Abstract
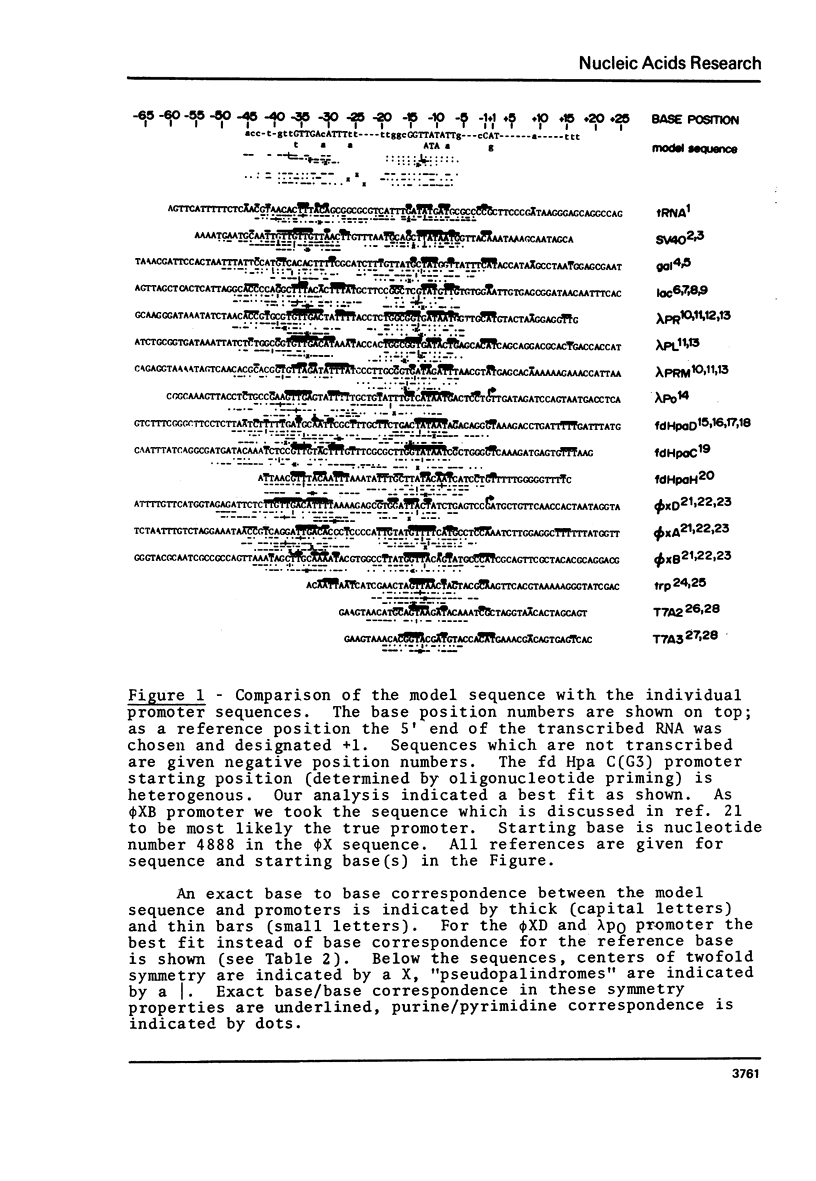
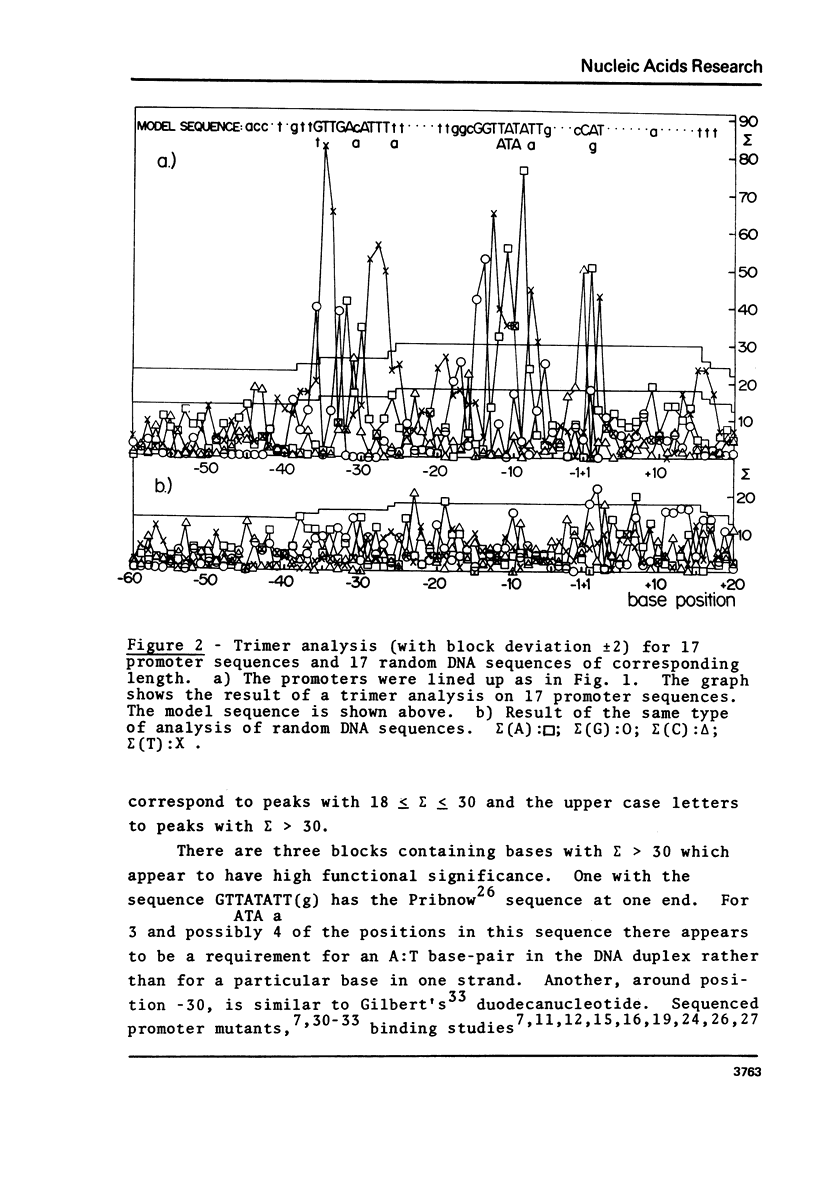
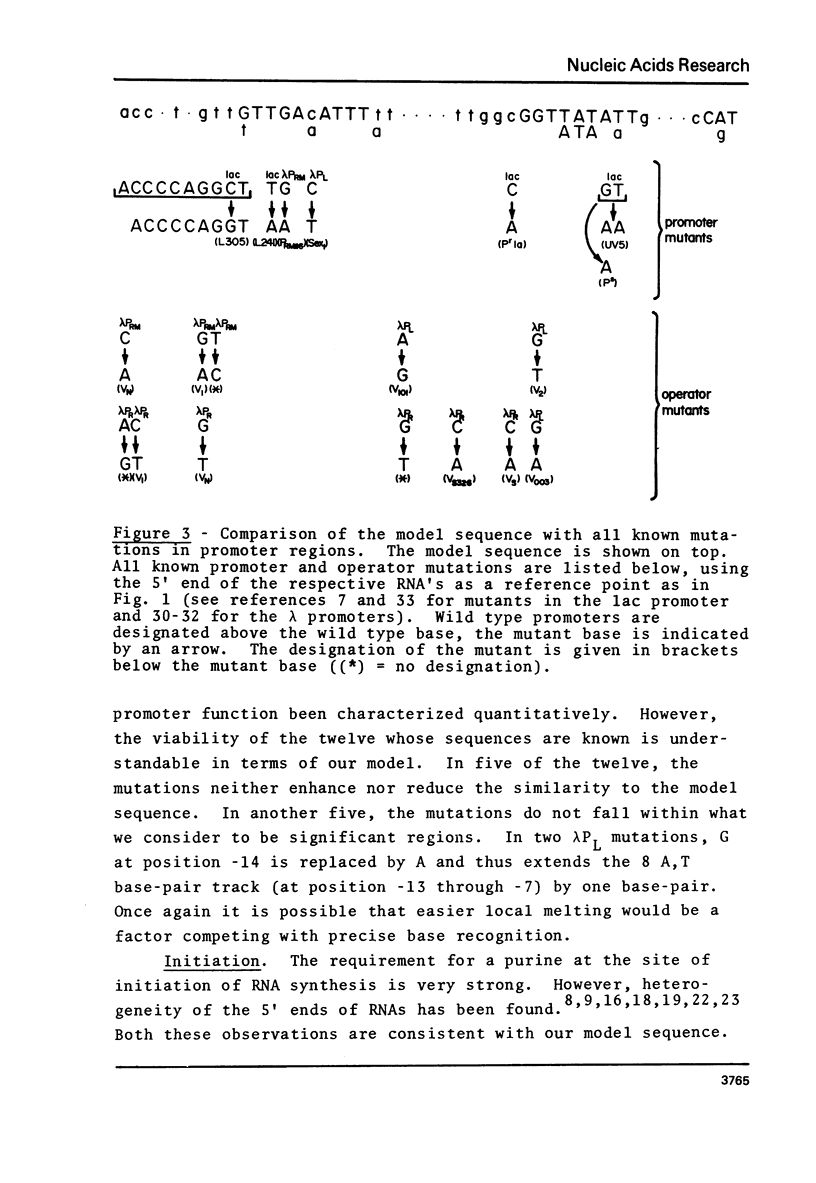
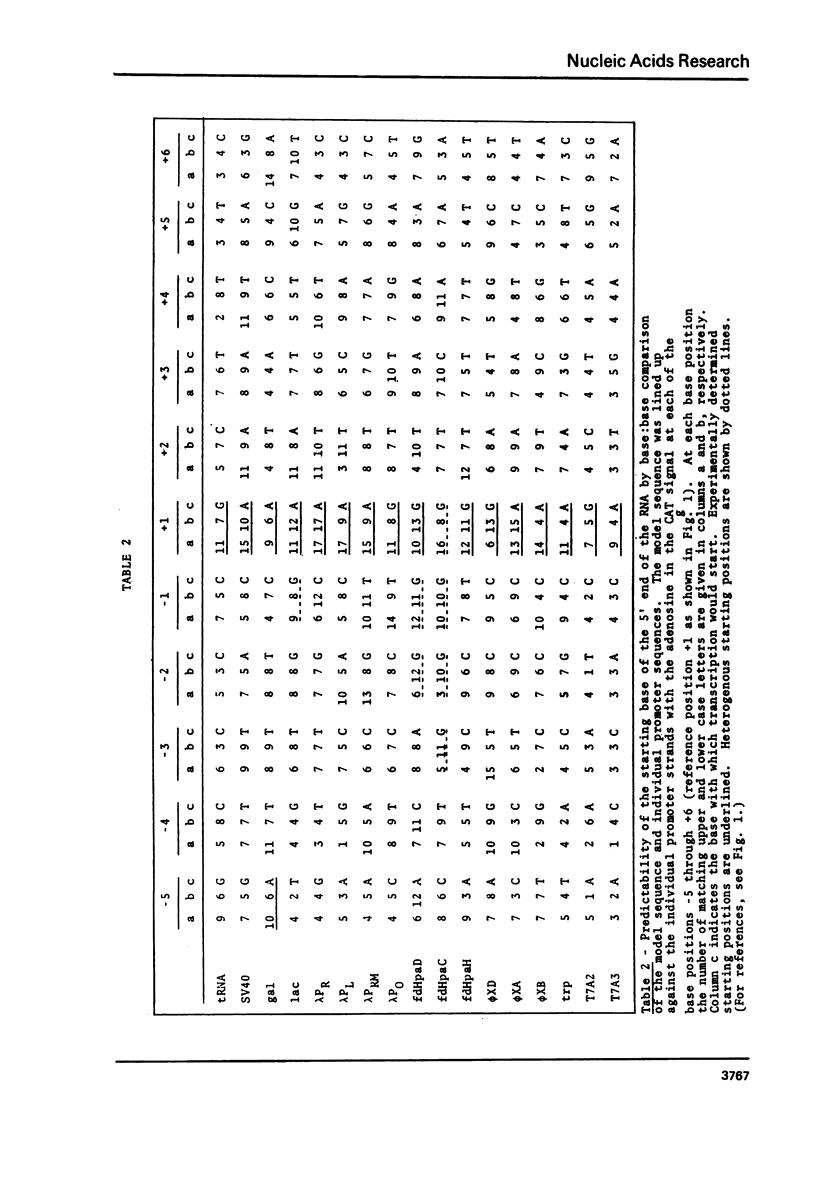
A novel computer procedure has been used to search for homology among 17 known procaryotic promoter sequences. A model sequence, :formula: (see text), is compatible with the properties of all known promoter and operator mutations, predicts base positions for the initiation of RNA synthesis coinciding with those determined experimentally, is compatible with current models for the regulation of transcription, suggests that RNA polymerase could recognize the DNA double helix firstly in the B conformation then in the A.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Roberts R. J., Gesteland R. F., Solem R. Class of promotor sites for Escherichia coli DNA-dependent RNA polymerase. Nature. 1974 May 17;249(454):217–221. doi: 10.1038/249217a0. [DOI] [PubMed] [Google Scholar]
- Arditti R., Grodzicker T., Beckwith J. Cyclic adenosine monophosphate-independent mutants of the lactose operon of Escherichia coli. J Bacteriol. 1973 May;114(2):652–655. doi: 10.1128/jb.114.2.652-655.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Bond P. J., Selsing E., Smith P. J. Models of triple-stranded polynucleotides with optimised stereochemistry. Nucleic Acids Res. 1976 Oct;3(10):2459–2470. doi: 10.1093/nar/3.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Fuller W., Hodgson A., Prutton I. Molecular conformations and structure transitions of RNA complementary helices and their possible biological significance. Nature. 1968 Nov 9;220(5167):561–564. doi: 10.1038/220561a0. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Transcription of bacteriophage phi-X174 in vitro: selective initiation with oligonucleotides. J Mol Biol. 1976 Dec 25;108(4):753–770. doi: 10.1016/s0022-2836(76)80115-5. [DOI] [PubMed] [Google Scholar]
- Bautz E. K. Ferdinaud Springer Lecture: initiation of transcription by RNA polymerases of E. coli and phage T3. FEBS Lett. 1973 Oct 15;36(2):123–129. doi: 10.1016/0014-5793(73)80352-7. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Brown K. D., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):139–152. doi: 10.1016/s0022-2836(78)80002-3. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of region preceding trp mRNA initiation site and its role in promoter and operator function. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2351–2355. doi: 10.1073/pnas.73.7.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):113–137. doi: 10.1016/s0022-2836(78)80001-1. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Yanofsky C. Sequence analysis of operator constitutive mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):179–192. doi: 10.1016/s0022-2836(78)80004-7. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E. RNA synthesis startpoints in bacteriophage lambda: are the promoter and operator transcribed? Nat New Biol. 1972 Jun 21;237(77):227–232. doi: 10.1038/newbio237227a0. [DOI] [PubMed] [Google Scholar]
- Bram S., Tougard P. Polymorphism of natural DNA. Nat New Biol. 1972 Oct 4;239(92):128–131. doi: 10.1038/newbio239128a0. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Bennett G. N., Lee F., Schweingruber M. E., Yanofsky C. RNA polymerase interaction at the promoter--operator region of the tryptophan operon of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):153–177. doi: 10.1016/s0022-2836(78)80003-5. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J. A proposed model for interaction of polypeptides with RNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):283–287. doi: 10.1073/pnas.71.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Sussman J. L., Kim S. H. Secondary structural complementarity between DNA and proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1458–1462. doi: 10.1073/pnas.74.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Dykes G., Bambara R., Marians K., Wu R. On the statistical significance of primary structural features found in DNA-protein interaction sites. Nucleic Acids Res. 1975 Mar;2(3):327–345. doi: 10.1093/nar/2.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentiev V. L., Ivanov V. I. RNA polymerase: two-step mechanism with overlapping steps. Nature. 1970 Nov 7;228(5271):519–522. doi: 10.1038/228519a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden B., Nüsslein C., Schaller H. Initiation of transcription within an RNA-polymerase binding site. Eur J Biochem. 1975 Jun 16;55(1):147–155. doi: 10.1111/j.1432-1033.1975.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Kleid D., Humayun Z., Jeffrey A., Ptashne M. Novel properties of a restriction endonuclease isolated from Haemophilus parahaemolyticus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):293–297. doi: 10.1073/pnas.73.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Backman K., Kield D., Flashman S., Jeffrey A., Maurer R. Recognition sequences of repressor and polymerase in the operators of bacteriophage lambda. Cell. 1975 Jun;5(2):109–113. doi: 10.1016/0092-8674(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Maurer R., Maniatis T., Ptashne M. Promoters are in the operators in phage lambda. Nature. 1974 May 17;249(454):221–223. doi: 10.1038/249221a0. [DOI] [PubMed] [Google Scholar]
- Meyer B. J., Kleid D. G., Ptashne M. Lambda repressor turns off transcription of its own gene. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4785–4789. doi: 10.1073/pnas.72.12.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., de Crombrugghe B., Pastan I., Sklar J., Yot P., Weissman S. The 5'-terminal nucleotide sequence of galactose messenger ribonucleic acid of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4940–4944. doi: 10.1073/pnas.71.12.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R., Di Lauro R., Rosenberg M., de Crombrugghe B. Nucleotide sequence of the operator-promoter region of the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jan;74(1):106–110. doi: 10.1073/pnas.74.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Studies on the mechanism of action of the gal repressor. J Biol Chem. 1973 Sep 10;248(17):5937–5942. [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Hobom G., Kössel H. DNA base sequence of the po promoter region of phage lamdba. Nature. 1977 Jan 13;265(5590):117–121. doi: 10.1038/265117a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Gait M. J., Noris K., Ramamoorthy B., Khorana H. G. The nucleotide sequence in the promoter region of the gene for an Escherichia coli tyrosine transfer ribonucleic acid. J Biol Chem. 1976 Aug 10;251(15):4481–4489. [PubMed] [Google Scholar]
- Smith L. H., Sinsheimer R. L. The in vitro transcription units of bacteriophage phiX174. III. Initiation with specific 5' end oligonucleotides of in vitro phiX174 RNA. J Mol Biol. 1976 Jun 5;103(4):711–735. doi: 10.1016/0022-2836(76)90205-9. [DOI] [PubMed] [Google Scholar]
- Squires C., Lee F., Bertrand K., Squires C. L., Bronson M. J., Yanofsky C. Nucleotide sequence of the 5' end of tryptophan messenger RNA of Escherichia coli. J Mol Biol. 1976 May 15;103(2):351–381. doi: 10.1016/0022-2836(76)90317-x. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Ptashne M. In vitro repression of RNA synthesis by purified lambda phage repressor. Nat New Biol. 1971 Mar 17;230(11):76–80. doi: 10.1038/newbio230076a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Okamoto T., Sugisaki H., Takanami M. The nucleotide sequence of an RNA polymerase binding site on bacteriophage fd DNA. Nature. 1975 Feb 6;253(5491):410–414. doi: 10.1038/253410a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. III. Nucleotide sequence preceding the RNA start-site on a promoter-containing fragment. Nucleic Acids Res. 1975 Nov;2(11):2091–2100. doi: 10.1093/nar/2.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami M., Sugimoto K., Sugisaki H., Okamoto T. Sequence of promoter for coat protein gene of bacteriophage fd. Nature. 1976 Mar 25;260(5549):297–302. doi: 10.1038/260297a0. [DOI] [PubMed] [Google Scholar]
- Walz A., Pirrotta V., Ineichen K. Lambda repressor regulates the switch between PR and Prm promoters. Nature. 1976 Aug 19;262(5570):665–669. doi: 10.1038/262665a0. [DOI] [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Jacobsen J. H., Saucier J. M. Physiochemical studies on interactions between DNA and RNA polymerase. Unwinding of the DNA helix by Escherichia coli RNA polymerase. Nucleic Acids Res. 1977;4(5):1225–1241. doi: 10.1093/nar/4.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C. K., Schaller H. RNA polymerase-promotor complex stability on supercoiled and relaxed DNA. FEBS Lett. 1977 Mar 1;74(2):215–219. doi: 10.1016/0014-5793(77)80849-1. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Ghosh S., Echols H., Spiegelman W. G. Repression by the cI protein of phage lambda: in vitro inhibition of RNA synthesis. J Mol Biol. 1972 Jun 28;67(3):407–421. doi: 10.1016/0022-2836(72)90459-7. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Weissman S. M., Dhar R., Pan J. The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 1. The sequence of the late strand transcript. Nucleic Acids Res. 1974 Apr;1(4):577–594. doi: 10.1093/nar/1.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]



