Abstract
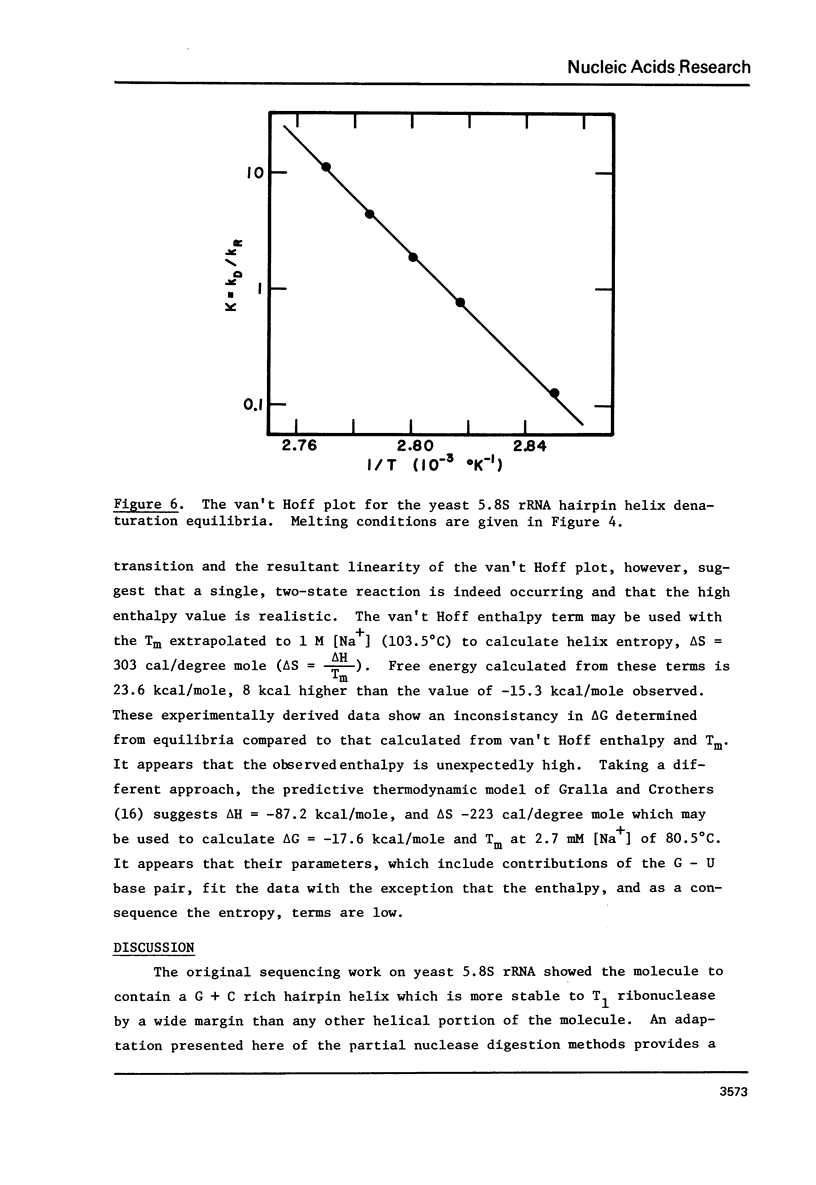
The 5. 8S ribosomal RNA of bakers yeast contains one particularly stable hairpin helix which is isolated by partial T1 ribonuclease digestion. Thermal hyperchromism analysis of the hairpin fragment showed that it dissociates cooperatively with 18% hyperchromism, with a Tm of 83 degree C at 2.7 mM sodium ion concentration, and with a hyperchromic difference spectrum indicative of over 90% G + C content. The probable secondary structure for the fragment was used to predict a helix free energy, delta G = -16.2 kcal/mole, which was the same as that determined from the melting equilibrium. The predicted enthalpy however, was 77% of the value, delta H = -114 kcal/mole, determined from the van't Hoff relationship. The effect on these data of a G.U base pair within the 9 base pair helix is discussed.
Full text
PDF

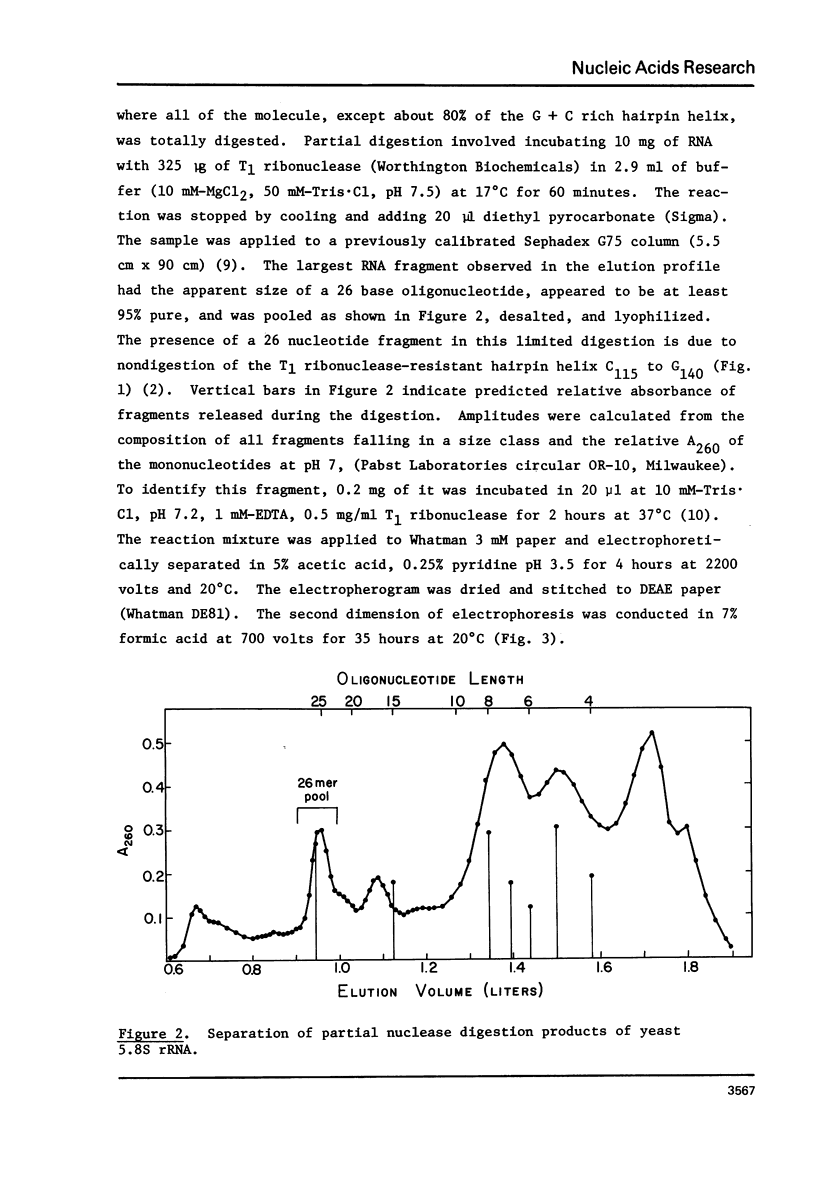



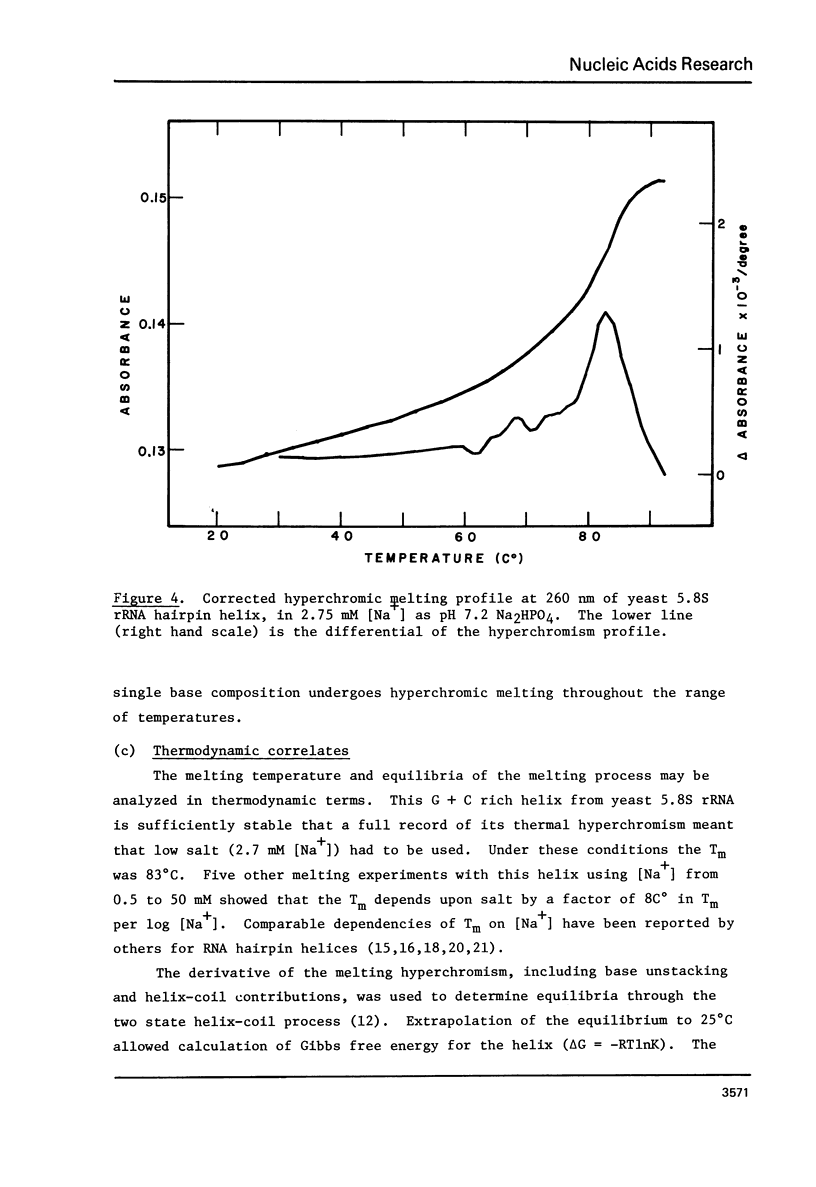
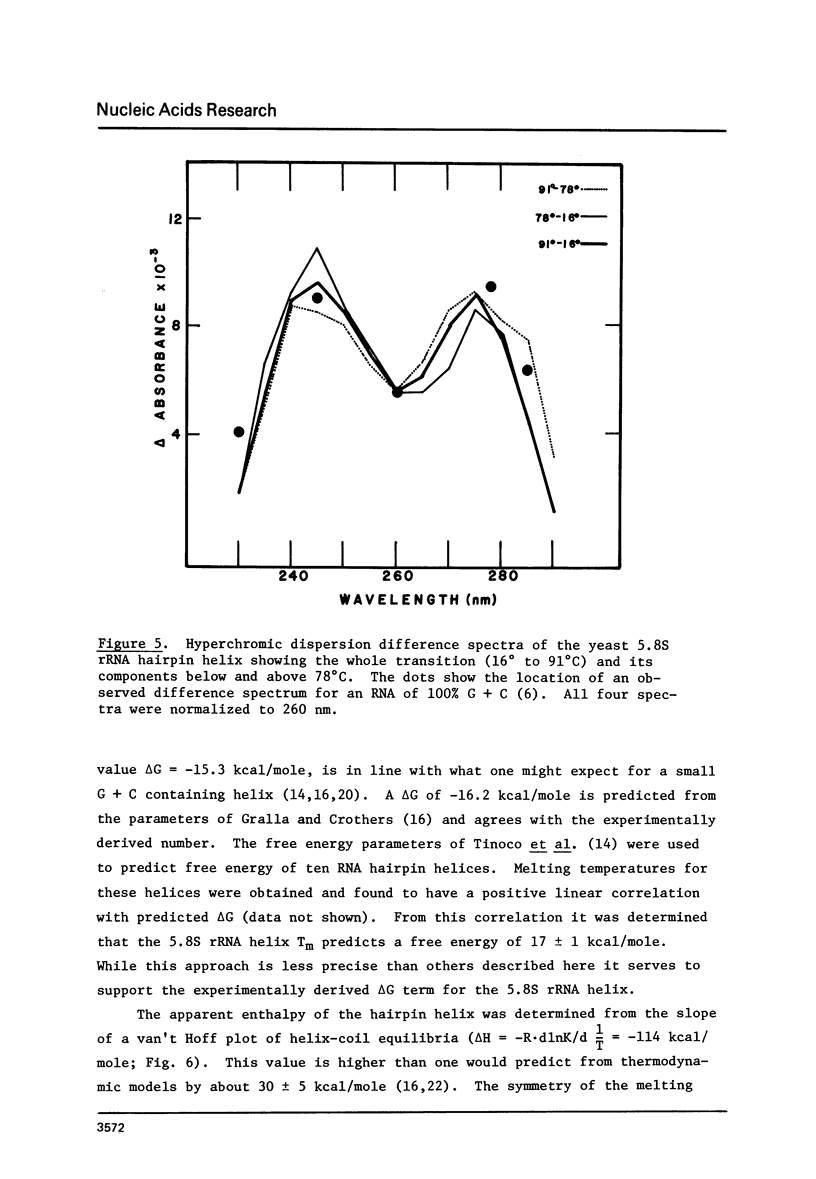




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Coutts S. M., Gangloff J., Dirheimer G. Conformational transitions in tRNA Asp (brewer's yeast). Thermodynamic, kinetic, and enzymatic measurements on oligonucleotide fragments and the intact molecule. Biochemistry. 1974 Sep 10;13(19):3938–3948. doi: 10.1021/bi00716a019. [DOI] [PubMed] [Google Scholar]
- Coutts S. M. Thermodynamics and kinetics of G-C base pairing in the isolated extra arm of serine-specific transfer RNA from yeast. Biochim Biophys Acta. 1971 Feb 25;232(1):94–106. doi: 10.1016/0005-2787(71)90494-1. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R., Ma R. I., Ofengand J., Siddiqui M. A. Thermal transitions in E. coli +RNA fMet and two of its molecular fragments. Biopolymers. 1973 Jun;12(6):1247–1257. doi: 10.1002/bip.1973.360120605. [DOI] [PubMed] [Google Scholar]
- Lightfoot D. R., Wong K. L., Kearns D. R., Reid B. R., Shulman R. G. Assignment of the low field proton nuclear magnetic resonance spectrum of yeast phenylalanine transfer RNA to specific base pairs. J Mol Biol. 1973 Jun 25;78(1):71–89. doi: 10.1016/0022-2836(73)90429-4. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Sitz T. O., Busch H. Homologies in eukaryotic 5.8S ribosomal RNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):736–743. doi: 10.1016/0006-291x(75)90461-1. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Sitz T. O., Busch H. Sequence homologies in mammalian 5.8S ribosomal RNA. Biochemistry. 1976 Feb 10;15(3):505–508. doi: 10.1021/bi00648a008. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Walker T. A., Schroeder E. Structure of the 5.8S RNA component of the 5.8S-28S ribosomal RNA junction complex. Biochemistry. 1977 Nov 29;16(24):5321–5328. doi: 10.1021/bi00643a025. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Zachau H. G. Preparation of transfer RNA fragments by limited degradation with spleen exonuclease. Methods Enzymol. 1974;29:473–477. doi: 10.1016/0076-6879(74)29040-2. [DOI] [PubMed] [Google Scholar]
- Reid B. R., Ribeiro N. S., McCollum L., Abbate J., Hurd R. E. High-resolution nuclear magnetic resonance determination of transfer RNA tertiary base pairs in solution. 1. Species containing a small variable loop. Biochemistry. 1977 May 17;16(10):2086–2094. doi: 10.1021/bi00629a006. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Römer R., Riesner D., Maass G., Wintermeyer W., Thiebe R., Zachau H. G. Cooperative helix-coil transitions in half molecules of phenylalanine specific tRNA from yeast. FEBS Lett. 1969 Sep;5(1):15–19. doi: 10.1016/0014-5793(69)80281-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Pachler P. F. Discontinuity of 26 s rRNA in Acanthamoeba castellani. J Mol Biol. 1972 May 14;66(2):225–237. doi: 10.1016/0022-2836(72)90475-5. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Baller J., Doty P. Complementary oligonucleotide binding to the anticodon loop of fMet-transfer RNA. Nature. 1970 Feb 7;225(5232):508–510. doi: 10.1038/225508a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Van N. T., Nazar R. N., Sitz T. O. Comparative studies on the secondary structure of eukaryotic 5.8S ribosomal RNA. Biochemistry. 1977 Aug 23;16(17):3754–3759. doi: 10.1021/bi00636a004. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Tinoco I., Jr The stability of RNA hairpin loops containing A-U-G: An-U-G-Um. Biopolymers. 1974 Nov;13(11):2367–2383. doi: 10.1002/bip.1974.360131116. [DOI] [PubMed] [Google Scholar]
- Woledge J., Corry M. J., Payne P. I. Ribosomal RNA homologies in flowering plants: comparison of the nucleotide sequences in 5.8-S rRNA from broad bean, dwarf bean, tomato, sunflower and rye. Biochim Biophys Acta. 1974 May 31;349(3):339–350. [PubMed] [Google Scholar]