Abstract
Duchenne muscular dystrophy (DMD), the commonest form of muscular dystrophy, is caused by lack of dystrophin. One of the most promising therapeutic approaches is antisense-mediated elimination of frame-disrupting mutations by exon skipping. However, this approach faces two major hurdles: limited applicability of each individual target exon and uncertain function and stability of each resulting truncated dystrophin. Skipping of exons 45–55 at the mutation hotspot of the DMD gene would address both issues. Theoretically it could rescue more than 60% of patients with deletion mutations. Moreover, spontaneous deletions of this specific region are associated with asymptomatic or exceptionally mild phenotypes. However, such multiple exon skipping of exons 45–55 has proved technically challenging. We have therefore designed antisense oligo (AO) morpholino mixtures to minimize self- or heteroduplex formation. These were tested as conjugates with cell-penetrating moieties (vivo-morpholinos). We have tested the feasibility of skipping exons 45–55 in H2K-mdx52 myotubes and in mdx52 mice, which lack exon 52. Encouragingly, with mixtures of 10 AOs, we demonstrated skipping of all 10 exons in vitro, in H2K-mdx52 myotubes and on intramuscular injection into mdx52 mice. Moreover, in mdx52 mice in vivo, systemic injections of 10 AOs induced extensive dystrophin expression at the subsarcolemma in skeletal muscles throughout the body, producing up to 15% of wild-type dystrophin protein levels, accompanied by improved muscle strength and histopathology without any detectable toxicity. This is a unique successful demonstration of effective rescue by exon 45–55 skipping in a dystrophin-deficient animal model.
Keywords: personalized medicine, nucleic acid therapy, molecular therapy, oligonucleotides, gene therapy
Duchenne muscular dystrophy (DMD), the commonest form of muscular dystrophy, is characterized by progressive deterioration of muscle function (1). DMD is caused mainly by frame-shifting deletion or nonsense mutations in the DMD gene, which encodes the protein dystrophin (2). At the milder end of the disease spectrum, Becker muscular dystrophy (BMD) is a form of dystrophin deficiency that presents with a large spectrum of severities, from borderline DMD to almost asymptomatic cases. BMD typically results from in-frame deletions in the DMD gene that allow the expression of limited amounts of an internally truncated but partly functional protein (3).
Skipping of exons in DMD muscle so as to restore an in-frame and asymptomatic or very mild Becker-like transcript is among the more promising therapeutic approaches to treatment of DMD (4). To this end, systemic administration of antisense oligonucleotides (AOs) targeting specific exon(s) in the DMD gene has been shown to restore the reading frame and induce body-wide production of partially functional BMD-like dystrophin in mouse and dog models of DMD (5–7). Recently, phase I/II human clinical trials with AOs targeting exon 51 have been completed (8, 9).
Although promising, future development of exon skipping to encompass a wider range of mutations faces two major hurdles. First, each exon must be targeted by a specific bespoke antisense sequence. This strategy requires the design and testing of many different antisense reagents to treat all of the different mutation patterns, entailing substantial investment in time and money to perform toxicology and safety assessments (10). Second, although in-frame mutations are associated with milder BMD forms in 80% of cases, the function and stability of each resulting truncated dystrophin are still unknown (11).
A potential solution for these two issues arises from the observation that exon 45–55 deletions are overwhelmingly associated with remarkably mild clinical phenotypes, sometimes almost asymptomatic, with elevated serum creatine kinase (CK) levels as the main symptom (12) (Fig. S1A). It has also been noted that exons 45–55 cover the main mutation “hotspot” of the DMD gene so that, theoretically, up to 63% of DMD patients with dystrophin deletion mutations could be treated if we were able to skip the entire exon 45–55 region, to generate an asymptomatic or remarkably mild BMD-like protein that appears to retain most of the function of the intact protein (13) (Fig. 1 A and B and Fig. S1B). Although several investigations have demonstrated successful skipping of two or three exons both in vitro and/or in vivo (5, 14, 15), there is no report of 10 exons being efficiently skipped by administration of antisense sequences; where attempted, the levels of multiple exon-skipped products have been very low (16). We therefore redesigned mixtures of phosphorodiamidate morpholino oligomer (PMO) sequences targeting exons 45–55, paying attention to the minimization of self- and cross-annealing. Additionally we maximized the efficiency of delivery of these improved sequences by combining them with a cell-penetrating moiety (vivo-morpholino or vPMOs) (Fig. S2).
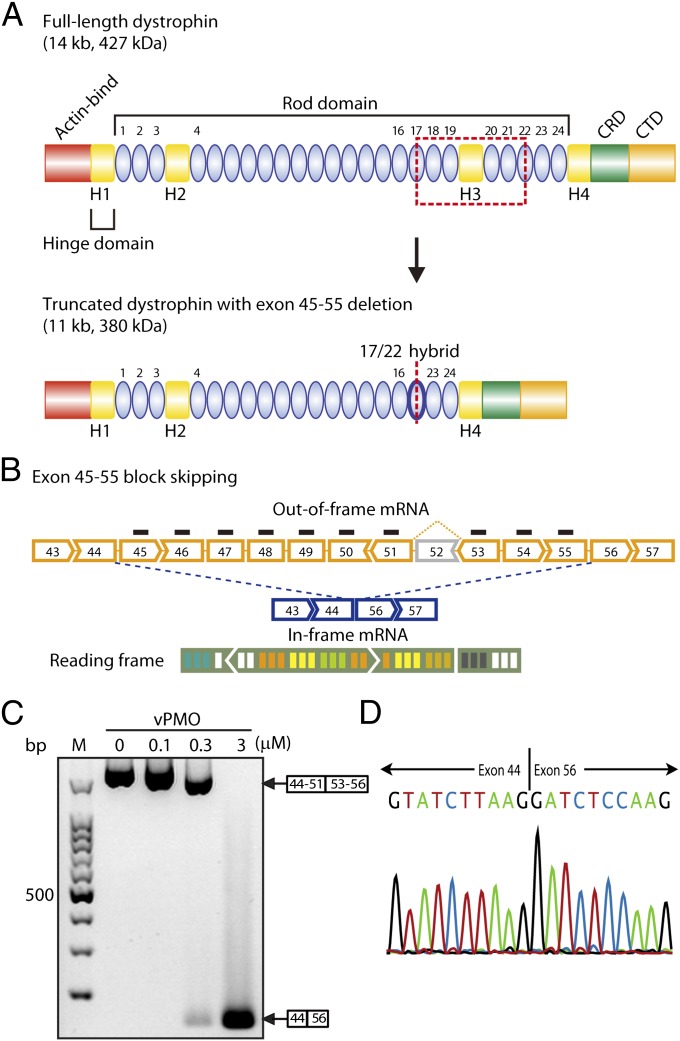
Fig. 1.
Efficacy of exon 45–55 multiskipping in H2K-mdx52 cells in vitro. (A) Structure of full-length and quasidystrophin. The quasidystrophin produced by exon 45–55 deletion (skipping) has a hybrid rod repeat of rods 17 and 22. Actin-bind, actin-binding domain; CRD, cysteine-rich domain; CTD, C-terminal domain. (B) Mdx52 mouse lacks exon 52 in the mRNA of the murine Dmd gene, leading to out-of-frame products (yellow broken line). Exon 45–55 skipping with mixture vPMOs (blue broken line) restores the reading frame of dystrophin mRNA. (C) RT-PCR results after 0.1, 0.3, or 3 μM in total of mixture vPMO transfected into H2K-mdx52 myotubes as indicated. M, molecular marker; 0, no vPMO transfection. (D) Confirmation of correct exon 45–55 block skipping by direct sequencing of the PCR products. Sequencing of the most intense band shows exon 45–55 skipped dystrophin mRNA sequence.
We demonstrate here that intramuscular or systemic injections of a vPMO mixture of these improved reagents generated extensive dystrophin expression in dystrophic skeletal muscles of mice harboring a deletion mutation of exon 52 (mdx52), unaccompanied by any detectable toxicity.
Results
In Vitro Evaluation of the vPMO Sequences in H2K-mdx52 Myotubes.
To examine the feasibility of skipping exons 45–55 in mdx52 mice, we designed AO sequences against the 10 exons between exons 45 and 55, which target the exonic splice enhancers (ESEs) or the exon/intron boundaries of each exon except exon 52 (Table S1 and Fig. S3). We initially transduced H2K-mdx52 myotubes with specific AO sequence to each separate targeted exon between exons 45 and 55. Skipping efficiency varied between exons, and although good levels of skipping were obtained for exons 45, 46, 47, 48, 49, 50, 51, 54, and 55, only a faint band was detected in the case of exon 53 after 0.1 or 1 μM in total of single vPMO (45A, 46A, 47A, 48A, 49A, 50A, 51A, 53A, 54A, or 55A) transfection (Fig. S4A).
As a next step, we sought to skip the entire exon 45–55 region with 10 vPMO mixtures in vitro. Efficient exon 45–55 skipping of the entire exon 45–55 region was detected by RT-PCR with a forward primer 44F at exon 44 and a reverse primer 56R at exon 56 after transfections of the mixture-ESE2 (Table S2 and Fig. 1C). The targeted splicing products with skipped exons 45–55 were confirmed by directly sequencing the RT-PCR band using both primer pairs (Fig. 1D). RT-PCR results after transfections with other mixture combinations are also shown in Fig. S4B.
Local Injections for Exon 45–55 Block Skipping.
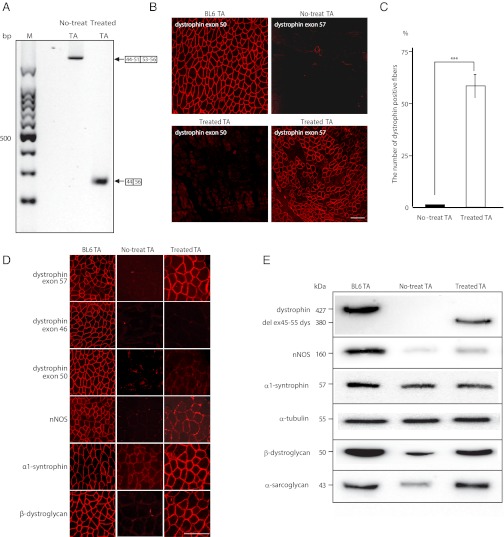
To restore dystrophin expression with exon 45–55 skipping in vivo, we injected the mixtures of 10 vPMOs, at 1.5 μg in total, into tibialis anterior (TA) muscle of 5-wk-old mdx52 mice and took muscle samples 2 wk after the injection. We detected the PCR product that was equivalent to the mRNA lacking the exon 45–55 region most efficiently after injection with mixture-ESE2 (Fig. 2A and Table S2 and Fig. S4C). Taking these results together, we concluded that the mixture-ESE2 can skip exons 45–55 of the murine Dmd gene efficiently both in vitro and in vivo. We also observed extensive dystrophin expression by immunohistochemistry after the mixture-ESE2 injection (Fig. 2 B and C). Dystrophin expression following exon 45–55 skipping was detected by P7 against exon 57, but not by MANEX46B against exon 46 or MANEX50 against exon 50 (Fig. 2D). Western blotting revealed a 380-kDa band that conformed to the size of the product estimated to be derived from deletion of exons 45–55; a truncated quasidystrophin found in the very mild Becker muscular dystrophy associated with deletion of these same exons as well as dystrophin-associated proteins (Fig. 2E and Fig. S5A). These results suggest that exon 45–55 block skipping is feasible in vivo.
Fig. 2.
Exon 45–55 multiskipping and rescue of dystrophin expression in mdx52 mice by local mixture-ESE2 injections. (A) Detection of exon 45–55 skipped dystrophin mRNA by RT-PCR with primers flanking exons 44 (44F1) and 56 (56R1) at 2 wk after injection of mixture-ESE2, targeting exons 45–55 except exon 52 into tibialis anterior (TA) muscles. Representative data are shown. M, molecular marker; no-treat TA, untreated TA muscles from mdx52 mice; treated TA, treated TA muscles from mdx52 mice. (B) Immunohistochemical staining of dystrophin exon 50 in the TA muscle of WT and treated mdx52 mice (Left) and dystrophin exon 57 in the TA muscle of untreated and treated mdx52 mice (Right). Representative data are shown. BL6 TA, TA muscle from a wild-type C57/BL6. (Scale bar, 100 μm.) (C) Percentage of dystrophin-positive fibers after local injections with the 10 vPMO cocktail. Data (n = 6) are presented as mean ± SD ***P < 0.001. (D) Recovery of dystrophin-associated proteins with exon 45–55 skipping. Immunohistochemical staining of dystrophin exons 57, 46, and 50, neuronal nitric oxide synthase (nNOS), α1-syntrophin, and β-dystroglycan in the TA muscle of WT, untreated, and treated mdx52 mice. Representative data are shown. BL6TA, TA muscle from a wild-type C57/BL6; no-treat TA, untreated TA muscles from mdx52 mice. (Scale bar, 100 μm.) (E) Western blotting after the mixture 10 vPMOs local injections to detect the expression of full-length dystrophin, 380-kDa quasidystrophin, nNOS, α1-syntrophin, α-tubulin, β-dystroglycan, and α-sarcoglycan in TA muscles of WT, untreated, and treated mdx52 mice. Representative data are shown.
Recovery of Dystrophin-Associated Proteins with Exon 45–55 Skipping.
We examined the expression of components included in the dystrophin–glycoprotein complex in the TA muscle by immunohistochemistry and Western blotting. In all dystrophin-positive fibers, the expression of α1-syntrophin and β-dystroglycan at the subsarcolemma was evident; however, the expression of neuronal nitric oxide synthase (nNOS) at the subsarcolemma was minimal compared with that of WT (Fig. 2D). Western blotting reveals that the expression levels of α1-syntrophin, β-dystroglycan, and α-sarcoglycan in the TA muscle were at 80–100% of normal levels, whereas the expression level of nNOS was 20% of normal levels (Fig. 2E and Fig. S5B).
Repeated Systemic Delivery of the Mixture-ESE2 Induces Efficient Exon 45–55 Skipping in Whole Body Skeletal Muscles.
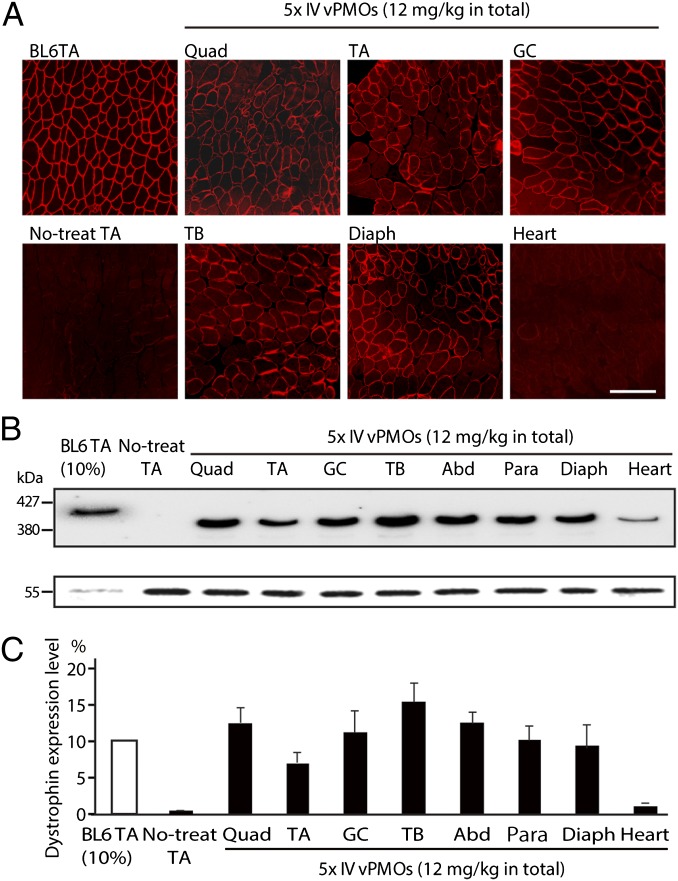
Next, we performed serial i.v. injections of the mixture-ESE2 into 5-wk-old mdx52 mice. After five biweekly (every 2 wk) i.v. injections of 12 mg/kg of the mixture per injection, we detected dystrophin-positive fibers in all skeletal muscles by immunohistochemistry (Fig. 3A). Dystrophin expression was evaluated by semiquantitative Western blotting (Fig. 3B). The dystrophin expression levels in the quadriceps, TA, gastrocnemius, triceps brachii, abdominal, paraspinal, and diaphragm muscles were ∼8–15% of normal levels, whereas the dystrophin expression level in the heart muscle was 2% of normal levels (Fig. 3C).
Fig. 3.
Systemic i.v. injections of the mixture-ESE2 in mdx52 mice restore dystrophin expression in body-wide skeletal muscles. (A) Immunohistochemical staining of dystrophin exon 57 in quadriceps (Quad), TA, gastrocnemius (GC), triceps brachii (TB), diaphragm (Diaph), and heart muscles in mdx52 mice after five consecutive biweekly systemic injections of 12 mg/kg of the mixture-ESE2. Representative data are shown. BL6TA, TA muscle from wild-type C57/BL6; no-treat TA, untreated TA muscle from mdx52 mice. (Scale bar, 100 μm.) (B) Western blotting analysis with mouse monoclonal antibody DYS2 after the repeated vPMOs systemic injections into mdx52 mice. Representative data are shown. vPMO-injected muscles show 380-kDa quasidystrophin bands (Upper) and α-tubulin (Lower) in Quad, TA, GC, TB, abdominal (Abd), paraspinal (Para), Diaph, and heart muscles of treated mdx52 mice. BL6TA (10% wt/wt), TA muscle from a 10% (wt/wt) extract of wild-type C57/BL6 mice. (C) Semiquantitative analysis of dystrophin expression after AO injection. Data (n = 4) are presented as mean ± SD *P < 0.05; **P < 0.01.
Exon 45–55 Skipped Quasidystrophin Ameliorates Skeletal Muscle Pathology.
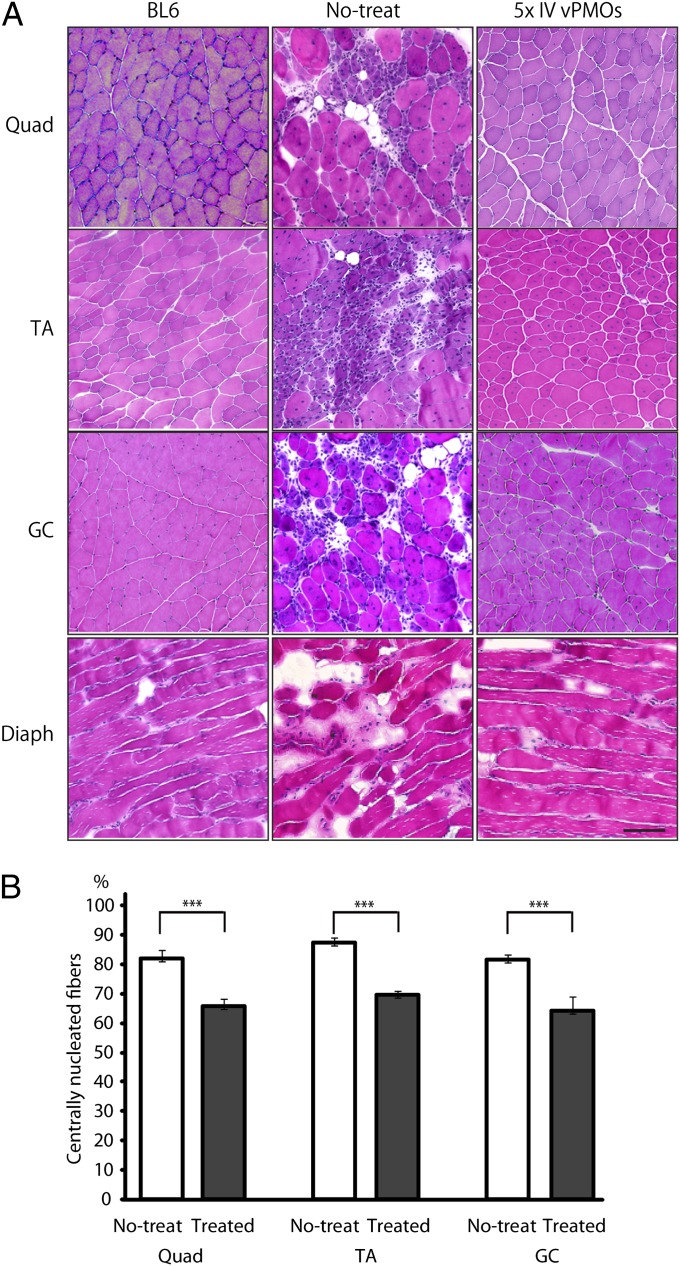
We then evaluated the detailed histological changes in quadriceps, TA, gastrocnemius, and diaphragm muscles of treated mice compared with the changes in those of untreated mice. After five i.v. mixture-ESE2 injections, we observed less muscle degeneration and fewer cellular infiltrates in the treated quadriceps, TA, gastrocnemius, and diaphragm muscles compared with the untreated muscles by H&E staining (Fig. 4A). We found that the percentage of centrally nucleated fibers (CNFs) were significantly lower in the quadriceps, TA, and gastrocnemius muscles compared with the untreated mice (Fig. 4B). These changes reflect the amelioration of dystrophic changes in the treated mdx52 mice.
Fig. 4.
Exon 45–55 skipped quasidystrophin ameliorates skeletal muscle pathology in mdx52 mice. (A) H&E staining in quadriceps (Quad), TA, gastrocnemius (GC), and diaphragm (Diaph) muscles of WT (BL6), untreated (no-treat), and treated mdx52 mice (five times i.v. vPMOs). Representative data are shown. (B) Measurement of centrally nucleated fibers (CNFs) after systemic exon 45–55 skipping (treated) and nontreated mdx52 muscles (no-treat) in Quad, TA, and GC muscles. Data (n = 4) are presented as mean ± SD ***P < 0.001.
Exon 45–55 Skipped Quasidystrophin Ameliorates Skeletal Muscle Function.
To examine the function of the exon 45–55 skipped quasidystrophin, we performed a battery of physiological and blood tests after five biweekly i.v. injections with the mixture-ESE2. Serum CK levels were significantly reduced in the treated mice, suggesting the protection of muscle fibers against degeneration (Fig. S6A). In addition, significant improvement in maximum forelimb grip force and improvement tendency in treadmill endurance and time latency to fall in rotarod test were observed in treated mdx52 mice compared with untreated mdx52 mice (Fig. S6 B–D).
No Detectable Toxicity After Repeated Delivery of AOs into mdx52 Mice.
To further monitor any potential toxicities in the major organs induced by treatment with AOs, we compared a series of standard serum markers as indicators of liver and kidney dysfunction in WT, untreated, and treated mdx52 mice after five biweekly 12 mg/kg injections with the mixture-ESE2. Serum aspartate amino transferase was reduced in the treated mice (Fig. S7). No significant differences were detected between untreated and treated mdx52 mice groups in the levels of aspartate transaminase, alanine aminotransferase, total bilirubin, alkaline phosphatase, blood urea nitrogen, creatinine, sodium ion, chloride ion, and potassium ion (Fig. S7). These data confirm that this AO combination was nontoxic in vivo.
Discussion
In this paper, we demonstrated that rescue of dystrophin expression by skipping exons 45–55 is feasible both in H2K-mdx52 myotubes in vitro and in dystrophic mdx52 mice in vivo. As far as we know, this is a unique report of a successful rescue of dystrophin using this technique to skip a large block of 10 exons. Currently, trials of exon 51 skipping using systemic delivery of PMO and 2′O-methyl phosphorothioate antisense oligonucleotides (2′O-MePS) AOs are underway (8, 9). Although exon 51 is the single target exon in the DMD gene whose skipping would restore ORF to the largest proportion of DMD mutations, it would still be applicable to only some 10% of DMD patients (or 17% of DMD patients with deletion mutations) (Fig. S1B) (17). The goal of treating a broader range of DMD patients with a single treatment has fostered interest in skipping multiple exons. In particular block skipping of exons 45–55 commends itself as a most promising approach (13, 18). Exon 45–55 skipping is known to have two major advantages. First, it would be applicable to ∼63% of DMD patients with dystrophin deletion mutations (13). Second, the majority of individuals with a deletion of exons 45–55 of the DMD gene fall into the category of BMD patients with exceptionally mild, sometimes almost asymptomatic skeletal muscle involvement (12, 13). Nakamura et al. reported two Japanese patients with exon 45–55 deletions (28 and 42 y old) showing no symptoms except for high blood CK level (12). Similarly, Ferreiro et al. reported a 69-y-old individual with exon 45–55 deletion showing only high CK level without showing any symptoms (19). Such observations raise the hope that antisense-mediated exon 45–55 skipping will have the potential not only to convert DMD patients to a mild BMD clinical phenotype but also to ameliorate the condition of some of the more severe BMD patients with mutations within the exon 45–55 hotspot region.
It is not known why deletion of exons 45–55 leads to very mild phenotypes. The Leiden database used in this study consists of reports from many different countries and sites, raising the possibility of inconsistencies arising from incorrect mapping of some patients or differences in the criteria of diagnosis for Duchenne, Becker, and intermediate cases. However, we have no reasons to suspect any systematic bias with this large dataset. The resulting products of exon 45–55 skipping lead to a truncation at the middle of two rod spectrin repeats (rod repeats 17 and 22) as we have previously pointed out (Fig. 1A) (11). Interestingly, the number of rod repeats in this quasidystrophin between the remaining adjacent hinge domains H2 and H4 (16 spectrin repeats) is exactly the same as that between H2 and H3 in full-length dystrophin, which might indicate a requirement of such a spacing for protein function or stability (Fig. 1A).
Our results indicate that the truncated dystrophin induced by skipping exons 45–55 restored all of the dystrophin-associated proteins except nNOS. It was reported that nNOS is anchored at the subsarcolemma through its binding to the rod domain of dystrophin at the 16 and 17 rod repeats encoded by exons 42–45 and by interaction with α1-syntrophin (20, 21). The truncated dystrophin induced by skipping exons 45–55 would lack half of the 17 rod repeat, thus disturbing the site-responsible anchoring of nNOS and possibly rendering its subsarcolemmal localization unstable. Although nNOS was not restored after the mixture oligo injections, it is encouraging to note that mild BMD patients with deletions of exons 45–55 also lack nNOS at the subsarcolemma (22).
A previous in vitro study by van Vliet et al. showed that the exon 45–55 skipping frequencies with 2′O-MePS AOs were minimal and comparable to those observed in untreated myocytes of DMD patients with exon 45–47 deletions (16). Here, we redesigned the mixture of vPMOs using online software called Human Splicing Finder and ESEfinder to detect ESEs (23, 24), but paying attention to the avoidance of formation of self- or heteroduplex of the AOs, which, we reasoned, could diminish the efficacy of multiple exon skipping. With this aim, we used OligoAnalyzer 3.1 (25) to design the mixture in which most of combinations of ΔG forces of different AOs were above −5 kcal/mole.
After i.v. injections of our mixture vPMOs into mdx52 mice, we observed extensive dystrophin-positive fibers and an average of some 8–15% of wild-type levels of dystrophin protein, as determined by Western blotting analysis in a range of skeletal muscles (Fig. 3). The pathology of skeletal muscles was ameliorated; but the skeletal muscle function was only marginally recovered, probably due to the incomplete restitution of quasidystrophin (Fig. 4 and Fig. S6).
Whereas conventional PMOs are relatively safe, high-dose administration is required to induce exon skipping systemically (5, 7). Here, we observed high efficacy for systemic rescue with vPMOs that contain a cell-penetrating moiety of octaguanidine dendrimer (Fig. S2B). Although the sequences we have used are specific to the mouse, our data validate the principle that carefully designed AOs may be used to realize block skipping of exons 45–55 and, by this means, generate effective amounts of quasidystrophin of near-optimal structure in some 60% of DMD deletion patients. This multiple exon skipping therapy was achieved by use of vPMOs, which produced no evidence of toxicity. The most significant barrier to translating the “mixture approach” to a therapeutic for DMD is the lack of adequate delivery, especially to the heart. Nevertheless, we suggest that a similar approach might pave the way for use of a single-mixture antisense drug that could be applied to treatment of 40–45% of DMD patients.
Materials and Methods
Animals.
Exon 52-deficient X chromosome-linked muscular dystrophy mice (mdx52 mice) were produced by a gene-targeting strategy and are maintained in our facility (26); they have been backcrossed to the C57BL/6J (WT) strain for more than eight generations. Five-week-old male mdx52 and WT mice were used in this study. The mice were allowed ad libitum access to food and drinking water. The Experimental Animal Care and Use Committee of the National Institute of Neuroscience, National Center of Neurology and Psychiatry (NCNP), Japan, approved all experimental protocols in this study.
Antisense Oligos.
AOs for targeted skipping of exons 45–55 in the mouse Dmd gene were designed using ESEfinder and Human Splicing Finder software to anneal to the ESEs of each exons (Table S1) (23, 24). All antisense mixtures consist of equal amounts of each antisense oligo. Unmodified morpholinos (PMOs) or octaguanidine dendrimer-conjugated morpholinos (vivo-morpholinos or vPMOs) were used in this study (Gene-Tools) (27, 28).
H2K-mdx52 Myoblasts.
H-2Kb-tsA58 × mdx52/mdx52 F1 male mice yielded dystrophin-deficient H2K-mdx52 myoblasts (29). H2K-mdx52 myoblasts were grown at 33 °C in medium containing γ-IFN at a concentration of 20 units/mL and 20% (vol/vol) FBS. After the treatment, the cells were grown in differentiation medium containing 5% (vol/vol) horse serum at 37 °C for 1 d.
AO Transfection.
Myotubes were differentiated from H2K-mdx52 cells and were transfected with the vPMO or the PMOs as previously reported (30). In the differentiation medium, the final concentration of the vPMO was a total of 0.1–3 μM and that of the PMO was a total of 10 μM for 10 sequences. After 48 h incubation with the vPMO or the PMO, total RNA was extracted from myotubes using TRIzol (Invitrogen).
AO Injections.
Animals were anesthetized by inhalation of sevoflurane (Wako Pure Chemical Industries) for injections. A total of 1.5 μg of vPMOs or 10 μg of PMOs targeting exons 45–55 in a total volume of 36 μL of saline were used for each TA muscle in the mdx52 mice. Muscle samples were obtained 2 wk after the intramuscular injection.
A total of 12 mg/kg dose of vPMOs in 100 μL of saline was injected into the tail vein of mdx52 mice, five times at biweekly (every 2 wk) intervals. The mice were examined 2 wk after the final injection. Muscles were obtained immediately, snap frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C for immunohistochemistry and Western blotting. Skeletal muscle tissues were cut and collected in microtubes and snap frozen in liquid nitrogen for reverse transcription PCR (RT-PCR).
RT-PCR and Sequencing of cDNA.
Total RNA from the muscles of WT, untreated, or mdx52 mice were extracted as previously described (7). Two hundred nanograms of RNA template was used for a 20-μL RT-PCR using a QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer’s instructions. The cDNA product (1 μL) was then used as the template for PCR in a 20-μL reaction with 0.10 μL of Ex Taq Hot Start Version (Takara). The reaction mixture composed of 10× PCR buffer (Roche), 10 mM of each dNTP (Qiagen), and 10 μM of each primer. The primer sequences for the PCR were designed using Primer3 (http://frodo.wi.mit.edu/) and described in Table S2. The cycling conditions were at 95 °C for 4 min, then 94 °C for 0.5 min, 60 °C for 0.5 min, 72 °C for 1.2 min for 35 cycles, and at 72 °C for 7 min. PCR products were separated on a 2% (wt/wt) agarose gel. Bands of the expected size for the transcript were extracted by using a gel extraction kit (Qiagen). Direct sequencing for PCR products was performed at Operon Biotechnologies.
Immunohistochemistry and Histology.
At least ten 7-μm cryosections were cut at 100-μm intervals from the quadriceps, the TA, the gastrocnemius, the triceps brachii, the diaphragm, and the heart muscles. The serial sections were stained with antidystrophin antibody such as monoclonal mouse antibody MANEX46B against exon 46, monoclonal mouse antibody MANEX50 against exon 50, and polyclonal rabbit antibody P7 against exon 57 (provided by Qi-Long Lu, Carolinas Medical Center, Charlotte, NC) anti–α-sarcoglycan monoclonal mouse antibody (Novocastra Laboratories), anti–β-dystroglycan monoclonal mouse antibody (Novocastra Laboratories), anti–anti-alpha1-syntrophin polyclonal rabbit antibody (Abcam), and antineuronal nitric oxide synthase polyclonal rabbit antibody (Zymed). Alexa 568 (Invitrogen) was used as a secondary antibody. The maximum number of dystrophin-positive fibers in one section of TA was counted under a BZ-9000 fluorescence microscope (Keyence). H&E staining was performed using Harris H&E.
Western Blotting Analysis.
Western blot analysis was performed as previously described (7). Two to 20 μg of protein from the TA muscle of a WT mouse as a positive control, 20 μg of protein from the TA muscle of untreated mdx52 as a negative control, and 20 μg of protein from the muscles of treated mdx52 mice were loaded onto a 5–15% (wt/vol) XV Pantera gel (DRC). The samples were transferred onto an Immobilon PVDF membrane (Millipore) by semidry blotting at 5 mA/mm2 for 1 h. The membrane was then incubated with the C-terminal monoclonal antibody DYS2 (Novocastra Laboratories), anti–α-sarcoglycan monoclonal mouse antibody (Novocastra Laboratories), anti–β-dystroglycan monoclonal mouse antibody (Novocastra Laboratories), anti–anti-alpha1-syntrophin polyclonal rabbit antibody (Abcam), and antineuronal nitric oxide synthase polyclonal rabbit antibody (Zymed) at room temperature for 1 h. Anti–α-tubulin (Abcam) was used as loading controls. The intensity of the bands obtained from the treated mdx52 muscles was analyzed using ImageJ software (http://rsb.info.nih.gov/ij/) and compared with that from normal WT muscles.
Blood Analysis and Muscle Functional Testing.
The blood analysis, grip strength, treadmill, and rotarod tests of the mice were performed as previously described (7).
Statistical Analysis.
Statistical differences were assessed by one-way analysis of variance with differences among the groups assessed by a Tukey comparison, or χ2 test. All data are reported as mean values ± SD or ± SEM. The level of significance was set at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by Intramural Research Grant (22-5) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP); Health and Labour Sciences Research Grants for Translation Research (H21-Translational Research-011); Health and Labour Sciences Research Grants for Translation Research (H21-Clinical Research-015); Comprehensive Research on Disability Health and Welfare (H23-Neuromuscular Disease-005) from the Ministry of Health, Labour, and Welfare of Japan; Foundation to Eradicate Duchenne; US Department of Defense (W81XWH-09-1-0599); the National Institutes of Health (1P50AR060836, 5T32AR056993, U54HD071601, R24HD050846, and K26OD011171); Muscular Dystrophy Association; University of Alberta; The Friends of Garrett Cumming Research; HM Toupin Neurological Science Research; and Muscular Dystrophy Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204638109/-/DCSupplemental.
References
- 1.Duchenne The pathology of paralysis with muscular degeneration (Paralysie Myosclerotique), or paralysis with apparent hypertrophy. British Medical Journal. 1867;2:541–542. doi: 10.1136/bmj.2.363.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Koenig M, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: Correlation of severity with type of deletion. Am J Hum Genet. 1989;45:498–506. [PMC free article] [PubMed] [Google Scholar]
- 4.Yokota T, et al. A renaissance for antisense oligonucleotide drugs in neurology: Exon skipping breaks new ground. Arch Neurol. 2009;66:32–38. doi: 10.1001/archneurol.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokota T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu QL, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki Y, et al. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol Ther. 2010;18:1995–2005. doi: 10.1038/mt.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goemans NM, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 9.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman EP, et al. Restoring dystrophin expression in duchenne muscular dystrophy muscle progress in exon skipping and stop codon read through. Am J Pathol. 2011;179:12–22. doi: 10.1016/j.ajpath.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokota T, Duddy W, Partridge T. Optimizing exon skipping therapies for DMD. Acta Myol. 2007;26:179–184. [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura A, et al. Follow-up of three patients with a large in-frame deletion of exons 45-55 in the Duchenne muscular dystrophy (DMD) gene. J Clin Neurosci. 2008;15:757–763. doi: 10.1016/j.jocn.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Béroud C, et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 2007;28:196–202. doi: 10.1002/humu.20428. [DOI] [PubMed] [Google Scholar]
- 14.Aartsma-Rus A, Janson AA, van Ommen GJ, van Deutekom JC. Antisense-induced exon skipping for duplications in Duchenne muscular dystrophy. BMC Med Genet. 2007;8:43. doi: 10.1186/1471-2350-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClorey G, Moulton HM, Iversen PL, Fletcher S, Wilton SD. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–1381. doi: 10.1038/sj.gt.3302800. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet L, de Winter CL, van Deutekom JC, van Ommen GJ, Aartsma-Rus A. Assessment of the feasibility of exon 45-55 multiexon skipping for Duchenne muscular dystrophy. BMC Med Genet. 2008;9:105. doi: 10.1186/1471-2350-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aartsma-Rus A, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura A, Takeda S. Exon-skipping therapy for Duchenne muscular dystrophy. Lancet. 2011;378:546–547. doi: 10.1016/S0140-6736(11)61028-3. [DOI] [PubMed] [Google Scholar]
- 19.Ferreiro V, et al. Asymptomatic Becker muscular dystrophy in a family with a multiexon deletion. Muscle Nerve. 2009;39:239–243. doi: 10.1002/mus.21193. [DOI] [PubMed] [Google Scholar]
- 20.Lai Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kameya S, et al. alpha1-syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J Biol Chem. 1999;274:2193–2200. doi: 10.1074/jbc.274.4.2193. [DOI] [PubMed] [Google Scholar]
- 22.Anthony K, et al. Dystrophin quantification and clinical correlations in Becker muscular dystrophy: Implications for clinical trials. Brain. 2011;134:3547–3559. doi: 10.1093/brain/awr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmet FO, et al. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owczarzy R, et al. DT SciTools: A suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res Jul. 2008;36:W163–W169. doi: 10.1093/nar/gkn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki E, et al. Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1997;238:492–497. doi: 10.1006/bbrc.1997.7328. [DOI] [PubMed] [Google Scholar]
- 27.Summerton J, Weller D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 28.Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: A non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008;45:613–614, 616, 618 passim. doi: 10.2144/000113005. [DOI] [PubMed] [Google Scholar]
- 29.Morgan JE, et al. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: A model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–498. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- 30.Saito T, et al. Antisense PMO found in dystrophic dog model was effective in cells from exon 7-deleted DMD patient. PLoS ONE. 2010;5:e12239. doi: 10.1371/journal.pone.0012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.