, Kitajima T. Production of a biologically active epidermal growth factor fusion protein with high collagen affinity. J Biochem. 2001;129:627–33. doi: 10.1093/oxfordjournals.jbchem.a002900. [DOI] [PubMed] [Google Scholar]
- 108.Sun W, Lin H, Xie H, et al. Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors. 2007;25:309–18. doi: 10.1080/08977190701803885. [DOI] [PubMed] [Google Scholar]
- 109.Zhao W, Chen B, Li X, et al. Vascularization and cellularization of collagen scaffolds incorporated with two different collagen-targeting human basic fibroblast growth factors. J Biomed Mater Res A. 2007;82:630–6. doi: 10.1002/jbm.a.31179. [DOI] [PubMed] [Google Scholar]
- 110.Kotch FW, Raines RT. Self-assembly of synthetic collagen triple helices. Proc Natl Acad Sci U S A. 2006;103:3028–33. doi: 10.1073/pnas.0508783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6(30):1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg. 2010;34:632–4. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 113.Pandit A, Feldman D, Listinsky C, Thompson A. The effect on wound healing by a modified fibrin scaffold delivering acidic fibroblast growth factor (FGF-1) J Bioactive Compatible Polymers. 1997;12:99–111. [Google Scholar]
- 114.Quirinia A, Viidik A. The effect of recombinant basic fibroblast growth factor (bFGF) in fibrin adhesive vehicle on the healing of ischaemic and normal incisional skin wounds. Scand J Plast Reconstr Surg Hand Surg. 1998;32:9–18. doi: 10.1080/02844319850158903. [DOI] [PubMed] [Google Scholar]
- 115.Ahn ST, Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg. 1990;24:17–23. doi: 10.1097/00000637-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105:249–59. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 117.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol. 2005;167:1575–86. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–82. [PubMed] [Google Scholar]
- 119.Gwak SJ, Kim SS, Sung K, Han J, Choi CY, Kim BS. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplant. 2005;14:809–17. doi: 10.3727/000000005783982521. [DOI] [PubMed] [Google Scholar]
- 120.Mogford JE, Tawil B, Jia S, Mustoe TA. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen. 2009;17:405–10. doi: 10.1111/j.1524-475X.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 121.Mazlyzam AL, Aminuddin BS, Fuzina NH, et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33:355–63. doi: 10.1016/j.burns.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 122.Hojo M, Inokuchi S, Kidokoro M, et al. Induction of vascular endothelial growth factor by fibrin as a dermal substrate for cultured skin substitute. Plast Reconstr Surg. 2003;111:1638–45. doi: 10.1097/01.PRS.0000053842.90564.26. [DOI] [PubMed] [Google Scholar]
- 123.Borenstein JT, Megley K, Wall K, et al. Tissue equivalents based on cell-seeded biodegradable microfluidic constructs. Materials. 2010;3:1833–44. [Google Scholar]
- 124.Ono K, Saito Y, Yura H, et al. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res. 2000;49:289–95. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 125.Obara K, Ishihara M, Ishizuka T, et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials. 2003;24:3437–44. doi: 10.1016/s0142-9612(03)00220-5. [DOI] [PubMed] [Google Scholar]
- 126.Swarbrick J. Encyclopedia of pharmaceutical technology. 3. New York: Informa Healthcare; 2007. [Google Scholar]
- 127.Piaggesi A, Baccetti F, Rizzo L, Romanelli M, Navalesi R, Benzi L. Sodium carboxyl-methyl-cellulose dressings in the management of deep ulcerations of diabetic foot. Diabet Med. 2001;18:320–4. doi: 10.1046/j.1464-5491.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 128.Ladin D. Becaplermin gel (PDGF-BB) as topical wound therapy. Plastic Surgery Educational Foundation DATA Committee. Plast Reconstr Surg. 2000;105:1230–1. doi: 10.1097/00006534-200003000-00065. [DOI] [PubMed] [Google Scholar]
- 129.Castronuovo JJ, Jr, Ghobrial I, Giusti AM, Rudolph S, Smiell JM. Effects of chronic wound fluid on the structure and biological activity of becaplermin (rhPDGF-BB) and becaplermin gel. Am J Surg. 1998;176(2A Suppl):61S–67. doi: 10.1016/s0002-9610(98)00175-5. [DOI] [PubMed] [Google Scholar]
- 130.Kuhn MA, Page L, Nguyen K, Ko F, Wang X, Wachtel TL, Robson MC. Basic fibroblast growth factor in a carboxymethylcellulose vehicle reverses the bacterial retardation of wound contraction. Wounds. 2001;13(2):73–80. [Google Scholar]
- 131.Blokhuis TJ. Formulations and delivery vehicles for bone morphogenetic proteins: latest advances and future directions. Injury. 2009;40(Suppl 3):S8–11. doi: 10.1016/S0020-1383(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 132.Lai WF, Lin MC. Nucleic acid delivery with chitosan and its derivatives. J Control Release. 2009;134:158–68. doi: 10.1016/j.jconrel.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 133.Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–84. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 134.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–8. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 135.Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–15. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 136.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9:857–79. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park CJ, Clark SG, Lichtensteiger CA, Jamison RD, Johnson AJ. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009;5:1926–36. doi: 10.1016/j.actbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 139.Hong JP, Kim YW, Lee SK, Kim SH, Min KH. The effect of continuous release of recombinant human epidermal growth factor (rh-EGF) in chitosan film on full thickness excisional porcine wounds. Ann Plast Surg. 2008;61:457–62. doi: 10.1097/SAP.0b013e31815bfeac. [DOI] [PubMed] [Google Scholar]
- 140.Kühtreiber WM, Lanza RP, Chick WL. Cell encapsulation technology and therapeutics. Boston, MA: Birkhauser; 1999. p. 450. [Google Scholar]
- 141.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 142.Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300:2647–62. doi: 10.1001/jama.2008.778. [DOI] [PubMed] [Google Scholar]
- 143.Lee WR, Park JH, Kim KH, et al. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen. 2009;17:505–10. doi: 10.1111/j.1524-475X.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 144.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs. 2001;25:558–65. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 145.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216–21. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 146.Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25:1407–14. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Wnek GE, Bowlin GL. Encyclopedia of biomaterials and biomedical engineering. 2. New York: Informa Healthcare USA; 2008. [Google Scholar]
- 148.Simamora P, Chern W. Poly-L-lactic acid: an overview. J Drugs Dermatol. 2006;5:436–40. [PubMed] [Google Scholar]
- 149.Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 150.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 151.Atala A, Lanza RP. Methods of tissue engineering. San Diego, CA: Academic Press; 2001. p. 1285. [Google Scholar]
- 152.Kanczler JM, Barry J, Ginty P, Howdle SM, Shakesheff KM, Oreffo RO. Supercritical carbon dioxide generated vascular endothelial growth factor encapsulated poly(DL-lactic acid) scaffolds induce angiogenesis in vitro. Biochem Biophys Res Commun. 2007;352(1):135–41. doi: 10.1016/j.bbrc.2006.10.187. [DOI] [PubMed] [Google Scholar]
- 153.Satchi-Fainaro R, Duncan R. Polymer therapeutics I: polymers as drugs, conjugates and gene delivery systems. Vol. 192. New York: Springer; 2006. p. 204. [DOI] [PubMed] [Google Scholar]
- 154.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–43. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zisch AH, Lutolf MP, Ehrbar M, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 156.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–44. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 157.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704–16. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 158.Hee Na D, Seok Youn Y, Bok Lee I, Ji Park E, Jeon Park C, Choon Lee K. Effect of molecular size of PEGylated recombinant human epidermal growth factor on the biological activity and stability in rat wound tissue. Pharm Dev Technol. 2006;11:513–9. doi: 10.1080/10837450600941053. [DOI] [PubMed] [Google Scholar]
- 159.Lee PY, Cobain E, Huard J, Huang L. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther. 2007;15:1189–94. doi: 10.1038/sj.mt.6300156. [DOI] [PubMed] [Google Scholar]
- 160.Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bashir R, Vale PR, Isner JM, Losordo DW. Angiogenic gene therapy: pre-clinical studies and phase I clinical data. Kidney Int. 2002;61(1 Suppl):S110–4. doi: 10.1046/j.1523-1755.2002.0610s1110.x. [DOI] [PubMed] [Google Scholar]
- 162.Liu L, Sang M, Shan B, Liu S. Advances in viral-vector systemic cytokine gene therapy against cancer. Vaccine. 2010;28:3883–7. doi: 10.1016/j.vaccine.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 163.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol. 1999;112:297–302. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 165.Nanney LB, Paulsen S, Davidson MK, Cardwell NL, Whitsitt JS, Davidson JM. Boosting epidermal growth factor receptor expression by gene gun transfection stimulates epidermal growth in vivo. Wound Repair Regen. 2000;8:117–27. doi: 10.1046/j.1524-475x.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 166.Dileo J, Miller TE, Jr, Chesnoy S, Huang L. Gene transfer to subdermal tissues via a new gene gun design. Hum Gene Ther. 2003;14:79–87. doi: 10.1089/10430340360464732. [DOI] [PubMed] [Google Scholar]
- 167.Eriksson E, Yao F, Svensjo T, et al. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- 168.Vranckx JJ, Yao F, Petrie N, et al. In vivo gene delivery of Ad-VEGF121 to full-thickness wounds in aged pigs results in high levels of VEGF expression but not in accelerated healing. Wound Repair Regen. 2005;13:51–60. doi: 10.1111/j.1067-1927.2005.130107.x. [DOI] [PubMed] [Google Scholar]
- 169.Lin MP, Marti GP, Dieb R, et al. Delivery of plasmid DNA expression vector for keratinocyte growth factor-1 using electroporation to improve cutaneous wound healing in a septic rat model. Wound Repair Regen. 2006;14:618–24. doi: 10.1111/j.1743-6109.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 170.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009;17:651–7. doi: 10.1038/mt.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun L, Xu L, Chang H, et al. Transfection with aFGF cDNA improves wound healing. J Invest Dermatol. 1997;108:313–8. doi: 10.1111/1523-1747.ep12286471. [DOI] [PubMed] [Google Scholar]
- 172.Jeschke MG, Herndon DN. The combination of IGF-I and KGF cDNA improves dermal and epidermal regeneration by increased VEGF expression and neovascularization. Gene Ther. 2007;14:1235–42. doi: 10.1038/sj.gt.3302972. [DOI] [PubMed] [Google Scholar]
- 173.Jeschke MG, Klein D. Liposomal gene transfer of multiple genes is more effective than gene transfer of a single gene. Gene Ther. 2004;11:847–55. doi: 10.1038/sj.gt.3302229. [DOI] [PubMed] [Google Scholar]
- 174.Romano Di Peppe S, Mangoni A, Zambruno G, et al. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–7. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- 175.Liechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated overexpression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J Invest Dermatol. 1999;113:375–83. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 176.Deodato B, Arsic N, Zentilin L, et al. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 2002;9:777–85. doi: 10.1038/sj.gt.3301697. [DOI] [PubMed] [Google Scholar]
- 177.Galeano M, Deodato B, Altavilla D, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546–55. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- 178.Eming SA, Medalie DA, Tompkins RG, Yarmush ML, Morgan JR. Genetically modified human keratinocytes overexpressing PDGF-A enhance the performance of a composite skin graft. Hum Gene Ther. 1998;9:529–39. doi: 10.1089/hum.1998.9.4-529. [DOI] [PubMed] [Google Scholar]
- 179.Doukas J, Chandler LA, Gonzalez AM, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther. 2001;12:783–98. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 180.Gu DL, Nguyen T, Phillips ML, Chandler LA, Sosnowski B. Matrix-Immobilized Growth Factor Gene Therapy Enhances Tissue Repair. Wounds. 2004;16:34. [Google Scholar]
- 181.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 182.Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine. 2004;29:E284–8. doi: 10.1097/01.brs.0000131217.61390.2c. [DOI] [PubMed] [Google Scholar]
- 183.Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348(9024):370–4. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 184.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 185.Hirshberg J, Coleman J, Marchant B, Rees RS. TGF-beta3 in the treatment of pressure ulcers: a preliminary report. Adv Skin Wound Care. 2001;14:91–5. doi: 10.1097/00129334-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 186.Occleston NL, O’Kane S, Goldspink N, Ferguson MW. New therapeutics for the prevention and reduction of scarring. Drug Discov Today. 2008;13:973–81. doi: 10.1016/j.drudis.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 187.Eming SA, Lee J, Snow RG, Tompkins RG, Yarmush ML, Morgan JR. Genetically modified human epidermis overexpressing PDGF-A directs the development of a cellular and vascular connective tissue stroma when transplanted to athymic mice—implications for the use of genetically modified keratinocytes to modulate dermal regeneration. J Invest Dermatol. 1995;105:756–63. doi: 10.1111/1523-1747.ep12325550. [DOI] [PubMed] [Google Scholar]