Abstract
Purpose
We evaluated adjuvant trial E1694 to more precisely define the prognostic significance of serum S100B in patients with high-risk resected melanoma.
Patients and Methods
Sera from 670 E1694 patients banked at baseline and three additional time points were tested for S100B protein using chemiluminescence.
Results
S100B testing results showed that the higher the S100B level is, the higher the risk of relapse and death, regardless of the cutoff value. Univariate analysis showed that baseline S100B ≥ 0.15 μg/L is significantly correlated with overall survival (OS; P = .01). Multivariate analysis was performed adjusting for significant prognostic factors (ulceration and lymph node status) and treatment. Baseline S100B was a significant prognostic factor for survival (hazard ratio = 1.39; 95% CI, 1.01 to 1.92; P = .043). S100B values measured at later time points over 1 year were also demonstrated to be significant prognostic factors for relapse-free survival (RFS) and OS. Lower S100B values at baseline and during follow-up were associated with longer survival. A changing S100B from low at baseline to high on follow-up seemed to be associated with the worst RFS and OS.
Conclusion
For patients with high-risk surgically resected melanoma, a high baseline or increasing serum S100B is an independent prognostic marker of risk for mortality that may allow us to refine the application of adjuvant therapy in the future.
INTRODUCTION
High-risk melanoma, as defined in the past four Eastern Cooperative Oncology Group (ECOG) and Intergroup trials of adjuvant high-dose interferon alfa-2b (HDI), is a disease that, after surgery, is associated with a recurrence and/or mortality risk higher than 35% to 50%; as formulated by the most recent sixth edition American Joint Committee on Cancer (AJCC) system, this is equivalent to ≥ stage IIB disease.1-3
ECOG and Intergroup trials of adjuvant treatment for melanoma conducted since 1984 (E1684, E1690, and E1694) have examined the effect of therapy with HDI on relapse-free survival (RFS) and overall survival (OS) in patients with AJCC stage IIB or III disease (except current AJCC stage IIIC disease).4-7 Intergroup trial E1694 was a randomized comparison of the GM2 ganglioside vaccine (GMK) versus HDI that accrued 880 patients. This trial was closed early based on an interim analysis in April 2000 indicating that GMK was significantly inferior to HDI in relation to both relapse and mortality end points.6
Serum S100B (isoforms S100AB and S100BB) is a protein of the S100 family that has shown promise as a prognostic marker for melanoma relapse and mortality risk. S100B is shed by melanoma cells,8 and its level in the peripheral blood has been investigated as a melanoma biomarker,9-11 where its prognostic potential is supported by many reports in the literature.12-30 Serum S100B has been demonstrated to be similarly reliable to serum lactate dehydrogenase (LDH) with respect to the prediction of clinical outcome in a recent study of 179 patients with AJCC stage III to IV melanoma tested at diagnosis. Survival analysis indicated that initially elevated LDH and S100B levels in patients with stage IV disease predict comparably short survival.14 S100B has been shown to be an independent prognostic factor and to be valuable for the detection of tumor progression or metastases.17,18,31 The mean serum concentration of S100B protein has been reported to be significantly related to the clinical stage in melanoma and to have a high sensitivity and specificity for the detection of metastatic melanoma.13,15,32 Therefore, we have evaluated blood sera collected in the context of the E1694 trial to more precisely define the prognostic significance of serial serum S100B protein levels in high-risk surgically resected melanoma patients.
PATIENTS AND METHODS
Patients
Sera from E1694 patients banked before treatment and after initiation of therapy at weeks 4 to 6, weeks 12 to 14, and weeks 48 to 52 were used.
Chemiluminescence
Sera from 670 patients enrolled onto E1694 were analyzed for S100B levels by chemiluminescence using the LIAISON Sangtec 100 diagnostic system (DiaSorin, Inc, Saluggia, Italy), a quantitative fully automated immunoassay analyzer (range, 0.02 to 30 μg/L; lowest detection level = 0.02 μg/L; normal reference range, < 0.15 μg/L, as established in feasibility studies performed at DiaSorin, Inc). After the development of a test for determination of the isoforms S100-A1B and S100-BB in serum (S100B), several sensitive assays for the detection of serum S100B have become available. One study compared four S100B assays, two automated assays (LIAISON Sangtec 100 and Elecsys S100 [Roche Diagnostics GmbH, Penzberg, Germany]) and two manual ones (Sangtec 100 enzyme-linked immunosorbent assay and CanAg S100 EIA [CanAg Diagnostics AB, Gothenburg, Sweden]), with respect to clinical data, reference values, and correlation. The interassay and intra-assay coefficients of variation were best for the automated assays. Method comparison revealed satisfactory correlation coefficients of 0.9 or higher. The overall sensitivity for melanoma was highest with LIAISON Sangtec 100 (47% for all stages of melanoma and reaching 77% for stage IV). Receiver operating characteristic curves showed the best accuracy for the LIAISON Sangtec 100 assay (area under the curve, 0.744; 95% CI, 0.652 to 0.837).33
Statistical Analysis
Using OS as the primary end point and RFS as a secondary end point of this study, we assessed the effect of elevated serum S100B level at baseline and during therapy. The Kaplan-Meier method34 was used to evaluate RFS and OS in univariate analysis. The difference in RFS or OS by the baseline S100B level was compared using the log-rank test. Cox proportional hazards regression models35 were used to evaluate the significance of S100B levels adjusting for treatment assignment (HDI v GMK) and other established prognostic factors (sex, age, ECOG performance status [PS], pigmentation of primary melanoma lesion [melanotic v Amelanotic], ulceration, Breslow depth, Clark level, nodal involvement, and AJCC stage). The categoric variables were used as presented in Table 1, and the continuous variables (age, size of primary melanoma lesion, and Breslow depth) were dichotomized using the median value. S100B value was dichotomized using the established upper limit of normal value of 0.15 μg/L.
Table 1.
Summary of Baseline Characteristics and Clinical Data for E1694 Patients
| Characteristic | No. of Patients* | % |
|---|---|---|
| OS, years | ||
| Median | 7.2 | |
| 95% CI | 6.0 to NR | |
| RFS, years | 3.1 | |
| Median | 3.1 | |
| 95% CI | 2.4 to 3.7 | |
| Baseline S100B level, μg/L | ||
| Median | 0.08 | |
| Range | 0.02 to 1.54 | |
| Age, years (n = 669) | ||
| Median | 51 | |
| Range | 19 to 81 | |
| Size of primary melanoma, cm (n = 470) | ||
| Median | 1.2 | |
| Range | 0.1 to 11.5 | |
| Breslow depth, mm (n = 594) | ||
| Median | 3.28 | |
| Range | 0.05 to 45 | |
| Sex (n = 669) | ||
| Male | 426 | 64 |
| Female | 246 | 36 |
| ECOG PS (n = 669) | ||
| 0 | 536 | 80 |
| 1 | 133 | 20 |
| Pigmentation (n = 528) | ||
| Melanotic | 482 | 91 |
| Amelanotic | 46 | 9 |
| Ulceration (n = 553) | ||
| No | 321 | 58 |
| Yes | 232 | 42 |
| Clark level (n = 582) | ||
| I | 4 | < 1 |
| II | 24 | 4 |
| III | 112 | 19 |
| IV | 340 | 58 |
| V | 101 | 17 |
| AJCC stage | ||
| IIB | 161 | 24 |
| III | 509 | 76 |
| No. of positive nodes (n = 676) | ||
| 0 | 162 | 24 |
| 1 | 273 | 40 |
| 2-3 | 152 | 23 |
| 4+ | 89 | 13 |
| Treatment | ||
| GMK | 329 | 49 |
| HDI | 341 | 51 |
Abbreviations: OS, overall survival; NR, not reached; RFS, relapse-free survival; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AJCC, American Joint Committee on Cancer; GMK, GM2 ganglioside vaccine; HDI, high-dose interferon alfa-2b.
Total No. of patients was 670 unless otherwise noted.
S100B levels at baseline and at subsequent time points were also evaluated in the Cox model. Repeated measurements of S100B were considered as time-varying covariates in the Cox model. To adjust for a possible bias in favor of patients with later time point S100B samples, a landmark analysis36 was also considered. All of the P values presented are based on two-sided significance tests.
RESULTS
E1694
Six hundred seventy patients were included in this analysis. Of these, 334 patients were alive as of May 2007. The median follow-up time of these patients was 7.8 years (range, 1.4 to 10.6 years). The median OS time is 7.2 years (95% CI, 6.0 years to not reached). RFS was assessed in 667 patients. Of these, 408 patients have experienced relapse thus far. The median RFS time was 3.1 years (95% CI, 2.4 to 3.7 years).
Baseline patient characteristics are listed in Table 1. At baseline, the median serum S100B value was 0.08 μg/L (range, 0.02 to 1.54 μg/L; n = 670). The higher the S100B level was, the higher was the risk of relapse and death. At baseline (Figs 1A and 1B), 582 patients (87%) had an S100B level less than 0.15 μg/L (normal), and 88 patients (13%) had an S100B level ≥ 0.15 μg/L (high).
Fig 1.
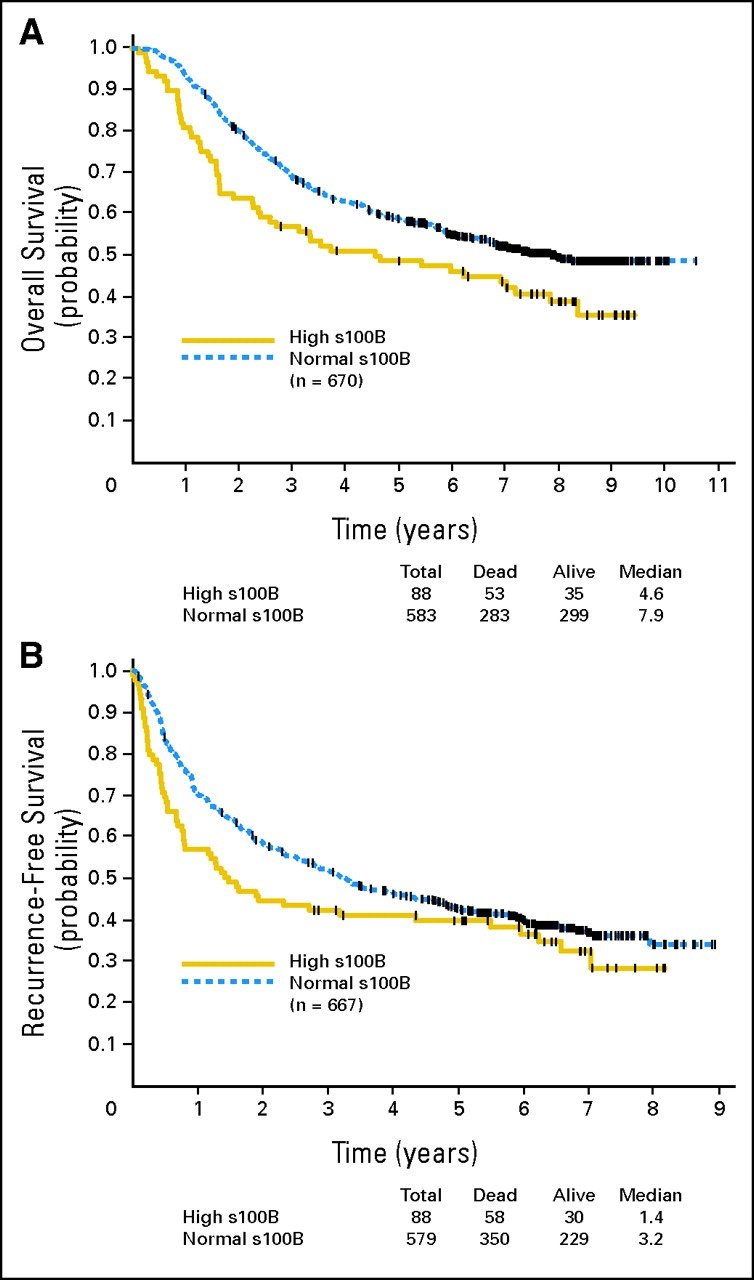
(A) Overall survival for E1694 patients (n = 670) with baseline S100B level of ≥ 0.15 μg/L (high) compared with less than 0.15 μg/L (normal). (B) Recurrence-free survival for E1694 patients (n = 667) with baseline S100B level of ≥ 0.15 μg/L (high) compared with less than 0.15 μg/L (normal).
In the univariate analysis (log-rank test), a baseline S100B level ≥ 0.15 μg/L significantly correlated with OS (P = .010), although it correlated less significantly with RFS (P = .062). Figures 1A and 1B display the Kaplan-Meier plots of OS and RFS by the baseline S100B level (normal v high).
In developing a multivariate model, first, univariate analyses were carried out using baseline S100B (< v ≥ 0.15 μg/L), sex (male v female), ECOG PS (0 v 1), pigmentation of primary melanoma (no v yes), ulceration (no v yes), size of primary melanoma (< v ≥ 1.2 cm), Clark level (I, II, III, IV, or V), Breslow depth (< v ≥ 3.28 mm), lymph node involvement at random assignment (0, 1, 2-3, or 4+), AJCC stage (II v III), age (< v ≥ 51 years), and treatment arm (HDI v GMK) for OS and RFS. Note that the categoric variables, as defined in Table 1, and the continuous variables (age, size, and Breslow depth) were dichotomized using the median value. Baseline S100B (P = .010), node involvement (P < .001), age (P = .009), PS (P = .044), stage (P < .001), and ulceration (P = .007) proved to be significant prognostic factors for OS. Treatment was not significant (P = .312) for OS; however, in patients with a high baseline S100B level (≥ 0.15 μg/L), patients treated with HDI experienced a longer survival (P = .028).
The multivariate Cox model for OS was selected in the following way. Initially, all six significant covariates and treatment were included in the model; the least significant covariate in this model was stage (P = .795), and stage was eliminated. In the model with five significant covariates and treatment, the least significant covariate was PS (P = .227), and PS was eliminated. In the next model with four covariates and treatment, age was the least significant covariate (P = .088) and was eliminated. This process led to the final model summarized in Table 2. The Cox model for OS included the baseline S100B level, node involvement, ulceration, and treatment. S100B was significantly associated with OS (P = .043) after adjusting for the other significant prognostic factors.
Table 2.
Multivariate Cox Model for Baseline S100B, Treatment, Ulceration, and Lymph Node Status in E1694
| Variable | RFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| S100B | 1.18 | 0.87 to 1.50 | .293 | 1.39 | 1.01 to 1.92 | .043 |
| Treatment | 0.81 | 0.65 to 1.01 | .056 | 0.88 | 0.69 to 1.44 | .265 |
| Ulceration | 1.66 | 1.34 to 2.05 | < .001 | 1.54 | 1.22 to 1.94 | < .001 |
| Lymph node | 1.36 | 1.22 to 1.51 | < .001 | 1.52 | 1.35 to 1.71 | < .001 |
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, hazard ratio.
In univariate analyses for RFS, ulceration (P < .001), size (P = .026), stage (P = .003), and node involvement (< 0.001) were significant, and S100B (P = .062) and treatment (P = .106) were marginally significant. Patients with a baseline S100B level of ≥ 0.15 μg/L experienced a longer RFS when treated with HDI (P = .018). A similar final model selection process as described for the final model for OS was used for the RFS model. Table 2 shows the final multivariate model for OS and RFS.
There were four time points when serum samples were collected and assayed for S100B; these were at baseline (n = 670), weeks 4 to 6 (n = 615), weeks 12 to 14 (n = 562), and weeks 48 to 52 (n = 395). These S100B values were also evaluated. The baseline proportion of patients with an S100B level ≥ 0.15 μg/L was 13%. Subsequently, this proportion increased to 21%, 20%, and 20% at weeks 4 to 6, 12 to 14, and 48 to 52. Of the 670 patients, 611 survived at least 1 year. To adjust for a possible lead time bias, only the 611 patients who survived at least 1 year were included in evaluating S100B values collected over 1 year. In these 611 patients, the changes in S100B values over time were categorized into the following three groups: group A = 378 patients (62%) with an S100B value of less than 0.15 μg/L at baseline and any of the later time points; group B = 71 patients (12%) with a baseline S100B value ≥ 0.15 μg/L; and group C = 162 patients (26%) with an S100B value of less than 0.15 μg/L at baseline that then increased to ≥ 0.15 μg/L at any one of the later time points. The median OS has not been reached for group A (95% CI, 7.0 years to not reached). The median OS was 7.8 years (95% CI, 4.7 years to not reached) for group B and 7.0 years (95% CI, 4.5 to not reached) for group C. The OS distribution was marginally significantly different in these three groups (P = .099 by log-rank test). The comparison of OS between groups A and C was significant (P = .048). Of the 611 patients, 608 had RFS assessed. The median RFS was 4.6 years (95% CI, 3.5 to 6.0 years) for group A, 4.3 years (95% CI, 1.5 to 7.0 years) for group B, and 2.4 years (95% CI, 1.7 to 5.0 years) for group C. The RFS distribution was significantly different in these three groups (P = .058). The comparison of RFS between groups A and C was significant (P = .017). Figures 2A and 2B display the Kaplan-Meier plots for OS and RFS by the three groups.
Fig 2.
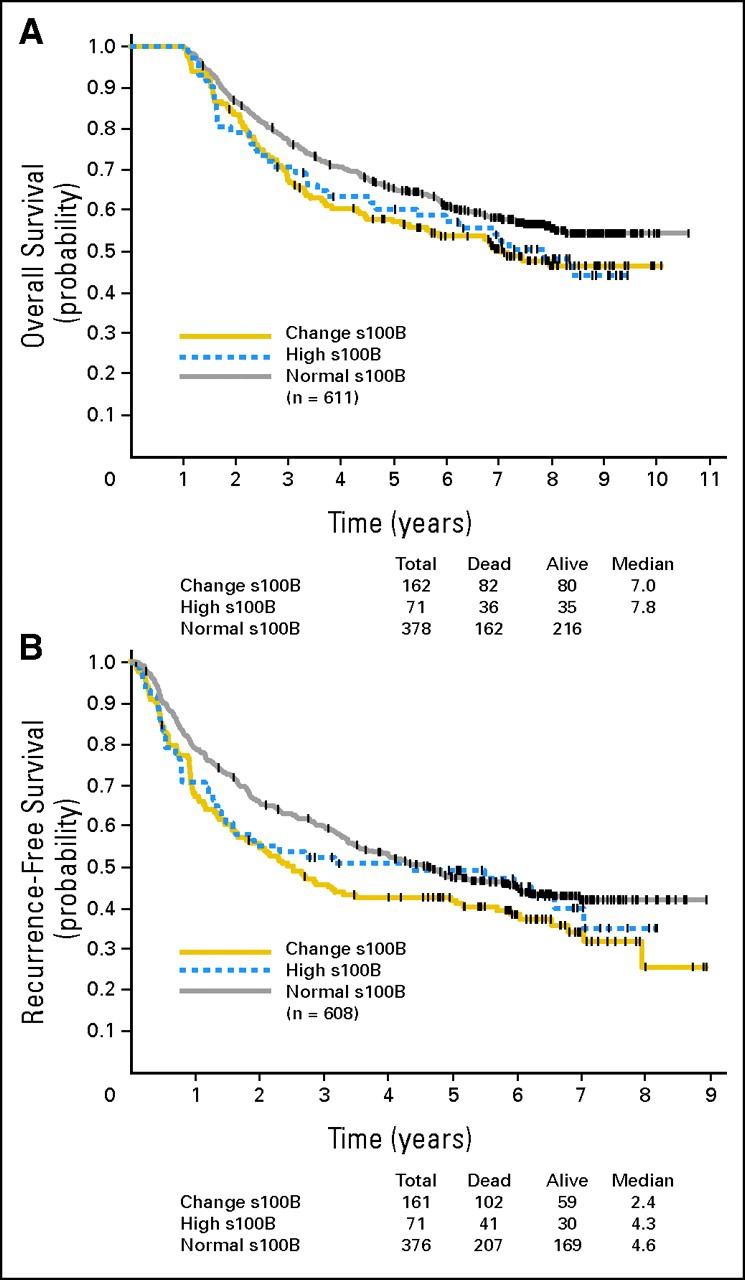
(A) Overall survival and (B) for recurrence-free survival for E1694 patients by S100B trend (normal, high, or changing from normal to high at later time points).
Subset analyses were conducted to evaluate the survival prognostic value of S100B at later time points. For weeks 4 to 6, to adjust for the possibility of lead time bias, only patients who survived at least 4 weeks were included. In the Cox model including both baseline and week 4 to 6 values as covariates, only the week 4 to 6 S100B level was significant (P = .039; hazard ratio [HR] = 1.33). Similarly, including patients who survived at least 12 weeks, the S100B level at weeks 12 to 14 was significant (P = .001; HR = 1.62) after adjusting for the baseline S100B level. Including patients who survived at least 48 weeks, the S100B level at week 48+ was a significant predictor (P < .001; HR = 2.21) after adjusting for the baseline level. S100B level at week 48+ remains significant (P = .002; HR = 2.10) when adjusted for nodal status, ulceration, treatment, and baseline S100B value in the Cox model. For RFS, subset analyses evaluating the later time point S100B levels, while adjusting for baseline values, indicated that these are significant predictors of relapse (P = .001 for weeks 4 to 6, P ≤ .001 for weeks 12 to 14, and P = .051 for week 48).
Given the larger portion of data missing for the last week 48+ measurement (266 of 661 patients; 40%), we used the S100B data from the first three time points (baseline, weeks 4 to 6, and weeks 12 to 14) as time-varying covariates in the Cox regression model while adjusting for the other significant prognostic factors (node involvement, ulceration, and treatment). S100B level proved to be a significant predictor of both death and relapse. HR for death was 1.44 (95% CI, 1.06 to 1.95; P = .0210), and HR for relapse was 1.70 (95% CI, 1.21 to 1.92; P < .001). Table 3 lists these results.
Table 3.
Multivariate Cox Model for S100B Values (at baseline, weeks 4 to 6, and weeks 12 to 14 as time-varying covariate), Treatment, Ulceration, and Lymph Node Status in E1694
| Variable | RFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| S100B values | 1.70 | 1.21 to 1.91 | < .001 | 1.44 | 1.06 to 1.95 | .021 |
| Treatment | 0.80 | 0.64 to 1.00 | .060 | 0.99 | 0.76 to 1.28 | .918 |
| Ulceration | 1.65 | 1.31 to 2.07 | < .001 | 1.48 | 1.14 to 1.93 | .003 |
| Lymph node | 1.37 | 1.21 to 1.54 | < .001 | 1.46 | 1.28 to 1.67 | < .001 |
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, hazard ratio.
DISCUSSION
No serum biomarkers have reliably demonstrated the capacity to predict disease outcome in the adjuvant setting of high-risk melanoma. The sixth edition (2002) AJCC staging system has recognized the importance of serum LDH as an independent prognostic factor in advanced inoperable melanoma.2,37-40 Accumulating data suggest a potential role of serum S100B protein as a promising prognostic marker for relapse and mortality risk in melanoma.12-15,17,18,31,32
The results of this study demonstrate that higher serum S100B levels are associated with poorer prognosis in terms of relapse and survival. To demonstrate the relationship between higher S100B values and higher risk of relapse and death, in Table 4, 5-year OS and 3-year RFS rates are listed by 25th percentile (0.06 μg/L), 50th percentile (0.08 μg/L), 75th percentile (0.11 μg/L) values of S100B and the upper limit of normal value of 0.15 μg/L. Using the specified cutoff values, low and high categories were generated, and the clinical outcome is summarized in each category. As the S100B cutoff value increases (0.06, 0.08, and 0.11 μg/L), the 5-year OS and 3-year RFS rates under the high category become smaller, and the difference in 5 year-OS and 3-year RFS rates between low and high groups becomes larger. The cutoff value of 0.15 μg/L also demonstrates a similar trend.
Table 4.
Five-Year OS and 3-Year RFS Rates by 25th (0.06 μg/L), 50th (0.08 μg/L), 75th (0.11 μg/L) Percentile Values of Baseline S100B and ULN Value of 0.15 μg/L
| Survival | Cutoff Level (μg/L) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.06 |
0.08 |
0.11 |
0.15 |
|||||
| Low | High | Low | High | Low | High | Low | High | |
| 5-Year OS, % | 60 | 57 | 59 | 55 | 61 | 47 | 59 | 48 |
| 3-Year RFS, % | 49 | 51 | 52 | 50 | 53 | 44 | 51 | 41 |
Abbreviations: OS, overall survival; RFS, relapse-free survival; ULN, upper limit of normal.
We chose 0.15 μg/L as our cutoff value because this was the upper limit of normal demonstrated earlier in feasibility studies performed by the manufacturer using the chemiluminescence assay (LIAISON Sangtec 100 diagnostic system; DiaSorin Inc).33 This is also justified given the distribution of S100B values noted in this study. We evaluated the prognostic significance of S100B independently at baseline and then at all time points simultaneously as time-varying repeated covariates in the Cox model.
In E1694, baseline S100B (≥ 0.15 μg/L by chemiluminescence) significantly correlated with OS, and there was a strong trend toward correlation with RFS (P = .062). In the multivariable assessment of the results of E1694, after adjusting for treatment, lymph node status, age, PS, ulceration, and baseline S100B level of ≥ 0.15 μg/L were significant adverse prognostic markers for death, with lesser correlations to relapse. Using S100B variables simultaneously analyzed as time-varying repeated covariates in the Cox regression model (three time points: baseline, weeks 4 to 6, and weeks 12 to 14; week 48+ was not used given the large degree of missing data for the week 48+ S100B measurement), the S100B value proved to be a significant predictor for OS and RFS. These results indicate that baseline S100B is a significant prognostic marker for mortality as shown in E1694. This biomarker is more powerful when considered using repeated measurements and, in this setting, has prognostic value both for relapse and for death, becoming more significant with time for the later time points. An increasing S100B value may be an indication for thorough evaluation to detect subclinical relapsed disease, where early detection may allow more effective interventions to be applied. The prognostic value of S100B in relation to OS was also evident in E1694 when classifying patients based on their baseline S100B value and its change over time. Group A (S100B < 0.15 μg/L at baseline and any of the later time points) patients had significantly longer survival than group C patients (S100B < 0.15 μg/L at baseline that then changed to ≥ 0.15 μg/L at any of the later time points; P = .048). The comparison of RFS between groups A and C was also significant (P = .017). As shown in Figures 2A and 2B, patients in group C (S100B changing from normal to high) seem to have the worst RFS and OS. These findings support the survival prognostic value of S100B when tested at baseline and in follow-up.
Patients who continue to have a low S100B value have better survival compared with patients whose S100B level starts to increase at a later time point, where it may be associated with recurrent disease. Patients who have a high S100B at baseline seem to have a worse prognosis compared with patients with a low S100B value. Therefore, a high S100B level at baseline or an increasing S100B level during follow-up is associated with a higher risk of relapse and mortality and might be considered an indication for early intervention.
Another potential value of S100B is in the stratification of patients into higher and lower risk groups that may best be studied separately in terms of benefit from adjuvant therapy in future studies. Such a role for a serum biomarker has emerged for LDH in advanced metastatic melanoma patients, and S100B may fulfill a similar role in the future design of adjuvant clinical trials. LDH has been shown to have strong, incremental prognostic value (P < .0001) based on the analysis of a recent phase III trial with oblimersen (Bcl-2 antisense; n = 771) and the large European Organisation for Research and Treatment of Cancer 18951 Biochemotherapy trial (n = 365) in advanced melanoma.41
In addition, testing the prognostic value of this biomarker for disease outcome and the predictive value for therapeutic response is obviously warranted in combination with other promising biomarkers in melanoma including serum C-reactive protein, serum melanoma-inhibitory activity, and serum LDH. Additional new biomarkers of autoimmunity and antitumor immunity also need to be evaluated; recent evidence suggests that the development of signs of autoimmunity is strongly correlated with therapeutic benefit of interferon alfa-2b.42 With these approaches, it is hoped that we will learn how to improve and refine adjuvant therapy of melanoma, which has evolved little since 1996.
Serum protein S100B is a valuable prognostic biomarker that is significantly correlated with mortality risk when assessed at baseline as well as at later time points among patients with high-risk resectable melanoma. The prognostic value of elevated S100B in relation to survival is most significant at baseline, when tested after surgical resection of disease; it is also of use on follow-up testing during and after therapy. Additional testing to confirm the prognostic value of S100B is planned as part of ongoing (ECOG 1697) and planned studies of adjuvant therapy in melanoma.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ahmad A. Tarhini, John M. Kirkwood
Administrative support: Ahmad A. Tarhini, Cindy Sander, John M. Kirkwood
Provision of study materials or patients: Ahmad A. Tarhini, Joseph Stuckert, Cindy Sander, John M. Kirkwood
Collection and assembly of data: Ahmad A. Tarhini, Joseph Stuckert, John M. Kirkwood
Data analysis and interpretation: Ahmad A. Tarhini, Joseph Stuckert, Sandra Lee, John M. Kirkwood
Manuscript writing: Ahmad A. Tarhini, John M. Kirkwood
Final approval of manuscript: Ahmad A. Tarhini, Joseph Stuckert, Sandra Lee, Cindy Sander, John M. Kirkwood
Footnotes
published online ahead of print at www.jco.org on December 1, 2008
Both A.A.T. and J.S. contributed equally to this work.
Supported by DiaSorin.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2007. CA Cancer J Clin 57:43-66, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Manola J, Atkins M, Ibrahim J, et al: Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18:3782-3793, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Agarwala SS: Systemic cytotoxic and biologic therapy of melanoma, in DeVita VT, Hellman S, Rosenberg SA (eds): Cancer: Principles and Practice of Oncology. Philadelphia, PA, Lippincott, 1993, pp 1-16
- 4.Kirkwood JM, Strawderman MH, Ernstoff MS, et al: Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 14:7-17, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Ibrahim JG, Sondak VK, et al: High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 18:2444-2458, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Ibrahim JG, Sosman JA, et al: High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol 19:2370-2380, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Manola J, Ibrahim J, et al: A pooled analysis of Eastern Cooperative Oncology Group and Intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res 10:1670-1677, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ghanem G, Loir B, Morandini R, et al: On the release and half-life of S100B protein in the peripheral blood of melanoma patients. Int J Cancer 94:586-590, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Harpio R, Einarsson R: S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin Biochem 37:512-518, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Kounalakis N, Goydos JS: Tumor cell and circulating markers in melanoma: Diagnosis, prognosis, and management. Curr Oncol Rep 7:377-382, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Salama I, Malone PS, Mihaimeed F, et al: A review of the S100 proteins in cancer. Eur J Surg Oncol 34:357-364, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Domingo-Domènech J, Molina R, Castel T, et al: Serum protein s-100 predicts clinical outcome in patients with melanoma treated with adjuvant interferon: Comparison with tyrosinase rt-PCR. Oncology 68:341-349, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Andrés R, Mayordomo JI, Zaballos P, et al: Prognostic value of serum S-100B in malignant melanoma. Tumori 90:607-610, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bánfalvi T, Boldizsar M, Gergye M, et al: Comparison of prognostic significance of serum 5-S-cysteinyldopa, LDH and S-100B protein in stage III-IV malignant melanoma. Pathol Oncol Res 8:183-187, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Mårtenson ED, Hansson LO, Nilsson B, et al: Serum S-100b protein as a prognostic marker in malignant cutaneous melanoma. J Clin Oncol 19:824-831, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hauschild A, Michaelsen J, Brenner W, et al: Prognostic significance of serum S100B detection compared with routine blood parameters in advanced metastatic melanoma patients. Melanoma Res 9:155-161, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hauschild A, Engel G, Brenner W, et al: Predictive value of serum S100B for monitoring patients with metastatic melanoma during chemotherapy and/or immunotherapy. Br J Dermatol 140:1065-1071, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hauschild A, Engel G, Brenner W, et al: S100B protein detection in serum is a significant prognostic factor in metastatic melanoma. Oncology 56:338-344, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Bonfrer JM, Korse CM, Nieweg OE, et al: The luminescence immunoassay S-100: A sensitive test to measure circulating S-100B—Its prognostic value in malignant melanoma. Br J Cancer 77:2210-2214, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buer J, Probst M, Franzke A, et al: Elevated serum levels of S100 and survival in metastatic malignant melanoma. Br J Cancer 75:1373-1376, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao MG, Auge JM, Molina R, et al: Melanoma inhibiting activity protein (MIA), beta-2 microglobulin and lactate dehydrogenase (LDH) in metastatic melanoma. Anticancer Res 27:595-599, 2007 [PubMed] [Google Scholar]
- 22.Curry BJ, Farrelly M, Hersey P: Evaluation of S-100beta assays for the prediction of recurrence and prognosis in patients with AJCC stage I-III melanoma. Melanoma Res 9:557-567, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Faries MB, Gupta RK, Ye X, et al: A comparison of 3 tumor markers (MIA, TA90IC, S100B) in stage III melanoma patients. Cancer Invest 25:285-293, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Juergensen A, Holzapfel U, Hein R, et al: Comparison of two prognostic markers for malignant melanoma: MIA and S100 beta. Tumour Biol 22:54-58, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Miliotes G, Lyman GH, Cruse CW, et al: Evaluation of new putative tumor markers for melanoma. Ann Surg Oncol 3:558-563, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Mohammed MQ, Abraha HD, Sherwood RA, et al: Serum S100beta protein as a marker of disease activity in patients with malignant melanoma. Med Oncol 18:109-120, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Rebmann V, Ugurel S, Tilgen W, et al: Soluble HLA-DR is a potent predictive indicator of disease progression in serum from early-stage melanoma patients. Int J Cancer 100:580-585, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Schultz ES, Diepgen TL, Von Den Driesch P: Clinical and prognostic relevance of serum S-100 beta protein in malignant melanoma. Br J Dermatol 138:426-430, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Smit LH, Korse CM, Hart AA, et al: Normal values of serum S-100B predict prolonged survival for stage IV melanoma patients. Eur J Cancer 41:386-392, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ugurel S, Bell N, Sucker A, et al: Tumor type M2 pyruvate kinase (TuM2-PK) as a novel plasma tumor marker in melanoma. Int J Cancer 117:825-830, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Schmitz C, Brenner W, Henze E, et al: Comparative study on the clinical use of protein S-100B and MIA (melanoma inhibitory activity) in melanoma patients. Anticancer Res 20:5059-5063, 2000 [PubMed] [Google Scholar]
- 32.Ortiz B, Vazquez C, Martinez C, et al: S100 protein as tumoral marker in melanoma patients: Comparative study with sentinel node biopsy and whole body FDG-PET. Rev Esp Med Nucl 22:87-96, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Smit LH, Korse CM, Bonfrer JM: Comparison of four different assays for determination of serum S-100B. Int J Biol Markers 20:34-42, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 35.Cox DR: Regression models and life tables. J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 36.Anderson JR, Cain KC, Gelber RD: Analysis of survival by tumor response. J Clin Oncol 1:710-719, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Eton O, Legha SS, Moon TE, et al: Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 16:1103-1111, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Keilholz U, Conradt C, Legha SS, et al: Results of interleukin-2-based treatment in advanced melanoma: A case record-based analysis of 631 patients. J Clin Oncol 16:2921-2929, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Franzke A, Probst-Kepper M, Buer J, et al: Elevated pretreatment serum levels of soluble vascular cell adhesion molecule 1 and lactate dehydrogenase as predictors of survival in cutaneous metastatic malignant melanoma. Br J Cancer 78:40-45, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deichmann M, Benner A, Bock M, et al: S100-beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol 17:1891-1896, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Keilholz U, Suciu S, Bedikian AY, et al: LDH is a prognostic factor in stage IV melanoma patients (pts) but is a predictive factor only for bcl2 antisense treatment efficacy: Re-analysis of GM301 and EORTC18951 randomized trials. J Clin Oncol 25:485s, 2007. (suppl, abstr 8552) [Google Scholar]
- 42.Gogas H, Ioannovich J, Dafni U, et al: Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 354:709-718, 2006 [DOI] [PubMed] [Google Scholar]


