Abstract
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder associated with progressive memory loss, severe dementia, and hallmark neuropathological markers, such as deposition of amyloid-β (Aβ) peptides in senile plaques and accumulation of hyperphosphorylated tau proteins in neurofibrillary tangles. Recent evidence obtained from transgenic mouse models suggests that soluble, nonfibrillar Aβ oligomers may induce synaptic failure early in AD. Despite their undoubted value, these transgenic models rely on genetic manipulations that represent the inherited and familial, but not the most abundant, sporadic form of AD. A nontransgenic animal model that still develops hallmarks of AD would be an important step toward understanding how sporadic AD is initiated. Here we show that starting between 12 and 36 mo of age, the rodent Octodon degus naturally develops neuropathological signs of AD, such as accumulation of Aβ oligomers and phosphorylated tau proteins. Moreover, age-related changes in Aβ oligomers and tau phosphorylation levels are correlated with decreases in spatial and object recognition memory, postsynaptic function, and synaptic plasticity. These findings validate O. degus as a suitable natural model for studying how sporadic AD may be initiated.
Keywords: memory dysfunction, neural plasticity, aging, T-maze, hippocampus
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by the accumulation of abnormally processed proteins in neurofibrillary tangles (NFTs) and senile plaques (1). These lesions are present in both familial and sporadic forms of AD. Familial AD is linked to inherited mutations in AD-related genes and represents a small percentage of AD cases, whereas sporadic AD represents the vast majority of cases and is not inherited. Results from transgenic mice bearing mutations in APP, PSEN1/2, and TAU show synaptic dysfunction in early stages of AD, before overt neurodegeneration (2, 3). More recent studies have demonstrated a critical role for soluble Aβ oligomers as an early trigger for AD, as well as associations with memory and neural plasticity loss (4–8).
Although transgenic mice have been extremely useful in elucidating the pathological mechanisms of AD, they have some substantial limitations. Examples include the absence of tau mutations linked to AD except for a triple transgenic mouse 3xTg-AD, bearing mutations for APP, PSEN1/2, and TAU (9); inability to develop the whole spectrum of the disease; overexpression of transgenes into a nonphysiological scenario; and the fact that the manipulated genes represent only familial, not sporadic forms of AD (10, 11). It would be highly desirable to have a nontransgenic model of AD to complement the existing models. Several species naturally develop features of AD with age; however, the usefulness of these species is limited, because none exhibits the full spectrum of AD-related alterations (12–14). For example, the Aβ peptide sequences of Cavia porcellus (guinea pig) and Microcebus murinus are similar to that of human (15, 16), but the first fails to develop senile plaques and NFTs (15), and experiments examining synaptic function and memory have not been carried out in such models. A promising candidate model for sporadic AD is the rodent Octodon degus (degus), which naturally develops the histochemical hallmarks of AD, including intracellular and extracellular accumulation of amyloid plaques, tau deposition in NFT (17), and hippocampal disconnection and brain parenchyma pathology (18). Prompted by these preliminary observations, we examined the neuropathological spectrum of AD in degus. Here we report that degus exhibits an age-related accumulation of soluble Aβ oligomers and tau protein phosphorylation that correlates with cognitive decline in spatial memory (T-maze) and object recognition memory (ORM), as well as synaptic and neural plasticity dysfunction. Based on these findings, we propose that (i) Aβ dodecamers (Aβ*56) may associate with phosphorylated tau proteins, constituting an early candidate for the neural toxicity and synaptic dysfunction that occurs before the appearance of fibrillar forms of Aβ, which are common to familial and sporadic forms of AD, and (ii) degus is a suitable nontransgenic model of sporadic AD.
Results
We evaluated the degree of neuropathology at the behavioral, synaptic, and molecular levels in degus at different ages. This approach allowed us to establish interpretative correlations to identify those animals suffering from AD (Table S1).
Age-Related Cognitive Decline with Aging in O. degus.
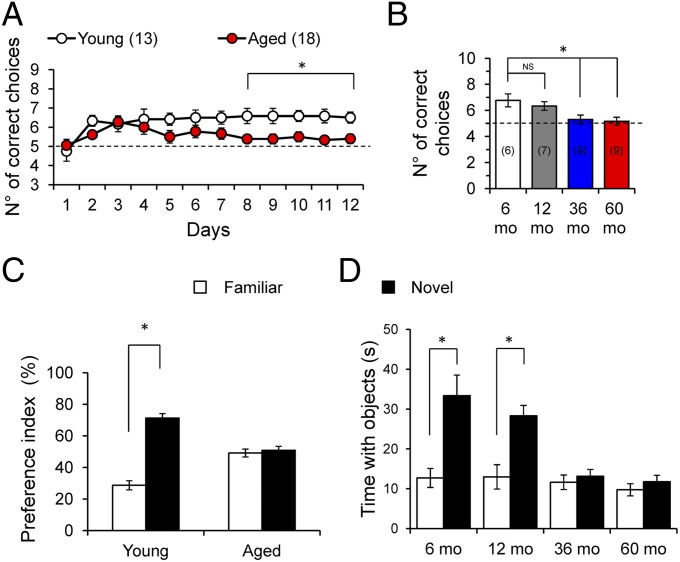
We first evaluated memory capacity with ORM and T-maze tests in 6-, 12-, 36-, and 60-mo-old degus. The results showed no significant difference between 6- and 12-mo-old degus and between 36- and 60-mo-old degus, and thus we classified the animals as either young (6 and 12 mo-old) or aged (36 and 60 mo-old) (Fig. 1 B and D). In the T-maze, the aged degus had poorer performance than the young degus (Fig. 1 A and B; P < 0.0001, ANOVA). On the ORM test, the aged degus explored less (total time and number of visits) and had longer latency than the younger degus (Table S2). Moreover, unlike young degus, aged degus did not demonstrate a preference between new objects and familiar objects (Fig. 1 C and D; P < 0.0001, t test). In general, we observed an age-dependent decline in memory performance beginning at 36 mo-old and persisting through 60 mo-old (Fig. 1B).
Fig. 1.
Age-dependent decline in cognitive performance in O. degus. (A) Average number of correct choices per day in the T-maze test. Two-way ANOVA [F(11,33) = 34.59, *P < 0.0001], followed by the Bonferroni post hoc test (P < 0.05) in the last 3 d for aged (36–60 mo-old) vs. young (6–12 mo-old). (B) Average correct choices at the end of the experimental phase for 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. One-way ANOVA [F(3,26) = 6.509, *P = 0.002], followed by Tukey´s post hoc test (P < 0.05). (C) Preference index for object recognition. Paired two-tailed t test (*P < 0.01), novel vs. familiar objects. (D) Average exploration time for novel vs. familiar objects. Paired two-tailed t test (*P < 0.01) vs. familiar objects. The values in parentheses indicate the number of animals.
Selective Postsynaptic Dysfunction Induces Impairments in Synaptic Transmission and Plasticity.
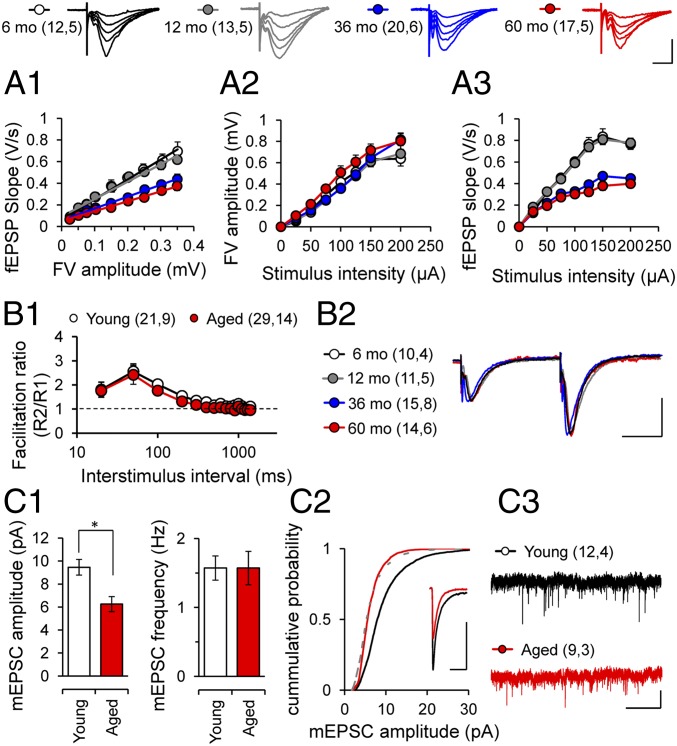
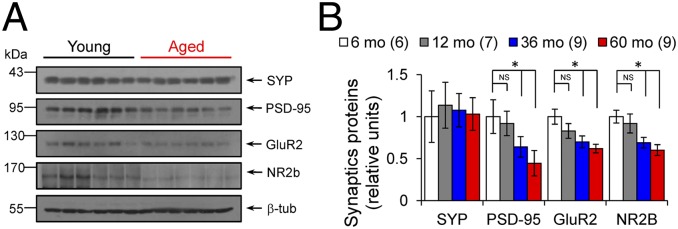
We next examined the synaptic basis of these learning deficits by evaluating the strength and plasticity of the CA3–CA1 synapses in hippocampal slices prepared from behaviorally tested animals. Basal excitatory synaptic transmission was reduced in aged degus compared with young degus (Fig. 2A1; P < 0.0001, ANOVA). Importantly, the field excitatory postsynaptic potential (fEPSP) slopes, but not fiber volley amplitude, were significantly reduced in aged animals (Fig. 2 A2 and A3; P < 0.0001, repeated-measures ANOVA). This is consistent with findings reported in AD mouse models overexpressing mutant forms of amyloid precursor protein (APP) (19, 20; reviewed in ref. 21). The reduced transmission could be due to a reduced postsynaptic responsiveness or to a decreased probability of neurotransmitter release. We ruled out the latter possibility, because we found no difference in the paired-pulse facilitation ratio (Fig. 2 B1 and B2; P > 0.05, two-way ANOVA). To confirm the likely postsynaptic basis for the reduced fEPSPs, we recorded AMPA receptor (AMPAR)-mediated miniature excitatory postsynaptic currents (mEPSCs). Consistent with previous reports in AD models (22, 23), we found that the significantly reduced amplitude, but not frequency, of mEPSCs in aged degus (Fig. 2 C1–C3; P < 0.01, t test), suggesting a change in AMPAR function or number. The electrophysiological analysis indicated that aging preferentially affects postsynaptic processes in degus. Thus, we further assessed the integrity of presynaptic and postsynaptic elements by measuring the levels of critical synaptic proteins extracted from hippocampal slices by Western blot analysis. We found a selective reduction in PSD-95, AMPAR subunit GluR2, and NMDA receptor (NMDAR) subunit NR2B expression (Fig. 3 A and B; P < 0.05, ANOVA), but not of synaptophysin, in accordance with the observation that measures of presynaptic function (fiber volley and paired pulse facilitation) are not affected in aged degus (Fig. 2 A2 and B1). These data support the observation of more vulnerable postsynaptic integrity in aged degus.
Fig. 2.
Altered synaptic transmission and postsynaptic deficits in the Schaffer collateral–CA1 pathway. Representative traces of fEPSP at different stimulus intensities from 6-mo-old (white), 12 mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. (Scale bars: 1 mV, 10 ms.) AMPAR-mediated input–output curves from 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. One-way ANOVA [F(3,58) = 157.3, *P < 0.0001], followed by Tukey's post hoc test (P < 0.05). (A2 and A3) Relationship between stimulus intensity and fiber volley amplitude (A2) and fEPSP slope (A3) in 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. Repeated-measures ANOVA [F(15,45) = 61.06, *P < 0.0001], followed by the Bonferroni post hoc test (P < 0.05). (B1) Normal paired-pulse facilitation between groups. (B2) Representative traces at interstimulus intervals of 50 ms are shown. (Scale bars: 1 mV, 20 ms.) (C1) Amplitudes and frequencies of AMPAR-mediated mEPSCs from young (white) and aged (red) degus. (C2) Cumulative probability plots for mEPSC amplitude size. (Inset) Representative superposed events. Calibration: 5 pA, 20 ms. (C3) Representative traces of mEPSCs. (Scale bars: 20 pA, 2 s.) Calibration: 1 mV, 10 ms. Unpaired two-tailed t test (*P < 0.01) for young vs. aged. The values in parentheses indicate the number of hippocampal slices (first number) and the number of animals (second number) used.
Fig. 3.
Postsynaptic proteins affect synapse during degus aging. (A) Representative blot of synaptic proteins from hippocampus extracts (age indicated above lanes). Arrows indicate respective migration positions. (B) Relative levels of synaptophysin (SYP), PSD-95, GluR2-AMPAR subunit, and NR2b-NMDAR subunit in the hippocampus from 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. Mean values of synaptic proteins are relative to β-tubulin levels. One-way ANOVA [F(3,20) = 6.626, *P = 0.0027 for PSD-95; F(3,20) = 3.968, *P = 0.0227 for GluR2; F(3,21) = 2.662, *P = 0.0745 for NR2b], followed by Tukey´s post hoc test (P < 0.05), young vs. aged.
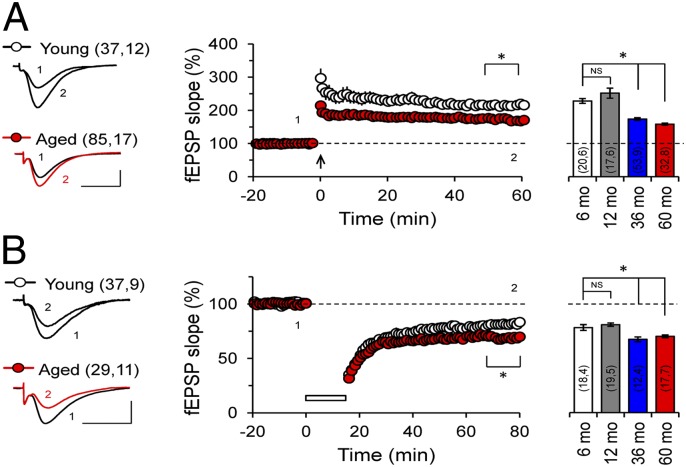
We next assessed whether synaptic plasticity was affected at the CA3–CA1 synapses of the aged degus. We measured long-term potentiation (LTP) induced with theta-burst stimulation (TBS) and long-term depression (LTD) induced with paired-pulse low-frequency stimulation (ppLFS). The LTP amplitude was significantly decreased after 60 min in aged degus compared with young degus (Fig. 4A; P < 0.0001, two-way ANOVA), whereas LTD was slightly but significantly increased in aged degus compared with young degus (Fig. 4B; P < 0.0001, two-way ANOVA). Prompted by the fact that neural plasticity was similar in the 36- and 60-mo-old degus, suggesting slowed progression of neurodegeneration, we examined an additional group of 72-mo-old degus. At this age, LTP continued to decrease and LTD to increase (Fig. S1A), indicating further progression of the neuropathology associated with AD.
Fig. 4.
Impaired hippocampal synaptic plasticity in aged O. degus. (A) TBS-induced LTP in the Schaffer collateral–CA1 synapse. (Left) Representative fEPSPs recorded 1 min before TBS (1) and 60 min after TBS (2). LTP protocol was delivered at the time indicated by the arrow. Averaged LTP magnitudes during the last 10 min of recording in 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus are shown as well. Two-way ANOVA [F(1,19) = 1841, *P < 0.0001], followed by the Bonferroni post hoc test (P < 0.05) in the last 10 min, for aged vs. young. (B) ppLFS-induced LTD in the Schaffer collateral–CA1 synapse. (Left) Representative fEPSPs recorded 1 min before ppLFS (1) and 60 min after ppLFS (2). LTD protocol was delivered at the time indicated by the horizontal open bar. Averaged LTD magnitudes during the last 10 min of recording for 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus are also as well. Two-way ANOVA [F(1,19) = 435.6, *P < 0.0001], followed by the Bonferroni post hoc test (P < 0.05) in the last 10 min for aged vs. young. (Scale bars: 1 mV, 10 ms.) The values in parentheses indicate the number of hippocampal slices (first number) and the number of animals (second number) used.
Synaptic plasticity measurements were recorded in behaviorally characterized degus, raising the possibility that synaptic modifications induced during the learning tasks might have affected the subsequent induction of LTP and LTD in hippocampal slices (24). However, the magnitude of LTP and LTD were similar (Fig. S2) in slices from naïve and trained degus, indicating that the effects of previous behavioral testing were minimal.
Increased Soluble Aβ Oligomers and Tau Phosphorylation in Aged Degus.
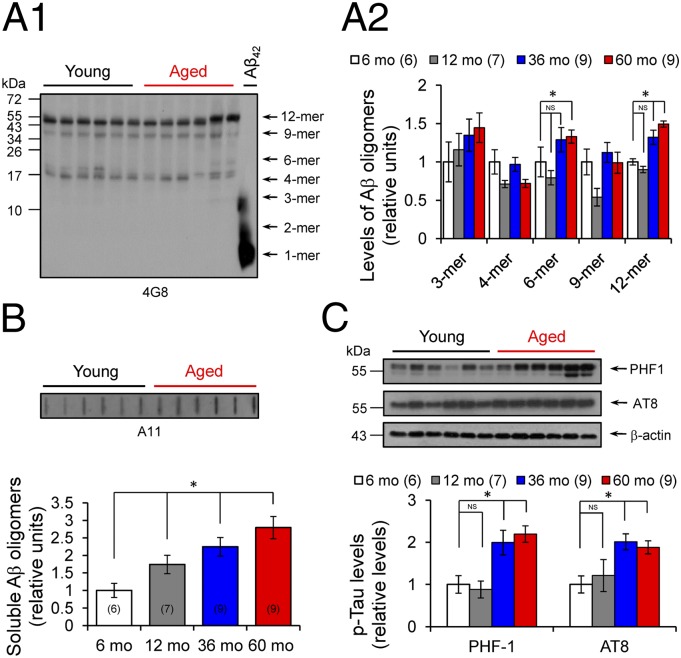
Accumulating evidence indicates that soluble Aβ oligomers are involved in synapse destruction and memory impairment in AD (5–7). To identify the nature of different types of Aβ species in hippocampal extracts from young and aged degus, we used specific mouse anti-β peptide 4G8, followed by immunoblot analysis (7). Young and aged degus exhibited similar forms of small Aβ peptides, including trimers (∼12 kDa), tetramers (∼16 kDa), and hexamers (∼27 kDa), whereas aged degus displayed higher levels of Aβ dodecamers (∼56 kDa) (Fig. 5 A1 and A2). The presence of these Aβ dodecamers in the hippocampus was confirmed by a slot-blot assay using the specific antioligomeric antibody A11, which detects soluble amyloid assemblies larger than 40 kDa (25). Levels of A11 were higher in the aged degus (Fig. 5B).
Fig. 5.
Accumulation of large soluble Aβ oligomers and tau phosphorylation during aging. (A1) Representative blot for amyloid oligomers using anti-Aβ peptide antibody 4G8. Arrows indicate respective migration positions of hexamers (6-mer), nonamers (9-mer), and dodecamers (12-mer). Synthetic Aβ42 peptide was used as size marker and positive control (right lane). (A2) Identification and relative levels of different Aβ oligomeric associations in hippocampal extracts from 6-mo-old (white), 12-mo-old (gray), 36-mo-old (blue), and 60-mo-old (red) degus. One-way ANOVA [F(3,21) = 17.21, *P < 0.0001 for 6-mer; F(3,19) = 5.439, *P = 0.0026 for 12-mer], followed by Tukey's post hoc test (P < 0.05) for aged vs. young. (B) Relative levels of soluble Aβ oligomers. (Inset) Representative slot blot from hippocampal extracts using antioligomeric antibody A11. One-way ANOVA [F(3,19) = 5.439, P = 0.0072], followed by Tukey's post hoc test (P < 0.05) for aged vs. young. (C) Determination of phosphorylated tau protein levels using PHF-1 and AT8 antibodies. (Inset) Representative blot for PHF-1, AT8, and β-actin. One-way ANOVA [F(3,20) = 23.21, *P < 0.0001 for PHF-1; F(3,20) = 5.95, *P = 0.0059 for AT8], followed by Tukey´s post hoc test (P < 0.05) for aged vs. young. The values in parentheses indicate the number of animals used.
The number and localization of NFT, but not of senile plaques, have been correlated with the level of dementia in patients with AD (26). To identify the presence of NFTs, we measured tau phosphorylation at sites known to be present in paired helical filaments (PHFs). The PHF-1 antibody detects tau phosphorylation at serine residues 396 and 404 (27). Compared with young degus, aged degus exhibited increased phosphorylation levels detected by this antibody (Fig. 5C). A similar result was obtained using AT8 antibody, which detects tau phosphorylation at serine 202 and threonine 205 (28). Tau phosphorylation at these sites was only slightly greater in aged degus, including 72-mo-old degus (Fig. S1 B and C), compared with young degus (Fig. 5C). The increased tau phosphorylation was not simply related to an increase in tau protein levels, given that the total tau level (Tau-5) remains unchanged up to age 72 mo (Fig. S1 B and C). Furthermore, pathological phosphorylated tau begins to appear first in the cortex and then in the hippocampus, where neurons containing hyperphosphorylated tau were preferentially detected in aged degus (see Fig. 7; also see figure 1 in ref. 17).
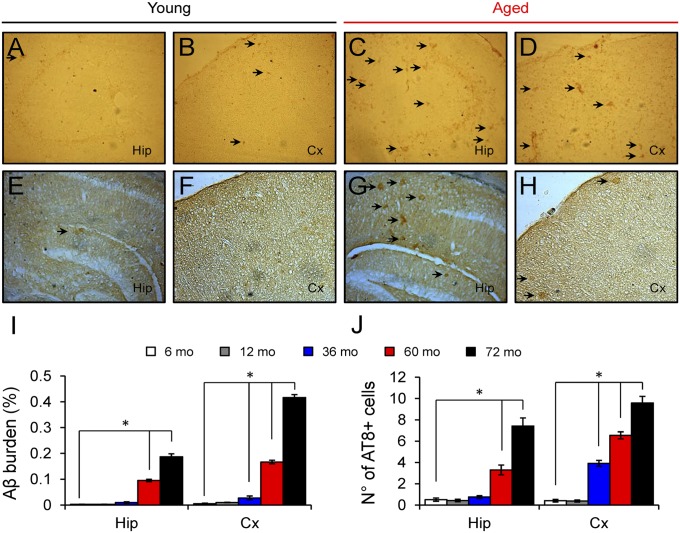
Fig. 7.
Amyloid deposition and tau phosphorylation begins in the cerebral cortex of O. degus. (A–D) Immunoreactivity for Aβ peptides using the specific antibody 6E10 showing extracellular (black arrows) staining in the hippocampus and the cerebral cortex in degus. A greater number of extracellular insoluble deposits were observed in aged degus (72 mo-old; C and D) compared with young degus (12 mo-old; A and B). (E–H) Immunodetection of pathological phosphorylated tau using the specific antibody AT8 in the hippocampus and cerebral cortex from degus. A greater number of positively stained somas (black arrows) were observed in aged degus (G and H) compared with young degus (E and F). (I and J) Quantification of Aβ burden (I; percentage of area occupied by 6E10-positive plaques) and the number of AT8-positive cells (J; number of neurons per area) in 6-mo-old (white, n = 2), 12-mo-old (gray, n = 2), 36-mo-old (blue, n = 2), 60-mo-old (red, n = 2), and 72-mo-old (black, n = 2) degus showing a significant increase after 36 mo in cortex and after 60 mo in hippocampus. One-way ANOVA [F(4,88) = 238.8, *P < 0.0001 for 6E10-Hip; F(4,88) = 646.4, *P < 0.0001 for 6E10-Cx; F(4,88) = 266.0, *P < 0.0001 for AT8-Hip; F(4,88) = 134.9, *P < 0.0001 for AT8-Cx], followed by Tukey's post hoc test (P < 0.05) compared with 6 mo-old.
Increased Levels of Soluble Aβ Oligomers and Tau Phosphorylation Correlate with Behavioral Impairment and Reduced Synaptic Plasticity.
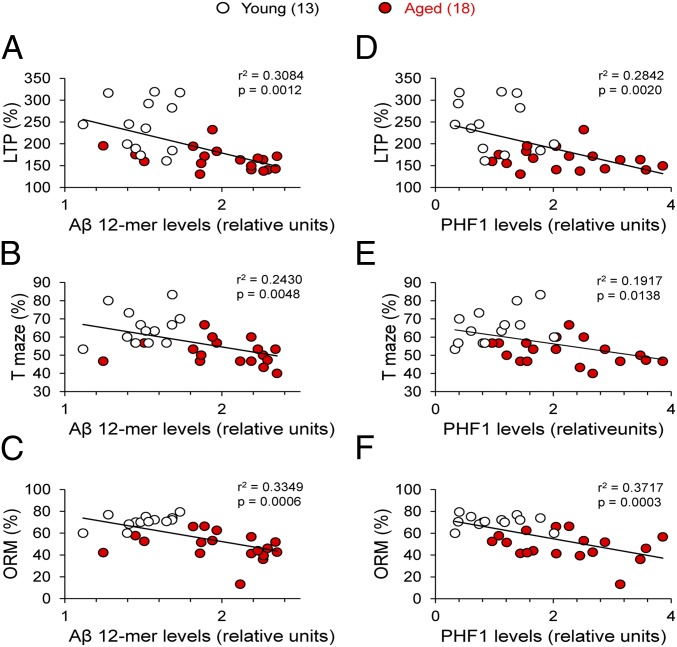
Table S1 shows that the 12mer dodecamer (Aβ*56) is highly negatively correlated with LTP magnitude (r2 = 0.31, P = 0.0012; Fig. 6A) and performance on T-maze (r2 = 0.24, P = 0.0048; Fig. 6B) and ORM (r2 = 0.34, P = 0.0006; Fig. 6C) tasks. Similarly, higher levels of PHF-1 are correlated with lower levels of LTP (r2 = 0.28, P = 0.0020; Fig. 6D), poorer ORM test performance (r2 = 0.37, P = 0.0003; Fig. 6F), and, to a lesser degree, poorer T-maze performance (r2 = 0.19, P = 0.0138; Fig. 6E). Comparable correlations were obtained when A11 and AT8 were compared with LTP values and T-maze and ORM test performance (Fig. S4). Further analysis using Principal Component Analysis is presented in supplementary information.
Fig. 6.
Large Aβ oligomers and tau phosphorylation correlate with LTP and memory impairments. (A–C) Relationships between soluble dodecamer (Aβ*56) level and LTP magnitude (A), T-maze (B), and ORM (C) in young and aged degus. (D–F) Relationships between PHF-1 epitope tau phosphorylated level and LTP magnitude (D), T-maze (E), and ORM (F) in young and aged degus. High correlation for LTP and ORM can be seen. The values in parentheses indicate the number of animals used.
Although these results suggest a similar time course and plausible causal links among the increases in soluble Aβ oligomers and phosphorylated tau and the incidence of synaptic and cognitive alterations, we cannot rule out the possibility that insoluble amyloid fibrils also participate in this cascade of neurotoxic events. For this reason, we analyzed the presence of amyloid deposition by immunohistochemistry and thioflavin S (ThS) staining (Fig. 7 and Fig. S3). Amyloid plaques were almost absent in young degus and began to appear in the cortex of aged degus at 36–60 mo-old, whereas plaques did not appear in the hippocampus until after 60 mo-old (Fig. S3). Just as with ThS-positive plaques, extracellular Aβ immunoreactivity (Fig. 7I) and pathological phosphorylated tau were first observed in the cortex at 36 mo-old and in the hippocampus after 60 mo-old (Fig. 7J).
Our findings indicate that degus develop a neurodegenerative AD-like condition with aging. In the initial stages, increased Aβ oligomers and phosphorylated tau levels could explain the impairments in learning, memory, and neural plasticity capacity. In later stages (after 72 mo-old), progressive deposition of plaques and tangles, likely leading to neurodegeneration, may aggravate or accelerate the symptomatology of AD in degus (Fig. 7 and Figs. S1 and S3).
Throughout this study, we noted that the behavioral, functional, and molecular alterations did not proceed at a uniform pace during aging. Rather, most of the changes occurred between 12 and 36 mo-old. To identify the most critical factors involved in the progression of AD, we performed a principal components analysis using variables with correlation ≥0.50: LTP, T-maze, ORM, Aβ*56, PHF-1, GluR2, and NR2b (Table S1). Interestingly, the plot in the space of the first two principal components (PC1 vs. PC2) clearly segregates two different classes of individuals: young degus and aged degus (Table S3–S5 and Fig. S5), supporting the importance of the variables examined here. Finally, it is important to note that although most of the deficits develop between 12 and 36 mo-old, the changes are not homogeneous in this population. Approximately 25% of individuals aged 36 mo exhibit “unimpaired” performance on either in the T-maze or ORM test.* This variability is expected in a group of nontransgenic animals with genetic backgrounds from a controlled but natural population.
Discussion
Synaptic and cognitive dysfunction in AD models has revealed significant impairments before neurodegeneration becomes evident (19, 20, 22, 23). We have shown a clear correlation between high levels of Aβ*56 oligomers and tau phosphorylation and reductions in synaptic strength and plasticity (Fig. 6 and Fig. S4); however, the exact mechanism by which Aβ oligomers or phosphorylated tau might impair synaptic function remains under debate. Some previous studies have reported that the addition of Aβ oligomers is sufficient to affect synaptic function in vitro (5, 29, 30) and also alter cognitive function (7, 31). Other studies have provided evidence suggesting that chemical reactions occurring during the process of Aβ aggregation produce toxic species, such as reactive oxygen species (32, 33). In that context, degus could provide a valuable “natural model” for testing toxicity mechanisms.
Recent evidence suggests that soluble Aβ oligomers (also referred to as ADDLs) may induce synaptic failure as an early event related to memory deficits in AD (6, 34). Indeed, soluble Aβ has been found to affect synapses by selectively targeting postsynaptic components (34, 35). More recently, it has been reported that cellular prion protein functions as a receptor for Aβ oligomers (36). Once bound to the membrane, these oligomers tend to accumulate at excitatory synapses, forming clusters with metabotropic glutamate receptors (mGluR5) and causing impairments in synaptic plasticity (37). Many different lengths and conformational states of Aβ peptide are generated during its biosynthesis, including highly mobile soluble Aβ oligomers and prefibrillar and fibrillar aggregates. These diverse assemblies have been associated with the disruption of memory and synaptic plasticity. For instance, dimers (8, 38), trimers (39), and dodecamers (7) derived from diverse sources (including chemical synthesis, transfected cells, and mouse and human AD brains) potently impair synaptic plasticity and memory. However, it has been shown that compared with other assemblies, Aβ*56 has the greatest effect on memory (7, 40, 41) and has been proposed as the key neurotoxic nonfibrillar assembly in AD because it is highly stable and prone to aggregation (42). In the same way, degus exhibited a stronger inverse relationship between higher Aβ*56 and LTP levels and improved T-maze and ORM test performance, which increases with aging (Fig. 6). Strikingly, aged degus also exhibited increased levels of phosphorylated tau (Fig. 5C and Fig. S3), suggesting a functional link between Aβ processing and tau phosphorylation, given that phosphorylated tau residues are also negatively correlated with LTP and cognitive impairment (Fig. 6). Several previous studies have shown that in mouse hippocampus, an increase in soluble Aβ by local administration increases the level of phosphorylated tau proteins, producing cognitive impairment (43–45). Furthermore, when the levels of both proteins decreased, recovery of cognitive abilities was observed (44). Interestingly, direct interaction between tau proteins and Aβ peptides induces tau aggregation and hyperphosphorylation (45). Furthermore, Aβ oligomers have been related to missorting and phosphorylation of tau (46), destabilization of microtubules, and disruption of axonal transport (47). On the other hand, Aβ-induced impairments in LTP are mediated by tau phosphorylation, suggesting that tau proteins are required for the synaptotoxic effects of Aβ oligomers (48).
Age-related reductions in spontaneous and evoked AMPAR-mediated currents also have been reported in AD models, attributed to a reduced number of these receptors (22, 23). We found no differences in the frequency of mEPSCs, suggesting that the number of functional synapses is not reduced in 60-mo-old degus. A possible explanation for the observed decrease in mEPSC amplitude is that the content of AMPARs available for trafficking is impaired in aged degus. Certainly, Aβ peptides prevent recruitment and anchoring of AMPARs to the postsynaptic compartment by reducing CAMKII activation and distribution as well as PSD-95 levels (49–51). Taken together, these results suggest that postsynaptic compartments are more susceptible to the effects of Aβ peptides compared with presynaptic elements, considering that both PSD-95 and glutamate receptors were reduced in aged degus (Figs. 2 and 4). However, presynaptic mechanisms cannot be totally excluded, given that APP (23), Aβ (52), and presenilin 1 and 2 (53) have been reported to be localized presynaptically.
An interesting comparison can be made between degus and 3xTg-AD mice (9). In both models, the decline in synaptic plasticity may be associated with an increase in Aβ peptides before the increase in tau phosphorylation. Because this process occurs naturally in degus, it represents a unique opportunity to examine physiological mechanisms and evaluate rescue therapies during sporadic AD.
Another motivating comparison here is with Microcebus murinus, a small nocturnal primate that lives to age 8–14 y in captivity, in which 20% of elderly adults exhibit neurodegenerative hallmarks of spontaneous AD, including brain amyloid plaques, tau pathology, decreased number of ACh neurons, and behavioral changes, with loss of sensory and cognitive functions (reviewed in ref. 16). Like Microcebus, 72-mo-old degus develop a discrete number of Aβ deposits, detectable first in the cortex and later in the hippocampus (Fig. 7 and Fig. S3; also see figure 1 in ref. 17). We have established changes in neural plasticity (LTP and LTD) in degus that correlate with the presence of soluble Aβ oligomers and phosphorylated tau proteins.
A recurrent and difficult question to address while studying neurodegeneration is its close association with the natural aging process. For example, aged rats show modest decreases in basal synaptic transmission, NMDAR-mediated response, and deficits in synaptic plasticity (54–56) similar to the decreases in both synaptic transmission and plasticity seen in degus (Figs. 2 and 3). However, aged rats do not demonstrate decreased spatial (T-maze) (56) or ORM test performance (57, 58), in clear contrast to degus (Fig. 1).
Taken together, our findings suggest that degus provides a strong and naturalistic model for the study of early neurodegenerative process associated with sporadic AD. Of note, degus can live approximately 9–10 y in captivity, and so the present work represents only half of the degus lifespan. Finally, although the precise mechanism remains unclear, our data are consistent with the concept that soluble Aβ oligomers at prefibrillar stages can act as toxic ligands at postsynaptic compartments, driving the synaptic and memory disruption seen in early AD models.
Materials and Methods
Animals.
O. degus were obtained from a breeding colony at the animal facility of the University of Valparaiso. All experiments were approved by the bioethics committee of the Universidad de Valparaiso and complied with the international NIH Approved Animal Welfare Assurance A5823-01.
Protocols.
More detailed information on the study procedures is provided in SI Materials and Methods. The different groups of degus were submitted to a complete characterization including behavioral tests, electrophysiological recordings, and biochemical measures. All animals received the same manipulations, regardless of age. Before evaluation of cognitive capacity, the animals were habituated to an open field over 5 consecutive days. Then they were submitted to the ORM test for 5 d, followed by the T-maze test for 17 d. Once the behavioral characterization was complete, degus were killed to obtain hippocampal slices in which to study synaptic transmission and plasticity. After completion of the electrophysiological experiments, the hippocampal slices were immediately frozen for biochemical characterization. Finally, the tissue was collected to obtain homogenates from the hippocampus to quantify different proteins levels by immunoblot analysis.
Behavioral Tests.
ORM was assessed using an open-field arena constructed of black Plexiglas (50 cm × 40 cm × 63 cm) over 5 d. Each ORM session consisted of three phases (180 s each): (i) familiarization, where degus explored a pair of identical objects; (ii) retention, where degus were removed for object cleaning and changing; and (iii) recognition, where degus explored a pair of different objects: a familiar object (FO) (extra copy of familiar object) and a novel object (NO). To quantitate OMR, a preference index (PI) was calculated as PI = NO/NO + FO.
Spatial working memory was assessed in a T-maze task using a training protocol to search for a reward over 12 consecutive days. Each session consisted of 10 trials composed of three parts (60 s each): (i) forced choice memory learning, with no food reward and one arm remaining closed; (ii) retention time, where the animal was removed for cleaning and the closed arm opened; (iii) free choice for memory recognition, where the two arms were open and the reward was placed in the previously closed arm. A correct response corresponded to a visit to the closed arm during part (i), in which case the animal was rewarded with a sunflower seed.
Electrophysiological Assessment.
Extracellular and whole-cell patch-clamp recordings were performed in hippocampal slices obtained from behaviorally characterized degus as described previously (54, 55).
Immunoblot Analysis.
Proteins were run on gradient denaturing gels, blotted, and probed with appropriate antibodies.
Histology.
PFA-fixed brains were sectioned into 20-μm slices, and free-floating slides were processed following immunohistochemical and ThS staining procedures.
Statistics.
All data are presented as mean ± SE or deviation of the mean (SEM or SD). Data were analyzed using Prism software (GraphPad).
Supplementary Material
Acknowledgments
We thank H. K. Lee and A. Megill (Johns Hopkins University), J. Ewer (Universidad de Valparaíso), A. Chavez (Albert Einstein University), A. Reichenbach (University of Leipzig), and L. Peichl (Max Planck University) for discussion and comments; C. Elgueta (University of Freiburg) for software assistance; and T. Lee (University of Michigan) for degus specimens from the United States. This work was supported by National Institutes of Health Fogarty International Research Collaboration Awards R03 TW007171-01A1 and R01 AG034606 (to A.K.); Chilean National Commission for Scientific and Technological Research Grant ANR-47 (to A.G.P. and F.A.), Grant PFB 12/2007 (to N.C.I.), and Fellowship AT-24091109 (to A.O.A.); and Interdisciplinary Center for Neuroscience of Valparaiso Millenium Scientific Initiative P09-022-F (to A.G.P.).
Footnotes
The authors declare no conflict of interest.
*Ponce A, Cerpa W, Inestrosa N, Palacios AG, Aging and spatial memory in the rodent Octodon degus. Annual Meeting of the Chilean Neuroscience Society, September 27, 2006, Curico, Chile.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201209109/-/DCSupplemental.
References
- 1.Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Götz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 3.Philipson O, et al. Animal models of amyloid-beta–related pathologies in Alzheimer’s disease. FEBS J. 2010;277:1389–1409. doi: 10.1111/j.1742-4658.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 7.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 8.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oddo S, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 10.Duff K, Suleman F. Transgenic mouse models of Alzheimer’s disease: How useful have they been for therapeutic development? Brief Funct Genomics Proteomics. 2004;3:47–59. doi: 10.1093/bfgp/3.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer’s disease? Genes Brain Behav. 2002;1:142–155. doi: 10.1034/j.1601-183x.2002.10302.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 13.Sarasa M, Pesini P. Natural non-transgenic animal models for research in Alzheimer’s disease. Curr Alzheimer Res. 2009;6:171–178. doi: 10.2174/156720509787602834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braidy N, et al. Recent rodent models for Alzheimer's disease: Clinical implications and basic research. J Neural Transm. 2011;119:173–195. doi: 10.1007/s00702-011-0731-5. [DOI] [PubMed] [Google Scholar]
- 15.Beck M, Bigl V, Rossner S. Guinea pigs as a nontransgenic model for APP processing in vitro and in vivo. Neurochem Res. 2003;28:637–644. doi: 10.1023/a:1022850113083. [DOI] [PubMed] [Google Scholar]
- 16.Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH. Microcebus murinus: A useful primate model for human cerebral aging and Alzheimer’s disease? Genes Brain Behav. 2006;5:120–130. doi: 10.1111/j.1601-183X.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- 17.Inestrosa NC, et al. Human-like rodent amyloid-beta peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol Aging. 2005;26:1023–1028. doi: 10.1016/j.neurobiolaging.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 18.van Groen T, et al. Age-related brain pathology in Octodon degu: Blood vessel, white matter and Alzheimer-like pathology. Neurobiol Aging. 2011;32:1651–1661. doi: 10.1016/j.neurobiolaging.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Fitzjohn SM, et al. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia AY, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Megill A, He K, Kirkwood A, Lee H-K. Consequences of inhibiting amyloid precursor protein processing enzymes on synaptic function and plasticity. Neural Plast. 2012;2012:272374. doi: 10.1155/2012/272374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang EH, et al. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double-knockin mice. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc Natl Acad Sci USA. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middei S, et al. Learning discloses abnormal structural and functional plasticity at hippocampal synapses in the APP23 mouse model of Alzheimer’s disease. Learn Mem. 2010;17:236–240. doi: 10.1101/lm.1748310. [DOI] [PubMed] [Google Scholar]
- 25.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 26.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 27.Otvos L, Jr, et al. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 28.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 29.Palop JJ, Mucke L. Amyloid-beta–induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson ME, Lesné SE. Soluble Aβ oligomer production and toxicity. J Neurochem. 2012;120(Suppl 1):125–139. doi: 10.1111/j.1471-4159.2011.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 32.De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 33.Cenini G, et al. Generation of reactive oxygen species by beta amyloid fibrils and oligomers involves different intra/extracellular pathways. Amino Acids. 2010;38:1101–1106. doi: 10.1007/s00726-009-0319-7. [DOI] [PubMed] [Google Scholar]
- 34.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacor PN, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renner M, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 39.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng IH, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- 41.Reed MN, et al. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2011;32:1784–1794. doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavía J, de Ceballos ML, Sanchez de la Cuesta F. Alzheimer’s disease: Relationship between muscarinic cholinergic receptors, beta-amyloid and tau proteins. Fundam Clin Pharmacol. 1998;12:473–481. doi: 10.1111/j.1472-8206.1998.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 44.Oddo S, et al. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 45.Rank KB, et al. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002;514:263–268. doi: 10.1016/s0014-5793(02)02376-1. [DOI] [PubMed] [Google Scholar]
- 46.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shipton OA, et al. Tau protein is required for amyloid beta-induced impairment of hippocampal long-term potentiation. J Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Z, Liu W, Yan Z. Beta-amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida CG, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 52.Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, et al. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boric K, Muñoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- 56.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 57.Willig F, et al. Short-term memory, exploration and locomotor activity in aged rats. Neurobiol Aging. 1987;8:393–402. doi: 10.1016/0197-4580(87)90033-9. [DOI] [PubMed] [Google Scholar]
- 58.Lukaszewska I, Radulska A. Object recognition is not impaired in old rats. Acta Neurobiol Exp (Warsz) 1994;54:143–150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.