Abstract
Osteoblasts sense and respond to mechanical stimuli in a process involving influx and release of large ions and signaling molecules. Unapposed gap junction hemichannels formed of connexin43 (Cx43) have been proposed as a major route for such exchange, and in particular for release of ATP and prostaglandin E2 (PGE2) in osteocytes. However, we have found that Cx43-null osteoblasts have unaltered mechanically-induced PGE2 release and ATP-induced YoPro dye uptake. In contrast, PGE2 release in response to fluid shear stress is abolished in P2X7 receptor (P2X7R) null osteoblasts and ATP-induced dye uptake is attenuated following treatment of wildtype cells with a P2X7R or Pannexin1 (Panx1) channel blocker. These data indicate that Panx1 channels, in concert with P2X7R, likely form a molecular complex that performs the hemichannel function in osteoblast mechanosignaling.
Keywords: P2X7R, ATP, osteoblast, gap junction, dye uptake
Introduction
Gap junctions in vertebrates are formed by connexins and in invertebrates by innexins; remarkably, these functionally equivalent proteins display no homology at the level of amino acid sequence, but, as shown forty years ago by Ross Johnson, their intramembrane particles look pretty similar (Johnson & Sheridan, 1971; Johnson, Herman & Preus, 1973). Pannexins were discovered in searches of the vertebrate genome for similarities to innexin cDNA sequences. In contrast to connexins and innexins, pannexins likely do not form junctional channels; however, at least one of the three pannexins, Pannexin1 (Panx1), forms large conductance, mechanosensitive and highly permeable channels in nonjunctional membranes of mammalian cells [for reviews, see (Iglesias et al., 2009a; Sosinsky et al., 2011)]. Certain connexin hemichannels can also open when unpaired, forming pores permeable to large molecules, similar to Panx1 channels and gap junctions (Spray, Ye & Ransom, 2006).
Thus, whereas both connexins and Panx1 are involved in intercellular communication, they appear to have different roles (Scemes et al., 2007). Connexins mainly provide junctional coupling, whereas Panx1 channels assist autocrine/paracrine signaling by providing a pathway for controlled release of signaling molecules such as ATP (Dahl & Locovei, 2006; Scemes et al., 2007; MacVicar & Thompson, 2010; Sosinsky et al., 2011). In this paper we report largely unpublished studies of osteoblasts in vitro, focusing on these different roles of connexins and Panx1 in bone cells. We conclude that, under the conditions of our studies, functions previously attributed to Cx43 hemichannels are likely mediated by Panx1 channels instead.
Bone cells are coupled into a functional syncytium by gap junction channels formed mainly by Cx43 (Donahue, 2000; Civitelli, 2008). Intercellular signals transmitted through gap junction channels formed by these connexins are believed to play key roles in bone embryogenesis, differentiation and mineralization (Minkoff et al., 1994; Donahue, 2000; Schiller et al., 2001; Civitelli, 2008; Kar et al., 2012). Studies of Cx43 deficient mice (Lecanda et al., 2000; Civitelli, 2008) and our recent study with immortalized wildtype and Cx43-null osteoblasts (Thi et al., 2010b) have clearly demonstrated that presence of Cx43 is essential during early phases of osteoblast differentiation and maturation.
Signaling through gap junction channels is also believed to be essential in bone remodeling. This life-long process is crucial for maintenance of bone mass and integrity, and consists of continuous bone resorption and deposition whereby aging tissue is replaced and injuries are repaired. While it is well established that bone remodeling is regulated by the mechanical loading imposed to the bone by daily physical activity, it is still unclear how these load-generated mechanical signals are translated into the cellular and biochemical events that ultimately result in bone remodeling. There is accumulating evidence that non-junctional Cx43 could actively participate in these events, where Cx43 hemichannels would open in response to mechanical stimulation and provide an efflux pathway for mechanosignaling molecules, such as ATP or prostaglandin E2 (PGE2) (Romanello & D’Andrea, 2001; Jiang & Cherian, 2003; Cherian et al., 2005; Genetos et al., 2007). Cx43 hemichannels are not mechanosensitive and their response to mechanical stimuli has been proposed to be mediated by their interaction with integrins (Batra et al., 2012a). Besides Cx43 hemichannels, a role for ATP receptors (ionotropic P2X7 receptors) in bone cell mechanotransduction and signaling has also been proposed (Li et al., 2005). Activation of P2X7 receptors (P2X7Rs) has been shown to mediate ATP-induced ATP release from certain cell types (Anderson, Bergher & Swanson, 2004; Suadicani, Brosnan & Scemes, 2006), and P2X7R deletion abrogates PGE2 release from osteoblasts in response to fluid-shear stress (Li et al., 2005). Moreover, studies by us and others have shown that P2X7Rs functionally interact with Panx1 channels to provide the permeabilization pathway for P2X7R-induced ATP and IL-1β release (Pelegrin & Surprenant, 2006; Locovei et al., 2007). In addition, Panx1 channels possess both mechanosensitivity (Bao, Locovei & Dahl, 2004) and activation by extracellular K+ (Silverman et al., 2009; Suadicani et al., 2012) both of which may be important in bone pathophysiology.
The main goal of the studies described here was to determine the relative role of Cx43 and Panx1 in bone cell mechanotransduction and formation of the ATP-induced dye-uptake pathway that facilitates PGE2 release. Use of pharmacological approaches to discriminate the participation of connexin hemichannels and Panx1 channels is somewhat complicated by the overlapping effects of pharmacological blockers. In this study we combined use of these drugs with that of the newly generated MOB cell line (wildtype and Cx43-null) (Thi et al., 2010b), allowing us to specifically address the participation of Cx43 in fluid shear stress-induced PGE2 release from osteoblast and ATP-induced dye-uptake.
Materials and Methods
Materials
Minimal Essential alpha Medium (α-MEM), fetal bovine serum (FBS), penicillin-streptomycin, and YoPro-1 Iodide (491/509) were purchased from Invitrogen Corporation (Carlsbad, CA, USA). Collagenase Type II was purchased from Worthington Biochemical Corporation (Lakewood, NJ, USA), protease inhibitor cocktail was purchased from Roche (Mannheim, Germany), nitrocellulose membranes were purchased from Whatman GmbH (Dassel, Germany) and Immobilon Western detection kit were purchased from Millipore (Billerica, MA, USA). All other chemicals were from Sigma-Aldrich Corp. (St Louis, MO, USA) unless otherwise stated.
Cell line Culture
Osteoblastic MOB-C and 43KO-MOB-C cells (mouse osteoblast cell lines derived from wildtype and Cx43-null calvaria; Thi et al., 2010b) and MC3T3-E1 cells (subclone 4) (ATCC, Manassas, VA, USA) were cultured in α-MEM containing 1% penicillin-streptomycin and 10% FBS at 37°C with 95% air/5% CO2.
Primary osteoblast culture
As described in our previous work (Thi et al., 2010b) osteoblasts were isolated from calvaria of newborn (P0), embryonic (E19-20) wildtype and Cx43-null mice obtained from in-house mating of Cx43 heterozygous mice (C57BL/6J-Gja1tm1Kdr) (Reaume et al., 1995) and from newborn P2X7R-null mice (B6.129P2-P2rx7tm1Gab/J). All animal procedures and experimental protocols were approved by the Institute for Animal Studies of the Albert Einstein College of Medicine in accordance with NIH Guidelines. Briefly, the pups were euthanized by decapitation, the periosteum and endosteum of individual calvaria were carefully removed, cleaned and thoroughly diced into small pieces, pooled for each pup and digested in 1X PBS containing 4 mg/ml of collagenase Type II at 37°C for 10 min. Supernatant from the second and third sequential digestions at 37°C were collected. Cells were then collected by centrifugation, resuspended in α-MEM supplemented with 10% FBS and 1% penicillin-streptomycin and seeded in culture dishes. Primary osteoblasts isolated from wildtype and from Cx43-null and P2X7R-null calvaria tissue were termed PMOB, 43KO-PMOB and P2X7RKO-PMOB, respectively.
Pulsatile fluid shear stress (PFSS) treatment
For flow experiments the immortalized wildtype (MOB-C), Cx43-null (43KO-MOB-C) and MC3T3-E1 osteoblastic cell lines were seeded at 104 cells/cm2 and primary osteoblasts were seeded at 2x104 cells/cm2 and grown on glass slides for 3 days. The fluid flow setup consisted of a parallel plate flow chamber (Cytodyne, La Jolla, CA, USA) and a re-circulating flow circuit as previously described (Thi, Suadicani & Spray, 2010a). Briefly, the flow loop included a variable speed Masterflex pump (Cole-Palmer Instrument Company, Vernon Hills, IL, USA) and a reservoir with culture medium (α-MEM + 1% FBS) maintained at 37°C with 95% air/5% CO2. This system produces pulsatile flow over a cell monolayer with average shear stress of 10 dyne/cm2 at 1 Hz frequency. Control cells were kept under static conditions at 37°C with 95% air/5% CO2.
Quantification of prostaglandin E2 (PGE2) release
Supernatants from control (static) and pulsatile flow-conditioned media were collected immediately after one hour exposure of the cells to fluid shear stress. Supernatants were stored at −80°C and then assayed for PGE2 using a Prostaglandin E2 EIA Kit (Cayman chemical Company, Ann Arbor, MI, USA). Average OD values were acquired at wavelength 415nm using a FLUOStar Omega plate reader (BMG Labtech, Ortenberg, Germany). PGE2 concentrations in the media were determined from the standard curve obtained for each set of experiments. The amount of PGE2 release was normalized to respective cellular protein levels. Total protein concentration from the samples was determined using the BCA Assay Kit (Thermo Scientific, Waltham, MA, USA).
Western Blot Analysis
Cells were seeded at 1500 cells/cm2 and cultured for 10 days. Cells were then harvested, sonicated in 70μl of lysis buffer (1mM NaHCO3, 2mM PMSF, 1mM Na Orthovanadate, 5mm EDTA and 1X protease inhibitor) and Western blotting was performed as previously described (Thi et al., 2010b). Briefly, protein samples were loaded onto 10% SDS-PAGE gels for separation and electrophoretically transferred to nitrocellulose membranes. The membranes were probed with primary polyclonal antibodies against Cx43 (1:10,000; Sigma-Aldrich), P2X7R (1:1000; Alomone Labs, Jerusalem, Israel) and Panx1 (mid, 1:100; Invitrogen), and monoclonal antibody against β-actin (1:25,000; Sigma-Aldrich), followed by incubation with the secondary antibody, horseradish peroxidase (HP)-conjugated anti-rabbit IgG or anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The protein bands were detected using the Immobilon Western detection kit and exposed on In-Vivo FX PRO imaging system (Carestream, Rochester, NY, USA).
YoPro-1 Dye Uptake Analysis
MOB-C, 43KO-MOB-C and MC3T3-E1 cells were seeded at 2000 cells/cm2 and grown for 3 days on MatTek glass bottom dishes (MatTek Corporation, Ashland, MA). All dye uptake experiments were performed in low divalent cation PBS (LDPBS, Ca2+/Mg2+ free), a condition that has been routinely used in experiments with connexin hemichannels and also P2X7Rs to maximize channel activation (Liu et al., 1996; Virginio et al., 1997; North & Surprenant, 2000; Contreras et al., 2003; Jiang & Cherian, 2003; Parpura, Scemes & Spray, 2004; Cherian et al., 2005; Burra et al., 2010; Batra et al., 2012a). Cells were pretreated with the P2X7R blocker brilliant blue G (BBG, Sigma-Aldrich), and the connexin/pannexin blockers carbenoxolone (Sigma-Aldrich) and mefloquine (QU-024, Bioblocks, CA, USA) in serum-free α–MEM for 20 minutes prior to the application of 5μM YoPro-1 (Invitrogen) dye with or without the P2 receptor agonist, ATP (Sigma-Aldrich) for 10 minutes. YoPro-1 dye was used because it is non-fluorescent in solution and only fluoresces when permeating the cells and interacting with nucleic acids. Three to four images per treatment were taken from each set of experiments using a Nikon Eclipse TE300 microscope (Nikon Instruments Inc., Melville, NY, USA) and a Spot-RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) with fixed gain and exposure time.
Statistical Analysis
YoPro uptake was quantified as number of YoPro positive cells divided by total number of cells x100 (% YoPro positive cells),counted using ImageJ software (NIH, Maryland, MD, USA). Data were analyzed from three to four independent sets of experiments using GraphPad Prism 5 software (San Diego, CA, USA). Statistical differences between PGE2 released amounts and YoPro-1 dye uptake were determined by one way ANOVA followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Participation of Cx43 hemichannels and P2X7Rs in fluid shear stress induced PGE2 release from osteoblasts
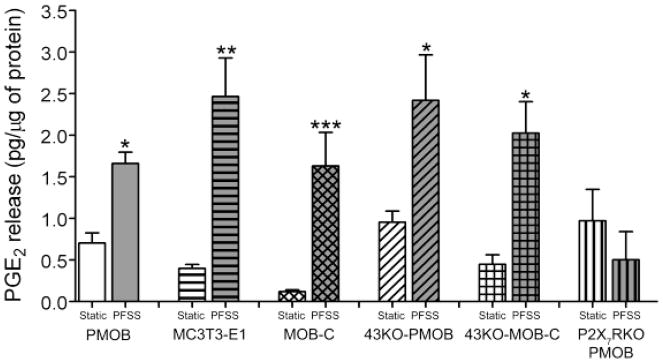
Most previous studies on the role of Cx43 hemichannels in shear-induced ATP or PGE2 release have relied on the use of compounds that do not pharmacologically discriminate Cx43 from the Panx1-P2X7R (Cherian et al., 2005; Genetos et al., 2007). Therefore, we have used Cx43-null or P2X7R-null osteoblasts to specifically address each channel’s role. As shown in Fig. 1, significant PGE2 is released from MC3T3-E1 cells, primary osteoblasts (PMOB),primary Cx43-null osteoblasts (43KO-PMOB) and from hTERT-immortalized wildtype (MOB-C) and Cx43-null (43KO-MOB-C) osteoblasts in response to pulsatile fluid shear stress (PFSS). In contrast, PFSS-induced PGE2 release was completely absent in primary osteoblasts lacking P2X7R (P2X7RKO-PMOB), as had been shown previously by another group (Li et al., 2005). PGE2 released amounts under static conditions were not significantly different among all the cell types. This finding shows that under these conditions P2X7R rather than Cx43 plays a crucial role in shear induced PGE2 release from osteoblasts.
Figure 1.
Analysis of pulsatile fluid shear stress (PFSS) induced PGE2 release from MC3T3-E1 cells, primary osteoblast (PMOB), primary Cx43-null osteoblast (43KO-PMOB), P2X7R-null osteoblasts (P2X7RKO-PMOB) and hTERT-immortalized wildtype (MOB-C) and Cx43-null (43KO-MOB-C) osteoblasts. Supernatants from static and PFSS conditioned media were assayed for PGE2 using the Prostaglandin E2 EIA Kit. PGE2 concentrations in the media were determined from standard curves obtained for each set of experiments, and amount of PGE2 release was normalized to respective cellular protein content. All data are presented as mean ± SEM, n = 4. P values were obtained using one way ANOVA followed by Tukey’s multiple comparison test. (***P < 0.0005, **P < 0.005, *P < 0.05 for static control vs. respective PFSS = 10 dyne/cm2 at 1 Hz for 1 h)
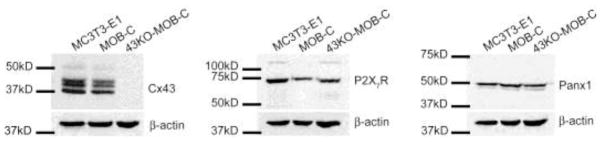
To evaluate the extent to which the cell lines used in these studies express the channels of interest, we screened MC3T3-E1, MOB-C and 43KO-MOB-C cells for expression of Cx43, P2X7R and Panx1 using Western blot analysis. As shown in Fig. 2, all three lines expressed P2X7R, as was expected from the well established role of these receptors in bone resorption and mechanotransduction (Gallagher, 2004; Li et al., 2005). In addition, Panx1 was found in all cell lines, consistent with its reported expression in primary osteoblasts (Penuela et al., 2007), and Cx43 was found in both MC3T3-E1 and MOB-C, but not in 43KO-MOB-C (Thi et al., 2010b).
Figure 2.
Western blot assessment of Cx43, P2X7R and Panx1 expression levels in MC3T3-E1, MOB-C and 43KO-MOB-C cells. Equal amounts of protein from each cell type were used. Western blot analysis was performed using antibodies against Cx43, P2X7R, Panx1 and β-actin. β-actin was used as a constitutively expressed protein for loading control.
Cx43 hemichannels do not mediate dye uptake in low divalent cation solution
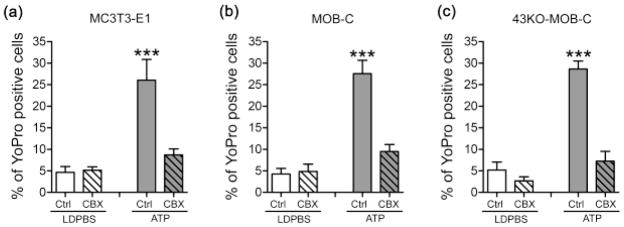
To study whether Cx43 was involved in dye uptake resulting from the mechanical stimulation induced by medium displacement in the imaging dish, we performed control basal level YoPro dye uptake experiments in low divalent cation PBS (LDPBS), a condition reported to favor hemichannel opening (Contreras et al., 2003; Cherian et al., 2005; Burra et al., 2010; Batra et al., 2012a). Our results indicate that mechanically induced YoPro dye uptake in LDPBS solution was virtually identical in MC3T3-E1, MOB-C and 43KO-MOB-C, and this dye uptake was thus independent of whether Cx43 was present or absent (Fig. 3).
Figure 3.
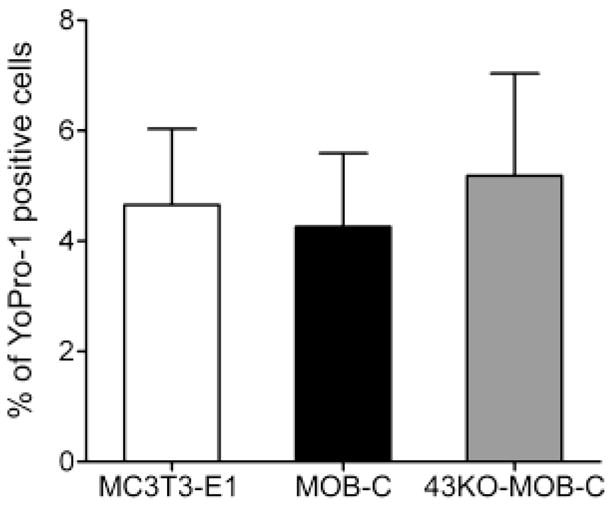
Assessment of basal level YoPro-1 uptake resulting from the mechanical stimulation induced by medium displacement in the imaging dish in (a) MC3T3-E1, (b) MOB-C and (c) 43KO-MOB-C cells. Cells were bathed in α-MEM at 37°C for 20 min prior to 5μM YoPro incubation for 10 min in low divalent cation PBS (LDPBS). (d) Percent of YoPro-1 positive cells was calculated by normalizing dye positive cells with total number of cells in each image field x100. All data are presented as mean ± SEM, n = 4 for each cell types and compared using one way ANOVA analysis followed by Tukey’s multiple comparison test.
When we treated all three cell types with 1mM ATP, YoPro uptake significantly increased (Fig. 4, solid gray bars), indicating that ATP could induce large pore formation in osteoblasts regardless of whether Cx43 was present or absent. Next, we examined the extent to which Cx43 channel blockers inhibited dye uptake into MC3T3-E1, MOB-C and 43KO-MOB-C cells. For these studies, we used two compounds originally shown to block gap junction channels but subsequently found to be much more potent inhibitors of Panx1 channels at much lower concentrations. Carbenoxolone (CBX) is a widely used gap junction blocker effective at concentrations of 100μM and higher (Cherian et al., 2005; Batra et al., 2012a); we found a radical decrease in ATP induced dye uptake not only in wild type cells but also in Cx43-null cells, using a lower concentration that blocks Panx1 [20μM, see (Bruzzone et al., 2005; Iglesias et al., 2008; Poornima et al., 2012)] (Fig. 4, gray colored striped bars). This finding of blockade of dye uptake by low CBX concentration in both wildtype and Cx43-null osteoblast cells strongly suggests that Cx43 hemichannels do not play a critical role in large pore formation induced by ATP. This also suggests that a channel other than Cx43, likely Panx1, is responsible for the dye uptake.
Figure 4.
Effects of carbenoxolone treatment on YoPro-1 uptake induced either by mechanical stimulation caused by liquid displacement or by ATP application in (a) MC3T3-E1, (b) MOB-C, and (c) 43KO-MOB-C osteoblasts. For these studies, cells were bathed in 5μM YoPro-1 and effects of 20μM carbenoxolone (CBX) were evaluated 20 min after treatment. For the ATP stimulated group, 1mM ATP was added 10 min after CBX. All experiments were performed in low divalent cation PBS (LDPBS). Percent of YoPro-1 positive cells was calculated by normalizing dye positive cells with total number of cells in each image field x100. All data are presented as mean ± SEM, n = 3. P values were obtained using one way ANOVA analysis followed by Tukey’s multiple comparison test. (***P < 0.0005, ATP treated cells vs. all other treatments)
To test the participation of Panx1 in ATP-induced pore formation, we also used mefloquine (MFQ). MFQ was originally shown to block gap junction channels [IC50 for Cx43 ~25μM: (Cruikshank et al., 2004)], but is now known to be a much more potent blocker for Panx1, being effective at submicromolar concentrations (Iglesias, Spray & Scemes, 2009b). We used three low concentrations of MFQ (10, 50 and 90nM) that were far below those with effects on Cx43 channels on other cell types (Cruikshank et al., 2004). We found that while MFQ only slightly inhibited basal dye influx in LDPBS (Fig. 5A, B, C, hatched bars), ATP-induced dye uptake was substantially reduced in MC3T3-E1 cells (Fig. 5A, gray colored hatched bars) and in both MOB-C and 43KO-MOB-C (Fig. 5B and C, gray colored hatched bars). In particular, ATP-induced dye uptake was completely eliminated in the presence of 90nM MFQ in all cell lines. These results imply that Panx1 rather than Cx43 is the mechanosensitive channel being activated by ATP that provides the influx pathway for YoPro uptake.
Figure 5.
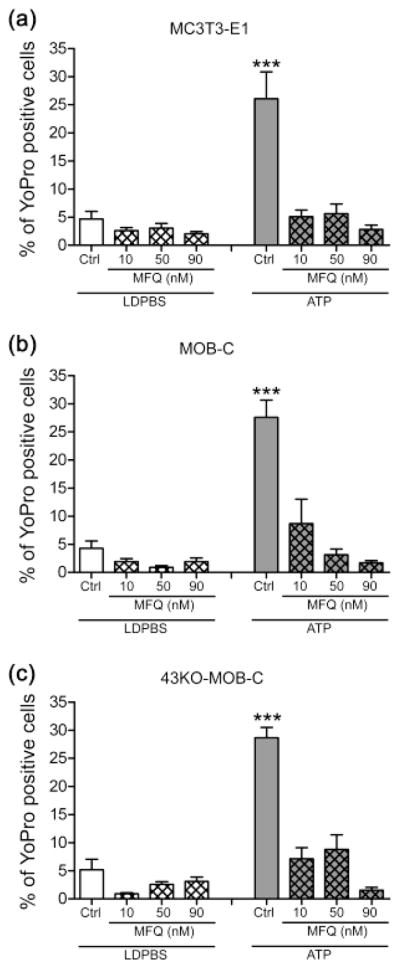
Effects of mefloquine on YoPro-1 uptake induced either by mechanical stimulation caused by liquid displacement or by ATP application in (a) MC3T3-E1, (b) MOB-C, and (c) 43KO-MOB-C osteoblasts. For these studies, cells were bathed in 5μM YoPro-1 and effects of three different concentrations of mefloquine (MFQ, 10, 50 and 90nM) were evaluated 20 min after treatment. For the ATP stimulated group, 1mM ATP was added 10 min after MFQ. All experiments were performed in low divalent cation PBS (LDPBS). Percent of YoPro-1 positive cells was calculated by normalizing dye positive cells with total number of cells in each image field x100. All data are presented as mean ± SEM, n = 3. P values were obtained using one way ANOVA analysis followed by Tukey’s multiple comparison test. (***P < 0.0005, ATP treated cells vs. all other treatments)
Participation of the Panx1-P2X7R complex in ATP-induced dye uptake
It has been shown that mice lacking P2X7R have osteopenia in load bearing bones, implying a critical role for P2X7R as a key mediator in the skeletal response to mechanical loading (Ke et al., 2003). Moreover, ATP signaling through P2X7R has been implicated in fluid shear stress induced release of PGE2 in bone cells (Li et al., 2005). There is accumulating evidence that P2X7R functionally interacts with Panx1, providing a conduit for ATP-induced ATP release (Locovei et al., 2007; Iglesias et al., 2008). Therefore, to test the hypothesis that the P2X7R-Panx1 complex in osteoblasts provides the pathway for ATP induced dye influx we tested the effects of brilliant blue G (BBG), a P2X7R blocker. As shown in Fig. 6, all cell lines showed inhibition of ATP-induced dye uptake by BBG in a dose-dependent manner. At higher concentrations (5 and 10 μM) BBG completely abolished ATP induced dye uptake in both wildtype osteoblastic cells and in 43KO-MOB-C cells, demonstrating that P2X7Rs play a role in such uptake. These collective findings strongly indicate that a pathway other than Cx43 hemichannels, likely the P2X7R-Panx1 complex, participates in dye uptake under static conditions.
Figure 6.
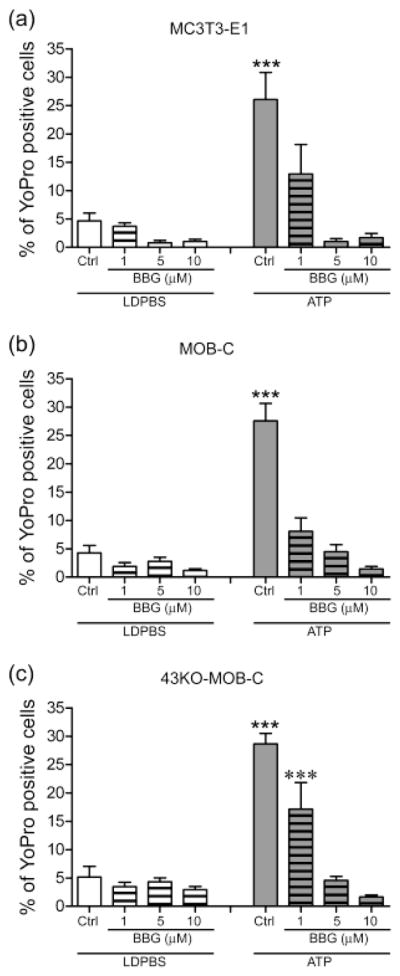
Effects of brilliant blue G treatment on YoPro-1 uptake induced either by mechanical stimulation caused by liquid displacement or by ATP application in (a) MC3T3-E1, (b) MOB-C, and (c) 43KO-MOB-C osteoblasts. For these studies, cells were bathed in 5μM YoPro-1 and effects of three different concentrations of brilliant blue G (BBG, 1, 5 and 10μM) were evaluated 20 min after treatment. For the ATP stimulated group, 1mM ATP was added 10 min after BBG. All experiments were performed in low divalent cation PBS (LDPBS). Percent of YoPro-1 positive cells was calculated by normalizing dye positive cells with total number of cells in each image field x100. All data are presented as mean ± SEM, n = 3. P values were obtained using one way ANOVA analysis followed by Tukey’s multiple comparison test. (***P < 0.0005, ATP treated cells vs. all other treatments, ***P < 0.0005, 1μM BBG vs. 1μM BBG + ATP)
Discussion
There are several types of channels in osteoblasts and other cell types that are permeable to molecules as large as 1 kDa (Spray et al., 2006). The most prominent of those are channels formed by the gap junction protein Cx43 and the P2X7R-Panx1 complex. Gap junction hemichannels have been proposed to underlie the large conductance anion channel in skeletal myocytes and elsewhere (Blatz & Magleby, 1983; Schwarze & Kolb, 1984) and were thought to be responsible for permeabilization of J774 cells by high ATP concentration (Beyer & Steinberg, 1991). This permeabilization by ATP now appears to be the result of P2X7R activation in association with Panx1 (Pelegrin & Surprenant, 2006; Locovei et al., 2007), and the permeability of these channels to fluorescent dyes has been shown to be very similar to that of gap junction channels [compare (Flagg-Newton, Simpson & Loewenstein, 1979; Di Virgilio et al., 1996)]. Moreover, pharmacological blockade of connexins and Panx1 is achieved by most of the same agents. It is thus possible that roles attributed to one of these classes of molecules could be performed by the other.
In bone cells, fluid shear stress induces the release of extracellular mechanosignaling molecules such as prostaglandins E2 (PGE2), nitric oxide (NO), ATP and VEGF that are essential for bone homeostasis (Reich & Frangos, 1993; Klein-Nulend et al., 1995; Genetos et al., 2005; Thi et al., 2010a). However, the cellular pathways that are involved in the release of these substances are not well characterized in bone cells. Studies with other cell types suggest that Cx43 hemichannels, Panx1 channels, and the ionotropic purinergic P2X7 receptor (Fig. 7) most likely assist in the release of these substances (Cherian et al., 2005; Li et al., 2005; Locovei, Bao & Dahl, 2006).
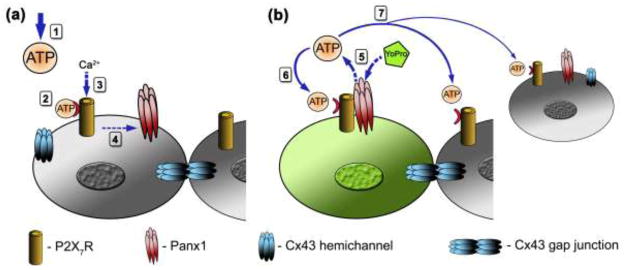
Figure 7.
Schematic diagram depicting the sequence of events triggered by ATP-induced activation of P2X7Rs in osteoblasts and the distribution of P2X7Rs, Panx1 channels and Cx43 when forming gap junction channels and hemichannels. (a) Extracellular ATP (exogenously added or release from mechanically stimulated cells) diffuses (1), binds and activates ionotropic P2X7Rs (2), resulting in influx of Ca2+ (3) and P2X7R interaction with Panx1 channels (4). (b) P2X7 activation induces opening of Panx1 channels, providing a pathway for ATP efflux and for YoPro dye uptake (5). Autocrine P2X7R activation by released ATP triggers further ATP-induced ATP release from stimulated cells (6) and enhances paracrine signaling (7) to the neighboring cells. Gap junction channels formed of Cx43 also play a role in intercellular signaling through exchange of second messengers.
Despite the existence of a small pool of unpaired Cx43 connexons (“hemichannels”) on the unopposed cell surface (Dermietzel et al., 2003), there is little evidence for opening of these channels under physiological conditions. Nevertheless, most reports of functional hemichannels use as evidence dye uptake measurements at normal resting potentials, with or without divalent cation chelation, and validation by use of gap junction channel blockers (Hofer & Dermietzel, 1998; Stout et al., 2002; Contreras et al., 2003; Goodenough & Paul, 2003). However, dye uptake mediated by the P2X7R-Panx1 complex is Ca2+ and mechanosensitive, increased by membrane depolarization and even more sensitive to blockade by carbenoxolone than are gap junctions (Bao et al., 2004; Suadicani et al., 2012). The activation of P2X7Rs was shown to mediate ATP release in astrocytes (Anderson et al., 2004; Suadicani et al., 2006) and PGE2 release from osteoblasts in response to fluid-shear stress is absent in P2X7R-null mice (Li et al., 2005). Studies on cells in which Panx1 expression was manipulated have shown that Panx1 provides the channel for P2X7R-induced release of ATP (Locovei et al., 2007), which has been confirmed using the Panx1-null mouse (Suadicani et al., 2012). Perhaps most interestingly of all, activation of Panx1 channels has been shown to be mechanosensitive (Bao et al., 2004). Therefore, although Cx43 hemichannels have been the focus of many studies in bone mechanotransduction (Jiang & Cherian, 2003; Cherian et al., 2005; Genetos et al., 2007; Burra et al., 2010; Batra, Kar & Jiang, 2012b), we here provide evidence for the P2X7R-Panx1 complex involvement in mechanically-induced ATP release from osteoblasts (as illustrated in Fig. 7) and PGE2 release via an unidentified pathway that does not require Cx43.
The combined use of pharmacological blockers and of osteoblasts lacking Cx43 or P2X7R in studies described here has allowed discrimination of the specific role of each of these molecular mediators of intercellular communication. While we find that mechanically induced PGE2 release and ATP-induced dye uptake are not dependent on the presence or function of Cx43, further dissection of roles of components of the P2X7R-Panx1 complex await the use of Panx1-null osteoblastic cell lines, which are currently being generated in our laboratory.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Disease [grant numbers DK091466 (to M.M.T), DK081435 (to S.O.S)] and National Institute of Arthritis and Musculoskeletal and Skin Diseases [grant number AR057139 (to D.C.S)].
References
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–56. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Letters. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, Desimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012a;109:3359–64. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012b;1818:1909–18. doi: 10.1016/j.bbamem.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Steinberg TH. Evidence that the gap junction protein connexin-43 is the ATP- induced pore of mouse macrophages. J Biol Chem. 1991;266:7971–4. [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Single voltage-dependent chloride-selective channels of large conductance in cultured rat muscle. Biophys J. 1983;43:237–41. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–53. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Archives of Biochemistry and Biophysics. 2008;473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Locovei S. Pannexin: To gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Meier C, Bukauskas F, Spray DC. Following tracks of hemichannels. Cell Commun Adhes. 2003;10:335–340. doi: 10.1080/cac.10.4-6.335.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Ferrari D, Falzoni S, Chiozzi P, Munerati M, Steinberg TH, Baricordi OR. P2 purinoceptors in the immune system. Ciba Found Symp. 1996;198:290–302. doi: 10.1002/9780470514900.ch17. discussion 302–5. [DOI] [PubMed] [Google Scholar]
- Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- Flagg-Newton J, Simpson I, Loewenstein WR. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979;205:404–7. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Gallagher JA. ATP P2 receptors and regulation of bone effector cells. J Musculoskelet Neuronal Interact. 2004;4:125–127. [PubMed] [Google Scholar]
- Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid Shear-Induced ATP Secretion Mediates Prostaglandin Release in MC3T3-E1 Osteoblasts. Journal of Bone and Mineral Research. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: The Molecular Substrate of Astrocyte “Hemichannels”. Journal of Neuroscience. 2009a;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–60. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Spray DC, Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes. 2009b;16:131–7. doi: 10.3109/15419061003642618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Herman WS, Preus DM. Homocellular and heterocellular gap junctions in Limulus: a thin-section and freeze-fracture study. J Ultrastruct Res. 1973;43:298–312. doi: 10.1016/s0022-5320(73)80040-1. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Sheridan JD. Junctions between cancer cells in culture: ultrastructure and permeability. Science. 1971;174:717–9. doi: 10.1126/science.174.4010.717. [DOI] [PubMed] [Google Scholar]
- Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012 doi: 10.1016/j.abb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WSS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 Nucleotide Receptor Reveals Its Regulatory Roles in Bone Formation and Resorption. Molecular Endocrinology. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. The Journal of Cell Biology. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- Liu TF, Li HY, Atkinson MM, Johnson RG. Comparison of lucifer yellow leakage and cell-to-cell transfer following intracellular injection in normal and antisense Novikoff cells under treatment with low extracellular Ca2+ Methods Find Exp Clin Pharmacol. 1996;18:493–7. [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: Function without a gap. Proceedings of the National Academy of Sciences. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Letters. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Minkoff R, Rundus VR, Parker SB, Hertzberg EL, Laing JG, Beyer EC. Gap junction proteins exhibit early and specific expression during intramembranous bone formation in the developing chick mandible. Anat Embryol (Berl) 1994;190:231–241. doi: 10.1007/BF00234301. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochemistry International. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin- 1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. Journal of Cell Science. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- Poornima V, Madhupriya M, Kootar S, Sujatha G, Kumar A, Bera AK. P2X7 receptor-pannexin 1 hemichannel association: effect of extracellular calcium on membrane permeabilization. J Mol Neurosci. 2012;46:585–94. doi: 10.1007/s12031-011-9646-8. [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Reich KM, Frangos JA. Protein kinase C mediates flow-induced prostaglandin E2 production in osteoblasts. Calcif Tissue Int. 1993;52:62–66. doi: 10.1007/BF00675628. [DOI] [PubMed] [Google Scholar]
- Romanello M, D’Andrea P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: a role for gap-junctional hemichannels. J Bone Miner Res. 2001;16:1465–1476. doi: 10.1359/jbmr.2001.16.8.1465. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biology. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PC, D’Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–369. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- Schwarze W, Kolb HA. Voltage-dependent kinetics of an anionic channel of large unit conductance in macrophages and myotube membranes. Pflugers Arch. 1984;402:281–91. doi: 10.1007/BF00585511. [DOI] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–51. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–7. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–73. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 Receptors Mediate ATP Release and Amplification of Astrocytic Intercellular Ca2+ Signaling. Journal of Neuroscience. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP Signaling Is Deficient in Cultured Pannexin1-Null Mouse Astrocytes. Glia. 2012 doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Suadicani SO, Spray DC. Fluid Flow-induced Soluble Vascular Endothelial Growth Factor Isoforms Regulate Actin Adaptation in Osteoblasts. Journal of Biological Chemistry. 2010a;285:30931–30941. doi: 10.1074/jbc.M110.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Urban-Maldonado M, Spray DC, Suadicani SO. Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria. AJP - Cell Physiology. 2010b;299:C994–1006. doi: 10.1152/ajpcell.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–94. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]