Abstract
Acquired aortic valve disease and valvular calcification is highly prevalent in adult populations worldwide and is associated with significant cardiovascular morbidity and mortality. At present, there are no medical therapies that will prevent or regress aortic valve calcification or stenosis and surgical or transcatheter aortic valve replacement remain the only effective therapies for treating this disease. In the setting of valve injury as a result of exposure to biochemical mediators or hemodynamic forces, normal homeostatic processes are disrupted resulting in extracellular matrix degradation, aberrant matrix deposition and fibrosis, inflammatory cell infiltration, lipid accumulation, and neoangiogenesis of the valve tissue and, ultimately, calcification of the valve. Calcification of the aortic valve is now understood to be an active process that involves the coordinated actions of resident valve endothelial and interstitial cells, circulating inflammatory and immune cells, and bone marrow-derived cells. These cells may undergo a phenotype transition to become osteoblast-like cells and elaborate bone matrix, endothelial-to-mesenchymal transition, and form matrix vesicles that serve as a nidus for microcalcifications. Each of these mechanisms has been shown to contribute to aortic valve calcification suggesting that strategies that target these cellular events may lead to novel therapeutic interventions to halt the progression or reverse aortic valve calcification.
Keywords: Calcification, Aortic Valve
The earliest description of calcific aortic stenosis is attributed to the French physician Lazare Rivière who, in 1663, reported the necropsy findings from a patient with palpitations, progressive dyspnea and loss of peripheral pulses. He noted left ventricular enlargement and identified large caruncle-like masses obstructing the left ventricular outflow to the aorta.1 While other reports followed, the debate about the etiology of aortic valve calcification (AVC) did not begin until much later.1 Although calcification was originally attributed to endocarditis, in 1846 Hasse challenged this theory by suggesting that a degenerative process occurred within the valve itself to initiate ossification.1 In 1904, Moenckeburg authored the first detailed description of AVC in which he reported that this pathology likely occurred as a result of calcium deposition on valve cusps that had become sclerotic. He described two mechanisms to explain this phenomenon: degeneration within the layers of the valve leaflets nearest the sinuses of Valsalva that propagated towards the tips of the cusps or sclerotic changes of the aorta that extended to involve the valve cusps.1 Within the past 20 years, there has been a tremendous resurgence in interest in advancing these early hypotheses to identify the cellular and molecular mechanisms that initiate AVC owing to the high prevalence of this disease, its associated morbidity and mortality, and the emerging idea that therapies that target these processes could increase the durability of surgically implanted and transcatheter bioprosthetic valves.
Epidemiology of aortic valve disease
Recent estimates indicate that aortic valve disease is common in the United States adult population and contributes to more than 28,000 deaths and 48,000 hospitalizations annually.2 Worldwide, population-based studies have revealed that aortic valve disease is the most frequently observed valve pathology and is present in up to 43% of patients presenting with valvular heart disease.3 In individuals ≥ 65 years of age, aortic sclerosis is present in 29% of individuals while the prevalence of aortic stenosis has been estimated to be between 2%-9%, depending upon the population studied.4, 5 Aortic sclerosis is not a benign finding as it is associated with a ~50% increased risk of myocardial infarction and cardiovascular death and the rate of progression to aortic stenosis is ~1.7% per year.4, 6 AVC is not limited to native aortic valves and has been shown to be the principal cause of bioprosthetic aortic valve failure with calcification occurring in areas of high biomechanical stress.7, 8 Longitudinal studies have shown that at 15 years following implantation, there is a 30-60% incidence of structural valve degeneration, in part, as a result of calcification.9 With the advent of transcatheter aortic valve replacement, it remains unknown if these valves will also be subject to the same rate of calcific valve degeneration and failure owing to the absence of long-term follow-up.
Aortic valve structure and resident cell types
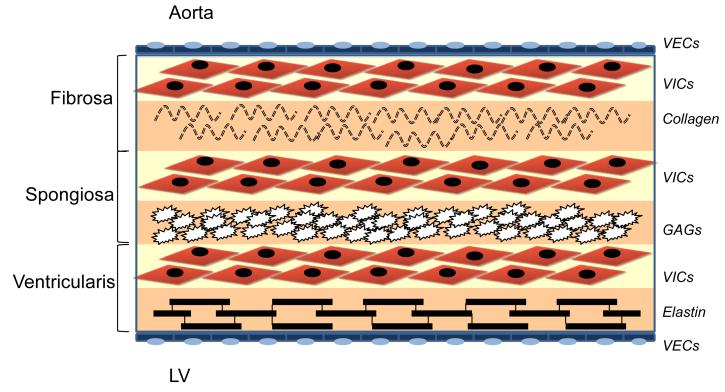
In humans, the aortic valve is an avascular trileaflet structure that is attached to the aorta via a fibrous annulus (Fig. 1). Typically, the leaflets are ≤ 1 mm in thickness and are comprised of an outer layer of valve endothelial cells (VEC) and three internal layers made up of valve interstitial cells (VIC), <5% smooth muscle cells, and myofibroblasts. These layers are known as the fibrosa, spongiosa, and ventricularis to reflect their anatomic location, cellular and extracellular matrix composition, and biomechanical properties.10, 11
Figure 1.
Cellular architecture of the aortic valve. Valve endothelial cells (VECs) line the outer surface of the valve and functions as a barrier to limit inflammatory cell infiltration and lipid accumulation. The three middle layers of the valve are the fibrosa, spongiosa, and ventricularis. These layers contain valve interstitial cells (VICs) as the predominant cell type. The fibrosa is nearest the aortic side of the valve, contains type I and type III fibrillar collagen, and serves a load-bearing function. The spongiosa contains glycosaminoglycans (GAGs) that lubricate the fibrosa and ventricularis layers as they shear and deform during the cardiac cycle. The ventricularis contains elastin fibers to decrease radial strain.92
The VECs maintain valve homeostasis by regulating permeability, inflammatory cell adhesion, and paracrine signaling. The VICs are arranged in subpopulations within the fibrosa, spongiosa, and ventricularis layers and secrete extracellular matrix, including collagen, elastin, and glycosaminoglycans, that provide strength and elasticity to the valve. The fibrosa layer is located on the aortic side of the valve, is formed by circumferentially arranged type I and type III fibrillar collagen, and serves a load-bearing function. The middle spongiosa layer contains glycosaminoglycans and lubricates the fibrosa and ventricularis layers as they shear and deform during the cardiac cycle. The ventricularis layer, which is closest to the ventricular inflow side, contains collagen and radially oriented elastin fibers that are arranged to reduce radial strain.11, 12
The normal valve cellular and matrix architecture may be disrupted by exposure to biochemical (i.e., hypercholesterolemia, hyperglycemia) or mechanical (i.e., hypertension) stress (Fig. 2). This results in a progressive thickening and fibrosis of the leaflets with evidence of inflammation, neovascularization, the appearance of ectopic mesenchymal tissue, and calcification. Prior to the appearance of calcification, early changes in the valve tissue include the infiltration of inflammatory cells, disruption of the elastic laminae, disorganized matrix deposition, and lipid accumulation at the valve surface with the appearance of microcalcifications.10, 11, 13 The calcification process may include formation of actual lamellar bone that contains osteoblasts and bone marrow. In addition, chondrocytes, cartilage, and endochondral calcification may also be present.14, 15 The calcific nodules and lesions tend to occur primarily in the fibrosa layer and extend to the aortic side of the valve where they ultimately compromise valve integrity and function.14
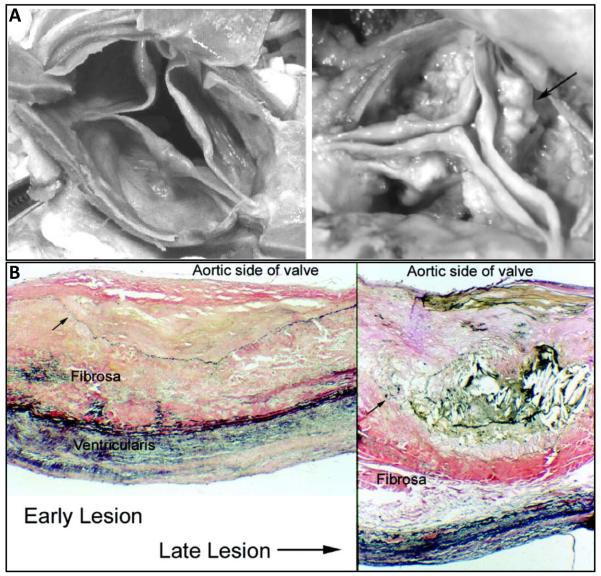
Figure 2.
Spectrum of aortic valve calcification. A) Gross anatomic specimen of a minimally diseased aortic valve demonstrating few nodules (left) and severely diseased calcified valve with evidence of nodular calcifications (arrow) on the valve cusps (right). B) Histological findings in the early stages of valve calcification include abundant subendothelial lipid and extracellular matrix with displacement of the elastic lamina (arrow)(left). As the disease progresses, lipid, cells, and extracellular matrix are increased with evidence of breakdown and displacement of the elastic lamina (arrow). Verhoff-van Gieson stain, 100X. Reprinted with permission from 93.
Cellular and molecular mechanisms that modulate AVC
It is now recognized that AVC does not result from simple passive deposition of calcium-phosphate complexes on the injured valve surface but, instead, is an active process that involves valve, circulating, and bone marrow-derived cells. Studies have implicated in situ transition of VICs to osteoblast-like bone forming cells,16-26endothelial-to-mesenchymal transition of VECs,17-19, 27 matrix vesicle formation giving rise to microcalcific nodules,28-31 and lipid accumulation as key mediators of AVC.32-41 These processes have been attributed to activation of VICs, dysfunction or denudation of VECs, infiltration of inflammatory and immune cells,16, 42-50 and downregulation of local or circulating inhibitors of calcification.51-58. These events, in turn, promote neoangiogenesis,59-63 extracellular matrix remodeling and fibrosis,11, 21, 64-67 and calcification of the valve leaflets.
These pro-calcific processes are counterbalanced by local and circulating inhibitors of calcification suggesting that decreased expression or activity of these mediators may also contribute to pathological cardiovascular calcification. Matrix Gla protein (MGP), a γ-carboxyglutamic acid-rich and vitamin K-dependent protein, prevents calcification by inhibiting bone morphogenetic protein (BMP) signaling.51 MGP levels have been shown to be significantly lower in patients with AVC as compared to individuals without valve disease.52 Experimental models of MGP deficiency develop early valve calcification while transgenic mouse models that overexpress MGP are protected, even in the setting of hypercholesterolemia.51, 53 These findings suggest that a decrease in MGP expression or activity may contribute to the progression of AVC. MGP activity is dependent upon its carboxylation status and vitamin K availability: warfarin, an inhibitor of γ-carboxylase and vitamin K epoxide reductase, inhibits MGP activity and use of this drug has been shown to be associated with AVC. One study of 1,155 patients found a significant association between the use of warfarin and the risk of calcification (unadjusted odds ratio = 1.71; 95% CI: 1.34-2.18).54
Fetuin-A is a liver-derived protein that is a potent circulating inhibitor of calcification.55 Fetuin-A acts by binding clusters of calcium and phosphate to stabilize these ions and prevent uptake by cells. Fetuin-A deficiency is known to be associated with soft tissue calcification in experimental models and humans and serum fetuin-A levels are lower in patients with AVC compared to control subjects.55, 56 Similarly, when stratified by fetuin A levels, individuals within the highest fetuin-A tertile had a significantly lower risk of developing of AVC and lower AVC scores on multislice spiral CT compared with those in the lowest tertile.57, 58 Together these findings suggest that valve remodeling and calcification occurs through the activation of pro-calcific cellular and molecular processes and that the activity of circulating inhibitors of calcification is insufficient to prevent AVC.
Cellular mechanisms of AVC
In situ transition of VICs to osteoblast-like bone forming cells
One mechanism implicated in the pathogenesis of AVC posits that normally quiescent VICs become activated and undergo a phenotype transition to become osteoblast-like bone forming cells. These activated VICs are responsive to typical osteogenic mediators, such as bone morphogenetic proteins (BMPs). BMPs are members of the transforming growth factor-β superfamily, stimulate osteoblasts to initiate skeletal bone formation, and have been implicated in vascular calcification (reviewed in 13).
It is likely that BMPs play an important role in the pathogenesis of AVC (Fig. 3). In experimental models, activated endothelial cells have been shown to secrete BMP-2 and BMP-4 in response to changes in laminar flow patterns and BMP-2 has been detected in VICs isolated from the aortic valve of aged rats.68-70 BMPs stimulate calcification by activating Smad and Wnt/β-catenin signaling as well as upregulating expression of the osteochondrogenic transcription factor Msx2. These signaling pathways converge to induce expression of the master osteoblast transcription factor Runx2.13 Once Runx2 is expressed, cells are committed to an osteoblast lineage, upregulate expression of calcification-related proteins, including osteopontin, bone sialoprotein II, and osteocalcin, and undergo calcification.20
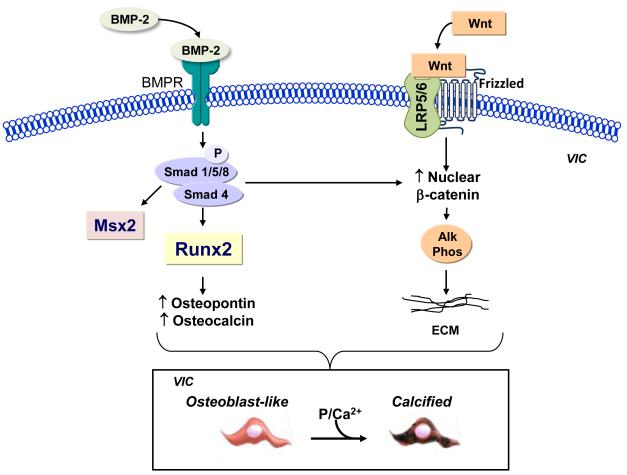
Figure 3.
Bone morphogenetic protein and Wnt signaling in VICs. Bone morphogenetic proteins (BMP) bind to the bone morphogenetic protein receptor (BMPR) to phosphorylate (P) and activate Smad signaling. Smad signaling increases transcription of the osteoblast transcription factor Runx2, which leads to upregulation of Runx2-dependent calcification proteins. Smad signaling also increases expression of Msx2 and participates in β-catenin-mediated gene transcription. BMPs also promote Wnt signaling. Wnt ligands bind to receptor complexes of frizzled protein/lipoprotein receptor-related protein (LRP) 5 or 6 to activate β-catenin signaling and upregulate expression of alkaline phosphatase. Together these signaling pathways promote transition of VICs to osteoblast-like cells that are able to calcify in the presence of phosphate and calcium. VICs, valve interstitial cells.
There is evidence to indicate that BMP signaling is activated in calcified valves. Smad 1/5/8 and Runx2 have been detected in calcified human aortic valves and levels of these markers have been shown to increase before there is evidence of valve leaflet calcification.21, 22 These findings correlate well with the observation that there is a significant increase in the Runx2-dependent calcification-related proteins osteopontin (7.4-fold) and bone sialoprotein II (5.8-fold) in calcified human valves.23
BMPs also activate the Wnt/β-catenin signaling pathway to increase the expression of alkaline phosphatase, which is also necessary to facilitate calcification.13 Wnt proteins belong to a family of secreted lipid-modified polypeptide ligands that bind to receptor complexes of frizzled protein/lipoprotein receptor-related protein (Lrp) 5 or 6 leading to an accumulation of β-catenin in the nucleus. Activation of this signaling pathway in experimental models of AVC and explanted human valves has been confirmed by demonstrating the expression of the Wnt ligand Wnt3a, the coreceptor lipoprotein receptor-related protein 5, and nuclear β-catenin in calcified valve tissue.15, 24, 25
BMPs also increase the expression of the osteochondrogenic transcription factor Msx2 that is important for intramembranous bone formation. Msx2 has been identified in valves from experimental models of AVC where it is localized to areas of calcification.24 Since Msx2-positive cells secrete Wnt ligands such as Wnt3a, these cells may upregulate Runx2 expression in adjacent VICs.26 Downstream of the BMP-Smad and BMP-Wnt/β-catenin signaling pathways, are the transcription factors osterix and NFATc1. These transcription factors have also been shown to be necessary for bone formation and have been detected in activated VICs and inflammatory cells in calcified human aortic valves.13, 22 Taken together, these studies indicate that BMPs are capable of driving VICs to an osteoblast-like phenotype where these cells express all of the markers of functional osteoblasts, elaborate bone matrix proteins, and mineralize to form calcific nodules typical of AVC.
Endothelial-to-mesenchymal transition
The primary function of VECs is to maintain valve homeostasis; however, valve endothelium may also undergo differentiation to osteoblast-like cells as a result of endothelial-to-mesenchymal-transition (EnMT). During this process, VECs lose their endothelial cell properties, no longer express endothelial-specific markers such as vascular endothelial cadherin, acquire phenotypic characteristics of mesenchymal cells or myofibroblasts, and express α-smooth muscle actin, type I collagen, and vimentin.19, 27These “transformed” VECs also exhibit increased motility and may migrate into surrounding tissues.19 This phenomenon may be stimulated by transforming growth factor-β, the transcription factor Msx2, and β-catenin signaling, all of which are present in calcifying aortic valves. Once EnMT occurs, VECs may participate in pathological fibrosis of the valve and/or undergo osteogenic differentiation through the same mechanisms as VICs exposed to BMPs, calcify, and contribute to AVC.13 Although EnMT is known to play a prominent role in the endocardial cushion during valve formation, much of the data to implicate EnMT in valve calcification has been derived from preclinical studies of the mitral valve.18 Experimental models using ovine mitral VEC clones have found that select clones underwent EnMT when stimulated with transforming growth factor-β and that these cells were capable of osteogenic and chondrogenic differentiation. Similarly, evidence of EnMT and bone-related proteins was observed in mitral leaflets following high levels of mechanical stretch.17 Further studies are required to determine if this mechanism of calcification is operative in human AVC.
Inflammation and immune response
Similar to atherosclerosis, AVC may also be considered an immune-inflammatory disease process. This is supported by the observation that explanted normal human aortic valves contain relatively few macrophages while there is abundant leukocyte and macrophage infiltration seen in explanted calcified human aortic valves.16 Valve macrophage and inflammatory cell infiltration is typically seen at sites of where VEC are activated and express adhesion molecules that facilitate the recruitment and transendothelial migration of monocytes and macrophages into the valve.42, 43, 71 Using molecular imaging, macrophage infiltration was identified as an early event in the development of AVC in atherosclerosis-prone mouse models and macrophage burden was shown to correlate with the degree of valve calcification.43, 44 In explanted human congenital bicuspid aortic valves, which are predisposed to early calcification, the density of infiltrating valve macrophages was greater than that observed for tricuspid valves.45 These areas of inflammatory cell infiltration were associated with the expression of pro-inflammatory cytokines that have been implicated in AVC, including tumor necrosis factor-α, interleukin-1β, and receptor activator of nuclear factor κ-B ligand (RANKL).43, 46, 47, 71 Once present, macrophages also release matrix metalloproteases and cysteine endoproteases that lead to degradation of collagen and elastin in the valve matrix to disrupt the normal valve architecture.17, 48
There is increasing evidence to suggest a role for immune modulatory cells in AVC as well. Calcified aortic valves have been shown to contain expanded populations of T cell clones that differed from peripheral CD8 or CD4 T cell subsets. A number of the T cell clones were of CD8 lineage (cytotoxic T cells or Natural Killer cells), suggesting that these T cells likely participated in the pathogenesis of AVC and were not merely a secondary response.49 Other studies using flow cytometry similarly identified increased levels of activated CD8 cells and memory effector cells in the peripheral blood of patients with calcified valves, indicating a systemic adaptive immune response may be associated with AVC.50 Whether this adaptive immune response modulates other cellular processes involved in AVC has not yet been determined.
Stem and progenitor cells
In addition to VECs and VICs, bone marrow-derived cells have been shown to populate normal aortic valves and it has been suggested that these progenitor cells may participate in AVC.72 For example, mesenchymal progenitor cells, which have been identified in porcine aortic valves, have the capacity to undergo differentiation to osteoblast-like cells.73 Using clonal analyses, a high frequency of these progenitor cells were shown to possess the capacity for self-renewal, undergo osteogenic differentiation, and elaborate bone matrix in the valve.73 Other studies have determined that the local environment drives osteogenic differentiation of mesenchymal progenitor cells. When these cells were co-cultured with explanted calcified valves they differentiated towards an osteoblastic lineage.74 Thus, under conditions that favor calcification, subpopulations of mesenchymal progenitors within the valve are capable of contributing to valve calcification
Endothelial progenitor cells have also been identified in diseased aortic valves. It is now believed that at sites of endothelial injury, the function of endothelial progenitor cells is to participate in the repair process by transiently establishing residence and secreting factors that facilitate proliferation and migration of resident endothelial cells.75 In AVC, there is evidence to demonstrate that endothelial progenitor cells are dysfunctional and the repair process is ineffective. Individuals with calcified aortic valves were found to have a significant reduction in endothelial progenitor cell number and ex vivo functional capacity as compared to control subjects.76 Moreover, endothelial progenitor cells isolated from AVC patients had increased pro-apoptotic caspase-3 activity and decreased telomere-repeating factor-2 expression indicating progenitor cell senescence.76 Thus, the combined effect of mesenchymal progenitor cell transition to osteoblast-like cells and endothelial progenitor cell dysfunction represents another cellular mechanism to promote AVC.
Other Processes Associated with AVC
Matrix vesicle formation and microcalcifications
AVC has also been associated with the formation and release of matrix vesicles that serve as a nidus for calcium deposition. Matrix vesicles are important for bone and cartilage mineralization and have been implicated in ectopic vascular calcification.28 In the presence of high levels of calcium and/or phosphate, smooth muscle cells have been shown to release vesicles of 100-300 nm that are derived from the plasma membrane.28, 29 These vesicles may be retained by the cells and attract calcium leading to areas of microcalcification.29 Although these vesicles have been shown to contain the calcification inhibitors matrix-Gla protein, fetuin-A, and osteoprotegerin, it is likely that encapsulation of these inhibitors in the vesicles serves as a mechanism to sequester them and render them inactive.30, 77 Microcalcifications have been shown to occur under inflammatory conditions suggesting that inflammation plays a role in their genesis.71
Other studies using electron microscopy have suggested that some of the 10-500 nm size particles present in calcified valves are actually derived from a type of bacteria referred to as nanobacteria. These particles stain positive for calcium-phosphate in a heterogeneous pattern, contain DNA, and appear to contain cell walls indicating a bacterial origin. Nanobacteria particles have been isolated from calcified aortic valves and one study found that 48 of 75 explanted calcified human valves contained calcifying nanoparticles consistent with nanobacteria.78,79 Despite these findings, the existence of nanobacteria has been challenged and it has been suggested that nanobacteria are actually calcium phosphate nanoparticles containing fetuin-A, albumin, or apolipoproteins and bind ions, nucleic acids, lipids, and carbohydrates. These calcifying nanoparticles nucleate, crystalize, and ultimately increase in size and become insoluble to function as a nidus for matrix calcification.80
Microcalcifications have also been shown to occur at sites of cell death and some amorphous calcium deposits isolated from calcified valves have a crystalline ultrastructure and do not contain live cells.31 This may result from either cell necrosis or apoptosis. In this manner, the cytoskeletal remains of apoptotic or necrotic cells may allow for calcium deposition, nodule formation, and expansion although the relative contribution of this process to AVC is not yet well understood. Importantly, valve calcification via these mechanisms (vesicle formation, nanobacteria-derived microparticles, and acellular calcium deposition) or via transition of valve cells to an osteoblast-like phenotype are not mutually exclusive processes and it is plausible that these osteoblast-like cells may actively generate vesicles or undergo apoptosis to accumulate calcium and form microcalcifications.
Extracellular matrix remodeling
One of the hallmarks of AVC is abnormal extracellular matrix remodeling. This occurs as a result of increased expression of matrix metalloproteinases-1, -2, -3, and -9 and cathepsins (S, K, V, and G) by inflammatory cells and VICs (reviewed in 11). Once activated, matrix metalloproteases and cathepsins degrade collagen and elastin to form pro-inflammatory peptides: this process compromises valve integrity, augments the inflammatory response, and allows for the expansion of calcified nodules.11
In addition to extracellular matrix degradation, there is also evidence of aberrant matrix deposition and valve fibrosis, which is extensive in AVC and contributes to the calcification of valves. Activated VICs secrete collagen, hyaluronan, and other extracellular matrix components; however, the deposition of these matrix proteins is often disorganized and alters the biomechanical properties of the valve.11 These changes in valve stiffness have also been shown to modulate the transition of VICs to osteoblast-like cells.81-83
The pro-fibrotic signaling molecule transforming growth factor-β is increased in experimental models of AVC and human valve tissue consistent with the degree of fibrosis seen in AVC.21, 64 Similar to what has been observed during myocardial repair processes where there is a return to the fetal gene program, expression of Twist1, which is important for endocardial cushion development and valve remodeling, has been identified in AVC in areas adjacent to calcium nodules.65 When overexpressed in mouse models, Twist1 leads to increased valve hypercellularity and cusp fibrosis.65 There is also evidence of increased thrombospondin-2, a matricellular protein that regulates extracellular matrix remodeling and fibrosis in human fibrocalcific aortic valve disease as well as the profibrotic enzyme neutral endopeptidase.66, 67
Angiogenesis and neovascularization
Another feature of AVC is angiogenesis and neovascularization of the valve. Healthy human aortic valves are avascular structures; however, in calcified valves, there is evidence of angiogenesis near calcified nodules, under the leaflet border, and in areas infiltrated with inflammatory cells (Fig. 4). In fact, calcified valves have been shown to contain small and medium size microvessels as well as organized arterioles.59 Histological analysis of calcified aortic valves has identified a subpopulation of cells that express the endothelial markers Tie-2 and VEGF receptor 2 as well as smooth muscle α-actin. These cells migrate and form capillary-like tubes in vitro and may represent a population of either activated VECs or VECs that have undergone EnMT.60
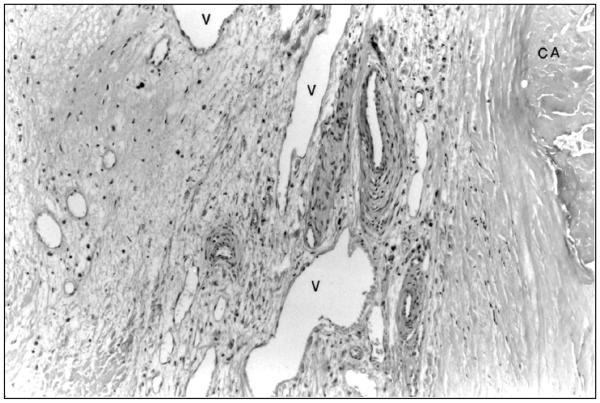
Figure 4.
Angiogenesis in a calcified valve. In calcifying valves, there is evidence of angiogenesis with the numerous vessels (V) in the valve. These vessels are located near an area of calcification (CA). Hematoxylin and eosin, 250X. Reprinted with permission from 14.
Valve neovascularization is enhanced by the release of pro-angiogenic factors by inflammatory cells and/or by downregulation of inhibitors of angiogenesis. Mast cells, which contain vascular endothelial growth factor (VEGF) were found to be present and degranulated in calcified valves. Furthermore, in vitro studies confirmed that mast cells secrete factors that stimulate VEGF production by myofibroblasts to augment local levels of this pro-angiogenic factor.59 VECs isolated from calcified valves have also been shown to express apelin and osteopontin, which both stimulate angiogenesis.61, 62
Neovascularization associated with AVC may also occur as a result of downregulation of local angiogenic inhibitors. In addition to the release of VEGF, mast cells also release tryptase, which degrades the angiogenesis inhibitor endostatin.59 Downregulation of chondromodulin-1, an antiangiogenic factor originally isolated from cartilage, has also been observed in human valve disease and associated with increased valve VEGF expression, angiogenesis, and calcification.45, 63 Downregulation of chronomodulin-1 is associated with increased expression of periostin by inflammatory cells and myofibroblasts. Periostin stimulates VEC capillary tube-like formation and high levels of periostin have been identified in calcified atherosclerotic aortic valves.84 Once neovascularization has occurred, the network of newly formed vessels facilitates rapid transit of inflammatory cells and pro-calcification signaling molecules.
Lipids
Lipid accumulation in calcified aortic valves may occur as a result of endothelial dysfunction in the setting of normal cholesterol levels or be associated with hypercholesterolemia and atherosclerosis. Individuals with significant fibrocalcific aortic valve disease have been shown to have elevated levels of circulating pro-atherogenic oxidized low-density lipoproteins.32 The aortic valve content of atheroprotective high-density lipoprotein is decreased in calcified human aortic valves and is dysfunctional as demonstrated by a decrease in the activity of the high-density lipoprotein-associated antioxidant enzyme paraoxonase-1.33, 34 The importance of pro-atherogenic lipids for valve injury and calcification was confirmed using a hypercholesterolemic mouse model with a genetic switch to induce rapid lipid lowering known as the “Reversa” mouse. 35 Using this model, investigators demonstrated that under hypercholesterolemic conditions, there was significant lipid infiltration of the valve with valve calcification. When the genetic switch was turned on and lipid levels lowered, valve calcium deposition decreased and progression of valve calcification was halted although this did not reverse established calcification or valve dysfunction.35 Lipid lowering with HMG CoA reductase inhibitors (statins) has also been shown to decrease inflammatory cell infiltration, osteoblast bone markers, including alkaline phosphatase and Runx2, and downregulated Lrp5/β-catenin signaling to decrease valve calcification in rabbit models of experimental hypercholesterolemia that have AVC.40, 41 Despite these findings, three large scale clinical trials, (SEAS, SALTIRE, and ASTONOMER) failed to show a benefit for statins to limit the progression of AVC. 36-38 This may result, in part, from the observation that lipid lowering therapy has no effect on valve fibrosis, a precursor for AVC, even after 6 months of treatment. Another possible explanation is that statins are known to promote osteoblast differentiation and calcification.39 Thus, in a valve where VICs have become osteoblast-like cells, statins may actually have a pro-calcifying effect.
Future directions
To date, there is no proven medical therapy to prevent or slow the progression of AVC and surgical or percutaneous valve replacement is the only curative treatment. Possible preventative strategies include medical therapy with inhibitors of the renin-angiotensin system or bisphosphonates. Observational studies have found that individuals with aortic stenosis taking angiotensin converting enzymes inhibitors or angiotensin receptor blockers have lower rates of all-cause mortality this finding has been attributed to improved left ventricular remodeling.85 Histopathological analyses of explanted calcific native valves have revealed that treatment with these drugs was associated with less pronounced valve remodeling.86 While bisphosphonates have been suggested as a therapy for AVC owing to the inverse relationship between skeletal bone and valve remodeling, a retrospective analysis of 801 female patients treated with bisphosphonates for a mean of 3.1 years revealed that the drug had no effect on freedom from aortic valve replacement or survival.87
A better understanding the cell biology of AVC may, therefore, identify rationale targets for the development of small molecule, gene transfer, or cell-based therapies to prevent or retard the calcification process or serve as adjuncts to limit bioprosthetic valve failure. Towards this end, investigators have utilized molecular and cell-based methodologies to modulate the cellular mechanisms of ectopic calcification with success in preclinical studies. For example, the expression of Runx2 in VICs commits these cells to an osteoblast-like phenotype. Gene transfer of small interfering RNA that target Runx2 using adenovirus or recombinant non-viral vectors has been shown to prevent calcification both in vitro and in vivo.88, 89 Other strategies have exploited the concept that ectopic calcification resembles orthotopic bone formation and focused on stimulating osteoclast differentiation and function. Osteoclasts resorb and remodel bone and osteoclasts or osteoclast-like cells have been demonstrated to be present in calcified vascular lesions where they are capable of resorbing mineral deposits.90 Despite this, ectopic vascular and valvular calcification does occur suggesting that either the cell number or function is insufficient to inhibit calcification. Thus, strategies to promote osteoclast recruitment or stimulate differentiation of monocytes and macrophages towards an osteoclast phenotype are areas of active investigation.
By employing techniques used to deliver stem or progenitor cells to the heart, it is feasible to see how these strategies could be adapted to treat the diseased aortic valve in the cardiac catheterization laboratory using catheter-based techniques. Cells have delivered successfully to ischemic myocardium using a NOGA-guided catheter system and direct injection.91 In the case of the aortic valve, NOGA guidance is not necessary and direct injection of small molecule inhibitors; osteoclasts, osteoclast-like cells, or molecules that promote transition of resident monocytes/macrophages towards an osteoclast lineage; or viral vectors encoding inhibitory RNA (Runx2, Msx2, Wnts) or RNA (MGP, fetuin-A) to the aortic side of the valve may be efficacious. Other possibilities include catheter-based delivery of a biocompatible biodegradable gel concentrated with bisphosphonates or other bone remodeling drugs to the valve leaflets; however, with this technique it would be necessary to ensure that the gel doesn’t interfere with leaflet performance.
Conclusion
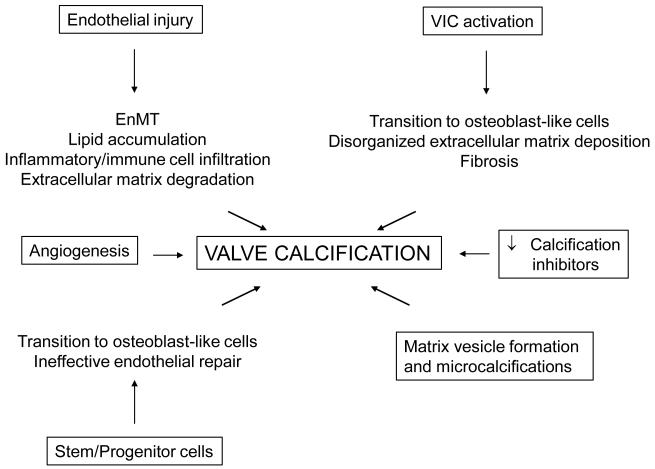
Although much progress has been made in understanding the pathogenesis of AVC, the disease process is exceedingly complex and involves several different resident, circulating, and bone marrow-derived cells (Fig. 5). These cells may become dysfunctional, transition to an osteoblast-like phenotype, and/or secrete pro-calcific factors. The net result of these cellular events is the infiltration of the valve by inflammatory or immune cells, angiogenesis and neovascularization, breakdown and aberrant matrix deposition and fibrosis, and lipid accumulation in the valve tissue. These pathological changes, in turn, are permissive for the formation of microcalcifications that ultimately form the calcific nodules typical of AVC. Therapies that target these cellular events may be readily adaptable for clinical use. Furthermore, many of these anti-calcification strategies may require novel catheter-based delivery systems to deliver cells or agents to the valve and avoid systemic effects.
Figure 5.
Key cellular mechanisms that promote aortic valve calcification.
Acknowledgments
Sources of Funding This work was supported by funding from the National Institutes of Health HL105301.
Footnotes
Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaslef SN, Roberts WC. Early descriptions of aortic valve stenosis. Am Heart J. 1993;125:1465–1474. doi: 10.1016/0002-8703(93)91036-e. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 4.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 5.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 6.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O’Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res. 2002;62:359–371. doi: 10.1002/jbm.10293. [DOI] [PubMed] [Google Scholar]
- 8.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Ruel M, Kulik A, Lam BK, Rubens FD, Hendry PJ, Masters RG, Bedard P, Mesana TG. Long-term outcomes of valve replacement with modern prostheses in young adults. Eur J Cardiothorac Surg. 2005;27:425–433. doi: 10.1016/j.ejcts.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ’degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 11.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 12.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 15.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Borggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 21.Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol. 2010;30:2482–2486. doi: 10.1161/ATVBAHA.110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos A, Bravou V, Peroukides S, Kaklamanis L, Varakis J, Alexopoulos D, Papadaki H. Bone regulatory factors NFATc1 and Osterix in human calcific aortic valves. Int J Cardiol. 2010;139:142–149. doi: 10.1016/j.ijcard.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Pohjolainen V, Taskinen P, Soini Y, Rysa J, Ilves M, Juvonen T, Ruskoaho H, Leskinen H, Satta J. Noncollagenous bone matrix proteins as a part of calcific aortic valve disease regulation. Hum Pathol. 2008;39:1695–1701. doi: 10.1016/j.humpath.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajamannan NM. Mechanisms of aortic valve calcification: the LDL-density-radius theory: a translation from cell signaling to physiology. Am J Physiol Heart Circ Physiol. 2010;298:H5–15. doi: 10.1152/ajpheart.00824.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–49. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125:1795–1808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;17:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 29.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 30.Wallin R, Schurgers LJ, Loeser RF. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: a fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2010;18:1096–1103. doi: 10.1016/j.joca.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cote C, Pibarot P, Despres JP, Mohty D, Cartier A, Arsenault BJ, Couture C, Mathieu P. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94:1175–1180. doi: 10.1136/hrt.2007.125740. [DOI] [PubMed] [Google Scholar]
- 33.Lommi JI, Kovanen PT, Jauhiainen M, Lee-Rueckert M, Kupari M, Helske S. High-density lipoproteins (HDL) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis. 2011;219:538–544. doi: 10.1016/j.atherosclerosis.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Cagirci G, Cay S, Karakurt O, Durmaz T, Yazihan N, Canga A, Aydin C, Acikel S, Kilic H, Topaloglu S, Aras D, Demir AD, Akdemir R. Paraoxonase activity might be predictive of the severity of aortic valve stenosis. J Heart Valve Dis. 2010;19:453–458. [PubMed] [Google Scholar]
- 35.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 37.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 38.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 39.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 40.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies MJ, Treasure T, Parker DJ. Demographic characteristics of patients undergoing aortic valve replacement for stenosis: relation to valve morphology. Heart. 1996;75:174–178. doi: 10.1136/hrt.75.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 44.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno PR, Astudillo L, Elmariah S, Purushothaman KR, Purushothaman M, Lento PA, Sharma SK, Fuster V, Adams DH. Increased macrophage infiltration and neovascularization in congenital bicuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;142:895–901. doi: 10.1016/j.jtcvs.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Butany J, Collins MJ, Demellawy DE, Nair V, Israel N, Leong SW, Borger MA. Morphological and clinical findings in 247 surgically excised native aortic valves. Can J Cardiol. 2005;21:747–755. [PubMed] [Google Scholar]
- 47.Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K. Tumor necrosis factor-alpha accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 2011;337:16–23. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 48.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 49.Wu HD, Maurer MS, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stewart AS, Winchester R. The lymphocytic infiltration in calcific aortic stenosis predominantly consists of clonally expanded T cells. J Immunol. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- 50.Winchester R, Wiesendanger M, O’Brien W, Zhang HZ, Maurer MS, Gillam LD, Schwartz A, Marboe C, Stewart AS. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koos R, Krueger T, Westenfeld R, Kuhl HP, Brandenburg V, Mahnken AH, Stanzel S, Vermeer C, Cranenburg EC, Floege J, Kelm M, Schurgers LJ. Relation of circulating Matrix Gla-Protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009;101:706–713. [PubMed] [Google Scholar]
- 53.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 54.Lerner RG, Aronow WS, Sekhri A, Palaniswamy C, Ahn C, Singh T, Sandhu R, McClung JA. Warfarin use and the risk of valvular calcification. J Thromb Haemost. 2009;7:2023–2027. doi: 10.1111/j.1538-7836.2009.03630.x. [DOI] [PubMed] [Google Scholar]
- 55.Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–1509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 56.Kaden JJ, Reinohl JO, Blesch B, Brueckmann M, Haghi D, Borggrefe M, Schmitz F, Klomfass S, Pillich M, Ortlepp JR. Systemic and local levels of fetuin-A in calcific aortic valve stenosis. Int J Mol Med. 2007;20:193–197. [PubMed] [Google Scholar]
- 57.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koos R, Brandenburg V, Mahnken AH, Muhlenbruch G, Stanzel S, Gunther RW, Floege J, Jahnen-Dechent W, Kelm M, Kuhl HP. Association of fetuin-A levels with the progression of aortic valve calcification in non-dialyzed patients. Eur Heart J. 2009;30:2054–2061. doi: 10.1093/eurheartj/ehp158. [DOI] [PubMed] [Google Scholar]
- 59.Syvaranta S, Helske S, Laine M, Lappalainen J, Kupari M, Mayranpaa MI, Lindstedt KA, Kovanen PT. Vascular endothelial growth factor-secreting mast cells and myofibroblasts: a novel self-perpetuating angiogenic pathway in aortic valve stenosis. Arterioscler Thromb Vasc Biol. 2010;30:1220–1227. doi: 10.1161/ATVBAHA.109.198267. [DOI] [PubMed] [Google Scholar]
- 60.Chalajour F, Treede H, Gehling UM, Ebrahimnejad A, Boehm DH, Riemer RK, Ergun S, Reichenspurner H. Identification and characterization of cells with high angiogenic potential and transitional phenotype in calcific aortic valve. Exp Cell Res. 2007;313:2326–2335. doi: 10.1016/j.yexcr.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 61.Peltonen T, Napankangas J, Vuolteenaho O, Ohtonen P, Soini Y, Juvonen T, Satta J, Ruskoaho H, Taskinen P. Apelin and its receptor APJ in human aortic valve stenosis. J Heart Valve Dis. 2009;18:644–652. [PubMed] [Google Scholar]
- 62.Poggio P, Grau JB, Field BC, Sainger R, Seefried WF, Rizzolio F, Ferrari G. Osteopontin controls endothelial cell migration in vitro and in excised human valvular tissue from patients with calcific aortic stenosis and controls. J Cell Physiol. 2011;226:2139–2149. doi: 10.1002/jcp.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, Yozu R, Sata M, Ogawa S, Hiraki Y, Fukuda K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 64.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 65.Chakraborty S, Wirrig EE, Hinton RB, Merrill WH, Spicer DB, Yutzey KE. Twist1 promotes heart valve cell proliferation and extracellular matrix gene expression during development in vivo and is expressed in human diseased aortic valves. Dev Biol. 2010;347:167–179. doi: 10.1016/j.ydbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pohjolainen V, Mustonen E, Taskinen P, Napankangas J, Leskinen H, Ohukainen P, Peltonen T, Aro J, Juvonen T, Satta J, Ruskoaho H, Rysa J. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis. 2012;220:66–71. doi: 10.1016/j.atherosclerosis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Helske S, Laine M, Kupari M, Lommi J, Turto H, Nurmi L, Tikkanen I, Werkkala K, Lindstedt KA, Kovanen PT. Increased expression of profibrotic neutral endopeptidase and bradykinin type 1 receptors in stenotic aortic valves. Eur Heart J. 2007;28:1894–1903. doi: 10.1093/eurheartj/ehm129. [DOI] [PubMed] [Google Scholar]
- 68.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 69.Seya K, Yu Z, Kanemaru K, Daitoku K, Akemoto Y, Shibuya H, Fukuda I, Okumura K, Motomura S, Furukawa K. Contribution of bone morphogenetic protein-2 to aortic valve calcification in aged rat. J Pharmacol Sci. 2011;115:8–14. doi: 10.1254/jphs.10198fp. [DOI] [PubMed] [Google Scholar]
- 70.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 71.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 72.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leskela HV, Satta J, Oiva J, Eriksen H, Juha R, Korkiamaki P, Ivaska KK, Soini Y, Lehenkari P. Calcification and cellularity in human aortic heart valve tissue determine the differentiation of bone-marrow-derived cells. J Mol Cell Cardiol. 2006;41:642–649. doi: 10.1016/j.yjmcc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50:266–272. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsumoto Y, Adams V, Walther C, Kleinecke C, Brugger P, Linke A, Walther T, Mohr FW, Schuler G. Reduced number and function of endothelial progenitor cells in patients with aortic valve stenosis: a novel concept for valvular endothelial cell repair. Eur Heart J. 2009;30:346–355. doi: 10.1093/eurheartj/ehn501. [DOI] [PubMed] [Google Scholar]
- 77.Schoppet M, Kavurma MM, Hofbauer LC, Shanahan CM. Crystallizing nanoparticles derived from vascular smooth muscle cells contain the calcification inhibitor osteoprotegerin. Biochem Biophys Res Commun. 2011;407:103–107. doi: 10.1016/j.bbrc.2011.02.117. [DOI] [PubMed] [Google Scholar]
- 78.Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, Yagubyan M, Rodgers JC, Cockerill FR, 3rd, Folk RL, Rzewuska-Lech E, Kumar V, Farell-Baril G, Lieske JC. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:H1115–1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 79.Bratos-Perez MA, Sanchez PL, Garcia de Cruz S, Villacorta E, Palacios IF, Fernandez-Fernandez JM, Di Stefano S, Orduna-Domingo A, Carrascal Y, Mota P, Martin-Luengo C, Bermejo J, San Roman JA, Rodriguez-Torres A, Fernandez-Aviles F. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008;29:371–376. doi: 10.1093/eurheartj/ehm592. [DOI] [PubMed] [Google Scholar]
- 80.Schlieper G, Kruger T, Heiss A, Jahnen-Dechent W. A red herring in vascular calcification: ’nanobacteria’ are protein-mineral complexes involved in biomineralization. Nephrol Dial Transplant. 2011;26:3436–3439. doi: 10.1093/ndt/gfr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yperman J, De Visscher G, Holvoet P, Flameng W. Molecular and functional characterization of ovine cardiac valve-derived interstitial cells in primary isolates and cultures. Tissue Eng. 2004;10:1368–1375. doi: 10.1089/ten.2004.10.1368. [DOI] [PubMed] [Google Scholar]
- 82.Stephens EH, Shangkuan J, Kuo JJ, Carroll JL, Kearney DL, Carberry KE, Fraser CD, Jr., Grande-Allen KJ. Extracellular matrix remodeling and cell phenotypic changes in dysplastic and hemodynamically altered semilunar human cardiac valves. Cardiovasc Pathol. 2011;20:e157–167. doi: 10.1016/j.carpath.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 84.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2011;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M, Pringle SD, Doney AD, Choy AM, Struthers AD, Lang CC. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. 2011;58:570–576. doi: 10.1016/j.jacc.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 86.Cote N, Couture C, Pibarot P, Despres JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest. 2011;41:1172–1179. doi: 10.1111/j.1365-2362.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 87.Aksoy O, Cam A, Goel SS, Houghtaling PL, Williams S, Ruiz-Rodriguez E, Menon V, Kapadia SR, Tuzcu EM, Blackstone EH, Griffin BP. Do bisphosphonates slow the progression of aortic stenosis? J Am Coll Cardiol. 2012;59:1452–1459. doi: 10.1016/j.jacc.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 88.Xue T, Mao Z, Lin L, Hou Y, Wei X, Fu X, Zhang J, Yu C. Non-virus-mediated transfer of siRNAs against Runx2 and Smad4 inhibit heterotopic ossification in rats. Gene Ther. 2010;17:370–379. doi: 10.1038/gt.2009.154. [DOI] [PubMed] [Google Scholar]
- 89.Lin L, Chen L, Wang H, Wei X, Fu X, Zhang J, Ma K, Zhou C, Yu C. Adenovirus-mediated transfer of siRNA against Runx2/Cbfa1 inhibits the formation of heterotopic ossification in animal model. Biochem Biophys Res Commun. 2006;349:564–572. doi: 10.1016/j.bbrc.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 90.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 92.Schoen FJ. Mechanisms of function and disease of natural and replacement heart valves. Annu Rev Pathol. 2012;7:161–183. doi: 10.1146/annurev-pathol-011110-130257. [DOI] [PubMed] [Google Scholar]
- 93.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]