Abstract
The anterior hypothalamus (AH) is a major integrator of neural processes related to aggression and defense, but cell types in the AH that selectively promote aggression are unknown. We here show that aggression is promoted in a very selective and potent manner by dorsal AH neurons that produce vasoactive intestinal polypeptide (VIP). Fos activity in a territorial finch, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) is positively related to aggression in the dorsal AH, overlapping a population of VIP-producing neurons. VIP is known to promote territorial aggression in songbirds, and thus we used antisense oligonucleotides to selectively block AH VIP production in male and female waxbills. This manipulation virtually abolishes aggression, reducing the median number of displacements in a 3-min resident–intruder test from 38 in control subjects to 0 in antisense subjects. Notably, most antisense and control waxbills exhibit an agonistic response such as a threat or agonistic call within 2 s of intrusion. Thus, antisense subjects clearly classify intruders as offensive, but fail to attack. Other social and anxiety-like behaviors are not affected and VIP cell numbers correlate positively with aggression, suggesting that these cells selectively titrate aggression. Additional experiments in the gregarious zebra finch (Estrildidae: Taeniopygia guttata) underscore this functional specificity. Colony-housed finches exhibit significant reductions in aggression (primarily nest defense) following AH VIP knockdown, but no effects are observed for social preferences, pair bonding, courtship, maintenance behaviors, or anxiety-like behaviors. To our knowledge, these findings represent a unique identification of an aggression-specific cell type in the brain.
Keywords: neuropeptide, reproduction
The anterior hypothalamus (AH) is a hub of neural processes related to aggression and agonistic communication in taxa ranging from fish to mammals (1–9). The AH is strongly interconnected with the ventrolateral subnucleus of the ventromedial hypothalamus (VMHvl) (10), and both areas are overlapped by the “hypothalamic attack area,” a region from which aggressive behavior is elicited by electrical stimulation in mammals (7, 9, 11, 12). A comparable organization is found in birds, as demonstrated by both electrical stimulation (1, 8) and immediate early gene expression (13, 14). The AH and VMHvl integrate information from many socially relevant forebrain regions and project to midbrain areas that regulate motivation and motor processes (10, 15–17). However, whereas recent studies in male mice show that overlapping but distinct neuronal populations of the VMHvl regulate fighting and mating (18), the neurochemical phenotypes of aggression-related neurons in the VMHvl and AH are largely unknown (7). AH vasopressin neurons that promote aggression in voles also influence affiliation (5), and the AH further regulates stress response and defense (12, 14, 19). Thus, the identification of AH neurons that selectively promote aggression requires that broad behavioral test batteries be used, as in the present experiments.
Although aggressive interactions induce immediate early gene responses in the AH, transcriptional responses are greater in subordinate rodents than in dominants (20, 21), and in male songbirds, both Fos and egr-1 immunoreactivity in the AH correlate negatively with the amount of aggression displayed (13). Hence the first hypothesis tested here is that there is spatial heterogeneity in the Fos responses of AH neurons. This hypothesis receives strong support and the spatial analysis focused our attention on an area defined by a population of vasoactive intestinal polypeptide (VIP) neurons. VIP neurons, fibers, and receptors are found in virtually every brain region that is known to be important for social behavior (22–25), but with the exception of the VIP neuronal populations in the suprachiasmatic nucleus and tuberal hypothalamus, which regulate circadian rhythms and prolactin release, respectively (26, 27), we know very little about the behavioral functions of VIP cell groups and their projection systems.
Because VIP infusions into the septum, an AH projection target (28), facilitate offensive aggression in male violet-eared waxbills (Estrildidae: Uraeginthus granatina; a highly territorial African finch) (29), we here test the additional hypotheses that (i) endogenous binding at VIP receptors promotes aggression in violet-eared waxbills, and (ii) production of VIP in the AH is necessary for the normal display of aggression. We then extend these studies to the highly gregarious zebra finch (Taeniopygia guttata). Unlike violet-eared waxbills, which aggressively defend large, exclusive territories (30), zebra finches primarily exhibit aggression only in the immediate vicinity of the nest (31).
Results
Fos Analyses of the AH.
Because immediate early gene activity in the AH is negatively correlated with aggression in songbirds (13), most neurons in the AH likely inhibit aggression, although offensive and defensive behaviors may be differentially influenced by constituent cell groups of the AH. Thus, to determine whether AH neurons that promote aggression and defense are spatially segregated to some degree (i.e., as opposed to being completely intercalated), we exposed male violet-eared waxbills to a resident–intruder encounter or control conditions for analyses of Fos protein expression using a grid of eight boxes (∼83 μm2 each; 2 across × 4 down; SI Methods).
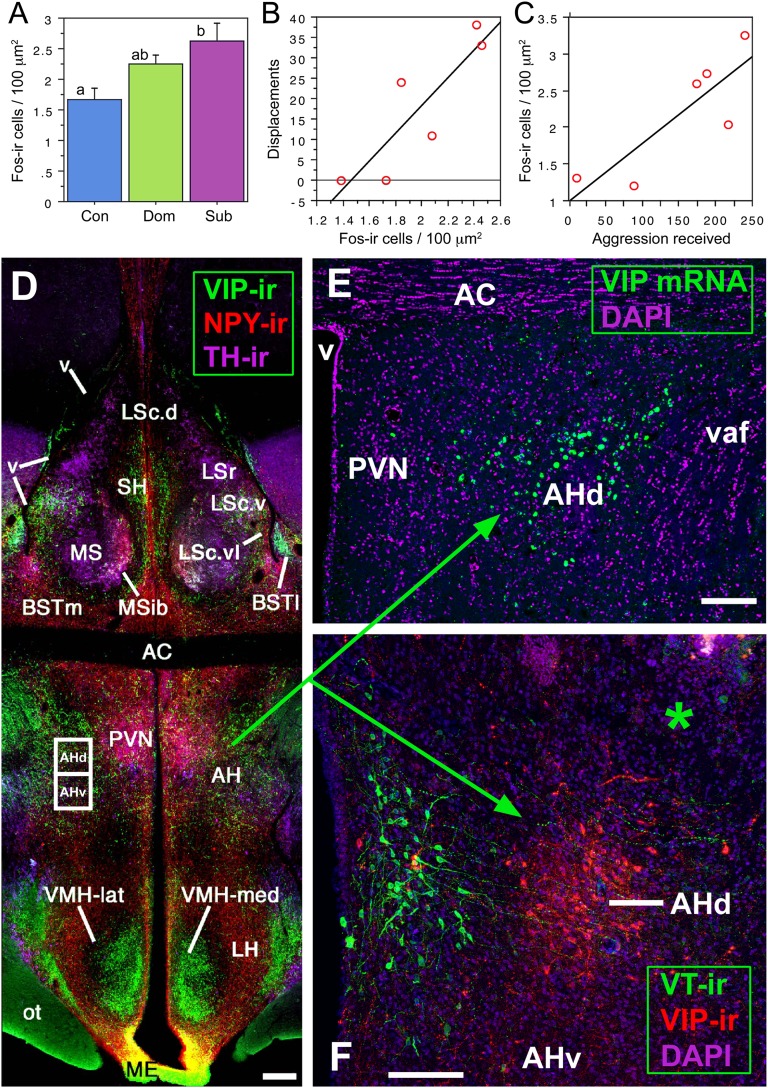
As predicted, the overall Fos response of the AH is greater in subordinates (Fig. 1A), but effects are spatially heterogeneous. Fos-immunoreactive (-ir) cell numbers in the dorsal AH (i.e., four dorsal boxes) of dominant males correlate positively with aggressive displacements (Fig. 1B), but this is not observed for the ventral AH. The total number of aggressive behaviors (threats, agonistic calls, and displacements) exhibit a similar but weaker trend in the dorsal AH (r = 0.752, P = 0.08), but no trend in the ventral AH (r = 0.011, P = 0.98). Conversely, the total aggression received by subordinates correlates positively with Fos-ir cell counts in the ventral AH (Fig. 1C), but not in the dorsal AH (r = 0.443, P = 0.38). Displacements received did not predict Fos-ir cell numbers for either the ventral AH (r = 0.626, P = 0.18) or dorsal AH (r = 0.154, P = 0.77). On the basis of these results, we subsequently sought to identify specific neuronal phenotypes of the dorsal AH that contribute to territorial aggression.
Fig. 1.
Histochemically distinct components of the AH exhibit differential responses to agonistic encounters. (A) Fos-ir cell numbers in the AH are significantly increased in subordinate (Sub) but not dominant (Dom) male waxbills relative to control subjects (Con); n = 6 per group. Different letters above the bars denote significant pairwise differences (P < 0.05). (B and C) Fos activation in the dorsal AH (AHd) of dominant birds correlates positively with the number of aggressive displacements given (r2 = 0.752, P = 0.02; B), whereas Fos activation in the ventral AH (AHv) of subordinate birds correlates positively with aggression received (r2 = 0.704, P = 0.04; C). (D–F) VIP elements in the septohypothalamic region of the zebra finch (D and E) and violet-eared waxbill (F; asterisk shows the injector tract in a scrambled oligonucleotide subject). The dorsal AH region is defined by VIP mRNA and peptide (E and F). (Scale bars, 200 μm in D and 100 μm in E and F.) AC, anterior commissure; AH, anterior hypothalamus; BSTl; lateral bed nucleus of the stria terminalis; BSTm, medial BST; LS, lateral septum (LSr. rostral LS division; LSc., caudal LS division: d, dorsal; v, ventral; vl, ventrolateral); ME, median eminence; MS, medial septum; MSib, internal band of the MS; ot, optic tract; PVN; paraventricular nucleus; SH, septohippocampal septum; v, ventricle; vaf, ventral amygdalofugal tract. D is modified from ref. 17.
AH Histochemistry and Gene Expression.
The position of the dorsal AH region identified in this study corresponds to an aggression locus identified through stimulation studies in domestic chickens (Gallus domesticus) (8) and Steller's jays (Cyanocitta stelleri) (1), which lies beneath the anterior commissure and lateral to the paraventricular nucleus (PVN; Fig. 1D). In both mammals and chickens, this area contains mRNA for VIP (22, 25), but VIP-ir cells have not been reported in the AH, perhaps reflecting rapid turnover. Thus, we first sequenced the zebra finch VIP gene and confirmed the presence of VIP mRNA in the dorsal AH (Fig. 1E) and subsequently demonstrated that a substantial number of VIP-ir neurons are visible in this area following colchicine pretreatment to block axonal transport (Fig. 1F).
VIP Antisense Production and Validation.
Using the sequence data from zebra finches and additional sequence data from violet-eared waxbills (a 300-bp 5′ fragment, including the start codon, and entire second exon) we identified a 16-nucleotide sequence spanning the start codon for antisense targeting. This sequence exhibits 100% identity with java finch (or java “sparrow”; Padda oryzivora, a basal estrildid) and only a single nucleotide difference with chickens. As shown in Fig. S1, hemispheres infused with VIP antisense contained 55% fewer VIP-ir neurons than did contralateral hemispheres infused with scrambled oligonucleotides. Injections were placed immediately dorsal to the AH VIP cell group. Aromatase production, immediately medial to the infusions in the medial bed nucleus of the stria terminalis, was not affected (P = 0.402). An additional comparison of scrambled oligonucleotides versus saline in three males showed no side differences in VIP cell numbers (P > 0.35).
VIP Antagonism and Antisense Knockdown in Waxbills.
We conducted antagonist and antisense experiments in the same group of violet-eared waxbills (n = 11 males, 11 females), which were fitted with bilateral guide cannulae that allowed infusions into either the lateral ventricles (for antagonist administrations, using short injectors that extended only as deep as the ventricles) or immediately dorsal to the AH VIP cell group (for antisense oligonucleotide infusions, using longer injectors that extended to the hypothalamus). Control subjects for antisense experiments were infused with scrambled oligonucleotides.
Cannulated male and female waxbills were housed individually and tested with both vehicle and a receptor antagonist with known selectivity for VIP binding sites in chickens (neurotensin6-11–mouse VIP7-28) (32). This was delivered at 125 ng per side in 0.5 μL saline in a counterbalanced order with 2 d between behavioral test batteries. This was followed by a sequence of five infusions of either antisense or scrambled oligonucleotides (1 μg in 0.25 μL isotonic saline) at 12-h intervals, a behavioral test battery, an infusion of a colchicine/oligonucleotide mixture, and a final oligonucleotide infusion ∼12 h before killing. Behavioral measures were resident–intruder aggression, anxiety-like behaviors (measured in exploration and novelty-suppressed feeding tests), and approach to cages containing either 10 or 2 Angolan blue waxbills (U. angolensis), a moderately gregarious congener that violet-eared waxbills occasionally associate with in the wild. In the latter test, subjects were placed in a 1-m-wide cage adjoined by a cage of 10 stimulus birds at one end and 2 stimulus birds at the other. Seven perches were provided in the central cage, and the side perches were 3.5 cm from the wall adjoining the stimulus cages (33, 34). This test provides a measure of “social contact” (time spent on the two furthest side perches combined) and group-size preference (defined as the percent of contact time spent with the larger group) (34).
ANOVA reveals no main effects of sex or sex × treatment interactions for any of the variables examined, and sexes were therefore pooled for further nonparametric analyses, given that aggression data were not normally distributed. As predicted, intraventricular infusions of a receptor antagonist significantly reduce aggressive displacements (aerial pursuits and supplants), but only modestly (∼20%; Wilcoxon P = 0.05). As described below, this weak effect may reflect the opposing influence of different hypothalamic cell groups. No effects are observed for other agonistic behaviors (Wilcoxon P = 0.75 for threats; P = 0.14 for agonistic calls) or the latency to respond by displacement, threat, or agonistic call (Wilcoxon P = 0.41). Likewise, no antagonist effects are obtained for anxiety-like behaviors such as approach to the novel object in the feeding assay (ANOVA P = 0.77) and exploration of a novel environment (ANOVA P = 0.53 for hops; P = 0.36 for branch visits) or for affiliation behaviors such as social contact with blue waxbills (ANOVA P = 0.50) and group size preference (ANOVA P = 0.12).
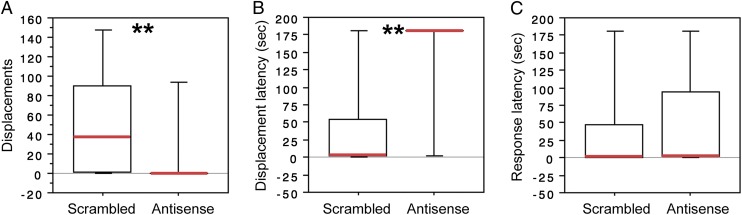
Antisense infusions produce a virtually identical pattern of results, with the exception that aggression in the resident–intruder test is decreased much more potently by knockdown of AH VIP than by intraventrical infusions of the antagonist. As shown by repeated measures ANOVA (using data from saline trials as a baseline), antisense infusions produce a significant reduction in aggressive displacements relative to infusions of scrambled oligonucleotides (P = 0.01). Fig. S2 shows these data, log transformed due to large variance, but because baseline data do not differ between sexes or treatment groups, posttreatment data were further analyzed by Mann–Whitney tests, allowing visualization of the absolute effect size in box plots (Fig. 2A). Remarkably, as shown in these plots, the median number of aggressive displacements in a 3-min test is reduced from 38 in the control group (scrambled oligonucleotides) to 0 in the antisense group, and hence the latency to exhibit a displacement is significantly increased following VIP knockdown (Fig. 2B). Nonetheless, knockdown does not impair the ability to rapidly assess the offensiveness of the intruder, as evidenced by the fact that most antisense and control subjects exhibit an agonistic response within 2 s of introduction of the intruder (Fig. 2C). For antisense subjects, this response was most often a threat or agonistic call that was never followed by pursuit. No effects are observed for other aggressive behaviors (threats, P = 0.58; agonistic calls, P = 0.63), approach to the novel object in the feeding assay (ANOVA P = 0.35), exploration (ANOVA P = 0.11 for hops; P = 0.62 for branch visits), social contact with blue waxbills (ANOVA P = 0.08), or group size preference (ANOVA P = 0.86).
Fig. 2.
Antisense knockdown of VIP production in the AH potently reduces territorial aggression in male and female violet-eared waxbills. (A and B) VIP antisense infusions immediately dorsal to the AH abolish aggressive displacements in most violet-eared waxbills, as measured in a 3-min resident–intruder test (A) and significantly increase the latency to displace the intruder (B). (C) Antisense infusions have no affect on short-latency appraisals of valence, as both antisense and control subjects exhibit an agonistic response (threat, agonistic call, or displacement) very rapidly. **P = 0.01. n = 12 antisense subjects (6 female, 6 male); 9 scrambled subjects (5 female, 4 male).
Finally, we quantified the number of AH VIP-ir cells to determine whether individual variation in cell number predicts individual differences in aggression. We find that VIP-ir cell number correlates positively with displacements in control subjects (Spearman ρ = 0.695, P = 0.05; Fig. S3A) and obtain similar results for displacement latency (ρ = −0.746, P = 0.03), threats (ρ = 0.613, P = 0.08), and agonistic calls (ρ = 0.661, P = 0.06). To determine whether the AH cell group is functionally distinct from other hypothalamic populations of VIP neurons, we also quantified the numbers of tuberal VIP-ir cells and find that the control subjects that exhibit no displacements (n = 2) have substantially more tuberal VIP-ir cells than do the more aggressive birds (n = 7) (Fig. S3B).
VIP Antisense Effects in Zebra Finches.
To further examine the functional specificity of the AH VIP neurons, we conducted two experiments in the highly gregarious zebra finch. Zebra finches provide an interesting contrast to violet-eared waxbills, because they form large colonies and do not defend exclusive home ranges, although they can be very aggressive when competing for mates and in defense of the immediate nest area (31, 35). The latter context is the primary context of aggression in wild zebra finches and is somewhat similar to territorial behavior in waxbills. However, whereas waxbill nests are typically hundreds of meters apart (30), zebra finches defend at most a single nesting bush (and not the ground around it), and multiple nests per bush are common (31).
In the first experiment, we tested male subjects (n = 7 control, 8 antisense) for social contact, group size preferences, novel–familiar social preferences, and anxiety-like behaviors following infusions of antisense or scrambled oligonucleotides. Consistent with the data from violet-eared waxbills, unpaired t tests demonstrate that VIP antisense infusions in male zebra finches produce no effects on anxiety-like behaviors (novelty suppression of feeding, P = 0.29; exploration, P = 0.97 for hops and P = 0.73 for branch visits) or affiliation behaviors (social contact with same-sex conspecifics, P = 0.48; group size preference, P = 0.26; and novel–familiar social preferences, P = 0.09).
For the second experiment, which focused on aggression and other behaviors in a group environment, we prescreened male zebra finches following 1 d in a colony cage containing females, nest cups, and nesting material. Only subjects that displaced other birds at least three times in a 5-min period were retained as subjects (n = 8 per treatment). Following the fifth oligonucleotide infusion, we introduced four subjects (two scrambled, two antisense) into cages containing five females, four nest cups, and nesting material. To give each subject an equal opportunity to be dominant, males that exhibited few displacements during screenings (4.75 ± 0.45; mean ± SEM) were placed into “low aggression cages” and males that exhibited many displacements in the screenings (16.63 ± 6.12) were assigned to “high aggression cages” (n = 2 cages of each type). We conducted focal observations upon introduction (capturing the initial burst of courtship) and ∼4 h and 30 h later. These observations allow the quantification of directed courtship singing, aggressive behaviors (displacements, threats, pecks, beak fences), maintenance behaviors (preening, eating, drinking), and the establishment of pair bonds (35). Pair bonds are readily quantified on the basis of side-by-side perching, following, allopreening, and typically occupation of a nest cup. Aggression in the colony cages was relatively low and thus aggressive behaviors were pooled for analysis. A single subject in each cell (treatment × cage type) was excluded due to inaccurate cannula placement.
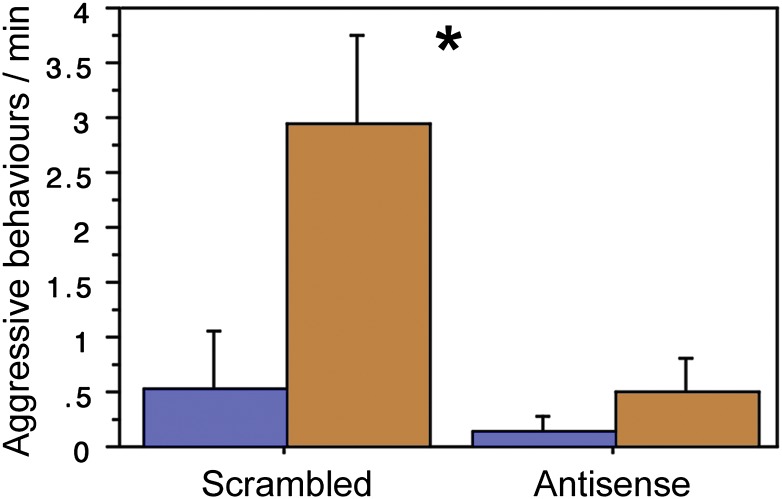
As shown in Fig. 3, antisense infusions strongly reduced aggression on the second day of testing, when aggression is primarily focused on nest defense. Comparable effects were not observed on the first day, when most aggression is focused on competition for mates (P = 0.91). Likewise, no effects were observed for directed courtship singing (P = 0.54), maintenance behaviors (preening, eating, drinking; P = 0.16, combined analysis), and the establishment of pair bonds (χ2 = 0.00, P > 0.99). Most subjects in both treatment groups formed a pair bond. Beak wipes, a high-frequency behavior associated with general arousal, were also not affected (P = 0.61).
Fig. 3.
Antisense knockdown of VIP production in the AH reduces aggression in male zebra finches housed in a colony environment. Data shown were collected after 1 d (30 h) of housing in a mixed-sex colony cage, when most males have paired and are defending nest sites. On the basis of behavior in prescreenings, males were housed in low-aggression (blue) or high-aggression (orange) colony cages. *P = 0.02, main effect of treatment; n = 6 per treatment.
Discussion
Although the AH has long been viewed as a major locus for agonistic behavior, the constituent cell groups that promote offensive aggression have remained largely unknown. Both electrical stimulation (1, 8) and Fos studies (present data) demonstrate that the dorsal AH plays an important role in avian agonistic behavior. We now show that VIP neurons in this area potently promote aggression that is focused on territory or nest defense. This effect is extraordinarily specific, as many other social and anxiety-like behaviors were not altered by VIP knockdown. In addition, because antisense-treated violet-eared waxbills rapidly classify intruders as offensive (based on agonistic response latencies), the present results also demonstrate that AH VIP neurons are not required for decisions about stimulus valence and significance, but rather that these neurons specifically generate a decision about aggressive response. We find that this basic process occurs not only in territorial finches, but in gregarious ones, as well. The present findings also suggest a functional heterogeneity of hypothalamic VIP populations, with VIP neurons of the AH and tuberal hypothalamus exhibiting opposing relationships to aggression, although effects in the tuberal hypothalamus are not strong. Similarly, recent studies in sparrows (Emberizidae) show that, whereas VIP immunolabeling in the AH and caudal septum is positively related to aggression, immunolabeling in the mediobasal hypothalamus is negatively related aggression (36).
A finding of particular interest is that knockdown of AH VIP has no effect on zebra finch aggression on the first day of testing in the colony cages (i.e., on the day of introduction to the cages), but severely impairs aggression on the second day. Previous findings likewise suggest that aggression in these contexts is modulated in distinct ways. For instance, chronic blockade of arginine vasotocin receptors in male zebra finches reduces aggression on the first day of testing in a new colony, when aggression is primarily focused on access to potential partners, but the direction of the effects reverses on subsequent days as birds form pairs and defend a nest site (35). Considered in the light of our present results, these observations suggest that mate competition and territorial behavior (including nest defense) are regulated very differently. Consistent with this idea are findings that intraseptal infusions of vasotocin and VIP exert differential effects when animals are tested in a territorial context versus the context of competition for mates (although VIP only weakly suppresses aggression in the latter context) (29, 37). Further support comes from correlations of midbrain Fos expression with behavior, which suggest that aggression in the context of mate competition may actually be much more similar to defensive behavior than to offensive territorial aggression (38). If this hypothesis is correct, then aggression in the context of mate competition should correlate positively with Fos expression in the ventral AH, as shown here for aggression received by subordinate waxbills. Indeed, this is the pattern that is observed in the medial portion of the midbrain nucleus intercollicularis (homolog of the dorsal periaqueductal gray). Dominant waxbills show a very different pattern of Fos expression, with Fos expression in the lateral portion of intercollicularis correlating positively with displacements (38).
Interestingly, whereas intraseptal VIP infusions decrease spontaneous agonistic singing during the “dawn chorus” in field sparrows (Spizella pusilla) housed in their natural habitat (39), they nonetheless increase territorial aggression when a resident is faced with an actual intruder. In the field sparrow, which is not an exceptionally aggressive species, this effect does not reach significance (despite strong trends) (39), but VIP infusions significantly decrease response latency and significantly increase aggression in male violet-eared waxbills (29). Thus, because agonistic interactions require that birds choose at any given moment between singing and attack, we hypothesize that AH VIP neurons are central to this decision, but experiments focused on the role of those neurons in agonistic singing are required to test this idea.
In conclusion, we here show that AH VIP neurons selectively promote offensive aggression in both territorial and gregarious species. This is observed in the contexts of territorial aggression and defense of nesting space, but is not observed in the context of competition for mates. Knockdown of AH VIP production has no effect on a wide range of other social and anxiety-like behaviors. These results may be relevant to mammals, as well, given that both rodents and birds exhibit VIP cell groups in many of the same brain areas, including the AH (22, 25), and intrathecal infusions of VIP potentiate aggression in male rats (40). Relevant data are not yet available for other mammalian species.
Methods
Methods for housing, surgery, antisense validation, immunocytochemistry, photomicroscopy, and cell counts follow standard laboratory protocols and are fully described in SI Methods, as are details of in situ hybridization. Experiments were conducted in a humane manner and were approved by the Institutional Animal Care and Use Committee of Indiana University.
Fos Experiment in Violet-Eared Waxbills (Behavior).
Pairs of violet-eared waxbills were screened for aggression in short resident–intruder assays (most less than 1 min; none more than 2 min) to select subordinate (intruder) and dominant (resident) subjects. At least 1 mo was allowed between screenings and testing. On the basis of behavior in these screenings, we were able to create groups of control, subordinate, and dominant subjects that did not differ in their levels of aggression when tested as residents during the screenings. Before testing and brain collection, we acclimated subjects to a sound isolation booth (3 h per day for 2 d), and on the third day left the subjects in the booth overnight. Resident–intruder tests were conducted the following morning (7 min). Because subordinates were handled twice (to introduce them to the resident's cage and to remove them), other subjects were likewise handled twice. Birds were perfused 90 min after the start of testing. Total n's for this experiment are 6 per group (for data shown in Fig. 1 B and C, power = 0.88 and 0.80, respectively).
Behavior Testing in Cannulated Violet-Eared Waxbills.
Before surgery, violet-eared waxbills were screened for aggression in short resident–intruder tests. In most cases, these screenings were only 30 s, with the outcome that the resident was clearly dominating the intruder. If the intruder dominated the subject, additional screenings were conducted with other intruders. Screenings were allowed to continue for 2 min in the event that little or no aggression was exhibited before that time. Subordinate stimulus animals identified through this process were subsequently used for all resident–intruder tests with a given subject (three total tests).
On the first day of testing, subjects were bilaterally infused with either saline vehicle or the VIP receptor (VPAC receptor) antagonist neurotensin6-11–mouse VIP7-28 (125 ng per side in 0.5 μL saline) into the lateral ventricles and were then run through a battery of behavioral tests. For all direct observations of behavior, experimenters observed subjects through a small window cut from a curtain “blind.” Experimenters were blind to subject condition.
Social contact and grouping.
After exploration tests (described below), subjects were placed in a 1-m-wide central cage (43 cm high, 36 cm deep) containing seven perches with two 46-cm cages on the sides, one containing a mixed-sex group of 10 Angolan blue waxbills and the other containing 2 waxbills. The location of the subject was recorded every 15 s for 3 min. The cage containing 10 stimulus animals was then removed and the test continued for an additional 3 min. This second phase of the test allowed us to determine whether time spent near 2 birds in the first 3 min might simply be a function of avoiding the group of 10. This test yields a measure of “social contact” (time spent on the side perches closest to the stimulus animals) and group size preference (the percent of contact time spent near the larger group in the first 3 min). This latter measure has been used as an assay of gregariousness in zebra finches and is sensitive to pharmacological manipulations (33, 34). Sides of stimulus presentation were counterbalanced across subjects.
Resident–intruder tests.
A same-sex stimulus bird (as selected in screening) was placed into the subject's housing cage with the room lights off. The lights were then turned on and behavior was quantified for 3 min. We recorded the latency to respond (by threat, agonistic call, or displacement) and the number of threats, agonistic calls, and displacements. Violet-eared waxbills of both sexes are extremely aggressive. To some extent, this is not as evident in the Fos study, because rates of aggression were somewhat muted in the sound isolation booths, but aggression in the home environment is often intense. Using cages that are larger than those used here, we previously found that once initiated, aggressive pursuits (displacements) are unrelenting up to 40 displacements (29), at which point we terminated tests for ethical reasons. In the present case, we were very concerned about the rate of pursuit and possible attack because of the smaller cage size. The smaller cage size provides greater control over “stimulus delivery,” but also renders the intruder more vulnerable. For this reason we terminated tests at 15 displacements and prorated behavior for the remaining test time. Our previous experience suggests that this provides a reasonably accurate measure of behavior (see supplementary material in ref 41). We further used an extremely short test duration (3 min) to reduce the need to prorate.
Two days later, subjects were again tested following infusions of saline or antagonist with the order of treatments counterbalanced across subjects. Before the tests described above, we also conducted two assays of anxiety-like behavior (34). Data from these tests were therefore analyzed in a between-subjects manner.
Novelty suppression of feeding.
Food was removed from the subjects' cages before lights on on test days to ensure that birds were hungry for the novelty suppression of feeding assay. Immediately following infusion, a party favor was placed in the subject's food dish and behavior was filmed for 45 min. Unlike zebra finches, violet-eared waxbills virtually never feed during this test, but approach the novel object tentatively and then retreat. We therefore used the latency to approach (within one body length) as our measure of response.
Exploration test.
After the feeding test, subjects were individually transferred to a large housing cage containing a variety of novel tree branches. This cage was visually isolated from the rest of the housing room and subjects were observed for 3 min. The number of hops and the number of branch changes were recorded. We then proceeded to conduct the social contact/grouping and resident–intruder tests. The order of these tests was dictated by the need to minimize carry-over effects, and thus resident–intruder tests were conducted last.
Antagonist testing was followed by a sequence of five infusions of antisense or scrambled oligonucleotides (1 μg in 0.25 μL isotonic saline) at 12-h intervals. The fifth infusions occurred in the evening, and the next morning we conducted a behavioral test battery following the sequence described above (novelty suppression of feeding, exploration, social contact and grouping, and aggression). For the assays of anxiety-like behavior, we used a different novel object (a purple nitrile glove hung above the food dish) and different branches in the exploration assay (deciduous vs. evergreen). This test battery was followed by a mixture infusion containing 3% colchicine and oligonucleotides. A final oligonucleotide infusion was delivered ∼12 h before killing.
The final subject numbers for results reported here are 12 females and 11 males. One female subject was intensely subjugated by the intruder in all trials, including the saline control trial (her first test), and we therefore exclude her from analyses of aggression. An additional female escaped on the final day of testing, while being transferred back to her home cage in the dark. This subject hit the wall and was stunned, and was therefore not tested (yielding final n's of 5 scrambled females, 4 scrambled males, 6 antisense females, and 6 antisense males for the last aggression test).
Behavior Testing in Cannulated Zebra Finches.
Following five oligonucleotide infusions as just described, we conducted a behavioral test battery in male zebra finches that was virtually identical to the one conducted in violet-eared waxbills. However, because zebra finches are not naturally aggressive outside of reproductive contexts such as competition for mates or nest defense, we did not conduct resident–intruder tests (but see below for colony tests). In addition, we added an assay of novel–familiar social preferences. This latter assay was conducted using the same test cage as the group size assay, but instead of a choice between 2 or 10 same-sex conspecifics, subjects were given a choice between five novel, previously unfamiliar birds and five familiar birds that had been housed in a cage adjacent to the subjects for 1 wk. Following this test battery, subjects were infused with a colchicine–oligonucleotide mixture and then an additional oligonucleotide infusion ∼12 h before killing. Final n's for these tests were 7 scrambled male controls and 8 antisense oligonucleotide male subjects.
For the second experiment, we prescreened male zebra finches after spending 1 d in a colony cage containing females, nest cups, and nesting material. Only subjects that displaced other birds at least three times in a 5-min period were retained as subjects (n = 16). Following the fifth oligonucleotide infusion, we introduced 4 subjects, 2 scrambled and 2 antisense) into cages containing 5 females, 4 nest cups, and nesting material. To give each subject an equal opportunity to be dominant, subjects were placed into low aggression cages containing male subjects that exhibited few displacements in screening (4.75 ± 0.45; mean ± SEM) or high aggression cages containing male subjects that exhibited many displacements in screening (16.63 ± 6.12). We conducted focal observations upon introduction (capturing the initial burst of courtship) ∼4 and 30 h later. These observations allow the quantification of directed courtship singing, aggressive behaviors (displacements, threats, pecks, beak fences), maintenance behaviors (preening, eating, drinking), and the establishment of pair bonds (35). Pair bonds are readily quantified on the basis of side-by-side perching, following, allopreening, and typically occupation of a nest cup. The first and last observations (at introduction and 30 h later) were each 5 min; the second was 2.5 min. Behavioral activity in newly established colonies is robust and readily quantified in sessions of this length. The 5-min duration also allowed us to conduct all 16 observations at a comparable time of day, which is important given diel variation in behavior. Four subjects were excluded due to major damage to the anterior commissure and the AH VIP cell group. These losses were balanced across cages and treatment groups, and thus final n's were 6 scrambled control and 6 antisense oligonucleotide subjects.
Supplementary Material
Acknowledgments
We thank Sara Schrock for assistance with histology and cell counts and Kristin Hoffbuhr for assistance with sequencing and in situ hybridization. Support was provided by National Institutes of Health Grant MH092331 (to J.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207995109/-/DCSupplemental.
References
- 1.Brown JL. Behavior elicited by electrical stimulation of the brain of the Stellar's Jay. Condor. 1973;75:1–16. [Google Scholar]
- 2.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 3.Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): Evoked feeding, aggressive and reproductive behavior with representative frontal sections. J Comp Neurol. 1971;143:1–16. doi: 10.1002/cne.901430102. [DOI] [PubMed] [Google Scholar]
- 4.Ferris CF, et al. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci USA. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- 7.Hrabovszky E, et al. Neurochemical characterization of hypothalamic neurons involved in attack behavior: Glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Phillips RE, Youngren OM. Brain stimulation and species-typical behaviour: Activities evoked by electrical stimulation of the brains of chickens (Gallus gallus) Anim Behav. 1971;19:757–779. doi: 10.1016/s0003-3472(71)80180-x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 10.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 11.Kruk MR, et al. The hypothalamus: Cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev. 1998;23:163–177. doi: 10.1016/s0149-7634(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 12.Hess WS, Brugger M. The subcortical center for affective defense reactions. Helv Physiol Pharmacol Acta. 1943;1:33–54. [Google Scholar]
- 13.Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: Segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Berk ML, Butler AB. Efferent projections of the medial preoptic nucleus and medial hypothalamus in the pigeon. J Comp Neurol. 1981;203:379–399. doi: 10.1002/cne.902030305. [DOI] [PubMed] [Google Scholar]
- 16.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canteras NS. The medial hypothalamic defensive system: Hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 20.Motta SC, et al. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci USA. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonopoulos J, et al. VIP- and CCK-like-immunoreactive neurons in the hedgehog (Erinaceus europaeus) and sheep (Ovis aries) brain. J Comp Neurol. 1987;263:290–307. doi: 10.1002/cne.902630211. [DOI] [PubMed] [Google Scholar]
- 23.Bottjer SW, Alexander G. Localization of met-enkephalin and vasoactive intestinal polypeptide in the brains of male zebra finches. Brain Behav Evol. 1995;45:153–177. doi: 10.1159/000113547. [DOI] [PubMed] [Google Scholar]
- 24.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuenzel WJ, Mccune SK, Talbot RT, Sharp PJ, Hill JM. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus) J Comp Neurol. 1997;381:101–118. doi: 10.1002/(sici)1096-9861(19970428)381:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Kennett JE, Poletini MO, Freeman ME. Vasoactive intestinal polypeptide modulates the estradiol-induced prolactin surge by entraining oxytocin neuronal activity. Brain Res. 2008;1196:65–73. doi: 10.1016/j.brainres.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.vanderBeek EM, Swarts HJM, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology. 1999;69:227–237. doi: 10.1159/000054423. [DOI] [PubMed] [Google Scholar]
- 28.Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- 29.Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin D. Estrildid Finches of the World. Ithaca, NY: Cornell Univ Press; 1982. [Google Scholar]
- 31.Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 32.Nowak JZ, Sedkowska P, Zawilska JB, Gozes I, Brenneman DE. Antagonism of VIP-stimulated cyclic AMP formation in chick brain. J Mol Neurosci. 2003;20:163–172. doi: 10.1385/JMN:20:2:163. [DOI] [PubMed] [Google Scholar]
- 33.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AM, et al. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodson JL, Wilson LC, Schrock SE. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10685–10692. doi: 10.1073/pnas.1203394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 38.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 40.Beyer C, Caba M, Banas C, Komisaruk BR. Vasoactive intestinal polypeptide (VIP) potentiates the behavioral effect of substance P intrathecal administration. Pharmacol Biochem Behav. 1991;39:695–698. doi: 10.1016/0091-3057(91)90149-v. [DOI] [PubMed] [Google Scholar]
- 41.Goodson JL, Kabelik D, Schrock SE. Dynamic neuromodulation of aggression by vasotocin: Influence of social context and social phenotype in territorial songbirds. Biol Lett. 2009;5:554–556. doi: 10.1098/rsbl.2009.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.