Abstract
Nasal colonization by Staphylococcus aureus is the major risk factor for disease and transmission. Epidemiological studies have reported a reduced risk of S. aureus carriage in immunocompetent but not in immunocompromised children colonized by Streptococcus pneumoniae. We investigate the hypothesis that the immune response to pneumococcal colonization affects S. aureus colonization. We demonstrate that pneumococcal colonization in mice inhibits subsequent S. aureus acquisition in an antibody-dependent manner and elicits antibody that cross-reacts with S. aureus. We identify the staphylococcal target of cross-reactive antibody as 1-pyrroline-5-carboxylate dehydrogenase (P5CDH), and the homologous immunogen in S. pneumoniae as SP_1119, both of which are conserved dehydrogenases. These antigens are necessary and sufficient to inhibit the acquisition of S. aureus colonization in a mouse model. Our findings demonstrate that immune-mediated cross-reactivity between S. pneumoniae and S. aureus protects against S. aureus nasal acquisition and thus reveal a paradigm for identifying protective antigens against S. aureus.
Keywords: pneumococcus, methicillin-resistant S. aureus, vaccine
The Gram-positive bacterial pathogen Staphylococcus aureus is responsible for significant morbidity, mortality, and excess healthcare costs worldwide. The management of S. aureus disease has become increasingly difficult because of the rising prevalence of methicillin-resistant S. aureus (MRSA), which can account for 60% of S. aureus infections in hospital and community settings (1, 2). Given the limited treatment options for MRSA infection, novel preventative approaches are needed to protect against S. aureus infection and transmission.
A predominant risk factor for S. aureus infection and transmission is asymptomatic colonization of the anterior nares (3). Eighty percent of S. aureus invasive infections in humans are caused by the host’s colonizing strain (4). However, the specific host and bacterial determinants of S. aureus nasal carriage are not well understood (5). In children, significantly reduced S. aureus colonization rates have been associated with carriage of another member of the upper respiratory tract flora, Streptococcus pneumoniae (6–14). These large and geographically diverse cohorts have demonstrated reproducibly that colonization with S. pneumoniae reduces the risk of S. aureus carriage by approximately half. This interference phenomenon has been reported for both vaccine and nonvaccine serotypes of S. pneumoniae (13). Moreover, pneumococcal vaccination, which reduces S. pneumoniae carriage, has been associated with an increased incidence of S. aureus-induced otitis media in children (15).
The etiology of this interference phenomenon between S. pneumoniae and S. aureus colonization is unknown. Although in vitro studies have demonstrated that hydrogen peroxide secreted by S. pneumoniae is bactericidal to S. aureus in coculture (16–18), neither hydrogen peroxide secretion by S. pneumoniae nor hydrogen peroxide sensitivity of S. aureus is predictive of cocolonization patterns in vivo (19–21). Moreover, any direct competitive effect in vivo is unlikely, because S. aureus is found primarily in the anterior nares (5), whereas S. pneumoniae colonizes the nasopharynx (22). Instead, we and others (21) have hypothesized that an immunological mechanism may be involved, because the antagonistic effect of pneumococcal colonization on S. aureus carriage is observed in HIV-negative but not immunocompromised HIV-positive individuals within the same cohort (8, 9, 23). To date, the only study that has addressed the role of the immune system measured antibody titers to 17 predetermined pneumococcal proteins and found no correlation with S. aureus carriage in 57 infants (24). Therefore, a comprehensive examination of this hypothesis without preselection of candidate antigens has not yet been performed.
Here we investigate whether the host immune response to S. pneumoniae carriage can influence S. aureus colonization in vivo. We demonstrate that antibodies elicited during pneumococcal colonization in a mouse model cross-react with S. aureus, leading to a reduction in S. aureus nasal colonization. We identify the staphylococcal target of cross-reactive antibody and the homologous immunogen in S. pneumoniae and confirm that these antigens are necessary and sufficient to limit the acquisition of S. aureus nasal colonization in vivo.
Results
Pneumococcal Colonization in Mice Reduces Subsequent S. aureus Carriage in an Antibody-Dependent Manner.
To recapitulate the observed interference between S. pneumoniae and S. aureus colonization, we developed a mouse model of S. aureus nasal colonization using strain 502A, a clinical isolate known for superior nasal colonization in humans (25). Unlike previously described models of S. aureus nasal carriage, which are highly variable, nasal colonization by 502A is established reproducibly in naive C57BL/6 mice with higher and less variable densities than seen with other strains (Fig. S1 A and B). 502A colonization was achieved with a dose of 105 cfu, but levels were highest and most reproducible at day 1 postinoculation with a dose of 108 cfu (Fig. S1 C and D). For all further experiments, we chose to use these latter conditions, which reproducibly model the first step in colonization—nasal acquisition—but do not model the long-term human carrier state. Therefore, our studies with this model focus on the initial establishment of S. aureus nasal colonization rather than on persistent carriage. Under these conditions, the levels of S. aureus detected in our model are comparable to those recovered from experimentally colonized humans (26).
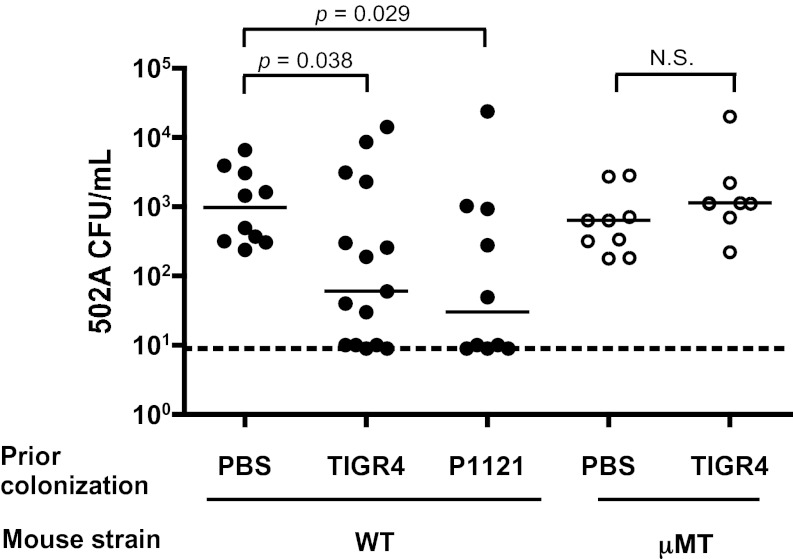
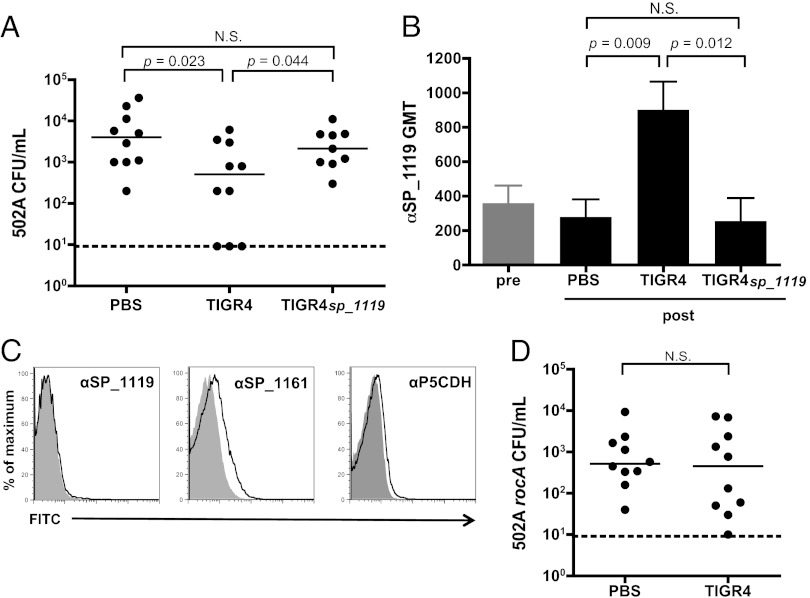
We next combined our 502A acquisition model with an established murine model of pneumococcal nasopharyngeal colonization that has colonization dynamics and immune responses similar to those observed in humans, including a robust antibody response to a diversity of pneumococcal antigens (27, 28). After colonizing mice with S. pneumoniae and allowing 5 wk for complete pneumococcal clearance, we challenged mice intranasally with S. aureus 502A. Compared with mock-colonized (PBS) controls, mice previously colonized with S. pneumoniae TIGR4 had significantly reduced levels of S. aureus 502A carriage (Fig. 1, closed circles), similar to observations made in children. The protective effect of prior pneumococcal colonization was not dependent on pneumococcal strain or serotype, because similar reductions in 502A colonization were seen following prior colonization with S. pneumoniae P1121 (Fig. 1, closed circles).
Fig. 1.
Pneumococcal colonization in mice reduces subsequent S. aureus carriage in an antibody-dependent manner. Colonization density of S. aureus 502A in C57BL/6 wild-type (closed circles) or antibody-deficient μMT mice (open circles) 5 wk after prior colonization with S. pneumoniae TIGR4, S. pneumoniae P1121, or sham (PBS) colonization. 502A carriage levels were assessed in lavages of the upper respiratory tract at day 1 postchallenge. Horizontal solid lines indicate median values; dotted line indicates limit of detection, N.S., not significant.
Because the effect of prior pneumococcal colonization was observed at a time when pneumococci no longer can be detected in the nasopharynx, we hypothesized that the reduction in S. aureus levels might be caused by the presence of anti-pneumococcal antibody. To test this hypothesis, we repeated the dual-species colonization experiment in antibody-deficient μMT mice and found no significant difference in 502A colonization levels between mice colonized previously with S. pneumoniae and mock colonized controls (Fig. 1, open circles). These data suggest that antibody is necessary for the protective effect of pneumococcal colonization on S. aureus colonization.
Pneumococcal Colonization Elicits Antibody That Cross-Reacts with S. aureus.
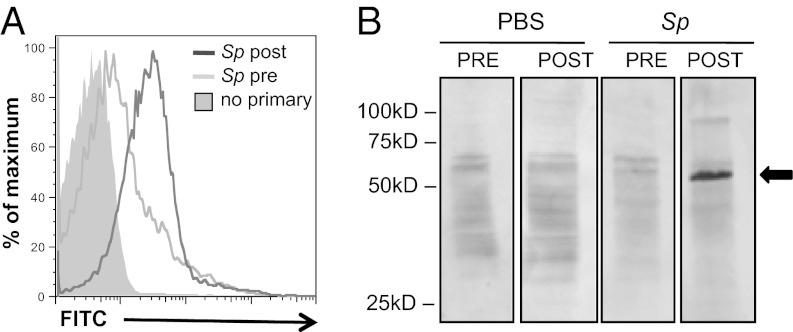
We next investigated whether the antibody response elicited by pneumococcal colonization was capable of recognizing S. aureus. Mice colonized with S. pneumoniae developed significantly increased levels of IgG that bound to the surface of live S. aureus, compared with levels in precolonization sera (P = 0.043 for five mice) (Fig. 2A). In Western blots of staphylococcal whole-cell lysates, sera from mice postcolonization with S. pneumoniae cross-reacted with a single prominent band of about 55 kD (Fig. 2B, Right two panels, arrow). In contrast, blots using sera after mock colonization with PBS resembled background levels of sera before colonization (Fig. 2B, Left two panels). After further separation by 2D gel electrophoresis and Western blot, the staphylococcal target of antibody induced by pneumococcal colonization was isolated for mass spectrometric analysis. Only two proteins, dihydrolipoamide dehydrogenase (DLDH, YP_499592) and 1-pyrroline-5-carboxylate dehydrogenase (P5CDH, YP_501325), were present at this position in equal abundance as determined by empirical protein-abundance index scores. For each staphylococcal protein, one closely homologous protein was identified in the S. pneumoniae TIGR4 genome by tBLASTn. The homologous loci in the TIGR4 genome are sp_1161 (E value = 1e−56) and sp_1119 (E value = 6e−68), respectively, and both encode putative but uncharacterized dehydrogenases which we refer to hereafter as “SP_1161” and “SP_1119.”
Fig. 2.
Pneumococcal colonization elicits serum antibody that cross-reacts with S. aureus. Sera were collected from C57BL/6 mice before (pre) and after (post) intranasal colonization with S. pneumoniae TIGR4. (A) Representative histogram of flow cytometric detection of antibody binding to S. aureus 8325–4spa incubated with mouse sera taken before (light gray line) and after (dark gray line) pneumococcal colonization. Control (no primary antibody) is shaded gray. (B) Western blot of S. aureus 8325–4spa lysates incubated with mouse sera taken before (PRE) and after (POST) pneumococcal (Sp) or sham (PBS) colonization. Data are representative of >10 biological replicates. Arrow indicates candidate target of cross-reactive antibody.
Candidate Antigen Is SP_1119 in S. pneumoniae and Its S. aureus Homolog, P5CDH.
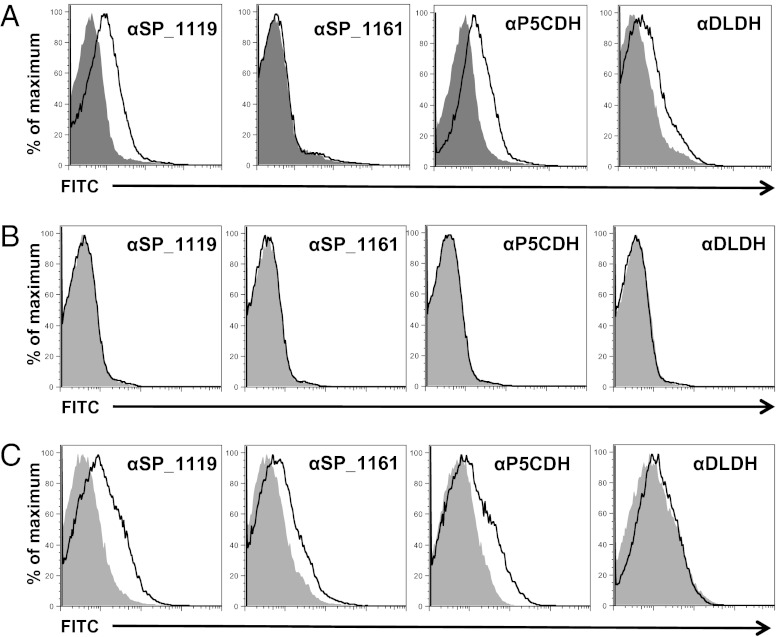
Each candidate antigen was cloned, recombinantly expressed, purified, and used to generate specific antisera. IgG to P5CDH and DLDH bound to the surface of live S. aureus, indicating that these proteins are antibody accessible (Fig. 3A). In contrast, incubation of S. pneumoniae TIGR4 with antisera specific to the pneumococcal proteins did not result in surface IgG binding (Fig. 3B). However, elimination of the antiopsonic capsular polysaccharide in TIGR4cps facilitated surface binding by anti-SP_1119 and anti-SP_1161 IgG, suggesting that these antigens are surface associated but masked by capsule (Fig. 3C).
Fig. 3.
Antisera to P5CDH and SP_1119, but not to DLDH and SP_1161, cross-react with the heterologous species. Flow cytometric detection of IgG binding to the surface of S. aureus 8325–4spa (A), S. pneumoniae TIGR4 (B), and S. pneumoniae TIGR4cps (C) after incubation with rabbit antisera raised to purified recombinant P5CDH, DLDH, SP_1119, or SP_1161 as indicated. Gray shaded area, preimmune rabbit sera; black line, immune rabbit sera.
We investigated whether antibodies raised against each candidate protein could cross-react with the heterologous species. When S. aureus was incubated with antisera to the pneumococcal proteins, we observed cross-reactive binding with anti-SP_1119 but not with anti-SP_1161 IgG (Fig. 3A). Similarly, antisera to the staphylococcal homolog of SP_1119, P5CDH, bound to the surface of unencapsulated S. pneumoniae, but antisera to DLDH did not (Fig. 3C). Together, these data suggest that antisera to the homologous pair P5CDH and SP_1119, but not to DLDH and SP_1161, cross-react with the surface of the heterologous species.
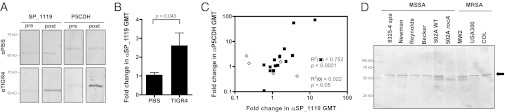
For SP_1119 to induce cross-reactive antibody in vivo, it must be immunogenic during pneumococcal colonization. We investigated whether pneumococcal colonization in mice elicited antibodies to SP_1119 by Western blot and ELISA. By Western blot we observed an increase in antibody binding to both SP_1119 and P5CDH in sera of mice after pneumococcal colonization as compared with sera from mice before pneumococcal colonization (Fig. 4A). No increase in binding was observed in mock-colonized animals (Fig. 4A). Similarly, by ELISA, mice colonized with TIGR4 had significantly elevated IgG titers to SP_1119, whereas mock-colonized control mice did not (Fig. 4B). There was a significant intraindividual correlation between elevated IgG tiers to SP_1119 and P5CDH, indicating that animals with a robust response to SP_1119 mounted commensurate responses to P5CDH (Fig. 4C, solid squares).
Fig. 4.
SP_1119 is immunogenic during S. pneumoniae colonization. (A) Western blot of purified recombinant SP_1119 or P5CDH incubated with mouse sera before (pre) and after (after) pneumococcal (TIGR4) or sham (PBS) colonization. (B) Quantification by ELISA of the increase in serum IgG titers to SP_1119 after pneumococcal (TIGR4) or sham (PBS) colonization. n = 10 mice per group. (C) Correlation between fold increase in serum IgG titers to SP_1119 and P5CDH in mice colonized with S. pneumoniae TIGR4 (closed squares) or S. pneumoniae TIGR4sp_1119 (open diamonds). (D) Detection of P5CDH (arrow) by specific anti-P5CDH sera in a Western blot of whole-cell lysates of the S. aureus strains indicated. MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus.
Because the clinical negative association between pneumococcal and S. aureus colonization appears to be independent of S. aureus strain, we reasoned that any target of cross-reactive antibody must be well conserved. In all publically available whole S. aureus genomes (n > 12), the amino acid sequence for P5CDH is at least 98% identical. We confirmed this widespread conservation by Western blot using a selection of methicillin-sensitive and methicillin-resistant S. aureus strains including the epidemic clinical isolate USA300. P5CDH was detected in all the strains tested but not in the unmarked, in-frame P5CDH deletion mutant (502ArocA), which was used as a negative control (Fig. 4D). Similarly, SP_1119 is broadly conserved across pneumococci with at least 99% amino acid identity in all the publically available whole S. pneumoniae genomes (n > 35).
SP_1119 and P5CDH Are Necessary to Reduce S. aureus Carriage in a Mouse Model.
We deleted the locus sp_1119 from S. pneumoniae TIGR4 to assess whether SP_1119 is necessary for the protective effect of pneumococcal colonization on subsequent S. aureus carriage. Although mice previously colonized with wild-type TIGR4 had significantly reduced levels of 502A carriage, mice previously colonized with TIGR4sp_1119 did not differ from mock (PBS)-colonized controls in 502A colonization density (Fig. 5A). Colonization with both the wild-type and mutant resulted in significant increases in antibody titers to whole pneumococci compared with PBS controls (Fig. S2), indicating that the lack of protection against 502A was not caused by an overall deficiency in the antibody response to the mutant. We confirmed by ELISA that animals colonized with TIGR4sp_1119 did not mount antibodies to SP_1119, and animals colonized with wild-type TIGR4 had significantly higher anti-SP_1119 titers than those seen in PBS-inoculated controls (Fig. 5B). Following colonization with TIGR4sp_1119, cross-reactive titers to P5CDH were not significantly higher than those in PBS-inoculated controls and no longer correlated with intraindividual titers to SP_1119 (Fig. 4C, open symbols). The requirement of SP_1119 for cross-reactivity was supported by flow cytometry using a TIGR4sp_1119cps double mutant, demonstrating that deletion of SP_1119 abrogates binding by P5CDH antisera (Fig. 5C). Similarly, the protective effect of previous colonization with wild-type S. pneumoniae TIGR4 was lost when animals were challenged with 502ArocA, which lacks P5CDH (Fig. 5D). These results provide evidence that cross-protection against S. aureus by S. pneumoniae requires SP_1119 as an immunogen and P5CDH as a target.
Fig. 5.
Deletion of SP_1119 or P5CDH from whole bacteria abrogates protective effect of prior pneumococcal colonization on S. aureus 502A carriage. (A) Colonization density of S. aureus 502A in C57BL/6 wild-type mice 5 wk after prior colonization with S. pneumoniae TIGR4, S. pneumoniae TIGR4sp_1119 or sham treatment (PBS). 502A carriage levels were assessed in upper respiratory tract lavages at day 1 postchallenge. Horizontal lines indicate median values. N.S., not significant. (B) Detection of SP_1119-specific IgG titers in mouse sera before (pre, gray bar) and after (post, black bars) colonization with S. pneumoniae TIGR4, S. pneumoniae TIGR4sp_1119 or sham treatment (PBS). (C) Flow cytometric detection of antibody binding to the surface of S. pneumoniae TIGR4sp_1119cps following incubation with rabbit antisera specific to SP_1119, SP_1161, and P5CDH as indicated. Gray shaded area, preimmune rabbit sera; black line, immune rabbit sera. (D) Colonization density of S. aureus 502ArocA in C57BL/6 mice 5 wk after prior colonization with S. pneumoniae TIGR4 or sham (PBS) inoculation. 502ArocA carriage levels were assessed in lavages of the upper respiratory tract at day 1 postchallenge. Horizontal solid lines indicate median values; dotted line indicates limit of detection. N.S., not significant.
Intranasal Immunization with SP_1119 or P5CDH Is Sufficient to Reduce S. aureus Colonization Levels.
Because SP_1119 and P5CDH were necessary for the protective effect of pneumococcal colonization on the acquisition of S. aureus carriage, we investigated whether immunization with these antigens alone was sufficient to recapitulate this effect. Mice were immunized intranasally with either adjuvant alone or in combination with purified recombinant SP_1119, P5CDH, or DLDH as a control protein. Mice immunized with SP_1119 had significantly lower levels of 502A colonization than those seen in controls administered adjuvant alone (Fig. 6A). Immunization with P5CDH resulted in a similar reduction in 502A colonization, but immunization with the control protein DLDH did not (Fig. 6A). As predicted, P5CDH or SP_1119 had no protective effect after challenge with 502ArocA, which lacks P5CDH (Fig. 6B). Complementation of the rocA deletion (using strain 502ArocA::pCL55-rocA+) restored expression of P5CDH (Fig. S3) and the protective effect of prior immunization with SP_1119 and P5CDH (Fig. 6C). Together, these data suggest that SP_1119 and P5CDH are necessary for the pneumococcal effect on S. aureus nasal carriage and are sufficient as mucosal immunogens to inhibit the acquisition of S. aureus 502A nasal carriage.
Fig. 6.
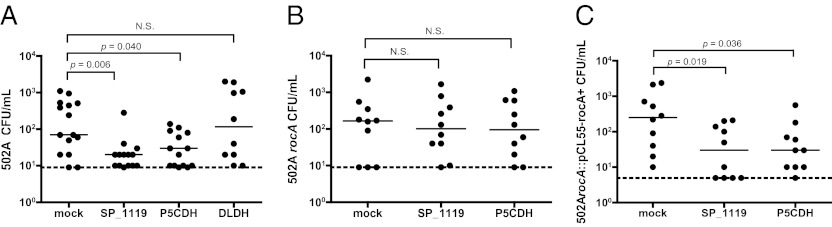
Reduction in density of S. aureus 502A colonization following intranasal immunization with purified SP_1119 and P5CDH. Colonization density of S. aureus 502A in C57BL/6 mice following intranasal immunization with cholera toxin alone (mock) or in combination with recombinant antigen, SP_1119, P5CDH, or DLDH. Carriage levels of 502A (A), 502ArocA (B), or 502ArocA::pCL55-rocA+ (C) were assessed in upper respiratory tract lavages at day 1 postchallenge. N.S., not significant.
Discussion
The concept of interspecies immune-mediated cross-reactivity is as old as vaccinology itself. Indeed, the first vaccine was based on Jenner’s observation of immune-mediated cross-reactivity between cowpox and smallpox. This seminal discovery was made by first identifying a naturally protected subset of the population. In that vein, we sought to investigate a subset of the population—healthy children colonized with S. pneumoniae—that was observed to be at reduced risk for S. aureus nasal carriage. This interspecies interference is one of the few epidemiological examples of protection against S. aureus acquisition, especially because exposure to S. aureus is not protective against future S. aureus carriage or infection in humans. Interspecies cross-reactive antibody is an important factor in natural immunity to other bacterial pathogens of the upper respiratory tract. For example, cross-reactivity between the capsular polysaccharides of certain enteric commensal Escherichia coli and Haemophilus influenzae type b has been implicated in the development of age-related natural immunity against this pathogen (29). Our study establishes that antibodies elicited in response to a specific protein during pneumococcal colonization cross-react with and inhibit S. aureus in vivo and thereby demonstrates the use of interspecies cross-reactivity to identify protective antigens.
Our findings implicate the antibody response to a homologous pair of putative dehydrogenases, P5CDH and SP_1119, in mediating cross-protection against S. aureus. SP_1119 elicits antibody to which the pneumococcus is resistant, whereas P5CDH may have limited immunogenicity during S. aureus colonization but still can be targeted by preexisting cross-reactive antibody. In humans, experimental colonization with S. aureus does not elicit antibody to P5CDH (30), although some antibody can be detected after invasive infection (31), indicating P5CDH is expressed in vivo. In addition to the identification of SP_1119 by in silico analysis, three lines of experimental evidence support the specific role of SP_1119 in inducing cross-protection: (i) antisera raised to SP_1119 cross-reacts with the surface of S. aureus in vitro; (ii) loss of SP_1119 in S. pneumoniae abolishes the protective effect of prior pneumococcal carriage on S. aureus colonization; and (iii) immunization with purified SP_1119 inhibits the establishment of S. aureus nasal carriage. The fact that SP_1119, like other protein antigens of S. pneumoniae, can be hidden from antibody by the antiopsonic capsular polysaccharide may explain the directional negative effect of pneumococcal colonization on S. aureus colonization and not vice versa. Preliminary data suggest that SP_1119 is immunogenic during childhood colonization with S. pneumoniae, and future studies will address whether these elevated antibody titers in childhood correlate with a reduced risk of nasal carriage of S. aureus.
SP_1119 shares extensive overall homology with P5CDH as well as a functional classification in the aldehyde dehydrogenase superfamily (32). Both proteins are highly conserved and can be detected on the bacterial surface, adding to the growing list of anchorless surface-exposed enzymes in Gram-positive bacteria (33). We predict that cross-reactivity between these two proteins is mediated by a region(s) of conformational similarity on a surface-exposed domain(s), given the lack of an identical stretch of amino acids indicative of a common linear epitope (Fig. S4). Further investigation will be needed to define the precise region(s) responsible for inducing cross-reactivity. It would be beneficial for future studies to identify the minimal epitope(s) required for protection to minimize any undesired impact on other members of the flora or cross-reactivity with human proteins. The biological function of the proteins SP_1119 and P5CDH has not been characterized in the context of S. pneumoniae or S. aureus, respectively, and our data indicate that these proteins are not essential during in vitro growth or murine colonization. Whether these proteins affect fitness during human nasal carriage remains to be tested. However, there appears to be selective pressure for these proteins to be maintained in vivo, given their extensive conservation among genome-sequenced strains. This conservation could account for the strain-independent interference between these two species observed in children (20).
Our study required a small animal model of S. pneumoniae and S. aureus nasal colonization to evaluate our hypothesis in vivo. However, models of S. aureus carriage have been limited by a lack of S. aureus strains capable of establishing reproducible colonization. S. aureus 502A was used throughout the 1960s to colonize adults with furunculosis and healthy newborns deliberately to prevent acquisition of other, more virulent S. aureus strains during nosocomial outbreaks (25). We reasoned that 502A might be more proficient than other S. aureus strains at establishing colonization in mice, as appeared to be the case in humans. Indeed, the reproducibility of S. aureus 502A nasal acquisition in mice at day 1 postinoculation enabled the current study of S. aureus colonization and may be a useful tool for studying other host and bacterial determinants of the acquisition of S. aureus nasal carriage. Because the protective effects of our antigens were observed during the establishment of carriage, we did not test them in other animal models where disease is created artificially by circumventing the carrier state.
For many bacterial pathogens of the upper respiratory tract, antibody functions to prevent the natural acquisition of carriage (34). In humans, pneumococcal conjugate vaccine is known to induce antigen-specific serum IgG, which is transported by transcytosis across epithelial barriers where it can be detected on the mucosa and is correlated with protection from the acquisition of colonization (35). However, the role of antibody in protection against S. aureus has been questioned, because S. aureus expresses protein A (Spa) which binds Ig nonspecifically. A Spa mutant often is used in vitro, especially whenever secondary antibody-detection methods are used. It has been assumed that the effect(s) of antibody in vivo would be negated similarly by Spa, but antibody-mediated protection has been demonstrated against nasal colonization with Spa-sufficient strains (31, 36). Passive i.p. immunization with a monoclonal antibody against clumping factor B resulted in reduced nasal carriage of S. aureus in mice (36), indicating that systemic antibody can protect against S. aureus colonization regardless of Spa. Our study provides another example of antibody-dependent inhibition of nasal carriage of a Spa-sufficient strain, suggesting that the immune-evasive effect ascribed to Spa may be of limited importance during colonization.
Much of the public health benefit of vaccines that target mucosal pathogens of the upper respiratory tract—including S. pneumoniae, Neisseria meningitidis, and H. influenzae type b—is the result of herd protection based on the inhibition of carriage in children and thus reduced transmission to unvaccinated members of the population (37). Clinical studies have demonstrated repeatedly that even modest (e.g., 50%) reductions in pathogen carriage following vaccination significantly reduce the risk of transmission, so that full protection (≥90%) from invasive disease is afforded to both vaccinated and unvaccinated individuals (37). Indeed, it has been calculated that pneumococcal conjugate vaccine prevented many more cases of invasive pneumococcal disease in unvaccinated individuals than in vaccinated children (37). These findings illustrate how nonsterilizing decreases in pathogen colonization can have vast ramifications on disease incidence and population-wide protection. In our mouse model, we observed a relative reduction in S. aureus carriage and hypothesize that, if similar reductions in carriage were observed in humans, significant morbidity and mortality caused by S. aureus invasive disease could be prevented by herd immunity. Moreover, the success of our current pediatric conjugate vaccines reveals the importance of childhood colonization as a reservoir for bacterial pathogens within the population and thus the importance of designing immunizations that inhibit carriage in children. We posit that a successful vaccine against S. aureus may benefit from the inclusion of antigens directed at reducing the acquisition of nasal carriage, such as SP_1119 and P5CDH. Future studies will be needed to address whether these antigens can protect against S. aureus in humans.
Materials and Methods
Bacterial Strains and Mutants.
S. pneumoniae was grown in tryptic soy (TS) broth at 37 °C in a nonshaking water bath. TIGR4 (a serotype 4 clinical isolate and genome-sequenced strain) and P1121 (a serotype 23F clinical isolate) were used because they colonize the murine nasopharynx efficiently (28). A TIGR4 mutant lacking sp_1119 was constructed using overlap extension PCR (see SI Materials and Methods for details). S. aureus was grown in TS or brain-heart infusion broth at 37 °C with shaking (strains and sources are identified in SI Materials and Methods). An unmarked, in-frame deletion mutant of rocA, which encodes P5CDH, was constructed in strain 502A using pKOR1-rocA and was complemented using pCL55 (see SI Materials and Methods for details).
Mouse Model of Nasopharyngeal Colonization and Challenge.
The murine model of pneumococcal nasopharyngeal colonization has been described previously (28) and is described in full in SI Materials and Methods. Mice received an intranasal dose of 107 cfu of S. pneumoniae at weeks 0 and 2 and were challenged at week 7, at which time no pneumococci remained in the nasopharynx (28). Control animals were subjected to the same protocol but were mock-colonized with PBS. Intranasal challenge of S. aureus consisted of 108 cfu. Colonization densities from nasal lavages were quantified on BBL CHROMagar Staph aureus (BD Diagnostics) 24 h postchallenge.
Identification of Candidate Antigens.
The targets of cross-reactive pneumococcal antibody were identified by Western blot analysis and mass spectrometry. See SI Materials and Methods for further details.
Measurement of Serum Antibody Binding.
Binding of total serum IgG to whole bacteria was detected by flow cytometry using a FITC-conjugated anti-mouse IgG secondary antibody. Antigen-specific serum IgG titers were quantified by ELISA. Both methods are detailed in SI Materials and Methods.
Recombinant Antigen Purification and Generation of Specific Antisera.
The coding sequences for each of the four candidate antigens were amplified from the appropriate chromosomal DNA using primers listed in SI Materials and Methods. Amplicons were ligated into pET29b (Novagen) for expression of recombinant antigens in E. coli BL21(DE3) and purification under native conditions. As appropriate, his-tags were removed by a thrombin cleavage capture system (Novagen) and dialysis. Polyclonal rabbit sera to each purified recombinant antigen were prepared commercially.
Immunization with Purified Antigens.
As previously described (38), mice were immunized intranasally with 4 μg of recombinant protein and 1 μg cholera toxin as adjuvant (List Biological Laboratories) per 20-μL dose. Control mice received adjuvant alone. Three immunizations were given at weekly intervals, followed by intranasal S. aureus challenge at week 5, as described above.
Statistical Analysis.
Colonization density was expressed as the log10 cfu/mL and analyzed for statistical significance using the Mann–Whitney U test. Paired t tests were used to compare pre- vs. posttreatment groups, and linear regressions were used to assess correlations. All other comparisons were made using the unpaired t test, as appropriate. A P value of less than 0.05 was considered significant. Statistical analyses were performed using Prism 4 (GraphPad).
Supplementary Material
Acknowledgments
This work was supported by a Commonwealth Universal Research Enhancement Grant from the Pennsylvania Department of Health and by US Public Health Service Grant AI-055400.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208075109/-/DCSupplemental.
References
- 1.Klevens RM, et al. National Nosocomial Infections Surveillance System Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis. 2006;42:389–391. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005;11:928–930. doi: 10.3201/eid1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim HF, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Study Group Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 5.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert D, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 7.Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect Dis. 2011;11:175–183. doi: 10.1186/1471-2334-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhi SA, et al. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-Infected and HIV-uninfected children. J Infect Dis. 2007;196:1662–1666. doi: 10.1086/522164. [DOI] [PubMed] [Google Scholar]
- 9.McNally LM, et al. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J Infect Dis. 2006;194:385–390. doi: 10.1086/505076. [DOI] [PubMed] [Google Scholar]
- 10.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regev-Yochay G, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA. 2004;292:716–720. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 12.Regev-Yochay G, et al. Maccabi Implementing Judicious Antibiotic Prescription Study Group Parental Staphylococcus aureus carriage is associated with staphylococcal carriage in young children. Pediatr Infect Dis J. 2009;28:960–965. doi: 10.1097/INF.0b013e3181a90883. [DOI] [PubMed] [Google Scholar]
- 13.van Gils EJ, et al. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS ONE. 2011;6:e20229. doi: 10.1371/journal.pone.0020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson K, et al. Kalgoorlie Otitis Media Research Project Team Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr Infect Dis J. 2006;25:782–790. doi: 10.1097/01.inf.0000232705.49634.68. [DOI] [PubMed] [Google Scholar]
- 15.Veenhoven R, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: A randomised study. Lancet. 2003;361:2189–2195. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 16.Park B, Nizet V, Liu GY. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J Bacteriol. 2008;190:2275–2278. doi: 10.1128/JB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selva L, et al. Killing niche competitors by remote-control bacteriophage induction. Proc Natl Acad Sci USA. 2009;106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margolis E. Hydrogen peroxide-mediated interference competition by Streptococcus pneumoniae has no significant effect on Staphylococcus aureus nasal colonization of neonatal rats. J Bacteriol. 2009;191:571–575. doi: 10.1128/JB.00950-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melles DC, et al. Nasopharyngeal co-colonization with Staphylococcus aureus and Streptococcus pneumoniae in children is bacterial genotype independent. Microbiology. 2007;153:686–692. doi: 10.1099/mic.0.2006/002279-0. [DOI] [PubMed] [Google Scholar]
- 21.Regev-Yochay G, et al. In vitro bactericidal activity of Streptococcus pneumoniae and bactericidal susceptibility of Staphylococcus aureus strains isolated from cocolonized versus noncocolonized children. J Clin Microbiol. 2008;46:747–749. doi: 10.1128/JCM.01781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. In: The Pneumococcus. Tuomanen E, editor. Washington, DC: ASM; 2004. pp. 136–147. [Google Scholar]
- 23.Bogaert D, Nouwen J, Hermans PW, Belkum A. Lack of Interference between Streptococcus pneumoniae and Staphylococcus aureus in HIV-infected individuals? J Infect Dis. 2006;194:1617–1618, author reply 1618–1619. doi: 10.1086/508886. [DOI] [PubMed] [Google Scholar]
- 24.Lebon A, et al. The inverse correlation between Staphylococcus aureus and Streptococcus pneumoniae colonization in infants is not explained by differences in serum antibody levels in the Generation R Study. Clin Vaccine Immunol. 2011;18:180–183. doi: 10.1128/CVI.00357-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Light IJ, Walton RL, Sutherland JM, Shinefield HR, Brackvogel V. Use of bacterial interference to control a staphylococcal nursery outbreak. Deliberate colonization of all infants with the 502A strain of Staphylococcus aureus. Am J Dis Child. 1967;113:291–300. doi: 10.1001/archpedi.1967.02090180051001. [DOI] [PubMed] [Google Scholar]
- 26.Iwase T, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 27.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roche AM, King SJ, Weiser JN. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun. 2007;75:2469–2475. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneerson R, Robbins JB. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975;292:1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- 30.Holtfreter S, et al. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol. 2009;16:1607–1614. doi: 10.1128/CVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke SR, et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 32.Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: The 2002 update. Chem Biol Interact. 2003;143-144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- 33.Chhatwal GS. Anchorless adhesins and invasins of Gram-positive bacteria: A new class of virulence factors. Trends Microbiol. 2002;10:205–208. doi: 10.1016/s0966-842x(02)02351-x. [DOI] [PubMed] [Google Scholar]
- 34.Barbour ML, Mayon-White RT, Coles C, Crook DW, Moxon ER. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis. 1995;171:93–98. doi: 10.1093/infdis/171.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Dagan R, et al. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J Infect Dis. 2005;192:367–376. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 36.Schaffer AC, et al. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun. 2006;74:2145–2153. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012;31:501–508. doi: 10.1097/INF.0b013e31824de9f6. [DOI] [PubMed] [Google Scholar]
- 38.Malley R, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001;69:4870–4873. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.