Abstract
Hormone ablation therapy (HALT) for breast or prostate cancer accelerates the development of osteoporosis in both men and women by causing estrogen deficiency, which increases the risk for fracture by promoting bone resorption mediated by osteoclasts. Denosumab, a fully human monoclonal antibody that inhibits osteoclast formation and function, increases bone mass in patients undergoing hormone ablation therapy. In the HALT study of 1,468 men with prostate cancer on androgen-deprivation therapy, denosumab significantly reduced the risk of new vertebral fractures, increased bone mineral density (BMD), and reduced markers of bone turnover. In a study of 252 women with breast cancer undergoing adjuvant aromatase inhibitor (AI) therapy, denosumab increased BMD at 12 and 24 months, overall and in all patient subgroups. The overall rates of adverse events were similar to placebo. Clinicians should consider fracture risk assessment and therapies such as denosumab to increase bone mass in patients on hormone ablation therapy who are at high risk for fracture.
Keywords: denosumab, treatment-induced bone loss, hormone-ablation therapy, breast cancer, prostate cancer
Hormone Ablation Therapy in Prostate Cancer and Breast Cancer
Prostate and breast cancer are among the most commonly diagnosed cancers worldwide, with 0.9 million diagnoses of prostate cancer and 1.4 million diagnoses of breast cancer every year.1,2 Diagnosis of these cancers in early stages and the development of effective therapies have reduced cancer mortality;1,2 10-year recurrence-free survival is estimated at up to 80% in women with breast cancer and 68% to 97% for men with prostate cancer.3,4 This trend has sharpened the focus on the overall health and quality of life of prostate and breast cancer survivors. Many patients with prostate cancer or breast cancer are treated with hormone therapy to reduce the risk of recurrence or progression.
In prostate cancer, androgen deprivation therapy (ADT) is widely used for men with hormone-sensitive cancer.5–9 ADT for prostate cancer includes orchiectomy, gonadotropin-releasing hormone (GnRH) agonists (eg, leuprolide, goserelin) and antagonists (eg, degarelix), given either alone or in combination with or androgen receptor antagonists (eg, flutamide, bucalutamide, nilutamide).10 The androgen biosynthesis inhibitor abiraterone will not be discussed in detail in this review, as this agent is indicated very late in the disease course, for metastatic castration-resistant prostate cancer after failure of chemotherapy.11
In breast cancer, up to 75% of cancers express either estrogen or progesterone receptors, and therefore would be expected to benefit from endocrine therapy.12–19 Hormone therapies for the adjuvant treatment of breast cancer include estrogen receptor modulators (SERMs; eg, tamoxifen), aromatase inhibitors (eg letrozole, anastrozole, exemestane), and luteinizing hormone-releasing hormone (LHRH) agonists (eg, goserelin, leuprolide). Additional endocrine therapies used in the metastatic setting include megesterol acetate, fulvestrant, and fluoxymesterone.
Effects of Hormone Ablation Therapy on Bone
While hormone ablation therapy can delay progression, prolong survival, or both in patients with prostate and breast cancer, it also accelerates the development of osteoporosis in both men and women. The risks for bone loss and resulting fractures are different for prostate and breast cancer and for various hormonal therapies. When given as monotherapy in prostate cancer, androgen receptor antagonists (antiandrogens) spare bone, but antiandrogen monotherapy is not an approved treatment in the US. In breast cancer, aromatase inhibitors and LHRH agonists, while often efficacious in preventing metastatic recurrence, cause decreases in bone density as they reduce circulating hormone levels.20–22 In post-menopausal women, SERMs carry less risk to bone than aromatase inhibitors and LHRH agonists, because although they antagonize estrogen receptors in breast tissue, they have inherent partial agonist activity in bone.23,24 In the case of post-menopausal women treated with SERMs, the benefit of this bone-sparing effect may be partly offset by a small increase in the risk of endometrial cancer and thromboembolic events.23
Mechanisms of bone turnover
Throughout life, bone undergoes a continuous process of formation (driven by osteoblasts) and resorption (driven by osteoclasts). In healthy adults, this process is balanced and coordinated.25,26 Osteoblasts arising from mesenchymal stem cells induce the formation of new bone; they also secrete factors that regulate bone metabolism, including macrophage colony stimulating factor (M-CSF) and RANK ligand (RANKL).27,28 RANKL, produced by bone marrow stromal cells and osteoblasts, binds to RANK on osteoclast precursors, inducing their differentiation from myeloid cells to osteoclasts.28–30 Activated osteoclasts attach to bone, secreting enzymes and acids that break down the bony matrix and dissolve bone minerals.28,30 The result is bone resorption. When resorption occurs more rapidly than formation, bone mass is reduced and bone tissue deteriorates, compromising bone strength and increasing the risk of fractures.
The mechanisms of bone turnover are similar in patients with bone metastases and cancer treatmentinduced bone loss (CTIBL), as both involve pathologically increased RANKL activity, but there are also important differences. The bone loss associated with hormonal therapies affects the total skeleton, whereas bone metastases are characterized by aggressive local bone destruction, fostered by growth factors released from bone matrix in response to osteoclast activity. This review is focused on bone loss and fractures associated with hormonal therapies, not the skeletal complications associated with bone metastases.
Effects of cancer treatment on bone turnover
In patients with cancer, the normal mechanisms of bone metabolism can be significantly disrupted by either disease or treatment. Estrogen (in the form of estradiol) is integral to the maintenance of bone mineral density in both men and women, with testosterone exerting only minor direct effects on the skeleton.31,32 In women, the effects of decreased estrogen levels in reducing trabecular bone mass are well known, observed as a mean annual rate of bone mineral density (BMD) loss of 1.9% in women undergoing natural menopause.33,34 Testosterone is converted to estradiol by aromatase; recent studies have demonstrated that estrogen deficiency occurs in the male skeleton if serum estradiol levels fall below a certain threshold, creating an independent risk factor for fracture.35 ADT reduces levels of testosterone and consequently, levels of estradiol. Estrogen stimulates the apoptosis of osteoclasts and suppresses the apoptosis of osteoblasts. The result is that, in estrogen deficiency such as that evoked by ADT, osteoclasts increase in number and osteoblasts decrease, tipping the balance toward bone resorption.36 Estrogen deficiency is also associated with increases in the levels of cytokines that promote bone resorption, including TNF-α and IL-1α. These cytokines increase the expression of RANKL, further promoting bone resorption.36
Bone loss that occurs in men receiving ADT is more rapid and severe than normal age-related bone loss: a decrease in BMD of up to 8.5% per year, compared with 0.5% to 1% per year.37 BMD continues to decrease as the duration of ADT increases.38,39 In women receiving aromatase inhibitors for the treatment of breast cancer, reduced levels of endogenous estrogen levels promote the formation of osteoclasts and the resorption of bone.21 In the ATAC trial of post-menopausal women receiving anastrozole or tamoxifen for breast cancer (n = 6,241), bone loss over 5 years of anastrozole therapy for women who had recently started menopause (≤4 years) was 11.3% at the lumbar spine and 7.5% at the total hip.21 Because circulating estradiol is involved in the regulation of bone turnover, the result of hormone ablation in both men and women is an increased risk of osteoporosis.40 Other agents used in adjuvant cancer treatment, including methotrexate, cyclophosphamide, doxorubicin, and dexamethasone, may have direct effects on bone, independent of their effects on hormone levels.41 In many patients, the risk of CTIBL compounds the risk of osteoporosis associated with age or reduced hormone levels.42
Fracture and Its Consequences
The reduction in bone strength associated with osteoporosis greatly increases the risk of fracture, often in the hip, distal forearm, and spine.41 In the year 2000, an estimated 9 million new osteoporotic fractures occurred worldwide, including 1.6 million at the hip, 1.7 million at the forearm and 1.4 million clinical vertebral fractures.43 By accelerating osteoporosis, hormone-ablation therapy increases fracture risk.
Fracture in patients with prostate and breast cancer
Among US men with nonmetastatic prostate cancer in the SEER and Medicare databases (N = 72,392), men on pharmacologic ADT had a 5.7% rate of fractures over 12 months, a 34% higher risk than for men not on ADT after adjusting for age, race, tumor grade, stage, comorbidities, and osteoporosis or fracture before prostate cancer diagnosis.39 The adjusted risk for fracture increased with the cumulative dose of ADT and was highest for men with nonmetastatic cancer who had an orchiectomy (adjusted hazard ratio [aHR], 1.62, 85% CI 1.42–1.84). Similar results were reported in a matched cohort study of more than 38,000 men in Ontario, Canada (mean age, 75 years). Those treated with ADT for prostate cancer experienced significantly more fractures of all types (17.2% versus 12.7%, P < 0.0001) and more fragility fractures (9.0% versus 5.9%, P < 0.0001) than matched patients not treated with ADT.44
Data from the Women’s Health Initiative and other studies indicate that post-menopausal breast cancer survivors have significantly lower bone mineral density (overall and total hip) and a resulting increased risk of clinical fracture.45,46 A 1999 World Health Organization (WHO) study found that women with non-metastatic breast cancer had more than triple the incidence of vertebral fracture compared with controls without breast cancer, irrespective of age (5.4% over 2.1 years vs. 1.5% over 2.9 years).47 In the ATAC trial, patients treated with anastrozole experienced fractures while on treatment at an annual rate of 2.93%, compared with a rate of 1.90% of those being treated with tamoxifen.48 After completion of treatment, the annual rate of fractures was similar in both groups: 1.56 in those treated with anastrozole and 1.51 in those treated with tamoxifen.48
Other risk factors for fracture
In addition to reduced bone density, other factors contribute to the risk for fractures. A 2001 study by Kanis et al showed that age is an independent risk factor for fracture in both men and women.49 ADT has been reported to decrease lean body mass in men with prostate cancer, with decreases of 2.7% to 3.6% over 12 months reported; lean body mass is associated with increased risk for fracture.50,51 The WHO notes that up to half of falls in elderly patients, a frequent cause of fractures, are associated with poor reflexes or vision, gait abnormalities, muscle weakness, chronic illnesses, and medications such as hypnotics, anti-depressants, sedatives,52 and potentially ADT.53 The National Comprehensive Cancer Network (NCCN) Task Force on Bone Health in Cancer Care notes that many non-oncologic factors are also associated with an increased risk of fracture, including smoking, excessive alcohol use, inadequate exercise, calcium and vitamin D deficiency, parental history of hip fracture, and the use of glucocorticoids, proton pump inhibitors, and anticoagulants.40
Consequences of fracture
The risk of mortality increases up to 50% for patients who experience a hip fracture, with a higher risk for men than women; the increased risk persists for months or years after the fracture.54,55 In the SEER-Medicare study of fracture risk in men who had ADT, the adjusted risk of death was twice as high for men who had a fracture as for those who did not (aHR = 2.05, 95% CI 1.98–2.12).39 Similar results were seen in a 2009 Swedish study, in which the age-adjusted mortality risk after fracture was doubled for men and increased by 81% for women compared with controls without fractures. In this study, after a hip fracture, the mortality rate was more than double the rate for all fractures in both men and women.55 Fractures also produce significant morbidity; a hip fracture may result in the inability to work, potentially leading to social isolation and loss of independence.56 Vertebral fractures may result in chronic, severe pain or vertebral compression that may compromise pulmonary function.57,58 Like hip fractures, vertebral fractures have been found to be associated with increased mortality, also at a higher rate in men than in women.55,59,60 The costs of fracture treatment impose significant financial burdens on the healthcare system; direct costs of hospital treatment and associated care were estimated at $17 billion for the US population aged 50–99 years in 2005.61
Risk Assessment and Fracture Prevention
Assessment
The WHO recommends assessment of fracture risk using both clinical and diagnostic tools for patients considered to be at risk of osteoporosis.62 The NCCN Task Force on Bone Health in Cancer Care recommends that patients for whom hormone ablation therapy is planned be evaluated at baseline with periodic follow-up using dual x-ray absorptiometry (DXA) scans to assess the risk of fracture.40 A patient’s bone density is described in comparison to a “young normal” adult; the result is called a T-score. WHO criteria define a T-score of ≥− 1.0 as normal, 1.0 to 2.5 standard deviations below normal (a T-score of −1.0 to −2.5) as osteopenia, and a T-score ≤−2.5 as osteoporosis.63 The FRAX tool, which can be calibrated for use in various countries and ethnic populations, was designed to assess the risk of fracture in general clinical practice.64 The probability of a fracture is calculated by the FRAX algorithm from age, sex, body mass index, prior fragility fracture, parental history of hip fracture, tobacco use, glucocorticoid use, high alcohol consumption, and other causes of secondary osteoporosis (eg, rheumatoid arthritis, prolonged immobility, thyroid disorders). These parameters are easy for clinicians to obtain; the tool also works if data for one or more parameters are missing. Measured BMD at the femoral neck can also be input as a variable. The FRAX tool is available at http://www.shef.ac.uk/FRAX.
Bone turnover markers are used to assess treatment effects in clinical trials, but they are not frequently used in clinical practice, as their validity for management of individual patients is not established.65–67 Markers of bone resorption include serum type 1 C-telopeptide (sCTX), urinary N-telopeptide (uNTX), and tartrate-resistant acid phosphatase 5b (TRACP-5b). Markers of bone formation include bone-specific alkaline phosphatase (BSAP), procollagen-1 N-terminal peptide (P1NP), and osteocalcin.
Fracture prevention
The goals of management to prevent fracture in patients undergoing hormone ablation therapy vary with the degree of bone loss. In patients with normal bone density,62 the goal of management is the preservation of bone density. In patients who already have osteopenia or osteoporosis, the goal of management is prevention of further bone loss and resulting fractures.68 An international interdisciplinary expert panel of clinical oncologists and specialists in metabolic bone diseases69 and the NCCN Task Force on Bone Health in Cancer 40 recommended that all patients at risk of CTIBL receive supplemental calcium and vitamin D. The NCCN also recommended lifestyle modifications: weight-bearing, muscle strengthening, and balance exercises; tobacco avoidance; and alcohol limitation.
Beyond these measures, pharmacologic options are recommended to increase bone mass in patients at high risk for fracture. Antiresorptive agents approved for prevention or treatment of osteoporosis, including denosumab, bisphosphonates, SERMs, estrogen, calcitonin, or recombinant parathyroid hormone (teriparatide) can be considered.40,69 Approval by the US Food and Drug Administration (FDA) of therapies for osteoporosis is based on demonstrated effectiveness in reducing the risk of vertebral fractures. Of these agents, only denosumab (60 mg given subcutaneously every 6 months) has been approved in many countries for treatment of CTIBL. No guidelines are currently available for the duration of therapy; the NCCN Bone Health in Cancer Care Task Force suggested that individual patients’ risk of fracture be considered when determining the appropriate therapy duration.
Pharmacologic Therapies
The role of pharmacologic therapy to treat or prevent osteoporosis in the general population is described elsewhere;15,40,42,52,69,71–82 key information will be summarized briefly here.
Bisphosphonates
Bisphosphonates reduce bone resorption by accumulating in bone and inhibiting the function of osteoclasts.83 Although none of these agents are currently approved by the US FDA for CTIBL, they are often used and have been recommended by the American Society of Clinical Oncology (ASCO), the NCCN, and an international expert panel for men and women receiving hormone ablation therapy.9,14,17,69 Oral bisphosphonates, which are often considered the first line of treatment for osteoporosis, include alendronate, generally taken weekly; risedronate, taken weekly or monthly; and ibandronate, taken monthly; ibandronate is also available as a solution for injection. Oral bisphosphonates have been shown in small studies to increase BMD in men and women undergoing hormone ablation therapy for breast or prostate cancer.78,80,84–89 Some patients may have difficulties using oral bisphosphonates, which require fasting and remaining upright for long periods in the morning and may be associated with esophagitis (sometimes severe), constipation, or stomach discomfort.90–92 Oral bisphosphonates are not recommended or are contraindicated for patients with renal impairment (creatinine clearance < 30 or 35 mL/min) or hypocalcemia.90–92
Intravenous bisphosphonates include pamidronate and zoledronic acid. Like oral bisphosphonates, they are not approved but are sometimes used for CTIBL. Pamidronate was shown to prevent bone loss in a trial of men with prostate cancer receiving ADT.93 Zoledronic acid has been demonstrated in numerous studies to increase BMD in men and women undergoing hormone ablation therapy for prostate cancer or breast cancer.94–108 Current product labeling recommends administration of zoledronic acid 5 mg as an intravenous infusion once a year to treat osteoporosis in men or postmenopausal women; in clinical trials evaluating zoledronic acid in patients undergoing hormone ablation therapy, zoledronic acid 4 mg was administered every 3 months94,95,101–103,108,109 or every 6 months.110,111 Like oral bisphosphonates, zoledronic acid is contraindicated in patients with renal impairment or hypocalcemia.112 Zoledronic acid has been associated with osteonecrosis of the jaw, atypical fractures of the femur, severe, incapacitating bone, joint, and/or muscle pain, and acute phase reactions (generally a first-dose effect).112
SERMs
SERMs, which selectively bind estrogen receptors, are used in both prostate and breast cancer, but with different objectives. Raloxifene and toremifene have been shown to increase BMD in men with prostate cancer receiving ADT.113,114 Toremifene was recently shown to reduce the incidence of new vertebral fractures compared with placebo at 2 years (1.0%, vs. 4.8%, P < 0.005),115 but development of toremifene for the reduction of fractures in men with prostate cancer on ADT was terminated in 2011.116 In breast cancer, tamoxifen and toremifene are used as adjuvant endocrine therapy19 and raloxifene is used to prevent and treat osteoporosis, but the NCCN Task Force on bone health recommends that SERMs not be used in combination with aromatase inhibitors outside of clinical trials,40 since the ATAC trial showed no benefit to combining tamoxifen and anastrozole as adjuvant therapy.
Estrogen
Estrogen has been used to reduce the risk of hot flashes in men with prostate cancer receiving ADT, and it may also reduce the risk of osteoporosis. However, side effects including gynecomastia and increased risk of thromboembolism limit its use in prostate cancer.117 Estrogen replacement therapy is likewise considered controversial in women with a history of breast cancer, including estrogen-receptor negative disease, because it may increase the risk of recurrence.118 If hot flashes require pharmacologic intervention, non-hormonal therapies (eg, gabapentin or anti-depressants) are generally used.
Calcitonin and teriparatide
Calcitonin, available as an injection or as nasal spray, is an antiresorptive agent approved for prevention and treatment of postmenopausal osteoporosis; the product labeling includes a warning of the possibility of severe allergic reactions including anaphylaxis.119 Evidence is limited regarding its use in patients with prostate or breast cancer. Teriparatide, administered by daily subcutaneous injection, is a recombinant human parathyroid hormone analog120 administered for a maximum of 2 years. Teriparatide has been shown to increase BMD and reduce fractures in men and postmenopausal women with osteoporosis.115,121,122 The product labeling for teriparatide includes a black-box warning stating that teriparatide is associated with an increased risk of osteosarcoma, particularly in patients who have received prior external beam or implant radiation therapy involving the skeleton. The NCCN Task Force on Bone Health in Cancer Care recommends avoiding teriparatide in patients who have received radiation therapy to the skeleton.40 Based on these concerns, teriparatide is rarely used in oncologic patients.
Denosumab
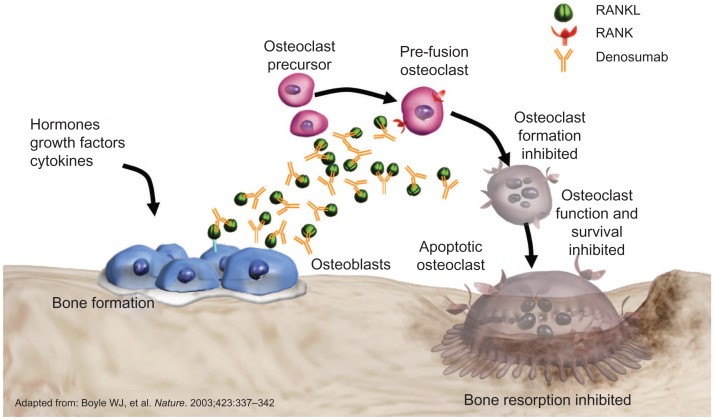
Denosumab is a fully human monoclonal IgG2 antibody against RANKL, a key mediator of osteoclast formation, function and survival.123 Denosumab inhibits bone resorption mediated by osteoclasts, with a mechanism of action different from that of bisphosphonates. By binding with high affinity and specificity to RANKL, denosumab prevents RANKL from activating its receptor RANK on the surface of osteoclasts and their precursors. Inhibition of RANK/RANKL interaction decreases bone resorption and increases bone strength (Fig. 1).
Figure 1.
Denosumab in CTIBL: proposed mechanism of action.28
Denosumab, under the brand name Prolia® (60 mg every 6 months), is approved in the US, Canada, Mexico, Europe, Russia, and Australia for treatment of postmenopausal women with osteoporosis at increased risk for fracture; to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer; and to increase bone mass in women at increased risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer. Check local product labeling for the wording of specific indications. Denosumab is also approved for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors in the US, Canada, the European Union, and several other countries under the brand name XGEVA® and in Japan under the brand name RANMARK®. Denosumab is not indicated for the prevention of SREs in patients with multiple myeloma except in Japan.
Dosing
For the treatment of men or women receiving hormone ablation therapy, a 60 mg dose of denosumab is administered once every 6 months by subcutaneous injection in the upper arm, upper thigh, or abdomen.70 All patients receiving denosumab should also receive daily supplements of 1,000 mg of calcium and at least 400 IU of vitamin D. (Denosumab 120 mg is administered every 4 weeks to patients with bone metastases for the prevention of skeletal related events.124)
Clinical studies in patients undergoing hormone ablation therapy
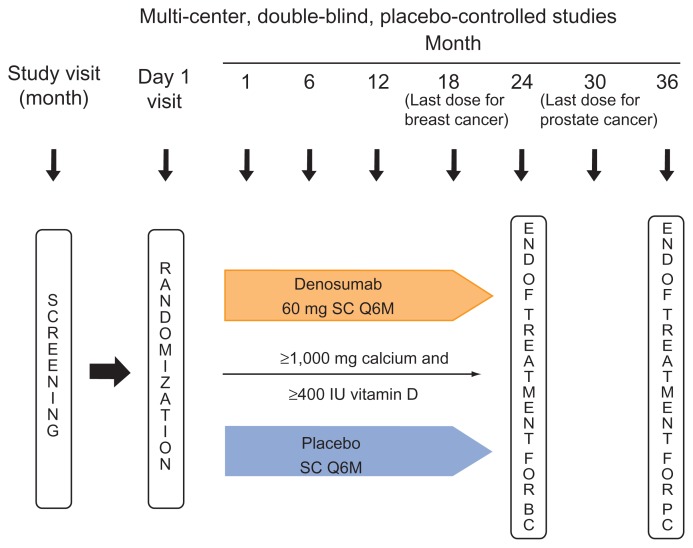
Denosumab was evaluated in patients with breast cancer or prostate cancer undergoing hormone ablation therapy in two placebo-controlled phase 3 studies that were similar in design (Fig. 2). Differences included study duration and the fact that the prostate cancer study was much larger and included vertebral fracture reduction as a secondary endpoint.125,126 In both studies, patients received subcutaneous denosumab 60 mg or subcutaneous placebo every 6 months. All patients were urged to take ≥ 1,000 mg of calcium and ≥400 IU of vitamin D daily. Patients in the prostate cancer study received their last dose of study drug at month 30 and the study ended at month 36. Patients in the breast cancer study received their last dose at month 18 and completed the study at month 24. The primary endpoint in both studies was the percent change from baseline in lumbar spine BMD, assessed at 24 months in the prostate cancer study 126 and at 12 months in the breast cancer study.125 BMD was assessed with DXA scans, using Hologic or Lunar machines calibrated across study centers with a set of standard phantoms; scans were centrally monitored. These studies were placebo-controlled because no standard of care was defined and no medications were approved for the treatment of bone loss associated with hormone ablation therapy.125,126 Key demographic characteristics of prostate and breast cancer patients receiving hormone ablation therapy in these studies are summarized in Table 1.
Figure 2.
Study designs: denosumab vs. placebo in men with prostate cancer and women with breast cancer receiving hormone ablation therapy.125,126
Abbreviations: BC, breast cancer; PC, prostate cancer; S, subcutaneous; Q6M, every 6 months.
Table 1.
| Characteristic | Breast cancer study | Prostate cancer study | ||
|---|---|---|---|---|
|
|
|
|||
| Placebo N = 125 | Denosumab N = 127 | Placebo N = 734 | Denosumab N = 734 | |
| Age, mean (SD) | 59.7 (9.7) | 59.2 (8.9) | 75.5 (7.1) | 75.3 (7.0) |
| Received prior hormone ablation therapy > 6 months, n (%) | 79 (63) | 80 (63) | 559 (76) | 559 (76) |
| White, n (%) | 119 (95) | 116 (91) | 609 (83) | 615 (84) |
| Body mass index, kg/m2, median (min, max) | 28.1 (18, 45) | 27.5 (18, 56) | 27.6 (18, 42) | 27.9 (15, 45) |
| ECOG status, n (%) | ||||
| 0 | 105 (84) | 114 (90) | 538 (73) | 552 (75) |
| 1 | 14 (11) | 13 (10) | 174 (24) | 154 (21) |
| BMD T-score, median (min, max) | ||||
| Lumbar spine | −1.20 (−2.9, 2.6) | −1.20 (−3.8, 1.9) | −0.60 (−4.8, 7.6) | −0.50 (−6.8, 7.3) |
| Total hip | −0.80 (−2.4, 0.8) | −1.00 (−2.4, 0.9) | −0.95 (−3.6, 3.1) | −0.90 (−3.6, 3.3) |
| Distal 1/3 of the radius | −2.40 (−4.4, 1.8) | −2.45 (−5.0, 1.5) | −2.60 (−6.6, 1.0)* | −2.35 (−6.8, 1.9)* |
Notes: N = the number of patients randomized in each group.
Substudy in prostate cancer; N = 309.
Prostate cancer study
The effects of denosumab treatment on the incidence of fractures, BMD, and bone turnover markers were assessed in the HALT study, a randomized, double-blind, placebo-controlled phase 3 study in 1,468 men with nonmetastatic prostate cancer receiving ADT.126 Eligible patients had histologically confirmed non-metastatic prostate cancer and were receiving ADT (bilateral orchiectomy or GnRH agonist with expected duration of on-study treatment ≥ 12 months). They had either a low baseline BMD (T-score < −1.0 at the lumbar spine, total hip, or femoral neck) or history of an osteoporotic fracture. Patients with very low BMD T-scores (<−4.0) at the lumbar spine, total hip, or femoral neck were excluded from the study. Randomization was stratified by age (<70 or ≥70 years) and duration of ADT (≤6 months or >6 months).
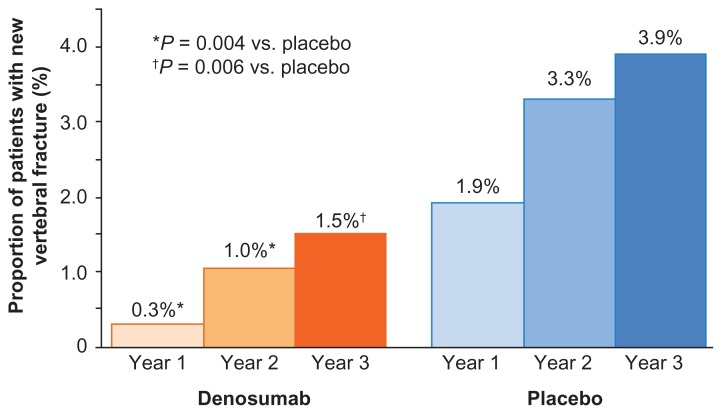
Denosumab was shown to reduce the risk of new vertebral fractures, increase BMD, and reduce markers of bone turnover among men with prostate cancer receiving ADT. The incidence of new vertebral fractures was reduced for the denosumab group after 1, 2, and 3 years. At 1 year, the percentage of new vertebral fractures was 0.3% with denosumab and 1.9% with placebo (relative risk, 0.15; P = 0.004); at 24 months, 1.0% vs. 3.3% (relative risk, 0.31; P = 0.004); and at 36 months, 1.5% with denosumab and 3.9% with placebo (relative risk, 0.38; P = 0.006) (Fig. 3).126
Figure 3.
Denosumab reduced the risk of vertebral fractures over 3 years in men with prostate cancer receiving ADT.126
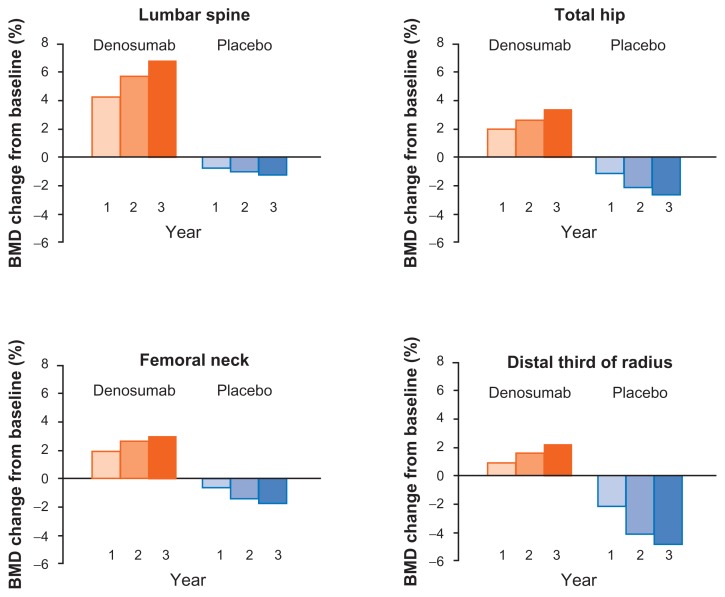
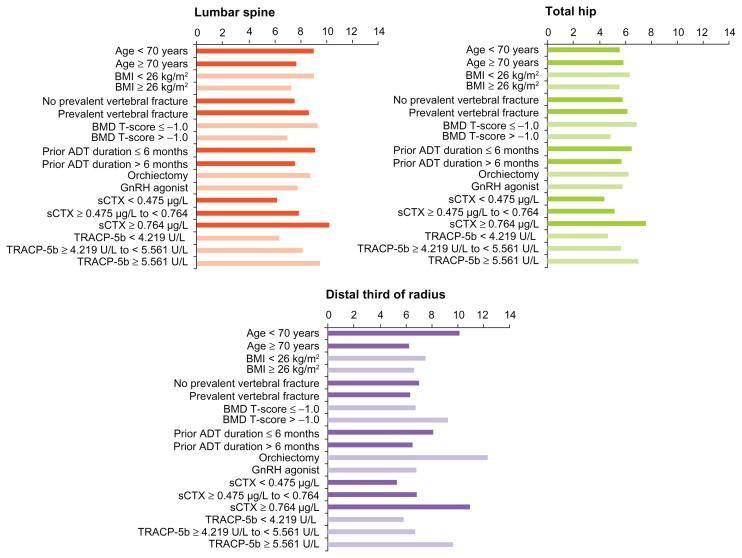
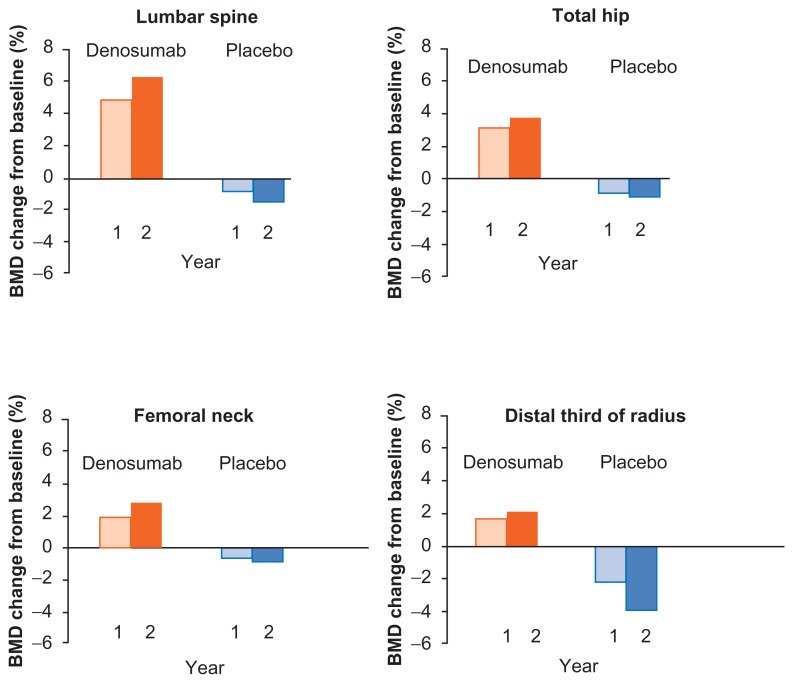
In the same study, denosumab also significantly increased mean BMD at the lumbar spine, total hip, femoral neck, and distal third of the radius at 12, 24, and 36 months (P ≤ 0.001) (Fig. 4).126 At 24 months (the primary endpoint), the difference between denosumab and placebo was 6.7% at the lumbar spine, 4.8% at the total hip, 3.9% at the femoral neck, and 5.5% at the distal third of the radius. These significant increases in BMD were consistent in all patient subgroups, including older men and those with lower BMD, higher levels of bone turnover markers, or a history of vertebral fracture at baseline (Fig. 5).127 The BMD increase in the lumbar spine with denosumab at 36 months was 9.1% for men < 70 years of age and 7.7% for those ≥70 years of age. In men with BMD T-scores at baseline ≤−1.0, the BMD increase at the lumbar spine was 9.3%, versus 7.0% for men with baseline BMD T-scores > −1.0. In men with prevalent vertebral fractures, BMD increased 8.7% over the 36 months of the study, compared with 7.6% for men without a prevalent vertebral fracture. Likewise, the duration of ADT at baseline did not have a marked effect on BMD increases. Men who had been on ADT for ≤6 months experienced an LS mean gain of 9.1% in lumbar spine BMD, compared with 7.6% for men who had been on ADT for >6 months. Denosumab also increased BMD at the total hip and distal third of the radius in all patient subgroups.
Figure 4.
Cumulative percent change in BMD from baseline, denosumab vs. placebo in men with prostate cancer receiving ADT.126
Notes: Results are presented as least-squares means.
Abbreviation: BMD, bone mineral density.
Figure 5.
Mean difference in BMD from placebo at 36 months, denosumab vs. placebo, in men with prostate cancer receiving ADT: subgroup analyses.127
Notes: Results are presented as least-squares means.
The effectiveness of denosumab in reducing bone resorption was also assessed in the HALT study using serum bone turnover markers.128 sCTX, TRACP-5b, and P1NP were assessed at baseline and 1 month postdose, and predose (denosumab trough level) at months 6, 12, 24, and 36. Denosumab treatment resulted in a rapid, sustained reduction of bone turnover markers from month 1 through the end of the last dosing interval at 36 months. As with BMD, denosumab’s effect on bone turnover was consistent across patients subgroups, including men aged ≥ 70 years, men with ADT duration at baseline > 6 months, and men with higher levels of bone turnover at baseline.128 The changes in bone turnover markers were associated with changes in BMD at 36 months.128
Breast cancer
The effects of denosumab treatment on BMD and bone turnover markers were assessed in a randomized, double-blind, placebo-controlled phase 3 study in 252 women with nonmetastatic breast cancer receiving aromatase inhibitors.125 Eligible patients were women ≥ 18 years of age with histologically or cytologically confirmed, hormone-receptor positive breast cancer, who were undergoing adjuvant aromatase inhibitor therapy after completion of surgery and/or radiation at least 4 weeks before study entry. All patients had T-scores from −1.0 to −2.5 (osteopenia). Women were excluded if they had prior vertebral fractures, T-scores < −2.5, or current use of bisphosphonates or any anticancer therapy except aromatase inhibitors. Randomization was stratified by duration of prior aromatase inhibitor therapy (≤6 months or >6 months).
Over the 24 months of the study, denosumab treatment was associated with numerically fewer major nonvertebral fractures and significant increases in BMD compared with placebo. No vertebral fractures were reported in either treatment group during the study. Major nonvertebral fractures (defined as fractures in the pelvis, distal femur, proximal tibia, ribs, proximal humerus, forearm and hip) occurred in 3 patients (2%) in the denosumab group and 5 patients (4%) in the placebo group. Nonvertebral fractures of all types (excluding pathologic fractures, those resulting from severe trauma, and fractures of the skull, face, mandible, and digits) were reported in 8 patients (6%) in each treatment group.
At 12 months, BMD in the lumbar spine increased by 5.5% in the denosumab group compared with the placebo group (denosumab 4.8%, placebo −0.7%, P < 0.0001) (Fig. 6). At 24 months, the difference between groups was 7.6%, P < 0.0001.125 Patients in the denosumab group also experienced an increase in BMD after 12 and 24 months at other measured sites; the difference from placebo at 24 months was 4.7% at the total hip, 3.6% at the femoral neck, and 6.1% at the distal third of the radius at 24 months.125
Figure 6.
Cumulative mean percent change in BMD from baseline, denosumab vs. placebo in women with breast cancer receiving aromatase inhibitors.129
Notes: Results are presented as least-squares means.
Abbreviation: BMD, bone mineral density.
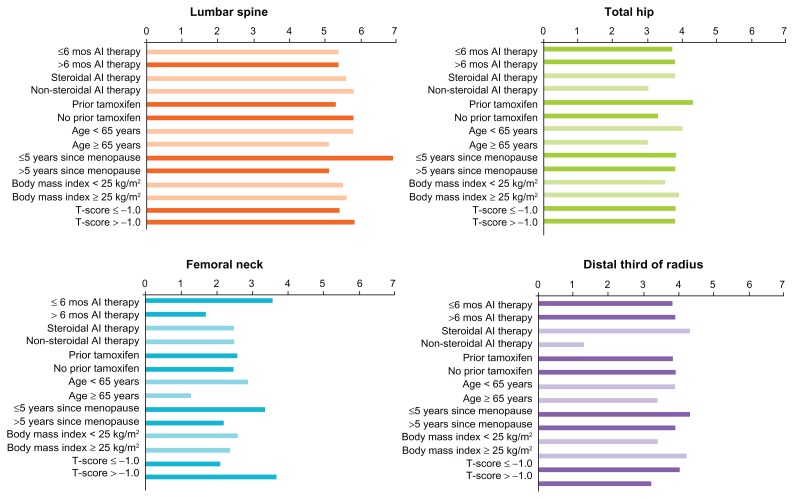
Gains in BMD were consistent across various patient subgroups in this study.129 For example, patients who had been on aromatase inhibitor therapy for less than 6 months at baseline had a difference of 5.4% from placebo in lumbar spine BMD at 12 months, compared with 5.6% for patients on aromatase inhibitors for more than 6 months. Gains at the total hip, femoral neck, and distal third of the radius were also similar (Fig. 7), and the treatment effect of denosumab on BMD was sustained through month 24. Denosumab was similarly effective at all BMD sites for patients regardless of type of AI therapy, prior use of tamoxifen, age, time since the onset of menopause, body mass index, and baseline T-score (Fig. 7). In the breast cancer study, 80% of patients treated with denosumab had a gain in BMD after 24 months of more than 3% at the lumbar spine, compared with 13% of patients receiving placebo; 50% of denosumab patients had a gain in BMD of more than 6%, compared with only 3% of placebo patients. Similar proportions of denosumab-treated patients had BMD gains at all measured sites.
Figure 7.
Least squares mean difference in BMD from placebo at 12 months in women with breast cancer receiving aromatase inhibitors: subgroup analyses.
Notes: Results are presented as least-squares means.129
Safety and tolerability
In clinical studies of denosumab in patients with prostate or breast cancer receiving hormone ablation therapy, the overall rates of adverse events were similar between the denosumab and placebo treatment groups. A summary of adverse events in these two studies is presented in Table 2. The denosumab product labeling notes that, in patients treated with denosumab for CTIBL or osteoporosis, hypocalcemia may be exacerbated and that all patients treated with denosumab should receive calcium and vitamin D supplementation.70 Hypocalcemia was reported in one patient (0.1%) in the prostate cancer study of patients receiving hormone ablation therapy and no patients in the breast cancer study. In the FREEDOM trial of more than 7,800 women with postmenopausal osteoporosis, no patients in the denosumab group were reported to have hypocalcemia during the first 3 years of the study and 1 patient during the 2-year extension phase.130 In patients with bone metastases, who received a higher dose of denosumab than is given to cancer patients without bone metastases or for osteoporosis, adverse events of hypocalcemia were reported in the prostate cancer study in 6% of patients on zoledronic acid and 13% on denosumab, 131 and in the breast cancer study, in 3.4% of patients on zoledronic acid and 5.5% of patients on denosumab.132
Table 2.
Summary of adverse events over 24 months in the breast cancer study and over 36 months in the prostate cancer study.125,126
| Breast cancer study | Prostate cancer study | |||
|---|---|---|---|---|
|
|
|
|||
| Placebo N = 120 | Denosumab N = 120 | Placebo N = 725 | Denosumab N = 731 | |
| Any adverse event, n (%) | 108 (90.0) | 117 (90.7) | 627 (86.5) | 638 (87.3) |
| Serious adverse events, n (%) | 11 (9.2) | 19 (14.7) | 222 (30.6) | 253 (34.6) |
| Adverse events related to investigational product,* n (%) | 31 (25.8) | 32 (24.8) | 65 (9.0) | 62 (8.5) |
| Any fatal adverse event, n (%) | 1 (0.8) | 1 (0.8) | 46 (6.3) | 44 (6.0) |
| Adverse events reported by > 10% of patients receiving denosumab in either study | ||||
| Arthralgia | 30 (25.0) | 31 (24.0) | 80 (11.0) | 92 (12.6) |
| Pain in extremity | 14 (11.7) | 19 (14.7) | 51 (7.0) | 66 (9.0) |
| Back pain | 15 (12.5) | 18 (14.0) | 74 (10.2) | 81 (11.1) |
| Fatigue | 17 (14.2) | 17 (13.2) | 45 (6.2) | 44 (6.0) |
| Constipation | 11 (9.2) | 15 (11.6) | 75 (10.3) | 73 (10.0) |
| Cough | 5 (4.2) | 13 (10.1) | 27 (3.7) | 33 (4.5) |
| Insomnia | 14 (11.7) | 12 (9.3) | 16 (2.2) | 23 (3.1) |
Notes: N = the number of patients randomized in each group.
Adverse events assessed by investigators as potentially related during the blinded clinical trials.
Another potential risk of denosumab treatment mentioned in the denosumab product labeling is serious infection leading to hospitalization, which was reported more frequently with denosumab in the FREEDOM trial of more than 7,800 women with postmenopausal osteoporosis. Serious adverse events related to infection were reported in 5.9% of denosumab- treated patients and 4.6% of placebo-group patients in the prostate cancer study of men receiving hormone ablation therapy, and in 2% of denosumab-treated patients and 1% of placebo patients in the breast cancer hormone ablation study.
Osteonecrosis of the jaw (ONJ) has been reported in patients receiving either bisphosphonates or denosumab to prevent bone resorption. No cases of ONJ were reported in patients in the two studies described here of denosumab for patients with prostate or breast cancer receiving hormone ablation therapy.125,126 The product labeling for denosumab recommends that a routine oral examination be performed by the prescriber and that appropriate preventive dentistry be considered before initiation of denosumab treatment.70
In the HALT study of men receiving ADT, cataracts developed in 4.7% of patients receiving denosumab vs. 1.2% of those receiving placebo. Cataract formation was not observed in other studies of denosumab, in which annual doses 12 to 13 times higher were administered to patients with castrate-resistant prostate cancer, including study of men with bone metastases receiving denosumab or zoledronic acid131 and a placebo-controlled denosumab study in men at high risk for bone metastases.133 Likewise, cataract formation was not observed in studies of women with breast cancer treated with denosumab.125,129,132
Pharmacokinetics and metabolism
To profile the pharmacokinetics of denosumab, data were pooled from 11 clinical studies of varied doses of denosumab that included 22,944 samples from 495 healthy subjects and 1069 postmenopausal women with osteopenia or osteoporosis.134 The age of participants ranged from 18 to 80 years for healthy subjects (men and women) and from 18 to 85 for post- menopausal women with bone loss. The subcutaneous bioavailability of denosumab was 64%, and the first-order absorption rate constant (ka) was 0.00883 h−1. The central volume of distribution was 2.49 L/66 kg; the linear clearance was 3.06 mL/h/66 kg. The variability between subjects was moderate. A fixed dose of 60 mg provided inhibition of RANKL similar to that achieved by equivalent body weight-based dosing. The effects of age and race were less than 15% on the area under the serum concentration-time curve of denosumab. Similar results were obtained in another study that included 581 subjects with advanced cancer.135 The antibody denosumab is metabolized through the reticuloendothelial system,136 without reliance on renal function, so potential renal impairment has no effect on the pharmacokinetics or pharmacodynamics of denosumab.70
Conclusions
Bone health is an important consideration in patients with prostate or breast cancer undergoing hormone ablation therapy. The increased survival afforded by effective therapies, including hormone ablation therapy, means that the effects of cancer therapy may influence patients’ health and well being for many years. Effective tools are available for the assessment of patients’ risk for loss of bone density and fracture, providing clinicians with the ability to monitor and promote patients’ bone health. Denosumab 60 mg administered subcutaneously every 6 months has been shown to increase BMD in breast cancer and prostate cancer and to reduce the risk of vertebral fractures in prostate cancer patients undergoing hormone ablation therapy, with an overall safety profile similar to placebo.125,127–129 To promote the overall health of their patients, clinicians treating patients with prostate or breast cancer using hormone ablation therapy should consider appropriate assessments and therapies such as denosumab to ensure optimal bone health in these long-term cancer survivors.
Acknowledgements
The authors acknowledge the medical writing and editorial assistance of Geoffrey R. Smith, PhD, of Amgen Inc. and Sue Hudson, BA, whose services were funded by Amgen.
Footnotes
Author Contributions
Conceived and designed the experiments: AL, MS, GE, CG. Analysed the data: AL, MS, GE, CG. Wrote the first draft of the manuscript: AL, MS, GE, CG. Contributed to the writing of the manuscript: AL, MS, GE, CG. Agree with manuscript results and conclusions: AL, MS, GE, CG. Jointly developed the structure and arguments for the paper: AL, MS, GE, CG. Made critical revisions and approved final version: AL, MS, GE, CG. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
Competing Interests
AL has received reimbursement for speaking, expert testimony, and research support from Novartis and Amgen. CG was an employee of Amgen during preparation of this manuscript and holds Amgen stock. MRS has received grants and consulting fees from Amgen. GKE has received consulting fees and financial support from Amgen.
References
- 1.International Agency for Research on Cancer (IARC) GLOBOCAN Cancer Fact Sheets 2008: Breast Cancer. GLOBOCAN 2008. 2010. [Accessed Jan 19, 2012]. http://globocan.iarc.fr/factsheets/cancers/breast.asp.
- 2.International Agency for Research on Cancer (IARC) GLOBOCAN Cancer Fact Sheets 2008: Prostate Cancer. GLOBOCAN 2008. 2010. [Accessed Jan 19, 2012]. http://globocan.iarc.fr/factsheets/cancers/breast.asp.
- 3.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Horwich A, Parker C, Bangma C, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v129–33. doi: 10.1093/annonc/mdq174. [DOI] [PubMed] [Google Scholar]
- 7.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 8.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol Esp. 2011;35:565–79. doi: 10.1016/j.acuro.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Prostate Cancer. V3.2012. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [DOI] [PubMed]
- 10.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson WF, Chatterjee N, Ershler WB, et al. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 13.Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi12–24. doi: 10.1093/annonc/mdr371. [DOI] [PubMed] [Google Scholar]
- 14.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–57. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Limburg CE. Screening, prevention, detection, and treatment of cancer therapy-induced bone loss in patients with breast cancer. Oncol Nurs Forum. 2007;34:55–63. doi: 10.1188/07.ONF.55-36. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast Cancer. V1.2012. [Accessed Jun 14, 2012]. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 18.Wolters R, Regierer AC, Schwentner L, et al. A comparison of international breast cancer guidelines—do the national guidelines differ in treatment recommendations? Eur J Cancer. 2012;48:1–11. doi: 10.1016/j.ejca.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso F, Fallowfield L, Costa A, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi25–30. doi: 10.1093/annonc/mdr372. [DOI] [PubMed] [Google Scholar]
- 20.Cheung AM, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(11)70389-8. [DOI] [PubMed] [Google Scholar]
- 21.Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–7. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 22.Aapro MS. Long-term implications of bone loss in breast cancer. Breast. 2004;13( Suppl 1):S29–37. doi: 10.1016/j.breast.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–53. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 25.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 26.Lipton A, Uzzo R, Amato RJ, et al. The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw. 2009;7(Suppl 7):S1–29. doi: 10.6004/jnccn.2009.0080. quiz S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodama H, Nose M, Niida S, et al. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991;173:1291–4. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 29.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 31.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 32.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5:437–43. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 33.Ahlborg HG, Johnell O, Turner CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–34. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–8. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosla S. Update in male osteoporosis. J Clin Endocrinol Metab. 2010;95:3–10. doi: 10.1210/jc.2009-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95:3569–77. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–9. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 38.Kiratli BJ, Srinivas S, Perkash I, et al. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–32. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 39.Beebe-Dimmer JL, Cetin K, Shahinian V, et al. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol Drug Saf. 2012;21:70–8. doi: 10.1002/pds.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw. 2009;7(Suppl 3):S1–32. doi: 10.6004/jnccn.2009.0076. quiz S33–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000;18:1570–93. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 42.Mackey JR, Joy AA. Skeletal health in postmenopausal survivors of early breast cancer. Int J Cancer. 2005;114:1010–5. doi: 10.1002/ijc.20826. [DOI] [PubMed] [Google Scholar]
- 43.International Osteoporosis Foundation. Facts and statistics about osteoporosis and its impact. 2011. [Accessed Jan 22, 2012]. http://www.iofbonehealth.org/facts-and-statistics.html.
- 44.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–8. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Maricic M, Pettinger M, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. 2005;104:1520–30. doi: 10.1002/cncr.21335. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Maricic M, Bassford TL, et al. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:552–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 47.Kanis JA, McCloskey EV, Powles T, et al. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–81. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 49.Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 50.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 51.Smith M. Changes in fat and lean body mass during androgen deprivation therapy for prostate cancer. Urology. 2004;63:742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization; Osteoporosis WSGotPaMo, editor. Geneva: World Health Organization; 2003. Prevention and management of osteoporosis: report of a WHO scientific group. [Google Scholar]
- 53.Bylow K, Mohile SG, Stadler WM, et al. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer. 2007;110:2604–13. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 54.Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 55.Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 56.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 57.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S–17. doi: 10.1016/s0002-9343(97)90022-x. discussion 17S–19. [DOI] [PubMed] [Google Scholar]
- 58.Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261–7. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 59.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 60.Smith M, Egerdie B, Sieber P, et al. Overall survival in men with and without prevalent vertebral fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer. Highlights of the Joint ECCO 15/34th ESMO Multidisciplinary Congress; 2009; [Accessed Mar 2, 2012]. http://www.esmo.org/fileadmin/media/pdf/2009/reports/meetings/Berlin2009-MeetingReport.pdf. [Google Scholar]
- 61.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–25. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: technical report 843. World Health Organization; 1994. [PubMed] [Google Scholar]
- 63.Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 64.Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395–411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 65.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 66.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 67.Costa L, Demers LM, Gouveia-Oliveira A, et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20:850–6. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 68.Michaud LB, Goodin S. Cancer-treatment-induced bone loss, part 2. Am J Health Syst Pharm. 2006;63:534–46. doi: 10.2146/ajhp050045.p2. [DOI] [PubMed] [Google Scholar]
- 69.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–32. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 70.Amgen Inc. PROLIA (denosumab) full prescribing information. May 20, 2012. [Accessed Jun 14, 2012]. http://pi.amgen.com/united_states/prolia/prolia_pi.pdf.
- 71.Body JJ. Prevention and treatment of side-effects of systemic treatment: bone loss. Ann Oncol. 2010;21(Suppl 7):vii180–5. doi: 10.1093/annonc/mdq422. [DOI] [PubMed] [Google Scholar]
- 72.Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11:384. doi: 10.1186/1471-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brufsky A. Management of cancer-treatment-induced bone loss in postmenopausal women undergoing adjuvant breast cancer therapy: a Z-FAST update. Semin Oncol. 2006;33:S13–7. doi: 10.1053/j.seminoncol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 74.Brufsky AM. Zoledronic acid for cancer therapy—induced and postmenopausal bone loss. Expert Opin Pharmacother. 2008;9:1013–28. doi: 10.1517/14656566.9.6.1013. [DOI] [PubMed] [Google Scholar]
- 75.Brufsky AM. Bone health issues in women with early-stage breast cancer receiving aromatase inhibitors. Curr Oncol Rep. 2008;10:18–26. doi: 10.1007/s11912-008-0005-z. [DOI] [PubMed] [Google Scholar]
- 76.Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist. 2008;13:187–95. doi: 10.1634/theoncologist.2007-0152. [DOI] [PubMed] [Google Scholar]
- 77.Coleman RE. Supportive care in oncology. Support Care Cancer. 2005;13:959–60. doi: 10.1007/s00520-005-0860-1. [DOI] [PubMed] [Google Scholar]
- 78.Greenspan SL, Nelson JB, Trump DL, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol. 2008;26:4426–34. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadji P, Body JJ, Aapro MS, et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–16. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 80.Lester JE, Dodwell D, Purohit OP, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–42. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 81.Theriault RL, Biermann JS, Brown E, et al. NCCN Task Force Report: Bone Health and Cancer Care. J Natl Compr Canc Netw. 2006;4(Suppl 2):S1–20. quiz S21–2. [PubMed] [Google Scholar]
- 82.von Moos R. Bisphosphonate treatment recommendations for oncologists. Oncologist. 2005;10( Suppl 1):19–24. doi: 10.1634/theoncologist.10-90001-19. [DOI] [PubMed] [Google Scholar]
- 83.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 84.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 85.Hines SL, Mincey BA, Sloan JA, et al. Phase III randomized, placebo-controlled, double-blind trial of risedronate for the prevention of bone loss in premenopausal women undergoing chemotherapy for primary breast cancer. J Clin Oncol. 2009;27:1047–53. doi: 10.1200/JCO.2008.19.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markopoulos C, Tzoracoleftherakis E, Polychronis A, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12:R24. doi: 10.1186/bcr2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28:967–75. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 88.Ishizaka K, Machida T, Kobayashi S, et al. Preventive effect of risedronate on bone loss in men receiving androgen-deprivation therapy for prostate cancer. Int J Urol. 2007;14:1071–5. doi: 10.1111/j.1442-2042.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- 89.Kearns AE, Northfelt DW, Dueck AC, et al. Osteoporosis prevention in prostate cancer patients receiving androgen ablation therapy: placebo-controlled double-blind study of estradiol and risedronate: N01C8. Support Care Cancer. 2010;18:321–8. doi: 10.1007/s00520-009-0655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merck & Co. I. Fosamax (alendronate sodium) tablets and oral solution full prescribing information. Jul, 2011. [Google Scholar]
- 91.Warner Chilcott Company L. Actonel (risedronate sodium) tablets full prescribing information. Feb, 2011. [Google Scholar]
- 92.Roche Laboratories I. Boniva (ibandronate sodium) tablets and solution for infection, full prescribing information. Jan, 2011. [Google Scholar]
- 93.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 94.Hines SL, Mincey B, Dentchev T, et al. Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast Cancer Res Treat. 2009;117:603–9. doi: 10.1007/s10549-009-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryan CW, Huo D, Demers LM, et al. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176:972–8. doi: 10.1016/j.juro.2006.04.078. discussion 978. [DOI] [PubMed] [Google Scholar]
- 96.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–45. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–8. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 98.Bertoldo F, Tonini G, Vincenzi B, et al. Medical oncology: zoledronic acid prevents bone loss in early-stage breast cancer. Nat Rev Clin Oncol. 2009;6:191–2. doi: 10.1038/nrclinonc.2009.24. [DOI] [PubMed] [Google Scholar]
- 99.Brufsky AM, Bosserman LD, Caradonna RR, et al. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9:77–85. doi: 10.3816/CBC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- 100.Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188–94. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 101.Shapiro CL, Halabi S, Hars V, et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: final results from CALGB trial 79809. Eur J Cancer. 2011;47:683–9. doi: 10.1016/j.ejca.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Israeli RS, Rosenberg SJ, Saltzstein DR, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5:271–7. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 103.Hershman DL, McMahon DJ, Crew KD, et al. Prevention of bone loss by zoledronic acid in premenopausal women undergoing adjuvant chemotherapy persist up to one year following discontinuing treatment. J Clin Endocrinol Metab. 2010;95:559–66. doi: 10.1210/jc.2009-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hines SL, Sloan JA, Atherton PJ, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy. Breast. 2010;19:92–6. doi: 10.1016/j.breast.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campbell SC, Bhoopalam N, Moritz TE, et al. The use of zoledronic acid in men receiving androgen deprivation therapy for prostate cancer with severe osteopenia or osteoporosis. Urology. 2010;75:1138–43. doi: 10.1016/j.urology.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 106.Casey R, Gesztesi Z, Rochford J. Long term zoledronic acid during androgen blockade for prostate cancer. Can J Urol. 2010;17:5170–7. [PubMed] [Google Scholar]
- 107.Polascik TJ, Given RW, Metzger C, et al. Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology. 2005;66:1054–9. doi: 10.1016/j.urology.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 108.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 109.Bhoopalam N, Campbell SC, Moritz T, et al. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol. 2009;182:2257–64. doi: 10.1016/j.juro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 110.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829–36. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 111.Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112:1001–10. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 112.Novartis Pharmaceuticals Corporation. Reclast (zoledronic acid) injection. Aug, 2011. [Google Scholar]
- 113.Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–6. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 114.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–5. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–21. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.GTX I. GTx provides corporate update and reports first quarter 2011 financial results. 2011. [Accessed Mar 2, 2012]. http://phx.corporate-ir.net/phoenix.zhtml?c=148196&p=irol-newsArticle_print&ID=1561101&highlight=
- 117.Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185:17–23. doi: 10.1016/j.juro.2010.08.094. [DOI] [PubMed] [Google Scholar]
- 118.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–5. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 119.Novartis Pharmaceuticals Corporation. Miacalcin (calcitonen salmon) (injection or nasal spray) full prescribing information. Jul, 2011. [Google Scholar]
- 120.Eli Lilly and Company. Forteo (teriparatide injection) full prescribing information. Jan, 2010. [Google Scholar]
- 121.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 122.Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510–6. doi: 10.1007/s00198-004-1713-3. [DOI] [PubMed] [Google Scholar]
- 123.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–66. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 124.Amgen Inc. XGEVA (denosumab) full prescribing information. Jun, 2012. [Accessed Jun 14, 2012]. http://pi.amgen.com/united_states/xgeva/xgeva_pi.pdf.
- 125.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. Journal of Clinical Oncology. 2008;26:4875–82. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 126.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith MR, Saad F, Egerdie B, et al. Effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2009;182:2670–5. doi: 10.1016/j.juro.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith MR, Saad F, Egerdie B, et al. Denosumab and changes in bone turnover markers during androgen deprivation therapy for prostate cancer. J Bone Miner Res. 2011;26:2827–33. doi: 10.1002/jbmr.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ellis GK, Bone HG, Chlebowski R, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 130.Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: Results from the first two years of the FREEDOM extension. J Bone Miner Res. 2011 doi: 10.1002/jbmr.1479. Epub Nov 23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 133.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sutjandra L, Rodriguez RD, Doshi S, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50:793–807. doi: 10.2165/11594240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 135.Gibiansky L, Sutjandra L, Doshi S, et al. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin Pharmacokinet. 2012;51:247–60. doi: 10.2165/11598090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 136.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]