Abstract
The physiological mechanisms leading to Scots pine (Pinus sylvestris L.) decline in the dry inner Alpine valleys are still unknown. Testing the carbon starvation hypothesis, we analysed the seasonal course of mobile carbohydrate pools (NSC) of Scots pine growing at a xeric and a dry-mesic site within an inner Alpine dry valley (750 m a.s.l., Tyrol, Austria) during the year 2009, which was characterized by exceptional soil dryness. Although, soil moisture content dropped to c. 10% at both sites during the growing season, NSC concentrations were rising in all tissues (branch, stem, root) till end of July, except in needles where maxima were reached around bud break. NSC concentrations were not significantly different in the analysed tissues at the xeric and the dry-mesic site. At the dry-mesic site NSC concentrations in the above ground tree biomass were significantly higher during the period of radial growth. An accumulation of NSC in roots at the end of July indicates a change in carbon allocation after an early cessation in above ground growth, possibly due to elevated below ground carbon demand. In conclusion our results revealed that extensive soil dryness during the growing season did not lead to carbon depletion. However, even though C-reserves were not exhausted, a sequestration of carbohydrate pools during drought periods might lead to deficits in carbon supply that weaken tree vigour and drive tree mortality.
Keywords: non-structural carbohydrates, Scots pine, drought, dry inner Alpine valley, carbon starvation, tree mortality
Introduction
During the last decades a decline of Scots pine (Pinus sylvestris L.) stands in the dry inner alpine valleys has been reported (e.g. Rigling & Cherubini 1999, Oberhuber 2001, Rebetez and Dobbertin 2004, Bigler et al. 2006). Close to the southern limits of distribution, in the Swiss Rhone Valley, locally half of the Scots pine died between 1995 and 2000 (Rigling & Cherubini 1999), but also in the western parts of the Inn valley (Tyrol) increased mortality rates of Scots pine were detected (Schwanninger 1998, Oberhuber 2001). These forest ecosystems are characterised by high summer temperatures and low precipitation and are regarded as sensitive to climate change. Therefore, high tree mortality has been related to a more frequent occurrence of severe drought and high temperatures during the last decades (e.g. Rebetez & Dobbertin 2004, Allen et al., 2010). Several tree ring studies conducted in the dry inner alpine valleys have confirmed that severe drought during the growing season results in long-lasting growth reductions and increased tree mortality (Oberhuber 2001, Bigler et al. 2006). Though, single extreme drought years seem to have minor impact (Pichler & Oberhuber 2007), whereas drought during several years might increase tree mortality (Bigler et al. 2006).
The physiological mechanisms underlying drought mortality are poorly understood. McDowell et al. (2008) formalized two hypotheses explaining physiological mechanisms for increasing world wide drought induced tree mortality: (1) hydraulic failure and (2) carbon starvation. However, these hypotheses are still highly controversial (e.g. Leutzinger et al. 2009, Sala 2009, Sala & Hoch 2009, Sala et al. 2010, Allen et al. 2010, McDowell & Sevanto 2010).
Zweifel & Zeugin (2008) found regular xylem cavitation in Scots pine under drought conditions in an inner alpine environment but whether the registered cavitation events can lead to hydraulic-failure (i.e. whole plant desiccation by widespread or total cavitation of the xylem) still has to be investigated. Results from other authors suggest, that only parts of the xylem are cavitated as mature Scots pine closes its stomata sufficiently to maintain water potential below cavitation thresholds and thus avoids runaway cavitation (Irvine et al. 1998, Perks et al. 2005). However, the carbon-starvation hypothesis could be relevant for an isohydric species like Scots pine. According to the hypothesis, stomatal closure to prevent desiccation causes deficits in photosynthetic carbon uptake, which together with continued metabolic demand, can deplete carbohydrate reserves. This can lead to direct starvation or weaken defence against insects or pathogens which subsequently causes tree mortality. For Scots pine stands in inner alpine dry valleys, such an amplifying of the negative effects of drought by pathogens and insects has been reported (Desprez-Loustau et al. 2006; Rouault et al., 2006, Wermelinger et al. 2008).
Non-structural carbohydrate (NSC) pools fuel tree metabolism in periods of low photosynthetic activity, but also provide energy for adaptive responses to soil water deficits and pathogen attacks (Lyr 1992, Barbaroux & Breda 2002, Barbaroux et al. 2003). Thus for trees suffering regular drought periods, these carbohydrate reserves can be crucial. Carbohydrate pools of Scots pine have been analysed in several studies and under different focuses (e.g. Saranpää & Höll 1989, Fischer & Höll 1991, 1992, Hansen & Beck 1990, 1994, Hansen et al. 1996, Terziev et al. 1997, Oleksyn et al. 2000, Hoch et al. 2002, 2003), but we are not aware of any study examining the NSC pools of Scots pine under drought stress conditions. Thus, we analysed the seasonal course of mobile carbohydrate pools of Scots pine growing at a xeric and a dry-mesic site to improve knowledge about the physiological mechanisms driving Scots pine decline in inner alpine dry valleys. We hypothesized that due to strained soil water availability at dry inner alpine sites, mobile carbohydrate pools are reduced, leading to periods of carbon shortage during drought.
Materials and methods
Study area
The study sites are situated in the area of a post glacial rockslide, within the inner Alpine dry valley of the upper Inn river (Tyrol, Austria, 47° 14′ 00″ N, 10° 50′ 20″ E) at c. 750 m a.s.l.. The climate is relatively continental with a mean annual temperature of 7,3°C and mean annual precipitation of 716 mm, with spring being the driest season in more than 30 % of years (long-term records (LTM) of total monthly precipitation and mean monthly temperatures since 1911 were available from a nearby meteorological station at Ötz, 812 m a.s.l, c. 5km from the study plots). Soils are predominantly of protorendzina type and consist of unconsolidated, coarse-textured materials (according to FAO 1998). Water balance is strained due to low water holding capacity of the shallow stony soils, which reach a maximum depth of c. 30 cm. The dominant plant community in the study area is a Spring Heath-Pine wood (Erico-Pinetum typicum, Ellenberg & Leuschner 2010), which is widespread on dolomitic and calcareous parent material within inneralpine dry valleys.
We selected two sites differing in aspect and soil development. A xeric open stand with trees of declined vigour (cf. Wermlinger et al. 2008) and scattered dead trees, at a south- to south-west facing slope with shallow stony soil (Syrosem) and pioneer vegetation prevailing in the ground flora. The second dry-mesic site was situated in a hollow (partly facing north) with deeper soils (Protorendzina) and higher stand density. Here the understory was dominated by crowberry (Vaccinium vitis-idaea L.) and a dense moss layer. Mean age and stem diameter of trees, were not significantly different but trees were about twice as high at the moderate compared to the xeric site (Table 1).
Table 1.
Site description and characteristics of selected stands (CC = canopy coverage, SDM = stem diameter, SD = standard deviation)
| Site | Aspect | Slope (°) |
Soil depth (cm) |
CC (%) |
Stand height (m) |
SDM1
(cm) mean ± SD |
Tree age1
(yr) mean ± SD |
|---|---|---|---|---|---|---|---|
| xeric | SW | 40 | 0-10 | 33 | 4-5 | 24.4 ± 4.9 | 150 ± 30 |
| dry-mesic | N | <10 | 20-30 | 66 | 10 | 29.3 ± 3.2 | 167 ± 23 |
Stem diameter and tree age were determined at 1 m height.
Microclimate records
Air temperature at 2 meters height (Onset, Pocasset, MA, USA), soil moisture (volumetric water content) and soil temperature at c. 10 cm soil depth were continuously monitored at a 30 minute interval (Cyclobios, proprietary development at University of Innsbruck, Austria). Due to small scale variability of soil structure, values of three sensors per plot were averaged. Daily means were calculated by averaging all measurements of the respective day (48 values/day). At the ridge at the xeric site daily precipitation was recorded at a 30 minute interval during the sampling year (Onset, Pocasset, MA, USA). Precipitation data were regarded representative for both sampling sites as they were located within 150 m linear distance.
Sampling for NSC analysis
Samples were taken from 6 to 7 pre-selected dominant trees of each site at 6 sampling dates (March 18, April 21, June 8, July 27, September 24, December 3) in 2009.
At each sampling date the following tissues were collected: (1) one year old needles, (2) branch wood with bark removed (diameter c. 1cm), (3) the sapwood fraction of stem xylem, (4) root wood from coarse roots. Although a study by Li et al. (2001) has shown that sampling position within the crown has no significant influence on NSC concentrations, needle and branch tissues were collected from sun exposed branches growing at the south facing side of the trees. Stem xylem at breast height and root tissue from 5 to 10 cm soil depth were sampled using a 5 mm increment corer. Samples were always collected around noon to minimise effects of diurnal NSC changes (Li et al. 2008).
The sampled material was stored in a cool box immediately after collection. Within three hours enzymes in the samples were denatured by heating the samples in a microwave at 600 W for 90 seconds (Hoch et al. 2002, Hoch & Körner 2003). Afterwards the samples were dried to weight constancy at 60°C, ground to powder and stored dry until they were analysed.
Analysis of NSC
For binding plant phenols 0,1 mg Polyvinylpyrrolidon was added to approximately 10 mg of fine ground plant material. Soluble carbohydrates were extracted from the weight in samples twice in 80% (v/v) acetone for 15 minutes at 50°C. After vaporizing the acetone, the residual of the soluble fraction was resolved in distilled water and the concentration of glucose was determined photometrical at 340nm, as NADPH+ H+ formation during enzymatic conversion of glucose-6-phosphate to gluconate-6-phosphate.
Aliquots of the resolved extract were treated with hexocinase and isomerase as well as invertase, to convert fructose and sucrose into glucose, which was subsequently measured as described above. For starch measurements, starch extraction was carried out by incubating the insoluble fraction with hydrochloric acid for 2 h at 60°C. After pH adjustment, starch was hydrolyzed enzymatically to glucose and subsequently measured. The photometric analyses were conducted using test combinations from Boehringer Mannheim (Mannheim, Germany).
Radial growth
Wood formation was monitored during the growing season 2009 by taking small punched cores from 5 trees/site of the outermost tree rings (micro-cores) with a diameter and length of 2.5 mm and c. 2 cm, respectively (Rossi et al. 2006). Micro-cores were taken at both study plots during March through October in weekly to 10 day intervals.
Because c. 20 samples were taken throughout the growing season from each selected tree, micro-cores were not sampled from the trees used for carbohydrate analyses, to avoid effects of wounding. Samples were taken starting at c. 1 m stem height on the slope-parallel side and following a spiral trajectory up the stem.
Micro-cores were embedded in glycolmethacrylate (Technovit 7100; Heraeus Kulzer GmbH, Germany) and transverse sections of c. 12 μm were cut with a rotary microtome. Afterwards the number of newly build tracheids was determined under the light microscope.
The mean radial ring widths of the years 2007 to 2009 were determined at the end of the growing season, by measuring four micro-cores taken from different aspects around the sampling trees.
Scaling to the tree level
NSC measurements from individual tissues were scaled up to tree level to provide estimates of NSC concentrations in the total above ground tree biomass. Therefore one tree per site typical in growth form and height was selected to determine above ground tree biomass distribution (i.e. biomass of needles, branches and stem-sapwood). Due to stony soils an evaluation of belowground biomass was not possible. As the study area is situated in a nature reserve, the felling of more than one tree per site was not possible.
Statistical analyses
Differences of NSC concentrations between sampling dates were tested for significance by applying post-hoc multiple comparison (Tukey HSD test). Site effects (xeric vs. dry-mesic) on NSC concentrations throughout the year were tested for significance for all sampled tissues and above ground biomass by repeated measure analysis applying a Bonferroni correction. All tests were performed using SPSS 18 (IBM, New York, NY, USA.
Results
Environmental variables during growing seasons 2009
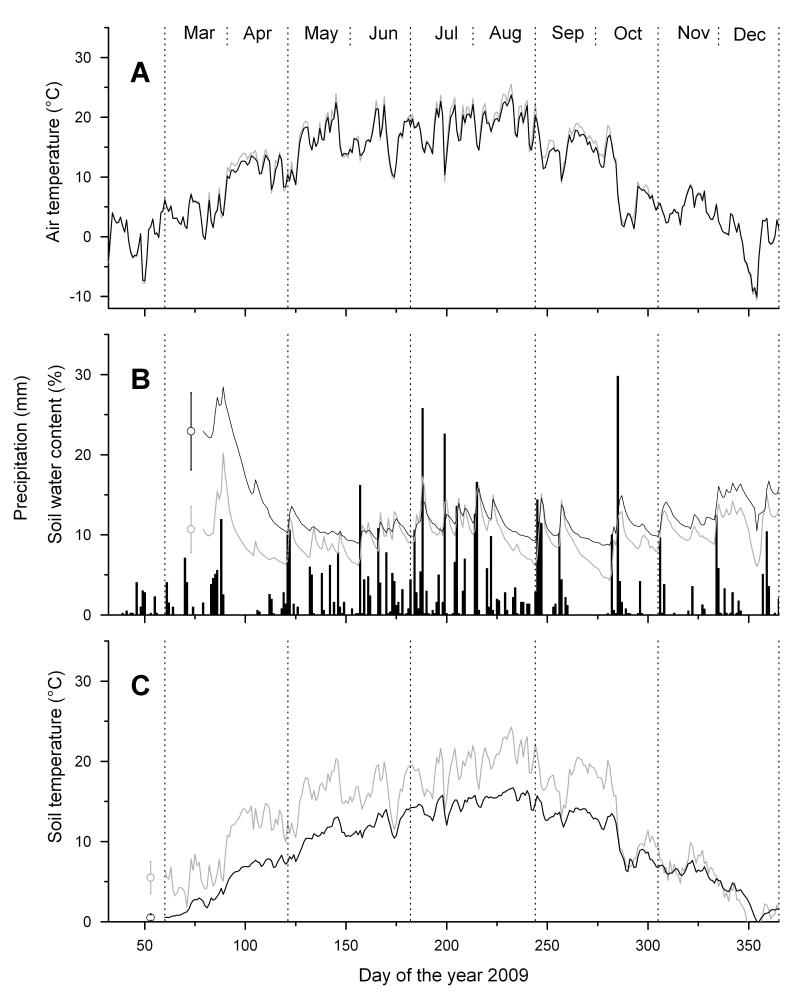
Climate in 2009 was characterized by the occurrence of exceptionally high temperatures in April and May (2.95°C and 1.95°C above LTM, respectively) and an almost continuous drought period during April (Fig. 1), when total monthly precipitation reached 2.5 mm only. From March to October mean daily temperatures were 13.0°C (LTM 11.9°C) and precipitation reached 579 mm (LTM 561 mm).
Fig. 1.
Climate variables recorded during the growing season 2009 within the study area. A Mean daily air temperature; B Daily precipitation sum (bars) and mean daily soil water content; C Mean daily soil temperature. Study sites are denoted by grey and black lines for the xeric and dry-mesic site, respectively. Mean standard deviations among soil moisture and soil temperature sensors (n = 3) are indicated.
Due to differences in aspect and canopy coverage (Tab. 1), mean air and soil temperatures in 2009 were lower at the dry-mesic site than at the xeric site (Fig. 1). From April to October mean air temperatures differed by only 0.59°C, while soil temperatures were 4.4°C higher at the xeric site.
During the drought period in April 2009 soil water content dropped to 9.8 vol. % at the dry-mesic and 9.0 vol. % at the xeric site (Fig. 1). Mean soil water content from April to October was 9.5 vol.% and 11.7 vol.% for the xeric and dry-mesic stand, respectively. Soil water content was significantly lower than in the two proceeding years, when values were more than 50 and 25% higher at the dry-mesic and xeric site, respectively (cf. Gruber et al. 2010).
Seasonal dynamics of NSC
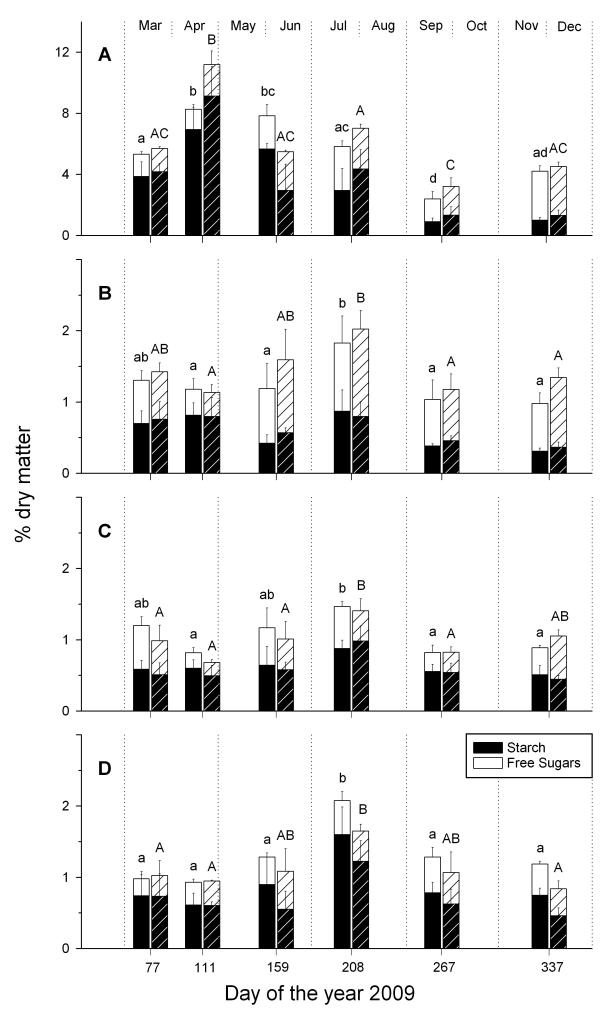
NSC concentrations changed significantly during the year in all sampled tissues (Fig. 2, Table S1). At both sites, one year old needles showed a strong increase of starch around bud break. Afterwards needle starch concentrations decreased continually and were lowest at the end of the season, whereas concentration of free sugars (e.g. sucrose, glucose and fructose) was highest in December. NSC and in particular concentrations of free sugars were higher in branch-wood than in stem-wood throughout the measuring period. In both branch- and stem-wood, variations of NSC concentrations during the year followed the same trends, though variations were less distinct in stem-wood. NSC values declined after beginning of radial growth and bud break in April. Afterwards they increased again till they reached a maximum at end of July, after which they declined markedly. Root-wood NSC concentrations were quite stable in spring and increased slightly at beginning of June and peaked at the end of July. Afterwards NSC concentrations decreased again till end of season. Average NSC concentrations in above and below ground woody tissues were below 1.5 % of dry matter throughout the year. The carbohydrate concentrations at the xeric and the dry-mesic site were not significantly different in the analysed tissues throughout the year.
Fig. 2.
Mean concentrations (% dry matter) of free sugars and starch (NSC) in (A) needles, (B) branches, (C) stems and (D) coarse roots of Scots pine, growing at a dry-mesic and xeric site (hedged bars), at six sampling dates during the year 2009. Different letters indicate significantly different NSC concentrations among sampling dates (P < 0.05, lower case letters: dry mesic site, upper case letters: xeric site). Note the difference in scaling of the y-axis between A (needles) and B-D (woody tissues).
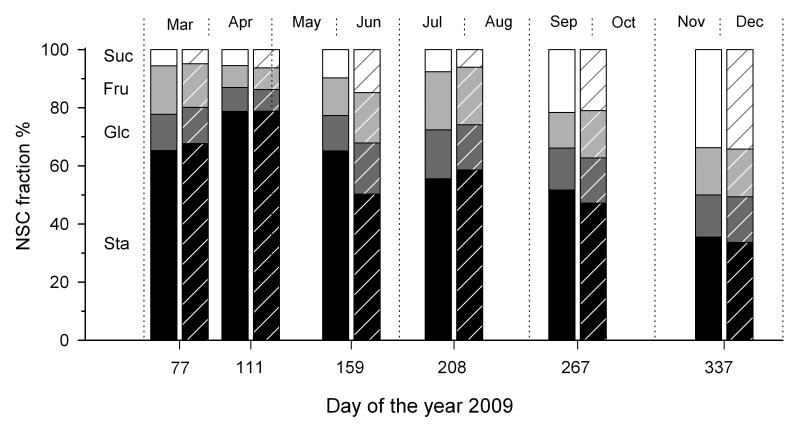
Starch was the major fraction throughout the growing season with a maximum percentage of 79% of total NSC (mean across all tissue-types) at both sites in April (Fig. 3) and a minimum in December (xeric site 34%, dry-mesic site 36%). Glucose and fructose reached a maximum percentage at the end of July (glucose 16% and 17 % at the xeric and dry-mesic site, respectively; fructose 20% at both sites). Sucrose concentrations were low till September, when sucrose started to accumulate in the above ground tissues (data not shown) till it reached a maximum in December (34 % of total NSC at both sites).
Fig. 3.
Relative changes in fractions of NSC (starch - Sta, glucose - Glc, fructose - Fru, sucrose - Suc) growing at the dry-mesic site and xeric site (hatched bars), at six sampling dates during the year 2009. Means across all tissue-types are shown.
Scaling to the tree level
Measured above ground tree biomass (i.e. stem sapwood, branch-wood and needles) accounted for 65kg and 64kg at the dry-mesic and xeric site, respectively. Biomass of stem-sapwood was higher at the dry-mesic (62% of analysed above ground biomass) compared to the xerix site (37% of analysed above ground biomass), whereas percentage of branch biomass was higher at the xeric site (xeric: 60%, mesic site: 29% of analysed above ground biomass). Trees at the dry-mesic site were well foliated while at the xeric site an increased crown transparency was evident. Needle mass accounted for 9% and 3% of analysed above ground biomass at the dry-mesic and the xeric site, respectively.
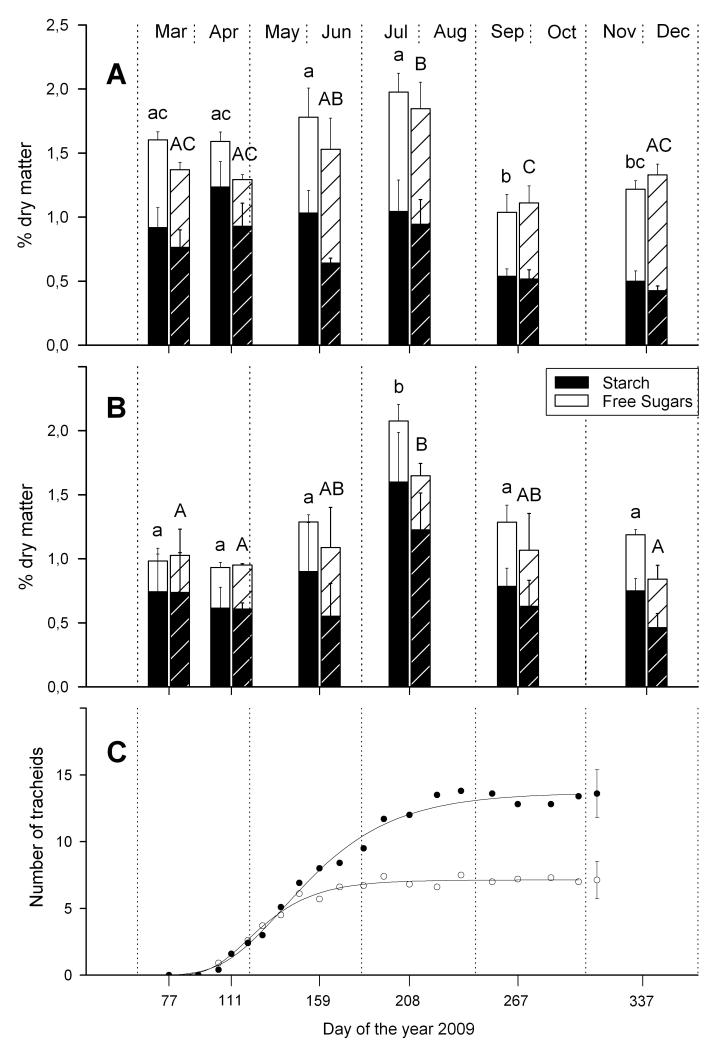
During the period of radial growth (sampling dates from March to end of July), NSC concentrations in the above ground tree biomass was significantly higher at the dry-mesic compared to the xeric site (+ 13,53 Diff.%, P = 0.022; Diff. % refers to the percentage increase in mobile carbon concentrations from the xeric to the dry-mesic site). However, differences between the two sites originated from the significantly higher starch concentrations at the dry-mesic site during this period (+ 22,98 Diff.%, P = 0.003) whereas the concentrations of free sugars were not significantly different. The starch concentration was significantly higher at the dry-mesic site across all sampling dates (+ 19,99 Diff.%, P = 0.009) (Fig. 4).
Fig. 4.
A Mean concentrations (% dry matter) of free sugars and starch (NSC) in above ground tree biomass of Scots pine, growing at a dry-mesic and xeric site (hedged bars). B. Mean concentrations (% dry matter) of free sugars and starch (NSC) in coarse roots of Scots pine, growing at a dry-mesic and xeric site (hedged bars). In A and B different letters indicate significantly different NSC concentrations among sampling dates (P < 0.05, lower case letters: dry mesic site, upper case letters: xeric site).C Dynamics of xylem growth in 2009 at the dry-mesic site (solid symbols) and xeric site (open symbols) modelled by applying the Gompertz function.
Radial growth and phenology in 2009
Bud break and onset of wood formation (first radial enlargement of tracheids) was detected in mid April on both, the xeric and dry-mesic site (Fig. 4). The formation of new tracheids was completed around the beginning of July and August at the xeric and dry-mesic site, respectively. The mean total number of new tracheids produced was lower at the xeric site (7.1 ± 1.4 cells) than at the dry-mesic site (13.6 ± 1.8 cells).
Discussion
In the upper Inn valley the growing season 2009 was characterized by exceptional soil dryness. Even though mean annual precipitation was around LTM, mean soil water contents were drastically reduced compared to the preceding years. Minor rain events after a drought period in April were not able to sufficiently replenish the desiccated soils. Hence, 2009 was quite appropriate for analysing the influence of soil dryness on mobile carbohydrate pools and for putting the starvation theory formulized by McDowell et al. (2008) to the test.
NSC concentrations in branch wood measured throughout 2009, where comparable to values measured by Hoch & Körner (2003) in Scots pine in often drained terrain near the treeline in northern Sweden, whereas Hoch et al. 2003 found twice the concentration in trees growing in a temperate forest, which is pointing to reduced NSC pools in branchwood under stress conditions. However NSC concentrations in stem wood of Scots pine seemed to be less affected by site conditions and was quite in the same range as data measured by other authors under different growth conditions (Terziev et al. 1997, Hoch & Körner 2003, Hoch et al. 2003). This is consistent to Hoch et al. 2003 who stated, that variation in NSC confined mainly to branches, while stem reserves are hardly involved. Even though NSC concentrations in one year old needles were reduced to less than half during the whole year if compared to data measured in Scots pine not exposed to drought stress (Hoch & Körner 2003 and Hoch et al. 2003), carbohydrate pools were never depleted.
Scots pine stomata have been found to close disproportionately more than those of neighbouring species under dry conditions (Zweifel et al. 2009), but direct starvation (cf. McDowell et al. 2008) was not detected in our study. Despite continuing soil dryness NSC concentrations were rising in all analysed tissues till end of July, except in needles were maxima are typically reached around bud break (cf. Fischer & Höll 1991, Oleksyn et al. 2000, Hoch et al. 2002, 2003). This is consistent with analysis of actual transpiration of Scots pine made during the drought year 2003 in an inner alpine dry valley in Switzerland, where transpiration was drastically reduced, but the trees were able to maintain a low but continuous activity throughout the summer (Zweifel et al. 2006). On the other hand, a reduction of photosynthetic activity due to more prevalent stomatal closure could explain the low carbohydrate concentrations in needles throughout the year (cf. Hoch & Körner 2003 and Hoch et al. 2003).
Sufficient C assimilation despite extensive soil dryness could to some extend be attributed to a partial release of low leaf water potential by the number of little rain events wetting the crown without replenishing the soil. Breshears et al. (2008) observed that foliar absorption of intercepted rainfall led to substantial improvement in water status of Juniperus monosperma during drought and for Scots pine Zweifel et al. (2005, 2006) noted, that wetting of the leaves alone seemed to be enough to accelerate growth.
Though NSC reserves were not depleted, above ground radial growth was drastically reduced in 2009 compared to the preceding years (cf. Gruber et al. 2010). This indicates that drought constrained carbon sinks more than carbon sources and several authors (e.g., Savidge 1996; Abe & Nakai 1999) found, that cell division is more sensitive to decreasing water potential than are photosynthesis and stomatal closure. In fact tree water status (Zweifel et al. 2006) and water availability (Eilmann et al. 2009) have been shown to limit radial growth within dry inner Alpine valleys. Hence, significantly higher radial growth rates in preceding years and almost twice as wide annual increments reported from inner Alpine dry stands in Switzerland (Rigling et al. 2002) can most likely be attributed to better soil water availability (Brunner et al. 2009, Gruber et al. 2010).
Körner (1989) stated that at (moderately) dry inner alpine stands, stand density and related differences in shading of needles had more influence on CO2 assimilation rates of Scots pine than soil dryness. Nevertheless, even though stand density at the xeric site was significantly lower (Tab. 1), not only growth rates but also whole tree NSC pools were significantly reduced compared to the dry-mesic site during the period of radial growth (Fig. 4). Because NSC concentrations (in particular starch) were considerably higher in needles than in other tissues, site-specific differences can mainly be attributed to reduced needle mass at the xeric site. Still, Zweifel et al. (2006) found that in Scots pine at dry inner alpine stands carbon assimilation was highest in spring, markedly reduced during dry conditions in summer but hardly ever ceased completely. Considering this an accumulation of NSC due to sink inhibition (reduced growth rates) could be expected (Hoch et al. 2002, Körner 2003, McDowell & Sevanto 2010, Sala et al. 2010). However, reduced NSC pools at both sites suggest, that not only carbon sink but also carbon gain (source) was hampered due to soil dryness.
In September an accumulation of sucrose in the above ground tissues (Fig. 3), which is associated with the development of winter freezing tolerance (e.g. Oleskyn et al. 2000, Hoch et al 2002), indicates the cessation of cambial activity and transition to dormancy stage. Even after above ground growth processes were finished (Fig. 4) no accumulation of carbohydrates in above ground organs was detected, which indicates that C assimilates were used for below ground growth and metabolism (Zweifel et al. 2006, Barbaroux et al., 2003). The early decrease in above ground growth rate within the study area can be regarded as an adaptation to cope with extreme environmental conditions, which might cause elevated carbon demand of the root system and mycorrhiza to ensure adequate water and nutrient supply (Gruber et al. 2010, Oberhuber et al. 2010). Increased carbon allocation to roots in response to drought and low nutrient supply is a well known phenomenon (Poorter & Nagel 2000, Millard et al. 2007, McDowell et al. 2008) and that the root system in Scots pine is a strong sink for carbohydrates under drought conditions is supported by results of Brunner et al. (2009), who reported a lack of increase in fine root biomass in response to irrigation treatment in a comparable dry inner Alpine environment.
The accumulation of NSC in course roots at the end of July (Fig. 4) indicates a change in carbon allocation due to a decrease in above ground sink demands at the end of the above ground growth period. In Scots pine a major part of carbohydrates is utilized for root growth after above ground growth is finished (Hansen & Beck 1994) and it is well known that the allocation of current photosynthate-carbon to below ground is at maximum in late summer and autumn (e.g., Ursino et al. 1968; Hansen & Beck 1990; Bhupinderpal-Singh et al. 2003). However, not all carbohydrates allocated below ground are infested into root growth. Up to one-third of the photoassimilates are estimated to be necessary for ectomycorrhizal functioning (for review, see Nehls et al. 2007). Especially under poor soil conditions where mycorrhizal symbiosis is a way to overcome nutrient deficiency of the substrate, reduced carbohydrate pools might originate from enhanced investment in mycorrhizal symbiosis, which is known to significantly reduce carbohydrate concentrations in Scots pine (Wallander & Nylund 1991).
Increasing mortality in the inner alpine dry valleys denotes that these Scots pine stands are at their physiological limit (Zweifel et al. 2009). Though radial growth was severely reduced the extensive soil dryness during the growing season 2009 did not lead to carbon depletion. However, there are hints that NSC pools in trees are never fully depleted (Millard et al. 2007), and Sala et al. (2010) put forward the hypotheses that stored C pools become inaccessible to transport under water stress and C starvation can occur even though the C-reserves are not exhausted. Such a sequestration of carbohydrate pools during drought periods can lead to temporary deficits in carbon supply that weaken tree vigour and drive tree mortality.
Supplementary Material
Acknowledgments
This work was supported by the Austrian Science Fund (FWF Project Nos. P19563-B16, “Dynamics of cambial activity and wood formation of Scots pine (Pinus sylvestris L.) exposed to soil dryness” and P22836-B16 “Growth response of Pinus cembra to experimentally modified soil temperatures at the treeline”) and the Daniel Swarovski donation. We greatly acknowledge Hydrographischer Dienst, Innsbruck, for providing us climate data and Gerhard Wieser from BFW for his support in analysing tree biomass.
References
- Abe H, Nakai T. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D Don. Trees. 1999;14:124–129. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bacheler D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg (Ted) EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Barbaroux C, Bréda N. Contrasting distribution and seasonal dynamics of carbohydrates reserves in the stem wood of adult ring porous (sessile oak) and diffuse porous (common beech) trees. Tree Physiology. 2002;22:1201–1210. doi: 10.1093/treephys/22.17.1201. [DOI] [PubMed] [Google Scholar]
- Barbaroux C, Bréda N, Dufrêne E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica) New Phytologist. 2003;157:605–615. doi: 10.1046/j.1469-8137.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- Bhupinderpal-Singh Nordgren A., Ottosson Löfvenius M, Högberg MN, Mellander PE, Högberg P. Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environm. 2003;26:1287–1296. [Google Scholar]
- Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems. 2006;9:330–343. [Google Scholar]
- Breshears DD, McDowell NG, Goddard KL, Dayem KE, Martens SN, Meyer CW, Brown KM. Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology. 2008;89:41–47. doi: 10.1890/07-0437.1. [DOI] [PubMed] [Google Scholar]
- Brunner I, Graf-Pannatier E, Frey B, Rigling A, Landolt W, Dobbertin M. Morphological and physiological responses of Scots pine fine roots to water supply in a climatic dry area in Switzerland. Tree Physiology. 2009;29:542–550. doi: 10.1093/treephys/tpn046. [DOI] [PubMed] [Google Scholar]
- Desprez-Loustau ML, Marcais B, Nageleisen LM, Piou D, Vannini A. Interactive effects of drought and pathogens in forest trees. Annals of Forest Science. 2006;63:597–612. [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Fonti P, Rigling A. Droughtinduced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiology. 2009;29:1011–1020. doi: 10.1093/treephys/tpp035. [DOI] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. ökologischer, dynamischer und historischer Sicht. Verlag Eugen Ulmer Stuttgart; 2010. Vegetation Mitteleuropas mit den Alpen. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 1998. [Google Scholar]
- Fischer C, Höll W. Food reserves of Scots pine (Pinus sylvestris L.) I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees. 1991;5:187–195. [Google Scholar]
- Fischer C, Höll W. Food reserves of Scots pine (Pinus sylvestris L.) II. Seasonal changes and radial distribution of carbohydrate and fat reserves in pine wood. Trees. 1992;6:147–155. [Google Scholar]
- Gruber A, Strobl S, Veit B, Oberhuber W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiology. 2010;30:490–501. doi: 10.1093/treephys/tpq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Beck E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees. 1990;4:16–21. [Google Scholar]
- Hansen J, Beck E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees. 1994;8:172–182. [Google Scholar]
- Hansen J, Vogg G, Beck E. Assimilation, allocation and utilization of carbon by 3-year-old Scots pine (Pinus sylvestris L.) trees during winter and early spring. Trees. 1996;11:83–90. [Google Scholar]
- Hoch G, Körner C. The carbon charging of pines at the climatic treeline: a global comparison. Oecologia. 2003;135:10–21. doi: 10.1007/s00442-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Hoch G, Popp M, Körner C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos. 2002;98:361–374. [Google Scholar]
- Hoch G, Richter A, Körner C. Non-structural carbon compounds in temperate forest trees. Plant, Cell and Environment. 2003;26:1067–1081. [Google Scholar]
- Irvine J, Perks MP, Magnani F, Grace J. The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiology. 1998;18:393–402. doi: 10.1093/treephys/18.6.393. [DOI] [PubMed] [Google Scholar]
- Körner C. Die Bedeutung von Wassermangel und winterlicher Schadgasbelastung für das Waldsterben. In: Führer E, Neuhuber F, editors. Waldsterben in Österreich: Theorien, Tendenzen, Therapien; FIW-Symposium; Universität für Bodenkultur, Wien. 1988.Oktober. 1989. pp. 127–138. [Google Scholar]
- Körner C. Carbon limitation in trees. Journal of Ecology. 2003;91:4–17. [Google Scholar]
- Leuzinger S, Bigler C, Wolf A, Körner C. Poor methodology for predicting large-scale tree die-off. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:E106. doi: 10.1073/pnas.0908053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Hoch G, Körner C. Spatial variability of mobile carbohydrates within Pinus cembra trees at the alpine treeline. Phyton. 2001;41:203–213. [Google Scholar]
- Li MH, Xiao WF, Wang SG, Cheng G, Cherubini P, Cal XH, Liu XL, Wang XD, Zhu WZ. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiology. 2008;28:1287–1296. doi: 10.1093/treephys/28.8.1287. [DOI] [PubMed] [Google Scholar]
- Lyr H. Physiologie und Ökologie der Gehölze. Gustav Fischer Publ; Jena, Germany: 1992. [Google Scholar]
- McDowell NG, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams D, Yepez EA. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Sevanto S. The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytologist. 2010;186:264–266. doi: 10.1111/j.1469-8137.2010.03232.x. [DOI] [PubMed] [Google Scholar]
- Millard P, Sommerkorn M, Grelet GA. Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytologist. 2007;175:11–28. doi: 10.1111/j.1469-8137.2007.02079.x. [DOI] [PubMed] [Google Scholar]
- Nehls U, Grunze N, Willmann M, Reich M, Küster H. Sugar for my honey: carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry. 2007;68:82–91. doi: 10.1016/j.phytochem.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Oberhuber W. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia. 2001;19:45–55. [Google Scholar]
- Oberhuber W, Gruber A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees. 2010;24:887–898. doi: 10.1007/s00468-010-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksyn J, Zytkowiak R, Karolewski P, Reich PB, Tjoelker MG. Genetic and environmental control of seasonal carbohydrate dynamics in trees of diverse Pinus sylvestris populations. Tree Physiology. 2000;20:837–847. doi: 10.1093/treephys/20.12.837. [DOI] [PubMed] [Google Scholar]
- Perks MP, Irvine J, Grace J. Xylem acoustic signals from mature Pinus sylvestris during an extended drought. Annals of Forest Science. 2004;61:1–8. [Google Scholar]
- Pichler P, Oberhuber W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. Forest Ecology and Management. 2007;242:688–699. [Google Scholar]
- Poorter H, Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water. Australian Journal of Plant Physiology. 2000;27:595–607. [Google Scholar]
- Rebetez M, Dobbertin M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theoretical and Applied Climatology. 2004;79:1–9. [Google Scholar]
- Rigling A, Bräker OU, Schneiter G, Schweingruber FH. Intra-annual tree-ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico-Pinion in the Valais (Switzerland) Plant Ecology. 2002;163:105–121. [Google Scholar]
- Rigling A, Cherubini P. Wieso sterben die Waldföhren im ’Telwald’ bei Visp? Schweizerische Zeitschrift für Forstwesen. 1999;150:113–131. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA Journal. 2006;27:89–97. [Google Scholar]
- Rouault G, Candau JN, Lieutier F, Nageleisen LM, Martin JC, Warzee N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Annals of Forest Science. 2006;63:613–624. [Google Scholar]
- Sala A. Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:E68. doi: 10.1073/pnas.0904580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A, Hoch G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant, Cell & Environment. 2009;32:22–30. doi: 10.1111/j.1365-3040.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Saranpää P, Höll W. Soluble carbohydrates of Pinus sylvestris L. sapwood and heartwood. Trees. 1989;3:138–143. [Google Scholar]
- Savidge RA. Xylogenesis, genetic and environmental regulation - a review. IAWA Journal. 1996;17:269–310. [Google Scholar]
- Schwanninger C. Kiefernsterben im Oberland. Tiroler Forstdienst. 1998;3:10. [Google Scholar]
- Terziev N, Boutelje J, Larsson K. Seasonal fluctuations of low-molecular-weight sugars, starch and nitrogen in sapwood of Pinus sylvestris L. Scandinavian Journal of Forest Research. 1997;12:216–224. [Google Scholar]
- Ursino DJ, Nelson DC, Krotlov G. Seasonal changes in the distribution of photoassimilated C in young pine plants. Plant Physiology. 1968;43:845–852. doi: 10.1104/pp.43.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander H, Nylund JE. Effects of excess nitrogen on carbohydrate concentration and mycorrhizal development of Pinus sylvestris L. seedlings. New Phytologist. 1991;119:405–411. [Google Scholar]
- Wermelinger B, Rigling A, Mathis ADS, Dobbertin M. Assessing the role of bark- and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecological Entomology. 2008;33:239–249. [Google Scholar]
- Zweifel R, Rigling A, Dobbertin M. Species-specific stomatal response of trees to drought - a link to vegetation dynamics? Journal of Vegetation Science. 2009;20:442–454. [Google Scholar]
- Zweifel R, Zeugin F. Ultrasonic acoustic emissions in drought-stressed trees-more than signals from cavitation? New Phytologist. 2008;179:1070–1079. doi: 10.1111/j.1469-8137.2008.02521.x. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Zimmermann L, Newbery DM. Modeling tree water deficit from microclimate: an approach to quantifying drought stress. Tree Physiology. 2005;25:147–156. doi: 10.1093/treephys/25.2.147. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Zimmermann L, Zeugin F, Newbery DM. Intra-annual radial growth and water relations of trees: implication towards a growth mechanism. Journal of Experimental Botany. 2006;57:1445–1459. doi: 10.1093/jxb/erj125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.