The auditory cortex plays a key role in auditory learning, speech perception, auditory attention, and cognitive analysis of sound. Like other neocortices, it consists of a layered network of excitatory cells and inhibitory interneurons. The inhibitory interneurons comprise only 15–20% of neocortical neurons but are much more diverse than the excitatory cells. Many distinct types of inhibitory interneurons have been identified based on differences in morphology, physiology, connectivity, and gene expression (1). These different interneuron types may have specialized functions within cortical circuits (2, 3). Moreover, certain brain disorders seem to be associated with dysfunction in particular interneuron classes. For example, schizophrenia appears to be linked to specific changes in cortical circuitry involving parvalbumin (PV)-positive interneurons (4); conversely, aging may have a disproportionate impact on somatostatin (SOM)-positive interneurons (5). How do chronic reductions in particular inhibitory interneuron populations affect cortical processing? In PNAS, Seybold et al. (6) address this question, exploring the effects of chronic, late-onset reduction in the number of dendrite-targeting interneurons (DTIs) in the auditory cortex of mice with a conditional KO of the gene Dlx1.
The Dlx family of homeobox transcription factors is involved in the development, migration, and survival of cortical interneurons. Constitutive KO of the Dlx1 gene in mice has no observed effect on interneuron density at postnatal day 20 (p20), after the critical period for development of tonotopy in mouse auditory cortex (7, 8). However, by p30, ∼30% of interneurons positive for somatostatin (SOM), neuropeptide Y (NPY), and calretinin (CR)—interneurons that preferentially target their synapses to the dendrites of cortical pyramidal cells—undergo apoptosis. Meanwhile, the density of PV-positive interneurons, which primarily target the soma and/or axon hillock, remains unchanged, and there is no observed alteration in the intrinsic properties of interneurons surviving after p30 (8). Therefore, in the Dlx1−/− mouse, populations of DTIs are chronically reduced after p30, whereas populations of soma-targeting interneurons (STIs) are spared. In principle then, the Dlx1−/− mouse could be used to address questions about how chronic, relatively late-onset reduction in the number of DTIs alters auditory cortical function, and therefore, perhaps, how reduction in the number of DTIs during aging in humans might contribute to increasing difficulty with speech-in-noise processing.
However, just as investigations of age-related changes in auditory cortical processing in humans are complicated by the fact that peripheral auditory processing also deteriorates with age, studies of the Dlx1−/− mouse are complicated by the fact that the mutation affects peripheral as well as central auditory processing. The middle ear bones fuse during development in Dlx1−/− mice, causing an elevation in auditory brainstem response (ABR) thresholds of ∼30 dB (9, 10). Similar levels of conductive hearing loss during development have previously been shown to alter spontaneous firing rates and the strengths of connections between excitatory and inhibitory neurons throughout the central auditory system, including in the auditory cortex (11, 12). Therefore, the only truly appropriate control animals for studies of auditory cortical changes in Dlx1−/− mice would be heterozygous littermates in which a comparable conductive hearing loss had been experimentally induced with exactly the same developmental time course—a very tall order indeed.
To circumvent this problem, Seybold et al. (6) use the toolkit of mouse genetics to disentangle peripheral and central auditory dysfunction. They developed a conditional deletion of Dlx1, restricting the mutation to forebrain GABAergic interneurons with a floxed conditional allele of Dlx1 and Cre-recombinase under the control of a Dlx1 enhancer element. This enhancer element is expressed in the forebrain but not in the developing middle ear. Like adult Dlx1−/− mice, adult conditional knockout (cKO) mice had fewer cortical inhibitory interneurons of the types typically classified as dendrite-targeting (SOM+, NPY+, and CR+ neurons, as well as neurons positive for vasoactive intestinal peptide), whereas the density of PV-positive inhibitory interneurons was similar to that in control animals (heterozygous littermates). However, unlike Dlx1−/− mice, cKO mice had normal ABR thresholds compared with controls (ref. 6, Supplementary Information). Therefore, the cKO animals allowed the authors to separate potential effects of the Dlx1 mutation on central auditory function from the confounding effects of peripheral hearing loss.
To examine central auditory processing, Seybold et al. (6) recorded extracellularly in vivo from (presumed pyramidal) neurons in the auditory cortex and compared neuronal response properties between cKO and control animals. In cKO mice, spontaneous firing rates were higher than in control animals, and tone-evoked responses were less sparse; these alterations in cortical response properties resemble those observed following acute pharmacological blockade of inhibition (Fig. 1B). However, there was no accompanying expansion of frequency-intensity receptive fields, as is observed following acute blockade of inhibition (13, 14). Instead, frequency-intensity receptive fields were smaller, with higher response thresholds and narrower bandwidths, in cKO mice than in control mice (Fig. 1C). Moreover, frequency-intensity receptive fields in auditory thalamus were not significantly different between cKO and control animals, suggesting that the differences observed in cortex were not inherited from the thalamus.
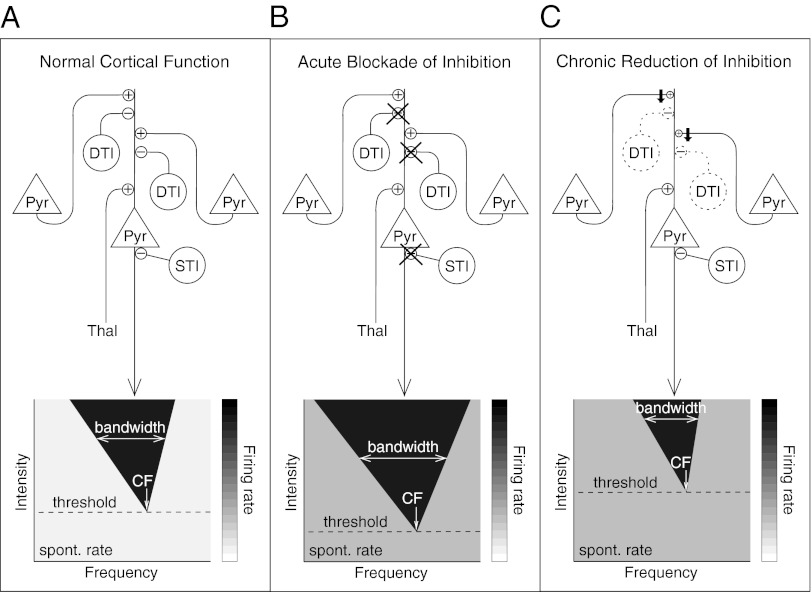
Fig. 1.
Cartoon illustration of the hypothesis proposed by Seybold et al. (6). (A) (Upper) Auditory responses of pyramidal cells (Pyr) are shaped both by thalamic input (Thal) and by corticocortical input from other pyramidal cells. STIs, such as PV-positive interneurons, form synapses on the pyramidal cell body or axon hillock, and are therefore in a good position to control pyramidal cell output. In contrast, DTIs, such as those positive for SOM, NPY, CR, and vasoactive intestinal peptide, presumably modulate the impact of pyramidal cell inputs. (Lower) Response properties of auditory neurons are typically characterized in terms of activity evoked by tones varying in frequency and intensity. Response measures analyzed by Seybold et al. (6) include the following: intensity threshold (dashed line), CF (defined as the tone frequency evoking a response at threshold intensity), bandwidth of the frequency-intensity receptive field at a fixed intensity increment above threshold (double-headed white arrow on black triangle), and spontaneous (spont.) rate (light gray; average firing rate below threshold or before stimulus onset). (B) Acute pharmacological blockade of inhibition with GABA receptor blockers interferes with synaptic transmission from both DTIs and STIs. Under these conditions, responses of presumed pyramidal cells in auditory cortex have been observed to show increased spontaneous rates, lower thresholds, and broader bandwidths (13, 14). (C) Chronic reduction in the number of DTIs (indicated by dotted outlines), such as occurs in the Dlx1 cKO mouse, may trigger a compensatory reduction in corticocortical excitatory drive (indicated by black downward arrows). Seybold et al. (6) compare response properties of presumed pyramidal cells in cKO and control animals and show that spontaneous rates were increased in cKO animals, just as would be expected after acute blockade of inhibition. However, in contrast to the effects of acute blockade of inhibition, thresholds were higher rather than lower and bandwidths were narrower rather than broader. These alterations in the frequency-intensity receptive field are consistent with reduced corticocortical excitatory drive, because corticocortical excitatory input contributes primarily to the edges of the frequency-intensity receptive field (15).
Furthermore, Seybold et al. (6) find that the likely cause of the auditory cortical abnormalities they observed in cKO animals was a reduction in the strength of corticocortical excitatory drive. Auditory cortical responses are shaped both by thalamic inputs and by corticocortical inputs (Fig. 1A). Responses to high-intensity tones near the characteristic frequency (CF) are driven primarily by thalamic inputs, but long-latency responses to low-intensity, off-CF tones are thought to be dominated by corticocortical inputs (15). These long-latency neuronal responses, although evident in control animals, appeared to be absent in cKO mice. Moreover, frequency-intensity receptive fields calculated from the early vs. late portions of tone-evoked responses were negatively correlated in control animals but positively correlated in cKO mice. Thus, the usual auditory cortex response pattern observed in control animals—early, presumably thalamocortical drive to the center of the receptive field, followed by late, likely corticocortical drive to the edges—was altered in cKO animals, in a manner consistent with loss of corticocortical excitatory drive.
The implication of these findings is that chronic reduction of inhibition in auditory cortex has very different effects from acute blockade of inhibition. Both chronic reduction of inhibition and acute blockade of inhibition increase spontaneous firing rates and decrease response sparsity, but the two manipulations appear to have opposite effects on the size of frequency-intensity receptive fields (Fig. 1). These results make intuitive sense; presumably, homeostatic plasticity mechanisms kick in to limit overall activity levels when hyperexcitability due to loss of inhibition is a chronic condition rather than an acute event. However, further experiments are needed to determine whether differences in the effects of chronic vs. acute loss of inhibition truly arise from the time course of the manipulation, or from differences in the affected interneuron populations [DTIs + STIs in previous acute blockade studies vs. DTIs alone in the study by Seybold et al. (6)]. Also, additional studies in awake animals are necessary to confirm that apparent effects of chronic reduction of inhibition on auditory cortical receptive fields do not arise, in part, from differences between cKO and control animals in responsiveness to anesthesia. Nevertheless, Seybold et al. (6) provide truly compelling evidence that chronic reduction of cortical inhibition leads to compensatory down-regulation of corticocortical excitatory drive, and they have created an excellent model system for exploring the mechanisms underlying this phenomenon.
Beyond its immediate relevance to studies of the role of inhibition in auditory cortical processing, the report by Seybold et al. (6) represents a significant step toward understanding how cortical function might be altered by the chronic changes in inhibitory interneuron populations observed in neuropsychiatric disorders, traumatic brain injury, tinnitus, and normal aging. Some of these conditions, such as schizophrenia, are thought to be associated with specific deficits in STI populations (4); others, such as aging, may primarily involve loss of DTIs (5). Compensatory down-regulation of corticocortical drive following chronic reductions in inhibitory interneuron populations could undermine cortical computation by limiting integration of information within the cortex. For different interneuron populations, in different brain areas, and at different times during development, the same fundamental process might give rise to disabilities ranging from cognitive deficits in neuropsychiatric disease to declining speech-in-noise comprehension in aging. In short, cortical compensation could have profound cognitive consequences.
Footnotes
The author declares no conflict of interest.
See companion article on page 13829.
References
- 1.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 5.Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging. 2012;33:431.e1–431.13. doi: 10.1016/j.neurobiolaging.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seybold BA, et al. Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex. Proc Natl Acad Sci USA. 2012;109:13829–13834. doi: 10.1073/pnas.1205909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 9.Qiu M, et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- 10.Polley DB, Cobos I, Merzenich MM, Rubenstein JLR. Severe hearing loss in Dlxl mutant mice. Hear Res. 2006;214:84–88. doi: 10.1016/j.heares.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: Implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hear Res. 2011;279:140–148. doi: 10.1016/j.heares.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foeller E, Vater M, Kössl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]