Abstract
Objective
To develop algorithms predicting serum 25 hydroxyvitamin D [s25(OH)D] for a large epidemiological study whose subjects come from large geographic areas, are racially diverse and have a wide range in age, skin types, and month of blood sample collection. This will allow a regression calibration approach to determine s25(OH)D levels replacing the more costly method of collection and analysis of blood samples.
Study design and setting
Questionnaire data from a sub-sample of 236 non-Hispanic whites (whites) and 209 blacks from the widely dispersed Adventist Health Study-2 (n = 96,000) were used to develop prediction algorithms for races separately and combined. A single blood sample was collected from each subject, at different times throughout the year.
Results
Models with independent variables age, sex, BMI, skin type, UV season, erythemal zone, total dietary vitamin D intake, and sun exposure factor explained 22 and 31% of the variance of s25(OH)D levels in white and black populations, respectively (42% when combined). UV season and erythemal zone determined from measured UV radiation produced models with higher R2 than season and latitude.
Conclusion
Combining races with a term for race and using variables with measured UV radiation capture the variance in s25(OH)D levels better than analyzing races separately.
Keywords: Cancer, Serum 25-hydroxyvitamin D, Predictors, Adventist health study-2, Blacks, Whites
Introduction
The number of chronic diseases tentatively associated with low serum 25 hydroxyvitamin D [s25(OH)D] levels has increased markedly during the last decade [1]. Further studies are needed to verify these associations. The three sources of vitamin D are diet, supplement intake, and ultraviolet B (UVB; 290–315 nm) radiation [2, 3]. Factors such as race [4–10], age [11–15], body mass index (BMI) [4–6, 9, 16–19], sun-reactive skin type/color [2, 20–22], sunscreen use [23], geographic location/latitude [22], time of year of blood sample collection [6, 7, 9, 24–27], and genetic factors [28] modify resulting s25(OH)D levels.
Previously published multivariate models for determinants of s25(OH)D vary considerably [4–7, 9, 10, 13, 19, 21, 23–26, 29–35]. The assortment in type and precision of data collection suggest that many of the variables contributing to s25(OH)D levels are difficult to ascertain accurately [36, 37]. This is especially true of exposure to UVB radiation [2, 22, 38, 39]. The effect of UVB light on any individual depends on a complex mix of personal and environmental factors [2, 3, 36, 37] such as geographic location of the subject and month/season of year. These are usually represented by the surrogates latitude and season, respectively [22]. However, strength of UV radiation does not vary in parallel lines across the United States as implied by the use of latitude. Rather, its convoluted patterns are such that the noon UV intensity could vary by as much as a factor of two in different parts of the country at the same latitude on the same fine day [40]. Furthermore, comparison of maps of the average monthly noon UV intensity throughout the year across the contiguous US demonstrates that seasonal changes in UV intensity do not occur in 3 monthly segments represented by the traditional seasons. Putting months with similar UV intensity patterns together results in different groupings representing the seasons [40].
Our main goal is to develop a prediction equation for s25(OH)D levels for the AHS-2 cohort, a large geographically dispersed population of racially diverse subjects. The blood samples used to develop the prediction equation were collected throughout the year from a representative sample of the calibration study subgroup of the AHS-2. Finding variables that predict s25(OH)D levels and refining their measurement would allow a regression calibration approach substituting E(s25(OH)D | questionnaire data) for measured serum values. This would enable this large epidemiological study to examine the effects of s25(OH)D at lower costs than collecting 90,000 blood samples. We will also compare and contrast regression models when blacks and non-Hispanic whites (whites) are analyzed separately and together, and search for differences in effect of the important variables.
Materials and methods
Parent study
The AHS-2 has been described in detail elsewhere [41]. In brief, it is a prospective epidemiological study of 96,000 Seventh-day Adventists designed to examine the relationship of lifestyle to risks of prostate, breast, and colon cancers. Enrollment to AHS-2 occurred between 2002 and 2007. More than 25,000 of the enrollees are black, and study members live in every state and province of the United States and Canada. Every 2 years, a questionnaire to gather information about hospitalizations is mailed, the second of which included additional detailed questions about sun exposure.
Study population
Subjects included in this report are a sample of members from the AHS-2 calibration study who were enrolled by June 2006, had provided detailed sun exposure information for the 2 months prior to their clinic attendance, and reported their race as either black (n = 209) or white (n = 236). These subjects had their blood samples analyzed for s25(OH)D. Details of the calibration study methods [41, 42] have been described elsewhere. Briefly, calibration subjects (n = 1,011) were randomly selected from among the 96,000 enrollees to the AHS-2. They were required to attend a clinic where weight and height were measured, and fasting blood samples collected. These clinics were held from November 2003 to May 2007 (excluding February, June, and July because of weather or vacation time). Calibration subjects also provided six 24-h telephone dietary recalls and completed a food frequency questionnaire (FFQ) within 1–3 months of blood sample collection. The study was approved by the institutional review board of Loma Linda University.
Determination and assessment of factors contributing to S25(OH)D Levels
BMI
BMI was determined from measured height (without shoes) and weight (with light clothing) of participants at time of blood collection.
Dietary, supplemental, and total vitamin D intake
Vitamin D intake was assessed from the AHS-2 FFQ which requested information about the previous 1 year and was validated against two blacks (each of three) of recalls taken 5–6 months apart to cover opposite seasons. The FFQ has corrected validity correlations of 0.60 and 0.64 against 24-h telephone recalls in black and white subjects, respectively [42]. Dietary vitamin D included D2 and D3. The naturally occurring and fortified vitamin D content of foods was obtained using the Nutrition Data System for Research (NDS-R) software version 5.03 database (The Nutrition Coordinating Center, Minneapolis, MN). Subjects were asked to name all supplements they were consuming, together with brand names and quantities. Values of vitamin D from supplements were verified from the manufacturers’ websites. No differentiation was made for D2 or D3 as this could not always be determined.
Dietary vitamin D was adjusted for energy intake using the residual method [43]. Supplemental intake was not energy adjusted. Total vitamin D intake was the sum of the population mean dietary intake, the energy-adjusted residual and supplemental intake.
Smoking history and alcohol consumption
Neither was included in our models as these habits are not part of the AHS-2 subjects’ lifestyle.
Skin pigmentation
Subjects were categorized according to Fitzpatrick sun-reactive skin types I through VI [44] based on their answer to the question “What happens to your skin if it is exposed many times to bright sunlight in the summer without protection?” Types I and II (no tan or tan very lightly) were collapsed for both blacks and whites to skin type I/II since there were only 11 white and 2 black subjects reporting skin type I. Fitzpatrick skin type III (tan moderately), IV (tan darkly), and V (already brown) and VI (already black) were coded skin type III, IV, and V/VI, respectively. These collapsed categories were scored 1, 2, 3, and 4, respectively, for the independent regression variable, skin type.
Percentage of body exposed
The detailed sun exposure questionnaire asked which parts of the body were typically exposed when in the sunshine on Sundays, Saturdays, and weekdays during the 2 months prior to clinic attendance. Adapting burn exposure charts [45], percentages of 4, 2, 6, 13, and 13 were assigned to face and neck, hands, most of the arms, most of the legs and upper torso, respectively. Our percentages are lower than Wachtel’s [45] because ours represent “most”, not “all” of that body part, and compensate for UVB radiation affecting only the side of the body facing the sun at any one time.
Duration of sun exposure
Subjects were asked how long they were in the sun on a typical weekday, Saturday, and Sunday during the 2 months prior to clinic attendance. Categories were 0, ≤29 min, 30–59 min, 1–2 h, and 2–3 h except for the hours 11 a.m. to 3 p.m., when the categories were 0, up to 14 min, 15–29 min, 30–59 min, 1–2.9 h, 3–4 h. Midpoints of each of these time categories were used to calculate total time per week which was converted to a daily average. Duration was also calculated weighting the hours from 11 a.m. to 3 p.m. by 2 to allow for higher intensity of UVB at that time of day [46, 47].
Sun exposure factor
The amount of vitamin D produced in the skin is the result of the percentage of skin surface exposed to the sun and duration of that exposure. Sun exposure factor, the product of these two variables, was calculated for (a) total time exposed, (b) 11 a.m. to 3 p.m., when the strength of UV radiation is strongest, and (c) when midday hours were weighted by 2 [46].
Latitude
Latitude is a surrogate for UVB exposure due to geographic location. Latitude categories 1–3 were defined in the baseline questionnaire by designating the state of subject’s residence as north, mid or south when more than 50% of the state fell between the latitudes of >40°N, 35–40°N, and <35°N, respectively.
Erythemal zone
Erythemal zones are based on UV index maps showing the convoluted patterns of UV radiation intensity across the United States. Using the maps available from the National Aeronautics and Space Administration (NASA) (August 1996–August 2003) [40], each subject was assigned an erythemal zone based on the average of those years of the average monthly strength of noon erythemal radiation during the two months prior to the date of blood sample collection at the location of their residence. Although erythemal radiance (which causes reddening or erythema of the skin) includes UVA and C, as well as UVB radiation, it is a more accurate indicator than latitude of relative UVB strength due to geographic location. The erythemal zones were coded 1–5, beginning at <60 mW/m2, and increasing by 60 W/m2 to 240–300 mW/m2. Erythemal zones are somewhat confounded by season, as they depend on the two months preceding the clinic visit.
Season
Season categories were designated using traditional breakpoints: Season 1, winter—December to February; Season 2, spring—March to May; Season 3, summer—June to August (although no samples were collected in June or July); Season 4, fall—September to November.
UV season
From the same maps used to determine Erythemal zone, UV seasons were formed by grouping together the months which had similar patterns of noon erythemal radiation strength [40] forming UV winter—November to February; UV spring—March; UV summer—April to August; and UV fall—September to October. UV winter and UV spring were collapsed to form UV season 1 since only 12 blood samples were collected in March, UV season 2 and 3 represent UV summer and UV fall, respectively. This modification for season was made to test whether it would be a more precise measure of UV exposure due to month of blood collection.
Sunscreen use and altitude
So few subjects in this cohort used sunscreen regularly or lived at altitudes greater than 3,000 feet that we did not include these variables in our analyses.
Biochemical methods
Plasma and cells were separated by centrifuge at the clinic sites. Blood collected at field clinics from calibration subjects was sent on frozen gel packs overnight to reach the processing lab at Loma Linda University, CA within 30 h of sample collection, then stored in liquid nitrogen. S25(OH)D was measured using a two-step radioimmunoassay procedure (Diasorin, Stillwater, MN). The selected samples were couriered on dry ice from the Loma Linda laboratory to the Reproductive Endocrine Research Laboratory, Department of Obstetrics and Gynecology, USC Keck School of Medicine, Los Angeles, and stored again in liquid nitrogen until time of assay which was carried out in three batches. Typical intra and interassay coefficients of variation at this laboratory are 10 and 16%, respectively.
Statistical analyses
All analyses were conducted using S-Plus software, version 7.0 (TIBCO software, Inc, Palo Alto, CA). Chi-square difference of means and two sample t-tests were used to determine the difference between the white and black populations for categorical and continuous variables, respectively. Partial Spearman correlations adjusted for age and sex were determined between all predictive variables. Blacks and whites were analyzed together and separately. Subjects with missing values for variables being tested were omitted from that analysis. Linear regressions were at first only age and sex adjusted, then multivariate models were used to examine the relationships between s25(OH)D levels and independent variables. Selected second-order terms were used to check evidence of non-linearity. Of these, skin type2 and UV season2 were statistically significant, and therefore included in the model as reported in Table 3. Sex and age-adjusted models were also used to examine the relationships between sun exposure variables, and skin types, and season.
Table 3.
Beta coefficients and p-values for variables in multivariate linear regression models predicting serum 25 hydroxyvitamin D levels (ng/mL) in non-Hispanic whites and blacks separately and combined
| Variables in final model | Non-Hispanic whites (n = 236) | Blacks (n = 209) | Blacks plus Non-Hispanic whites (n = 445) |
|---|---|---|---|
| R2 = 0.221 (p <0.0001) | R2 = 0.308 (p <0.0001) | R2 = 0.415 (p <0.0001) | |
| Beta (p) | Beta (p) | Beta (p) | |
| Age, years | −0.13 (0.02) | 0.04 (0.55) | −0.27 (0.02) |
| Age × Race | – | – | 0.15 (0.06) |
| Sexa | −0.22 (0.90) | −0.75 (0.6) | −0.69 (0.53) |
| Raceb | – | – | −15.92 (0.003) |
| BMI, Kg/m2 | −0.29 (0.04) | −0.18 (0.1) | −0.21 (0.02) |
| Skin typec alone | 1.97 (0.05) | – | – |
| Skin type | – | 6.98 (0.07) | 6.67 (0.01) |
| Skin type2 | −1.51 (0.04) | −1.37 (0.01) | |
| UV seasond | −17.05 (0.008) | −29.21 (<0.0001) | −21.32 (<0.0001) |
| UV season2 | 3.38 (0.03) | 6.08 (0.0004) | 4.30 (0.0001) |
| Erythemal zonee | 1.34 (0.09) | 1.82 (0.017) | 1.72 (0.001) |
| Total Vitamin D intake, mcgf | 0.20 (0.02) | 0.35 (0.0006) | 0.23 (0.0003) |
| Sun exposure factorg | 0.0016 (0.007) | 0.0001 (0.9) | 0.0031 (0.008) |
| Sun exposure factor × race | −0.0015 (0.04) |
Coded 1 for male and 2 for female
Coded 1 for whites, 2 for blacks; included only in model where blacks and non-Hispanic white data sets were combined
Adapted from Fitzpatrick skin type; coded 1–4, for Types I + II, III, IV, and V + IV, respectively
Months of year grouped according to similarity of erythemal zone patterns; coded 1–3 for months 11–3, 4–8 and 9–10), respectively
Average of average monthly erythemal radiance for subject’s location for 2 months prior to blood collection; coded 1–5 beginning at<60 mW/m2, and increasing by 60 W/m2 to 240–300 mW/m2
Sum of population mean dietary vitamin D intake, the energy-adjusted residual, and supplemental vitamin D intake. Conversion factor to IU = ×40
Product of duration of daily sun exposure and percentage of body exposed to sunshine
Regressions for blacks were not log-transformed to allow for easier comparison of effect between races, although distribution of their s25(OH)D levels was skewed slightly to the right. The same variables were significantly associated with s25(OH)D levels using either the transformed or non-transformed data.
All multivariate models included variables measuring age, sex, BMI, dietary vitamin D, supplemental vitamin D, month of blood sample collection, geographic location of the subject, skin type, and skin surface and duration of sun exposure. The model combining the races also included a term for race, and the product terms with race for those variables which were significantly different between the races. Low numbers of males in both white and black populations limited power when testing interactions with gender.
Results
Relevant baseline characteristics of the study population are described in Table 1. Of the 209 blacks and 236 whites, more than two-thirds were female. It was an older population, the mean age for whites being somewhat higher than for blacks. Mean s25(OH)D levels were 20.0 (SD 10.2) and 30.8 (SD 10.3) ng/mL, with 15.8 and 52.1% attaining sufficiency (≥30 ng/mL) [1] in blacks and whites, respectively. BMI was higher in blacks compared to whites. There were no significant differences between races for nutritional vitamin D intake or sun exposure variables.
Table 1.
Means (SD) and proportions of selected baseline characteristics of study population by racial group
| Characteristic | Non-Hispanic whites n = 236 |
Blacks n = 209 |
pa |
|---|---|---|---|
| Males, % | 36.9 | 26.3 | 0.016 |
| Age, years | 63.0 (13.8) | 58.3 (12.5) | 0.0002 |
| Range, years | 34–96 | 31–88 | |
| Serum 25 hydroxyvitamin D [1], ng/mL | 30.8 (10.3) | 20.0 (10.2) | <0.0001 |
| Severe deficiency (<25), % | 0.9 | 12.0 | |
| Deficiency (≥25–≤50), % | 14.4 | 45.5 | |
| Insufficiency (≥50–≤75), % | 32.6 | 26.8 | |
| Sufficiency (≥75), % | 52.1 | 15.8 | |
| BMI, Kg/m2 | 26.9 (5.2) | 30.2 (6.7) | <0.0001 |
| Unknownb, % | 4.2 | 3.8 | |
| Fitzpatrick skin types | |||
| I No tan/freckles, % | 8.9 | 1.0 | <0.0001 |
| II Tan lightly, % | 32.2 | 14.8 | |
| III Tan moderately, % | 42.4 | 15.3 | |
| IV Tan darkly, % | 16.5 | 21.1 | |
| V/VI skin brown or black, % | 0 | 43.5 | |
| Unknownb, % | 0 | 4.3 | |
| Dietary Vitamin D intakec, mcg | 3.4 (2.4) | 3.3 (2.4) | 0.60 |
| Unknownb, % | 5.9 | 14 | |
| Supplemental Vitamin D intake, mcg | 6.3 (7.9) | 5.0 (7.4) | 0.39 |
| Unknownb, % | 4.2 | 9.6 | |
| Total Vitamin D intaked, mcg | 9.9 (6.2) | 8.8 (7.9) | 0.22 |
| Unknownb, % | 5.9 | 20.6 | |
| Season of the yeare (for blood collection) | <0.0001 | ||
| Winter (months 12–2), % | 13.3 | 4.8 | |
| Spring (months 3–5), % | 14.4 | 35.4 | |
| Summer (months 6–8), % | 18.6 | 13.9 | |
| Fall (months 9–11), % | 53.8 | 45.9 | |
| UV seasonf (blood collection) | 0.0004 | ||
| UV season1 (months 11–3), % | 30.9 | 20.0 | |
| UV season 2 (months 4–8), % | 29.2 | 46.9 | |
| UV season 3 (months 9–10), % | 39.8 | 33.0 | |
| Latitude of residence | <0.0001 | ||
| >40°N, % | 42.4 | 25.4 | |
| 35–40°N, % | 49.6 | 43.5 | |
| <35°N, % | 8.0 | 31.1 | |
| Erythemal zoneg of residence | 0.20 | ||
| <60 mW/m2, % | 8.5 | 9.1 | |
| 60–119 mW/m2, % | 11.0 | 12.4 | |
| 120–179 mW/m2,% | 24.2 | 29.7 | |
| 180–240 mW/m2, % | 30.9 | 33.0 | |
| 240–300 mW/m2, % | 25.4 | 15.8 | |
| Duration of daily sun exposure (min) | 98.7 (93.1) | 91.2 (91.0) | 0.50 |
| Unknownb, % | 12.7 | 14.8 | |
| Percentage of body exposed to sunshineh | 9.3 (6.5) | 8.7 (6.9) | 0.31 |
| Unknownb, % | 9.7 | 1.9 | |
| Sun exposure factori | 1,005.7 (1,268.4) | 978.8 (1,471.6) | 0.9 |
| Unknownb, % | 12.7 | 14.8 |
Chi-square difference of means and two sample t-test comparing blacks to whites, for categorical and continuous variables, respectively
Percentage of cohort with unknown value for the variable
Calorie adjusted by residual method. Conversion factor to IU = ×40
Sum of population mean dietary vitamin D intake, the energy-adjusted residual and supplemental vitamin D intake. Conversion factor to IU = ×40
Traditional seasons: winter (months 12–2), spring (months 3–5), summer (months 6–8), fall (months 9–11)
Months of year grouped according to similarity of erythemal zone patterns
Average of average monthly erythemal radiance for subject’s location for 2 months prior to blood collection
Percentages of 4, 2, 6, 13, and 13 were assigned to face and neck, hands, most of the arms, most of the legs, upper torso, respectively. Adapted from burn exposure charts [46]
Product of duration of daily sun exposure and percentage of body exposed to sunshine
Spearman’s age and sex-adjusted (where appropriate) pair-wise correlations between all independent variables in the multivariate model revealed no significant correlations for blacks. For whites, sun exposure factor was found to be positively correlated with skin type (0.14, p = 0.05), UV season (0.22, p = 0.002) and erythemal zone (0.22, p = 0.002) and negatively correlated with age (−0.25, p = 0.0005). In age and sex-adjusted linear regression, positive associations were found between min spent in the sunshine (p = 0.03) and both the percentage of body exposed to the sunshine and UV season (p = 0.004).
In age and sex-adjusted linear regression, blacks and whites shared several variables significantly associated with s25(OH)D levels, including BMI, season, UV season, vitamin D from supplements and total vitamin D intake (Table 2). Age, skin type, and sun exposure factor were significant predictors in whites only. Erythemal zone was significantly associated with s25(OH)D levels for blacks but not whites. Sex, latitude, percentage of body exposed to sunshine, time spent in the sunshine, and vitamin D from food were not significant predictors for either racial group. Neither midday hours alone, midday hours weighted by 2, or sun exposure factor using midday hours alone or midday hours weighted by 2 were significant in any regression. We did find positive associations between sun exposure factor with skin type (p = 0.03) and percentage of body exposed with UV season (p = 0.004).
Table 2.
Beta coefficients, p-values, and R2 for predictor variables for serum 25 hydroxyvitamin D levels (ng/mL) in age and sex-adjusted linear regressions among blacks and non-Hispanic whites separately and combined
| Predictor variables | Non-Hispanic whites (n = 236)
|
Blacks (n = 209)
|
Combined Non-Hispanic whites and blacks (n = 445)
|
|||
|---|---|---|---|---|---|---|
| Beta (p) | R2 | Beta (p) | R2 | Beta (p) | R2 | |
| Agea (years) | −0.16 (0.001) | 0.044 | 0.10 (0.07) | 0.016 | 0.02 (0.61) | 0.006 |
| Sexb | −1.19 (0.40) | 0.044 | −0.47 (0.77) | 0.016 | −1.82 (0.12) | 0.006 |
| Racec | – | – | – | – | −10.95 (<0.0001) | 0.221 |
| Body mass index, Kg/m2 | −0.37 (0.006) | 0.077 | −0.24 (0.02) | 0.039 | −0.52 (<0.0001) | 0.081 |
| Skin typed alone | 2.79 (0.003) | 0.081 | – | – | – | – |
| Skin type | N/A | 7.17 (0.08) | 0.030 | 12.03 (<0.0001) | 0.088 | |
| Skin type2 | −1.26 (0.10) | −2.77 (<0.0001) | ||||
| Seasone | −10.66 (0.009) | 0.072 | −2.73 (0.001) | 0.09 | −14.77 (<0.0001) | 0.074 |
| Season2 | 1.97 (0.01) | – | 2.99 (<0.0001) | |||
| UV seasonf | −16.51 (0.006) | 0.082 | −27.46 (<0.0001) | 0.117 | −30.51 (<0.0001) | 0.105 |
| UV season2 | 3.77 (0.01) | 6.35 (<0.0001) | 7.12 (<0.0001) | |||
| Latitudeg | 0.27 (0.8) | 0.045 | 0.23 (0.81) | 0.016 | −1.88 (0.015) | 0.020 |
| Erythemal zoneh | 0.19 (0.70) | 0.045 | 1.83 (0.002) | 0.059 | 1.27 (0.006) | 0.023 |
| Vitamin D from foodi, mcg | 0.40 (0.17) | 0.052 | 0.50 (0.13) | 0.023 | 0.59 (0.017) | 0.018 |
| Vitamin D from supplements, mcg | 0.23 (0.008) | 0.074 | 0.35 (0.0005) | 0.075 | 0.32 (0.003) | 0.056 |
| Total vitamin D intakej, mcg | 0.25 (0.004) | 0.080 | 0.33 (0.001) | 0.072 | 0.31 (0.0001) | 0.044 |
| Time spent in sunshine, min per day | 0.01 (0.14) | 0.053 | 0.01 (0.2) | 0.034 | 0.01 (0.047) | 0.021 |
| Percentage of body exposed to sunshine | 0.16 (0.14) | 0.048 | 0.003 (0.97) | 0.016 | 0.15 (0.09) | 0.017 |
| Sun exposure factork | 0.002 (0.002) | 0.087 | 0.0007 (0.19) | 0.035 | 0.001 (0.004) | 0.032 |
Adjusted for sex only
Adjusted for age only; coded 1 for male and 2 for female
Coded 1 for whites, 2 for blacks
Adapted from Fitzpatrick skin type; coded 1–4, for Types I + II, III, IV, and V + IV, respectively
Traditional seasons: winter (months 12–2), spring (months 3–5), summer (months 6–8), fall (months 9–11); coded 1–4, respectively
Months of year grouped according to similarity of erythemal zone patterns; coded 1–3 for months 11–3, 4–8, and 9–10, respectively
Coded 1–3 for latitudes >40°N, 35–40°N, and <35°N, respectively
Average of average monthly erythemal radiance for subject’s location for 2 months prior to blood collection; coded 1–5 beginning at <60 mW/m2, and increasing by 60 W/m2 to 240–300 mW/m2
Calorie adjusted by residual method. Conversion factor to IU = ×40
Sum of population mean dietary vitamin D intake, the energy-adjusted residual and supplemental vitamin D intake. Conversion factor to IU = ×40
Product of duration of daily sun exposure and percentage of body exposed to sunshine
For multivariate analyses, all the variables that were significant in the age and sex-adjusted models for either racial group, plus sex, were included. Significant variables in the age and sex-adjusted models remained significant for whites. For blacks, BMI became non-significant. For the white cohort, this multivariate model explained 22% of the variance of s25(OH)D levels, and for the black cohort, 31% (Table 3). Significant differences in effects on s25(OH)D between races were found for age (p = 0.037) and sun exposure factor (p = 0.007). When the racial groups were combined and product terms between these two variables and race, as well as a term for race were included in the model, 42% of the variance in s25(OH)D levels was explained. Using season and latitude, the R2 for multivariate white, black, and combined races models were 0.20, 0.21, and 0.37, respectively. Thus, variables for UV exposure based on recorded UV radiation rather than season and latitude improved the R2 of these models by 11, 44, and 13% in whites, blacks, and combined races models, respectively.
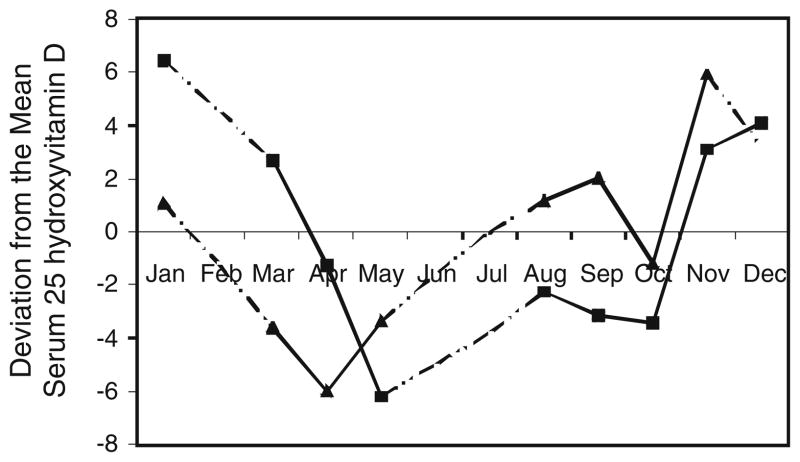
The relative effect of 1 SD change for the significant non-quadratic variables, age, BMI, skin type, total vitamin D intake, and sun exposure factor on s25(OH)D levels in whites was 0.7, 0.6, 0.5, 0.8, and 1.0 ng/mL, respectively; and for blacks, effects of 1 SD change of sun exposure factor and total vitamin D intake produced changes of 0.1 and 1.1 ng/mL. Changing to the next higher erythemal zone of residence predicted an increase of 0.9 ng/mL in blacks. Skin type in blacks had a curvilinear association with s25(OH)D, this being similar to whites for lighter skin tones, but when skin type was brown/black, there was dramatically less effect. The effect of season is also complex and is shown in Fig. 1. In our data, for both blacks and whites the highest s25(OH)D levels were found in fall or early winter, respectively.
Fig. 1.
Monthly change in serum 25 hydroxyvitamin D levels using deviations from multivariate-adjusted annual means. Black square non-Hispanic whites, Black up pointing triangle blacks, - -..- -.. No samples Collected
The three models shown in Table 3 can provide predicted levels of s25(OH)D when applied to the relevant populations. These predicted levels (ng/mL) are such that the 10th and 90th percentiles are 25.0 and 37.1 (white subjects alone); 13.5 and 27.3 (black subjects alone); and 16.6 and 35.3 (combined populations). For each race, separately, these percentile ranges correspond to vitamin D dietary intake differences of 60 mcg or 2,400 IU (whites), 34 mcg or 1,360 IU (blacks), according to these same regressions. These ranges of predicted values are sufficient to potentially detect differences in risk of some endpoint when such equations are used in regression calibration.
Discussion
We developed algorithms that explained 31, 22, and 42% of the variance in measured s25(OH)D levels in our black, white, and racially combined populations, respectively. The higher R2 of the combined model indicates that the race variable captures factors not accounted for, nor yet understood. Only age and sun exposure factor were found to have significantly different effects between races. Among blacks, sun exposure had a lesser impact than diet due to diminished cutaneous production of s25(OH)D. For both races, season had a higher impact on changes in s25(OH)D levels than any other factor, even after we included variables for sun exposure.
The difference we found in mean s25(OH)D levels between races is no doubt due in part to the differences in skin tone. Not only is the lighter toned skin of whites capable of greater production of s25(OH)D, cutaneously produced vitamin D3 has been reported to result in more sustained levels of s25(OH)D than does oral dosing of vitamin D2 [48, 49].
Decreased production of cutaneous 7 dehydroxy cholesterol with age has been reported in Caucasians with skin type III, the most common skin type in the United States [14]. This may contribute to the negative association of s25(OH)D levels with age which, in our white cohort, remained after adjustment for sun exposure factor, the only other variable found to decrease with age in sex-adjusted analyses. As in NHANES III reports [9], we found no association between s25(OH)D and age among blacks, this difference with white subjects being nearly statistically significant (p = 0.06).
For whites, we found a negative association between BMI and s25(OH)D levels which is consistent with the literature [8, 9, 50]. For blacks, the association in the age and sex-adjusted model disappeared in the multivariate model. In the literature, when only blacks are included in the model, the results vary [4, 9, 27, 51].
At first glance, the positive linear association that we found in whites between skin types I to IV and s25(OH)D levels and the curvilinear relationship in blacks appear to conflict with the understanding that the fairer the skin, the higher the conversion of pre-vitamin D to cholecalciferol per unit of UVB radiance [2, 22]. But photosensitive skin type is determined by the skin’s potential for tanning [44, 52] while skin color is determined by the amount of melanin in the skin [2]. Skin types I and II correlate well with skin color, but skin types III and IV can have fair as well as darker tones prior to tanning. We found, along with others [52, 53], that those with greater ability to tan spend more time in the sunshine (p = 0.03) explaining the higher levels of s25(OH)D with increasing skin type. That some effect of skin type on s25(OH)D persists after adjustment for sun exposure may be due to residual confounding.
Many studies have reported that s25(OH)D levels are lowest in winter and highest in summer when UV strength is at its peak [4, 5, 13, 17, 18]. At least one study reported highest levels in the fall [9]. Our study found levels in whites were lowest and began to rise when UVB intensity began its annual considerable increase in April [40], continuing past the summer peak of UVB strength until January (Fig. 1). The continued rise through fall may imply a cumulative effect, s25(OH)D continuing to be stored while UVB strength is high, thereafter being released from storage for a time. Additionally, a positive association between amount of body exposed and UV season (p = 0.004) in whites but not blacks indicates an increasing body exposure among whites during fall, which may explain the continuing rise in their s25(OH)D levels during this time. A similar trend in s25(OH)D levels in blacks occurred 1 and 2 months ahead of the whites (Fig. 1). The earlier start in decreasing s25(OH)D levels in blacks may be explained, at least in part, by the inability of their darker skin to produce vitamin D at the lower intensities of UVB radiation in the fall and by their lack of increased body exposure during that time.
The variables, UV season and erythemal zone which we constructed from maps of UV radiation weighted for erythemal reaction and which included UVA, B, and C were intended to improve the accuracy of season and latitude as surrogates for UVB exposure. While they did produce moderate improvements in R2, further improvement is likely with the use of recently published maps of UV radiation which have been refined and weighted for pre-vitamin D3 production [54] rather than erythemal reaction in human skin.
Whether our model will allow effective regression calibration remains to be seen in actual trials with disease endpoints. Giovannucci et al. [5] used this approach to predict colon cancer risk with an R2 for predicting s25(OH)D of 0.28 in a model that included race. Compared to the (unattainable) average of a large number of serum values, using our predictive equations would reduce power by about 50%. However, using a single serum measure without adjustment for its with-in person random error will also result in a 30–40% reduction in power, but in addition, will bias effect estimates toward the null by about 50% [55, 56].
There are several limitations to this study including the relative inaccuracy of measuring certain exposure variables. There are the well-known effects of errors in dietary questionnaires [57]. We did not separate cholecalciferol and ergocalciferol [58, 59], or account for other vitamin D metabolites found in animal products [60]. The nutrient database values for vitamin D in foods and supplements are inaccurate, a problem currently being addressed by the Nutrient Data Laboratory [61].
As with many questionnaire items, questions for duration of vitamin D-producing sun exposure and amount of body exposed requested ‘usual’, not ‘actual’, exposure times. We could not correct for the variation in strength of UVB that occurs throughout the day. We found neither advantage in separating exposure by time of day, nor weighting midday hours by 2 [46]. We were also unable to adjust for the point at which cutaneous production of s25(OH)D levels plateau for each individual. Total sun time may have exceeded this point for many, especially those with lighter skin and longer daily sun exposure times.
The dynamics of lag time between UV exposure/vitamin D intake and s25(OH)D levels has not yet been fully elucidated. No calculations were made to differentiate between cutaneous production of vitamin D which results in a more sustained supply of s25(OH)D compared to oral vitamin D [49]. Reports for the half-life of s25(OH)D vary from 2 weeks to 2 months [58, 62–64].
The R2s we obtained in our study compare favorably with other studies on US populations [4–7, 13, 18, 19, 26], but are still relatively low. Other factors such as the common genetic variants of vitamin D binding protein which result in as much as a threefold difference in s25(OH)D levels [28], and others yet unknown, may contribute to this.
Conclusion
A higher R2 for predicting s25(OH)D levels is obtained in the model where the races are combined, compared to separate models for blacks and whites. Studying the racial groups separately allowed us to determine that age and sun exposure factor affect s25(OH)D levels in blacks and whites differently. The higher R2 of the combined population results from the race variable and its significant product terms. This persists even after allowing for skin type and sun exposure. Other metabolic/genetic differences between the races may account for this. Seasonal changes are strong in both races. In whites, sun exposure was less in the summer than in the fall and this was probably reflected in serum levels. In blacks, dietary vitamin D has a proportionately greater influence than in whites. Skin type has a complex association with s25(OH)D. Mildly darker tones are associated with higher levels in both races, but blacks with the darkest skin tones have much lower levels. Replacing the usual surrogates of UV exposure, season, and latitude, with measured UV intensities determined from the erythemal index for the geographic location and season of each subject improves the R2. A predictive model developed by one study may not be transferable to other study groups. The common features of each study, nevertheless, provide useful insights into the factors that affect s25(OH)D levels.
Acknowledgments
Supported by National Institutes of Health Grants #5F32HL082435 and #RO1 CA94594.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10552-009-9481-1) contains supplementary material, which is available to authorized users.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–1086. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 4.Egan K, Signorello L, Munro H, Hargreaves M, Hollis B, Blot W. Vitamin D insufficiency among African–Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19:527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs ET, Alberts DS, Foote JA, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MA, Davey A, Park S, Hausman DB, Poon LW. Age, race and season predict vitamin d status in African American and white octogenarians and centenarians. J Nutr Health Aging. 2008;12:690–695. doi: 10.1007/BF03028616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 9.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third national health and nutrition examination survey, 1988–1994. Am J Clin Nutr. 2008;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer IM, Boeke AJP, Lips P, et al. Fatty fish and supplements are the greatest modifiable contributors to the serum 25-hydroxyvitamin D concentration in a multiethnic population. Clin Endocrinol. 2008;68:466–472. doi: 10.1111/j.1365-2265.2007.03066.x. [DOI] [PubMed] [Google Scholar]
- 11.Bolland MJ, Chiu WW, Davidson JS, et al. The effects of seasonal variation of 25-hydroxyvitamin D on diagnosis of vitamin D insufficiency. N Z Med J. 2008;121:63–74. [PubMed] [Google Scholar]
- 12.Holick MF. 25-OH-vitamin D assays. J Clin Endocrinol Metab. 2005;90:3128–3129. doi: 10.1210/jc.2005-0162. [DOI] [PubMed] [Google Scholar]
- 13.Jacques PF, Felson DT, Tucker KL, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66:929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 14.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739–2748. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 17.Lucas J, Bolland M, Grey A, et al. Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int. 2005;16:1641–1648. doi: 10.1007/s00198-005-1888-2. [DOI] [PubMed] [Google Scholar]
- 18.McKinney K, Breitkopf CR, Berenson AB. Association of race, body fat, and season with vitamin D status among young women: a cross-sectional study. Clin Endocrinol. 2008;69:535–541. doi: 10.1111/j.1365-2265.2008.03233.x. [DOI] [PubMed] [Google Scholar]
- 19.Rock CL, Thornquist MD, Kristal AR, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra post-marketing surveillance study. J Nutr. 1999;129:855–864. doi: 10.1093/jn/129.4.855. [DOI] [PubMed] [Google Scholar]
- 20.Clemens TL, Henderson SL, Adams JS, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;319:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 21.Gozdzik A, Barta JL, Wu H, et al. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health. 2008;8:336. doi: 10.1186/1471-2458-8-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77:51–58. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 24.Hill TR, O’Brien MM, Lamberg-Allardt C, et al. Vitamin D status of 51–75 year-old Irish women: its determinants and impact on biochemical indices of bone turnover. Public Health Nutr. 2006;9:225–233. doi: 10.1079/phn2005837. [DOI] [PubMed] [Google Scholar]
- 25.Hintzpeter B, Mensink GBM, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2007;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 26.Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr. 2006;25:395–402. doi: 10.1080/07315724.2006.10719551. [DOI] [PubMed] [Google Scholar]
- 27.Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 28.Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;40:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Andersen R, Molgaard C, Skovgaard LT, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr. 2005;59:533–541. doi: 10.1038/sj.ejcn.1602108. [DOI] [PubMed] [Google Scholar]
- 30.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86:97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 31.Brustad M, Alsaker E, Engelsen O, Aksnes L, Lund E. Vitamin D status of middle-aged women at 65–71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004;7:327–335. doi: 10.1079/PHN2003536. [DOI] [PubMed] [Google Scholar]
- 32.Burgaz A, Akesson A, Oster A, Michaelsson K, Wolk A. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am J Clin Nutr. 2007;86:1399–1404. doi: 10.1093/ajcn/86.5.1399. [DOI] [PubMed] [Google Scholar]
- 33.Hirani V, Mosdol A, Mishra G. Predictors of 25-hydroxyvitamin D status among adults in two British national surveys. Br J Nutr. 2009;101:760–764. doi: 10.1017/S0007114508023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lips P, van Ginkel FC, Jongen MJ, et al. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am J Clin Nutr. 1987;46:1005–1010. doi: 10.1093/ajcn/46.6.1005. [DOI] [PubMed] [Google Scholar]
- 35.van Dam RM, Snijder MB, Dekker JM, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn study. Am J Clin Nutr. 2007;85:755–761. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 36.McCarty CA. Sunlight exposure assessment: can we accurately assess vitamin D exposure from sunlight questionnaires? Am J Clin Nutr. 2008;87:1097–1101. doi: 10.1093/ajcn/87.4.1097S. [DOI] [PubMed] [Google Scholar]
- 37.Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87:1102–1105. doi: 10.1093/ajcn/87.4.1102S. [DOI] [PubMed] [Google Scholar]
- 38.Hoeppe P, Oppenrieder A, Erianto C, et al. Visualization of UV exposure of the human body based on data from a scanning UV-measuring system. Int J Biometeorol. 2004;49:18–25. doi: 10.1007/s00484-004-0211-9. [DOI] [PubMed] [Google Scholar]
- 39.Webb AR, Weihs P, Blumthaler M. Spectral UV radiance on vertical surfaces: a case study. Photochem Photobiol. 1999;69:464–470. doi: 10.1562/0031-8655(1999)069<0464:suiovs>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Ozone Processing Team. Monthly average local noon erythemal irradiance, v.8. NASA; 2006. ftp://toms.gsfc.nasa.gov/pub/eptoms/images/monthlyaverages/erythemal/ [Google Scholar]
- 41.Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: the adventist health study-2 (AHS-2) Int J Epidemiol. 2008;37:260–265. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 42.Jaceldo-Siegl K, Knutsen S, Sabate J, et al. Validation of nutrient intake using a food-frequency questionnaire and repeated 24 h recalls in Black and White subjects of the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2009 doi: 10.1017/S1368980009992072. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willett WC, Stampfer MJ. Implications of total energy intake for epidemiologic analyses. In: Willett W, editor. Nutritional epidemiology. 2. Oxford University Press; New York: 1998. pp. 273–301. [Google Scholar]
- 44.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 45.Wachtel TL, Berry CC, Wachtel EE, Frank HA. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns. 2000;26:156–170. doi: 10.1016/s0305-4179(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 46.Diffey BL. Solar ultraviolet radiation effects on biological systems. J Phys Medicine Biology. 1991;36:299–328. doi: 10.1088/0031-9155/36/3/001. [DOI] [PubMed] [Google Scholar]
- 47.Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82:1697–1703. doi: 10.1562/2005-09-01-RA-670. [DOI] [PubMed] [Google Scholar]
- 48.Fraser DR. The physiological economy of vitamin D. Lancet. 1983;1:969–972. doi: 10.1016/s0140-6736(83)92090-1. [DOI] [PubMed] [Google Scholar]
- 49.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 51.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malvy DJM, Guinot C, Preziosi P, et al. Relationship between vitamin D status and skin phototype in general adult population. Photochem Photobiol. 2000;71:466–469. doi: 10.1562/0031-8655(2000)071<0466:rbvdsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Fears TR, Bird CC, Guerry DIV, et al. Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Cancer Res. 2002;62:3992–3996. [PubMed] [Google Scholar]
- 54.Lee-Taylor J, Madronich S. NCAR Technical Note TN-474-STR. 2007. Climatology of UV-A, UV-B, and erythemal radiation at the earth’s surface, 1979–2000. [Google Scholar]
- 55.Al-Delaimy WK, Jansen EHJM, Peeters PHM, et al. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers. 2006;11:370–382. doi: 10.1080/13547500600799748. [DOI] [PubMed] [Google Scholar]
- 56.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 57.Fraser GE. A search for truth in dietary epidemiology. Am J Clin Nutr. 2003;78:521–525. doi: 10.1093/ajcn/78.3.521S. [DOI] [PubMed] [Google Scholar]
- 58.Bischoff-Ferrari HA, Dawson-Hughes B. Where do we stand on vitamin D? Bone. 2007;41:S13–S19. doi: 10.1016/j.bone.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr. 2008;88:507–510. doi: 10.1093/ajcn/88.2.507S. [DOI] [PubMed] [Google Scholar]
- 60.Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D:A vitamin D metabolite to be reckoned with? Ann Nutr Metab. 2003;47:107–113. doi: 10.1159/000070031. [DOI] [PubMed] [Google Scholar]
- 61.Holden JM, Lemar LE, Exler J. Vitamin D in foods: development of the US Department of Agriculture database. Am J Clin Nutr. 2008;87:1092–1096. doi: 10.1093/ajcn/87.4.1092S. [DOI] [PubMed] [Google Scholar]
- 62.Dlugos DJ, Perrotta PL, Horn WG. Effects of the submarine environment on renal-stone risk factors and vitamin D metabolism. Undersea Hyperb Med. 1995;22:145–152. [PubMed] [Google Scholar]
- 63.Holick MF. Vitamin D: biosynthesis, metabolism, and mode of action. In: DeGroot LJ, Cahill GF Jr, Martini L, et al., editors. Endocrinology. Grune and Stratton; New York: 1989. pp. 902–926. [Google Scholar]
- 64.Kobayashi T, Okano T, Shida S, et al. Variation of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 levels in human plasma obtained from 758 Japanese healthy subjects. J Nutr Sci Vitaminol (Tokyo) 1983;29:271–281. doi: 10.3177/jnsv.29.271. [DOI] [PubMed] [Google Scholar]