Abstract
The anti-angiogenic drug sunitinib is a receptor tyrosine-kinase inhibitor with significant, yet not curative, therapeutic impacts in metastatic renal cell carcinoma (mRCC). Sunitinib is also an immunomodulator, potently reversing myeloid-derived suppressor cell (MDSC) accumulation and T-cell inhibition in the blood even of non-responder RCC patients. We observed that sunitinib similarly prevented MDSC accumulation and restored normal T-cell function to spleens of tumor-bearing mice, independent of sunitinib's capacity to inhibit tumor progression (RENCA>CT26>4T1). Both monocytic and neutrophilic splenic MDSC were highly repressible by sunitinib. In contrast, MDSC within the microenvironment of 4T1 tumors or human RCC tumors proved highly resistant to sunitinib, and ambient T-cell function remained suppressed. Proteomic analyses comparing tumor to peripheral compartments demonstrated that GM-CSF predicted sunitinib resistance, and recombinant GM-CSF conferred sunitinib resistance to MDSC in vivo and in vitro. MDSC conditioning with GM-CSF uniquely inhibited STAT3 and promoted STAT5 activation, and STAT5ab(null/null) MDSC were rendered sensitive to sunitinib in the presence of GM-CSF in vitro. We conclude that compartment-dependent GM-CSF exposure in resistant tumors may account for sunitinib's regionalized impact upon host MDSC modulation, and hypothesize that ancillary strategies to decrease such regionalization will enhance sunitinib's potency as an immunomodulator and a cancer therapy.
Keywords: RCC, myeloid derived suppressor cells (MDSC), sunitinib, STAT, GM-CSF
Introduction
Angiogenesis is a crucial step in tumor progression; hence the rational development of several angiogenesis inhibitors. One such drug, sunitinib (Sutent, Pfizer), which inhibits signaling through receptor tyrosine kinases (RTKs) including VEGFR1-3, PDGFR, ckit, flt3, and m-CSF receptors, is typically first-line therapy for metastatic renal cell carcinoma (RCC) (1–3). Yet all patients eventually progress on sunitinib, which is thought to reflect an adaptive tumor resistance to angiogenesis inhibition (4–6). While the basis of such resistance is incompletely understood, accumulating evidence suggests that host myeloid-derived suppressor cells (MDSC) are recruited by tumors to mediate resistance to anti-angiogenic drugs (7,8). Indeed, MDSC themselves can promote angiogenesis (9–12), in addition to serving as pivotal agents of tumor-induced T2-type biasing and the escape from cell-mediated immunity (13–15).
We recently demonstrated that sunitinib therapy significantly reversed RCC-induced MDSC accumulation in patients' peripheral blood, correlating with improved peripheral T-cell function (16). Sunitinib inhibited peripheral accumulation of all currently identified CD33+ human-MDSC subsets, including immature (lineage negative), neutrophilic (CD15+CD14−), and monocytic (CD14+HLADR−/dim) MDSC (16–23). Such pan-inhibition was unlikely to be mediated solely through an indirect anti-tumor effect, since sunitinib therapy inhibited peripheral MDSC accumulation even when RCC patients displayed tumor progression (i.e., sunitinib treatment failure) (16). We therefore investigated whether sunitinib's remarkable impact on peripheral MDSC was also evident when animal tumors were treated with sunitinib, and whether sunitinib's seemingly decisive negative modulation of MDSC extended into the tumor compartment.
We here report that sunitinib is uniformly effective for suppressing peripheral (splenic) CD11b+Gr-1+ MDSC accumulation in all tested tumor models, despite widely ranging anti-tumor effects among these models. Sunitinib inhibited proliferation of Ly6G− monocytic (m-) MDSC and also impaired survival of Ly6G+ neutrophilic (n-) MDSC. We also observed, however, that sunitinib's dramatic abrogation of splenic MDSC and normalization of bystander T cell function did not extend to the tumor microenvironment in the most resistant 4T1 model or in human RCC. MDSC resistance to sunitinib corresponded to compartmental availability of GM-CSF, and recombinant GM-CSF itself conferred sunitinib resistance in vitro and in vivo. GM-CSF-induced sunitinib-resistance in MDSC was STAT5 mediated, as it was negated in STAT5ab(null/null) MDSC, thus providing a likely rescue mechanism for MDSC in the face of STAT3 inhibition by sunitinib (24). Such regional disparities may explain how residual intratumoral MDSC contribute to anti-angiogenic resistance despite pronounced drug-mediated declines in peripheral MDSC.
Materials and Methods
Mice, tumors, and treatment
Experiments performed under institutionally approved animal research committee protocols adhered to USDA guidelines. Female BALB/c mice from NCI, Frederick, MD were maintained pathogen-free (USDA) and studied at 8–12wks. 4T1-mammary, CT26-colonic, and RENCA-renal carcinomas syngeniec to BALB/c mice were maintained and injected into mice as previously described (25). Sunitinib 4, 20, or 40mg/kg/day i.p. was initiated for 9days total after tumors reached 7–10mm diameter. Intraperitoneal treatment yielded the same MDSC reductions as oral treatment. Spleens, BM and tumors were processed as previously (25). STAT5ab(null/null) and (WT) BM was obtained as previously (26).
Reagents
Culture medium consisted of RPMI-1640+10%FCS and conventional additives (16,27). Pan-T-cell isolation kit and magnetic beads conjugated to anti-CD11b, -FITC, or -APC were from Miltenyi Biotec (Auburn, CA). Functional grade anti-mouse-CD3e and −CD28 were from BD Pharmingen (San Jose, CA). rmflt3L, rmSCF, rmIL-6, rmG-CSF, rmGM-CSF were from Peprotech (Rocky Hill, NJ). CFSE was from Invitrogen (San Diego, CA). Luminex Bioplex Mouse Cytokine Arrays were from Biorad (Hercules, CA). Proteome Profile Arrays were from RnD Systems (Minneapolis, MN). Fluorochrome-coupled antibodies were purchased from ebiosciences (San Diego, CA) or BD Biosciences (San Jose, CA). FACS data was collected on FACSCalibur (BD) and analyzed using Cellquest software (BD) or FlowJo software (Tree Star, Ashland, OR).
Determination of T-cell response
IFN-gamma production was assayed following polyclonal stimulation as previously (16). Proliferation was assayed with either CFSE dilutions or tritiated thymidine incorporation as previously (25,28).
In vivo proliferation and viability assays
One hour following sunitinib treatment, mice were injected i.p. with 1mg of sterile BrdU/mouse. Four hours following BrdU injection, cells obtained from spleen, BM and in some cases tumor and blood were stained according to the BrdU-APC kit (BD). AnnexinV staining was done as previously (16).
MDSC subset isolation and staining
Splenic cell suspensions from 4T1+ mice were depleted of T-cells, B cells, macrophages, and dendritic cells using APC-labeled antibodies to CD3, CD4, CD8, and MHCII and anti-APC magnetic beads. Remaining cells contained mostly MDSC, and n-MDSC were isolated using anti-Ly6G-FITC and anti-FITC beads. m-MDSC were isolated from remaining cells using anti-CD11b magnetic beads. LS magnetic columns were from Miltenyi. Cells were then stained for FACS analysis or were adhered to glass slides by cytospin. Adherent cells were air dried then stained using Fisher Diagnostics Hema 3 Manual Staining Kit which is comparable to the Wright-Giemsa method.
Cytokine analysis using proteome profile array
Equal volumes of blood obtained via cardiac puncture were added to ETDA to prevent clotting. Cell-free plasma obtained following centrifugation at 4°C at 13,000 for 10 minutes was stored at −80°C. 500uL of pooled plasma was added to each membrane in the array. SuperSignal West Femto Maximum Sensitivity Substrate from ThermoScientific was used for development. Additionally, lysates from frozen tumor tissue were obtained using the provided lysis buffer recipe with protease (ThermoScientific) and phosphatase inhibitors (Pierce) added. Protein was assayed using the Biorad DC Protein Assay kit per the manual. 150ug of protein was added to each membrane.
Cytokine analysis using Bioplex array
Pooled plasma obtained as above. Spleen and tumor tissue was immediately frozen in eppendorf tubes on dry ice. Lysates were obtained from thawed, homogenized/sonicated tissue in the same lysis buffer as above and protein quantified as above. Plasma was assayed according to the Bio-Plex Pro custom assay (Biorad). Tissue lysates were brought to 1mg/mL using 1xPBS with 0.5% BSA, and 50uL added to each well. A separate standard curve was made using lysis buffer as diluent for the analysis of cell lysates. Data was acquired and analyzed on a Luminex device using the Biorad Bio-Plex System and Bio-Plex Manager software.
pSTAT signaling analysis in MDSC
BM-derived MDSC were cultured in stem cell factor+flt3L +/− either IL-6, G-CSF, or GM-CSF. pStat staining was done according to the BD Phosflow Fix Buffer I and Perm Buffer III products. Briefly, culture plates were spun down and supernatants removed. Fix buffer I was added to wells for 10min at 37°. Washed cells were surface stained (CD11b and Gr1), fixed/permeabilized with ice cold Perm Buffer III for 30 minutes on ice, then Fc blocked with 10% heat-inactivated rat serum, rat IgG, and anti-CD16/32 antibodies prior to staining. Anti-pStat antibodies and isotypes were Alexa-Fluor-647 conjugated.
Analysis of human RCC tumor tissue
Freshly explanted clear-cell RCC tumors were digested. Cells were stained for MDSC or cultured at 1 million lymphocytes/1mL in complete RPMI media and stimulated for IFNγ production as above. Some tumors (RCC- 0885, 0803, 0958, and 3081427) were cultured short-term (1–2 passages) and GM-CSF was quantified in resultant cell-free supernatants, or in supernatants derived from the long-term clear-cell RCC line, SK-RC26b, (previously acquired from Dr. Neil Bander at Cornell Medical Center (29)) in the presence or absence of sunitinib.
Biostatistics
Unless indicated otherwise, all experiments were completed at least 3 times, with at least 5 mice per group. Results were pooled and the mean +/− standard deviation were expressed graphically as columns. Treatment groups were compared using t-test for two samples assuming equal variances. A 2-tailed p-value less than 0.05 was deemed significant.
Results
Sunitinib reverses systemic MDSC-mediated immune suppression in mice bearing renal and non-renal tumors
We first studied sunitinib's ability to act as a broad immunopotentiator in several mouse tumor models. Treatment of either Renca-kidney, CT26-colon or 4T1-breast tumor-bearing mice with sunitinib (40mg/kg) daily for 9days significantly reduced both the percentage and total numbers of CD11b+Gr1+ MDSC detected in the spleen. Significant MDSC reductions also occurred in Renca+ or CT26+ with 20mg/kg sunitinib (Figure 1A). MDSC reductions were associated with significant disinhibition of T-cells which were otherwise suppressed in the tumor-bearing state. T-cells from tumor-bearing mice were less able to produce IFNγ in response to polyclonal stimulation with anti-CD3/28 when compared to naïve, non-tumor bearing mice. Such T-cell suppression was reversible with either in vivo MDSC depletion using sunitinib, or in vitro MDSC depletion using anti-Gr-1 magnetic beads; finally, bead-isolated MDSC could be introduced to suppress T-cells from naïve mice as well (Figure 1B). Deficits in T-cell proliferation were also attributable to the presence of MDSC (Figure 1C), and CD4+ and CD8+ T-cells regained function when MDSC were depleted with sunitinib or mechanically (Figure 1D). These findings suggest that MDSC are major mediators of T-cell suppression in tumor-bearing hosts, and that suppressed T-cells can be rendered functional if activated in the absence of MDSC. In addition, higher doses of sunitinib were required in 4T1+ mice to fully reverse immune suppression.
Figure 1. Sunitinib reverses immune suppression in tumor-bearing hosts.
(A) Mice were given 4T1, CT26, or Renca tumors (s.c.) and sunitinib initiated for 9days. MDSC analyzed by FACS at the end of treatment. (B) Splenocytes from naïve, untreated, or sunitinib-treated tumor-bearing mice; or, from 4T1+ mice depleted for MDSC (magnetic beads), or from naïve mice with isolated MDSC added in, were stimulated with anti-CD3/28, and IFNγ detected after 72 hours by FACS intracellular staining. (C) Proliferation induced specifically in response to CD3/28-stimulation in each of the above conditions is shown. (D) Representative data from 2 experiments showing CFSE dilutions in CD4 and CD8-gated splenocytes.
Sunitinib inhibits intrasplenic proliferation of m-MDSC
We next asked whether sunitinib functions to inhibit MDSC expansion in vivo. The proliferative rate of MDSC was kinetically quantified by the percentage of BrdU+ cells. Within the MDSC population, cells brightest for Gr1 staining (Gr1hi) were relatively non-proliferative in naïve and tumor-bearing mice (pink squares, <10%BrdU+, green squares and triangles, 10–20%BrdU+ respectively)(Figure 2A,left=spleen, right=bone marrow (BM)). In contrast, MDSC which were dim for Gr1 (Gr1lo) were very proliferative in the spleens and BM of tumor-bearing mice (blue squares and triangles, Figure2A-left and right, ~50%BrdU+) compared to naïve mice, where proliferation was only present in the BM but not in spleen (red circles, <10%BrdU+, Figure 2A-left, p<.0002 compared to tumor-bearing), indicating pronounced proliferative pathology of splenic Gr1lo MDSC in the tumor-bearing state. Sunitinib reversed this pathology by significantly inhibiting the expansion of splenic Gr1lo MDSC on day 6 following treatment (blue triangles, p<.02, Figure 2A). The analysis undertaken is shown in Figure 2B. Sunitinib did not have a sustained impact on BM MDSC proliferation, which was reflected in sunitinib's (40mg/kg) lack of impact on the %MDSC in naïve or tumor+ mouse BM (data not shown).
Figure 2. Anti-proliferative and pro-apoptotic effects of sunitinib on MDSC subsets in the spleen.
(A,B) After 3, 6, and 9 days +/− sunitinib initiation to 4T1+ mice, mice were pulsed with BrdU (1 hour following sunitinib dosing) for 4 hours. Splenic and BM CD11b+Gr1+lo and CD11b+Gr1+hi cells were gated on and BrdU positivity determined by FACS. Cells from mice not given BrdU were used as staining controls. (C) 4T1+ splenocytes were stained for CD11b, Gr1, Ly6G, and F4/80 before and after MDSC subset isolation with magnetic beads. Representative FACS data and cytospins shown. n=2. (D) Mice were treated as above for (A) only splenocytes were stained for CD11b, Gr1, and annexinV and immediately ran for FACS.
These findings suggested that MDSC could be functionally divided into two groups based on their proliferative potential in vivo. Upon isolation, as previously reported (30,31) Ly6G+ n-MDSC were indeed Gr1hi, Ly6G+, and F4/80−, and displayed early or completed polymorphonuclear features on cytospins (Figure 2C), consistent with their lower rate of proliferation. Ly6G− m-MDSC were Gr1lo, Ly6G− and evenly distributed in regards to F4/80 expression and monocytic versus immature morphology in cytospins (Figure 2C), consistent which their higher rate of proliferation. Thus, splenic m-MDSC were highly proliferative compared to n-MDSC, and sunitinib inhibited this proliferation, significantly contributing to the overall reduction in splenic MDSC accumulation.
Sunitinib impairs the viability of splenic n-MDSC
We found over half the Gr1hi n-MDSC in the spleens of naïve mice were undergoing apoptosis, consistent with their being normal neutrophils with a rapid rate of turnover in naïve animals (Figure 2D). The rate of n-MDSC apoptosis in tumor-bearing mice was significantly reduced compared to naïve, indicating that they have a prolonged lifespan in vivo. Sunitinib significantly reduced the viability of splenic n-MDSC in vivo on day 6 following treatment (p<.00005, Figure 2D). In contrast, m-MDSC generally had lower rates of apoptosis that were only modestly affected by sunitinib in vivo (naive= 25%, 4T1=15%, 4T1+Sunitinib=32% on average, data not shown).
Compared to spleen, intratumoral MDSC are sunitinib-resistant in resistant tumor models
In contrast to the global effect of sunitinib to improve splenic T-cell function via reductions in MDSC, the drug's effect on tumor growth varied considerably. Renca kidney tumors were disproportionately sensitive to sunitinib, with tumor shrinkage occurring at both 20 and 40mg/kg drug. In contrast, CT26 tumors continued to progress, albeit more slowly, during sunitinib treatment, and 4T1 tumor growth was not significantly impacted by sunitinib treatment (Figure 3A). Tumor cell sensitivity to drug in vitro was inversely related to in vivo sensitivity. As such, sunitinib's variable effects on tumor growth in vivo could not be predicted by its variable toxicity to tumor cells lines in vitro (Figure 3B).
Figure 3. MDSC in 4T1 tumor bed are relatively protected from sunitinib-mediated downregulation.
(A) Tumor growth in Renca+, CT26+ and 4T1+mice +/−sunitinib treatment. (B) Renca, CT26, and 4T1 tumor cells were seeded and allowed to adhere overnight. Sunitinib was added to cultures at various doses the following morning for 72 hours. At the end of treatment, the recovery of adherent cells (cells counted following trypsonization of washed cells) was counted, as well as the viability of all cells (adherent and non-adherent combined) determined with Annexin V and 7AAD FACS staining. (C) MDSC were measured as a percentage of viable, scatter-gated cells in tumors digested following the various doses of sunitinib treatment in each tumor model. (D) Left- Cells from spleen and tumor-derived suspensions from untreated-or sunitinib-treated 4T1+mice were stimulated in vitro. IFNγ production in T-cells was assayed and compared to that produced by T-cells from naïve spleen suspensions. Right- MDSC from untreated-4T1+ spleens or from untreated- and sunitinib-treated 4T1+ tumors were isolated using either magnetic bead-bound antibodies to Gr1, or FACS flow sorting, and then added to naïve T-cells. Cultures were stimulated and assayed for IFNγ production.
We then examined sunitinib's impact on cellular tumor constituents which remained following a 4mg/kg (Renca tumors only), 20mg/kg, or 40mg/kg daily treatment schedule. Unlike Renca and CT26 tumors, 4T1 tumors retained statistically equal amounts of MDSC following treatment at 20mg/kg; and, compared to splenic MDSC in 4T1+ mice, there was a much more modest decline in tumor-associated MDSC (Figure 3C) in response to 40mg/kg drug. In this relatively resistant model, tumor-infiltrating T-cells from sunitinib-treated mice did not function significantly better than those from untreated mice, in marked contrast to the improved splenic T-cell function obtainable from the same mice (Figure 3D-left). Indeed, intratumoral MDSC from sunitinib-treated 4T1+ mice retained T-cell suppressive capabilities equivalent to those from untreated mice (Figure 3D-right). Our findings suggest that even when sunitinib broadly reverses peripheral MDSC accumulation (16), intratumoral MDSC can be significantly less impacted.
GM-CSF is selectively expressed in resistant tumor microenvironment in vivo and uniquely protects MDSC in the presence of sunitinib in vitro
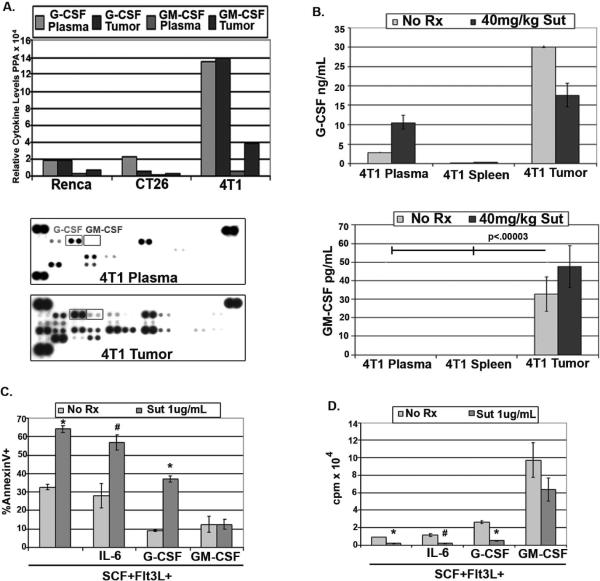
Despite their disparate susceptibilities to in vivo sunitinib exposure, purified splenic- and tumor-derived MDSC proved equally sensitive to sunitinib-mediated apoptosis in culture (not shown), prompting us to investigate regionally produced factors which could account for in vivo disparities. To determine whether the presence of a particular cytokine or group of cytokines could predict intratumoral sunitinib resistance, we used arrays to compare the relative levels of numerous cytokines in the plasma and tumor lysates of mice bearing Renca, CT26, or 4T1 tumors. 4T1+ mice contained an abundance of systemic and intratumoral G-CSF compared to mice bearing other tumor types (Figure 4A). In addition, GM-CSF was selectively present in the tumor bed of 4T1+ mice only (Figure 4A, representative blots below). Luminex assays of untreated and sunitinib-treated 4T1+ mice confirmed the selective presence of GM-CSF in 4T1 tumors versus plasma, and spleen (not shown) and quantified the amount of G- and GM-CSF in the plasma and tumors of 4T1+ untreated and sunitinib-treated mice (Figure 4B). The need to flush and disaggregate BM did not permit its direct comparison to the other tissues.
Figure 4. GM-CSF is unique to resistant 4T1-tumor microenvironment and selectively confers sunitinib resistance to MDSC in vitro.
(A) Relative levels of cytokines/chemokines from pooled plasma or tumor lysates from tumor-bearing mice using proteome profile array. Representative data shows G-CSF squared left, and GM-CSF squared right. (B) G- and GM-CSF quantification by Luminex in plasma and tumor lysates from untreated or sunitinib-treated 4T1+-mice. (C) BM containing MDSC was cultured in flt3L+SCF (25ng/mL each) either alone, or with IL-6 (25–50ng/mL), G-CSF (100ng/mL), or GM-CSF (10ng/mL) for 72 hours in the absence or presence of 1ug/mL of sunitinib. After 72 hours, gated CD11b+Gr1+ cells were assessed for apoptosis using annexinV and FACS. *p<.0003, #p<.02. (D) BM containing MDSC was treated as above and proliferation assayed. *p<.00002, #p<.004.
Because several host- and tumor-produced factors such as stem cell factor (SCF), IL-6, G-CSF, and GM-CSF have been implicated in MDSC accumulation (32), we cultured fresh BM containing MDSC in the presence of SCF and flt3 ligand to provide basal support, with the further addition of either IL-6, G-CSF, or GM-CSF +/− sunitinib. Sunitinib significantly impaired the viability of CD11b+Gr1+ MDSC, but GM-CSF, and to a lesser extent G-CSF, could partially restore MDSC viability when added to cultures (Figure 4C). Indeed, 10ng/mL of GM-CSF protected MDSC significantly better than G-CSF added at 100ng/mL. In addition, G-CSF and GM-CSF, when added to SCF and Flt3L, induced the strongest proliferative response in these cultures; and, while sunitinib totally prevented MDSC expansion in the presence of G-CSF, it had a relatively modest effect in the presence of GM-CSF (Figure 4D).
GM-CSF confers sunitinib-resistance to peripheral MDSC and relies on STAT5 signaling to rescue MDSC from sunitinib toxicity
4T1+ mice received systemic GM-CSF (i.p.) with and without sunitinib treatments to test whether systemic resistance to sunitinib would result. Indeed, daily GM-CSF significantly inhibited the remarkable reductions in splenic MDSC otherwise seen in response to sunitinib in 4T1+ mice (Figure 5A). The persistence of 30% MDSC in GM-CSF-treated mice was sufficient to significantly inhibit the recovery in T-cell function that was otherwise induced in sunitinib-treated 4T1+ mice (Figure 5B).
Figure 5. GM-CSF renders MDSC resistant to sunitinib via STAT5 signaling.
(A) Recombinant murine GM-CSF was given (10ug/mouse/day) i.p. for 10 days, beginning the day prior to sunitinib initiation. MDSC were quantified at the end of treatment as above. (B) T cell function following the treatments described in (A) was measured using intracellular cytokine staining for IFNγ as above. (C)BM containing MDSC was cultured as above in Figure 4C, and gated-CD11b+Gr1+ cells were assessed for pSTAT staining using intracellular phospho-flow and FACS (BD). Representative overlays of phopho-STAT staining from n=4 experiments. (D) BM from either CD45.2+ WT or STAT5ab (null/null) mice was allowed to engraft into lethally irradiated recipient CD45.1+ mice. Donor CD45.2+ BM cells were FACS sorted and cultured under the conditions listed for 72 hours. AnnexinV was measured in harvested cells via FACS.
To test whether a particular signaling profile could distinguish sunitinib-sensitive from sunitinib-insensitive MDSC, we performed intracellular staining for steady state levels of pY705STAT3 and pY694STAT5 expression in MDSC cultured in non-protective (SCF,Flt3L, +/− IL-6 or G-CSF) or protective (SCF,Flt3L, + GM-CSF) conditions. Consistent with our previous studies (24), MDSC maintained in the absence of GM-CSF displayed a STAT3-driven signaling signature (Figure 5C); and, consistent with prior reports, these STAT3-driven MDSC showed sensitivity to sunitinib, resulting in pSTAT3 downregulation (25). In contrast, GM-CSF dominantly activated pSTAT5 (Figure 5C) rather than pSTAT3, suggesting that STAT5 could provide an alternate survival signal for MDSC in the presence of sunitinib-mediated pSTAT3 inhibition in MDSC. This was formally tested by comparing sunitinib sensitivity in the presence of GM-CSF in WT versus STAT5ab(null/null) MDSC. We found that the absence of STAT5 rendered MDSC sensitive to sunitinib-mediated toxicity in the presence of GM-CSF, while having no significant effect on cultures driven by pSTAT3-promoting IL-6 and/or G-CSF (Figure 5D).
Tumor microenvironment limits sunitinib's anti-MDSC effect in RCC patients
We next quantified MDSC subsets in RCC patients' tumor specimens (described in Supplementary Table 1), using the gating methods shown for HLADR−CD33hiCD15−CD14+ monocytic, HLADR−CD33hiCD15−CD14− lineage-negative, and HLADR−CD33loCD15+CD14− neutrophilic MDSC. Figure 6A shows the average percentage of each MDSC subset detected in tumors from untreated patients, as well as the amount detected in two tumors from sunitinib-treated patients. Unlike the pronounced decline in peripheral blood MDSC observed in RCC patients treated with sunitinib (16), tumors obtained from sunitinib-treated patients have not demonstrated declines in MDSC. The continued presence of MDSC in the tumors of sunitinib-treated patients was associated with continued T-cell suppression, as measured by IFNγ production, compared to normal donor T-cells and T-cells from untreated tumors (Figure 6B). In addition, several short-term tumor cell lines derived from surgical patients' RCC tumors (1–2 passages) produced abundant GM-CSF in vitro, and this production was not significantly reduced in the presence of sunitinib (Figure 6C). GM-CSF levels equivalent to those detected in RCC supernatants could also induce pSTAT5 activation in patient-derived MDSC (Figure 6D). This suggests that compartmental constraints limit sunitinib's ability to act as an immunopotentiator, and that locally produced GM-CSF may promote local sunitinib resistance.
Figure 6. Tumor microenvironment limits local anti-MDSC effect of sunitinib in RCC patients.
(A) MDSC subsets in tumors of untreated patients, as well as % found in two patients treated with sunitinib prior to surgery. (B) IFN-gamma+ tumor-infiltrating T-cells (FACS) following anti-CD3/28 stimulation from tumors of untreated patients (compared to normal donor T-cells), as well as patients treated with sunitinib prior to tumor removal. (C) GM-CSF levels (ELISA) in short-term and long-term RCC cultures. The impact of sunitinib on SK-RC-26b tumor cell production of GM-CSF. (D) pSTAT5 activation in gated CD33+HLADR− RCC patient-derived MDSC was measured with intracellular phosphoflow staining 48 hours into culture with varying concentrations of GM-CSF.
Discussion
While the role of immunosurveillance to inhibit tumor progression is well documented, there are many obstacles which prevent the destruction of advanced tumors by immune cells, one of which is MDSC accumulation. Sunitinib targets several RTKs and has experienced relative clinical success, especially in the setting of mRCC (1). We recently reported sunitinib's ability to reverse immune suppression in mRCC patients via MDSC inhibition (16), yet sunitinib's broad potential to modulate immune function independently of its anti-tumor effect was unknown. We here report that sunitinib, when used at clinically relevant doses (33,34), can inhibit MDSC accumulation, and thereby restore normal T-cell function, in the spleens of mice bearing both sunitinib-sensitive and sunitinib-insensitive tumors. This suggests that sunitinib's immunomodulatory activities occur independently of its anti-tumor potency. As such, sunitinib may prove a useful adjunct agent in immunotherapy trials (35).
We here demonstrate two mechanisms by which sunitinib inhibits MDSC accumulation. Pilot studies identified the spleen and BM as sites of MDSC proliferation, with BrdU-positive cells appearing after only a 1hour BrdU pulse. Using in vivo BrdU administration, Gr1lo m-MDSC were found to proliferate rapidly in the BM of naïve and tumor-bearing mice, and in the spleens of tumor-bearing mice, suggesting that the extramedullary proliferation is the most pathological proliferation, because it does not normally occur in naïve mice under steady-state conditions. Sunitinib had an anti-proliferative effect on splenic m-MDSC in vivo, and this effect could be duplicated in vitro.
The anti-proliferative effect of sunitinib is unlikely to account solely for the remarkable declines seen in MDSC in response to drug. Because previous studies had shown sunitinib to have a toxic effect on RCC patient MDSC in vitro (16), its effect on Gr1hi, neutrophilic MDSC (n-MDSC) viability was also studied in vivo. Our findings show that Gr1hi n-MDSC were relatively non-proliferative, and suggest that the accumulation of this MDSC subset in tumor-bearing mice is more related to an abnormally prolonged lifespan. Indeed, n-MDSC from tumor-bearing mice were much less likely to be undergoing apoptosis at any given time, compared to naïve mice. Sunitinib seemed to normalize this in vivo by increasing the frequency of n-MDSC apoptosis, as measured by AnnexinV staining, an effect which was reproducible in vitro. The data thus show that sunitinib acts to inhibit both the abnormal expansion of m-MDSC and the abnormally extended survival of n-MDSC. An alternative interpretation is that sunitinib prevents the expansion of mononuclear MDSC which may, in part, represent n-MDSC precursors (36). Such activity alone could lead to the perceived increase in n-MDSC apoptosis, because terminal n-MDSC would eventually undergo spontaneous apoptosis and fail to be replaced. This possibility is currently being tested.
In addition to being MDSC subset-specific, our data strongly suggest that sunitinib's inhibition of MDSC is direct. Proteome profile and luminex arrays did not identify a decline in any of the factors implicated in MDSC expansion or activation. In fact, several cytokines such as G-CSF and IFNγ were present at increased amounts in sunitinib treated mice, consistent with Ebos et. al's parallel observations in naïve mice (37).
The receptors involved in sunitinib's anti-MDSC activity are currently under investigation. Putative RTK's inhibited by sunitinib include targets of VEGF, SCF (ckit), Flt3L (flt3), m-CSF and PDGF (1–3). SCF has recently been implicated in MDSC accumulation (38), and sunitinib inhibits its receptor, ckit. However, culture with RTK ligands such as SCF and/or flt3L produced only limited MDSC expansion and viability unless combined with proliferatively synergizing cytokines such as IL-6, G-CSF or GM-CSF (25). This suggests that while the sunitinib RTK targets ckit and/or flt3 may provide permissive signals for MDSC expansion, tumor-promoted excessive MDSC accumulation requires synergy from additional cytokines.
G-CSF and GM-CSF have both previously been implicated in MDSC expansion (21,32,39,40), and vaccines which produce higher concentrations of GM-CSF have been reported to be less effective as a consequence of promoting MDSC (39). Additionally, however, our studies show the divergent abilities of G-CSF and GM-CSF to confer resistance to sunitinib in vitro and in vivo, paralleling the compartmental disparities of these cytokines in vivo. Because the Renca model is paradoxically far more vulnerable to sunitinib treatment than human RCC, we believe other models such as 4T1 are potentially more predictive of escape mechanisms relevant to human cancer. Notably, both mouse 4T1 and human RCCs displayed disparately greater GM-CSF production than sunitinib-sensitive peripheral compartments (plasma), whereas all compartments were similarly rich in G-CSF.
We hypothesize that the striking ability of GM-CSF but not other pro-proliferative cytokines to promote sunitinib resistance is due to its preferential activation of STAT5, which cannot be appreciably repressed by sunitinib. Our previous studies showed that treatment of CD34+ hematopoieitic progenitors with GM-CSF induced proliferative synergy with Flt3L/IL-6, despite GM-CSF-mediated inhibition of STAT3 activation in favor of dominant STAT5 programming (25). Despite preventing STAT3 activation, GM-CSF continued to display proliferative synergy with Flt3L and IL-6, which was undiminished even for STAT3 knockout BM (25). We here demonstrate a similar pSTAT5-dominated signaling pathway for MDSC exposed to GM-CSF. Conversely, in the absence of GM-CSF, combinations of SCF, Flt3L, IL-6 and/or G-CSF all promoted STAT3-activated MDSC which were highly susceptible to inhibition by sunitinib. While previous studies have highlighted the importance of STAT3 signaling in MDSC accumulation and function (41,42), as well as in emergency granulopoiesis (43), the present report is the first to demonstrate generation of MDSC in a steady state of STAT5 rather than STAT3 activation, the consequence of GM-CSF exposure. Because sunitinib's capacity to inhibit MDSC was recently shown to depend upon STAT3 blockade (24), we hypothesize that GM-CSF, when available, can rescue MDSC by reprogramming them to function independently of STAT3, rendering them resistant to sunitinib. Indeed, in vitro experiments with STAT5ab(null/null) mice furthermore confirmed an important role for STAT5 in MDSC rescue from sunitinib-toxicity.
MDSC-mediated diminutions in T-cell-mediated immunity may contribute to the currently limited effectiveness of immunotherapy in RCC and other tumors (16,44–47). Our previous studies in RCC patients showed that MDSC declines and improvements in T-cell function were not contingent upon tumor shrinkage in response to sunitinib, and even sunitinib-induced tumor cytoreduction is not associated with cure (16,47). Our data thus far obtained in human RCC patients supports the model established in 4T1 tumor-bearing mice, where peripheral compartment reductions in MDSC ubiquitously occur in response to sunitinib, but where the drug's anti-MDSC activity is much less pronounced intratumorally as a result of a relative abundance of non-sunitinib-targeted growth factors, such as GM-CSF, which provide alternative sunitinib-resistant survival signals. We are investigating ancillary strategies to abrogate such regional resistance, in order to enhance sunitinib's potency both as an immunomodulator and as a cancer therapy.
Supplementary Material
References
- 1.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI. Sunitinib. Expert Opin Pharmacother. 2007;8:2359–2369. doi: 10.1517/14656566.8.14.2359. [DOI] [PubMed] [Google Scholar]
- 3.Roskoski R., Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356:323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 8.Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 16.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 17.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Schmielau J, Finn OJ. Activated granulocytes and granulocyte- derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 19.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 20.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 21.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients wit modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandruzzato S, Solito S, Falisi E, et al. IL4Ralpha+ myeloid-derivedsuppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 24.Xin H, Zhang C, Herrmann A, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen PA, Koski GK, Czerniecki BJ, et al. STAT3- and STAT5-dependent pathways competitively regulate the pan-differentiation of CD34pos cells into tumor-competent dendritic cells. Blood. 2008;112:1832–1843. doi: 10.1182/blood-2007-12-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Miskimen KL, Wang Z, et al. STAT5 requires the N-domain for suppression of miR15/16, induction of bcl-2, and survival signaling in myeloproliferative disease. Blood. 2009 doi: 10.1182/blood-2009-07-234963. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng L, Kjaergaard J, Plautz GE, et al. Tumor-induced L-selectinhigh suppressor T cells mediate potent effector T cell blockade and cause failure of otherwise curative adoptive immunotherapy. J Immunol. 2002;169:4811–4821. doi: 10.4049/jimmunol.169.9.4811. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JP, Kudoh S, Melsop KA, et al. T-cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- 29.Ebert T, Bander NH, Finstad CL, Ramsawak RD, Old LJ. Establishment and characterization of human renal cancer and normal kidney cell lines. Cancer Res. 1990;50:5531–5536. [PubMed] [Google Scholar]
- 30.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 31.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 34.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 35.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steimer DA, Boyd K, Takeuchi O, et al. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serafini P, Carbley R, Noonan KA, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 40.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panopoulos AD, Zhang L, Snow JW, et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 46.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.