Abstract
Throughout history, poor quality medicines have been a persistent problem, with periodical crises in the supply of antimicrobials, such as fake cinchona bark in the 1600s and fake quinine in the 1800s. Regrettably, this problem seems to have grown in the last decade, especially afflicting unsuspecting patients and those seeking medicines via on-line pharmacies. Here we discuss some of the challenges related to the fight against poor quality drugs, and counterfeits in particular, with an emphasis on the analytical tools available, their relative performance, and the necessary workflows needed for distinguishing between genuine, substandard, degraded and counterfeit medicines.
Introduction
Universal access to affordable healthcare and quality medication is a fundamental right that remains elusive to large segments of the population in developing countries. In 2009, world leaders, predominantly from Africa, released the “Cotonou Declaration”, recognizing counterfeit (“falsified”) drugs as a key culprit preventing adequate access to quality medication,1 a key part of at least three of the Millennium Development Goals2 proposed by the United Nations.
Recently published evidence in the scientific literature3,4 and periodic reports in the printed and electronic media5 strongly suggest that accelerated globalization of pharmaceutical manufacturing and distribution at the turn of the 20th century has also greatly facilitated access, for criminal counterfeiters, to technologies required to manufacture copies of genuine pharmaceutical products.6 Moreover, the porosity of the pharmaceutical supply chain in many developing countries has made distribution channels easily accessible for counterfeit drugs. The Internet, with all its positive effects on worldwide economy, has also served as a conduit to market diverse counterfeit products, including pharmaceuticals, to millions of unsuspecting customers in developed countries.7,8 This new “business opportunity” has made drug counterfeiting an appealing income source for organized criminals.9 Several research agencies, think tanks and non-profit organizations have recognized the growing threat to public health resulting from this criminal trade. In a recent opinion formers’ conference, for example, the Wellcome Trust called for a better evaluation of the extent of the problem of counterfeit drugs, and for increased multiagency cooperation.10 Along these lines, international efforts to tackle counterfeit medicines have been spearheaded by the International Medical Products Anti-Counterfeiting Taskforce (IMPACT),11 a World Health Organization-associated body created in parallel with successful collaborative efforts against counterfeit antimalarials in SE Asia.12
Although more rampant in the developing world, counterfeit drugs know no borders, and cases are constantly uncovered in both developed and developing countries. For example, in June 2010 the US Food and Drug Administration issued a warning of online pharmacies selling a fake version of the flu treatment Tamiflu (oseltamivir) that could be dangerous to people allergic to penicillin.13 The so-called “generic Tamiflu” sold online was found to contain cloxacillin, a penicillin that could cause unexpected and inexplicable severe allergic reactions. In January 2010, INTERPOL released the results of a large multi-country police operation targeting the manufacture and distribution of counterfeit medicines in SE Asia named “STORM II”.14 This operation resulted in more than 30 arrests and the seizure of 20 million fake and illegal medicines. It also led to the closure of more than 100 pharmacies and illicit drug outlets. In Argentina, a high profile case involving a “medicine mafia” that sold fake, expired and stolen medication to trade-union run hospitals was recently widely publicized.15 Doctors and cancer patients became suspicious when anti-cancer medications did not cause hair loss. We argue that if chemical detection technologies became more widely available, user friendly and affordable, it may be more difficult for counterfeit drugs to compromise the end user’s health.
Defining the problem
As it becomes increasingly evident that the problem of poor quality medicines is of enormous proportions, it has also become evident that harmonizing the definition of what constitutes a substandard, counterfeit or degraded drug is a critical task to effectively combat this problem from a legal standpoint.10 Accurate definitions are not only important as a framework for governments to develop their own legal instruments, but also from the perspective of developing appropriate analysis methods with the necessary ability to distinguish different types of poor quality medicine.
Poor quality medicines can be classified into three different main types: substandard, counterfeit and degraded. There seems to be some consensus on the use of the term ‘substandard’ medicine16 to describe a medicine, produced by a registered, traceable, manufacturer, which contains the stated active ingredient(s) and excipients but does not fulfill one or more criteria of content, purity or other pharmaceutical properties. Hence, substandard medicines arise from poor quality control at factories and, unless severe negligence was involved, are not criminal matters. However, ‘counterfeit’ and ‘substandard’ are mutually exclusive categories if the definitions used by WHO are considered. “Substandard medicines (also called out of specification (OOS) products) are genuine medicines produced by manufacturers authorized by the National Medicine Regulatory Agency (NMRA) which do not meet quality specifications set for them by national standards. Normally, each medicine that a manufacturer produces has to comply with quality standards and specifications. These are reviewed and assessed by the national medicines regulatory authority before the product is authorized for marketing”.17
The current definition of counterfeit medicines used by WHO since 1992 reads as follows: “A counterfeit medicine is one which is deliberately and fraudulently mislabelled with respect to identity and/or source. Counterfeiting can apply to both branded and generic products and counterfeit products may include products with the correct ingredients, wrong ingredients, without active ingredients, with insufficient quantity of active ingredient or with fake packaging”. Hence counterfeits are, by definition, the products of criminals. The importance of the distinction was vividly illustrated recently by the discovery that an epidemic of falciparum malaria on the Pakistan/Afghanistan border was caused by substandard sulfadoxine–pyrimethamine (SP) with very poor dissolution,18 hence inspection of the manufacturer and assistance in improving manufacturing practices, rather than police action, would be required.
As more drug counterfeiting cases are uncovered, this definition has shown some limitations, as it does not contemplate scenarios where other medical products (e.g. counterfeit blood glucose test strips19) have been counterfeited and that counterfeit drugs may sometimes contain a larger amount of active ingredient than the genuine product.20 In order to clarify the definition and to provide a much needed framework that countries could consider adapting into national law, the International Medical Products Anti-Counterfeiting Taskforce (IMPACT) has proposed a new definition to the WHO stating that: “A medical product is counterfeit when there is a false representation in relation to its identity and/or source. This applies to the product, its container or other packaging or labeling information. Counterfeiting can apply to both branded and generic products. Counterfeits may include products with correct ingredients/components, with wrong ingredients/components, without active ingredients, with incorrect amounts of active ingredients, or with fake packaging. Violations or disputes concerning patents must not be confused with counterfeiting of medical products. Medical products (whether generic or branded) that are not authorized for marketing in a given country but authorized elsewhere are not considered counterfeit. Substandard batches or quality defects or non-compliance with Good Manufacturing Practices/Good Distribution Practices in legitimate and medical products should not be confused with counterfeiting”. This proposed new definition has generated considerable controversy—with tugs of war between different interests—especially those related to generic medicine versus innovator medicine intellectual property issues.6,21,22 An additional development that has caused understandable concern within the generics industry has been the seizures of generic medicines in the European Union in transit, due to suspicions that they infringed intellectual property law,23 even though the definitions used by WHO were apparently not referenced. Medical product counterfeiting should be treated as a criminal issue targeting public health and that the end goal of new analytical methodologies for detecting fakes should be first and foremost the protection of the patient’s welfare.
Confusion is even more rampant regarding degraded medicines, as the analytical methods required to distinguish these are more advanced, and few suppliers furnish products appropriately tested for stability in tropical countries. Stability tested products labeled for the WHO Climatic Zones I/II markets are not necessarily appropriate for these Climatic Zone III/IV markets. A medicine classified as genuine following packaging investigation, and found to contain an insufficient amount of active ingredient by standard chemical detection methods, could be either substandard or degraded, i.e. the low AI could have arisen before or after dispatch from the manufacturer. If found in a wholesaler using Good Pharmaceutical Practice24 (GPP) it is likely that the medicines are substandard and that there is a manufacturing problem. However, if found in an outlet in a hot/humid market, the medicine could be substandard or it could be degraded post-manufacture due to inability to observe GPP in more remote locations.
Technological and human resource limitations
Considering that an estimated 30% of the world’s medicine regulatory authorities (MRAs) “have no drug regulation or a capacity that hardly functions”,3 ensuring the quality of the drug supply requires efforts that involve technology development and transfer and, vitally, capacity building. That only two laboratories in malarious Africa are WHO pre-qualified for the analysis of anti-malarial medicines25 strongly suggests that interventions should not only focus on deploying more effective and affordable analysis technologies to secure the supply chain, but also on facilitating access to analytical chemistry and appropriate training in Africa. Without increasing such capacity in Africa, sustainable, long-term solutions against poor quality medicines will not be achieved. The availability of local teams with the capacity of generating high quality analytical data would also ensure that new policies and interventions are based on accurate, statistically valid evidence. Additional difficulties involve the limited after-sales support for analytical instrumentation, and difficulties in obtaining high purity solvents, reagents, and compressed gases.
Researchers with interests in interdisciplinary detection technology and scientists trained in pharmaceutical analysis play a central role in ensuring the authenticity of the pharmaceutical products consumed by patients. However, the analytical workflow—from sampling to data collection and reporting—presents some particular hurdles, mostly when surveys are being carried out in developing countries. Some of the key difficulties include:
Insufficient financial resources and insurmountable logistical obstacles, preventing true randomized drug sampling for estimating the prevalence of counterfeit/substandard/degraded drugs.
Difficulty in acquiring examples of genuine medicines (and their packaging) from legitimate manufacturers as references for chemical and packaging analysis.
Limited availability of field deployable techniques based on orthogonal physicochemical detection principles to accurately and inexpensively detect poor quality medicines.
Lack of consensus on sample collection, analysis and sharing to build appropriate data repositories to better support MRAs and law enforcement agencies in their actions.
The analytical toolbox and workflow
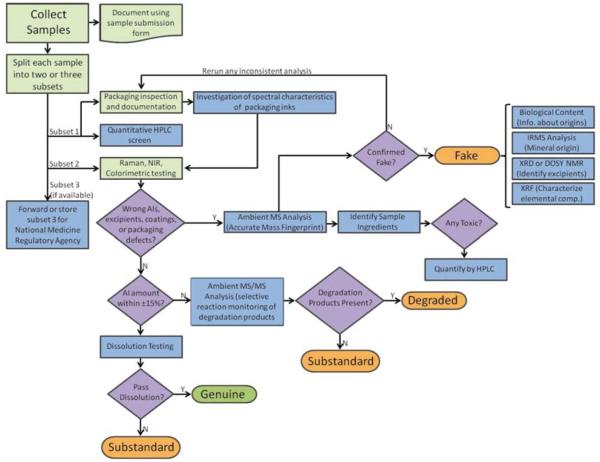
Deciding if a medicine sample is substandard, counterfeit or degraded requires a defined analytical workflow so the distinction can be made accurately and economically. Fig. 1 shows a decision tree stemming from the analytical workflow used by the authors in countrywide surveys carried out as part of the antimalarial drug quality activities of the Artemisinin Combination Therapy (ACT) Consortium26 through the Counterfeit Drug Forensic Investigation Network (CODFIN),27 a network of research laboratories collaborating on the investigation of poor quality pharmaceuticals in developing countries. The first critical step in this workflow is the collection of samples in a statistically valid manner with preservation of the chemical integrity of the sample. Several approaches ranging from convenience sampling to fully randomized stratified sampling can be undertaken, depending on the question being asked. Following sampling, the packaging is inspected to note typographical and/or grammatical errors that might suggest that the sample is not genuine. If genuine packaging is available, the sample is compared to investigate any differences in logos, colors and any other overt or covert anti-counterfeiting measures known to the investigators. Any leaflets accompanying the drug sample should also be checked for differences. From this point, analytical workflows can be very diverse, depending on the depth of the chemical analysis pursued and available resources. The simplest approach is to follow packaging investigation with some form of rapid test involving colorimetry, thin layer chromatography (TLC), refractive index testing,28 and/or a simplified dissolution test. Colorimetric tests can be evaluated visually,29,30 or with the aid of simple field photometers that provide semi-quantitative information about the active ingredient content. In addition to rapid testing, spectroscopic techniques such as IR and Raman can also be used to obtain a molecular signature of the sample’s chemical composition. Both field-friendly and laboratory versions of IR and Raman spectrometers are currently available, with the latter providing improved signal-to-noise, resolution and spatially resolved measurement capabilities when combined with microscope set-ups.31 In CODFIN we perform high performance liquid chromatographic (HPLC) screening with UV detection to quantify the expected active ingredients, which obviates the need for rapid testing. The active ingredient’s identity is assessed on a first approximation by retention time matching. HPLC was chosen as the first laboratory test to perform on all samples due to its wide availability in many drug quality analysis laboratories, and its wide acceptance in national and international pharmacopeias32 (European, British, the United States and the WHO International). These pharmacopeias contain recognized instructions for testing many pharmaceutical products using specific and validated methods. Also, rapid “fingerprinting” analysis, based on Raman and IR spectroscopy, can be carried out directly on tablets and other pharmaceuticals at this stage.31,33-35 Their main advantage is the lack of sample preparation and the capability of producing quantitative (or at least semi-quantitative) AI content information,36 and detection of degradation products. In our workflow, we incorporate these techniques to further build a database of NIR and Raman spectra of well characterized genuine and poor quality medicines as potential future alternatives to more costly analytical techniques. Differences in NIR and Raman fingerprints between genuine and suspect samples may also point at the presence of wrong excipients, triggering more in-depth investigation via mass spectrometric techniques (Fig. 1).
Fig. 1.
Analytical workflow currently in use by CODFIN to test samples collected in country-level drug quality surveys. Steps colored with light green background can be performed in the field or in the laboratory depending on the logistics of the study.
If any reasons have been found to suspect that the sample under investigation is poor quality, the following step in our workflow is to investigate the sample composition using direct ionization “ambient” mass spectrometry. We typically perform all or parts of the following procedure12 on all samples investigated:
(1) Direct Analysis in Real Time Time-of-flight (DART TOF) MS:37 this procedure enables identification of the main constituents of the sample under investigation by obtaining elemental formulas from the accurate masses of protonated molecules and matching those to entries in custom-built or online databases such as PubChem, ChemSpider etc. Determination of the compound elemental formula makes use not only of the accurate mass of the ion, but also the relative isotopic ion abundances of its isotopic clusters. Mass accuracies of at least 10 ppm are required for this task, but less than 5 ppm is desirable to reduce the number of formula candidates. In terms of resolving power, a minimum of 6000 (FWHM) is desirable. The higher resolving power of higher performance TOFs is desirable, but comes at higher financial cost and instrument sophistication. Distinction between isomeric species in fake drugs requires tandem MS experiments together with retention time matching approaches.38
(2) Desorption Electrospray Ionization (DESI) MS:39 in addition to DART TOF MS analysis, we perform DESI MS in both reagentless and reactive modes40,41 to detect molecules not detected by DART due to their lower volatility.42 DESI-MS is also a powerful technique that allows quantitation of pharmaceutical AIs,43 directly from tablets, but this method is not yet implemented in a routine fashion in our workflow.
Following DESI and DART MS investigation, select samples that show unique spectral characteristics are forwarded to a network of collaborating laboratories.27 We have performed analysis by several techniques, including (a) microscopic investigation of the material (debris, insect parts, pollen) trapped in the tablet body during fabrication,12,44 (b) isotope ratio MS to identify the mineral phases present as excipients and pinpoint their geographical origin,12 (c) X-ray fluorescence to investigate elemental composition (unpublished), (d) FT-IR and or DESI imaging for obtaining spatially resolved information on the surface of the sample,42,45 and (e) Two-Dimensional Diffusion-Ordered Nuclear Magnetic Resonance Spectroscopy (2D DOSY NMR)42 for validating DESI and DART MS results. The purpose of this final “forensic” analysis is to try to determine the origin of the fakes by establishing a link between the geographical origin of the samples and their similarities, as shown by their spectral characteristics. From the analytical perspective, this procedure is similar to fingerprinting analysis carried out for purposes of authenticating regional products such as olive oils46 or red wines.47
If no wrong AIs or excipients are suspected by NIR/Raman or HPLC, and packaging inspection does not reveal differences with known genuine packaging samples, the next question is to consider if the active ingredient content is outside specifications. Monographs state the allowed range of % AI for a good quality sample, usually ~90 to 110%. These acceptable ranges are based on large samples (20–30) of dosage units that may not be available in medicine quality surveys. However, there is no consensus on what acceptable ranges of % AI are permitted for smaller samples. For example, if one tablet in a blister pack of 6 tablets has a content of 80% AI, it is unclear if the medicine could be classified as substandard based only on that one analysis, taking into account that performing more analytical replicates may not be feasible due to lack of tablets and/or resources.
If the AI content is found to be acceptable by HPLC, the next step is to perform dissolution testing, which measures the amounts of AI released in vitro as a function of time, as a reflection of in vivo bioavailability. Dissolution tests have been successfully used to assess the quality of antimalarial drugs.48 Detailed protocols (official monograms) set out for most drugs in all pharmacopoeias describe dissolution solvent/buffer, stirring speed, dissolution profile of given AIs, and temperature for the assay. The use of incorrect excipients, as well as inadequate manufacturing processes, may contribute to poor dissolution resulting in much lower or higher bioavailability, rendering these drugs substandard. Poor storage conditions resulting in decomposition products may also influence dissolution, but these samples should be considered “degraded” and not substandard. Sulfadoxine–pyrimethamine is especially at risk of reduced dissolution, despite acceptable % AI.18
If the AI content is found to be outside accepted limits, further investigation is needed to determine if the sample is substandard or the amount of active ingredient has changed (decreased) due to improper storage of the medicines. The major problem with distinguishing substandard from degraded medicines is the very limited data on degradation products of genuine medicines, as this task requires that one or several of the degradation products of the investigated AI are known and have been chemically identified in terms of elemental formula and, perhaps, structure.49 Mass spectrometry (MS) and Nuclear Magnetic Resonance (NMR) spectroscopy are useful techniques at this stage, the latter being less sensitive, but allowing quantification without chemical standards. Detection of degradation products can also be carried out by HPLC with single-wavelength or diode array detection if the degradation processes for a certain AI are well understood. In our workflow, we employ direct “ambient” mass spectrometric approaches further described below to determine artemisinin-based antimalarial degradation products. For example, the antimalarial drug artesunate degrades through dihydroartemisinin (DHA) as an intermediate product to give β-artesunate, artesunate dimers, 9,10-anhydrodihydroartemisinin, a DHA β-formate ester, and smaller amounts of other products.50 These products can be rapidly determined with techniques such as desorption electrospray ionization mass spectrometry (DESI-MS) carried out in artemisinin-specific “reactive” mode,41 directly from tablets, using selected reaction targeted monitoring to enhance selectivity. These targeted selective reaction monitoring (SRM) experiments can be accommodated in a variety of mass spectrometers such as ion traps, triple quadrupoles etc. In SRM, the product ion currents resulting from collision-induced dissociation (CID) of precursor drug ions selected in the first mass analysis step yield specific fragment ions that can be monitored with high sensitivity, ensuring the selectivity of the measurement through the specificity of the precursor ion to fragment ion transition.
Sampling strategies
In most peer reviewed literature, “convenience sampling” has been used to investigate the existence of poor quality medicine in a certain geographical region. Researchers collect samples in locations that they have direct access to, or compile results from samples forwarded by collaborators in various locations. The design of this sampling strategy is guided by what is possible, and not by what is acceptable by statistical or power calculations. For example, “convenience surveys” conducted in South East Asia in 2000/1 and 2002/3 suggested that 38% and 53%, respectively, of artesunate blister packs obtained from pharmacies and shops were counterfeit.51-53 This sampling strategy is useful as a preliminary investigation that requires minimum resources, analogous to case reports of drug adverse reactions, but lacks the proper experimental design to provide an accurate estimate of the prevalence of poor quality drugs with confidence intervals. There is also a strong probability for bias depending on whether the collector consciously or subconsciously set out to procure or not procure poor quality drugs. If the sellers realize that their goods are being investigated, there is a risk that they will either decline to take part or only sell what they believe is the authentic or “best quality” drug. This suggests that medicine surveys should be carried out covertly by “mystery shoppers”.54 Sampling frameworks, based on random sampling, either conventional random population sampling or random lot quality assurance sampling (LQAS) have been proposed as the way forward.55 In these approaches, sampling locations are randomly chosen from a pre-compiled list of outlets. This list may be stratified to reflect differences, such as between districts, provinces, and urban vs. rural regions. The number of outlets to be sampled is determined using power calculations based on poor drug quality prevalence estimates.56 Conscious or subconscious biases are minimized and an estimate of poor drug quality prevalence with confidence intervals is produced. Repeated randomized sampling of drugs in one area would enable monitoring of the effectiveness of interventions, and dynamic changes in the pharmaceutical supply chain. A difficulty with this approach is that lists of pharmaceutical outlets may not be available, especially for unlicensed outlets. The method of randomization should be stated and pseudo-randomization, not using random number tables or software, should be avoided. Proposed guidelines for the sampling and reporting of medicine quality have recently been published (MEDQUARG).55
Packaging inspection
Inspection of the packaging is a crucial, although often overlooked step, in the scientific investigation of a suspect medicine. Ideally this should be performed blinded to the chemistry results but should subsequently be reviewed in conjunction with all other data on the sample. Packaging is vital in assessing whether a poor quality medicine is substandard/degraded or counterfeit. There are examples of counterfeit medicines containing the correct % AI but with clearly fake packaging.57 Although the finding of a sample with no or minimal % AI suggests that the medicine is counterfeit this is not necessarily the case if severe quality control problems occurred at the factory. Counterfeits may fraudulently state the name of a genuine manufacturer (e.g. artesunate12) or that of a manufacturer that apparently does not exist.58
Investigation involves measuring packaging dimensions, inspection with a hand lens, looking for features only visible under UV light using an inexpensive bank note checker, weighing packet and tablets, scanning packets, tablets and leaflets and quantifying colors, comparing manufacturer, expiry dates and batch numbers with genuine details from the manufacturer, looking for spelling mistakes and differences in symbols and fonts and formal analysis of paper, card, foil and holograms.53 Sometimes poor quality inks may be easily rubbed off with a moist finger.59 Comparison of the packaging of counterfeits may suggest linkage of samples collected in different locations.12 However, as illustrated by the great diversity of fake artesunate holograms12 and change through time of both counterfeits and genuine products, packaging is very dynamic. A crucial step for packaging inspection and major difficulty is collecting genuine samples direct from manufacturers. We suggest that the pharmaceutical industry should be more responsive to such requests. There is great need for up to date genuine and counterfeit high-resolution packaging scans, and details of the packaging from different batches to be made available via secure websites so that samples can be compared.
Rapid field testing methods
Developing countries that do not have the technical, financial, or human resources required to inspect and protect the drug supply chain can use simple and affordable field methods. Measuring physicochemical sample characteristics such as pH, tablet weight, the viscosity of syrups, and density of suspensions or solutions can be the simplest approach to detect fakes, but the results from these tests should not be considered conclusive. Measurement of tablet color may be an interesting approach for some highly colored or coated drugs.60 AI-specific colorimetric methods for artemisinin-based antimalarials have been developed.29,30,61 These can be used in pass–fail mode, or coupled to inexpensive hand-held LED photometers for producing semi-quantitative data. Despite its low selectivity, refractometry has also been demonstrated as a useful means for detecting simple fake drugs,28 but false positive results are highly likely.
Portable labs, in particular the GPHF-MiniLab®, provide a cost-effective means for screening of most antimalarial formulations and other drugs without extensive user training.62 The MiniLab® uses visual inspection followed by simplified disintegration testing, colorimetric reactions and thin-layer chromatography63 to test for the quality of drugs. The Tanzanian Food and Drugs Authority piloted the use of the MiniLab® kits and found it to be relatively inexpensive and rapid, but that only grossly substandard or drug samples containing wrong AIs could be detected.64
Handheld field instrumentation approaches: Raman, IR and other techniques
Because of their ‘point-and-shoot’ capabilities, portable Raman and near-infrared (NIR) instrumentation are being adopted for testing of suspect counterfeit drugs in the field in developing countries.33,65 Authorities in the People’s Republic of China have used mobile NIR to investigate medicine quality66 and NAF-DAC in Nigeria has recently used portable Raman spectroscopy to screen and detect counterfeit antimalarials entering the country.67 However, there have been no detailed comparisons of these new technologies in the field and it remains unclear which are the most accurate, appropriate and cost effective in different situations and how much training they require. A common concern with both fieldable NIR and Raman instrumentation is its relatively high capital cost compared to TLC and colorimetric approaches, which limits the number of units deployed in the field. However, they will become more cost effective with long term use as they require no consumables. Raman spectroscopy is based on the notion that light is scattered when it interacts with the different vibrational modes of molecules present in a sample analyzed (the Raman effect). Advantages of this technique include its portability and non-destructiveness, allowing complimentary testing by other methods and that tablets can be examined through packaging. However, several drawbacks have been reported, including the fact that only the surface of the sample is examined, which could result in mislabeling a sample as counterfeit whereas it is substandard. This drawback can be mitigated by more complex spatially offset34 or transmission approaches,68 but these are not yet available as portable instrumentation. Because Raman is most commonly used as a fingerprinting technique, the signal resulting from the AI cannot be easily deconvoluted from the rest of the Raman spectrum. Another issue is that Raman relies on brand-specific spectral libraries for identification, which may not be available for medicines vital in developing countries or specific drugs produced by non-Western companies.65 Moreover, if excipients in a given drug sample are slightly different than those in the genuine samples previously included in the database, a genuine produced at a different plant, or different batch, may be falsely categorized as a counterfeit. Because of the fingerprinting nature of this technique, every genuine drug expected in the market should be included prior to its use in any survey, which could be very time and resource consuming, or even impossible to accomplish. Background fluorescence is the most typical interference encountered when using field Raman instrumentation, but advances in miniaturization of different excitation laser sources may mitigate this problem in the near future. NIR spectroscopy, which takes advantage of the feature that different drug molecules interact in different ways when excited by infrared light, may be used when fluorescent interferents prevent Raman analysis. NIR spectrometers excite transitions with a net dipole moment change, which are either very slightly or not Raman active. NIR penetrates samples further than Raman, allowing for the advantage that slightly more sample volume will be examined, but does not consistently penetrate packaging and blister packs, forcing the user to examine tablets and other samples outside of their packaging. Like Raman, NIR spectroscopy utilizes the fingerprinting method to identify samples, also requiring a database for identification.69,70 Under controlled laboratory settings, NIR spectroscopy has been shown to be able to distinguish differences as small as 2.5% (w/w) in AI content and 1.0% (w/w) in excipient or coating variation.71 NIR in imaging mode has been shown to be a powerful tool for characterizing fake drugs,45,72 but this approach is not well suited for field use. Other portable techniques commonly used for chemical analysis in the field, such as ion mobility spectrometry (IMS), have been largely absent from the fight against drug counterfeiting. It would be expected that the large resources and extensive research performed towards using IMS for narcotics and explosive detection could be successfully leveraged in detecting fake drugs. Portable Gas Chromatography-Mass Spectrometry (GC-MS) and MS technologies are currently under development.
Analysis of contaminants and excipients
Pollen, spores, fungal hyphae, insects, fibers, charcoal, leaf cuticles, other plant and animal cells and other contaminants are incorporated into genuine and counterfeit pharmaceuticals during production, and can be used as a tool for obtaining forensic clues about their geographical origin.12 Such contamination can be obtained from the site of manufacture, the source of individual ingredients or both, and only the most highly processed foods or pharmaceuticals lack contaminants. Disadvantages of this type of analysis are that pharmaceuticals are destroyed during processing, large numbers of tablets are required to recover a diverse range of palynomorphs, and the high cost of detailed examination of samples that are processed minimally to avoid loss of important evidence. Extraction processes differ considerably according to the excipient used and some added chemicals react adversely with the acids used for pollen extraction.
Maize starch is mainly used to hold the active ingredient in tablets but counterfeiters use anything white and able to hold shape. Talc, calcite, dolomite, aragonite, calcium sulfate, and gypsum are a few of the minerals detected by X-ray Diffraction (XRD) and these also provide clues to source or environment. Stable carbon and oxygen isotope analyses of calcite can determine whether the source is hydrothermal, natural precipitation or medical and has been used to determine where calcite was mined.12 Stable sulfur isotope analyses can be used to determine the source of gypsum, derived from the evaporation of seawater, as sulfur isotope ratios through geological time are well known.73
Palynological analysis is undertaken and identification of palynomorphs made by comparison with reference slides and published pollen databases. Most palynomorphs recovered are anemophilous allowing comparison with published annual and seasonal air-borne pollen data from countries or areas of interest. There is pollen evidence that the production environment is becoming more sanitized as production of counterfeit pharmaceuticals becomes more sophisticated. However, combined with XRD and stable isotope analyses, palynology can provide strong evidence, but not proof, of the source or sources of manufacture of counterfeit pharmaceuticals. These analyses are carried out independently of all other laboratories in the network to avoid unintentional bias in the interpretation of the results obtained.
Recent advances in mass spectrometry sample introduction techniques for detecting poor quality drugs
A new family of sampling/ionization techniques known collectively as “ambient” mass spectrometry (MS) promises to be of great utility for detection of poor quality drugs and characterization of degraded and substandard pharmaceutical products. This family consists of at least 25 different approaches that combine various desorption (laser, plasma, thermal, liquid jet) and ionization (gas-phase proton transfer, electrospray, photoionization) methods in a one- or two-step fashion.74 A recent mini-review in this Journal described the basic principles of these techniques.75 Two of the most widely adopted ambient MS techniques used for pharmaceutical analysis are DESI and DART. Both allow investigating pharmaceuticals without any sample preparation, requiring only a few seconds per sample in their basic operation modes. If high resolution mass spectrometers are used in combination with these techniques, accurate mass experiments to identify sample components are possible. Generally, experiments are first carried out in positive ion mode to detect basic AIs, but negative ion mode experiments are also useful to detect specific drug families.
DART is a popular high-throughput ionization method for MS. In DART MS, a special type of ion source that produces a heated stream of protonated reactant ions is used. This stream is directed towards the tablet under investigation. The heat from this stream desorbs chemical species from the tablets, simultaneously ionizing them. These ions are generated in the open air between the ion source and the mass spectrometer inlet, and sampled by the inlet of the mass spectrometer. DART has the advantage of producing simpler spectra than DESI, but the disadvantage of relying on thermal desorption which can cause some degree of fragmentation if the temperature settings are not chosen correctly.76
In DESI MS analysis, a high-speed electrically charged liquid spray is directed at the tablet under inspection. The tablet is kept at atmospheric pressure, outside of the mass spectrometer. The DESI spray progressively dissolves material from the tablet, giving a qualitative depth profile. The charged droplets containing tablet material are sampled downstream by a mass spectrometer, providing a spectrum of the sample components. No tablet dissolution is required making this technique well-suited for screening large number of drug samples.
A unique capability of DESI is that it easily allows spatially resolved measurements on various sample parts. Therefore, several points on the tablet surface and interior are typically sampled during DESI analysis of suspicious drug samples to obtain an accurate picture of the components that might be present. Depth profiling experiments may also be carried out to investigate the presence of specific AIs that may have been introduced on the tablet surface during the tablet pressing step of tablet manufacture. In our investigations of counterfeit antimalarials, depth profiling DESI MS is performed with a quadrupole ion trap MS detector which allows various types of scans to be performed sequentially as the DESI jet drills into the tablet (Table 1).
Table 1.
Method used to investigate suspect poor-quality artesunate samples with DESI-MS
| Scan time | Scan type | Comments |
|---|---|---|
| 0–0.2 min | High repetition rate scan in 100–1000 m/z range (TurboScan) |
Superficial species on the tablet outermost layers are monitored |
| 0.2–0.7 min | Full scan in 400–110 m/z range at normal rate |
High sensitivity scan for artesunate [M + Na]+ (m/z = 407) in inner tablet layers |
| 0.7–1 min | Data dependent MS2 scan in 100–1000 m/z range |
Structural information (identification) of unknowns via MS2 experiments. Background ions are excluded |
While DESI is most often used as a surface ionization technique, the jet of charged solvent used to ionize the analyte dissolves a small area on the tablet and, over time, removes enough mass so that it effectively drills into the tablet, resulting in analysis of compounds located deeper within the sample. High repetition rate scans are used to rapidly monitor the first few layers of the sample as it is being ablated. The high repetition rate limits resolution but maximizes spectral acquisition speed. The next scan step focuses on specifically detecting artesunate that might be present only in the outer layers of the sample,41 indicating the possibility of fakes being produced at a facility that also manufactures the same-type of genuine artemisinin-based pharmaceutical. The last step in the depth profiling experiment performs MS/MS analysis on the most intense signals in the DESI MS spectrum, while simultaneously excluding known background ions produced by the solvent mixture. These experiments, in combination with DART MS, are useful to identify wrong active ingredients used in the manufacture of fakes.
To further validate standard (“reagentless”) DESI experiments, DESI MS can also be performed in “reactive” mode. For artemisinin-based antimalarials, this is done by addition of 100 μM dodecylamine (DDA) to the spray solution. DDA forms stable complexes with artemisinins that enable the direct and sensitive detection in positive ion mode with minimal fragmentation. Our group has also demonstrated the use of reactive DESI for detecting and quantifying AIs on and in other types of commonly counterfeited drugs, such as oseltamivir.40 Reactive DESI allows detecting AIs with high sensitivity, but may preclude detection of other wrong AIs at trace levels due to ionization suppression of trace compounds caused by the addition of highly ionizable alkylamines to the spray. Therefore, it is recommended only in combination with conventional DESI or DART MS analysis.
Accurate mass DART and DESI mass spectra can be processed via a variety of approaches, including searches via Excel macros against in-house libraries of exact masses for protonated molecules derived from the Model List of Essential Drugs published by the WHO77 or others in the literature,78 elemental formula matching using the “seven golden rules” approach,79 and vendor-specific elemental formula elucidation software followed by online database searches.
Information management—the CODFIN database
The key final step is the reporting of results to the national MRA, before scientific publication, so that appropriate action can be taken to try to improve the medicine supply. CODFIN has developed a database containing detailed information about each sample investigated (Fig. 2). The information obtained through examination and testing of each sample is stored in the online database, which is hosted in a Microsoft SharePoint server. This environment allows for Excel spreadsheets containing laboratory data and sample collection information data, and word documents with embedded packaging images to be stored directly into a web-accessible file sharing environment. A hierarchy of user permissions restricts dissemination of confidential data, while allowing all contributors to view and upload various types of information. The built in version-tracking system prevents two users from entering data or modifying the same spreadsheet simultaneously.
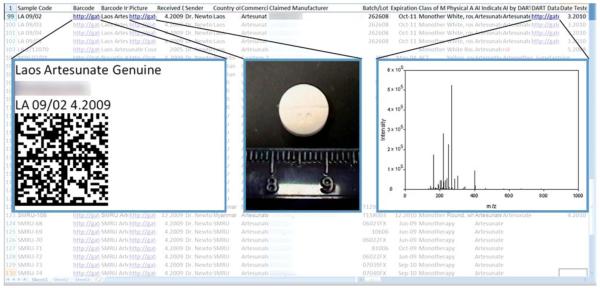
Fig. 2.
Sample screenshot of the CODFIN database. This database contains information about each investigated sample, identified by a unique sample code. In the entry for each sample are links to documents containing a barcode, photos of the sample, and ASCII data which may be used to plot spectral information (MS, Raman, NIR).
Upon arrival, each sample is identified by a sample code. The codes assigned to each sample identify the country or manufacturer from which the sample was collected/received followed by a sample number and year separated by a forward slash. For example, a sample assigned the code VN 09/01 would have been collected in Vietnam in 2009 and would have been the 1st sample received from that country of origin. For each sample, the database includes information about its origin, physical appearance, the active ingredient stated on the label, and any active ingredient detected using Raman, NIR and DART or DESI MS. The information concerning the origin of the sample includes its country of origin, claimed manufacturer, and commercial name, as well as the sender and date received. Information on AI content determined by HPLC and other information produced by XRD etc. is also added progressively to the database as it becomes available.
Another important feature is the use of 2D barcodes for sample identification. Each barcode encodes a text string that concatenates some of the basic, important information from the database about each sample including sample code, country of origin, claimed manufacturer, claimed active ingredient, and whether the active pharmaceutical ingredient claimed on the packaging was detected. These barcodes can be printed and placed on the physical sample in order to more accurately and readily identify samples, so if the barcode of a misplaced sample is scanned, the user can immediately link that barcode to an entry in the database. A document with the barcode and the decoded information contained in it is linked to each sample’s entry in the database. Other documents linked to each sample entry include a document containing photos of the sample and scans of its packaging as well as the actual raw spectral data from each analysis method.
Conclusions
From the analytical chemist’s perspective, the fight against poor quality drugs requires a wide range of technologies. These technologies are used for two main tasks: (1) the vital classing of samples as genuine, substandard, degraded or counterfeit, and (2) providing clues about their possible origin and interrelationships. Innovative fieldable and “low tech” analysis methods are likely to play significant roles in the new future, providing key answers in resource poor settings. Further decrease in the cost of handheld, ruggedized instrumentation, and improvement of their built-in libraries should further empower local MRAs and law enforcement agents. Analytical chemists should also play a role in capacity building efforts targeted at improving local human resources to make the fight against poor quality drugs more sustainable.
Acknowledgements
The authors are grateful to the Artemisinin Combination Therapy Consortium for funding their project through a grant from the Bill and Melinda Gates Foundation to the London school of Hygiene and Tropical Medicine. PNN is supported by the Wellcome Trust of Great Britain. FMF also thanks the US National Science Foundation for financial support though CAREER award #CHE-0645094.
Footnotes
This article is part of a themed issue on Grand Challenges.
References
- 1. http://www.fondationchirac.eu/en/programs/access-to-medicine/international-mobilization-against-the-traffic-of-falsified-medicines/
- 2. http://www.un.org/millenniumgoals/
- 3.Newton PN, Green M, Fernandez FM, Day NPJ, White NJ. Lancet Infect. Dis. 2006;6:602–612. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 4.Newton PN, Green MD, Fernandez FM. Trends Pharmacol. Sci. 2010;31:99–101. doi: 10.1016/j.tips.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. http://www.notofakes.com/
- 6.Shepherd M. Nat. Med. (N. Y., NY, U. S.) 2010;16:366–366. doi: 10.1038/nm0410-366. [DOI] [PubMed] [Google Scholar]
- 7.Veronin MA, Youan BBC. Science. 2004;305:481–481. doi: 10.1126/science.1097355. [DOI] [PubMed] [Google Scholar]
- 8.Weiss AM. Cleve. Clin. J. Med. 2006;73:282–288. doi: 10.3949/ccjm.73.3.282. [DOI] [PubMed] [Google Scholar]
- 9.Bate R. Making a Killing: the Deadly Implications of the Counterfeit Drug Trade. The AEI Press; Washington, DC: 2008. [Google Scholar]
- 10.Wellcome Trust; Opinion Formers’ Conference on Counterfeit Medicines-Perspectives and Action; London. 2009. [Google Scholar]
- 11. http://www.who.int/impact/en/
- 12.Newton PN, Fernandez FM, Plancon-Lecadre A, Mildenhall D, Green MD, Ziyong L, Christophel EM, Phanouvong S, Howells S, MacIntosh E, Laurin P, Blum N, Hampton CY, Faure K, Nyadong L, Soong CWR, Santoso B, Zhiguang W, Newton J, Palmer K. PLoS Med. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm216009.htm.
- 14. http://www.interpol.int/Public/ICPO/PressReleases/PR2010/PR007.asp.
- 15. http://www.youtube.com/watch?v = Y6W5uUAlENQ.
- 16.Caudron JM, Ford N, Henkens M, Mace C, Kiddle-Monroe R, Pinel J. Trop. Med. Int. Health. 2008;13:1062–1072. doi: 10.1111/j.1365-3156.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 17. http://www.who.int/medicines/services/counterfeit/faqs/06/en/index.html.
- 18.Leslie T, Kaur H, Mohammed N, Kolaczinski K, Ord RL, Rowland M. Emerging Infect. Dis. 2009;15:1753–1759. doi: 10.3201/eid1511.090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. http://www.cbc.ca/health/story/2006/10/16/counterfeit-test.html.
- 20. http://apps.who.int/gb/ebwha/pdf_files/EB124/B124_14-en.pdf.
- 21. http://www.safemedicines.org/2008/07/defining-the-problem.html.
- 22.Shukla N, Sangal T. J. Intellect. Prop. Rights. 2009;14:236–240. [Google Scholar]
- 23. http://www.unitaid.eu/en/20090304156/News/UNITAID-statement-on-Dutch-confiscation-of-medicines-shipment.html.
- 24. http://www.fip.org/files/fip/Statements/latest/Dossier%20004%20total.PDF.
- 25. http://apps.who.int/prequal/lists/PQ_QCLabsList.pdf.
- 26. www.actconsortium.org.
- 27. www.codfin.org.
- 28.Green MD, Nettey H, Villalba-Rojas O, Pamanivong C, Khounsaknalath L, Grande Ortiz M, Newton PN, Fernandez FM, Vongsack L, Manolin O. J. Pharm. Biomed. Anal. 2007;43:105–110. doi: 10.1016/j.jpba.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Ioset JR, Kaur H. PLoS One. 2009;4:e7270. doi: 10.1371/journal.pone.0007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green M, Mount DL, Wirtz RA. Trop. Med. Int. Health. 2001;6:980–982. doi: 10.1046/j.1365-3156.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 31.Witkowski MR. Am. Pharm. Rev. 2005;8(56):58–62. [Google Scholar]
- 32. www.uspnf.com/uspnf/login.
- 33.Ricci C, Nyadong L, Yang F, Fernandez FM, Brown C, Newton PN, Kazarian S. Anal. Chim. Acta. 2008;632:178–186. doi: 10.1016/j.aca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Ricci C, Eliasson C, Macleod NA, Newton PN, Matousek P, Kazarian SG. Anal. Bioanal. Chem. 2007;389:1525–1532. doi: 10.1007/s00216-007-1543-1. [DOI] [PubMed] [Google Scholar]
- 35.De Veij M, Vandenabeele P, Alter Hall K, Fernandez FM, Green MD, White NJ, Dondorp AM, Newton PN, Moens L. J. Raman Spectrosc. 2007;38:181–187. [Google Scholar]
- 36.Dowell FE, Maghirang EB, Fernandez FM, Newton PN, Green MD. J. Pharm. Biomed. Anal. 2008;48:1011–1014. doi: 10.1016/j.jpba.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Cody R, Laramee J, Durst H. Anal. Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 38.Wolff JC, Thomson LA, Eckers C. Rapid Commun. Mass Spectrom. 2003;17:215–221. doi: 10.1002/rcm.893. [DOI] [PubMed] [Google Scholar]
- 39.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 40.Nyadong L, Hohenstein EG, Johnson K, Sherrill CD, Green MD, Fernandez FM. Analyst. 2008;133:1513–1522. doi: 10.1039/b809471c. [DOI] [PubMed] [Google Scholar]
- 41.Nyadong L, Green M, De Jesus V, Newton PN, Fernandez FM. Anal. Chem. 2007;79:2150–2157. doi: 10.1021/ac062205h. [DOI] [PubMed] [Google Scholar]
- 42.Nyadong L, Harris GA, Balayssac S, Galhena AS, Malet-Martino M, Martino R, Parry RM, Wang MDM, Fernandez FM, Gilard V. Anal. Chem. 2009;81:4803–4812. doi: 10.1021/ac900384j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyadong L, Late S, Green M, Banga A, Fernandez FM. J. Am. Soc. Mass Spectrom. 2008;19:380–388. doi: 10.1016/j.jasms.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Mildenhall D. Rev. Palaeobot. Palynol. 1990;64:227–234. [Google Scholar]
- 45.Ricci C, Nyadong L, Fernandez FM, Newton PN, Kazarian S. Anal. Bioanal. Chem. 2007;387:551–559. doi: 10.1007/s00216-006-0950-z. [DOI] [PubMed] [Google Scholar]
- 46.Alonso-Salces RM, Moreno-Rojas JM, Holland MV, Reniero F, Guillou C, Heberger K. J. Agric. Food Chem. 2010;58:5586–5596. doi: 10.1021/jf903989b. [DOI] [PubMed] [Google Scholar]
- 47.Casale M, Oliveri P, Armanino C, Lanteri S, Forina M. Anal. Chim. Acta. 2010;668:143–148. doi: 10.1016/j.aca.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Kaur H, Goodman C, Thompson E, Thompson KA, Masanja I, Kachur SP, Abdulla S. PLoS One. 2008;3:e3403. doi: 10.1371/journal.pone.0003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keoluangkhot V, Green M, Nyadong L, Fernandez FM, Mayxay M, Newton PN. Am. J. Trop. Med. Hyg. 2008;78:552–555. [PMC free article] [PubMed] [Google Scholar]
- 50.Haynes RK, Chan HW, Lung CM, Ng NC, Wong HN, Shek LY, Williams ID, Cartwright A, Gomes MF. ChemMedChem. 2007;2:1448–1463. doi: 10.1002/cmdc.200700064. [DOI] [PubMed] [Google Scholar]
- 51.Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, Chotivanich K, Mayxay M, Looareesuwan S, Farrar J, Nosten F, White NJ. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 52.Newton PN, Dondorp A, Green M, Mayxay M, White NJ. Lancet. 2003;362:169–169. doi: 10.1016/S0140-6736(03)13872-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall KA, Newton PN, Green MD, De Veij M, Vandenabeele P, Pizzanelli D, Mayxay M, Dondorp A, White NJ, Fernandez FM. Am. J. Trop. Med. Hyg. 2006;75:804–811. [PubMed] [Google Scholar]
- 54.Newton PN, Hampton CY, Alter-Hall K, Teerwarakulpana T, Prakongpan S, Ruangveerayuth R, White NJ, Day NPJ, Tudino MB, Mancuso N, Fernandez FM. Am. J. Trop. Med. Hyg. 2008;79:662–669. [PMC free article] [PubMed] [Google Scholar]
- 55.Newton PN, Lee SJ, Goodman C, Fernandez FM, Yeung S, Phanouvong S, Kaur H, Amin AA, Whitty CJ, Kokwaro GO, Lindegardh N, Lukulay P, White LJ, Day NP, Green MD, White NJ. PLoS Med. 2009;6:e52. doi: 10.1371/journal.pmed.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machin D, Campbell MJ, Tan S-B, Tan S-H. Sample Size Tables for Clinical Studies. 3rd edn BMJ Books; 2008. [Google Scholar]
- 57.Newton PN, McGready R, Fernandez FM, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJM, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NPJ, Greenwood BM, Nosten F, White NJ. PLoS Med. 2006;3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lon CT, Tsuyuoka R, Phanouvong S, Nivanna N, Socheat D, Sokhan C, Blum N, Christophel EM, Smine A. Trans. R. Soc. Trop. Med. Hyg. 2006;100:1019–1024. doi: 10.1016/j.trstmh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 59. http://www.pharmacyboardkenya.org/
- 60.Rodomonte AL, Gaudiano MC, Antoniella E, Lucente D, Crusco V, Bartolomei M, Bertocchi P, Manna L, Valvo L, Alhaique F, Muleri N. J. Pharm. Biomed. Anal. 2010;53:215–220. doi: 10.1016/j.jpba.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 61.Green M, Mount DL, Wirtz RA, White NJ. J. Pharm. Biomed. Anal. 2000;24:65–70. doi: 10.1016/s0731-7085(00)00360-5. [DOI] [PubMed] [Google Scholar]
- 62.Jäahnke RWO, Küsters G. Mekong Malaria Forum. 2001;8:118–124. [Google Scholar]
- 63.Sherma J. Acta Chromatogr. 2007;19:5–20. [Google Scholar]
- 64.Risha PG, Msuya Z, Clark M, Johnson K, Ndomondo-Sigonda M, Layloff T. Health Policy. 2008;87:217–222. doi: 10.1016/j.healthpol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Bate R, Tren R, Hess K, Mooney L, Porter K. Afr. J. Pharm. Pharmacol. 2009;3:165–170. [Google Scholar]
- 66.Mullard A. Nat.Med. (N. Y., NY, U. S.) 2010;16:361. doi: 10.1038/nm0410-361a. [DOI] [PubMed] [Google Scholar]
- 67. http://AllAfrica.com.
- 68.Macleod NA, Matousek P. Pharm. Res. 2008;25:2205–2215. doi: 10.1007/s11095-008-9587-2. [DOI] [PubMed] [Google Scholar]
- 69.Lei Y, Luo Z-Y, Hu C-Q. J. Near Infrared Spectrosc. 2008;16:349–355. [Google Scholar]
- 70.Rodionova OY, Houmoller LP, Pomerantsev AL, Geladi P, Burger J, Dorofeyev VL, Arzamastsev AP. Anal. Chim. Acta. 2005;549:151–158. [Google Scholar]
- 71.Storme-Paris I, Rebiere H, Matoga M, Civade C, Bonnet PA, Tissier MH, Chaminade P. Anal. Chim. Acta. 2010;658:163–174. doi: 10.1016/j.aca.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Dubois J, Wolff J-C, Warrack JK, Schoppelrei J, Lewis EN. Spectroscopy. 2007;22:36–41. [Google Scholar]
- 73.Claypool GE, Holser WT, Kaplan IR, Sakai H, Zak I. Chem. Geol. 1980;28:199–260. [Google Scholar]
- 74.Chen H, Gamez G, Zenobi R. J. Am. Soc. Mass Spectrom. 2009;20:1947–1963. doi: 10.1016/j.jasms.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 75.Harris GA, Nyadong L, Fernandez FM. Analyst. 2008;133:1297–1301. doi: 10.1039/b806810k. [DOI] [PubMed] [Google Scholar]
- 76.Harris GA, Hostetler DM, Hampton CY, Fernandez FM. J. Am. Soc. Mass Spectrom. 2010;21:855–863. doi: 10.1016/j.jasms.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 77. http://www.who.int/medicines/publications/essentialmedicines/en/
- 78.Gergov M, Ojanpera I, Vuori E. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2003;795:41–53. doi: 10.1016/s1570-0232(03)00498-7. [DOI] [PubMed] [Google Scholar]
- 79.Kind T, Fiehn O. BMC Bioinf. 2007;8:105–125. doi: 10.1186/1471-2105-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]