Abstract
We investigated how the perceptual visibility of a target influences the pattern of microsaccadic eye movements expressed during generalized flash suppression. We found that the microsaccade rate was highly dependent on the reported visibility of the target. In the visible trials, the microsaccade rate promptly rebounded to the pre-onset level, whereas on the invisible trials the rate remained low, reaching pre-onset levels hundreds of milliseconds later. In addition, the directional distributions of microsaccades biased to the target positions in the visible condition. The present findings indicate that the microsaccade behavior is highly correlated with the perceptual state of target visibility, and suggest that the measured microsaccade rate and direction are reliable indicators of the perception.
Keywords: Microsaccade, Generalized flash suppression, Multistable perception, Fixation, Visual attention
1. Introduction
The largest eye movements during normal fixation are microsaccades that are rapid and involuntary jerks of the eyes (generally < 1°). They are generally considered to share the same neurological control system with larger saccades, as they both fall onto the so-called “main sequence” for saccadic eye movement (Bridgeman & Palca, 1980; Zuber, Stark, & Cook, 1965). Microsaccades typically occur at rates of 1 – 2 per sec, interspersed by slower fixational drifts (Winterson & Collewijn, 1976).
The role of fixational eye movements in visual perception has been debated for more than five decades (for a review see Martinez-Conde, Macknik, & Hubel, 2004). Once disregarded as nervous tics presenting a potentially interesting “evolutionary puzzle” (Kowler & Steinman, 1980; Steinman, Haddad, Skavenski, & Wyman, 1973), these tiny eye movements have recently received new attention, and been the object of considerable research. This revival is primarily due to new findings in neurophysiological and behavioral studies, thanks to technical advances in neuronal signal recording in vivo and in detecting miniature eye movements (Engbert & Kliegl, 2003; Santini, Redner, Iovin, & Rucci, 2007). Recent neurophysiological evidence in awake monkeys has shown that microsaccades modulate neural activity in various areas along the visual pathway (Bair & O’Keefe, 1998; Gur, Beylin, & Snodderly, 1997; Leopold & Logothetis, 1998; Martinez-Conde, Macknik, & Hubel, 2000, 2002; Snodderly, Kagan, & Gur, 2001), indicating that these movements may have a fundamental role in visual processing.
More recently, behavioral studies have established links between microsaccade and cognitive activity in two aspects. First, the investigations have found important roles for miniature eye movements, including drifts, in enhancing fine spatial details (Rucci, Iovin, Poletti, & Santini, 2007). In addition, the rate of microsaccades correlated with the visibility of peripheral stimuli during prolonged fixation (Martinez-Conde, Macknik, Troncoso, & Dyar, 2006). Both studies indicate that the perceptual improvement originates from the modulations introduced by microsaccades in visual input to the retina, suggesting a bottom-up flow of information leading to microsaccade-driven perception. Second, clear evidence supports the notion that some kinds of microsaccade behavior are influenced by attention and other cognitive variables, e.g. (Steinman, Cunitz, Timberlake, & Herman, 1967). Several recent studies have reported that (1) the absolute microsaccade rate (number per sec) is modulated by spatial attention (Engbert & Kliegl, 2003; Galfano, Betta, & Turatto, 2004; Hafed & Clark, 2002; Rolfs, Engbert, & Kliegl, 2005), working memory (Betta, Galfano, & Turatto, 2007; Turatto, Valsecchi, Tame, & Betta, 2007; Valsecchi, Betta, & Turatto, 2007; Valsecchi & Turatto, 2007), ocular (Rolfs, Laubrock, & Kliegl, 2006) and manual (Betta & Turatto, 2006) motor preparation, and (2) the microsaccade direction is modulated by a shift of spatial attention induced by either endogenous (Engbert & Kliegl, 2003; Laubrock, Engbert, & Kliegl, 2005) or exogenous (Galfano et al., 2004; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs, Engbert, & Kliegl, 2004) cues. This body of literature raises the hypothesis that the behavioral characteristics of microsaccades (e.g. amplitude, duration, velocity, rate and direction) may also be reliable indicators of cognitive states of perception during visual suppression (Wilke, Logothetis, & Leopold, 2003) and other multistable perception (Ito et al., 2003; Leopold & Logothetis, 1999; Sabrin & Kertesz, 1980, 1983; van Dam & van Ee, 2006). On the one hand, changes of microsaccade behavior (e.g. microsaccade rate) may influence the process of vision at lower level (e.g. visual fading) and may thus contribute to the maintenance or suppression of the dominant stimulus at higher level. On the other hand, the stimulus in perceptual dominance may concurrently bias a subject’s attention, which, in turn, could be reflected in the variation of microsaccade behavior (e.g. rate and direction).
In this work, we report the results of microsaccade behavior observed in non-human primates experiencing general flash suppression (GFS) (Wilke et al., 2003; Wilke, Logothetis, & Leopold, 2006). The investigation had been planned as a follow-up study to examine microsaccadic patterns induced in GFS paradigm. We chose non-human primates as the subjects, since it is interesting to obtain a comparison of microsaccade behavior between humans and monkeys. This comparison is important as the study can provide information on whether it is valid to use monkeys as models of the human oculomotor system in neurophysiological studies that cannot be conveniently adapted to human subjects. In the GFS, a salient, monocular target is induced to subjectively disappear in an all-or-none and sustained fashion following the sudden presentation of a binocular surrounding pattern. We show that in this paradigm the pattern of microsaccades is distinctly different according to the state of visibility of the target. Specifically, we found that when fixating subjects experienced GFS, the occurrence rate of microsaccades always dropped to near-zero after the surround onset. After this initial decrease, the microsaccade rate in the next several hundred milliseconds was highly dependent on the reported visibility of the target. In the visible trials the microsaccade rate promptly rebounded to the pre-onset level, whereas on the invisible trials the rate remained low, reaching pre-onset levels hundreds of milliseconds later. In addition, microsaccade directions were affected, in that they showed different distributions between the visible and invisible trials. Amplitude, microsaccade duration, and peak velocity did not show significant correlation with perception. In the Discussion, we speculate on the links between the behavior of microsaccades and perceptual suppression, and on the underlying neural mechanism of the phenomenon. Our results indicate that microsaccade behaviors are primarily influenced by higher cognitive activities.
2. Material and Methods
2.1. Experimental method
Since the GFS paradigm and the experimental setups have been reported in detail elsewhere (Wilke et al., 2003, 2006), we provide only a brief description here. The GFS paradigm refers to a specific stimulus sequence (see below) to induce a complete disappearance of a salient target for at least hundreds of milliseconds upon the onset of a surround. It combines the principles of binocular rivalry flash suppression (Wolfe, 1984) and motion-induced blindness (Bonneh, Cooperman, & Sagi, 2001) to allow for experimental control over the time course of perception.
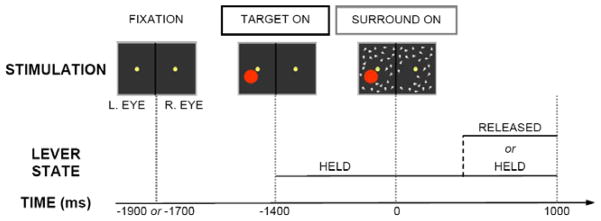
The experimental sequence of GFS paradigm as used in the current study is shown in Figure 1. The monkey was placed in front of a stereoscope. During each session, a typical trial started with a warning tone to cue the monkey to maintain fixation at a small central disk (0.15°). The spatial resolution of each monitor was 1280 × 800 pixels with an eye-screen distance of 110 cm and a refresh rate of 90 Hz. A circular aperture of 14.5° diameter restricted the visible portion of the screen. The head of the monkey was fixed with a custom-made titanium head post. The monkey was required to maintain fixation within a fixation window (0.6°) throughout the whole trial. 1400 ms before surround onset, a target was turned on at a parafoveal location on the screen. The target consisted of a uniform red monocular disk or a Gabor patch with a size of 1.0°. In the sessions included in this analysis, all targets were displayed in the lower half of the screen. The additional surround pattern, consisting of randomly moving white dots, was suddenly presented binocularly to the regions surrounding the target. Monkeys indicated the visibility of the target by means of a lever. They were required to hold the lever at least in the interval between target and surround onset. A monkey had to hold it as long as the target was visible and to release it as soon as the target was invisible. If the target became invisible (either by perceptual suppression or physical removal), the monkey had to release the lever within 1000 ms after surround onset and keep fixation for another 800 ms. Catch trials, in which the target was physically removed, or the parameters were adjusted to make it unambiguously visible, were continually interleaved with ambiguous trials in order to ensure correct behavioral performance. In the present study, the probability of perceptual suppression of the target after surround onset was adjusted to about 50% as determined with psychophysical testing (Wilke et al., 2006). Specifically, for each monkey the parameters of surround density (number of moving dots per unit area of the surround pattern, dots/deg2) and target-surround distance (the distance between the target and the closest moving dot, deg) were varied. In agreement with the previous study, the monkeys reported target disappearance more frequently when the density of the surround pattern was increased, or when its inner edge was closer to the target. Based on monkeys’ reports, the parameter values of the test stimulus fixed so that the target would subjectively disappear in approximately half the trials.
Figure 1.
Illustration of generalized flash suppression (GFS) experimental paradigm. The figure shows the stimulation sequence and the states (held or released) of the lever provided to monkeys. Monkeys were required to maintain fixation after the appearance of fixation spots (FIXATION). They fixated on the central spots for 300 ms (in some sessions 500 ms) before the presentation of the target stimulus (red disk). After 1400 ms of the target only presentation (TARGET ON), a surrounding pattern consisting of randomly moving dots was added to the presentation (SURROND ON). Monkeys were required to hold the lever as long as the target was visible. If the target became invisible, either through perceptual suppression or physical removal, they had to release the lever within 800 ms. (Adapted from Fig. 1. in (Wilke et al., 2006) with permission)
Three adult Macaca mulatta monkeys (ER, WA and DA) participated in the experiments. In total 28 sessions of data have been analyzed (ER = 13, WA = 10, DA = 5). The trials were classified into two categories according to the two perceptual states, namely, the “invisible”, in which the monkeys released the lever to indicate the subjective disappearance of the target, and the “visible”, in which the target was perceived to be visible throughout the whole trial. The total number of trials involved in the invisible condition was 795 (ER = 403, WA = 244, DA = 148) and in the visible condition 783 (ER = 244, WA = 414, DA = 125). Thus, the proportions of “invisible” trials of each monkey are ER = 62.3%, WA = 37.1% and DA = 54.2% and the proportion of total “invisible” trials is 50.4%. In addition, a control condition, namely “physical disappearance”, in which the target was physically removed from the screen simultaneously with the onset of the surround pattern was examined (totaling 1297 trials: ER = 353, WA = 595, DA = 349) to further establish the relationship between perceptual suppression and microsaccade rate.
2.2. Eye movement recording
Eye positions were monitored by means of a single scleral search coil (CNC Engineering, Seattle, WA) with a high spatial resolution less than 0.01° (Collewijn, 1998). At the beginning of each session, the offset of the eye positions was zeroed to the animal’s center of gaze. The gains of horizontal and vertical movements were then calibrated by having the monkey repeatedly saccade to one of the seven small squares (0.1° per side) placed on the screen. The monkey’s eye positions were checked on-line. When the monkey moved the eyes outside of the fixation window (radius 0.5° – 0.6°), trials were automatically aborted and the on-line fixation check introduced a penalty to the animals in the form of less reward. Horizontal and vertical eye position was digitized at a sampling rate of 1 kHz.
2.3. Microsaccade detection
Then, the algorithm proposed by Engbert and Kliegl (Engbert & Kliegl, 2003) was employed to identify the microsaccades that occurred between the display of the targets and 1000 ms after the onset of surround patterns (2400 ms interval). Since the search coil was implanted only to one eye, the algorithm was adapted for detecting microsaccades monocularly. The time series of eye positions was transformed into velocities calculated over a moving window of five samples. A microsaccade process was defined as the period in which the following conditions were satisfied: (1) the angular eye velocity exceeded a combined threshold, which was four times the median-based standard deviation of the velocity distribution (λ = 4 ), computed independently for horizontal and vertical components and separately for each trial; (2) the minimum microsaccade duration was set to 8 ms (Leopold & Logothetis, 1998); (3) the amplitude of a microsaccade was between 1′ (Engbert & Kliegl, 2003) and 36′ (Wilke et al., 2006); and (4) the maximum peak velocity was set to 110 °/s (Martinez-Conde, Macknik, & Hubel, 2004). Since the fixation window was small (0.6°), the upper limit of velocity can effectively exclude outliers to keep the detected microsaccades close to the main sequence. In addition, the microsaccades that happened less than 100 ms after their predecessors were discarded, in order to prevent the corrective movements (Moller, Laursen, Tygesen, & Sjolie, 2002) from being counted as a new microsaccade.
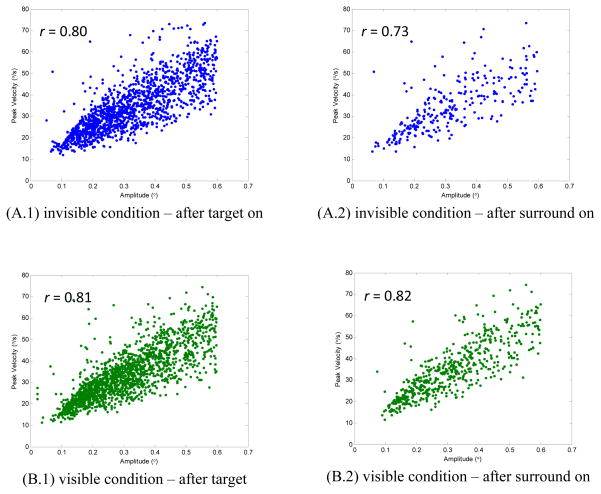
To verify the validity of the detected microsaccades, we examined whether the eye movements classified as microsaccades satisfied the main-sequence criterion (Zuber et al., 1965): a linear correlation between peak saccadic velocity and amplitude. The peak velocity-amplitude relationship of microsaccades in both conditions is depicted in Figure 2. Panels (A.1) and (B.1) show the relationship of the microsaccades detected after target onset from the pool of all three monkeys in the “invisible” and “visible” condition, respectively. To facilitate the understanding of the analysis of microsaccade dynamics posterior to the surround onset (see below), the velocity-amplitude relationships of the microsaccades in this period are shown in panels (A.2) and (B.2) for the two conditions, respectively. The results showed evident linear relationship supported by high correlation coefficients, which are consistent with the previous findings (Betta et al., 2007; Engbert & Kliegl, 2003; Galfano et al., 2004; Moller et al., 2002). Therefore, the linear relationship verifies that the events detected by the algorithm are valid microsaccades.
Figure 2.
Peak velocities of microsaccades as a function of amplitude (“main sequence”). Panel A shows the results in the invisible condition. The plot (A.1) contains 1440 microsaccades from all three monkeys, which were detected between target on and 1000 ms posterior to the surround onset. The correlation coefficient was 0.80. Specifically, the relationship of the 279 microsaccades detected after surround onset in the same condition was shown in the plot (A.2) with the correlation coefficient of 0.73. Panel B shows the results in the visible condition. The plot (B.1) contains 1770 microsaccades (r = 0.81), while the plot (B.2) contains 581 microsaccades (r = 0.82).
3. Results
Here we examined the effect of target visibility on the pattern of microsaccades. Following the onset of a surrounding pattern of randomly moving dots in the GFS experiments (Wilke et al., 2006), a salient target can have a high probability of complete disappearance, remaining perceptually suppressed for several seconds. The parameters of the visual stimuli were adjusted to induce disappearance of the target in approximately 50% of the trials, providing half of the trials in which the target remained visible and half in which it became invisible (Wilke et al., 2003, 2006).
3.1. Amplitude, duration and peak velocity
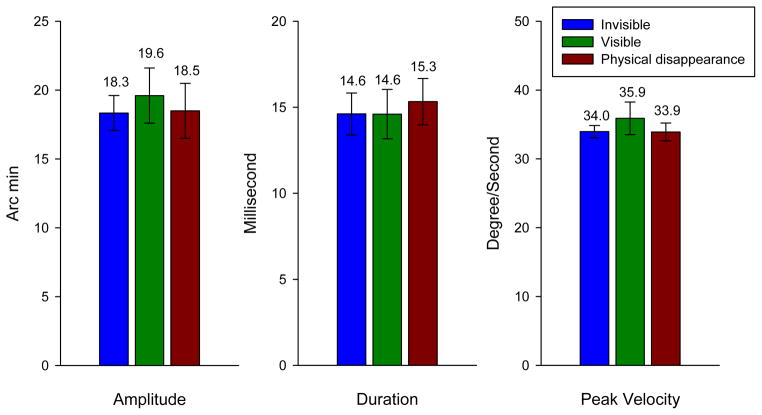
We first examined the parameters of microsaccade amplitude, duration and peak velocity after surround onset. Figure 3 depicts the mean values of these parameters for “invisible”, “visible” and “physical disappearance” conditions. The average measures were first calculated for each monkey in both conditions and then the between-subject mean was obtained. The error bars represent one standard error of means (±1 SEM). As shown in the figure, there is no significant difference of the means in these three parameters for the three perceptual conditions. The results indicate that the microsaccade amplitude, duration and peak velocity in general are not sensitive to different perceptual states of the monkeys. Note that Martinez-Conde et al (Martinez-Conde et al., 2006) reported an increase of microsaccade amplitude before intensifying visibility in Troxler’s effect experiments. However, their method was event-triggered average by aligning the measures at the moment of percept transit. Thus, their results of amplitude increase reflect the microsaccade dynamics before perceptual transition. The results in Figure 3 are grand averages.
Figure 3.
Microsaccade parameters of amplitude, duration and peak velocity in the “invisible”, “visible” and “physical disappearance” conditions. The microsaccades were detected after the onset of the surround patterns. The between-subject mean value of each parameter was first obtained for each monkey and then averaged across the three monkeys, which are shown above the bars for each condition. Error bars indicate 95% confidence intervals based on the standard error of mean (SEM).
3.2. Microsaccade rate
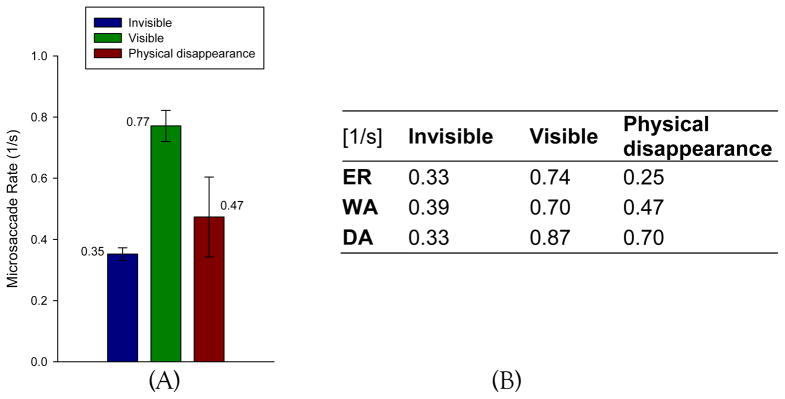
We investigated the effect of different perceptual states on microsaccade rate posterior to surround onset. In contrast to the above parameters, the absolute microsaccade rate was strongly influenced by the target visibility. We calculated the overall mean microsaccade rates in both conditions by averaging the mean rates of the three monkeys (Figure 4(A)). For each monkey, the mean rate was obtained by averaging the total number of microsaccades across trials after surround onset. As shown in Figure 4(B), all three monkeys display a decrease of microsaccade rate in the “invisible” and “physical disappearance” condition compared with the visible condition. Single-sided paired (“invisible” rate – “visible” rate) t-test was conducted to demonstrate significant lower mean rate in the “invisible” condition than that in “visible” condition (p = 0.012, T statistic = −6.3060, degree of freedom = 2).
Figure 4.
Average microsaccade rates after surround onset by perceptual state. Panel (A) depicts the mean rates, shown above the bars, in three conditions. Error bars indicate ±1 SEM. Average microsaccade rates of individual monkeys are summarized in the table of panel (B).
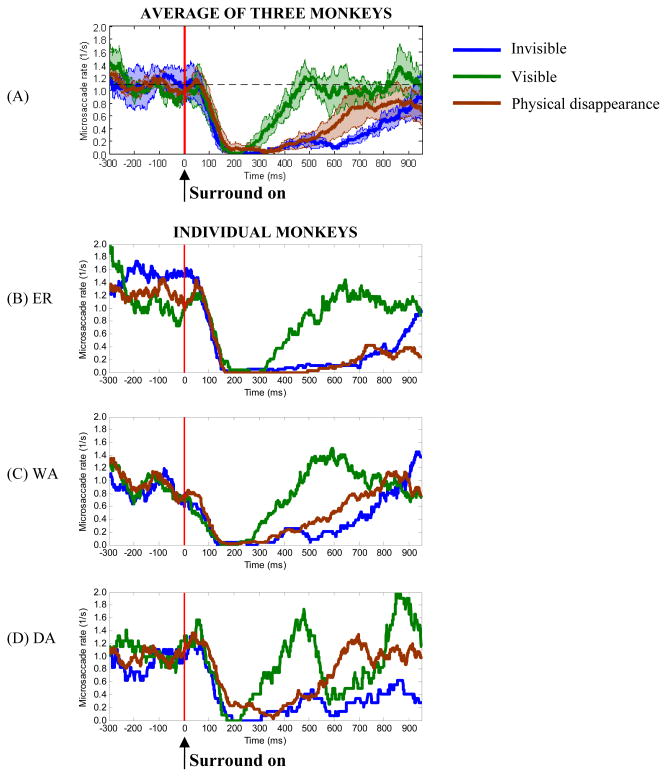
We then examined the variations of microsaccade rate over time as a function of perceptual states. A pre-onset mean rate of 1.08 microsaccades per second was obtained (computed in [−300, 0] ms) as the baseline rate, which is in good agreement with previous reports (Bridgeman & Palca, 1980; Engbert & Kliegl, 2003; Galfano et al., 2004). As shown in Figure 5, modulation of microsaccade rate exhibits a clear separation of rate curves corresponding to different conditions. In panel (A) a strong inhibition of microsaccade rate (i.e. a rapid drop that began around 50 ms and reached its minimum close to zero at about 200 ms) is evident in both conditions. This stereotyped suppression of microsaccades immediately following a change of display has been repeatedly observed in a number of studies, e.g. (Betta & Turatto, 2006; Bridgeman & Palca, 1980; Engbert & Kliegl, 2003; Galfano et al., 2004; Laubrock et al., 2005; Valsecchi et al., 2007). In our study, however, we did not observe an obvious rebound of microsaccade rate over the pre-surround-on baseline (≈1.08/s). In the visible condition, microsaccade rate following the inhibition quickly returned to the baseline in about 250 ms posterior to the rate minimum (slope = 1.08/0.25 ≈ 4.32/s). It subsequently stayed at this level without significant deviation from the baseline (i.e. the baseline was generally in the ±1 SEM band). The trace of rate enhancement in the invisible condition is different. Following the decrease, the microsaccade rate slowly recovered to the baseline level at least 700 ms posterior to the rate minimum (slope = 1.08/0.70 ≈ 1.54/s). A period of significant separation of the two average rate curves can be identified between 228 ms and 889 ms after the surround onset. The significance is defined as the distance between the two curves is not less than the sum of one “visible” SEM and one “invisible” SEM.
Figure 5.
Evolution of microsaccade rates in the conditions of “invisible”, “visible” and “physical disappearance” (physical removal of the target). Panel (A) shows the average microsaccade rates between 300 ms before and 950 ms after surround onset as a function of time. The horizontal dashed line represents the average microsaccade rate in the interval of −300 ms to 0 ms (1.08 1/s). The vertical red line displays the onset of surrounding patterns. Shadows around the mean indicate confidence bands of ± 1 SEM between monkeys (n = 3). The average rates from individual monkeys per condition are shown in panels (B) – (D). The rate of microsaccades has been calculated in a window of 100 ms width moving in 1 ms steps.
To study whether the modulation of microsaccade rate is significant, we focused on four consecutive 300-ms time windows to obtain statistical analysis of microsaccade rate, one before and three after surround onset, covering the range from −300 to +900 ms. Mean rate was calculated for each monkey for each combination of perceptual condition (invisible and visible) and window position. A repeated measures analysis of variance with the factors of perceptual condition and window position (two-way ANOVA) was conducted to show that differences between time windows, F(3,16) = 15.61, p < 0.001, and between perceptual conditions, F(1,16) = 22.49, p < 0.001, were highly significant. In addition, the effect of perceptual condition × window position interaction was also significant, F(3,16) = 6.89, p < 0.01. The results demonstrate that the difference observed in post-surround-onset microsaccade rates is statistically significant.
In order to further investigate the relationship between perceptual suppression and microsaccade rate, we examined the rate dynamics in a control condition of “physical disappearance”. For average microsaccade rates (Figure 5(A)), we can see that after surround onset, the rate trace in the “physical disappearance” condition closely followed the trace in the “invisible” condition until around 600 ms, while it was significantly separated from that in the “visible” condition in this interval. The pattern was reversed between about 600 ms and 800 ms. The microsaccade rate in the “physical disappearance” condition did not show statistically significant difference from the rates in the “invisible” and “visible” condition after about 800 ms. Similarity between the rate traces was measured by calculating their correlation coefficients. We found that, after surround onset, the coefficient between the “invisible” and “physical disappearance” conditions (r = 0.9) was higher than that between the “visible” and “physical disappearance” conditions (r = 0.6). The results suggest that the perceptual suppression of the target is unlikely caused by the reduction of microsaccade rate (see Discussion).
In summary, we have observed a common drop of microsaccade rate immediately after surround onset, but different processes of rate enhancement in different perceptual conditions. In the visible condition, the initial inhibition of microsaccade rate was followed by a relatively quick rate recovery to the pre-surround-onset baseline level. The rate then stayed at this level for the rest of the observation. In the invisible condition, a rather long period of rate enhancement followed the rate inhibition and didn’t return to the baseline level until ≈900 ms posterior to surround on. However, in neither condition did we observe a rebound of microsaccade rate over the baseline level following the microsaccade inhibition. The evolution of microsaccade rate in the “physical disappearance” condition is close to that in the “invisible” condition.
3.3. Microsaccade direction
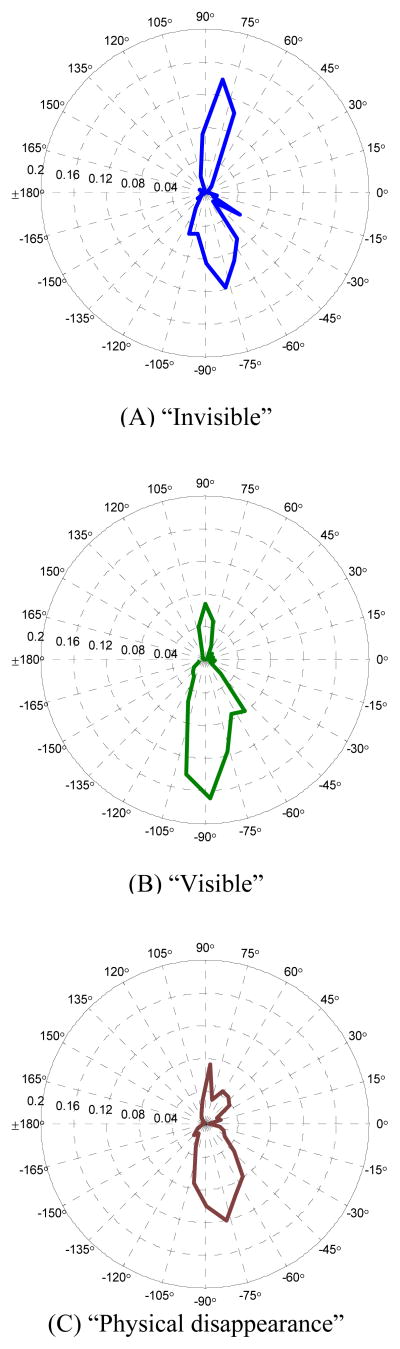
To explore possible interaction between perceptual condition and microsaccade direction, we first analyzed polar plot histogram of microsaccade directions in the conditions of “invisible”, “visible” and “physical disappearance”. The direction of a microsaccade was defined as the angle between the microsaccade vector and x-axis in the screen coordinate. In Figure 6 a comparison of histograms of microsaccade directions in both conditions reveals a tendency of microsaccades to be predominantly directed downward in the visible condition after the onset of surrounding patterns. Since all the targets located in the lower half of the screen, the microsaccades may be divided into two categories: “toward-target (downward) microsaccades” whose directions were in the range −180° ≤ θ < 0° and “opposite-to-target (upward) microsaccades” for the rest. The histograms in the polar coordinates show that the toward-target microsaccades occupied a higher proportion (76.4%) in the “visible” condition than that (60.5%) in the “invisible” condition. A two-sample Kolmogorov-Smirnov (KS) distribution test was performed for the null hypothesis that the directions of microsaccades under the “invisible” and “visible” conditions were drawn from same continuous distributions at the 5% level of significance. For the pre-surround-onset 300-ms interval, we cannot reject the null hypothesis, while the distributions are significantly different (K = 0.207, p < 0.001) after surround onset (from 0 ms to 1000 ms). In the control condition of “physical disappearance”, the proportion of downward microsaccade (66.6%) is approximately 6% higher than that in “invisible” condition and 10% lower than that in “visible” condition.
Figure 6.
The directional distributions of microsaccades detected after surround onset. Polar plots of microsaccade direction probability density were computed from 30 equally spaced directional bins for three conditions (“invisible”, “visible” and “physical disappearance”). The distance from the center of the plot represents the proportion of the microsaccade contained in a 12° bin. Direction is defined by the angle between the microsaccade vector and the x-axis of the screen coordinates (0° in the polar coordinates). The distribution in the “invisible” condition shows a smaller proportion of downward microsaccades (60.5%) than that in the “visible” condition (76.4%). About 2/3 of the microsaccades in the “physical disappearance” condition were downward (66.6%).
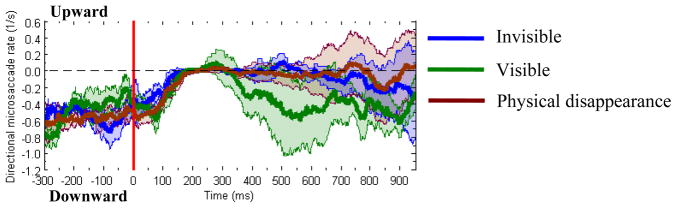
Next, we analyzed the change of microsaccade directions as a function of time. The directional frequency was computed in a 100-ms time window moving by 1-ms steps as the difference between the rate of downward (toward-target) microsaccades and the rate of upward (opposite-to-target) microsaccades. Figure 7 shows that in all perceptual conditions the average rate of microsaccade directed downwards was higher before surround onset. Similar to the change of microsaccade rate, a rapid change of directional rate started around 100 ms and reached zero level at about 200 ms after surround onset. In the “visible” condition, the trace of average rate after about 350 ms was lower than zero, indexing a bias toward the target locations. On the other hand, the average directional rate generally did not deviate from the zero level significantly in the “invisible” and “physical disappearance” conditions, although a tendency of negative directional rate was observed at the later portion of the recording. This result suggests that when the targets disappeared, either through perceptual suppression or physical removal, the monkeys produced the microsaccades directed in both directions at approximately equal rates. Although the directional rate traces in all three conditions were not significantly separated in general after surround onset, the “invisible” or “physical disappearance” rate kept higher after around 350 ms, implying that less downward microsaccades were produced in these conditions.
Figure 7.
Directional microsaccade rate in three conditions (“invisible”, “visible” and “physical disappearance”). The directional rate was calculated in a window of 100 ms width moving in 1 ms steps as the difference between the rate of microsaccades directed upwards and the rate of microsaccades in the opposite direction. The figure shows the average directional microsaccade rates as a function of time. Shadows around the means indicate between-monkey confidence bands of ±1 SEM (n = 3). The horizontal dashed line represents the level of zero. The vertical red line represents the time stamp of surround onset. A positive value corresponds to the higher rate of upward microsaccades. A zero directional rate indicates that the rates of microsaccades in both directions are equal.
4. Discussion
The aim of the present study was to investigate the pattern of microsaccades during perceptual suppression of a salient visual stimulus. A fast initial decrease of the (absolute) microsaccade rate in response to abrupt onset of the surrounding patterns was observed in both perceptual conditions. This effect of microsaccade inhibition has been noted in previous studies to occur irrespective to cue properties (e.g. color, symbol or flash) (Engbert & Kliegl, 2003; Laubrock et al., 2005; Valsecchi et al., 2007), cue class (endogenous or exogenous) (Engbert & Kliegl, 2003; Laubrock et al., 2005) and sensory modality of the stimuli (visual or auditory) (Rolfs et al., 2005; Valsecchi & Turatto, 2008). In GFS experiment, this microsaccade rate signature also occurred in response to surround onset, irrespective to the resulting visibility states. Our results thus support the notion that initial microsaccade inhibition is a fast reflex of the oculomotor system to a sudden display change. Similar findings of rate suppression have also been reported for larger saccades (Graupner, Velichknovsky, Pannasch, & Marx, 2007; Pannasch, Dornhofer, Unema, & Velichknovsky, 2001; Reingold & Stampe, 2000), but it was reported that the inhibition of large saccade could not be induced by irrelevant auditory stimuli (Reingold & Stampe, 2004).
However, the traces of rate increase immediately following rate inhibition are remarkably different. They were significantly separated from about 200 ms to 900 ms after surround onset. In the visible condition, the microsaccade rate returned to its baseline level at about 450 ms and subsequently stayed at this level. Since the target was presented parafoveally, the increase in the microsaccade rate might be relevant to information processing within these areas. One potential function of these tiny saccadic movements was to counteract fading of the target, possibly contributing to the sustained perception of the target. Our findings, however, do not support the suggestion that microsaccades actually cause target reappearance in the visible condition, because both human (Wilke et al., 2003) and monkey (Wilke et al., 2006) experiments only reported an all-or-none fashion of target visibility, rather than the presumed perception switch, i.e. target disappearance followed by its reappearance. The results thus indicate that microsaccades are primarily driven by the stimulus and task.
In contrast to the previous reports (Betta et al., 2007; Engbert & Kliegl, 2003; Galfano et al., 2004; Rolfs et al., 2004), we did not observe an over-baseline rebound effect. Our results are not necessarily in contradiction to their findings. The experimental paradigms were entirely different. The rebound effect was generally observed after spatial cue onset in the previous experiments. Because of the requirement of fixation, one hypothesis suggests that the enhancement of the microsaccade is the “leakage” due to the cancellation of reflexive cue-directed saccade (Laubrock et al., 2005). Interestingly, the microsaccades in this period were usually directed opposite to the cue, which might be explained by the “saccadic inhibitory hypothesis” (Betta et al., 2007; Rolfs et al., 2004), that mandatory fixation leads to a general inhibition of all (micro)saccades congruent with the direction of peripheral cue, which in turn might result in a majority of microsaccades directing opposite to the cue. In GFS, the recovery process was observed after the surround onset instead of cue. Therefore, the inhibition of reflexive large saccade was not necessary, which might account for the absence of the over-baseline rebound. Moreover, the monkeys were required to maintain fixation with high precise (within a 0.6° window) in the tasks, in which the on-line fixation check introduced a penalty to the animals for moving the eye outside window. Thus, our paradigm resembled a high-acuity observation task for which strong microsaccade inhibition was reported (Bridgeman & Palca, 1980). It is possible that top-down or attentional influences was sufficient to eliminate the tendency for over-baseline rebound. Finally, the inhibition-rebound pattern of microsaccade rate was mainly reported in human subjects so far. Although microsaccades are quite similar between humans and macaque monkeys (Martinez-Conde et al., 2004), it is not clear whether the monkeys will produce the same effect. In addition, we have checked that the absence of over-baseline rebound was not caused by excluding those microsaccades following their predecessors within 100 ms.
In the invisible condition, the process of microsaccade rate recovery was relatively slow. It is significantly smaller than the rate under the visible condition until around 900 ms after surround onset, showing a strong inhibition of microsaccade during this period. Because of the identical visual stimuli under the two conditions, this extended period of microsaccade inhibition is clearly correlated to the perceptual state of target invisibility. Prolonged microsaccade inhibition was observed in the studies of visual oddballs (Valsecchi et al., 2007; Valsecchi & Turatto, 2007), in which the microsaccade rate in response to active oddball stimuli took longer time (about 300 – 400 ms longer than that in standard stimuli) to recover from the minimum to the overall rate in oddball trials. It was suggested that the slow process of rate increase was caused by high cognitive load that was thought to be linked to information updating in working memory, but the possibility that the rate was modulated by the level of attention load could not be excluded (Valsecchi et al., 2007). In GFS, however, the perceptual suppression of a salient visual stimulus does not seem to incur high load of cognitive process, although widespread activity in visual cortexes is expected (Leopold & Logothetis, 1999; Wilke et al., 2006). Furthermore, the period of the prolonged inhibition is much longer than that observed in oddball paradigm (300 – 400 ms longer). These evidences suggest that the modulation of microsaccade rate in the invisible condition might be driven by top-down influences other than working memory. The underlying mechanisms that account for the rate inhibition are not clear. One potential mechanism may be the spatial attention shift, when monkeys shifted their attention to the surrounding patterns in periphery while their eyes were kept fixation. This is supported by the observation that the microsaccade directions were biased away from the targets in the “invisible” condition (Figure 7, since the modulated microsaccade direction correlates to shifts in spatial attention (Engbert & Kliegl, 2003; Hafed & Clark, 2002). However, Valsecchi et al. (2007, 2008) excluded the effects of the shift of spatial attention on the prolonged rate inhibition observed in oddball stimuli. A more speculative explanation might be derived from the hypothesis that multistable vision (or multistable perception in general) is a behavioral action of the brain involving higher cognitive process, rather than a passive sensory response confined in the visual system (Leopold & Logothetis, 1999). According to this hypothesis, motor-directed actions and perceptual transients might work together to achieve a meaningful perception in response to a challenging stimulus. Thus, the prolonged inhibition of microsaccade in “invisible” condition could be an optimal strategy of the brain to maintain the enduring perceptual state, i.e. to keep the target invisible. Since one of the functions of microsaccade is to prevent image fading on the retina, it is suggested that the decrease of microsaccade rate may reflect the cancellation of the retinal refreshing process (Galfano et al., 2004). Finally, the similarity of the rate evolution between the “physical disappearance” and “invisible” condition does not support the notion that the microsaccade behavior leads to the invisibility of the target, as the physical removal of the stimuli cannot be induced by microsaccade activity. Thus, the result favors an alternative interpretation that the visibility states themselves, or associated motor preparation/attentional factors, influence the patterns of microsaccade behavior. However, it is possible that microsaccade activity with higher frequency in the “visible” condition contributes to the maintenance of the formed perceptual state by refreshing retina images and counteracting fading.
Since the invisible state of the monkey’s perception was indicated by the lever release, it was suspected that the observed rate reduction in the “invisible” condition could also reflect the influence from the motor to the oculomotor area. One previous study (Betta & Turatto, 2006) associated the reduction of microsaccade rate with manual motor preparation. However, their experimental paradigm was different and it is not clear whether the same effect exists in the paradigm of visual suppression. Note also that in the “visible” condition the monkey hold the lever throughout the trial, preparing lever release as soon as the target became invisible. If motor response preparation plays a major role in rate suppression, we would expect a similar rate inhibition in this condition, since the monkey was in the mode of motor preparation as well. However, we have not observed such inhibition in the “visible” condition. Moreover, an additional analysis of the data collected under a training condition of “physical disappearance (fixation only)” has been made. In this condition, the stimulus sequence was the same as that in the condition of “physical disappearance”. However, the lever was not provided to the monkey, who was required to keep fixation within the fixation window throughout the trial. We found that the overall microsaccade rate after surround onset (in the interval of [0, 1000] ms) in this condition was 0.52/s. This value is close to the rate in “physical disappearance” (0.47/s) and apparently lower than that in “visible” (0.77/s) condition (see Figure 4). Because of the absence of the lever, response preparation effects on rate should be minimal, which suggests that the observed prolonged rate inhibition may be mainly correlated to visibility states of the subjects. Though it has been interpreted that the prolonged inhibition of microsaccade rate after surround onset is mainly associated with the perceptual state, the contribution of motor preparation to microsaccade rate reduction is also possible. Further investigation is necessary to clearly disentangle these two influences.
The dynamics of the microsaccade direction differed in the average directional rates between the two perceptual conditions, although they were not significantly separated. When the target was subjectively perceived, the trace of directional microsaccade rate was generally negative after surround onset, which indicates that more microsaccades directed toward the target than those opposite to it were produced in a unit time. On the other hand, in trials where the target became perceptually suppressed, after an initial increase, the directional rate generally did not deviate from the zero level. This means that the microsaccades directed to both directions occurred at an approximately equal rate. Recently, microsaccade direction has been linked to the shift of attention (Engbert & Kliegl, 2003; Galfano et al., 2004; Hafed & Clark, 2002; Laubrock, Engbert, Rolfs, & Kliegl, 2007; Rolfs et al., 2004, 2005) (but see Horowitz, Fencsik, Fine, Yurgenson, & Wolfe, 2007; Horowitz, Fine, Fencsik, Yurgenson, & Wolfe, 2007), that the vector of spatial attention is congruent with the directional vector of microsaccades. According to this hypothesis, our results imply that the target was more attended by the monkey when it was visible than it was not. Although there is a possibility that the observed downward directional distribution could be also attributed to the compensation of microsaccades for an upward drift, which is a common pattern for monkeys during fixation (Skavenski, Robinson, Steinman, & Timberlake, 1975), this effect may only play a minor role, as the directional rates in both “invisible” and “physical disappearance” conditions did not show clear downward bias (Figure 7).
What are the implications for our understanding the physiological aspects of the relation between microsaccade and perceptual suppression? It was shown that low level manipulations that directly impact early sensory representations can exert large influences on the ultimate perception of the target (Wilke et al., 2003). These manipulations involved ocular and spatial configuration of the visual stimulus. We suspect that the configuration might contribute to attention shift to the surroundings and thus disturb the global representation of the target, together with other factors (Wilke et al., 2003), in higher level of the cortex.
To summarize, our findings demonstrate that the visual system displays specific patterns of oculomotor activity that reflects different states of visibility in GFS experiments. In general, during the perceptual disappearance of a salient target, the microsaccades were strongly inhibited and they were less directed towards the target. Because the visual stimuli are identical under either condition, it seems that the microsaccade rate and direction are well modulated by the subjective visibility states. Finally, the present results indicate that the oculomotor behavior may be used as a new method to study the phenomena of multistable perception, as our results suggest that microsaccade rate and direction can be indicators of the inner perceptual states.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bair W, O’Keefe LP. The influence of fixational eye movements on the response of neurons in area MT of the macaque. Visual Neuroscience. 1998;15(4):779–786. doi: 10.1017/s0952523898154160. [DOI] [PubMed] [Google Scholar]
- Betta E, Galfano G, Turatto M. Microsaccadic response during inhibition of return in a target-target paradigm. Vision Research. 2007;47(3):428–436. doi: 10.1016/j.visres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Betta E, Turatto M. Are you ready? I can tell by looking at your microsaccades. Neuroreport. 2006;17(10):1001–1004. doi: 10.1097/01.wnr.0000223392.82198.6d. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature. 2001;411(6839):798–801. doi: 10.1038/35081073. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Palca J. The role of microsaccades in high acuity observational tasks. Vision Research. 1980;20(9):813–817. doi: 10.1016/0042-6989(80)90013-9. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Eye movement recording. In: Carpenter RHS, Robson JH, editors. Vision research: a practical guide to laboratory methods. Oxford; UK: 1998. pp. 245–285. [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Research. 2003;43(9):1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Galfano G, Betta E, Turatto M. Inhibition of return in microsaccades. Experimental Brain Research. 2004;159(3):400–404. doi: 10.1007/s00221-004-2111-y. [DOI] [PubMed] [Google Scholar]
- Graupner ST, Velichknovsky BM, Pannasch S, Marx J. Surprise, surprise: Two distinct components in the visually evoked distractor effect. Psychophysiology. 2007;44:251–261. doi: 10.1111/j.1469-8986.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. Journal of Neuroscience. 1997;17(8):2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Research. 2002;42(22):2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Ito J, Nikolaev AR, Luman M, Aukes MF, Nakatani C, van Leeuwen C. Perceptual switching, eye movements, and the bus paradox. Perception. 2003;32(6):681–698. doi: 10.1068/p5052. [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. Small saccades serve no useful purpose - Reply to a letter by R. W. Dichburn. Vision Research. 1980;20(3):273–276. doi: 10.1016/0042-6989(80)90113-3. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, Kliegl R. Microsaccade dynamics during covert attention. Vision Research. 2005;45(6):721–730. doi: 10.1016/j.visres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, Rolfs M, Kliegl R. Microsaccades are an index of covert attention: Commentary on Horowitz, Fine, Fencsik, Yurgenson, and Wolfe (2007) Psychological Science. 2007;18(4):364–366. doi: 10.1111/j.1467-9280.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Experimental Brain Research. 1998;123(3):341–345. doi: 10.1007/s002210050577. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences. 1999;3(7):254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nature Neuroscience. 2000;3(3):251–258. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13920–13925. doi: 10.1073/pnas.212500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience. 2004;5(3):229–240. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006;49(2):297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Moller F, Laursen ML, Tygesen J, Sjolie AK. Binocular quantification and characterization of microsaccades. Graefes Archive for Clinical and Experimental Ophthalmology. 2002;240(9):765–770. doi: 10.1007/s00417-002-0519-2. [DOI] [PubMed] [Google Scholar]
- Pannasch S, Dornhofer SM, Unema PJA, Velichknovsky BM. The omnipresent prolongation of visual fixations: Saccades are inhibited by changes in situation and in subject’s activity. Vision Research. 2001;41:3345–3351. doi: 10.1016/s0042-6989(01)00207-3. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition and gaze contingent research paradigms. In: Kennedy A, Radach R, Heller D, Pynte J, editors. Reading as a perceptual process. Amsterdam: Elsevier; 2000. pp. 119–145. [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in reading. Journal of Experimental Psychology-Human Perception and Performance. 2004;30(1):194–211. doi: 10.1037/0096-1523.30.1.194. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Microsaccade orientation supports attentional enhancement opposite a peripheral cue - Commentary on Tse, Sheinberg, and Logothetis (2003) Psychological Science. 2004;15(10):705–707. doi: 10.1111/j.0956-7976.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Crossmodal coupling of oculomotor control and spatial attention in vision and audition. Experimental Brain Research. 2005;166(3–4):427–439. doi: 10.1007/s00221-005-2382-y. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Laubrock J, Kliegl R. Shortening and prolongation of saccade latencies following microsaccades. Experimental Brain Research. 2006;169(3):369–376. doi: 10.1007/s00221-005-0148-1. [DOI] [PubMed] [Google Scholar]
- Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447(7146):851–854. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- Sabrin HW, Kertesz AE. Microsaccadic Eye-Movements and Binocular-Rivalry. Perception & Psychophysics. 1980;28(2):150–154. doi: 10.3758/bf03204341. [DOI] [PubMed] [Google Scholar]
- Sabrin HW, Kertesz AE. The Effect of Imposed Fixational Eye-Movements on Binocular-Rivalry. Perception & Psychophysics. 1983;34(2):155–157. doi: 10.3758/bf03211341. [DOI] [PubMed] [Google Scholar]
- Santini F, Redner G, Iovin R, Rucci M. EyeRIS: A general-purpose system for eye-movement-contingent display control. Behavior Research Methods. 2007;39(3):350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
- Skavenski AA, Robinson DA, Steinman RM, Timberlake GT. Miniature Eye-Movements of Fixation in Rhesus-Monkey. Vision Research. 1975;15(11):1269. doi: 10.1016/0042-6989(75)90173-x. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Kagan I, Gur M. Selective activation of visual cortex neurons by fixational eye movements: Implications for neural coding. Visual Neuroscience. 2001;18(2):259–277. doi: 10.1017/s0952523801182118. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cunitz RJ, Timberlake GT, Herman M. Voluntary control of microsaccades during maintained monocular fixation. Science. 1967;155:1577–1579. doi: 10.1126/science.155.3769.1577. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Turatto M, Valsecchi M, Tame L, Betta E. Microsaccades distinguish between global and local visual processing. Neuroreport. 2007;18(10):1015–1018. doi: 10.1097/WNR.0b013e32815b615b. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Betta E, Turatto M. Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research. 2007;177(2):196–208. doi: 10.1007/s00221-006-0665-6. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Turatto M. Microsaccadic response to visual events that are invisible to the superior colliculus. Behavioral Neuroscience. 2007;121(4):786–793. doi: 10.1037/0735-7044.121.4.786. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Turatto M. Microsaccadic responses in a bimodal oddball task. Psychol Res. 2008 doi: 10.1007/s00426-008-0142-x. [DOI] [PubMed] [Google Scholar]
- van Dam LCJ, van Ee R. Retinal image shifts, but not eye movements per se, cause alternations in awareness during binocular rivalry. Journal of Vision. 2006;6(11):1172–1179. doi: 10.1167/6.11.3. [DOI] [PubMed] [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Generalized flash suppression of salient visual targets. Neuron. 2003;39(6):1043–1052. doi: 10.1016/j.neuron.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Local field potential reflects perceptual suppression in monkey visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17507–17512. doi: 10.1073/pnas.0604673103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterson BJ, Collewijn H. Microsaccades during finely guided visuomotor tasks. Vision Research. 1976;16:1387–1390. doi: 10.1016/0042-6989(76)90156-5. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Reversing ocular dominance and suppression in a single flash. Vision Research. 1984;24(5):471–478. doi: 10.1016/0042-6989(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Cook G. Microsaccades and the velocity–amplitude relationship for saccadic eye movements. Science. 1965;150:1459–1460. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]