Abstract
Retinoic acid, an active metabolite of vitamin A, plays essential signaling roles in mammalian embryogenesis. Nevertheless, it has long been recognized that overexposure to vitamin A or retinoic acid causes widespread teratogenesis in rodents as well as humans. Although it has a short half-life, exposure to high levels of retinoic acid can disrupt development of yet-to-be formed organs, including the metanephros, the embryonic organ which normally differentiates into the mature kidney. Paradoxically, it is known that either an excess or a deficiency of retinoic acid results in similar malformations in some organs, including the mammalian kidney. Accordingly, we hypothesized that excess retinoic acid is teratogenic by inducing a longer lasting, local retinoic acid deficiency. This idea was tested in an established in vivo mouse model in which exposure to excess retinoic acid well before metanephric rudiments exist leads to failure of kidney formation several days later. Results showed that teratogen exposure was followed by decreased levels of Raldh transcripts encoding retinoic acid-synthesizing enzymes and increased levels of Cyp26a1 and Cyp26b1 mRNAs encoding enzymes that catabolize retinoic acid. Concomitantly, there was significant reduction in retinoic acid levels in whole embryos and kidney rudiments. Restoration of retinoic acid levels by maternal supplementation with low doses of retinoic acid following the teratogenic insult rescued metanephric kidney development and abrogated several extrarenal developmental defects. This previously undescribed and unsuspected mechanism provides insight into the molecular pathway of retinoic acid-induced teratogenesis.
Keywords: congenital malformations, mouse embryos, retinoids
All-trans retinoic acid (RA), a bioactive vitamin A metabolite, is a signaling molecule indispensable for the formation of many organs, including eyes, heart, and kidneys (1). In embryos, cellular RA is synthesized from retinol, the circulatory form of vitamin A, via two oxidation steps, the first catalyzed by alcohol/retinol dehydrogenases and the second by retinaldehyde dehydrogenases (RALDHs). Inactivation of RA is catalyzed by CYP26, a cytochrome P450 enzyme. In target cells, RA acts as a ligand for nuclear retinoic acid receptors (RARs), which form heterodimers with retinoid X receptors (RXRs). The complex binds to a regulatory DNA segment, the retinoic acid response element (RARE), to control transcription of RA target genes. Local RA availability depends on RALDH and CYP26, each of which has three subtypes respectively encoded by Raldh1, Raldh2, and Raldh3, and Cyp26a1, Cyp26b1, and Cyp26c1 (2, 3). Within embryos, too little or too much vitamin A/RA causes malformations. Rat fetuses in mothers reared on vitamin A-deficient diets demonstrate an array of anomalies collectively known as “fetal vitamin A deficiency” (VAD) syndrome, which comprises hindbrain, eye, ear, heart, lung, diaphragm, kidney, testis, limb, and skeletal defects (4). Similarly, mice with compound null mutations of RA nuclear receptors (5, 6) and RA-synthesizing enzymes (7) have malformations resembling the VAD syndrome.
Notably, excess vitamin A/RA in humans and animal models causes malformations resembling the fetal VAD syndrome (8, 9). Shenefelt (9) administered RA to pregnant hamsters to determine the gestational windows for the induction of specific malformations. In about a quarter of the malformations identified, “the period during which administration of RA produced a given malformation began well before there was any morphologically identifiable precursor of the structure destined to be affected.” For example, although in hamsters the metanephric rudiment, the primordium of permanent kidney, does not form until embryonic day (E) 9.5, RA administered at E8 caused fetuses to lack kidneys. We reported that exposure of mouse embryos to RA at E9 (the anatomical equivalent to E8 in hamsters) resulted in fulminant apoptosis in nascent metanephroi at E11 (10). Instead of differentiating, the rudiments regressed in vivo, resulting in absent kidneys (bilateral renal agenesis). RA has a short half-life, and a teratogenic dose of 100 mg/kg administered orally to pregnant mice at E11 is eliminated from embryos within 12 h (11). Thus, a teratogenic dose of RA administered at E9 should be removed before the metanephros begins at E11. Therefore RA-induced renal agenesis is unlikely to be explained by RA’s known direct teratogenic mechanisms, including induction of cell death, inhibition of proliferation, and alteration of cell migration, identity, and fate (12, 13). Importantly, renal agenesis and/or hypoplasia also occur in rat fetuses gestating in vitamin A-deficient mothers (14) and in mouse embryos that are null mutants for RARα/γ, RARα/β2, or RARα/RXRα (5, 6). Accordingly, we hypothesized that, paradoxically, excess RA might perturb renal development via a secondary, longer-term induction of RA deficiency.
Results
Rapid Changes in Raldhs and Cyp26s mRNA Levels in Mouse Embryos After RA Overexposure.
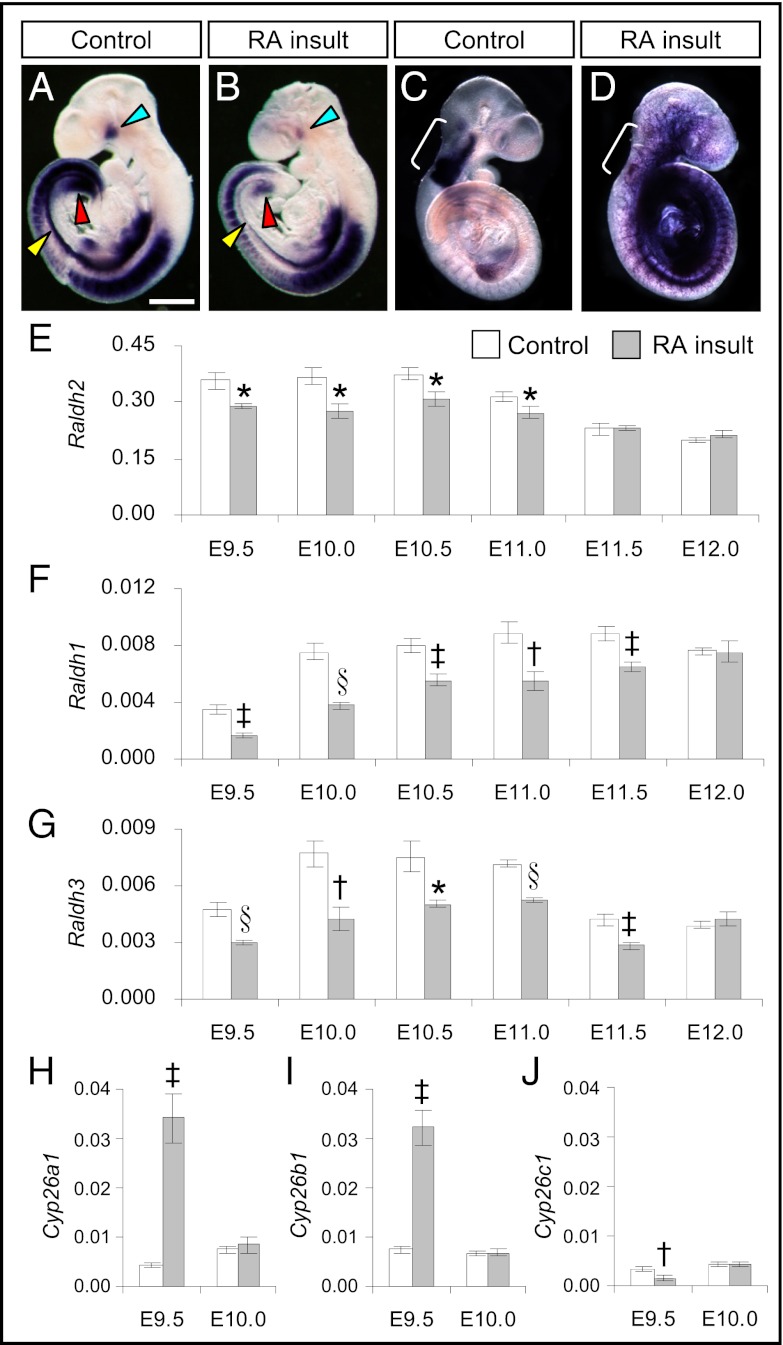
To determine whether there was any change in gene-expression patterns of RA-synthesizing enzymes after maternal administration of a teratogenic dose of 125 mg/kg body weight (b.w.) of all-trans RA at E9.0 (hereafter called “RA insult”), in situ hybridization (ISH) was performed on E9.5–E11.0 whole mouse embryos. Raldh2 is the most widely expressed of the three subtypes and is the major enzyme for generating RA in the embryo (7). At E9.5, Raldh2 was expressed strongly at multiple sites in control embryos exposed to suspension vehicle only (Fig. 1A). In RA-overexposed embryos, the expression level of Raldh2 appeared to be attenuated at E9.5 (Fig. 1B). This attenuation was especially marked in perioptic mesenchyme, in a strip of tissues ventral to the somites that was identified in tissue sections as the dorsolateral edges of the intraembryonic coelomic cavity containing tissues that form the urogenital ridge, and in the cloacal region that contains the nephrogenic cord tissue. At E10.5 reduced expression of Raldh2 was observed clearly in the otic vesicle, the precursor of the ear, and in the maxillomandibular region, the precursor of the jaw (Fig. S1). Consistent with the ISH results, real-time RT-PCR showed that levels of Raldh2 transcripts in whole embryos were significantly reduced in RA-overexposed embryos from E9.5–E11.0 (Fig. 1E). By E11.5, levels had returned to those found in time-matched controls. Here, we quantified Raldh2 expression in whole embryos only from 12 h after treatment, but in a previous experiment from our laboratory embryonic Raldh2 down-regulation was detected as soon as 2 h after a lesser dose (50 mg/kg b.w.) of all-trans RA had been administered to the mother at E8.5 (Fig. S2), showing that changes in the expression level of Raldh2 in response to excess RA are very rapid.
Fig. 1.
Prolonged reduction of Raldhs but transient increase of Cyp26a1 and Cyp26b1 expression in the embryo after teratogenic RA insult. (A–D) Whole-mount ISH patterns of Raldh2 and Cyp26b1 in control (A and C) and RA-overexposed embryos (B and D) at E9.5. A decrease in Raldh2 expression was observed in the whole embryo (A and B) and was especially prominent in the perioptic mesenchyme (blue arrowhead), the dorsolateral edges of the intraembryonic coelomic cavity (yellow arrowhead), and the cloacal region (red arrowhead). In contrast, Cyp26b1 was expressed ectopically at multiple sites throughout the embryo (C and D) but was reduced at its normal site of expression in the rhombomeres (r2–6, marked by white bracket). (Scale bar: 0.6 mm.) (E–J) Real-time quantitative RT-PCR of gene-expression levels of Raldh2 (E), Raldh1 (F), Raldh3 (G), Cyp26a1 (H), Cyp26b1 (I), and Cyp26c1 (J) relative to β-actin in control and RA-overexposed embryos at different time points after RA insult. (Data are shown as mean ± SEM, n = 5–6; embryos in each litter were pooled as one sample. *P < 0.05; †P < 0.01; ‡P < 0.005; §P < 0.001 vs. time-matched control, independent samples t test.)
Next we determined transcript expression levels of the two less abundant RA-synthesizing enzymes, Raldh1 and Raldh3, in the whole embryo. Significant reductions of Raldh1 (Fig. 1F and Fig. S3 A and B) and Raldh3 (Fig. 1G and Fig. S4 A and B) were found in RA-overexposed embryos from E9.5–E11.5. For Raldh1, reduced expression was noticeable in the otic vesicle and dorsal optic vesicle as early as E9.5 and continued to E11.0 (Fig. S3 C–H). For Raldh3, reduced expression was observed in the surface ectoderm overlying the optic vesicle and the olfactory region at E9.5. At E10.0, prominent reduction was visible in the nephrogenic cord at the caudal region. A lower expression of Raldh3 in the olfactory region still was seen clearly at E11.0 (Fig. S4 C–H).
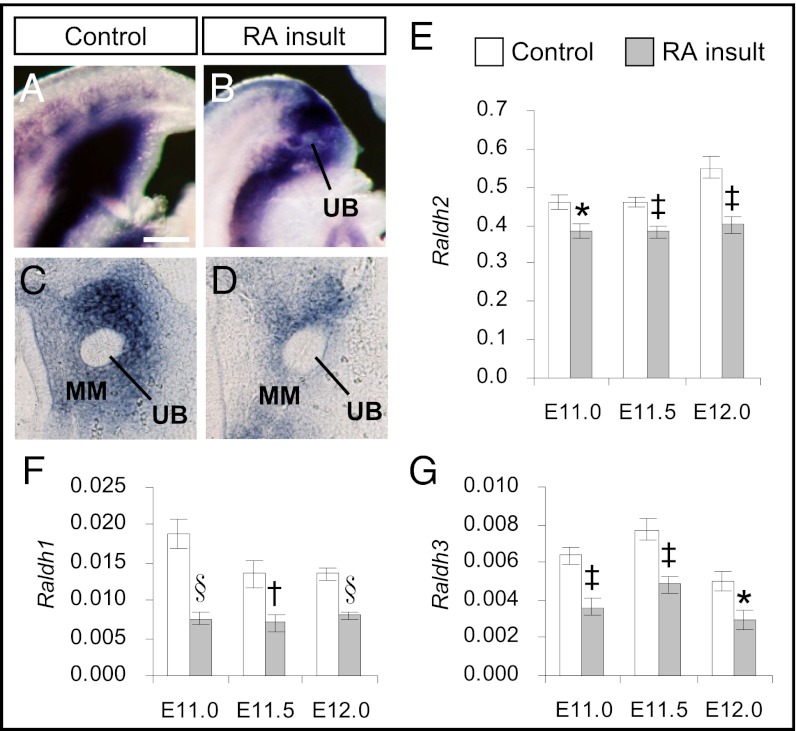
We performed further analyses to establish whether reduced gene expression of RA-synthesizing enzymes also could be observed in the developing metanephros, which at E11 is comprised of a ureteric bud (UB) penetrating the metanephric mesenchyme (MM). Subsequently, in normal development, these two tissues differentiate into collecting ducts and nephrons, respectively. RALDH2 is the major enzyme that generates RA in the metanephros (15). Whole-mount ISH of control and RA-overexposed embryos at E11.0 showed reduced expression of Raldh2 in the caudal region where the metanephros resides (Fig. 2 A and B). Histology revealed that Raldh2 expression was prominently reduced in the MM of RA-overexposed embryos (Fig. 2 C and D). Further quantification by real-time RT-PCR confirmed that the expression level of Raldh2 was reduced significantly, by an average of 20%, in metanephroi of RA-overexposed embryos from E11.0–E12.0 (Fig. 2E). Moreover, although they were expressed at lower levels than Raldh2, the expression of Raldh1 and Raldh3 also was reduced significantly, by 41–59% and 38–44%, respectively, in these same kidney rudiments as compared with time-matched controls (Fig. 2 E–G).
Fig. 2.
Reduction of Raldhs expression in the metanephric rudiment after teratogenic RA insult. (A and B) Whole-mount ISH patterns of Raldh2 in control (A) and RA-overexposed embryos (B) at E11.0 in the caudal region where the metanephros resides. (C and D) Vibratome sections of control (C) and RA-overexposed embryos (D) showing the expression patterns of Raldh2 in the metanephros comprising the metanephric mesenchyme (MM) and ureteric bud (UB). (Scale bar: A and B, 0.3 mm; C and D, 40 mm.) (E–G) Real-time quantitative RT-PCR of gene-expression levels of (E) Raldh2, (F) Raldh1, and (G) Raldh3 relative to β-actin in the metanephros of control and RA-overexposed embryos from E11.0–E12.0. (Data are shown as mean ± SEM, n = 5–6; isolated metanephroi from embryos in each litter were pooled as one sample. *P < 0.05; †P < 0.01; ‡P < 0.005; §P < 0.001 vs. time-matched control, independent samples t test.)
In addition to RA-synthesizing enzymes, CYP26 enzymes, which are responsible for catabolic removal of RA, showed marked changes following RA insult. Interestingly, Cyp26a1 and Cyp26b1 transcripts were increased extensively, by eightfold and fourfold, respectively, whereas Cyp26c1 showed reduced expression in RA-overexposed embryos at E9.5 (Fig. 1 H–J). Notably, a previous experiment in our laboratory found that significant changes in all three subtypes of Cyp26 were evident 2 h after treatment with a lesser dose (50 mg/kg b.w.) of all-trans RA at E9.0 (Fig. S5 C, F, and I). ISH study revealed dramatic changes in the expression patterns of Cyp26a1 (Fig. S5 A and B) and Cyp26b1 (Fig. S5 D and E). Instead of being expressed in restricted tissues, they became ectopically expressed at multiple sites throughout the embryo. Paradoxically, expression of Cyp26b1 at its normal domain in the rhombomeres was almost switched off (Fig. 1 C and D). The expression of Cyp26c1 in the cranial region was attenuated, and the normal lack of expression caudally was unchanged by RA insult (Fig. S5 G and H). However, unlike Raldh, the changes in Cyp26 expression in response to excess RA, although extensive, were short lived. By E10.0, transcript expression of all three subtypes of Cyp26 was restored to levels similar to those in time-matched controls (Fig. 1 H–J).
Together, these results demonstrated a transient increase in transcript levels of RA-catabolizing CYP26A1 and CYP26B1 enzymes together with a prolonged reduction of mRNA levels of all three subtypes of RA-synthesizing RALDH enzymes in the embryo after the RA insult.
Reduction of RA Levels in Whole Embryos and Metanephroi After RA Insult.
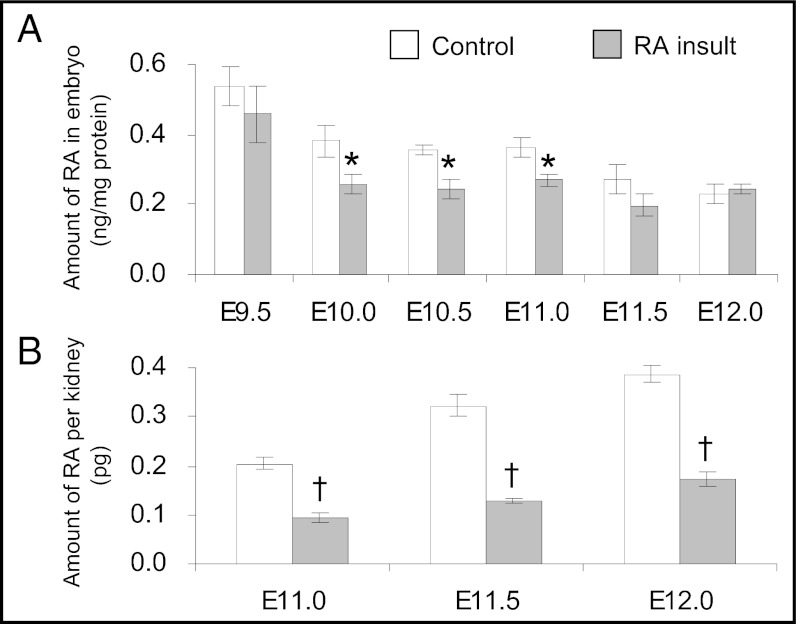
To determine whether changes in transcript expression of RA-synthesizing and -catabolizing enzymes led to a longer-term deficiency in RA, concentrations of all-trans RA in control and RA-overexposed embryos were quantified by HPLC. Six hours after RA insult (E9.25), the amount of RA in the embryo was 1,800-fold higher than in control embryos (Fig. S6). However, after another 6 h (E9.5), the RA content in RA-overexposed embryos had reduced dramatically, to a level similar to that in time-matched controls (Fig. 3A), showing that the exogenous RA was removed from the embryo rapidly, within 12 h after maternal injection of RA. Moreover, by E10.0 the amount of RA detected in RA-overexposed embryos was significantly lower, by 33%, than in control embryos. A significant depletion still was found in RA-overexposed embryos at E11.0 but was no longer evident by E11.5 and onwards. Further quantification of RA content in the metanephros by using a RA-responsive cell line that has high sensitivity to all-trans RA showed that the endogenous RA level in the metanephros of the control embryo increased gradually from E11.0 to E12.0. In RA-overexposed embryos, metanephric RA levels were reduced significantly, by 55–60% versus controls. Such reduction was found at all stages from E11.0–E12.0.
Fig. 3.
Reduction of RA levels in the embryo after teratogenic RA insult. (A) Quantification of all-trans RA levels in control and RA-overexposed embryos from E9.5–E12.0 by HPLC. (Data are shown as mean ± SEM, n = 5, three litters of E9.5 control embryos or one litter of embryos in other groups were pooled as one sample.) (B) Quantification of RA levels in the metanephros of control and RA-overexposed embryos from E11.0–E12.0 using an RA-responsive cell line. (Data are shown as mean ± SEM, n = 7–17; two metanephroi were pooled as one sample. *P < 0.05; †P < 0.001 vs. time-matched control, independent samples t test.)
In summary, a teratogenic dose of RA administered at E9.0 resulted in the reduction of RA levels in the embryo by 24 h after administration. This reduction also was observed in the metanephros from the day it initiates at E11.0, a time point well after the excess RA was removed in the embryo.
Supplementation with Low Doses of RA Rescued Renal Development.
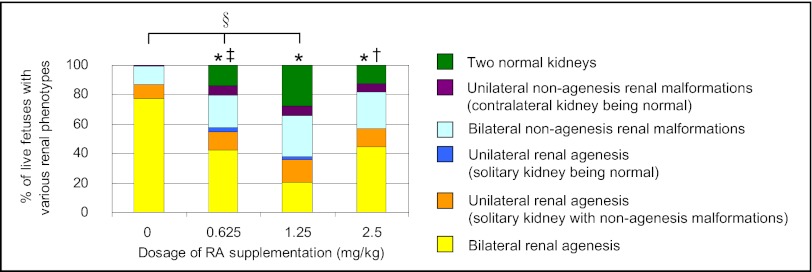
To determine whether reduction of RA levels following the RA insult indeed played a role in causing congenital malformations, low doses of RA were supplemented in vivo to test whether RA compensation could rescue kidney development. It has been shown that maternal oral administration of 2.5 mg/kg b.w. of all-trans RA at 12-h intervals from E6.75–E10.25 could bypass lethality and rescue developmental defects effectively in Raldh2-null mutant mouse embryos (7). Thus, in an attempt to restore renal development, we adopted a similar dosing strategy for RA-overexposed embryos via maternal oral supplementation with 0.625, 1.25, or 2.5 mg/kg b.w. of all-trans RA at 12-h intervals from E10.0–E11.0 (corresponding to the period in which we documented RA-induced RA deficiency). Following in vivo supplementation, the renal phenotypes in near-term fetuses of various treatment groups were examined at E18 and were graded in a severity spectrum (legend of Fig. 4), the most marked phenotype being bilateral renal agenesis. In the control group without RA supplementation and fed with suspension vehicle only, 100% of the 107 RA-overexposed fetuses had renal malformations. Of these fetuses, 78% had bilateral renal agenesis; the remainder had hypoplastic, dysplastic, hydronephrotic, and cystic kidneys (Fig. S7). Following oral supplementation with low doses of RA, the incidence and severity of renal malformations were ameliorated significantly. Moreover, a significant dose-dependent restoration of renal development was observed in RA-overexposed fetuses supplemented with 0, 0.625, and 1.25 mg/kg b.w. of RA. In particular, at the dosing regimen of 1.25 mg/kg b.w. of RA, in a total of 150 fetuses, the incidence of bilateral renal agenesis was reduced to 21% and, strikingly, 28% of fetuses had a pair of normal kidneys. However, when the supplemented dosage of RA was increased further, to 2.5 mg/kg b.w., the rescue effect on kidney development was reduced significantly compared with the effect of 1.25 mg/kg b.w. of RA, showing that the dosage of 2.5 mg/kg b.w. of RA was too high and was less effective for restoration of renal development. Based on these results, the dosing regimen of 1.25 mg/kg b.w. of RA administered at 12-h intervals from E10.0–E11.0 was adopted for subsequent rescue experiments.
Fig. 4.
Supplementation with low doses of RA reduced renal malformations as visualized in E18 fetuses that had undergone prior exposure to a teratogenic dose of RA. The graph shows the percentage of live fetuses with various renal phenotypes (categorized according to the severity of malformations) in the different treatment groups Following RA insult at E9.0, the conceptuses received three low doses (0.625, 1.25, or 2.5 mg/kg b.w.) of all-trans RA or suspension vehicle (0 mg/kg) at 12-h intervals from E10.0–E11.0. (*P < 0.001 vs. control; †P < 0.05 and ‡P < 0.005 vs. 1.25 mg/kg RA supplementation, Jonckheere–Terpstra test; §rs = −0.52 and P < 0.001, Spearman’s rank correlation; n = 107, 64, 150, and 72 fetuses for the control, 0.625-, 1.25-, and 2.5-mg/kg groups, respectively.)
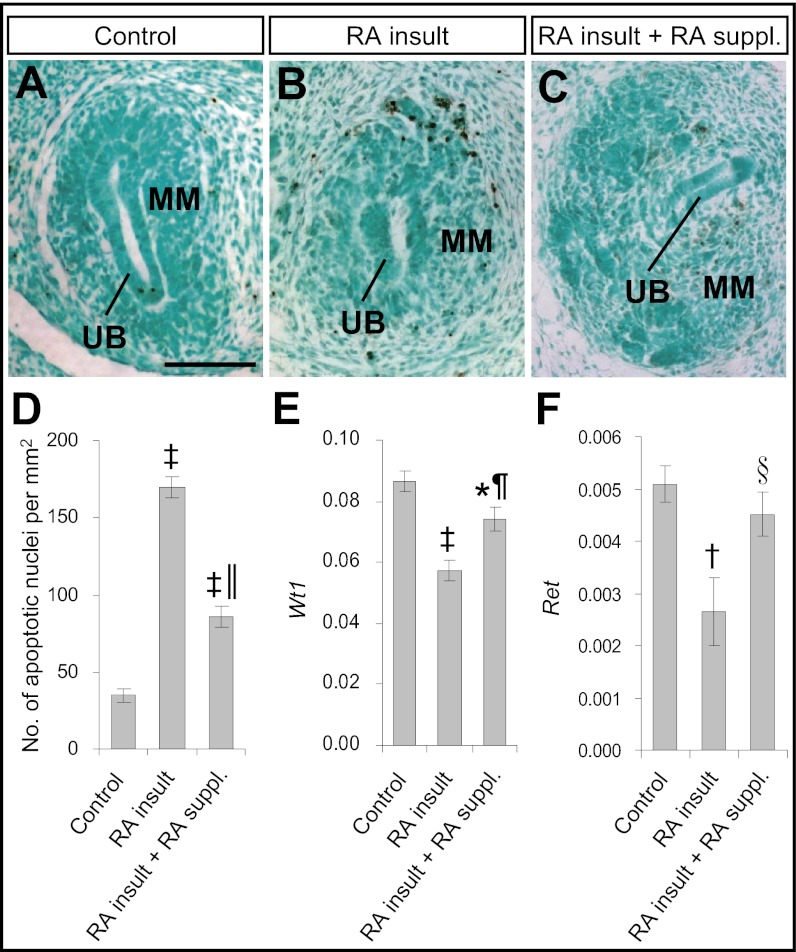
In the mouse model of RA-induced renal agenesis, fulminant apoptosis was observed in the metanephros of RA-overexposed embryos, and the metanephros subsequently degenerated (10). To determine whether RA supplementation could reduce apoptosis in the metanephros of RA-overexposed embryos, TUNEL assay was performed on E12.0 embryos. In the control metanephros without exposure to any exogenous RA, apoptotic nuclei were found only occasionally in the metanephros (Fig. 5A). However, the metanephros of RA-overexposed embryos demonstrated fulminant apoptosis, especially in the MM (Fig. 5B). The number of apoptotic nuclei in RA-overexposed embryos was significantly (fivefold) greater than in control embryos (Fig. 5D). Supplementation with low doses of RA reduced the number of apoptotic nuclei significantly, by 50% (Fig. 5 C and D).
Fig. 5.
Supplementation with low doses of RA ameliorated apoptosis and partially restored Wt1 and Ret expression in metanephric rudiments of embryos exposed to a teratogenic dose of RA. (A–C) TUNEL staining of apoptotic nuclei (stained brown) in paraffin sections of the metanephros of an E12.0 control embryo without exposure to any exogenous RA (A) and of RA-overexposed embryos without (B) and with (C) supplementation with low doses of RA. (Scale bar: 100 μm.) (D) Quantification of the number of apoptotic nuclei in paraffin-embedded sections of the metanephros of various treatment groups. (Data are shown as mean ± SEM, n = 5–9 metanephroi.) (E and F) Real-time quantitative RT-PCR of gene-expression levels of Wt1 (E) and Ret (F) relative to β-actin in metanephroi of E12.0 embryos of various treatment groups. (Data are shown as mean ± SEM, n = 6 or 7; isolated metanephroi from embryos in each litter were pooled as one sample. *P < 0.05; †P < 0.005; ‡P < 0.001 vs. control; §P < 0.05; ¶P < 0.005; ║P < 0.001 vs. RA insult without RA supplementation, independent samples t test.)
Apoptosis is a normal physiological process in kidney development. Wilms tumor 1 (WT1), encoding a transcription factor, is a key regulator of apoptosis in the MM and is indispensible for progression of early kidney development. In homozygous Wt1-knockout mouse embryos, fulminant apoptosis occurred in the MM, eventually leading to complete failure of kidney development (16). In the mouse model of RA-induced renal agenesis, there was significant down-regulation of Wt1 expression in the metanephros at E11.0 and E12.0. This down-regulation was implicated directly in the pathogenic pathway, because Wt1+/− mouse embryos, which originally had no renal defects, demonstrated greater susceptibility to RA-induced renal malformations than did their wild-type littermates (10). Accordingly, we tested whether Wt1 expression in the metanephros of RA-overexposed embryos could be restored by supplementation with low doses of RA. Consistent with previous findings, the expression level of Wt1 was reduced considerably in the metanephroi of RA-exposed embryos as compared with controls (Fig. 5E). RA supplementation increased Wt1 expression levels in the RA-overexposed group, although levels still were somewhat lower than in controls. We observed similar trends in transcript levels for Ret (Fig. 5F), a receptor tyrosine kinase expressed in the UB which transduces MM-derived growth signals (17). Thus, supplementation with RA in the days after RA insult markedly rescued kidney initiation and subsequent morphogenesis, with significant amelioration of apoptosis and restoration toward normal of Wt1 and Ret expression.
Supplementation with Low Doses of RA Prevented Certain Extrarenal Malformations.
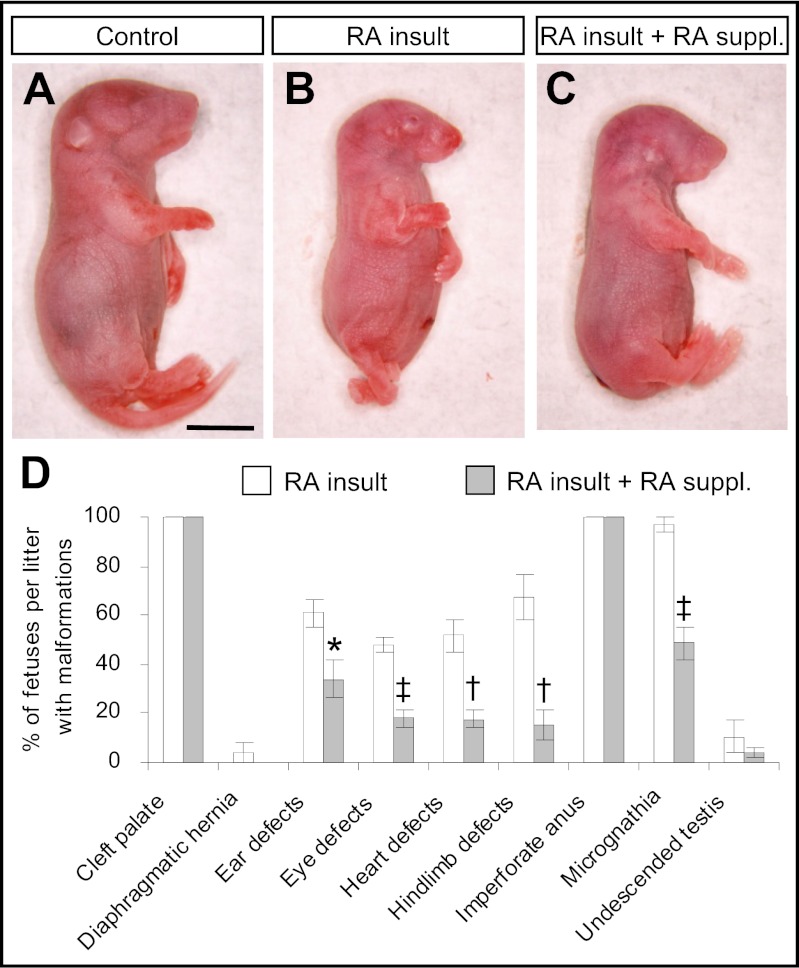
Compared with time-matched control fetuses, RA-overexposed fetuses assessed at E18 were smaller in size, with significant reductions in head length, crown–rump length, and weight (Fig. 6 A and B and Table S1). They exhibited multiple external defects affecting the eye, ear, jaw, and hindlimb, and they lacked tails (Fig. 6D and Fig. S8 A–E). They also had internal extrarenal defects affecting the palate, diaphragm, heart, testis, and anus (Fig. S8 E–I). Notably, supplementation with low doses of RA to RA-overexposed embryos during the period of RA deficiency not only rescued renal development but also normalized various growth parameters and prevented certain extrarenal malformations (Fig. 6D and Table S1). Most of the external defects were ameliorated, although fetuses remained tailless (Fig. 6C and Table S1). There was significant reduction in the incidence of micrognathia (jaw hypoplasia) as well as defects of the eye, ear, heart, and hindlimb. However, some malformations, such as cleft palate and imperforate anus, which occurred in 100% of RA-overexposed fetuses, could not be prevented by RA supplementation.
Fig. 6.
Supplementation with low doses of RA prevented a number of developmental defects as visualized in E18 fetuses that had undergone prior exposure to a teratogenic dose of RA. (A–C) External morphology of the control fetus without exposure to any exogenous RA (A) and RA-overexposed fetuses without (B) and with (C) supplementation with low doses of RA. (Scale bar: 50 mm.) (D) The incidence rate of various types of developmental defects in RA-overexposed fetuses with and without supplementation with low doses of RA. (Data are shown as mean ± SEM, n = 12–14 litters. *P < 0.05; †P < 0.005; ‡P < 0.001 vs. no RA supplementation, independent samples t test.)
Discussion
In this study we show that maternal administration of excess RA at midgestation causes a reduction of RA content in whole embryos and metanephric rudiments. With supplementation with low doses of RA, renal development could be rescued, with partial restoration of Wt1 and Ret expression and amelioration of apoptosis.
RA signaling is essential for kidney development. Homozygous RARα/RARγ (5) and RARα/RXRα (6) compound-null mutant mice exhibit renal agenesis, whereas renal hypoplasia occurs in Raldh2/Raldh3 double-null mutants (15). Previous studies have shown that Wt1 and Raldh2 are coexpressed in several tissues, including the metanephros, during embryogenesis (15, 16). More recently, a RARE has been discovered in the zebrafish Wt1a, a homolog of the mammalian gene (18). The RARE consensus sequence is highly conserved among zebrafish, human, and mouse. In the current study, concomitant with a decrease in transcript levels of Raldhs and a lowering of RA levels, levels of Wt1 transcripts were reduced in metanephroi of RA-overexposed embryos, but supplementation with low doses of RA tended to restore levels of expression. These findings suggest that in early kidney development the expression of Wt1, which is important for suppressing apoptosis in the MM (16), may be regulated by RA. Taken together, the results suggest that a teratogenic dose of RA leads to RA deficiency, which reduces Wt1 expression in the metanephros. As a result, the metanephric rudiment undergoes fulminant apoptosis and degenerates, thereby leading to renal agenesis. Moreover, with RA supplementation we found similar patterns of restoration of Ret in metanephroi of RA-overexposed embryos, consistent with previous observations that Ret expression is dependent on vitamin A (19).
In addition to renal defects, our results showed that teratogenic RA insult at E9.0 resulted in a spectrum of malformations in the fetus. It is interesting that some of the malformations could be prevented by supplementation with low doses of RA, but some could not. Together with renal malformations, the incidence of defects of the eye, ear, jaw, heart, and hindlimb was reduced significantly with supplementation. This finding prompts speculation that these different types of RA-induced malformations may be caused by an RA teratogenic mechanism similar to that causing RA-induced renal malformations, i.e., excess RA leading to RA deficiency. Indeed, similar defects in these organs (e.g., eye malformations, such as open or rudimentary eyelids, microphthalmia, and exophthalmia; external ear malformations, such as unfused auricular hillocks, microtia, and anotia; cardiovascular anomalies, such as small heart, persistent truncus arteriosus, and transposition of great vessels; diaphragmatic hernia; undescended testis; and inward turning of hindlimbs and clubfeet) exhibited in RA-overexposed embryos also are commonly associated with vitamin A or RA deficiency (4, 9). In the current study, prolonged reduction of the Raldhs was observed in RA-overexposed embryos in the optic vesicle, otic vesicle, and branchial arch, which are precursors of the eye, ear, and jaw, respectively. Although specific quantification of RA levels in these embryonic tissues has not been conducted, the finding of the ISH study suggests that RA-induced malformations of these organs also may be caused by RA deficiency. For instance, the most common macroscopically identifiable eye defects of RA-overexposed fetuses were open or rudimentary eyelids. RA is required for morphogenetic movements that form the eyelids (20). Raldh2 is transiently expressed in the perioptic mesenchyme, whereas Raldh1 and Raldh3 are expressed in the dorsal and ventral retina. RA synthesized by Raldh1 and Raldh3 diffuses from the retina to the perioptic mesenchyme, and RA is required to stimulate apoptosis in the perioptic mesenchyme for forming the eyelid.
Although low doses of RA supplementation reduced the incidence of certain anomalies in RA-overexposed fetuses, caudal regression, imperforate anus, and cleft palate were not rescued. Perhaps the dosing regimen was not optimal to rescue these defects, but a more probable explanation is that they were caused by the direct teratogenic actions of RA rather than via RA-induced RA deficiency. Indeed, RA-induced caudal regression is caused by early-onset apoptosis in tail bud mesenchyme which contains vertebral and anal progenitors (12). Based on previous findings and this study, we propose that RA can cause a spectrum of malformations via two nonmutually exclusive modes: (i) direct action on progenitors of target tissues, and (ii) indirect action by the resultant prolonged RA deficiency after the excess RA is removed.
How does excess RA lead to prolonged RA deficiency? Within 6 h after RA insult, RA levels in the embryo were elevated 1,800-fold, but the exogenous RA was removed completely by 12 h. Extensive up-regulation of Cyp26a1 and Cyp26b1 ectopically at multiple sites throughout the embryo probably contributed to the rapid removal of RA from the embryo and possibly even led to initial overreduction of RA. However, this effect was not long-lasting, because Cyp26s expression returned to baseline levels by E10.0. It is reported that Cyp26a1 is highly inducible by RA via the synergistic responses of two RAREs within its promoter region (21). However, no RARE has been identified in Cyp26b1 and Cyp26c1. The finding that Cyp26b1 was up-regulated in ectopic tissues but down-regulated in its normal site of expression suggests that regulation of Cyp26b1 transcription by RA is indirect and likely involves different sets of regulators in various tissues. In addition to inducing rapid up-regulation of RA-degrading enzymes, RA insult also led to rapid down-regulation of RA-synthesizing Raldhs in the embryo as soon as 2 h after treatment. Reduction in transcript levels of Raldhs continued to E11.5 in the whole embryo and to at least E12.0 in specific tissues, including the metanephros. These findings have led to the speculation that the expression of RA-synthesizing enzymes may be regulated negatively by RA. Indeed, a nonconsensus RARE, with affinity similar to that of the consensus RARE, has been identified in the human ALDH1 and the mouse Raldh1 and Raldh2 promoter region (22). In this study, although the overall reduction of RA in the whole embryo after RA insult was 29% on average, reduction in specific tissues such as the metanephric kidney was more than 55%. Moreover, it has been proposed that RA is a morphogen and that its action is highly concentration dependent (23). A small reduction in RA levels will have a large influence on tissues that are distant from the site of synthesis but reliant on low RA concentrations. Thus, prolonged RA deficiency can affect the expression of vital RA-induced developmental genes in progenitor cells of an organ that forms well after the exogenously applied RA is removed, thereby causing RA deficiency-related congenital malformations.
Our study enhances the understanding of RA’s teratogenic mechanisms and also may have wider implications for understanding RA’s roles in postnatal biology. In humans, overexposure to vitamin A can result from excessive use of health supplements and consumption of liver products. Moreover, RA is the active component in some drugs used to treat skin diseases and cancer. Thus, a better understanding of the feedback regulation between externally supplied RA and its endogenous synthesis may help in the designing of improved dietary and medical treatments.
Materials and Methods
Animals.
All animal experimentation was conducted according to the guidelines for the care and use of laboratory animals set by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong. Experiments were conducted with ICR mice kept under a 14:10-h light:dark cycle with the dark period beginning at 1:00 AM. Timed mating was performed by pairing male and female mice for 2 h starting at 9:00 AM. Fertilization was assumed to have occurred at 10:00 AM, which was designated E0.
Administration of RA.
For teratogenic RA insult, 125 mg/kg b.w. of all-trans RA (Sigma) suspended in peanut oil with 10% (vol/vol) absolute ethanol was injected intraperitoneally into pregnant mice at E9.0. Control mice received an equivalent volume of suspension vehicle. For the rescue experiment, following RA insult, pregnant mice were fed orally at 12-h intervals from E10.0–E11.0 with one of three low doses (0.625, 1.25, or 2.5 mg/kg b.w.) of all-trans RA or an equivalent volume of suspension vehicle.
Experimental details on the detection of gene expression by in situ hybridization and real-time quantitative RT-PCR, quantification of RA levels in whole embryos and metanephroi by HPLC and the RA-responsive cell line, respectively, detection and quantification of apoptosis by TUNEL staining, and examination of malformations in E18 fetuses are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Ursula Dräger for the gift of Raldh1 and Raldh3; Dr. Nicholas Hastie for Wt1; Dr. Hiroshi Hamada for Cyp26a1, -b1 and -c1 mouse cDNA plasmids; Dr. Michael Wagner for the RA-responsive cell line; and Dr. Jamie Davies and Dr. Nicholas Hastie for helpful general discussions. This work was supported by Hong Kong Research Grants Council General Research Fund project reference CUHK 473807 (to A.S.W.S., P.J.M., C.-C.W., and A.S.W.). A.S.W. received support from the Manchester Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200872109/-/DCSupplemental.
References
- 1.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederreither K, Fraulob V, Garnier JM, Chambon P, Dollé P. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002;110:165–171. doi: 10.1016/s0925-4773(01)00561-5. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Zolfaghari R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr. 2011;31:65–87. doi: 10.1146/annurev-nutr-072610-145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn C, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 6.Kastner P, et al. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 7.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 8.Rothman KJ, et al. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333:1369–1373. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 9.Shenefelt RE. Morphogenesis of malformations in hamsters caused by retinoic acid: Relation to dose and stage at treatment. Teratology. 1972;5:103–118. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]
- 10.Tse HK, et al. Implication of Wt1 in the pathogenesis of nephrogenic failure in a mouse model of retinoic acid-induced caudal regression syndrome. Am J Pathol. 2005;166:1295–1307. doi: 10.1016/S0002-9440(10)62349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satre MA, Kochhar DM. Elevations in the endogenous levels of the putative morphogen retinoic acid in embryonic mouse limb-buds associated with limb dysmorphogenesis. Dev Biol. 1989;133:529–536. doi: 10.1016/0012-1606(89)90055-9. [DOI] [PubMed] [Google Scholar]
- 12.Shum AS, et al. Retinoic acid induces down-regulation of Wnt-3a, apoptosis and diversion of tail bud cells to a neural fate in the mouse embryo. Mech Dev. 1999;84:17–30. doi: 10.1016/s0925-4773(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 13.Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JG, Warkany J. Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat. 1948;83:357–407. doi: 10.1002/aja.1000830303. [DOI] [PubMed] [Google Scholar]
- 15.Rosselot C, et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreidberg JA, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 17.Schuchardt A, D’Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- 18.Bollig F, et al. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development. 2009;136:2883–2892. doi: 10.1242/dev.031773. [DOI] [PubMed] [Google Scholar]
- 19.Batourina E, et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 20.Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem J. 2005;392:241–248. doi: 10.1042/BJ20050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elizondo G, Medina-Díaz IM, Cruz R, Gonzalez FJ, Vega L. Retinoic acid modulates retinaldehyde dehydrogenase 1 gene expression through the induction of GADD153-C/EBPbeta interaction. Biochem Pharmacol. 2009;77:248–257. doi: 10.1016/j.bcp.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaller C, Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987;327:625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.