SUMMARY
Germline mutations in the RAS/ERK signaling pathway underlie several related developmental disorders collectively termed neuro-cardio-facial-cutaneous (NCFC) syndromes. Patients with these disorders manifest varying degrees of cognitive impairment, but the developmental basis of their brain abnormalities remains largely unknown. Among NCFC syndromes, neurofibromatosis type 1 (NF1) is an exception, as it is caused by loss-of-function heterozygous mutations. Here, we show that bi-allelic Nf1 inactivation promotes Erk-dependent, ectopic Olig2 expression specifically in transit-amplifying progenitors, leading to increased gliogenesis at the expense of neurogenesis in neonatal and adult subventricular zone (SVZ). Nf1-deficient brains exhibit enlarged corpus callosum - a structural brain defect recently linked to severe learning deficits in NF1 patients. Strikingly, these NF1-associated developmental defects are rescued by transient treatment with an MEK/ERK pathway inhibitor during neonatal stages. These studies reveal a critical role for Nf1 in maintaining postnatal SVZ-derived neurogenesis, and identify a potential therapeutic window for treating NF1-associated brain abnormalities.
Keywords: Neurofibromatosis type 1, NF1, tumor suppressor gene, neural stem cells, glial cells, subventricular zone (SVZ)
INTRODUCTION
RAS genes are the key intracellular mediators that transmit signals from cell surface receptors to downstream effector pathways, regulating cell proliferation, survival and differentiation (Bos, 1989; Downward, 2003). One of the major RAS-regulated downstream effector pathways is mediated by the extracellular-signal regulated kinase (ERK) subfamily of mitogen-activated protein kinases (MAPK), which is comprised of a kinase cascade of RAF, MEK and ERK (Rubinfeld and Seger, 2005). Somatic activating mutations in RAS and RAF are among the most frequent gain-of-function alterations in human cancer, highlighting a critical role for the RAS-RAF-MEK-ERK pathway in human oncogenesis (Bos, 1989; Downward, 2003). Recent studies have revealed that germline mutations in the components of the RAS/ERK pathway are associated with a group of clinically related developmental disorders including Noonan, LEOPARD, cardio-facio-cutaneous (CFC), Costello, Legius and neurofibromatosis type 1 (NF1) syndromes, which has been collectively referred to as neuro-cardio-facio-cutaneous (NCFC) syndromes or “RASopathies” (Bentires-Alj et al., 2006; Samuels et al., 2009; Schubbert et al., 2007; Tidyman and Rauen, 2009). One of the most important unknown aspects of NCFC syndromes is the mechanism(s) underlying the substantial neurocognitive burden of these diseases. Particularly, the developmental basis of brain abnormalities in NCFC syndrome patients remains largely unknown.
Most NCFC syndromes are caused by dominant activating mutations in the RAS/ERK signaling pathway in the germline. However, NF1 is arguably an exception, as it is caused by germline recessive loss-of-function mutations in one of the two NF1 alleles (NF1+/-) (Bentires-Alj et al., 2006; Samuels et al., 2009; Schubbert et al., 2007; Tidyman and Rauen, 2009). The NF1 gene encodes a RAS GTPase activating protein (GAP) neurofibromin, which promotes the conversion of an active RAS-GTP bound form to an inactive RAS-GDP form, thus functioning as a negative regulator of RAS/ERK signaling (Cichowski and Jacks, 2001). Between 30-70% of NF1 patients have learning disabilities - the most significant cause of lifetime morbidity associated with these patients (Hyman et al., 2005). Recent studies using genetically engineered mouse (GEM) models have shown that heterozygous Nf1 inactivation (Nf1+/-) causes behavioral abnormalities with similarities to learning disabilities in NF1 patients (Cui et al., 2008). However, Nf1+/- mice exhibit no evidence of structural brain defects such as enlarged corpus callosum (CC), which has been recently associated with severe learning disabilities observed in a subpopulation of NF1 patients (Moore et al., 2000; Pride et al., 2010). The CC is comprised of neuronal fibers that connect the two brain hemispheres, as well as the two primary glial cell types, oligodendrocytes that form myelin of axons and astrocytes that provide trophic and functional support for neurons (Rowitch and Kriegstein, 2010). The clinical observations that only a subpopulation of NF1 patients exhibit an enlarged CC argue that mono-allelic NF1 inactivation (NF1+/-) is not sufficient to induce these structural brain defects. It has recently been shown that some NF1-related non-tumor manifestations such as skin hyperpigmentation and bone abnormalities result from bi-allelic NF1 inactivation (NF1-/-) caused by “second-hit” mutations in the remaining wild-type NF1 alleles of somatic cells (De Schepper et al., 2008; Stevenson et al., 2006). Accordingly, we hypothesize that the structural brain defects such as the enlarged CC observed in a subset of NF1 patients could be caused by bi-allelic NF1 inactivation in developing neural stem cells. Compared to other cells, stem cells have greater potentials for self-renewal and consequently, one or few NF1-/- stem cells could produce a greater number of progeny that manifest clinical symptoms (Kriegstein and Alvarez-Buylla, 2009). Therefore, NF1-associated structural brain abnormalities may provide a unique opportunity to define the timing and cellular targets of somatic “second-hit” NF1 mutations and more importantly, the phenotypic consequences of hyperactive RAS/ERK signaling in developing stem cell lineages underlying these brain abnormalities.
Most glial cells in the CC arise from neural progenitor cells that are specified by a basic helix-loop-helix transcription factor, Olig2, in the subventricular zone (SVZ) of the lateral ventricle during perinatal stages (Marshall et al., 2005; Richardson et al., 2006; Rowitch and Kriegstein, 2010). Olig2 is both sufficient and necessary for specifying SVZ progenitors to adopt glial fates. Olig2 promotes their differentiation to both oligodendrocytes and astrocytes during neonatal stages, but only to oligodendrocytes in adulthood (Cai et al., 2007; Hack et al., 2005; Marshall et al., 2005; Menn et al., 2006). Loss of Olig2 leads to a nearly complete absence of glial cells including both oligodendrocytes and astrocytes in the postnatal CC (Cai et al., 2007). During mouse embryonic development, the SVZ initially is comprised of neuron-restricted progenitors, also known as transit-amplifying progenitors or intermediate progenitor cells (TAP/IPCs), which express Tbr2 and give rise to excitatory projection neurons in the cerebral cortex from embryonic day 14.5-17.5 (E14.5-17.5) (Molyneaux et al., 2007). When neurogenesis ceases at E17.5 in the cerebral cortex, gliogenesis ensues and persists into postnatal stages. However, one multipotent neural stem cell population with neurogenic activity in the SVZ persists into adulthood and this region forms the largest germinal zone in the adult brain (Ihrie and Alvarez-Buylla, 2011; Kriegstein and Alvarez-Buylla, 2009). The SVZ stem cell lineage is organized in a hierarchy: type B cells (SVZ-B) are multipotent stem cells expressing glial fibrillary acidic protein (GFAP), which give rise to type C cells (SVZ-C) that are multipotent TAP/IPCs. The SVZ-C TAP/IPCs subsequently differentiate into two lineage-restricted progenitors: (1) neuron-restricted type A neuroblasts (SVZ-A) that migrate along rostral migratory stream (RMS) and differentiate into inhibitory neurons in the olfactory bulb (OB) and (2) glia-restricted progenitors that migrate and differentiate into glial cells populating the overlying CC (Ihrie and Alvarez-Buylla, 2011; Kriegstein and Alvarez-Buylla, 2009). The cellular output of neonatal and adult SVZ stem and progenitor cells is dramatically different. While neonatal SVZ cells simultaneously produce a large number of neurons and glial cells in the OB and CC, respectively, adult SVZ cells predominately generate neurons in the OB (>90%) (Hack et al., 2005; Marshall et al., 2005; Menn et al., 2006). The mechanism(s) by which postnatal SVZ stem and progenitor cells suppress Olig2 expression and maintain high levels of neurogenesis in an otherwise gliogenic environment of the postnatal brain is not well understood.
RESULTS
Bi-allelic inactivation of Nf1 leads to an enlarged corpus callosum
To determine whether bi-allelic inactivation of Nf1 leads to structural brain defects observed in a subpopulation of NF1 patients, we targeted an Nf1 conditional mutation into radial glia by using a Cre transgenic strain under the control of the human GFAP promoter (hGFAP-cre) (Zhu et al., 2001; Zhuo et al., 2001). During embryonic development, radial glia are the primary neural stem cell populations, which give rise to neurons, glia, and adult neural stem cells in the SVZ (Kriegstein and Alvarez-Buylla, 2009). The onset of hGFAP-cre expression occurs in ~95% of E12/13 radial glia in the forebrain, thus inactivating Nf1 in both embryonic and adult neural stem cells as well as their differentiated progeny (Malatesta et al., 2003). Nf1 conditional knockout mice with the genotypes of hGFAP-cre+;Nf1flox/flox or hGFAP-cre+;Nf1flox/- were referred to as Nf1hGFAPCKO (Nf1-/-), as both genotypes showed similar phenotypes analyzed in this study. Germline (Nf1+/-) or conditional (hGFAP-cre+;Nf1flox/+) heterozygous mice exhibited no overt structural or cellular defects in the brain, indicating that mono-allelic Nf1 inactivation is not sufficient to induce the structural brain defects observed in human NF1 patients. In contrast, Nf1 conditional bi-allelic inactivation caused a 10-15% increase in the size of cerebral cortical surface area, a manifestation often observed in human NF1 patients (Figure 1A, B). We next measured and compared the size of the CC at three different sagittal positions along the midline to lateral hemisphere of control and Nf1hGFAPCKO brains at 2 months of age (Figure 1C, C’). At all three positions that are directly associated with the SVZ of the lateral ventricle (P1-P3), Nf1 inactivation caused a significant increase in the thickness of the CC (Figure 1D, E; S1A-C). Of note, the CC from the midline section, which is not directly associated with the SVZ, showed no increase in thickness (Figure 1E; S1D). These observations demonstrate that bi-allelic, but not heterozygous, inactivation of Nf1 leads to an enlarged CC specifically associated with the SVZ.
Figure 1. Nf1-deficient adult brain exhibits enlarged corpus callosum and reduced olfactory bulb.
(A, A’, B) Whole-mount images and size quantification of control and Nf1hGFAPCKO brains and OB. (C, C’) Schematic demonstration of different histological planes used for analysis and the SVZ/RMS/CC/OB system and the orientation of brains analyzed (A, anterior; P, posterior; D, dorsal; V, ventral). (D) Sagittal sections from control and Nf1hGFAPCKO brains at 2 months were analyzed at P2 position, stained with hematoxylin & eosin (H&E) and imaged at 3 increasingly higher magnifications. The cells that form chain-like structures (arrowheads) were exclusively observed in the mutant CC. (E) The thickness of the control and mutant CC was quantified. (F, G) Coronal sections from control and mutant brains were stained with NF200/Olig2 and MBP/Olig2. Lower panels in (F) are the high magnification view of delineated CC areas in the upper panels. (H, I) Ultrathin sections were subjected to EM analysis from dorsal to ventral CC. Representative EM images from two histological planes are shown in (H) and (I). * indicates degenerating tissues only observed in the mutant CC. (J, K) Two sets of EM images covering the entire thickness of the CC at two parallel positions (column 1 and column 2) at comparable histological planes were analyzed. The total number of axons (J) and cell bodies (K) in each column from 3 controls and 3 mutants were quantified. (L) Total cell density in the CC was quantified and compared at P1, P2, P3 and M positions based on H&E-stained sections. (M) Control and mutant CC were stained for GFAP/Olig2, and their density was quantified in (N). The number of cells expressing both GFAP and Olig2 (arrows, M) was significantly increased in mutant CC (O). Dashed lines delineate the border of the CC. All the quantification data are presented as mean ± SEM. LV, *, lateral ventricle. Scale bars: 50 μm except for (H) and (I) at 2 μm. See also Figure S1.
To determine the mechanism underlying the enlarged CC, we first examined whether there was an increase in the number of neurons in the cerebral cortex that project their axons to the CC (Molyneaux et al., 2007; Richards et al., 2004). The number of NeuN+ cortical neurons, Ctip2+ deep- or Cux1+ upper-layer neurons was not significantly different between control and Nf1hGFAPCKO brains (Figure S1E-E”). Second, we examined whether there was an increase in the number of axons in the Nf1hGFAPCKO CC. From the coronal sections, we confirmed that the enlargement of the CC occurred specifically in the areas directly associated with the SVZ, but not in the midline (Figure 1F; S1F, F’). Compared to controls, neurofilament 200 (NF200, an axonal marker) staining revealed a more diffused and reduced expression pattern in the Nf1hGFAPCKO CC (Figure 1F). At the ultrastructural level, only a marginal, but not statistically significant, increase in the number of axons was observed in the Nf1hGFAPCKO CC compared to that of controls (Figure 1H-J; S1G, H). In contrast, the number of cell bodies was significantly increased on the same sections analyzed by electron microscopy (EM) (Figure 1K). Consistently, a robust increase in both the absolute number and density of cells in the SVZ-associated CC of Nf1hGFAPCKO brains was observed on thicker sections compared to controls (Figure 1D, L; S1A-C). The increased number in GFAP+ and Olig2+ glial lineage cells appeared to be the major contributing factor for the increased cellular density (Figure 1M-O). Together, these results suggest that an increase in the number of glial cells, but not the number of axons or cortical neurons, is mainly responsible for the enlarged SVZ-associated CC in Nf1hGFAPCKO brains. In support of this conclusion, no CC alteration was observed in neuron-specific Nf1 conditional knockout mice driven by Synapsin I-cre (Figure S1F”) (Zhu et al., 2001). It is worth noting that despite relatively normal appearance of myelination (Figure 1G), varying degrees of tissue degeneration was observed in the CC of Nf1hGFAPCKO brains (Figure 1I).
Nf1-deficient adult brain exhibits an enlarged SVZ with increased glial differentiation
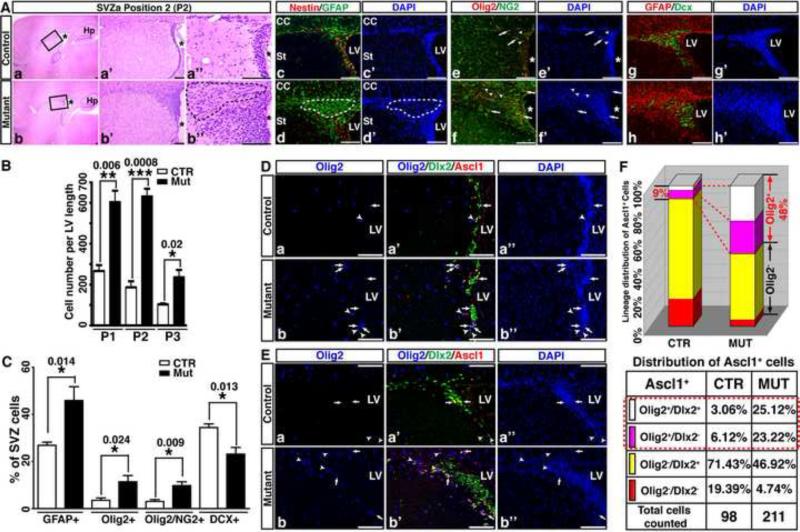
Despite a disproportionally enlarged SVZ-associated CC, one discernible structural difference between control and Nf1hGFAPCKO forebrains was the significantly reduced size of the OB, the destination of SVZ-derived neurons (Figure 1A, A’). The opposite size alterations observed in the two brain structures related to SVZ-derived gliogenesis and neurogenesis raise the possibility that these phenotypes could result from functional impairments of Nf1-deficient SVZ stem and progenitor cells. We therefore analyzed the SVZ from these mice at the same three positions used for the CC studies. At each of these three positions, Nf1hGFAPCKO SVZ exhibited a 2- to 3-fold size increase compared to controls (Figure 2Aa-b”, 2B; S2A-C). To investigate the relative contribution of SVZ cells to its increased size, we determined the number of individual SVZ cell populations by using lineage markers. Compared to controls, the percentage of SVZ cells expressing glial-lineage markers including GFAP, Olig2 and NG2 was markedly increased with a concomitant reduction in Doublecortin (Dcx)-expressing neuroblasts in the Nf1hGFAPCKO SVZ (Figure 2Ac-h’, 2C). Furthermore, a greater number of GFAP+ cells accumulated and formed a less cellular area in the anterior-dorsal part of the Nf1hGFAPCKO SVZ, which was not identified in the control SVZ (dotted areas in Figure 2Ab’’, 2Ad,d’; S2A). These GFAP+ cells exhibited the features of differentiated astrocytes including the presence of multiple long cellular processes, and a lack of expression of stem/progenitor marker Nestin or Ki67 (Figure 2Ad, S3A). Together, these results demonstrate that Nf1 inactivation leads to an enlarged SVZ with accumulation of differentiated glial cells.
Figure 2. Nf1hGFAPCKO SVZ shows increased glial differentiation and ectopic Olig2 expression in Ascl1+ TAP/IPCs.
(A) Representative sections of the control and mutant SVZ at P2 position were stained with H&E and imaged at 3 magnifications (a-b”). The high magnification views of the boxed area in (a) and (b) are shown in (a’, a’’) and (b’, b’’), respectively. Adjacent sections were stained for Nestin/GFAP (c, d), Olig2/NG2 (e, f) and GFAP/Dcx (g, h). Arrows identify Olig2+/NG2+ cells and arrowheads mark Olig2+/NG2- cells (e-f’). (B) The number of cells per unit length of LV at P1-P3 was quantified. (C) The percentage of SVZ cells expressing different lineage markers (Ac-h’) was quantified. (D, E) Olig2/Dlx2/Ascl1 triple immunofluorescence labeling was performed on sections from control and mutant brains. In the ventral (D) and dorsal (E) SVZa, Olig2+/Dlx2+/Ascl1+ triple-positive cells (white) and Olig2+/Dlx2-/Ascl1+ double-positive cells (pink) are marked by arrows and arrowheads, respectively. (F) The percentage of Ascl1+ cells expressing Olig2 and/or Dlx2 was quantified and compared between the control and mutant SVZ. All the quantification data are presented as mean ± SEM. LV and *, lateral ventricle. St, striatum. Scale bars: 50 μm. See also Figures S2 and S3.
Nf1 inactivation impairs the balance of glial versus neuronal output in the adult SVZ
Abnormally increased numbers of glial lineage cells observed in the Nf1hGFAPCKO SVZ/CC system could result from an increase in proliferation or a reduction in apoptosis of SVZ stem and/or progenitor cells as expected from loss of Nf1 tumor suppressor function. To investigate this possibility, we performed an in vivo bromodeoxyuridine (BrdU) labeling assay and in vitro neurosphere assays (Figure S3 and see Supplemental text for details). These studies demonstrate that alterations in proliferation or apoptosis in adult Nf1hGFAPCKO SVZ unlikely contribute to increased glial lineage cells in the enlarged SVZ. We next investigated whether Nf1 inactivation altered glial versus neuronal fate-specification by examining Olig2 expression in the SVZ stem cell lineages. No Olig2 expression was observed in GFAP+ SVZ-B stem cells or lineage-restricted Dcx+ SVZ-A neuroblasts in the adult control or Nf1hGFAPCKO SVZ (Figure S2E and data not shown). Previous studies showed that Ascl1+ progenitor cells in the embryonic and neonatal SVZ selectively express the Dlx1/2 homeodomain transcription factors and Olig2, directing neural stem and progenitor cells to adopt neuronal and glial fates, respectively (Marshall et al., 2005; Parras et al., 2004; Petryniak et al., 2007). Consistent with the previous studies indicating that glial output of adult SVZ stem cells is minor (5-10%) (Hack et al., 2005; Menn et al., 2006), approximately 9% of multipotent Ascl1+ TAP/IPCs expressed Olig2 in the control adult SVZ (Figure 2D-F). Strikingly, nearly half of Ascl1+ TAP/IPCs expressed Olig2 in the Nf1hGFAPCKO SVZ (48.3%, Figure 2D-F). These results are most consistent with a model wherein Nf1 inactivation promotes ectopic expression of Olig2 specifically in SVZ-C TAP/IPCs that normally undergo neuronal differentiation, leading to an increased output of glial cells at the expense of neuronal differentiation. This hypothesis is supported by the observation that Nf1 inactivation leads to an enlarged CC with a concomitant reduction in OB size.
To further test this hypothesis, we performed a long-term BrdU pulse-chase experiment on 2-month-old control and Nf1hGFAPCKO mice. Thirty days after BrdU pulses, we analyzed the number and the identity of newly generated SVZ-derived BrdU+ cells in the SVZ/RMS/OB/CC system. Compared to control brains, there was a more than two-fold increase of BrdU+ cells in the SVZ/RMS/CC and surrounding areas of Nf1hGFAPCKO brains (Figure 3A, B). All of the BrdU+ cells in the CC of both control and Nf1hGFAPCKO brains expressed Olig2, but not other lineage markers including GFAP, Pax6 or Dcx (Figure 3C and data not shown). The rate of proliferation or apoptosis of Olig2+ cells was not significantly different between the control and mutant CC (Figure 3D, D’ and data not shown). These results indicate that the increase of glial cells in the Nf1hGFAPCKO CC is unlikely contributed by locally increased proliferation or reduced apoptosis in the mutant CC. Strikingly, we observed a 9-fold increase of BrdU+ cells in the Nf1hGFAPCKO RMS compared to controls (Figure 3E, F). While a small number of BrdU+ cells observed in the control RMS were Pax6- or Dcx-expressing migrating neuroblasts, over half of the BrdU+ populations in the Nf1hGFAPCKO RMS expressed Olig2 or GFAP (Figure 3E-G). Consistently, a nearly 3-fold increase in Olig2- and NG2-expressing cells was observed in the Nf1hGFAPCKO RMS, some of which expressed myelin basic protein (MBP) (Figure S4A-C). The abnormal myelination in the mutant RMS may contribute to the progressive disruption of neuronal migratory chain over time (Figure S4D). Consistently, Pax6+/BrdU+ migrating neuroblasts were accumulated and significantly increased in the Nf1hGFAPCKO RMS compared to controls (Figure 3E, F). In contrast to a marked increase in gliogenesis in the CC and RMS, the number of BrdU+ cells in the OB of Nf1hGFAPCKO mice was greatly reduced compared to controls (Figure 3A). Most of the BrdU+ cells in the control OB were newly generated NeuN+ neurons, which were reduced by 50% in the mutant OB (Figure 3H, H’). No significant difference in the BrdU+/NeuN- populations was observed, indicating that local gliogenesis in the OB was not affected by Nf1 inactivation. Taken together, these observations demonstrate that Nf1 plays a critical role in regulating glial versus neuronal output in the adult SVZ stem cell niche by suppressing Olig2 expression in Ascl1+ SVZ-C TAP/IPCs.
Figure 3. Nf1 inactivation leads to increased gliogenesis in the CC at the expense of neurogenesis in the OB.
A BrdU pulse-chase assay from P60-P90 was performed to label newly differentiated neurons and glia. (A) Low magnification view and (B) the quantification of the overall distribution of BrdU+ cells in P90 control and mutant brains. Arrows identify BrdU+ cells in the CC and arrowheads mark the abnormal increase of BrdU+ cells in the mutant RMS. The dashed line marks the border of the OB and the forebrain. (C) Increased Olig2+/BrdU+ cells (arrows) were observed in the mutant CC compared to controls. (D, D’) A short-term BrdU proliferation assay was performed on P60 mice. (E) 30 days after BrdU pulse, the identity of BrdU+ cells in the RMS was revealed by triple or double labeling of BrdU with Pax6/GFAP (a, b), Dcx/GFAP (c, d), or Olig2 (e, f). Arrows and arrowheads: BrdU double- and single-labeling cells. (F, G) The number and ratio of BrdU+ cells that coexpressed Pax6, GFAP or Olig2 in the control and mutant RMS was quantified. (H) Sections of the control and mutant OB were stained for BrdU, NeuN and DAPI. The number of BrdU+/NeuN+ (arrows) and BrdU+/NeuN- cells per OB surface area was quantified in (H’). All the quantification data are presented as mean ± SEM. *, lateral ventricle. Scale bars: 50 μm. See also Figure S4.
Acute inactivation of Nf1 is sufficient to induce glial/neuronal fate switch in the adult SVZ
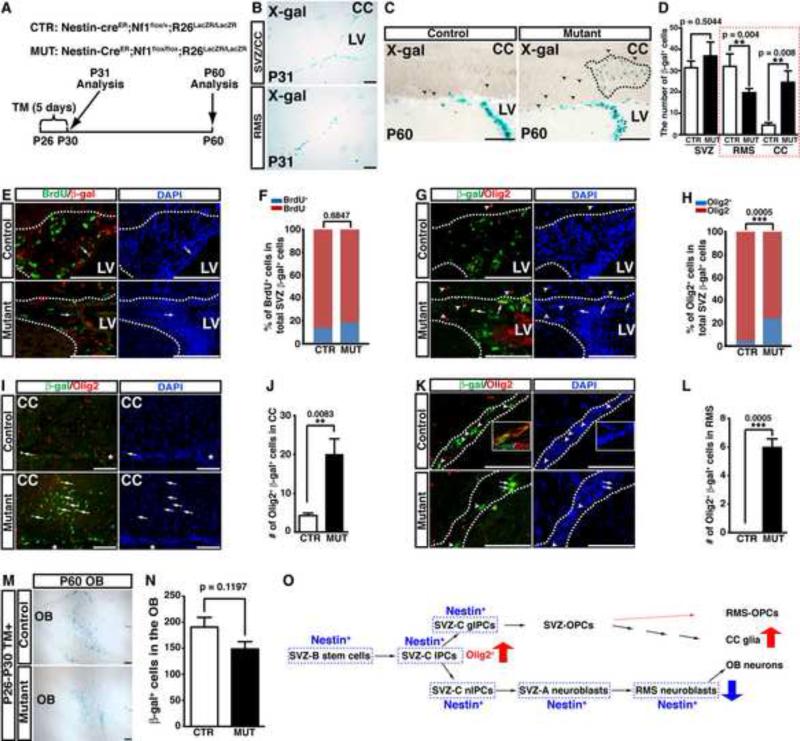
To determine whether abnormal glial/neuronal fate-specification reflects an ongoing cell-autonomous requirement for Nf1 function in adult SVZ progenitors or abnormal SVZ niche environment, we employed an inducible system in which Cre recombinase (Nestin-creER) can be activated in Nestin-expressing cells by tamoxifen (TM) treatment (Figure S5) (Burns et al., 2007). To target adult SVZ cells, we established a 5-day TM induction protocol from postnatal day 26 (P26) to P30 (Figure 4A). TM-induced Nestin-creER -mediated recombination specifically targets neural stem and progenitor cells in the SVZ as well as neuroblasts in the RMS, but not the CC or OB of the adult brain (Figure 4B). This genetic approach allowed us to perform long-term lineage-tracing analysis for SVZ-derived glial cells in the CC and neurons in the OB, as no cells in these two structures were directly targeted by this Nestin-creER transgene (Figure 4O). We injected TM into control and Nf1NcreERCKO mice from P26 to P30, and analyzed the distribution and identity of Nestin-creER-targeted SVZ/RMS-derived cells one month later. At P60, the number of control and Nf1-deficient SVZ cells, marked by β-gal expression, was not significantly different between TM-treated control and Nf1NcreERCKO mice (Figure 4C, D). Moreover, the percentage of proliferating BrdU+ cells in the β-gal+ cell populations of the control and Nf1NcreERCKO SVZ was also comparable, confirming that Nf1 inactivation confers no growth advantage to adult SVZ cells (Figure 4E, F). However, the percentage of β-gal+ cells expressing Olig2 was greatly increased, indicating that acute inactivation of Nf1 in the normal SVZ niche is sufficient to induce ectopic expression of Olig2 in adult SVZ cells (Figure 4G, H). Consistently, β-gal+ cells that all expressed Olig2 were dramatically increased and often formed clusters in the CC of TM-treated Nf1NcreERCKO brains (Figure 4C, D, I, J). Together, these results demonstrate that acute Nf1 inactivation promotes ectopic Olig2 expression in adult SVZ cells, and subsequently enhances SVZ-derived gliogenesis in the CC.
Figure 4. Acute inactivation of Nf1 induces a glia/neuron fate switch in the adult SVZ.
(A) The summary of the genotypes of inducible mouse models and the time frame of tamoxifen (TM) induction and analysis. (B) Young adult mice were induced by TM from P26-P30 and analyzed at P31. The cells undergoing Cre-mediated recombination revealed by X-gal staining were restrictedly distributed in the SVZ and RMS. (C-N) Control and mutant brains induced by TM from P26-P30 were analyzed at P60. (C) X-gal staining of the SVZ/RMS/CC system shows the cluster of β-gal+ cells (dashed lines) exclusively identified in the mutant CC. Arrowheads identify individual β-gal+ cells in the CC. (D) The number of β-gal+ cells in the control and mutant SVZ/RMS/CC was quantified. (E, F) The proliferation rate of β-gal+ cells marked by BrdU staining was not significantly different between the control and mutant SVZ. (G-L) The β-gal+ cells coexpressing Olig2 in the control and mutant SVZ (G, H), CC (I, J) and RMS (K, L) are identified by arrows and quantified. Arrowheads label non-colocalizing cells. The inset in (K) shows the coexpression of β-gal and Dcx in the control RMS. Dashed lines delineate the SVZ and/or RMS region. (M) The OB from TM-induced control and Nf1NcreERCKO brains was stained with X-gal and compared. The number of β-gal+ cells was quantified (N). (O) An illustration summarizes the cell lineages targeted by Nestin-creER and their respective derivatives. All the quantification data are presented as mean ± SEM. LV, lateral ventricle. Scale bars: 50 μm. See also Figure S5.
Consistent with the notion of the Nf1-deficiency dependent glial/neuronal fate switch, the number of β-gal+ cells in the RMS was significantly reduced in TM-induced Nf1NcreERCKO brains (Figure 4D). Moreover, a subpopulation of the β-gal+ cells expressed Olig2 in the mutant RMS whereas all the β-gal+ cells in the control RMS were Dcx+/Olig2- neuroblasts (Figure 4K, L). These results suggest that Nf1 inactivation not only increases SVZ-derived gliogenesis in the CC, but also alters the migratory pattern of some glial progenitor cells that abnormally enter into the RMS. Since it requires about 14 days for a neuroblast to complete its migration from the SVZ to OB (Ihrie and Alvarez-Buylla, 2011), the β-gal+ neuroblasts observed in the RMS 30 days after TM induction would be exclusively derived from multipotent neural stem and progenitor cells in the SVZ (Figure 4K). In contrast, a considerable number of β-gal+ neurons in the OB were derived from Nestin-creER-targeted neuroblasts in the RMS. Thus, a 2-fold decrease in the number of SVZ-derived neuroblasts in the mutant RMS opposed to a marginal reduction in SVZ-derived neurogenesis in the mutant OB could reflect the fact that Nf1 has a dispensable role in neuroblast migration and differentiation (Figure 4M-O). Collectively, these observations demonstrate that acute inactivation of Nf1 confers no proliferative advantage to adult SVZ cells, but instead alters glial/neuronal fate-specification by promoting Olig2 expression in adult SVZ progenitor cells in a cell-autonomous manner.
Nf1 suppresses Olig2 expression in neonatal SVZ progenitor cells
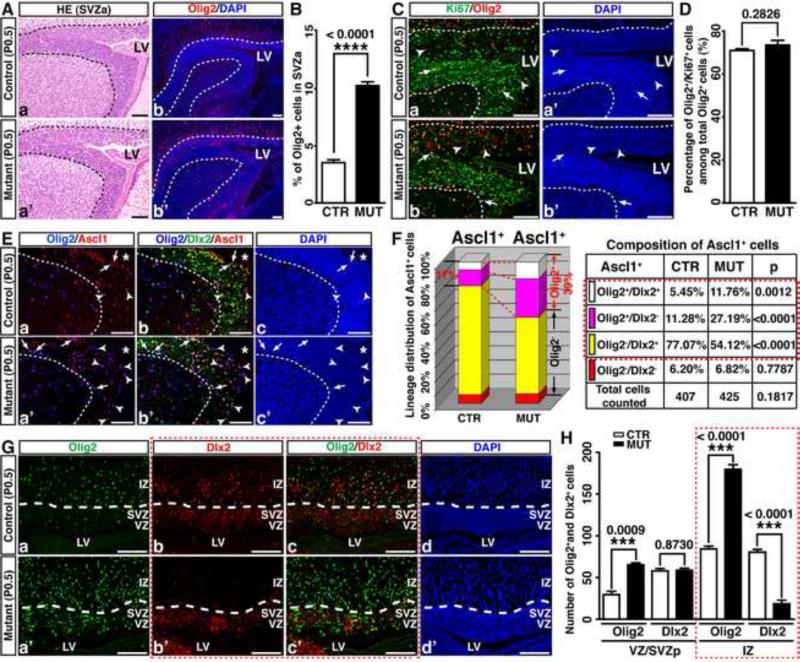
Despite an ongoing requirement for Nf1 function in regulating fate-specification of adult SVZ progenitor cells, acute inactivation of Nf1 in adult SVZ cells did not produce any of the structural defects (e.g., enlarged CC or SVZ) observed in Nf1hGFAPCKO brains. We therefore sought to investigate the timing and mechanism(s) by which Nf1 inactivation leads to impaired fate-specification of SVZ cells and enlarged CC during development. We measured the size and cell number of the anterior SVZ (SVZa) and posterior SVZ (SVZp), and no significant difference was identified between control and Nf1hGFAPCKO forebrains at birth (Figure 5Aa, a’; Figure S6A, D). The overall rate of proliferation or apoptosis in the SVZ was not significantly altered (Figure S6B, C). Compared to controls, however, one striking phenotype observed was a nearly 3-fold increase in the number of cells that expressed Olig2 in the Nf1hGFAPCKO SVZa (Figure 5Ab,b’, 5B). No increase in the percentage of Ki67+ proliferating cells in the Olig2+ lineage was observed, indicating that Olig2+ cells were not hyperproliferative in the Nf1hGFAPCKO SVZa (Figure 5C, D). Given no alteration in proliferation or apoptosis observed in the Nf1hGFAPCKO SVZa, we investigated the possibility of whether Nf1 inactivation leads to ectopic Olig2 expression in SVZa stem and progenitor cells in neonatal brains. Although the number of Ascl1+ cells was comparable between control and Nf1hGFAPCKO SVZa (p = 0.104), the percentage of Olig2-expressing cells in Ascl1+ populations (Olig2+/Ascl1+) was greatly increased from 16.7% in controls to 38.7% in mutants (Figure 5E, F). Concomitantly, the Dlx2+/Ascl1+ progenitor populations were reduced from 82.5% to 65.3% (Figure 5E, F). Moreover, the percentage of Ascl1+ cells that expressed both Olig2 and Dlx2 was increased from 5.5% to 11.8% (Figure 5Eb, b’, 5F). These observations suggest that similar to its role in the adult SVZ, Nf1 inactivation promotes ectopic Olig2 expression in Ascl1+ SVZ progenitors in neonatal stages. Similarly, Nf1 inactivation leads to ectopic Olig2 expression in the SVZp, which suppresses Dlx2 expression as these cells become more differentiated and migrate to the IZ, leading to overproduction of Olig2+ lineage cells at the expense of the Dlx2+ lineages (Figure 5G, H; S6E-K and see Supplemental text for details).
Figure 5. Nf1 inactivation leads to ectopic expression of Olig2 in neural stem/progenitor cells in neonatal brains.
(A) Sections from P0.5 control and Nf1hGFAPCKO brains were stained with H&E (a, a’) and Olig2 (b, b’). (B) The percentage of Olig2+ cells in the anterior SVZ (SVZa) was quantified. (C, D) The proliferation rate of Olig2+ cells was not significantly different between the control and mutant SVZa. Arrows and arrowheads: Olig2+/Ki67+ and Olig2+/Ki67-cells. (E, F) The percentage of Ascl1+ cells coexpressing Olig2 and/or Dlx2 in the control and mutant SVZa was analyzed and quantified. Arrows and arrowheads: Olig2+/Dlx2+/Ascl1+ triple-positive cells and Olig2+/Dlx2-/Ascl1+ double-positive cells. (G, H) The expression of Olig2 and Dlx2 in the posterior control and mutant VZ/SVZ and IZ was analyzed and quantified. Dashed lines demarcate the SVZa (A, C, E) or VZ/SVZ regions from the IZ (G). All the quantification data are presented as mean ± SEM. LV and *, lateral ventricle. IZ, intermediate zone. Scale bars: 50 μm. See also Figure S6.
Nf1-related structural brain defects are established during neonatal development
We observed no significant difference in the size of control and Nf1hGFAPCKO brains including OB at P0.5 and P8 (Figure 6A-C). However, by P18, the size of the mutant OB was significantly reduced to a similar degree as that observed in the adult (Figure 6D; S8E). Notably, neuronal clusters, which could be readily identified in the control OB, were largely absent in mutants (Figure 6E). These observations suggest that the glial/neuronal fate-specification defect caused by Nf1 inactivation is largely established during postnatal stages from P8 to P18. To test this, we pulsed control and Nf1hGFAPCKO mice with BrdU at P8, and analyzed the BrdU-treated mice at P18. SVZ-derived newborn neurons labeled by BrdU and NeuN (BrdU+/NeuN+) were markedly reduced in the Nf1hGFAPCKO OB compared to controls (Figure 6E-G). It is worth noting that the number of BrdU+ cells coexpressing the neuronal-lineage marker Pax6 was not significantly different between the control and Nf1hGFAPCKO RMS (Figure S7A, B). This observation suggests that in contrast to adulthood, the reduced neurogenesis in the neonatal mutant OB is unlikely contributed by migratory defects in the RMS. Compared to controls, the number of BrdU+ cells was increased by more than 3-fold in the Nf1hGFAPCKO RMS and CC, where most of the BrdU+ cells expressed glial markers, GFAP and Olig2 (Figure 6H-J; S7B-D). These observations demonstrate that Nf1 inactivation leads to increased gliogenesis at the expense of neurogenesis in the neonatal SVZ/RMS/OB/CC system. Interestingly, the ratio of newly generated GFAP+/BrdU+ astrocytes and Olig2+/BrdU+ oligodendrocytes was not significantly different in the CC of control and Nf1hGFAPCKO brains, indicating that Nf1 inactivation only promotes overproduction of Olig2+ progenitors, but does not alter the potential of these Olig2+ progenitors to differentiate into astrocytes or oligodendrocytes (Figure 6K) (Cai et al., 2007; Marshall et al., 2005). It is worth noting that the size and cell number of Nf1hGFAPCKO SVZ was already significantly enlarged at P8, and this was accompanied by a transient increase in proliferation of GFAP+ stem cells, but not Olig2+ cells (Figure 6L-R and see Supplemental text for details). Taken together, these results demonstrate that ectopic expression of Olig2 in Ascl1+ progenitors caused by Nf1 inactivation manifests during neonatal stages (Figure 6R), leading to increased gliogenesis in the CC at the expense of neurogenesis in the OB. Consequently, Nf1-related structural brain defects including enlarged CC and reduced OB are both established during early postnatal stages.
Figure 6. Reduced neurogenesis is accompanied by increased gliogenesis in Nf1hGFAPCKO brains during neonatal SVZ development.
(A-C) The size of P0.5 (A) and P8 (B) control and mutant OB was measured and quantified from the dorsal view and the lateral view. (D) The size of P18 control and Nf1hGFAPCKO OB was quantified. (E-K) Control and Nf1hGFAPCKO mice were pulsed with BrdU at P8 and analyzed at P18. (E, F) SVZ-derived neurogenesis was analyzed and quantified by BrdU/NeuN double labeling (arrows) in the granule cell layer (GCL) and periglomerular layer (PGL) of the OB. (G) The density of NeuN+ cells in the OB was quantified. (H-K) The number of newly generated astrocytes (BrdU+/GFAP+) and oligodendrocytes (BrdU+/Olig2+) in the control and mutant CC are illustrated (H, I, arrows) and quantified (J). Arrowheads label non-colocalizing cells. (K) Quantification of the proportion of GFAP+ versus Olig2+ cells in the total BrdU+ cell population in the control and mutant CC. (L, M) Proliferating GFAP+ cells are shown (arrows) and quantified in the control and mutant SVZa at P8. (N, O) The quantification of total cells and the percentage of Ki67+ proliferating cells are shown for P8 control and mutant SVZ. (P, Q) proliferating Olig2+ (Olig2+/Ki67+, arrows) and differentiated Olig2+/Ki67- (arrowheads) cells were shown and quantified in control and mutant P8 SVZa. Dashed lines demarcate the SVZ/RMS. (R) Quantification and characterization of Ascl1+ cells in P8 SVZa based on their expression of Olig2 and/or Dlx2. All the quantification data are presented as mean ± SEM. *, lateral ventricle. Scale bars: 1 mm (A, B); 50 μm (E-P). See also Figure S7.
Neonatal Erk pathway inhibition rescues structural brain defects caused by Nf1 inactivation
To investigate the molecular mechanism underlying ectopic Olig2 expression in Nf1-deficient SVZ progenitors, we performed a series of in vitro and in vivo experiments. We showed that activation of Erk signaling pathway, but not phosphatidylinositol 3-kinase (PI3K)/Akt or mammalian target of rapamycin complex 1 (mTORC1), was associated with ectopic Olig2 expression in Ascl1+ TAP/IPCs (Figure S8A-C and see Supplemental text for details). These results suggest that Nf1-mediated negative regulation of Erk signaling suppresses Olig2 expression and consequently, glial fates in neurogenic Ascl1+ TAP/IPCs. To test this model, we treated mice from P0.5-P18 with a potent MEK/ERK pathway inhibitor (MEKi), PD0325901 (Sebolt-Leopold and Herrera, 2004). Despite relatively normal at birth, Nf1hGFAPCKO mice became hunched, scruffy and exhibited growth retardation within a week. Consequently, Nf1hGFAPCKO mice can be readily identified from control littermates from P8 on, and weighed only half as much as control littermates by P18 (Figure 7A, B). Strikingly, this P0.5-P18 treatment protocol completely rescued these phenotypes. For example, the appearance of the MEKi-treated Nf1hGFAPCKO mice was indistinguishable from control littermates at P18 (Figure 7A). In the brain, the level of Erk activation in MEKi-treated Nf1hGFAPCKO mice at P18 was reduced to the level of control SVZ and more importantly, the number of Olig2+ cells in the mutant SVZ was rescued to the level of vehicle-treated controls (Figure 7C-C”). For the Ascl1+ progenitors, the percentage of Olig2+ cells in MEKi-treated Nf1hGFAPCKO SVZ was also reduced to control levels (Figure 7C”). In the CC, the density of Olig2+ cells and GFAP+ cells in MEKi-treated Nf1hGFAPCKO brains, albeit not completely rescued, was reduced to nearly the levels of vehicle-treated controls (Figure 7D-D”). Most importantly, the enlarged CC phenotype was completely rescued in the MEKi-treated Nf1hGFAPCKO brains (Figure 7D, D’). Conversely, neuronal density and the size of the OB were significantly increased in mutants after MEKi treatment, particularly evidenced by the reappearance of neuronal clusters that were only observed in the control OB (Figure 7E, E’; S8E-E”). These MEKi treatment experiments demonstrate a potential therapeutic window for preventing and treating NF1-associated structural brain defects.
Figure 7. Neonatal MEKi treatment rescues brain abnormalities caused by Nf1 inactiviation.
(A) Control and Nf1hGFAPCKO mice were treated with vehicle and MEKi from P0.5-P18 and analyzed at P18. (B) The body weight of treated control and mutant mice was quantified. (C) Triple labeling of p-Erk/Olig2/Ascl1 was performed on brain sections of vehicle-treated control and Nf1hGFAPCKO as well as MEKi-treated Nf1hGFAPCKO mice. Arrows and arrowheads: Olig2+/Ascl1+ and Olig2+/Ascl1- cells. Of note, p-Erk+/Olig2+/Ascl1+ cells were only identified in vehicle-treated mutants. (C’, C’’) The number of p-Erk+ cells and the percentage of Olig2+ cells among total SVZ Ascl1+ cells were quantified among vehicle and MEKi-treated control and mutant mice. (D) GFAP/Olig2 double-labeling of the CC from treated control and mutant mice was performed. Dashed lines mark the border between the CC and cerebral cortex (Ctx). (D’, D”) The thickness of the CC and the density of GFAP+ and Olig2+ cells in the CC of treated control and mutant mice were quantified. NeuN staining in the treated control and mutant OB is shown (E) and quantified (E’). Bottom panels in (E) are the higher magnification view of the boxed areas in the top panels. (F) Proposed model for the mechanism by which Nf1-regulated Erk signaling pathway regulates neuronal/glial fate specification of SVZ progenitors. SVZ progenitor cells most affected by Nf1 inactivation are labeled as red (see main text). All the quantification data are presented as mean ± SEM. Scale bars: 50 μm. See also Figure S8.
DISCUSSION
Given the well-documented role of ERK activity in synaptic transmission and memory formation, pharmacological intervention of RAS/ERK signaling could provide an attractive therapeutic strategy to ameliorate the substantial neurocognitive burden associated with the NCFC syndromes (Davis and Laroche, 2006; Samuels et al., 2009). However, it is unclear to what degree development-related structural brain abnormalities contribute to the cognitive deficits in NCFC syndrome patients. Recent studies have revealed that the structural brain abnormalities, particularly, an enlarged CC, are identified in a subpopulation of NF1 patients with severe learning disabilities (Moore et al., 2000; Payne et al., 2010; Pride et al., 2010). These observations provide strong support for the hypothesis that these structural brain abnormalities could be responsible for the severe forms of cognitive impairment observed in a subset of NCFC patients. Thus, our study elucidates the timing, cellular and molecular mechanism(s) by which hyperactive RAS/ERK signaling leads to the development-related structural brain defects caused by bi-allelic Nf1 inactivation. More importantly, we demonstrate the neonatal stage(s) as a critical therapeutic window for treating these brain abnormalities.
Bi-allelic inactivation as an NF1-related non-tumor disease mechanism
The mechanism underlying the extremely high level of variability in disease manifestation among NF1 patients, even for those with the same germline heterozygous mutations, is poorly understood (Riccardi, 1992). Our study demonstrates that bi-allelic, but not heterozygous, inactivation of Nf1 leads to structural brain defects including enlarged CC. Moreover, our study shows that bi-allelic inactivation of Nf1 in developing, but not adult, neural stem cells, results in an enlarged CC. The results are consistent with the observations that (1) most glial cells in the CC are generated by SVZ stem cells during perinatal stages, and (2) only a minor population of the progeny derived from adult SVZ stem cells contribute to glial cells in the CC (Marshall et al., 2005; Richardson et al., 2006; Rowitch and Kriegstein, 2010). Therefore, these observations suggest that the timing and cellular target(s) of somatic “second-hit” events could profoundly impact on the severity of disease manifestations, thus providing a potential mechanism underlying high levels of disease variability observed among NF1 patients. It has been well established that benign and malignant tumors arising in NF1 patients result from bi-allelic inactivation of NF1 (Cichowski and Jacks, 2001). NF1-/- tumor cells exhibit growth advantage, leading to clonal expansion and thus clinically identifiable diseases in NF1 patients. Similarly, because of the substantial self-renewal capacities possessed by developing stem and progenitor cell populations, a relatively small number of somatic “second-hit” events in these cells could produce a greater number of NF1-/- progeny, leading to more severe phenotypic consequences than those with the “second-hit” events in differentiated cells. It would be of particular interest to determine whether somatic “second-hit” NF1 mutations indeed occur in cells within the enlarged CC from human specimens, as previously demonstrated in other NF1-related non-tumor lesions including skin hyperpigmentation and bone abnormalities (De Schepper et al., 2008; Stevenson et al., 2006). Alternatively, a subset of NF1+/- cells could phenotypically behave like NF1-/- cells when they have aberrantly high activity of a recently identified ubiquitin-mediated NF1 degradation machinery (Tan et al., 2011).
The role Nf1 in SVZ stem and progenitor cells in the brain
During embryonic development, neurogenesis and gliogenesis are temporally regulated and occur sequentially in the developing VZ and SVZ (Kriegstein and Alvarez-Buylla, 2009; Molyneaux et al., 2007). Recent studies using the same hGFAP-cre driver show that Erk1 and Erk2 double knockout mice exhibit a significantly thinner VZ and SVZ and a reduction in the size of the CC, and Erk2 loss results in reduced proliferation of SVZ TAP/IPCs during embryonic cortical development (Samuels et al., 2008; Satoh et al., 2011). These mouse genetic studies support the notion that ERK/Erk signaling pathway has an essential role in both human and mouse embryonic cortical development. However, our study shows that Nf1 has a dispensable role in embryonic cortical development. Specifically, the number of deep- and upper-layer neurons as well as laminar organization of the cerebral cortex appears to be relatively normal in Nf1hGFAPCKO brains. In the neonatal and adult SVZ, neurogenesis and gliogenesis occur simultaneously (Kriegstein and Alvarez-Buylla, 2009). Our study indicates that the percentage of Ascl1+ TAP/IPCs expressing Olig2 is highly correlated with the glial output of neonatal and adult SVZ stem cell niches, ~20% and ~9%, respectively. This observation is consistent with previous studies showing that the regulation of Olig2 expression in the Ascl1+ TAP/IPC compartment appears to be a critical mechanism controlling the balance between gliogenesis and neurogenesis in the postnatal SVZ stem cell niche (Cai et al., 2007; Hack et al., 2005; Marshall et al., 2005; Menn et al., 2006). Although Olig2 expression is activated by sonic hedgehog (Shh) signaling in developing ventral spinal cord and forebrain, the signaling pathway(s) regulating Olig2 is relatively less understood in the dorsal forebrain (Lagace et al., 2007; Lu et al., 2000; Zhou et al., 2000). In cultured neural precursor cells, fibroblast growth factor 2 (FGF2) promotes Olig2 expression in an Erk-dependent, but Shh-independent manner (Bilican et al., 2008; Chandran et al., 2003; Kessaris et al., 2004). In light of these in vitro studies, our study provides compelling in vivo evidence supporting that Nf1-regulated Erk signaling plays a critical role in regulating Olig2 in both neonatal and adult SVZ cells. Based on these observations, we propose that FGF-mediated Ras/Erk signaling pathway induces Olig2 expression, triggering the transition from neurogenesis to gliogenesis at the end of cortical development. The critical role of Nf1 is to inhibit Ras/Erk activity and in turn suppress Olig2 expression in a subset of Ascl1+ TAP/IPCs, thereby ensuring persistent neurogenesis in otherwise gliogenic environment of the perinatal and adult brain (Figure 7F). This model is supported by the observations that hyperactivative Erk signaling and ectopic Olig2 expression in Nf1hGFAPCKO brains are almost exclusively observed in SVZ-C Ascl1+ TAP/IPCs, but not in any other SVZ lineage cells. Thus, our study demonstrates that the regulation of Erk signaling pathway by Nf1 mainly occurs at the stages of SVZ TAP/IPC cells. It is worth noting that Nf1 has functions that are critical for glia and neurons in the other regions of the brain (Hegedus et al., 2007; Lee da et al., 2010; Zhu et al., 2005). Together, these studies suggest that individual ERK pathway components (e.g. NF1) may be rate-limiting only in specific cell types and/or during specific developmental stages, and this could contribute to phenotypic heterogeneity among NCFC syndromes.
Clinical implication – a therapeutic window of opportunity
The most striking finding of this study is the robust therapeutic response when an 18-day MEKi treatment protocol was delivered to neonatal Nf1hGFAPCKO mice, which suffer from structural brain defects (e.g., enlarged CC and reduced OB), weight loss to deteriorating health. Remarkably, this MEKi treatment protocol not only rescues SVZ-associated glial versus neuronal fate-specification defects and the size abnormalities observed in the CC and OB, but also dramatically improve the overall health of Nf1hGFAPCKO mice (Figure 7A). These observations suggest that Nf1-related brain pathologies are mainly caused by hyperactivation of the Erk/MAPK signaling pathway, but not other Ras-mediated signaling pathways (e.g., PI3K/Akt). Furthermore, clinical studies indicate that optic nerve gliomas only develop in younger NF1 children (<6 years) and that nearly half of plexiform neurofibromas are identified in NF1 children younger than 5 years (Listernick et al., 1999; Waggoner et al., 2000). Taken together, we propose that this transient MEKi treatment protocol during early postnatal stages might represent an exciting opportunity to prevent and treat various NF1-associated tumor and non-tumor manifestations including structural brain defects, which are associated with severe learning disabilities and are previously thought to be “irreversible” due to their hypothetical origin during embryonic development.
EXPERIMENTAL PROCEDURES
Control and Mutant Mice
The control mice used in this study are a pool of phenotypically indistinguishable mice with genotypes of hGFAP-cre-;Nf1flox/flox, hGFAP-cre-;Nf1flox/+, hGFAP-cre+;Nf1flox/+ and hGFAP-cre±;Nf1KO/+. The mutant mice (Nf1hGFAPCKO) used were of the genotypes, hGFAP-cre+;Nf1flox/KO and hGFAP-cre+;Nf1flox/flox. For inducible experiments, Nestin-creER+;Nf1flox/+ (tamoxifen+) mice were used as controls while Nestin-creER+;Nf1flox/flox (tamoxifen+) mice were used as mutants (Nf1NcreERCKO). Both of inducible strains carry R26LacZR allele as a reporter. Nf1hGFAPCKO and control mice were maintained in the mixed backgrounds of C57Bl6, 129Svj and FVB, which improve overall health and lifespan of the mutant mice. Nestin-creER+;Nf1flox/flox and control mice were maintained on the mixed C57Bl6 and 129Svj backgrounds. Age- and littermate-matched control and mutant mice were used for analysis to minimize the impact of modifier genes. All mice in this study were cared for according to the guidelines that were approved by the Animal Care and Use Committees of the University of Michigan at Ann Arbor.
MEK Inhibitor Treatment
MEK inhibitor (PD0325901, Sigma) was dissolved in DMSO at a concentration of 25mg/ml and resuspended in vehicle (0.5% hydroxypropyl methyl-cellulose with 0.2% Tween80, Sigma) at a concentration of 1mg/ml. The solution was administered by oral gavage at the dosage of 5 mg/kg (body weight) daily to lactating females for the treatment of P0.5-P18 mice. MEK inhibitor treated mice were collected and compared to littermate control and Nf1hGFAPCKO mice treated with vehicle.
Histological, Molecular and Statistical Analyses
Detailed descriptions for the experimental procedures are provided in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Bi-allelic Nf1 inactivation recapitulates a structural brain defect in NF1 patients.
Nf1 regulates glial/neuronal fate-specification of neonatal and adult SVZ TAP/IPCs.
Nf1 maintains postnatal neurogenesis by suppressing Olig2 in neurogenic progenitors.
Neonatal treatment with a MEK inhibitor rescues Nf1-related structural brain defects.
ACKNOWLEDGEMENTS
We thank M. Best, L. Cregan and G. Tomasek for technical assistance; members of the Zhu lab for support; J. Harrison, D. Sorenson, S. Meshinchi at the MIL core facility for EM analysis; A. Messing for providing hGFAP-cre mice; and Dr. H. Song, D. Wellik, K.S. O'Shea, J. Sebolt-Leopold, E. Fearon for critically reading the manuscript. This work is supported by grants from the NIH (1R01 NS053900) and DOD (W81XWH-11-1-0251) (Y.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bentires-Alj M, Kontaridis MI, Neel BG. Stops along the RAS pathway in human genetic disease. Nature medicine. 2006;12:283–285. doi: 10.1038/nm0306-283. [DOI] [PubMed] [Google Scholar]
- Bilican B, Fiore-Heriche C, Compston A, Allen ND, Chandran S. Induction of Olig2 precursors by FGF involves BMP signalling blockade at the Smad level. PLoS One. 2008;3:e2863. doi: 10.1371/journal.pone.0002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review [published erratum appears in Cancer Res 1990 Feb 15;50(4):1352]. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex. 2007;17:2585–2592. doi: 10.1093/cercor/bhl164. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2003;130:6599–6609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes, brain, and behavior. 2006;5(Suppl 2):61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- De Schepper S, Maertens O, Callens T, Naeyaert JM, Lambert J, Messiaen L. Somatic mutation analysis in NF1 cafe au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol. 2008;128:1050–1053. doi: 10.1038/sj.jid.5701095. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell stem cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Jamen F, Rubin LL, Richardson WD. Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development. 2004;131:1289–1298. doi: 10.1242/dev.01027. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee da Y, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes & Development. 2010;24:2317–2329. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listernick R, Charrow J, Gutmann DH. Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet. 1999;89:38–44. doi: 10.1002/(sici)1096-8628(19990326)89:1<38::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–7298. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nature reviews Neuroscience. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Moore BD, 3rd, Slopis JM, Jackson EF, De Winter AE, Leeds NE. Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology. 2000;54:914–920. doi: 10.1212/wnl.54.4.914. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. The EMBO journal. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JM, Moharir MD, Webster R, North KN. Brain structure and function in neurofibromatosis type 1: current concepts and future directions. Journal of neurology, neurosurgery, and psychiatry. 2010;81:304–309. doi: 10.1136/jnnp.2009.179630. [DOI] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride N, Payne JM, Webster R, Shores EA, Rae C, North KN. Corpus callosum morphology and its relationship to cognitive function in neurofibromatosis type 1. J Child Neurol. 2010;25:834–841. doi: 10.1177/0883073809350723. [DOI] [PubMed] [Google Scholar]
- Riccardi VM. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Second Edition Johns Hopkins University Press; Baltimore and London: 1992. [Google Scholar]
- Richards LJ, Plachez C, Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clinical genetics. 2004;66:276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nature reviews Neuroscience. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Kobayashi Y, Takeuchi A, Pages G, Pouyssegur J, Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1149–1155. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nature reviews Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature reviews Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Stevenson DA, Zhou H, Ashrafi S, Messiaen LM, Carey JC, D'Astous JL, Santora SD, Viskochil DH. Double inactivation of NF1 in tibial pseudarthrosis. American journal of human genetics. 2006;79:143–148. doi: 10.1086/504441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Zhao Y, Kim SJ, Liu M, Jia L, Saunders TL, Zhu Y, Sun Y. SAG/RBX2/ROC2 E3 Ubiquitin Ligase Is Essential for Vascular and Neural Development by Targeting NF1 for Degradation. Developmental cell. 2011;21:1062–1076. doi: 10.1016/j.devcel.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Current opinion in genetics & development. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner DJ, Towbin J, Gottesman G, Gutmann DH. Clinic-based study of plexiform neurofibromas in neurofibromatosis 1. Am J Med Genet. 2000;92:132–135. [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.