Abstract
Elevated levels of the inflammatory cytokine interleukin-6 (IL-6) occur in a number of CNS disorders. However, little is known about how this condition affects CNS neuronal function. Transgenic mice that express elevated levels of IL-6 in the CNS show cognitive changes, increased propensity for hippocampal seizures and reduced number of inhibitory interneurons, suggesting that elevated levels of IL-6 can cause neuroadaptive changes that alter hippocampal function. To identify these neuroadaptive changes, we measured the levels of protein expression using Western blot analysis and synaptic function using field potential recordings in hippocampus from IL-6 transgenic mice (IL-6 tg) and their non-transgenic (non-tg) littermates. Western blot analysis showed enhanced levels of the GFAP and STAT3 in the IL-6 tg hippocampus compared with the non-tg hippocampus, but no difference for several other proteins. Field potential recordings of synaptic transmission at the Schaffer collateral to CA1 synapse showed enhanced dendritic excitatory postsynaptic potentials and somatic population spikes in the CA1 region of hippocampal slices from IL-6 tg mice compared with slices from non-tg littermate controls. No differences were observed for several forms of short-term and long-term synaptic plasticity between hippocampal slices from IL-6 tg and non-tg mice. These results demonstrate that elevated levels of IL-6 can alter mechanisms involved in the excitability of hippocampal neurons and synapses, an effect consistent with recent evidence indicating that elevated production of IL-6 plays an important role in conditions associated with seizure activity and in other impairments observed in CNS disorders with a neuroinflammatory component.
Keywords: cytokine, synaptic plasticity, long-term potentiation, post tetanic potentiation, electrophysiology, hippocampal slice, Western blot, field potential recording, population spike, field excitatory synaptic potential
1. Introduction
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that plays a crucial role in the host defense response to infection and trauma (Akira et al., 1990; Heinrich et al., 1990; Hirano, 1992). IL-6 is also an essential mediator of neuroimmune responses within the central nervous system (CNS), where it can play a neurotrophic, neuroprotective or neurotoxic role depending on conditions (Gadient and Otten, 1997; Gruol and Nelson, 1997; Spooren et al., 2011). The expression of elevated levels of IL-6 and other cytokines in the CNS is thought to be a contributing factor to the neuropathological and pathophysiological features associated with several CNS disorders such as AIDS dementia complex (Gallo et al., 1989; Tyor et al., 1992), Alzheimer’s disease (Bauer et al., 1991), multiple sclerosis (Maimone et al., 1991), epilepsy (Li et al., 2011), depression (Miller and O’Callaghan, 2005) and Parkinson’s disease (Mogi et al., 1994). Studies in experimental animals support a role for IL-6 in CNS disease states including seizure activity (Campbell et al., 1993; Kirkman et al.), infection (Sauder and de la Torre, 1999), chronic variable stress (Tagliari et al., 2011), Alzheimer’s disease (Ruan et al., 2009; Tehranian et al., 2001), and other CNS disorders (Spooren et al., 2011; Wu and Lin, 2008).
In addition to a role in CNS disease states, emerging evidence implicates a role for IL-6 in the normal regulation of physiological functions of CNS neurons. CNS neurons have been shown to express functional receptors for IL-6 (Gadient and Otten, 1994) and IL-6 production can be induced by neuronal activity (Sallmann et al., 2000). Basal levels of IL-6 in the CNS are low under normal conditions, but localized increases in IL-6 mRNA levels are produced by intense synaptic or neuronal activity such as occurs experimentally during induction of hippocampal long-term potentiation (LTP) (Balschun et al., 2004; Jankowsky et al., 2000; Jankowsky and Patterson, 1999). LTP is a form of synaptic plasticity that is thought to be a cellular mechanism underlying learning and memory (Bliss and Collingridge, 1993). Studies show that the endogenous IL-6 produced by the induction of hippocampal LTP acts as a negative regulator of LTP maintenance by limiting long-term storage of certain types of memory information (Balschun et al., 2004). Consistent with a role as a negative regulator of LTP, in vitro studies utilizing the hippocampal slice preparation showed that exogenous IL-6 applied shortly before high frequency synaptic stimulation inhibits LTP induction in the hippocampus (Li et al., 1997; Tancredi et al., 2000).
The association of IL-6 with neuronal activity may be an important contributing factor to its role in the pathological state. For example, several studies link increased levels of IL-6 in the CNS to seizure activity (for reviews see Vezzani et al., 2008a; Vezzani et al., 2008b). Moreover, elevated levels of IL-6 are produced in the brain shortly after seizure activity (Lehtimaki et al., 2004; Lehtimaki et al., 2003; Minami et al., 1991; Peltola et al., 1998) and seizure activity is a common complication of CNS conditions associated with increased levels of IL-6 such as in viral infections with febrile seizures (Getts et al., 2007; Millichap and Millichap, 2006). Acute IL-6 can induce seizures when injected directly into the CNS (Xiaoqin et al., 2005) and has a pro-convulsive effect in the CNS when applied shortly before experimentally-induced seizures in rats (Kalueff et al., 2004).
IL-6 tg mice that express elevated levels of IL-6 in the CNS show increased propensity for spontaneous and experimentally evoked seizures (Campbell et al., 1993; Samland et al., 2003), suggesting that IL-6 can produce neuroadaptive changes that enhance neuronal excitability. To address this possibility, we have investigated the levels of protein expression and characteristics of synaptically evoked neuronal activity in hippocampal slices obtained from IL-6 tg mice and their non-tg littermate controls. Increased CNS expression of IL-6 in the transgenic mice was achieved by genetic manipulation of astrocyte expression (Campbell et al., 1993), thus providing a model that simulates a normal route for IL-6 production in vivo both under normal and pathological conditions. Results show that exposure to increased levels of IL-6 in the transgenic mice enhances excitatory synaptic transmission in the hippocampus. Results also show that, with the exception of increased expression of GFAP and STAT3, the elevated levels of IL-6 did not result in prominent changes in the levels of several important proteins involved in neuronal function. This result suggests that the changes in excitability in the IL-6 tg hippocampus may reflect actions of IL-6 at specific targets rather than a general effect on neuronal survival or homeostasis.
2. Methods
2.1. Transgenic mice
Production of the IL-6 transgenic mice has been described in detail elsewhere (Campbell et al., 1993). Briefly, IL-6 expression in the CNS was targeted to astrocytes by an expression vector derived from the murine glial fibrillary acidic protein (GFAP) gene. Full-length murine IL-6 cDNA was modified and inserted into the GFAP gene. The genes were then microinjected into fertilized eggs of F1 generation hybrid mice (C57BL/6J × SJL). After weaning (3–4 weeks old) transgenic mice were identified by slot blot analysis of tail DNA. Heterozygous mice of the G167 transgenic line maintained for many years on a C57BL/6J background were used in this study. These mice expresses moderate levels of IL-6 and do not show prominent neuropathlogy at the ages we studied. Age-matched littermates that did not express the IL-6 transgene were used as controls. All animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2. Preparation of hippocampal tissue
The mice (1–6 months of age) were weighed, anesthetized with isoflurane and decapitated. Both females and males were used. Brains were rapidly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF). The right half of the brain was used for electrophysiological recordings and the left half was snap frozen on dry ice and used for protein measurements (Western blot). For the electrophysiological studies, slices of the dorsal hippocampus (400 μm) were cut using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd., Surrey, UK) and placed in a gas-fluid interface chamber maintained at approximately 33°C and wit3h an ACSF perfusion rate of 0.55 ml/min until use. The composition of the ACSF was (in mM): 130.0 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24.0 NaHCO3, 2.0 CaCl2, 1.3 MgSO4, and 10.0 glucose (all chemicals from Sigma-Aldrich, St. Louis, MO, USA). All solutions were gassed continuously with 95% O2/5% CO2 (pH 7.2–7.4). In some studies NBQX was tested.
2.3. Electrophysiological recordings
Hippocampal slices were transferred to a second gas-fluid recording chamber and allowed to stabilize for 30–60 minutes before starting experiments. The slices were continuously superfused with ACSF at a rate of 2 ml/min (33°C). A concentric bipolar stimulating electrode (FHC Inc., Bowdoin, ME, USA) was placed in the Schaffer collaterals at the border of the CA2 and CA1 regions of the hippocampus and synaptic responses were elicited by brief electrical stimulation of the fibers (50 μs duration; S88 Square Pulse Stimulator and PSIU6 Stimulus Isolation Unit, Grass Technologies, West Warwick, RI, USA). Extracellular recordings of field excitatory postsynaptic potentials (fEPSPs) and population spikes (PS) were recorded in area CA1 in the stratum radiatum (dendritic region of pyramidal neurons) and stratum pyramidale (somatic region of pyramidal neurons), respectively, using microelectrodes (1–3 MΩ) filled with ACSF. The signals were amplified with an Axoclamp-2A amplifier and acquired using the pClamp software program (both from Molecular Devices, Union City, CA, USA).
2.4. Experimental protocols for electrophysiological recordings
To determine the response parameters of the synapse, an input-output (I-O) protocol was performed for each slice. The Schaffer collaterals were stimulated at a rate of 0.05 Hz at a range of stimulus intensities (typically from 20 to 240 μA) starting at the threshold intensity required to elicit a presynaptic fiber volley (PSV) measured in the dendritic region of the CA1 pyramidal neurons. The stimulus strength was increased in steps (typically 10–20 μA) until the maximum PS amplitude was reached.
Short-term synaptic plasticity at the dendritic synapses was assessed in each slice by measuring paired-pulse facilitation (PPF) using a standard paired-pulse stimulation protocol applied to the Schaffer collaterals. Paired-pulse intervals of 40–200 ms were used at a test stimulus intensity that elicited a fEPSP equal to 50% of the maximal fEPSP amplitude, as determined from I-O protocols. Inhibitory influences at the somatic region were assessed using a paired-pulse stimulation protocol applied to the Schaffer collaterals at short intervals (10–20 ms). A test stimulus intensity was used that elicited a PS equal to 50% of the maximal PS amplitude, as determined from I-O protocols. For both protocols, three paired-pulse responses (1 minute between acquisitions) were averaged in each slice.
Synaptic plasticity elicited by high frequency stimulation was assessed in each slice including post-tetanic potentiation (PTP), short-term synaptic plasticity, (STP) and long-term synaptic plasticity (long-term potentiation; LTP). A stimulus intensity was used that elicited a fEPSP equal to 50% of the maximal fEPSP amplitude, as determined from I-O protocols. The high frequency stimulation (HFS) protocol consisted of 100 pulses for 1 sec (100 Hz), repeated a total of three times with a 20 sec interval between trains at a test stimulus intensity (50% half-maximal fEPSP).
2.5. Analysis of electrophysiological data
Data were analyzed off-line with the AxoGraph software program (AxoGraph Scientific, axograph.com). Measurements were made of the fEPSP slope and PS amplitude at each stimulus intensity tested starting at subthreshold stimulus intensity (i.e., no synaptic response) and ending at the maximum response. In some studies the PSV amplitude was also measured. Threshold stimulus intensity for eliciting a fEPSP and PS varied across slices for both the IL-6 transgenic (IL-6 tg) and non-transgenic (non-tg) hippocampus. To adjust for these differences, the stimulus intensities plotted in the I-O curves were standardized such that the stimulus intensity that elicited a threshold fEPSP (or PS depending on the graph) was the same in all slices. From the I-O data, the mean values at each stimulus intensity was determined for all slices and plotted as a function of the stimulus intensity to construct the I-O curves for mean values. Paired-pulse data were expressed as the ratio of the second response with respect to the first response in each slice. LTP, PTP and STP of the fEPSP were expressed as a percentage of the average of five baseline (pre-HFS) test responses in each slice. When data from different stimulus intensities or time points were combined, data was first averaged on an individual slice basis then the mean value for all slices was calculated.
2.6. Western blot
Relative levels of cellular and synaptic proteins in the hippocampal samples from the IL-6 tg and non-tg mice were determined by Western blot. To prepare the protein samples, the frozen hippocampi were place in individual tubes containing cold lysis buffer and the tubes placed on ice until the hippocampi were thawed. The lysis buffer contained: 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, a Protease Inhibitor Cocktail Tablet (Roche, Mannheim, Germany), and a cocktail of phosphatase inhibitors (4.5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1 mM sodium fluoride, 1 mM sodium orthovanadate). Each hippocampus was sonicated to disrupt the cells and then incubated on ice for 30 minutes. The resulting homogenates were centrifuged at 15,000 rpm (30 min), the supernatant collected and protein concentration determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Typically 8 individual hippocampi were processed at one time.
Proteins (20–50 μg of protein; in duplicate) were separated by SDS-PAGE using 4–12% Novex NuPage Bis-Tris gels (Invitrogen Life Technologies, Grand Island, NY, USA) and transferred overnight onto Immobilon-P membranes (Millipore, Billerica, MA, USA). Uniform transfer was confirmed by Ponceau S (Sigma-Aldrich) staining. After washing, the membranes were blocked with casein (Pierce Biotechnology, Rockford, IL, USA), transferred to phosphate buffered saline (PBS) or Tris buffered saline (TBS) containing 0.1% Tween-20, and incubated in primary antibody overnight at 4°C. After washing with PBS/Tween-20 or TBS/Tween-20, the membranes were incubated in secondary antibody coupled to horseradish peroxidase (HRP), washed and the immunoreactive bands visualized on photographic film (Kodak, VWR Scientific Products, San Diego, CA, USA) by chemiluminescence using the ECL system (Pierce Biotechnology). To determine if equal loading of protein was achieved, membranes were stripped with Pierce Restore Stripping buffer (Pierce Biotechnology), washed and reprobed for β-actin.
Protein signals were quantified from densitometry measurements of immunoreactive bands on photographic film using NIH Image software (http://rsb.info.nih.gov/nih-image/). The density of each band was quantified and normalized against the corresponding density of β-actin in the same lane to adjust for possible loading errors. Normalized data from IL-6-tg mice were then normalized to values from non-tg mice run on the same gel. Data were combined according to treatment group. Summarized results are the combined normalized data.
The following antibodies were used for Western blot studies: a monoclonal antibody to β-actin (AC-15, 1:5000; Sigma-Aldrich); a monoclonal antibody to glial fibrillary acidic protein (GFAP; MAB360; 1:10,000; Millipore); a mouse monoclonal antibody raised against a recombinant fragment of human glutamine synthetase (Glu syn; ab64613; 1:500; abcam, San Francisco, CA, USA); a purified rabbit antibody raised against a synthetic protein made to an internal region of the mouse CD11b protein (NB110-89474; 1–500; Novus Biologicals, Littleton, CO, USA); a monoclonal antibody raised against neuron specific enolase (MAB314; 1:5000; Millipore); a rabbit polyclonal antibody raised against a synthetic peptide to the C-terminus of rat GAD 65/67 (AB1511; 1:1000; Millipore); a purified rabbit polyclonal antibody raised against purified parvalbumin (PAV) from rat skeletal muscle (ab11427; 1:500; abcam); a purified rabbit polyclonal antibody raised against a peptide mapping at the carboxy terminus of C/EBP beta (C-19; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA); a purified rabbit polyclonal antibody raised against a synthetic peptide (KLH-coupled) corresponding to the sequence of mouse STAT3 (AB#9132; 1:1000; Cell Signaling Technology, Boston, MA, USA); a purified rabbit polyclonal antibody raised against a synthetic phosphopeptide corresponding to residues surrounding Tyr705 of mouse STAT3 (AB#9131; 1:500; Cell Signaling Technology); a purified rabbit polyclonal antibody raised against a synthetic peptide (KLH coupled) derived from rat p42 MAP kinase (AB#9102; 1–1000; Cell Signaling Technology); a purified rabbit polyclonal antibody raised against a synthetic phosphopeptide (KLH coupled) corresponding to sequences around Thr202/Thr204 of human p44 MAP kinase (AB#9102; 1–500; Cell Signaling Technology); a purified rabbit antibody raised against a synthetic peptide corresponding to amino acids 1–20 at the N-terminus of mouse VGAT (NB110-55238; 1–500; Novus Biologicals); a monoclonal antibody to VGlut 1 produced by fusion protein amino acids 494–560 (cytoplasmic terminal) of rat VGlut 1 (clone N28/9; 1:500; NeuroMab, UC Davis, Davis, Ca); a purified rabbit antibody to synapsin 1 (#51–5200; 1:5,000; Invitrogen Life Technologies); a rabbit monoclonal antibody to Postsynaptic Density Protein 95 (PSD 95) produced by immunizing rabbits with a KLH-coupled synthetic peptide corresponding to residues surrounding Gly99 of the human PSD95 (#3450; 1:500; Cell Signaling); a purified rabbit polyclonal antibody raised against a synthetic peptide of the rat GluA1 (GluR1) subunit of the AMPA receptor (AMPAR) conjugated to KLH with a cysteine added (07–660; 1:500; Millipore); a purified goat polyclonal antibody raised against a peptide corresponding to an amino acid sequence mapping to the C-terminus of the human GluN1 (NR1) subunit of the NMDA receptor (NMDAR; sc-1467; 1:500; Santa Cruz Biotechnology); a purified rabbit polyclonal antibody raised against a synthetic peptide corresponding to a sequence from the 2nd cytoplasmic domain of human GluN2A (NR2A) conjugated to an immunogenic carrier protein (ab84181;1–500; abcam); a purified rabbit polyclonal antibody raised against a carboxy terminus peptide of the rat mGluR2/3 conjugated to BSA with gluteraldehyde (AB1553; 1–1000; Millipore).
2.7. Statistical analysis
Summarized data are reported as the mean ± SEM. Statistical analyses were performed using the unpaired t-test. A p value of <0.05 was considered significant.
3. Results
3.1. Protein levels in the IL-6-tg hippocampus
Progressive neuropathology has been reported to occur in the IL-6 tg line (G167) used in the current study, with prominent changes evident at 12 months of age (Campbell et al., 1993; Chiang et al., 1994). To gain information on the status of the hippocampal tissue in the adult IL-6 tg mice used in our studies, we measured the levels of several important cellular and synaptic proteins by Western blot analysis. The mean age of the IL-6 tg and non-tg littermates used for Western blot studies was 4.4 ± 1 (n=43) and 4.3 ± 0.1 (n=40) months, respectively.
In the Western blot studies, relatively few differences in protein levels were observed between the IL-6 tg and non-tg hippocampus. The most prominent difference was a significant increase in the level of GFAP, an astrocyte structural protein, in the IL-6 tg hippocampus (Fig. 1A), consistent with the known action of IL-6 to increase GFAP levels in astrocytes (Sanz et al., 2008). Another astrocyte protein, glutamine synthetase, showed comparable levels in IL-6 tg and non-tg hippocampus, indicating that the effect of IL-6 on astrocytes did not involve a general increase in the level of all astrocyte proteins (Fig. 1A). The level of protein for β-actin, a structural protein found in all cells, and the level of cell specific markers for neurons and microglia, enolase and CD11b, respectively, were also similar in IL-6 tg and non-tg hippocampus (Fig. 1A), consistent with the lack of a general effect of IL-6 on protein expression. A small decrease in the level of GAD65/67, the synthetic enzyme for GABA and a marker for inhibitory interneurons, was observed in the IL-6 tg hippocampus, but there was no difference in the level of PAV, a Ca2+ binding protein expressed in certain population of interneurons (Andressen et al., 1993), between IL-6 tg and non-tg hippocampus (Fig. 1A). The level of STAT3, a protein known to be involved in IL-6 signal transduction was also significantly increased in the IL-6 hippocampus, but the levels of C/EBPβ and p42/44 MAPK, other signaling molecules know to be used by IL-6 (Cardinaux et al., 2000), were not altered (Fig. 1B). Both STAT3 and p42/44 MAPK are activated by phosphorylation and this activation can be detected in Western blots using commercially available antibodies that recognize the phosphorylated form of the protein. The levels of phosphorylated STAT3 (pSTAT3) and phosphorylated p42/44 MAPK (pp42/44 MAPK) were significantly increased in the IL-6 hippocampus compared with the non-tg hippocampus (Fig. 1B).
Figure 1.
Western blot analysis of protein levels in IL-6 tg and non-tg hippocampus. A variety of cellular (A), signal transduction (B) and synaptic (C) proteins were examined. Results are expressed as mean normalized values (see Methods). Representative Western blots are shown above the graphs for the protein level. In B, inset at right shows representative immunoblots for pSTAT3 (n=4 for IL-6 tg and non-tg each). Numbers in bars indicate the number of mice tested. * = significantly different from non-tg (unpaired t-test, p< 0.05)
We also measured the level of several synaptic proteins and found no prominent differences between the IL-6 tg and non-tg hippocampus (Fig. 1C). These proteins included VGAT, the transporter involved in uptake of GABA into synaptic vesicles, VGlut1, the transporter involved in uptake of glutamate into synaptic vesicles, synapsin I, a protein involved in transmitter release and a marker for presynaptic terminals, PSD95, a postsynaptic density protein, and several glutamate receptors (GluN1, GluA1, mGluR 2/3) (Fig. 1C). Taken together, these results indicate that in vivo exposure to elevated levels of IL-6 does not produce dramatic alterations in the cellular and molecular composition of the hippocampus at the age studied (with the exception of GFAP, STAT3 levels), at least with respect to the proteins measured.
3.2. Altered basal synaptic responses in IL-6 transgenic mice
The characteristics of basal synaptic transmission in the CA1 region of the hippocampus of IL-6 tg mice and their non-tg littermates was investigated in young mice 1–2 months of age (IL-6 tg = 1.8 ± 0.04 months old, n= 25; non-tg = 1.8 ± 0.04 months old, n=39) and in adult mice 3–6 months of age (IL-6 tg = 4.4 ± 0.1 months old, n= 37; non-tg = 4.3 ± 0.1 months old, n=30). Two age groups were studied to determine if effects of IL-6 showed age-dependency. Such an effect could result from age-related sensitivity to IL-6 or actions that progressed with age or duration of exposure. Synaptic transmission was evoked by electrical stimulation of the Schaffer collaterals and recorded in the CA1 region using field potential recordings following protocols described previously (Nelson et al., 2011). Electrical stimulation of the Schaffer collaterals produced three electrical events: (a) a presynaptic volley (PSV), which reflects a summation of the action potentials occurring in the stimulated Schaffer collateral afferents, (b) a dendritic field fEPSP, which reflects a summation of excitatory postsynaptic responses mediated primarily by glutamate receptors of the AMPA subtype (AMPAR) and occurring in the dendrites of CA1 pyramidal neurons, and (c) a population spike (PS), which represents a summation of the of action potentials evoked by the dendritic synaptic response and occurring in the somata of the pyramidal neurons. The properties of synaptic transmission were characterized by the input-output (I-O) relationships for the fEPSP slope and PS amplitude.
In both the young and adult mice we observed a significant enhancement of the fEPSP in hippocampal slices from IL-6 tg mice compared with slices from non-tg mice (Figs. 2,3). This enhancement was characterized by a leftward shift in the I-O curve showing the relationship between fEPSP slope and stimulus intensity (Fig. 2C,3C). The I-O relationship for the amplitude of PS recorded in the somatic region of CA1 also showed a significant enhancement in the hippocampal slices from the IL-6 tg mice compared with hippocampal slices from the non-tg mice, an effect that was observed in both the young (Fig. 2B,D) and adult hippocampus (Fig. 3B,D). Thus, in the IL-6 slices, neuronal excitability at the somatic level, as reflected by the amplitude of the PS, showed an increase that paralleled the increase in the synaptic response (i.e., fEPSP) observed in dendritic recordings. When the relationship between the dendritic fEPSP and somatic PS evoked by the fEPSP was expressed as a ratio (PS/fEPSP ratio) at each stimulus intensity, there was no difference in the ratio for the IL-6 tg hippocampus compared with the non-tg hippocampus in either the young or adult mice. These results indicate that the increase in the PS amplitude in the IL-6 tg hippocampus resulted from the larger fEPSP (Fig. 2A,3A).
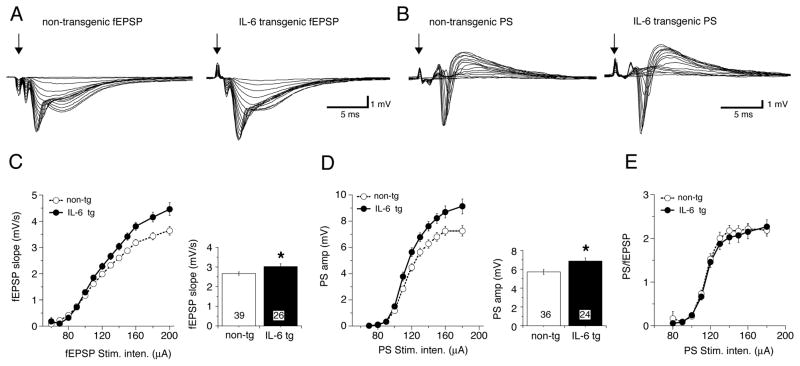
Figure 2.
Altered postsynaptic responses in hippocampal slices from young IL-6 tg mice (1–2 months of age). A–B. Representative field potential recordings in the dendritic (fEPSP; A) and somatic (PS; B) region of area CA1 of slices taken from IL-6 tg and non-tg mice. Each set of traces represents field responses to Schaffer collateral stimulation over a range of stimulus intensities. Input-output (I-O) relationships were plotted from the data (C,D). C,D. Slices from IL-6 tg mice exhibited a significantly larger fEPSP (C) and PS (D) at the higher stimulus intensities compared to the slices from non-tg mice. Graph of mean values for higher stimulus intensities (<120 μA) is shown to the right. Numbers in boxes show number of slices tested. * = significantly different from non-tg (unpaired t-test, p< 0.05). E. PS/EPSP relationship. When the PS amplitude was expressed as a function of fEPSP slope, there was no significant difference in the ratio between non-tg and IL-6 tg hippocampus. This result indicates that the increased size of the fEPSP is responsible for the larger PS observed in the IL-6 tg hippocampus. In this and other figures some error bars are smaller than the symbol that indicates the mean value.
Figure 3.
Altered postsynaptic responses in hippocampal slices from adult IL-6 tg mice. A–B. Representative field potential recordings (I-O curves) in the dendritic (fEPSP; A) and somatic (PS; B) region of area CA1 of slices taken from IL-6 tg and non-tg mice. Each set of traces represents field responses to Schaffer collateral stimulation over a range of stimulus intensities. Input-output (I-O) relationships were plotted from the data (C,D). C,D. Slices from IL-6 tg mice exhibited a significant larger fEPSP (C) and PS (D) at the higher stimulus intensities compared to the slices from non-tg mice. Graph showing mean values for higher stimulus intensities (<100 μA) is shown to the right. Numbers in boxes show number of slices tested. * = significantly different from non-tg (unpaired t-test, p< 0.05). E. PS/EPSP relationship. When the PS amplitude was expressed as a function of fEPSP slope, there was no significant difference in the ratio between non-tg and IL-6-transgenic hippocampus. These results indicate that the increased size of the fEPSP is responsible for the larger PS observed in the IL-6 tg hippocampus.
3.3. Paired-pulse facilitation and paired-pulse inhibition are unaltered in IL-6 transgenic mice
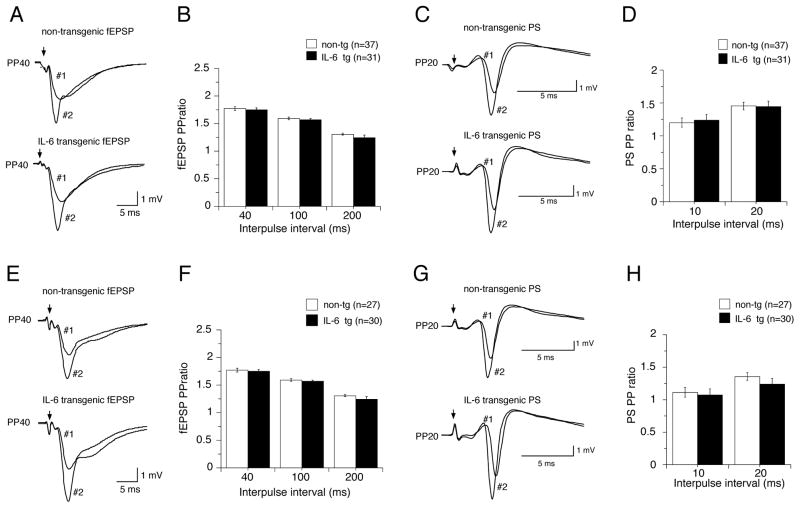
To determine if alterations in presynaptic neurotransmitter release contributed to the enhanced fEPSP produced in CA1 pyramidal neurons by IL-6 exposure, we compared presynaptic facilitation in hippocampal slices from non-tg and IL-6 tg mice. For these studies we used a standard paired-pulse protocol to measure paired-pulse facilitation (PPF) of the dendritic fEPSP slope. PPF was determined using a standardized stimulus intensity (50% maximum slope) and interpulse intervals of 40–200 ms and was calculated as the ratio of the fEPSP slope for the second (test) stimulation with respect to the fEPSP slope for the first (conditioning) stimulus (Fig. 4A,E). Differences in the PPF ratio are indicative of differences in the probability of neurotransmitter release from the presynaptic terminals (Andreasen and Hablitz, 1994; Creager et al., 1980; Debanne et al., 1996; Dobrunz and Stevens, 1997; Dunwiddie and Haas, 1985; Otmakhov et al., 1993). PPF was observed at all stimulation intervals tested in both IL-6 tg and non-tg slices. However, no differences in PPF were observed between control and IL-6 slices at any of the interpulse intervals studied for either the young or adult hippocampus (Fig. 4B,F).
Figure 4.
Paired-pulse studies in hippocampal slices from young and adult IL-6 tg and non-tg mice. A,C,E.G. Sample field recordings of CA1 pyramidal neurons showing conditioning (#1) and test (#2) responses to pairs of stimuli delivered to Schaffer collateral afferents. Paired-pulse protocols with either long (40–200 ms) or short (10–20 ms) interpulse intervals were used to examine PPF of dendritic fEPSPs (A,E) and inhibitory influences in the somatic region that influence the PS (C, G), respectively. B,D. PPF (fEPSP PP ratio) and PPRPS (PS PP ratio) were expressed as the ratio of the fEPSP slopes or PS amplitudes, respectively, of the second response (#2) with respect to the first response (#1). PPF and PPRPS were unaltered in slices from young or adult IL-6 tg mice compared with their age-matched non-tg littermate controls.
We also determined whether changes in inhibitory influences at the somatic region were produced by the in vivo IL-6 exposure, as would be suggested by the lower GAD65/67 level observed in the Western blot studies of the IL-6 tg hippocampus. Inhibitory influences were determined by a paired-pulse ratio protocol for the PS amplitude (PPRPS) using a standard stimulus intensity (50% maximum amplitude) and short interpulse intervals (10–20 ms). PPRPS was calculated as the ratio of the PS amplitude produced by the second stimulation relative to the PS amplitude produced by the first stimulation (Fig. 4C,G). The amplitude of the PS produced by the second stimulation is modulated by recurrent inhibitory feedback by GABAergic interneurons (i.e., basket cells) and intrinsic inhibitory currents in the somatic region. Inhibitory influences were not strong in either the IL-6 tg or non-tg slices, presumably due to the angle that the slice was cut, and facilitation of the PS was observed for the second stimulation rather than inhibition. However, no difference in PPRPS was observed between IL-6 tg and non-tg hippocampus at the two interpulse intervals tested in either the young or adult hippocampus (Fig. 4D,H).
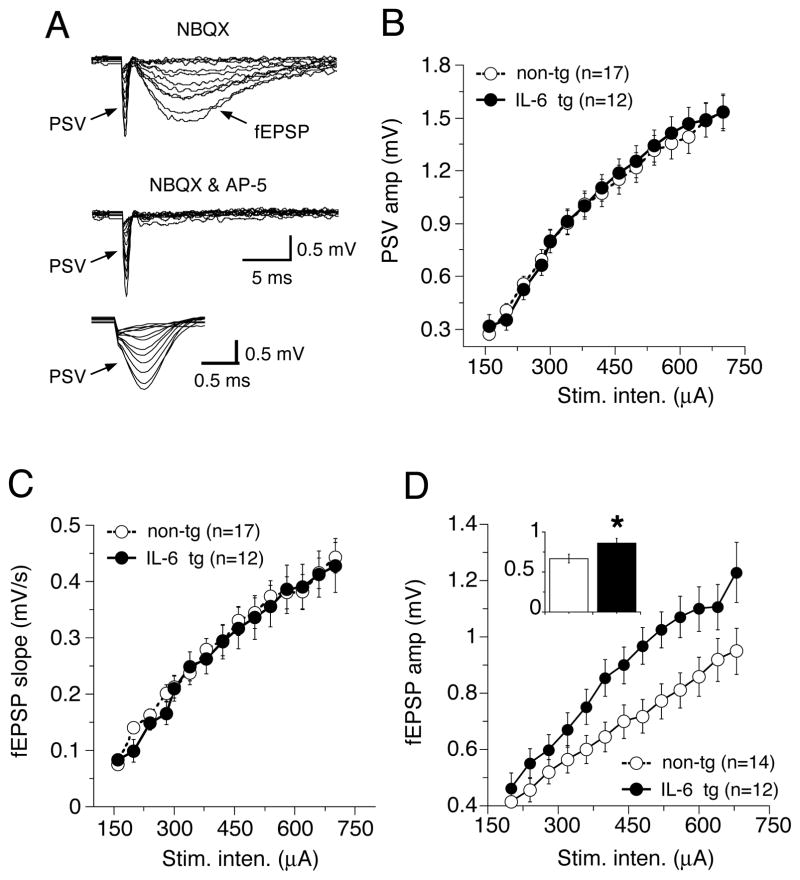
In additional studies of the adult hippocampus, we determined if changes in the magnitude of the PSV contributed to the enhanced fEPSP in the IL-6 tg hippocampus. These studies were carried out in the presence of NBQX to block the AMPAR-mediated fEPSP, which can interfere with measurement of the PSV. In the presence of NBQX, I-O curves for the amplitude of the PSV were comparable in the IL-6 tg and non-tg hippocampus (Fig. 5A,B), indicating that the larger fEPSP in the IL-6 tg hippocampus was not a result of a larger PSV. In the presence of NBQX, the NMDAR-mediated fEPSP could be observed at high stimulus intensities. The slope of the NMDAR-mediated fEPSP was similar in IL-6 tg and non-tg hippocampus, suggesting that the enhancement of the fEPSP in the IL-6 tg hippocampus primarily involved the AMPAR-mediated component of the synaptic response (Fig. 5C). However, the peak amplitude of the fEPSP was increased in the NBQX-treated slices. This increase may reflect a difference between IL-6-tg and non-tg hippocampus in the kinetics of the NMDAR current that underlies the synaptic response, intrinsic ionic conductances activated by the membrane depolarization produced by the NMDAR current or synaptic interactions.
Figure 5.
Synaptic transmission in the presence of NBQX. A. Sample field recordings from CA1 pyramidal neurons in the presence of NBQX to block the AMPA receptor mediated component of the synaptic response. The PSV and NMDA receptor-mediated fEPSP are evident. The PSV is shown at a faster timebase in the bottom panel. The NMDA receptor blocker AP-5 completely blocked the NMDA receptor-mediated fEPSP. B–D. Input-output curves for the PSV amplitude (B), fEPSP slope (C) and peak amplitude of the fEPSP in the IL-6-tg and non-tg hippocampal slices. The inset in D shows graph of mean values for all stimulus intensities. * = significantly different from non-tg (unpaired t-test, p< 0.05)
3.4. LTP is unaltered in IL-6 transgenic mice
Our above results show that in vivo exposure to elevated levels of IL-6 results in increased postsynaptic responses at Schaffer collateral-CA1 pyramidal neuron synapses in hippocampal slices from young and adult mice under condition of low frequency synaptic activity. We also determined if in vivo exposure to elevated levels IL-6 produced changes in synaptic function associated with high frequency synaptic activity. For these studies we tested the effect of high frequency stimulation (HFS) on fEPSP and PS magnitude in IL-6 tg and non-tg hippocampal slices. The HFS protocol produced short-term plasticity (PTP and STP) and long-term plasticity (LTP) of the fEPSP slope in both the IL-6 tg and the non-tg hippocampus from the young and adult mice. PTP was indicated by an initial peak increase in the magnitude of the fEPSP slope that occurred immediately after the termination of the HFS protocol and lasted for 2–3 min. The subsequent declining phase of the fEPSP slope, which lasts ~30 minutes, is referred to as STP. PTP is thought to result from a presynaptic enhancement of synaptic vesicle release from the reserve pool that lasts for seconds to minutes after termination of the HFS (Zucker, 1996). The mechanisms underlying STP are still not fully understood, but both presynaptic and postsynaptic sites appear to be involved (Erickson et al., 2009; Lauri et al., 2007). Both PTP and STP are thought to play a role in short-term memory (Erickson et al., 2009). LTP, the long-term enhancement of the fEPSP, was indicated by the stable, enhancement of the fEPSP that lasted up to 60 min following the induction protocol. LTP primarily results from increased membrane expression of AMPARs (Miyamoto, 2006) and is considered to be an important cellular model for learning and memory (Peng et al., 2010).
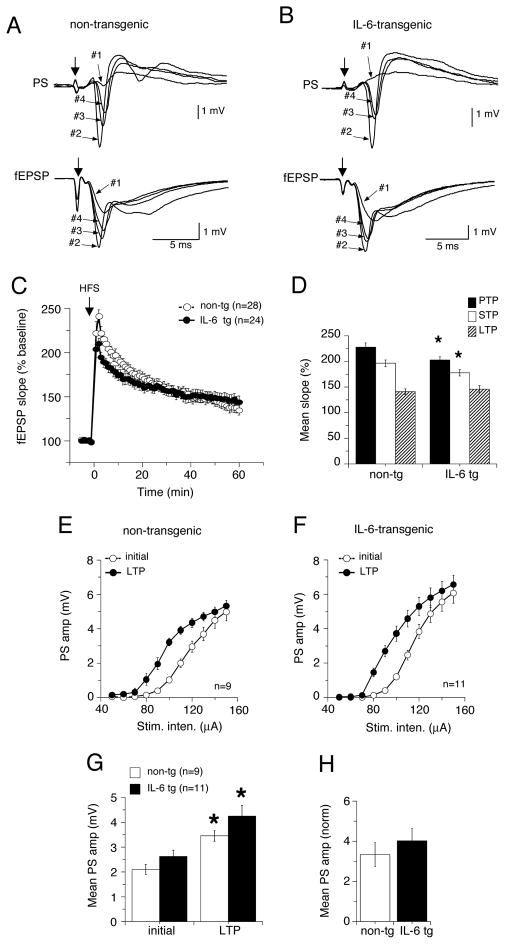
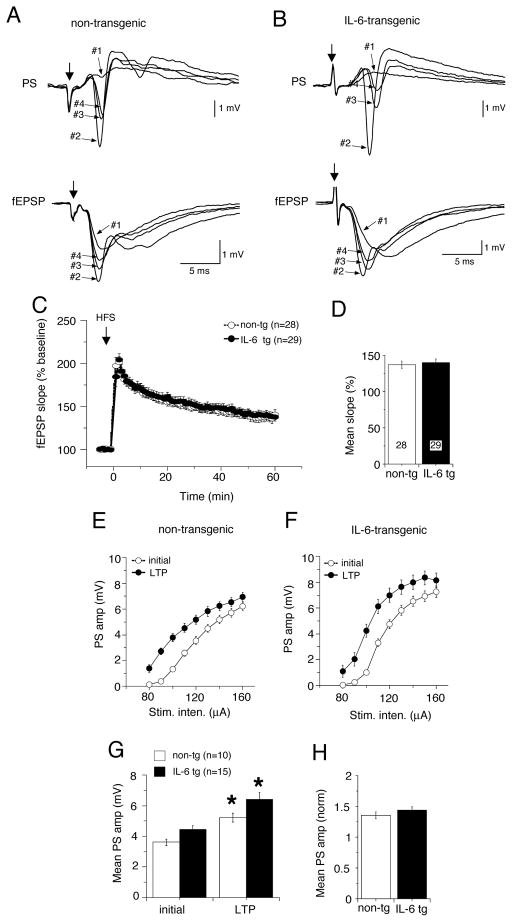
In the dendritic recordings, the magnitude of LTP of fEPSPs was similar in IL-6 tg and non-tg hippocampal slices from both young (Fig. 6A–C) and adult mice (Fig. 7A–C), indicating that mechanisms that mediate long-term synaptic plasticity were not altered by the in vivo exposure to elevated levels of IL-6. However, in slices from the young mice, both PTP and STP were significantly smaller in the IL-6 tg slices than in non-tg slices, an effect that was not observed in the slices from adult hippocampus (Fig. 6C, 7C).
Figure 6.
Long-term potentiation (LTP) in hippocampal slices from young IL-6 tg and non-tg mice. LTP was induced by high frequency stimulation (HFS) of Schaffer collaterals and synaptic responses were monitored for 60 min after HSF. A,B. Representative traces showing dendritic (fEPSP) and somatic (PS) field recordings of CA1 pyramidal neurons from non-tg (A) IL-6 tg (B) slices 5 min before (#1, baseline) HFS and after HFS during PTP (#2), STP (#3) and LTP (#4). C. Graph showing mean values for fEPSP slope expressed as a percent of baseline values measured a 1 min intervals in non-tg and IL-6 tg hippocampus. D. Graph showing mean values for fEPSP slope as a percent of baseline values during PTP, STP and LTP (1–3, 5–13 and 51–60 min post HFS, respectively). * = significantly different from non-tg values for the same measurement (unpaired t-test, p< 0.05). LTP of fEPSPs was unaltered in IL-6 tg slices relative to non-tg slices, but PTP and STP were reduced. E,F. I-O curves for the PS taken before and >60 min after LTP induction in non-tg and IL-6 tg hippocampus showed a leftward shift for both non-tg (E) and IL-6 tg (F) hippocampus, indicating LTP of the PS. G. Mean values for PS amplitude for stimulations of 80 to 140 μA. * = significantly different from initial values for the same measurement (unpaired t-test, p< 0.05). H. Graph showing relative increase in PS amplitude in slice from non-tg and IL-6 tg hippocampus determined by normalizing the amplitude of the PS >60 min after LTP induction to the value before LTP induction. No significant difference was observed between mean values for the PS indicating that the degree of LTP of the PS was similar for non-tg and IL-6 tg hippocampus.
Figure 7.
Long-term potentiation in hippocampal slices from adult IL-6 tg and non-tg mice. LTP was induced by high frequency stimulation (HFS) of Schaffer collaterals and synaptic responses were monitored for 60 min after HSF. A,B. Representative traces showing dendritic (fEPSP) and somatic (PS) field recordings of CA1 pyramidal neurons from non-tg (A) IL-6 tg (B) slices 5 min before (#1, baseline) HFS and after HFS during PTP (#2), STP (#3) and LTP (#4). C. Graph showing mean values for fEPSP slope expressed as a percent of baseline values measured a 1 min intervals in non-tg and IL-6 tg hippocampus. D. Graph showing mean values for fEPSP slope as a percent of baseline values during LTP (51–60 min post HFS). LTP of fEPSPs was of similar magnitude in IL-6 tg and non-g slices. E,F. I-O curves for the PS taken before and 60 min after LTP induction in non-tg and IL-6 tg hippocampus showed a leftward shift for both non-tg (E) and IL-6 tg (F) hippocampus, indicating LTP of the PS. G. Mean values for PS amplitude for stimulations of 90 to 160 μA. * = significantly different from initial values for the same measurement (unpaired t-test, p< 0.05). H. Graph showing relative increase in PS amplitude in slice from non-tg and IL-6 tg hippocampus determined by normalizing the amplitude of the PS >60 min after LTP induction to the value before LTP induction. No significant difference was observed between mean values for the PS indicating that the degree of LTP of the PS was similar for non-tg and IL-6 tg hippocampus.
The HFS protocol also produced an enhancement of the PS amplitude in both the IL-6 tg and the non-tg hippocampus from the young and adult mice. To determined if the enhancement differed for the IL-6 tg and non-tg hippocampus, we compared I-O curves for the PS before and 60 min after LTP induction. LTP of the PS was indicated by a leftward shift in the I-O relationship in the IL-6 tg and non-tg slices from both the young (Fig 6E–G) and adult mice (Fig 7E–G). However, there was no difference in the degree of LTP between the IL-6 tg and non-tg slices from both the young or adult mice (Fig. 6H,7H). Taken together, these results indicate the in vivo exposure to elevated levels of IL-6 did not alter the fundamental mechanisms underlying LTP in hippocampal CA1 pyramidal neurons but did have age-dependent effects on presynaptic mechanisms activated by HFS.
4. Discussion
The studies described here show that in vivo exposure to elevated levels of IL-6 in the CNS can result in enhanced synaptic transmission at the Schaffer collateral-pyramidal neuron synapse within area CA1 of the hippocampus. The enhancement of synaptic transmission was reflected in larger fEPSP slopes and PS amplitudes in the hippocampal slices from IL-6 tg mice compared with hippocampal slices from age matched non-tg mice. Other aspects of synaptic function were similar in the IL-6 tg and non-tg hippocampus. Thus, no difference was observed in short-term plasticity (PPF and PPRPS) induced by low frequency stimulation and long-term synaptic plasticity (LTP) induced by high frequency stimulation between IL-6 tg and non-tg hippocampus at young and adult ages. However, short-term plasticity (PTP and STP) produced by high frequency stimulation was reduced in the IL-6 tg hippocampus from young mice compared to their non-tg littermate controls. This difference was not observed in hippocampus from the adult mice, suggesting an age or IL-6 exposure-dependent neuroadaptive effect of IL-6. These changes in basal synaptic function produced by exposure to elevated levels IL-6 could significantly alter the normal flow of information through the hippocampus and interfere with critical behaviors that are known to involve the hippocampus such as learning, memory, cognition, and emotion (Bast, 2007; LeDoux, 2000; Small et al. 2010). Consistent with this possibility, studies by others have shown altered hippocampal-dependent behavior in the IL-6 tg mice (Heyser et al., 1997). The enhanced synaptic responses could also contribute to the reduced seizure threshold in the IL-6 tg mice reported in previous studies (Samland et al., 2003). However, the role of IL-6 in seizure activity appears to be complex because both increases (Libbey et al., 2011; Samland et al., 2003) and decreases (De Luca et al., 2004; De Sarro et al., 2004; Penkowa et al., 2001) in IL-6 levels have been reported to increase seizure susceptibility in mice.
The enhancement in synaptic transmission induced by elevated levels of IL-6 in the CNS was evident in hippocampal slices from both young mice 1–2 months of age and adult mice 3–6 months of age and was retained under conditions resulting in synaptic potentiation, such as occurs with the induction of LTP. The enhancement primarily affected the larger synaptic responses and was similar in magnitude in young and adult hippocampus. These results suggest that IL-6-induced changes in synaptic transmission are an early consequence of the increased in vivo expression of IL-6 in the CNS. Associated with the enhanced fEPSP in the IL-6 tg hippocampus was an enhancement of the PS in both the young and adult mice. Examination of the relationship between fEPSP slope and PS amplitude showed that the increase in the PS amplitude could be explained by the enhanced fEPSP.
The mechanisms mediating the enhanced fEPSP in the IL-6 tg hippocampus remains to be determined. Studies of the presynaptic volley elicited by Schaffer collateral stimulation showed similar amplitudes in the IL-6 tg and non-tg hippocampus, indicating that input to the presynaptic terminals was of similar magnitude for the IL-6 tg and non-tg hippocampus. PPF was also comparable in the IL-6 tg and non-tg hippocampus, indicating that the larger fEPSP in IL-6 tg hippocampus was not a result of alterations in the probability of neurotransmitter (glutamate) release from the presynaptic terminals. The slope of the NMDAR-mediated synaptic response, measured after blockade of the AMPAR-mediated synaptic response with NBQX, was similar for IL-6 tg and non-tg hippocampus, making it unlikely that an IL-6 induced change in the NMDAR-mediated synaptic response contributed to the enhanced fEPSP slope in the IL-6 tg hippocampus. These results suggest that the larger fEPSPs in the IL-6 tg hippocampal slices result from postsynaptic mechanisms that affect the AMPAR-mediated synaptic component. Measurement of protein levels in IL-6 tg and non-tg hippocampus by Western blot did not reveal significant changes in the levels of glutamate receptors that mediate the fEPSP. However, the peak amplitude of the NMDAR-mediated synaptic response was enhanced in the IL-6 tg hippocampus (adult mice studied) at strong stimulus intensities, an effect that could result from postsynaptic actions of IL-6 or actions of IL-6 at other synaptically connected neurons. The physiological consequence of the increased NMDAR-mediated synaptic response remains to be determined.
Previous reports noted a decrease in parvalbumin (PAV) containing inhibitory interneurons in the IL-6 tg hippocampus measured by immunohistochemistry (Campbell et al., 1993; Heyser et al., 1997). The number of GABA-containing neurons was not significantly altered, although there was a decrease (Samland et al., 2003). These results likely reflect the loss of a subpopulation of inhibitory interneurons in the IL-6 tg hippocampus, some of which contain PAV. Our results from Western blot studies do not show a difference in PAV levels between the IL-6 tg and non-tg hippocampus, but the Western blot technique may not be sensitive enough to detect a change if it occurred in a small number of neurons. A small but significant decrease in GAD65/67, the enzyme responsible for synthesis of GABA in inhibitory interneurons, was observed in our Western blot studies of the IL-6 tg hippocampus, but the level of neuron specific enolase, a marker for neurons, was not altered. These results suggest that the reduced level of GAD65/67 is not indicative of cell loss. Reduced levels of GAD65/67 could result in reduced levels of GABA in inhibitory interneurons and, consequently, reduced inhibitory influences. Measurement of PPRPS, which reflects the influence of recurrent inhibitory synaptic transmission and intrinsic excitability in the somatic region, did not show a difference between the IL-6 tg and non-tg hippocampus, suggesting that the level of GABA was not reduced in the inhibitory interneurons (basket cells) that provide the recurrent inhibitory synaptic input to the somatic region. Inhibitory influences were not strong in our studies and this may have affected our ability to detect a change in inhibitory synaptic transmission. However, our results are consistent with a previous in vivo study (Steffensen et al., 1994) of synaptic transmission in the IL-6 tg mice and their non-tg littermate controls that showed no apparent change in the function of GABAergic recurrent inhibitory circuitry in area CA1 of the IL-6 tg hippocampus. Feed-forward inhibitory interneurons, which synapse on CA1 pyramidal neuron dendrites and are well positioned to influence dendritic responses to excitatory input (Maccaferri and Dingledine, 2002), were not accessed in our studies and could be the neuronal targets of IL-6 that expresses lower levels of GAD65/67. Reduced output from these neurons would be expected to result in enhanced synaptic response. Future studies will be required to test this possibility.
Studies testing the effect of acute exposure to IL-6 on basal synaptic transmission in hippocampal slices from rats showed no effect of IL-6 on the fEPSP (Li et al., 1997) or PS (Tancredi et al., 2000) but a depression of PTP, STP and LTP of both the fEPSP and PS (Li et al., 1997; Tancredi et al., 2000). In contrast, in our studies prolonged in vivo expression of elevated levels of IL-6 enhanced the fEPSP and PS in the hippocampus from both the young and adult IL-6 tg mice with no apparent effect on LTP. PTP and STP were reduced in the hippocampus of the young IL-6 tg mice but not in the hippocampus of the adult mice, which may reflect an age-dependent sensitivity to IL-6 or a consequence of the differences in duration of IL-6 exposure. From these results we conclude that both acute and long-term exposure to IL-6 can alter synaptic functions in the hippocampus, but that the effects of IL-6 depend on the duration of exposure.
Neuroadaptive changes in the IL-6 tg mice are likely to result from alterations in neuron and glial function due to the persistent activation of the IL-6 signal transduction pathway. These neuroadaptive changes could involve changes in the signal transduction pathways linked to IL-6 receptor activation. For example, the effects of acute exposure to IL-6 were associated with an increase in the level of activation of STAT3 and reduction in the level of activation of p42/44 MAPK, as determined by Western blot analysis of the levels of phosphorylated (i.e., activated) and total protein for these enzymes (Tancredi et al., 2000). In our studies, increased activation of STAT3 was also observed of the IL-6-tg hippocampus but the level of activation of p42/44 MAPK was increased as well, in contrast to the decrease observed in the studies of acute IL-6 (Tancredi et al., 2000). p42/44 MAPK plays an important role in many neuronal functions including gene expression and the increased levels of the activated form produced by the elevated levels of IL-6 could have significant impact on these functions.
The difference between the effects of acute IL-6 and enhanced levels of IL-6 in the IL-6 tg mice on LTP are similar to that reported for the chemokine CXCL10. Acute CXCL10 inhibits LTP in hippocampus from wildtype mice (Vlkolinsky et al., 2004), as was observed for acute IL-6 in rat hippocampus (Tancredi et al., 2000). In contrast, LTP in hippocampal slices from transgenic mice that express elevated levels of CXCL10 through increased astrocyte expression (CXCL10 transgenic mice) did not differ from that observed in hippocampus from wildtype controls (Vlkolinsky et al., 2004), as was observed for the IL-6 tg and non-tg hippocampus. The mechanisms mediating the effects of CXCL10 remain to be determined, but could also involve neuroadaptive changes in signal transduction pathways. In a hippocampal culture preparation, chronic exposure to CXCL10 was shown to increase the levels of several signal transduction molecules including p42/44 MAPK, CREB and NF-κB (Bajova et al., 2008).
The ability of increased expression of IL-6 in the CNS to produced neuroadaptive changes in vivo was also demonstrated recently in studies using an adenoviral gene delivery approach (Wei et al., 2011a). In this model, mouse pups were injected with mouse GFP-IL-6 or GFP adenovirus on the day of birth. At 1–4 weeks of age, behavioral testing showed several alterations in the behavior of the GFP-IL-6 mice including impaired cognitive ability (Wei et al., 2011a). In addition, electrophysiological studies of synaptic transmission at the Schaffer collateral to CA1 hippocampus using paired-pulse protocols indicated reduced inhibitory influences and immunohistochemical studies showed an increase in VGlut 1 and a decrease in VGAT (Wei et al., 2011a). In out studies of synaptic transmission in the IL-6-tg and non-tg hippocampus, inhibitory influences measured in the somatic region were comparable in the IL-6-tg and non-tg hippocampus. In addition, our Western blots did not show a similar change in VGlut 1 or VGAT. These differences may be related to the type of cells expressing IL-6. The adenoviral gene delivery approach resulted in gene delivery into a variety of cell types, whereas in our studies increased IL-6 expression was limited to astrocytes
In contrast to our results at the Schaffer collateral-pyramidal neuron synapse, a previous study of synaptic transmission in hippocampal slices taken from the IL-6 tg mice and non-tg littermates showed impaired LTP at the perforant path-granule cell synapse in the dentate gyrus of IL-6 slices with no changes in basal synaptic transmission (Bellinger et al., 1995). These differences suggest that prolonged exposure to IL-6 exposure has variable effects on hippocampal synaptic transmission and plasticity (i.e., LTP) depending on the specific synapse under study. Alternatively, the differences could relate to the different transgenic lines studied and thus a difference in the degree of IL-6 expression. IL-6 transgene expression was shown to be lower in the heterozygous mice of the low-expressing line (G167) used in our study than in either the homozygous G167 mice or in the heterozygous mice of the high-expressing line of mice (G369) used in the study of dentate gyrus by Bellinger et al. (1995) (see Campbell et al., 1993).
Previous data suggests that acute or prolonged exposure to elevated levels of IL-6 leads to a disruption of the balance between excitatory and inhibitory neurotransmission in the brain, resulting in increased seizure susceptibility (Campbell et al., 1993; Samland et al., 2003; Xiaoqin et al., 2005). For example, mice that are homozygous for the IL-6 transgene show spontaneous seizures, suggesting altered neuronal excitability (Campbell et al., 1993). In contrast, the heterozygous low-expressing IL-6 transgenic mice do not exhibit spontaneously occurring seizures at the ages used in our studies but do show increased propensity for seizures induced by application of the glutamatergic receptor agonists kainic acid and NMDA (Samland et al., 2003). The decreased seizure threshold in IL-6 mice was selective for these glutamatergic agonists and did not occur with the cholinergic receptor agonist pilocarpine, suggesting that altered excitatory glutamatergic neurotransmission was involved. Results from our studies raise the possibility that neuroadaptive changes produced by IL-6 that enhance synaptic responses could be a key contributing factor in the reduced seizure threshold in the IL-6 mice reported in previous studies(Samland et al., 2003).
In addition to CNS conditions associated with seizure activity, IL-6 is known to be persistently upregulated in the CNS of other disorders including Alzheimer’s disease (Hull et al., 1996), Parkinson’s disease (Nagatsu et al., 2000), autism (Li et al., 2009; Wei et al 2011b) and clinical depression (Hayley and Anisman, 2005; Miller and O’Callaghan, 2005). Our results from the IL-6 tg mice raise the possibility that IL-6 induced alterations in synaptic function may also play a role in these disorders. Future studies are needed to address this possibility.
Acknowledgments
The authors wish to thank Floriska Chizer for her administrative assistance. This work was supported by NIH grant MH083723. We thank Katie Dutchman and Khanh Vo for carrying out some of the Western blot studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF) FASEB Journal. 1990;4:2860–2867. [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Andressen C, Blumcke I, Celio MR. Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res. 1993;271:181–208. doi: 10.1007/BF00318606. [DOI] [PubMed] [Google Scholar]
- Bajova H, Nelson TE, Gruol DL. Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kB in hippocampal neuronal cell culture. J Neuroimmunology. 2008;195:36–46. doi: 10.1016/j.jneuroim.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB Journal. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Bauer J, Strauss S, Volk B, Berger M. IL-6-mediated events in Alzheimer’s disease pathology. Immunol Today. 1991;12:422. doi: 10.1016/0167-5699(91)90148-M. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba SG, Campbell IL, Siggins GR. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci Lett. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. [PubMed] [Google Scholar]
- Chiang C-S, Stalder A, Samimi A, Campbell IL. Reactive gliosis as a consequence of interleukin-6 expression in the brain: Studies in transgenic mice. Dev Neurosci. 1994;16:212–221. doi: 10.1159/000112109. [DOI] [PubMed] [Google Scholar]
- Creager R, Dunwiddie T, Lynch G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J Physiol. 1980;299:409–424. doi: 10.1113/jphysiol.1980.sp013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca G, Di Giorgio RM, Macaione S, Calpona PR, Costantino S, Di Paola ED, De Sarro A, Ciliberto G, De Sarro G. Susceptibility to audiogenic seizure and neurotransmitter amino acid levels in different brain areas of IL-6-deficient mice. Pharmacol Biochem Behav. 2004;78:75–81. doi: 10.1016/j.pbb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- De Sarro G, Russo E, Ferreri G, Giuseppe B, Flocco MA, Di Paola ED, De Sarro A. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol Biochem Behav. 2004;77:761–766. doi: 10.1016/j.pbb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Maramara LA, Lisman J. A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J Cogn Neurosci. 2009;22:2530–2540. doi: 10.1162/jocn.2009.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neuroscience Letters. 1994;182:243–246. doi: 10.1016/0304-3940(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Getts DR, Matsumoto I, Muller M, Getts MT, Radford J, Shrestha B, Campbell IL, King NJ. Role of IFN-gamma in an experimental murine model of West Nile virus-induced seizures. J Neurochem. 2007;103:1019–1030. doi: 10.1111/j.1471-4159.2007.04798.x. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Hayley S, Anisman H. Multiple mechanisms of cytokine action in neurodegenerative and psychiatric states: neurochemical and molecular substrates. Curr Pharm Des. 2005;11:947–962. doi: 10.2174/1381612053381611. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. The biology of interleukin-6. Chem Immunol. 1992;51:153–180. [PubMed] [Google Scholar]
- Hull M, Strauss S, Berger M, Volk B, Bauer J. Inflammatory mechanisms in Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1996;246:124–128. doi: 10.1007/BF02189112. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learning and Memory. 2000;7:400–412. doi: 10.1101/lm.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Patterson PH. Cytokine and growth factor involvement in long-term potentiation. Mol Cell Neurosci. 1999;14:273–286. [PubMed] [Google Scholar]
- Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365:106–110. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Palmer M, Segerstrale M, Vesikansa A, Taira T, Collingridge GL. Presynaptic mechanisms involved in the expression of STP and LTP at CA1 synapses in the hippocampus. Neuropharmacology. 2007;52:1–11. doi: 10.1016/j.neuropharm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lehtimaki KA, Keranen T, Huhtala H, Hurme M, Ollikainen J, Honkaniemi J, Palmio J, Peltola J. Regulation of IL-6 system in cerebrospinal fluid and serum compartments by seizures: the effect of seizure type and duration. J Neuroimmunol. 2004;152:121–125. doi: 10.1016/j.jneuroim.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Lehtimaki KA, Peltola J, Koskikallio E, Keranen T, Honkaniemi J. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110:253–260. doi: 10.1016/s0169-328x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- Li AJ, Katafuchi T, Oda S, Hori T, Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997;748:30–38. doi: 10.1016/s0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hamer HM. Cytokines and epilepsy. Seizure. 2011;20:249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Dingledine R. Control of feedforward dendritic inhibition by NMDA receptor-dependent spike timing in hippocampal interneurons. J Neurosci. 2002;22:5462–5472. doi: 10.1523/JNEUROSCI.22-13-05462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimone D, Gregory S, Arnason BGW, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Depression, cytokines, and glial function. Metabolism. 2005;54:33–38. doi: 10.1016/j.metabol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006;35:165–172. doi: 10.1016/j.pediatrneurol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Satoh M. Effects of kainic acid on messenger RNA levels of IL-1 beta, IL-6, TNF alpha and LIF in the rat brain. Biochem and Biophys Res Commun. 1991;176:593–598. doi: 10.1016/s0006-291x(05)80225-6. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1b, interleukin-6, epidermal growth factor and transforming growth factor-a are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000:143–151. [PubMed] [Google Scholar]
- Nelson TE, Hao C, Manos J, Ransohoff RM, Gruol DL. Altered hippocampal synaptic transmission in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Brain Behav Immun. 2011;25(Suppl 1):S106–119. doi: 10.1016/j.bbi.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Shirke AM, Malinow R. Measuring the impact of probabilistic transmission on neuronal output. Neuron. 1993;10:1101–1111. doi: 10.1016/0896-6273(93)90058-y. [DOI] [PubMed] [Google Scholar]
- Peltola J, Hurme M, Miettinen A, Keranen T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Res. 1998;31:129–133. doi: 10.1016/s0920-1211(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Peng S, Zhang Y, Zhang J, Wang H, Ren B. Glutamate receptors and signal transduction in learning and memory. Mol Biol Rep. 2010;38:453–460. doi: 10.1007/s11033-010-0128-9. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Molinero A, Carrasco J, Hidalgo J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience. 2001;102:805–818. doi: 10.1016/s0306-4522(00)00515-7. [DOI] [PubMed] [Google Scholar]
- Ruan L, Kang Z, Pei G, Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2009;6:531–540. doi: 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M. Induction of interleukin-6 by depolarization of neurons. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-23-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- Sanz E, Hofer MJ, Unzeta M, Campbell IL. Minimal role for STAT1 in interleukin-6 signaling and actions in the murine brain. Glia. 2008;56:190–199. doi: 10.1002/glia.20602. [DOI] [PubMed] [Google Scholar]
- Sauder C, de la Torre JC. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Campbell IL, Henriksen SJ. Site-specific hippocampal pathophysiology due to cerebral overexpression of interleukin-6 in transgenic mice. Brain Res. 1994;652:149–153. doi: 10.1016/0006-8993(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Tagliari B, Tagliari AP, Schmitz F, da Cunha AA, Dalmaz C, Wyse AT. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem Res. 2011;36:487–493. doi: 10.1007/s11064-010-0367-0. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafe C, Giovedi S, Bue MC, D’Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Hasanvan H, Iverfeldt K, Post C, Schultzberg M. Early induction of interleukin-6 mRNA in the hippocampus and cortex of APPsw transgenic mice Tg2576. Neurosci Lett. 2001;301:54–58. doi: 10.1016/s0304-3940(01)01592-0. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008a;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008b;49(Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- Vlkolinsky R, Siggins GR, Campbell IL, Krucker T. Acute exposure to CXC chemokine ligand 10, but not its chronic astroglial production, alters synaptic plasticity in mouse hippocampal slices. J Neuroimmunol. 2004;150:37–47. doi: 10.1016/j.jneuroim.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2011a;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, Li X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011b;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TH, Lin CH. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav Brain Res. 2008;193:183–191. doi: 10.1016/j.bbr.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Xiaoqin Z, Zhengli L, Changgeng Z, Xiaojing W, Li L. Changes in behavior and amino acid neurotransmitters in the brain of rats with seizure induced by IL-1beta or IL-6. J Huazhong Univ Sci Technolog Med Sci. 2005;25:236–239. doi: 10.1007/BF02828129. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Exocytosis: a molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]